Abstract
Interleukin (IL)-10 is one of the most crucial immunoregulatory cytokines. Its short-term effects have been analysed extensively, but little is known about its long-term effects. This is of considerable importance, as high systemic IL-10 levels are present for long periods in patients with persistent viral infections, certain cancers and in critical care patients. Our study investigated the effects of the long-term presence of IL-10 on human peripheral blood monocytes. In vitro, IL-10 treatment of these cells for 7 days induced the development of a novel cell type characterized by unique phenotypical and functional characteristics. These cells showed high HLA-DR expression and low expression of CD86 and other co-stimulatory molecules on their surface. The mRNA levels of both HLA-DR and CD86 were high, but no intracellular accumulation of CD86 protein was observed. With respect to its function, these cells showed strongly diminished tumour necrosis factor-α production following lipopolysaccharide stimulation, strongly diminished allogenic CD4+ T cell stimulatory capacity, and even induced a hyporesponsive state in CD4+ T cells. The phenotype remained stable despite the removal of IL-10. In vivo, we found monocytic cells from patients exhibiting this phenotype after long-term IL-10 exposure. These results complement our knowledge further about the biological effects of IL-10 and may provide an explanation for the sustained immunodeficiency in cases of the persistent presence of systemic IL-10.
Keywords: cell differentiation, cytokines, human, monocytes/macrophages
Introduction
Interleukin (IL)-10 is one of the most crucial immunoregulatory cytokines. It was discovered in 1989 as a protein suppressing cytokine synthesis in murine Th1 cells [1]. Subsequent studies showed that IL-10 mediates a broad spectrum of activities on various cell types, whereby peripheral blood monocytes are one of the most important target cells [2,3]. Peripheral blood monocytes circulate typically in the bloodstream for 1 or 2 days before entering the tissue. Depending on the tissue-specific cytokine environment they either differentiate into macrophages or dendritic cells (DCs). Macrophages and DCs are essential mediators of non-adaptive and adaptive immune responses. The short-term presence of IL-10 (over hours) rapidly down-regulates the cell surface expression of major histocompatibility complex (MHC) class II and B7 co-stimulatory molecules such as CD86 and CD80 on monocytes and macrophages [4,5]. MHC class II molecules as well as CD86 and CD80, among others, are essential for the activation of naive CD4+ T cells and the initiation of an adaptive immune response. Moreover, IL-10 inhibits the production of soluble mediators such as the proinflammatory cytokines tumour necrosis factor (TNF)-α, IL-1β, IL-6 and IL-12 or the CXC chemokine ligand 8 (CXCL8) [6,7]. At the same time, IL-10 enhances the cell surface expression of CD14 and CD163 as well as of the Fcγ-receptors CD16, CD32 and CD64, accompanied by the up-regulation of phagocytosis of bacteria and apoptotic cells [8–10].
Numerous in vitro and in vivo experiments have demonstrated the effects of short-term IL-10 on the immune system [2]. However, several diseases are characterized by the long-term presence of IL-10. For example, persistently elevated systemic IL-10 levels are observed in patients with certain malignancies, such as some kinds of carcinoma, melanoma and lymphoma; in patients with chronic viral infections such as hepatitis B virus (HBV), Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV) infections, as well as in some intensive care unit (ICU) patients [11–17]. The long-lasting and predominant expression of IL-10 is believed to be largely responsible for the reduced immunocompetence seen in these patients [18–20].
In the present study, we investigated the in vitro effects of long-term IL-10 on human peripheral blood monocytes. We demonstrate that long-term IL-10 treatment leads to the generation of a new cell type with unique phenotypic and functional characteristics, which may explain parts of its biological effects.
Materials and methods
Cell isolation and cell culture
Human peripheral blood mononuclear cells (PBMCs) were obtained from voluntary healthy donors' venous blood by standard density gradient centrifugation using Ficoll-Paque (Pharmacia, Freiburg, Germany). Monocytes and CD4+ T cells were isolated from PBMCs using magnetic affinity cell sorting (MACS) systems not touching the relevant cell types (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of isolated cells was checked routinely and was greater than 90% for monocytes and greater than 95% for CD4+ T cells. Cells were cultured at 37°C in RPMI-1640 medium tested for very low (< 0·01 EU/ml) endotoxin content and supplemented with 10% (v/v) fetal bovine albumin and 2 mM glutamine (all reagents from Biochrom KG, Berlin, Germany) at a concentration of 1 × 106 cells per ml (PBMCs) or 2 × 105 cells per ml (monocytes), if not indicated otherwise. In all experiments, cell adherence was inhibited by coating the culture vessels with 100 ml/cm2 of 1% (w/v) poly (2-hydroxyethylmethacrylate) (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) prior to usage as described in the manufacturer's protocol. Cell vitality was checked at the respective study end-points by means of trypan blue (BioWhittaker, Walkersville, MD, USA) exclusion and was always greater than 93%.
