Abstract
YKL-40 is secreted by macrophages, neutrophils, chondrocytes, endothelial-, vascular smooth muscle- and cancer cells. Interleukin (IL)-6 stimulates YKL-40 production in human in vivo studies. High serum YKL-40 is associated with poor prognosis in patients with inflammatory diseases and cancer. We studied whether serum YKL-40 was associated with systemic low-level inflammation, an immune risk phenotype, and mortality in relatively healthy 80-year old humans. Serum YKL-40, IL-6 and tumour necrosis factor (TNF)-α were measured by enzyme-linked immunosorbent assays (ELISAs) in octogenarians (n = 151) and serum YKL-40 in 18–30-year-olds (n = 89). Fifty-one of the octogenarians died during the 6-year follow-up. Serum YKL-40 in octogenarians was higher compared to the level in young people (median 116 versus 31 μg/l, P < 0·0005). Serum YKL-40 correlated with serum IL-6 in elderly women (Spearman's rho = 0·30, P = 0·009) and men (rho = 0·25, P = 0·003), but only with serum TNF-α (rho = 0·23, P = 0·05) and C-reactive protein (CRP) (rho = 0·57, P < 0·0005) among the elderly women. In addition, high serum level of YKL-40 was associated with a low CD4 : CD8 cell ratio. Univariate analysis of serum YKL-40 (logarithmically transformed and divided by tertiles) showed significant association with all-cause mortality [tertile 3: hazard ratio (HR) = 2·38, 95% confidence interval (CI): 1·19–4·78, P = 0·02]. The effect persisted after adjusting for potential confounders (sex, smoking, body mass index, chronic disease and anti-inflammatory medicine). These results suggest that serum YKL-40 is a prognostic and sensitive biomarker of all-cause mortality in octogenarians.
Keywords: ageing, CHI3L1, IL-6, inflammation, YKL-40
Introduction
Ageing is associated with low-grade elevations in circulating levels of the cytokines tumour necrosis factor (TNF)-α and interleukin (IL)-6 and in the acute phase protein C-reactive protein (CRP). The elevations in these inflammatory biomarkers are related to functional disability, cognitive decline, morbidity and mortality in the elderly [1–7].
YKL-40, a member of ‘mammalian chitinase-like proteins’ is a phylogenetically highly conserved serum protein with homologues in vertebrates and invertebrates ([8–10] and Databases at the National Center for Biotechnology Information; NCBI) [YKL-40 is also named chitinase-3-like-1 protein (CHI3L1), human cartilage glycoprotein-39 (HC gp-39), 38-kDa heparin binding glycoprotein (Gp38k), breast regressing protein 39 Kd (brp-39) and Chondrex]. YKL-40 is expressed and secreted in vitro by cancer cells ([11], NCBI), arthritic chondrocytes [8] and vascular smooth muscle cells [12] and is expressed strongly by late stages of macrophages [9,13], by activated monocytes [14] and released from the specific granules of activated neutrophils [15]. In vivo YKL-40 is expressed by a subpopulation of macrophages in tissues with inflammation such as atherosclerotic plaques [16], arteritic vessels [17], inflamed synovial membranes [18], sarcoid lesions [19] and by peritumoral macrophages [20].
High serum concentration of YKL-40 is an independent prognostic biomarker of short survival in patients with primary or metastatic solid tumours such as breast, colorectal, ovary, lung, prostate, renal, melanoma and glioblastoma [11] and in patients with Streptococcus pneumoniae bacteraemia [21] and alcoholic liver disease [22].
YKL-40 can be regarded as an acute phase protein, as its serum concentration increases in patients by more than 25% following an inflammatory stimulus such as S. pneumoniae[21], active rheumatoid arthritis [23] and inflammatory bowel disease [24]. Human endotoxaemia, which is followed by increased plasma TNF-α and IL-6 levels, increases plasma YKL-40 [25]. IL-6 is the major regulator of acute phase protein synthesis during the acute-phase response [6,26]. We have demonstrated recently in IL-6 knock-out (KO) and IL-6 wild-type (WT) mice that YKL-40 is regulated by IL-6 (unpublished data).
We hypothesized that serum YKL-40 levels were correlated with markers of systemic low-grade inflammation such as IL-6, TNF-α and CRP and would be a risk factor of mortality in elderly people, and aimed to assess whether serum YKL-40 is a prognostic biomarker of all-cause mortality in octogenarians. In addition, it was tested if serum YKL-40 was associated with some of the markers in the immune risk phenotype, which has also been associated with mortality risk in elderly populations [27].