Generation of different monocyte-derived antigen-presenting cells (APCs)
In order to generate different monocyte-derived populations of APCs, isolated monocytes were cultured for 7 days with either IL-10 (generation of IL-10 APCs) or with macrophage colony-stimulating factor (M-CSF) (generation of macrophages) or with IL-4 plus granulocyte–macrophage colony-stimulating factor (GM-CSF) (generation of DCs). In order to analyse the stability of the IL-10 APC type, monocytes were incubated with IL-10 for 3 and 7 days, respectively, followed by extensive washing and division into two groups: one group was recultured with IL-10 as indicated, the other group was recultured without further IL-10 administration. All cytokines (recombinant human IL-10, M-CSF, IL-4 and GM-CSF) used in our studies were purchased from R&D Systems (Wiesbaden, Germany) and applied at 10 ng/ml. In the case of cell cultivation longer than 3 days, 50% of the medium with the respective supplements was replaced at days 3 and 5.
Analysis of lipopolysaccharide (LPS)-induced cytokine production
For determination of the cytokine production capacity of the various APC populations, cells were stimulated with 100 ng/ml LPS from Eschericha coli 0127 B8 (Sigma-Aldrich Chemie GmbH) for 4 or 24 h, respectively. Prior to stimulation, cells were washed to remove the respective cytokine supplements and cell numbers were re-adjusted to 2 × 105 cells per ml. Corresponding controls cultured without LPS were always carried out. The following cytokines were analysed: TNF-α (4 h), IL-12 p40 (24 h) and IL-10 (24 h).
Influence of IL-10 APCs on CD4+ T cell responses against alloantigen
To analyse further the functional state of IL-10 APCs, their capacity to stimulate allogenic CD4+ T cells was tested in a mixed lymphocyte reaction measuring allogenic CD4+ T cell proliferation. Prior to stimulation, isolated CD4+ T cells were stained with carboxyfluorescein succinimidyl ester (CFSE), according to the manufacturer's instructions (Molecular Probes, Inc., Eugene, OR, USA). CFSE-labelled CD4+ T cells (2 × 105 cells/well) were stimulated with IL-10 APCs or respective control APCs at APC : CD4+ T cell ratios of 1 : 100, 1 : 30 or 1 : 10 in flat-bottomed 96-well plates. Additionally, tests were repeated in the presence of cytotoxic T lymphocyte antigen-4 immunoglobulin (CTLA-4-IgG) (10 μg/ml; R&D Systems). CTLA-4 is a ligand for CD86 and CD80 and blocks CD86- and CD80-mediated T cell co-stimulation by APCs. Proliferation of CD4+ T cells was determined by flow cytometry after 6 days.
To analyse whether IL-10 APCs induce a hyporesponsive state in allogenic CD4+ T cells, isolated allogenic CD4+ T cells were cultured at 1 × 106 cells/ml with IL-10 APCs or control APCs at an APC : CD4+ T cell ratio of 1 : 100 for 3 days followed by restimulation with freshly prepared IL-10 APCs or control APCs from the same donor for another 3 days. Then, CD4+ T cells were re-isolated, stained with CFSE and stimulated with freshly isolated third-party monocytes in 96-well plates for up to 6 days (1 × 106 CD4+ T cells/well, APC : CD4+ T cell ratios tested: 1 : 1000, 1 : 300, 1 : 100, 1 : 30, 1 : 10). CD4+ T cell proliferation was determined by flow cytometric analysis after 4 and 6 days of culture. GM-CSF and interferon (IFN)-γ production were measured in the supernatants after 6 days of culture.
Flow cytometric analysis
The phenotypic characterization of cell surface molecule expression was performed by flow cytometry as described previously [21,22] using the following monoclonal antibodies: fluorescein isothiocyanate (FITC)-anti-CD14 (clone M5E2; Becton Dickinson, Heidelberg, Germany), FITC-anti-CD16 (clone 3G8; Coulter Immunotech, Hamburg, Germany), FITC-anti-CD64 (clone 22; Coulter Immunotech), FITC-anti-CD71 (clone YDJ.1·2.2.; Coulter Immunotech), FITC-anti-CD80 (clone BB1; Becton Dickinson), FITC-anti-CD86 (clone 2331 (FUN-1); Becton Dickinson), FITC-anti-human leucocyte antigen (HLA)-A/B/C (cloneG46-2·6; Becton Dickinson); phycoerythrin (PE)-anti-CD1A (clone BL6; Coulter Immunotech), PE-anti-CD40 (clone 5C3; Becton Dickinson), PE-anti-CD86 (clone 23311; Pharmingen GmbH, Hamburg, Germany), PE-anti-HLA-DR (clone L243; Becton Dickinson); R-phycoerythrin-cyanin-labelled (PC5)-anti-CD14 (clone RMO52; Coulter Immunotech) or the respective isotype controls: mouse FITC-IgG1 (clone 679·1Mc7; Coulter Immunotech), mouse FITC-IgM/κ (clone G155-228; Becton Dickinson), mouse FITC-IgG2a, κ (clone G155-178, Becton Dickinson), mouse PE-IgG1/κ (clone G18-145; Pharmingen GmbH) and mouse PE-IgG2a/κ (clone X39; Becton Dickinson). Ten thousand (monocytes or CD4+ T cells, respectively) to 30 000 (PBMCs) live cells (scatter properties) per measurement were analysed on a FACScan flow cytometer (Becton Dickinson). Monocyte gating was performed using CD14 staining and side-scatter properties.
For flow cytometric measurement of intracellular CD86 expression, CD86 surface molecules were blocked by incubation with unlabelled anti-CD86 monoclonal antibody (mAb) (clone 23311) at 100 times the concentration used for cell surface staining for 20 min at room temperature followed by fixation with 2% paraformaldehyde for another 20 min at room temperature. For permeabilization, cells were washed twice in phosphate-buffered saline (PBS), resuspended in 0·5 ml FACS permeabilizing solution 2 (Becton Dickinson), and incubated for 20 min at room temperature. They were then washed twice in PBS, resuspended in the same buffer and stained for another 20 min at 4°C with anti-CD86-FITC mAb (clone 23311) followed by washing with PBS prior to flow cytometric analysis.
Flow cytometric analysis of monocytic HLA-DR and CD86 expression in ICU patients and in psoriatic patients treated with recombinant human IL-10
From 20 March to 20 December 2000, ethylenediamine tetraacetic acid (EDTA) anti-coagulated blood samples from all ICU patients of the surgical ICU unit at the Charité Hospital, Berlin, who underwent immunological routine analysis at the Institute of Medical Immunology, Charité, Berlin, were analysed flow cytometrically for monocytic HLA-DR and CD86 expression using the Quantibrite system, as described recently [23]. Altogether, 1344 samples were analysed. The study was approved by the Institutional Review Board of the Medical Faculty.
Blood samples anti-coagulated with EDTA from 10 adult patients with moderate to severe chronic plaque psoriasis undergoing IL-10 therapy were taken 5 days prior to IL-10 therapy and at days 4, 50 and 57 after the initiation of IL-10 therapy. Monocytic HLA-DR and CD86 expression was measured flow cytometrically as described above. Patients received subcutaneous applications of recombinant human IL-10 (SCH 52000; provided by Essex Pharma, Munich, Germany/Schering Plough Research Institute, Kenilworth, NJ, USA) at either 8 μg per kg per day (n = 5) or 20 μg per kg three times per week (n = 5) over a period of 49 days. The study was approved by the Institutional Review Board of the Medical Faculty, and written informed consent was obtained from all patients. Histological, immunohistological and molecular biology findings of this study have been published previously [24].
Cytokine quantification assays
Detection of TNF-α, IL-12 p40 and IL-10 in cell culture supernatants was conducted using respective commercially available ELISA kits from BioSource Europe SA (Nivelles, Belgium). Detection of GM-CSF and IFN-γ was conducted with the protein analysis system Bio-Plex (Bio-Rad, Munich, Germany).
Quantitative gene expression analysis
Total RNA from the cultured monocytes and from the new cell type was prepared using Invisorb RNA Kit II (Invitec, Berlin, Germany). Reverse transcription of mRNA was performed as follows: 0·05 mg/ml total RNA of each sample were incubated in the presence of 5 ng/ml oligo-d(T)12–18 primers (Gibco brl, Eggenstein, Germany) at 75°C for 5 min and then cooled in an ice bath. After addition of the first-strand buffer (Gibco brl), 1 unit/ml RNasin ribonuclease inhibitor (Promega, Mannheim, Germany), 10 mM dithiothreitol (Gibco brl), 250 mM deoxyadenosine triphosphate (dATP), thymidine triphosphate (dTTP), deoxycytidine triphosphate (dCTP) and deoxyguanine triphosphate (dGTP) (Pharmacia Biotech, Uppsala, Sweden) and 5 units/ml of Moloney murine leukaemia virus reverse transcriptase (Gibco brl), a reaction took place at 42°C for 60 min and was stopped by heating to 95°C for 5 min. The samples were then analysed by TaqMan polymerase chain reaction (PCR) using TaqMan universal master mix (Applied Biosystems, Weiterstadt, Germany) in the ABI Prism 7700 Sequence Detector System (Perkin-Elmer Cetus, Foster City, CA, USA), as described previously [25,26]. The sequences and concentrations of primers and FAM/TAMRA double-labelled probes were as follows: HLA-DR: forward 5′-CTT ggA TgA gCC TCT TCT CAA gC-3′, 50 nM; reverse 5′-CAC CAC gTT CTC TgT AgT CTC Tg-3′, 50 nM; FAM-5′-ACT ggg AgT TTg ATg CTC CAA gCC CTC-3′-TAMRA, 200 nM; CD 86: forward 5′-ggA AAT ggA AgA AgA AgA AgC g-3′, 300 nM; reverse 5′-gTC TgT TCA CTC TCT TCC CTC TCC-3′, 300 nM; FAM-5′-CCT CgC AAC TCT TAT AAA TgT ggA ACC AAC ACA-3′-TAMRA, 200 nM; hypoxanthine phosphoribosyl-transferase 1: forward 5′-AgT CTg gCT TAT ATC CAA CAC TTC g-3′, 300 nM; reverse 5′-gAC TTT gCT TTC CTT ggT CAg g-3′, 300 nM; FAM-5′-TTT CAC CAg CAA gCT TgC gAC CTT gA-3′-TAMRA, 50 nM.