Materials and methods
Subjects
The present study included a subgroup of 151 relatively healthy people from the 1914 population [28], who participated both in a home visit, a health examination at Research Centre for Prevention and Health, Copenhagen County, Denmark and collection of blood samples at Rigshospitalet in 1995, when they were 80 years old. Most participants lived in their own homes, and none were demented. Polymyalgia rheumatica and giant cell arteritis were exclusion diagnoses. A questionnaire was used in 1995 to isolate octogenarians with comorbidity and intakes of anti-inflammatory medicine [acetylsalicylic acid (ASA), corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs)] (Table 1). Body mass index (BMI) was calculated as weight divided by height squared. Vital status and time of death were obtained from the Civil Registration System in October 2001 6 years after the survey of the octogenarians. The local ethical committee approved the study. Written informed consent was obtained from each participant. Results of serum IL-6 and TNF-α from the study group have been published previously [3]. Participants in the elderly population are more healthy than non-participants. Accordingly, the study population comprises relatively healthy, non-disabled elderly people [3]. Blood samples from 89 young, healthy volunteers aged 18–30 years (45 men and 44 women) were collected and stored in the same time-period and served as reference material for serum YKL-40.
Table 1.
Serum concentrations of YKL-40 in octogenarians according to demographic characteristics and comorbidity.
| Characteristics | Number | Serum YKL-40 μg/l | P-value |
|---|---|---|---|
| Gender | |||
| Men | 75 | 131 (68–194) | 0·02 |
| Women | 76 | 105 (72–138) | |
| Smoking status | |||
| Non-smokers | 119 | 113 (67–158) | 0·5 |
| Current | 32 | 119 (82–183) | |
| Body mass index | |||
| Men | 75 | R = 0·01 | 0·9 |
| Women | 76 | R = 0·02 | 0·9 |
| Chronic diseases | |||
| > 1 diagnosis | 134 | 116 (68–170) | 0·7 |
| No chronic disorders | 17 | 118 (76–155) | |
| Type 2 diabetes | |||
| Yes | 16 | 179 (83–237) | 0·09 |
| No | 135 | 113 (67–155) | |
| Cardiovascular disease | |||
| Yes | 51 | 120 (74–194) | 0·4 |
| No | 100 | 113 (67–161) | |
| Stroke/TCI | |||
| Yes | 7 | 103 (62–203) | 0·05 |
| No | 144 | 116 (69–161) | |
| Hypertension | |||
| Yes | 40 | 100 (64–146) | 0·7 |
| No | 111 | 118 (71–168) | |
| Cancer | |||
| Yes | 18 | 100 (66–153) | 0·5 |
| No | 133 | 117 (71–169) | |
| Chronic obstructive pulmonary disease | |||
| Yes | 18 | 105 (53–132) | 1·0 |
| No | 133 | 116 (72–170) | |
| Thyroid disorder | |||
| Yes | 5 | 112 (77–133) | 0·7 |
| No | 146 | 116 (68–168) | |
| Connective tissue disorders | |||
| Yes | 59 | 112 (79–158) | 0·9 |
| No | 92 | 118 (62–169) | |
| Gastrointestinal disorders | |||
| Yes | 29 | 118 (72–178) | 0·4 |
| No | 122 | 116 (68–163) | |
| Urogenitial disorders | |||
| Yes | 22 | 125 (59–225) | 0·1 |
| No | 129 | 116 (70–158) | |
| Anti-inflammatory medicine | |||
| Yes | 40 | 118 (89–163) | 1·0 |
| No | 111 | 113 (63–168) | |
Values are median (interquartile range) and two-tailed t-test. Serum YKL-40 is log transformed in the t-test. Never-smokers and previous smokers are pooled as non-smokers. Information about comorbidity is self reported in relation to a questionnaire and an interview. Disorders concerning connective tissue include osteoporosis and osteoarthritis as the major groups. Anti-inflammatory medicine: acetylsalicylic acid (ASA) (> 100 mg), non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids.