HLA-DR and CD86 expression data were calculated relative to that of the house-keeping gene hypoxanthine phosphoribosyl-transferase 1.
Statistical analysis
Statistical analyses were performed by Wilcoxon matched-pairs signed-ranks test using SPSS software. P-values < 0·05 were considered as statistically significant.
Results
Effects of long-term IL-10 treatment on human peripheral blood monocytes
To analyse the effects of long-term IL-10 treatment on peripheral blood monocytes we cultivated human PBMCs with or without (control group) IL-10 for up to 7 days. At 6 h, 24 h, 3 days and 7 days we determined the monocytic expression of various surface molecules and the capacity to produce LPS-induced TNF-α. As described in earlier studies [4–7], we observed a down-regulation of HLA-DR and CD86 expression and an inhibition of LPS-induced TNF-α production after 6 and 24 h following IL-10 treatment (short-term IL-10 effects, Fig. 1). Interestingly, whereas CD86 expression and LPS-induced TNF-α production remained suppressed until the end of the 7-day treatment, HLA-DR increased with time (Fig. 1). This resulted in a unique monocytic phenotype not described previously with bright HLA-DR but with dim CD86 surface expression. We analysed further the influence of IL-10 on the monocytic expression of IgG receptors (CD16, CD64) and the differentiation parameter CD71. As shown in Fig. 1, IL-10 led to a continuous increase in CD16 and CD64 expression until the end of the treatment and up-regulated CD71 expression until day 3.
Fig. 1.
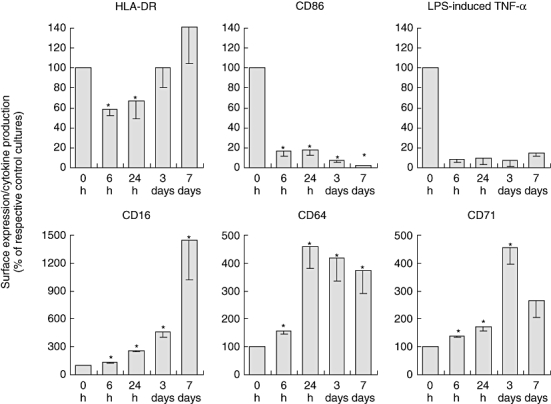
Effects of interleukin (IL)-10 treatment on monocytic cell surface marker and lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α secretion. Peripheral blood mononuclear cells (PBMCs) were treated with or without IL-10 (respective control cultures) for 6 h, 24 h, 3 days and 7 days. In 7-day-treated cultures 50% of the medium with the respective supplements was replaced at days 3 and 5. Mean monocytic cell surface expression of HLA-DR, CD86, CD16, CD64 and CD71 was analysed by flow cytometry and values of monocytes treated with IL-10 were related to respective values of control monocytes not treated with IL-10, which were set to 100%. TNF-α concentration was measured in culture supernatants after stimulation of PBMCs with LPS (100 ng/ml) for 4 h and values were related to respective values of control monocytes not treated with IL-10, which were set to 100%. Original fluorescence and TNF-α data can be seen in Fig. S1 (Supplementary material). Data from five independent experiments are given in relation to respective controls as the mean ± standard error of the mean (*P < 0·05 compared to respective control by Wilcoxon matched-pairs signed-ranks test).
Analysis of HLA-DR and CD86 mRNA expression and intracellular CD86 protein expression in IL-10 treated monocytes
In order to analyse the mechanisms underlying the different regulations of HLA-DR (increased) and CD86 (decreased) surface expression, we analysed the respective mRNA levels relative to the expression of the house-keeping gene hypoxanthine phosphoribosyl-transferase 1 in isolated monocytes after 7 days of IL-10 treatment. As shown in Fig. 2a, the expression levels of both HLA-DR as well as CD86 mRNA were increased in IL-10-treated monocytes compared to respective control cells. With regard to HLA-DR, the increased mRNA expression (Fig. 2) was in line with the slightly, although not statistically significantly, increased surface protein expression (Fig. 1). With respect to CD86, a discrepancy was found between the increased levels of CD86 mRNA (Fig. 2) and the strongly decreased cell surface protein expression (Fig. 1). We therefore analysed the intracellular levels of CD86 protein expression in comparison to its extracellular levels. In control monocytes cultured for 7 days without IL-10, we found about four times more CD86 molecules to be located at the cell surface compared to those located intracellularly. In IL-10 treated monocytes, no difference between intracellular and surface levels of the CD86 protein was observed and intracellular CD86 protein levels were comparable to respective control monocytes. These results let us assume that the low CD86 surface expression on 7-day IL-10-treated monocytes was not based on transcriptional regulation or accumulation of intracellular CD86 protein but may result from other mechanisms, such as inhibited translation or increased degradation of the protein.
Fig. 2.

Mechanism of regulation of HLA-DR and CD86 expression in long-term, interleukin (IL)-10-treated monocytes. Negatively separated monocytes were cultured for 7 days with or without (control) IL-10. (a) The mRNA expression of HLA-DR and CD86 was analysed by quantitative polymerase chain reaction. Data from six donors are presented as the fold expression of the house-keeping gene hypoxanthine phosphoribosyl-transferase 1 (HPRT). (b) Seven-day cultured monocytes were analysed flow cytometrically with respect to surface CD86 expression paralleled by intracellular measurement of CD86 protein expression. Individual data from three donors are shown (*P < 0·05 by Wilcoxon matched-pairs signed-ranks test).
Phenotypic and functional stability of long-term IL-10-treated monocytic cells
We then questioned the phenotypic stability of long-term IL-10-treated monocytes. To address this question, we removed IL-10 by washing at days 3 or 7, respectively, recultivated the cells with or without IL-10 for another 24 h followed by analysis of surface HLA-DR and CD86 expression. Removal of IL-10 at day 3 as well as at day 7 resulted in slightly more elevated monocytic HLA-DR expression (data not shown). With respect to CD86, IL-10 withdrawal at day 3 led to a complete recovery of CD86 expression (Fig. 3a) whereas at day 7, despite IL-10 withdrawal, CD86 expression remained down-regulated (Fig. 3b). For 7-day IL-10-treated monocytes we extended the time of recultivation in the absence of IL-10 for up to 2 additional days. We observed a slow progressive increase of CD86 expression, and after 3 days CD86 levels reached 60% of the expression in the control group (data not shown). These data showed that 7-day IL-10-treated peripheral blood monocytes constitute a novel and, with respect to HLA-DR and CD86, stable phenotype characterized by HLA-DR bright and CD86 dim expression. We named these cells ‘IL-10 APCs’.
Fig. 3.

The long-lasting presence of interleukin (IL)-10 induced a stable monocyte-derived phenotype. Peripheral blood mononuclear cells (PBMCs) were treated with or without IL-10 (control). After (a) 3 or (b) 7 days of treatment, cells were washed extensively. The control group was recultured without IL-10 for another 24 h, and the IL-10 treated group was divided into two subgroups and recultured with and without IL-10, respectively, for another 24 h. Cell surface expression of CD86 was then determined by flow cytometry. Data from six independent experiments are shown as the mean ± standard error of the mean (*P < 0·05 by Wilcoxon matched-pairs signed-ranks test).
Phenotypic and functional analysis of the IL-10 APCs in comparison to other monocyte-derived APCs
In the following experiments, we characterized the phenotype and function of IL-10 APCs in comparison to monocyte-derived macrophages and monocyte-derived DCs. In addition to HLA-DR and CD86, we analysed the surface expression of CD80 and CD40 (co-stimulatory molecules), HLA-A/B/C (MHC class I proteins), CD14 (LPS receptor), CD16 (Fcγ receptor III) and CD1a (myeloid DC marker). As demonstrated in Fig. 4a, the outstanding features of IL-10 APCs were strong HLA-DR expression and weak CD86 expression. The other co-stimulatory molecules were either not (CD80) or weakly expressed (CD40). The MHC class I molecule expression was comparable with that shown by DCs. Similar to macrophages (MΦ), IL-10 APCs were CD14- and CD16-positive and expressed only minimal amounts of CD1a.
Fig. 4.

Phenotypic and functional characterization of long-term interleukin (IL)-10 treated monocytes in comparison to freshly prepared monocytes, monocyte-derived macrophages and monocyte-derived dendritic cells (DCs). Negatively separated monocytes were used either immediately for measurement of the respective parameters or were cultured for 7 days with either IL-10 (10 ng/ml, generation of IL-10 APCs), macrophage colony-stimulating factor (M-CSF) (10 ng/ml, generation of MΦ) or IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) (10 ng/ml each, generation of DCs). (a) Surface molecule expression was measured by flow cytometry. Data from three experiments are shown as the mean ± standard error of the mean. (b) All cell populations were washed extensively and cytokine secretion was measured after 4 h [tumour necrosis factor (TNF)-α] or 24 h (IL-12 p40, IL-10) of culture with or without lipopolysaccharide (100 ng/ml). Data from three experiments are shown as the mean ± standard error of the mean.
For functional characterization, we analysed LPS-induced cytokine production. Following LPS stimulation, IL-10 APCs could produce TNF-α and IL-10, but at amounts less than monocytes or MΦ (Fig. 4b). Like monocytes and MΦ, IL-10 APCs secreted only minimal amounts of IL-12 p40. As expected, the main producers of IL-12 p40 were DC.
Influence of the IL-10 APCs on CD4+ T cell responses against alloantigen
To test the accessory function of IL-10 APCs we first analysed their capacity to activate allogenic CD4+ T cells. As shown in Fig. 5, in comparison to respective control APCs allogenic CD4+ T cell proliferation induced by IL-10 APCs was clearly diminished. Interestingly, proliferation induced by control APCs could be reduced to the same extent as by IL-10 APCs via addition of CTLA-4-IgG blocking the surface CD80/CD86 molecules (Fig. 5). Proliferation induced by IL-10 APCs could be abrogated completely by co-incubation with CTLA-4-IgG (data not shown).