Biomarker analysis
Serum samples for TNF-α and IL-6 measurements were obtained in 1995 at Glostrup Hospital and stored at −20°C until analysis. Serum samples for YKL-40 and high sensitive CRP measurements were obtained in 1995 at Rigshospitalet and stored at −80°C until analysis. Serum concentrations of YKL-40 were determined by a two-site, sandwich-type, enzyme-linked immunosorbent assay (ELISA) (Quidel, Santa Clara, CA, USA) [23]. The sensitivity of the ELISA was 10 μg/l. The intra- and interassay coefficients of variation (CV) were < 7·2% and < 8·6%, respectively. Serum concentrations of IL-6 were measured by ELISA (high sensitive, catalogue number HS600; R&D Systems, Minneapolis, MN, USA). The sensitivity of the ELISA was 0·10 ng/l. The intra- and interassay CV were 8·7% and 15·1%, respectively. Serum concentrations of TNF-α were measured by ELISA (catalogue number HSTA50; R&D Systems). The sensitivity of the TNF-α ELISA was 0·20 ng/l. The intra- and interassay CV were 15·7% and 25%, respectively. High sensitive serum CRP concentrations were measured by nephelometry. All analyses were run within 8 weeks. ELISA kits with the same batch number were used in order to limit interassay variation. Samples were analysed without knowledge of age, clinical, biochemical and survival data.
The measurements and data of proliferative responses, natural killer cell activity and the lymphocyte phenotype have previously been reported [29,30]. In brief, blood mononuclear cells were isolated by density gradient centrifugation. Cells were frozen in freezing medium and kept in liquid nitrogen until thawed for analysis. Lymphocyte phenotype was measured by flow cytometry, natural killer (NK) cell cytotoxicity was determined by a crom release assay and proliferative responses were measured by a thymidine incorporation assay.
Statistical analysis
Statistical analyses were performed by spss version 11·0 (SPSS Inc., Copenhagen, Denmark). Independent groups were compared by two-tailed t-tests or analyses of variance (continuous variables). Serum concentrations of inflammatory and immune parameters showed normal distribution after log transformations. The Spearman's correlation test was used to test for correlations. Survival curves were constructed using the Kaplan–Meier method. Cox regression models were used to explore effects of serum YKL-40 and other inflammatory markers (TNF-α, IL-6 and CRP) in univariate analyses and after adjustments of potential confounders (gender, smoking, BMI, comorbidity and anti-inflammatory medicine). All Cox regression models were stratified by sex. The model fit was evaluated by −2 log likelihood. P-values (two-sided) of < 0·05 were considered statistically significant.
Results
The median serum concentration of YKL-40 in the octogenarians was 116 μg/l [interquartile range (IQR): 69–166 μg/l, n = 151] and significantly higher compared to the level in 18–30-year-olds (31 μg/l, IQR 23–43 μg/l, n = 89, P < 0·0005) (Fig. 1). Seventy-seven (51%) of the octogenarians had a serum YKL-40 level above the upper range (113 μg/l) of the young.
Fig. 1.
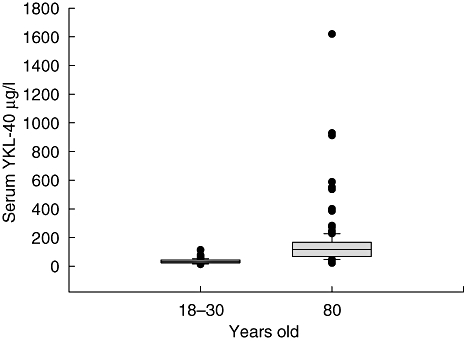
Box plots of serum YKL-40 in 18–30-year-olds (n = 89) and in octogenarians (n = 151). The box plots display the median, interquartiles range, the 5th and 95th percentile and outliers.
Elderly men had a higher serum YKL-40 compared to elderly women (Table 1), whereas no difference was found in serum YKL-40 between young men and young women (median P = 0·8). Cigarette smoking, BMI and intake of anti-inflammatory drugs or comorbidity were not associated with enhanced serum YKL-40 in the elderly population (Table 1).
Immune risk phenotype
Serum YKL-40 correlated with serum IL-6 in both elderly women (Spearman's rho = 0·30, P = 0·009, n = 76) and elderly men (rho = 0·25, P = 0·003, n = 75), whereas it was correlated with serum TNF-α only among the elderly women (women: rho = 0·23, P = 0·05, n = 76; men: rho = 0·03, P = 0·8, n = 75). Similarly, serum YKL-40 and CRP were correlated only in women (women: rho = 0·57, P < 0·0005, n = 74; men: rho = 0·03, P = 0·8, n = 73).