Fig. 5.

Interleukin (IL)-10 APCs induced diminished allogenic CD4+ T cell proliferation. Negatively separated monocytes were cultured for 7 days with (b) or without (a, c) IL-10 to generate IL-10 antigen-presenting cells (APCs) (b) and control APCs (a, c), respectively. These APCs were then used as stimulator cells in mixed leucocyte reaction to activate allogenic, negatively separated, carboxyfluorescein succinimidyl ester (CFSE)-labelled CD4+ T cells at APC : T cell ratios of 1 : 10, 1 : 30 and 1 : 100. In (c), stimulation of CD4+ T cells with control APCs was carried out in the presence of cytotoxic T lymphocyte antigen-4 immunoglobulin. Proliferation was determined by flow cytometry after 6 days. Proliferation of cells is marked by the loss of CFSE staining intensity. Results are representative of three independent experiments.
Secondly, we tested whether IL-10 APCs could induce a hyporesponsive state in allogenic CD4+ T cells. For this, we repeatedly incubated allogenic CD4+ T cells with IL-10 APCs followed by reseparation of the CD4+ T cells prior to stimulation with third-party alloantigen. Third-party alloantigen induced proliferation and production of GM-CSF and IFN-γ were analysed. As shown in Fig. 6, in contrast to respective control experiments repeated preincubation of CD4+ T cells with IL-10 APCs led to a hyporesponsive state of these cells demonstrated by diminished third-party alloantigen induced proliferation (Fig. 6a) as well as diminished GM-CSF and IFN-γ secretion (Fig. 6b).
Fig. 6.

Interleukin (IL)-10 antigen-presenting cells (APCs) induced a hyporesponsive state in allogenic CD4+ T cells. Negatively separated monocytes were cultured for 7 days with or without IL-10 to generate IL-10 APCs and respective control APCs. Purified CD4+ T cells were activated with IL-10 APCs or control APCs at an APC : T cell ratio of 1 : 100 for 3 days and were re-isolated and co-cultured under identical culture conditions with freshly prepared IL-10 APCs and control APCs from the same donor. The CD4+ T cells were then separated again, labelled with carboxyfluorescein succinimidyl ester and restimulated with freshly prepared third-party allogenic monocytes at the indicated APC : T cell ratios. (a) After 4 and 6 days of culture, proliferation of CD4+ T cells was determined by flow cytometry. (b) After 6 days of culture, the amount of granulocyte–macrophage colony-stimulating factor and interferon-γ in the culture supernatants was measured by the protein analysis system Bio-Plex. Data from one donor are shown.
Indications for the in vivo existence of IL-10 APCs
First indications for the in vivo existence of IL-10 APCs were found in surgical ICU patients with diminished immunocompetence [23]. In about 1% of the analysed blood samples (15 of 1344, data not shown) from these patients we found monocytic cells with the IL-10 APC phenotype.
Furthermore, in two of 10 psoriatic patients treated systemically with human recombinant IL-10 for 49 days [24], the phenotype of peripheral blood monocytes was similar to that of IL-10 APCs upon completion of the IL-10 therapy (Fig. 7). In fact, as a result of IL-10 therapy the HLA-DR/CD86 expression ratio increased strongly from 1·30 (day −5) to 1·75 (day 57) in patient 1 as well as from 1·49 (day −5) to 2·00 (day 57) in patient 2.
Fig. 7.

HLA-DR and CD86 expression in two psoriatic patients treated systemically with interleukin (IL)-10. The patients received subcutaneous applications of recombinant human IL-10 at 20 μg per kg three times per week over a period of 49 days. Blood samples anti-coagulated with ethylenediamine tetraacetic acid were taken 5 days prior to IL-10 therapy and at days 2, 50 and 57 after the initiation of IL-10 therapy. Monocytic HLA-DR and CD86 expression was analysed flow cytometrically and mean fluorescence values are shown.
Discussion
In contrast to short-term effects of the immunoregulatory cytokine IL-10, its long-term effects have only scarcely been analysed. In particular, there are no reports on long-term IL-10 treatment of susceptible target cell populations. This, however, is of interest in light of persistently enhanced IL-10 levels in some kinds of malignancies, infections or trauma [11–17]. Some of the main immunological target cells for IL-10 are monocytes. Monocytes are crucial for non-adaptive as well as adaptive immune responses. By incubating human peripheral blood monocytes from healthy donors with IL-10 for up to 7 days we tested the long-term effects of IL-10 on this cell type. Indeed, the effects of short-term and long-term IL-10 treatment were remarkably different. Long-term IL-10 treatment resulted in a novel, monocyte-derived cell type with the particular characteristics of (i) HLA-DR bright and CD86 dim expression (in contrast to short-term IL-10-treated monocytes, which are HLA-DR dim and CD86 dim) and (ii) the ability to induce hyporesponsive CD4+ T cells. The phenotype of the long-term IL-10-treated monocytic cells was stable, and we therefore named these in vitro-generated cells ‘IL-10 APCs’.
It is well known that the stimulation of CD4+ T cells via MHC class II molecules without adequate co-stimulation results in T cell anergy [27]. This is in line with our observation that IL-10 APCs induced hyporesponsive CD4+ T cells. We therefore hypothesize that IL-10 APCs act as inhibitory APCs via active down-regulation of adaptive immune responses to antigens presented by these IL-10 APCs. We assume further that locally generated IL-10 APCs would inhibit the immune reaction only to local antigens presented by them but would not induce general immunodeficiency. With respect to its action, IL-10 APCs would differ from inhibitory APCs already described [19,28–34]. For instance, IL-4-induced, alternatively activated mouse macrophages inhibit antigen-induced proliferation of CD4+ T cells [31]. Phenotypically, these cells are MHC class II dim and CD86 dim compared to the respective control macrophages, indicating that diminished antigen presentation rather than active down-regulation of the immune system is responsible for the inhibitory effect. However, respective experiments to analyse its ability to induce a hyporesponsive state in CD4+ T cells were not described. Two other groups recently described inhibitory DCs in mice and humans, respectively [32,33]. The phenotype of these inhibitory DCs was MHC class II bright and CD86 bright, again indicating that IL-10 APCs are different from these inhibitory APCs. All these facts suggest that different inhibitory APC populations may be present in the tissues of ICU patients, cancer patients and patients with chronic viral infections where they may contribute to the well-known reduced immune responses seen in these patients. This is supported by reports describing the induction of inhibitory APCs in some tumour patients [19,28].
First indications for the in vivo existence of IL-10 APCs came from our recently published study on ICU patients with diminished immunocompetence [23]. In about 1% of the analysed blood samples (15 of 1344) from such patients we found monocytic cells with the phenotype of IL-10 APCs. Secondly, two of 10 patients treated systemically with recombinant human IL-10 [24] demonstrated peripheral blood monocytes with a phenotype similar to that of IL-10 APCs. However, we assume that IL-10 APCs reside mainly in tissues other than peripheral blood, as is the case with other APC populations. Interestingly, neither of the two psoriatic patients with systemically detectable monocytic cells with the IL-10 APC phenotype developed infectious complications, indicating that the existence of IL-10 APCs alone does not necessarily lead to general immunodeficiency but, rather, general immunodeficiency is of multi-faceted genesis.
In summary, IL-10 had some heterogeneous effects on monocytes depending on its duration of administration. We have described a novel, monocyte-derived APC population generated by long-term IL-10 application (IL-10 APCs). IL-10 APCs differ from other APCs with respect to phenotype and function. They are HLA-DR bright and CD86 dim and induce hyporesponsive CD4+ T cells. The exact mechanisms by which the persistently low CD86 expression and the hyporesponsive state of the adaptive immune system are achieved as well as the in vivo occurrence and relevance of these cells need to be investigated further.
Acknowledgments
We would like to thank Elizabeth Wallace for accurate proofreading of the manuscript and the Deutsche Forschungsgemeinschaft (DFG) for financial support of our work.
Supplementary material
The following supplementary material is available for this article online:
Effects of interleukin (IL)-10 treatment on monocytic cell surface marker and lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α secretion. Peripheral blood mononuclear cells (PBMCs) were treated with or without IL-10 (respective control cultures) for 6 h, 24 h, 3 days and 7 days. In 7-day-treated cultures 50% of the medium with the respective supplements was replaced at days 3 and 5. Mean monocytic cell surface expression of human leucocyte antigen D-related, CD86, CD16, CD64 and CD71 was analysed by flow cytometry. TNF-a concentration was measured in culture supernatants after stimulation of PBMCs with or without LPS (100 ng/ml) for 4 h. Data from five independent experiments are given as the mean ± standard error of the mean (*P ≤ 0·05 compared to respective control by Wilcoxon matched-pairs signed-ranks test).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries other than missing material) should be directed to the corresponding author for the article.