CD4 : CD8 ratio, CD28-CD8+ lymphocyte count, proliferative responses to different stimulators [phytohaemagglutinin (PHA), pokeweed mitogen (PWM) and IL-2], NK cell count and NK cell cytotoxicity are parts of an immune risk phenotype, which constitutes a predictor of mortality in elderly populations [27]. Information about these parameters was available in most subjects in the present study. High serum YKL-40 was associated with a low CD4 : CD8 ratio, whereas there was no association with CD28- CD8+ lymphocyte count, proliferative responses, NK cell count or NK cell cytotoxicity (Table 2).
Table 2.
Serum YKL-40 and markers of the immune risk phenotype.
| Low YKL-40 | Medium YKL-40 | High YKL-40 | |
|---|---|---|---|
| CD4 : CD8 ratio | 2·3 (1·5–3·4) | 2·7 (1·8–3·5) | 1·7 (1·3–2·3)* |
| n = 50 | n = 49 | n = 47 | |
| CD28–CD8+ (cells/ml blood) | 135 (81–192) × 103 | 115 (60–160) × 103 | 154 (71–263) × 103 |
| n = 43 | n = 43 | n = 41 | |
| PHA proliferation (cpm) | 8392 (5161–11 078) | 7764 (5811–9003) | 7457 (5408–9542) |
| n = 48 | n = 47 | n = 48 | |
| PWM proliferation (cpm) | 1275 (884–2174) | 1158 (651–1823) | 1255 (764–2430) |
| n = 47 | n = 47 | n = 47 | |
| IL-2 proliferation (cpm) | 644 (307–890) | 518 (320–960) | 572 (392–860) |
| n = 47 | n = 45 | n = 44 | |
| NK (cells/ml blood) | 183 (104–317) × 103 | 159 (96–284) × 103 | 182 (97–281) × 103 |
| n = 44 | n = 44 | n = 39 | |
| NK cytotoxicity (%) | 10 (6–17) | 15 (8–21) | 12 (9–24) |
| n = 46 | n = 50 | n = 45 |
Denotes P < 0·02 in a one-way analysis of variance. Turkey post-hoc test shows significant difference between medium YKL-40 and high YKL-40. Natural killer (NK) cell cytotoxicity has the effector/target cell ratio 50 : 1. YKL-40 is divided by tertiles into low, medium and high. Median and quartiles are shown. The immune risk phenotype constitutes a predictor of non-survival in longitudinal studies. PHA, phytohaemagglutinin; PWM, pokeweed mitogen; IL-2, interleukin-2; cpm: counts per million.
Mortality risk
The octogenarians were followed-up in December 2003, i.e. the follow-up period was 5–6 years. Over this period,all-cause mortality was registered. There were 51 (34%) deaths. The median serum YKL-40 was higher in the octogenarians who died compared to those still living after 6 years (median 140 μg/l, IQR 80–211 μg/l versus 105 μg/l, IQR 65–143 μg/l, P = 0·009). Figure 2 illustrates the overall survival plots when the octogenarians were grouped by tertiles of serum YKL-40.
Fig. 2.
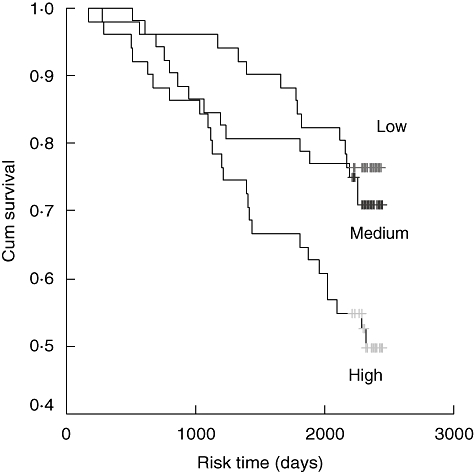
Kaplan–Meier survival estimates of death from serum YKL-40 in octogenarians. Patients were divided by tertiles of serum YKL-40. Low serum YKL-40 (≤ 80 μg/l, n = 50, 12 deaths), versus medium serum YKL-40 (> 80 μg/l and ≤ 140 μg/l, n = 51, 14 deaths) versus high serum YKL-40 (> 140 μg/l, n = 50, 25 deaths).
Cox analysis of serum YKL-40 (divided into tertiles as well as logarithmically transformed) showed significant association with all-cause mortality in a univariate analysis (Table 3). Similar results were found when serum YKL-40 was included as a continuous variable. Men had an increased mortality risk compared with women, and men had higher levels of serum YKL-40. Accordingly, all Cox regression analyses were stratified by gender. This means that different baseline hazard functions are assumed in men and women. The effect of serum YKL-40 was very robust, as it persisted after simultaneously adjustments for the effect of gender, smoking, BMI, anti-inflammatory drugs and comorbidity including hypertension, cardiovascular disease, type 2 diabetes, chronic obstructive pulmonary disease, cerebrovascular events, a history of cancer, thyroid disorders, disorders in connective tissues, gastrointestinal disorders and urogenital disorders in this relatively small population (Table 3).
Table 3.
Univariate and multivariate Cox regression analysis of serum YKL-40 and all-cause mortality in octogenarians.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Serum YKL-40 | ||||||
| Tertile 1 | ||||||
| Tertile 2 | 1·22 | 0·57–1·19 | 0·6 | 1·05 | 0·46–2·38 | 0·9 |
| Tertile 3 | 2·38 | 1·19–4·78 | 0·02 | 2·20 | 1·05–4·63 | 0·04 |
| Log YKL-40 (continuous variable) | 2·51 | 1·15–5·52 | 0·02 | 2·39 | 0·98–5·85 | 0·06 |
All models (including univariate) are stratified by gender. Multivariate models are adjusted for the effect of smoking, body mass index, anti-inflammatory medicine and comorbidity (se Table 1). n = 151; 51 deaths within the 6 years of follow-up. HR: hazard ratio.
Serum IL-6, but not serum CRP or TNF-α, was also associated significantly with mortality in the present study population (data not shown).
Discussion
In this population-based study of octogenarians we found that a high serum concentration of YKL-40 was associated with increased mortality. This was independent of comorbidity, cigarette smoking and BMI, but not of serum concentrations of IL-6. Men had higher serum levels of YKL-40, and serum YKL-40 correlated with serum IL-6 in both 80-year-old women and men, but with serum TNF-α and CRP only in the elderly women. Serum YKL-40 was also associated weakly with a low CD4 : CD8 ratio but not with other parameters of the immune risk phenotype associated with increased mortality risk in elderly populations.
IL-6 is a multi-functional cytokine with varied system functions and plays an important role in inflammatory processes, regulates the acute phase response, induces cell differentiation, regulates haematopoiesis and bone metabolism and is involved in the regulation of solid tumour growth in both a paracrine and an autocrine manner [7,31,32]. Ongoing studies of IL-6 KO and IL-6 WT mice show that YKL-40 is regulated by IL-6 with high YKL-40 mRNA expression in blood, lung and adipose tissue following an inflammatory stimulus. However, in contrast to CRP that is produced by hepatocytes in response to high IL-6, no YKL-40 expression was found in mouse livers in response to IL-6 (unpublished data). Circulating YKL-40 may therefore reflect other aspects of the inflammatory process than CRP. The YKL-40 coding chitinase 3-like 1 (CHI3L1) gene bears a number of polymorphic sites, some of which are potentially functional [33,34]. A significant association is found between schizophrenia and haplotypes within the promoter region of CHI3L1[33], whereas there is no difference in haplotype frequencies between patients with sarcoidosis and controls [34]. A-329 G/A polymorphisms influence serum YKL-40 levels significantly in healthy subjects but not in patients with sarcoidosis [34]. The YKL-40 gene [9] is located on the same arm of chromosome 1 (1q32) as the IL-6 receptor gene (lq21) and the CRP gene (1q21–1q23). Multiple genes on chromosome 1 may influence inflammatory biomarker levels and have a role in development of cardiovascular disease [35].