References
- 1.Fiorentino DF, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Waal Malefyt R, Moore KW, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 3.Kunz S, Wolk K, Witte E, et al. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol. 2006;15:991–1004. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 4.de Waal Malefyt R, Spits H, Roncarolo MG, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willems F, Delville JP, Gerard C, et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994;24:1007–9. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino DF, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 7.de Waal Malefyt R, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Waal Malefyt R, te Velde AA, Huijbens RJ, de Vries JE, Figdor CG. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992;149:4048–52. [PubMed] [Google Scholar]
- 9.Calzada-Wack JC, Ziegler-Heitbrock HW. Interleukin-10 drives human monocytes to CD16 positive macrophages. J Inflamm. 1996;46:78–85. [PubMed] [Google Scholar]
- 10.Lingnau M, Hoflich C, Volk HD, Sabat R, Docke WD. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Hum Immunol. 2007;68:730–8. doi: 10.1016/j.humimm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Gotlieb WH, Watson JM, Velu TJ, Berek JS, Martinez-Maza O. Presence of interleukin 10 (IL-10) in the ascites of patients with ovarian and other intra-abdominal cancers. Cytokine. 1992;4:385–90. doi: 10.1016/1043-4666(92)90082-3. [DOI] [PubMed] [Google Scholar]
- 12.Asadullah K, Haeussler A, Sterry W, Volk HD. Progression of mycosis fungoides is associated with increasing cutaneous expression of interleukin-10 mRNA. J Invest Dermatol. 1996;107:833–7. doi: 10.1111/1523-1747.ep12330869. [DOI] [PubMed] [Google Scholar]
- 13.Fortis C, Gianotti L, Galli L, et al. Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett. 1996;104:1–5. doi: 10.1016/0304-3835(96)04213-9. [DOI] [PubMed] [Google Scholar]
- 14.Ameglio F, Solmone M, Bonifati C, et al. Serum IL-10 levels in HIV-positive subjects: correlation with CDC stages. J Biol Regul Homeost Agents. 1994;8:48–52. [PubMed] [Google Scholar]
- 15.Herbst H, Samol J, Araujo I, et al. Frequent expression of interleukin-10 by Epstein–Barr virus-harboring tumor cells of Hodgkin's disease. Blood. 1996;87:2918–29. [PubMed] [Google Scholar]
- 16.Berney T, Gasche Y, Robert J, et al. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas. 1999;18:371–7. doi: 10.1097/00006676-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Woiciechowsky C, Asadullah K, Nestler D, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4:808–13. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- 18.Heper Y, Akalin EH, Mistik R, et al. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis. 2006;25:481–91. doi: 10.1007/s10096-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 19.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock. 2002;17:443–50. doi: 10.1097/00024382-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Wolk K, von Baehr V, Volk HD, Sabat R. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96:218–23. [PubMed] [Google Scholar]
- 22.Wolk K, Grutz G, Witte K, Volk HD, Sabat R. The expression of legumain, an asparaginyl endopeptidase that controls antigen processing, is reduced in endotoxin-tolerant monocytes. Genes Immun. 2005;6:452–6. doi: 10.1038/sj.gene.6364224. [DOI] [PubMed] [Google Scholar]
- 23.Wolk K, Hoflich C, Zuckermann-Becker H, Docke WD, Volk HD, Sabat R. Reduced monocyte CD86 expression in postinflammatory immunodeficiency. Crit Care Med. 2007;35:458–67. doi: 10.1097/01.CCM.0000254724.54515.2F. [DOI] [PubMed] [Google Scholar]
- 24.Asadullah K, Hanneken S, Rohrbach C, et al. Effects of systemic interleukin-10 therapy on psoriatic skin lesions: histologic, immunohistologic, and molecular biology findings. J Invest Dermatol. 2001;116:721–7. doi: 10.1046/j.0022-202x.2001.01317.x. [DOI] [PubMed] [Google Scholar]
- 25.Wolk K, Witte E, Reineke U, et al. Is there an interaction between interleukin-10 and interleukin-22? Genes Immun. 2005;6:8–18. doi: 10.1038/sj.gene.6364144. [DOI] [PubMed] [Google Scholar]
- 26.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JG, Jenkins MK. The role of anergy in peripheral T cell unresponsiveness. Life Sci. 1994;55:1767–80. doi: 10.1016/0024-3205(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 28.Van Ginderachter JA, Meerschaut S, Liu Y, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525–35. doi: 10.1182/blood-2005-09-3777. [DOI] [PubMed] [Google Scholar]
- 29.Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–13. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 30.Schebesch C, Kodelja V, Muller C, et al. Alternatively activated macrophages actively inhibit proliferation of peripheral blood lymphocytes and CD4+ T cells in vitro. Immunology. 1997;92:478–86. doi: 10.1046/j.1365-2567.1997.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald KP, Rowe V, Clouston AD, et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–50. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 33.Rutella S, Bonanno G, Pierelli L, et al. Granulocyte colony-stimulating factor promotes the generation of regulatory DC through induction of IL-10 and IFN-alpha. Eur J Immunol. 2004;34:1291–302. doi: 10.1002/eji.200324651. [DOI] [PubMed] [Google Scholar]
- 34.Bellone G, Carbone A, Smirne C, et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol. 2006;177:3448–60. doi: 10.4049/jimmunol.177.5.3448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of interleukin (IL)-10 treatment on monocytic cell surface marker and lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α secretion. Peripheral blood mononuclear cells (PBMCs) were treated with or without IL-10 (respective control cultures) for 6 h, 24 h, 3 days and 7 days. In 7-day-treated cultures 50% of the medium with the respective supplements was replaced at days 3 and 5. Mean monocytic cell surface expression of human leucocyte antigen D-related, CD86, CD16, CD64 and CD71 was analysed by flow cytometry. TNF-a concentration was measured in culture supernatants after stimulation of PBMCs with or without LPS (100 ng/ml) for 4 h. Data from five independent experiments are given as the mean ± standard error of the mean (*P ≤ 0·05 compared to respective control by Wilcoxon matched-pairs signed-ranks test).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries other than missing material) should be directed to the corresponding author for the article.