The octogenarians had a higher serum YKL-40 compared to the young, in accordance with previous studies of healthy humans showing increasing serum YKL-40 with age [23,36]. The elevated serum YKL-40 (compared to healthy young humans) in approximately 50% of the octogenarians may reflect the chronic low-level inflammation in the elderly humans [2,3,6,7,37] or an indirect indicator of a specific underlying condition that increases mortality risk, such as cardiovascular disease and cancer. Chronic inflammation is involved in the pathophysiology of cardiovascular disease and cancer [5,7,31]. A chronic age-related, progressive stimulation of macrophages towards a proinflammatory status has been postulated [38]. Activated macrophages from many different tissues are probably a major source of the circulating YKL-40 in octogenarians. Macrophages in atherosclerotic plaques express YKL-40 mRNA, particularly macrophages that have infiltrated deeper in the lesion, and the highest YKL-40 expression is found in macrophages in the early lesion of atherosclerosis [16]. YKL-40 is also expressed by a subpopulation of macrophages in biopsies of inflamed arthritic synovial membrane [18], giant cell arteritis [17], pulmonary sarcoidosis [19] and peritumoral macrophages in biopsies of small cell lung cancer [20]. In patients with rheumatoid arthritis YKL-40 is expressed by CD16+ monocytes with a dim expression of CD14 [18]. This CD14+ CD16+ proinflammatory phenotype can differentiate from classic CD142+ monocytes by maturation in vitro and is supposed to be a more mature version of monocytes with properties of tissue macrophages and are a major source of TNF [39].
Serum concentrations of YKL-40 have been found recently to be elevated in patients with coronary artery disease, and there was an association between serum YKL-40 and the extent of coronary artery disease defined by the number of diseased vessels assessed by coronary angiography [40]. The present study, with only a limited number of participants with known cardiovascular disease, cannot be conclusive. One hypothesis is that a high serum YKL-40 may reflect ongoing atherosclerosis. YKL-40 protein expression is found in human smooth muscle cells in atherosclerotic plaques [12]. YKL-40 promotes vascular smooth muscle cell attachment, spreading and migration, suggesting that YKL-40 has a role in the process of atherosclerotic plaque formation, where smooth muscle cells are induced to migrate through the intima in response to exogenous signals [12]. In vitro YKL-40 is synthesized by vascular smooth muscle cells isolated from the thoracic aorta of pigs during the time of transition from a proliferating monolayer culture to a non-proliferating differentiated multi-layer culture [41]. YKL-40 secretion continues as the cells reorganize and form multi-cellular nodules in which cells re-express markers of differentiated vascular smooth muscle cells [41,42]. This in vitro nodule forming process mimics some of the characteristics of the in vivo changes that occur in smooth muscle cells of the vascular wall following injury, where media smooth muscle cells dedifferentiate, migrate and contribute to the process of restenosis and neointima formation. YKL-40 also modulates vascular endothelial cell morphology by promoting the formation of branching tubules, indicating that YKL-40 has a role in angiogenesis by stimulating the migration and reorganization of vascular endothelial cells [42]. In the future, large, prospective, epidemiological studies should evaluate whether serum YKL-40 is an independent predictor of future cardiovascular events, both in patients with a history of coronary heart disease and in apparently healthy subjects.
Studies have demonstrated that high serum YKL-40 in patients with different types of solid cancer is an independent biomarker of short survival [11]. The exact biological functions of YKL-40 in cancer diseases are unknown. YKL-40 may play a role in proliferation and differentiation of the malignant cells, may protect the cells from undergoing apoptosis, stimulate angiogenesis and could have an effect on extracellular tissue remodelling, although in vivo proof of this is yet to be obtained. In vitro studies of glioblastoma cell lines showed that diverse types of stress resulted in YKL-40 mRNA and protein expression, suggesting an involvement of YKL-40 as a cellular survival factor [43].
The molecular processes governing the induction of YKL-40 and its precise functions are unknown. YKL-40 is a growth factor for fibroblasts and chondrocytes [44–46], acts synergistically with IGF-1 [45], is regulated by TNF-α[46] and requires sustained activation of nuclear factor (NF)-kappaB [47]. YKL-40 initiates mitogen-activated protein (MAP) kinase and PI-3K signalling cascades in fibroblasts leading to the phosphorylation of both the extracellular signal-regulated kinase (ERK)-1/2, MAP kinase and protein kinase B (AKT)-mediated signalling cascades, which are associated with the control of mitogenesis [46]. YKL-40 treatment of fibroblasts can counteract the inflammatory response to TNF-α and IL-1 by phosphorylation of AKT, thereby attenuating ASK1 mediated signalling pathways [46]. This leads to decreased levels of the metalloproteinase and IL-8 expression [46]. Cellular receptors mediating the biological effects of YKL-40 are currently not known, but the activation of cytoplasmic signal-transduction pathways suggests that YKL-40 interacts with signalling components on the cell membrane.
In conclusion, we have found that a high serum YKL-40 in relatively healthy octogenarians is a prognostic biomarker of all-cause mortality. This effect is probably related to effects of IL-6. Prospective epidemiological studies should evaluate whether serum YKL-40 is an independent predictor of future cardiovascular events and development of cancer in community-based population studies.
Acknowledgments
The expert technical assistance of Tonni Løve Hansen and Debbie Nadelmann, Herlev Hospital for the measurement of serum YKL-40 are gratefully acknowledged. The study was supported by grants from ‘Direktør Jens Aage Sørensen og Hustru Edith Ingeborg Sørensens Mindefond’ (J. S. J.) and the Novo Nordic Foundation. The Centre of Inflammation and Metabolism is supported by a grant from the Danish National Research Foundation (no. 02-512-55). Quidel provided YKL-40 ELISA kits.
References
- 1.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 2.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JvB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115:278–83. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 3.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krabbe S, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–75. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:17–28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 7.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificient pathway. J Gerontol Med Sci. 2006;61A:575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–10. [PubMed] [Google Scholar]
- 9.Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–5. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 10.Funkhouser JD, Aronson NN. Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007;7:1–16. doi: 10.1186/1471-2148-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Rev Cancer Epidemiol Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa KC, Millis AJT. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res. 2003;287:79–87. doi: 10.1016/s0014-4827(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 13.Renkema GH, Boot RG, Au FL, et al. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–9. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto S, Suzuki T, Dong H-Y, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood. 1999;94:837–44. [PubMed] [Google Scholar]
- 15.Volck B, Price PA, Johansen JS, et al. YKL-40, a mammalian member of the bacterial chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians. 1998;110:351–60. [PubMed] [Google Scholar]
- 16.Boot RG, van Achterberg AE, van Aken BE, et al. Strong induction of members of the chitinase family of proteins in atherosclerosis. Chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687–94. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- 17.Johansen JS, Baslund B, Garbarsch C, et al. YKL-40 in giant cells and macrophages from patients with giant cell arteritis. Arthritis Rheum. 1999;42:2624–30. doi: 10.1002/1529-0131(199912)42:12<2624::AID-ANR17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Baeten D, Boots AMH, Steenbakkers PGA, et al. Human cartilage gp-39+,CD16+ monocytes in peripheral blood and synovium. Correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000;43:1233–43. doi: 10.1002/1529-0131(200006)43:6<1233::AID-ANR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Johansen JS, Milman N, Hansen M, Garbarsch C, Price PA, Graudal N. Increased serum YKL-40 in patients with pulmonary sarcoidosis − a potential marker of disease activity? Respir Med. 2005;99:396–402. doi: 10.1016/j.rmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Junker N, Johansen JS, Andersen CB, Kristjansen PEG. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer. 2005;48:223–31. doi: 10.1016/j.lungcan.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Kronborg G, Østergaard C, Weis N, et al. Serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated to the outcome of the disease. Scand J Infect Dis. 2002;34:323–6. doi: 10.1080/00365540110080233. [DOI] [PubMed] [Google Scholar]
- 22.Nøjgaard C, Johansen JS, Christensen E, et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39:179–86. doi: 10.1016/s0168-8278(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 23.Harvey S, Weisman M, O'Dell J, et al. Chondrex: new marker of joint disease. Clin Chem. 1998;44:509–16. [PubMed] [Google Scholar]
- 24.Vind I, Johansen JS, Price PA, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 2003;38:599–605. doi: 10.1080/00365520310000537. [DOI] [PubMed] [Google Scholar]
- 25.Johansen JS, Krabbe KS, Møller K, Pedersen BK. Circulating YKL-40 levels during human endotoxaemia. Clin Exp Immunol. 2005;140:343–8. doi: 10.1111/j.1365-2249.2005.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 27.DelaRosa O, Pawelec G, Peralbo E, et al. Immunological biomarkers of ageing in man: changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology. 2006;7:471–81. doi: 10.1007/s10522-006-9062-6. [DOI] [PubMed] [Google Scholar]
- 28.Schroll M. A ten-year prospective study, 1964–1974, of cardiovascular risk factors in men and women from the Glostrup population born in 1914. Dan Med Bull. 1982;29:213–52. [PubMed] [Google Scholar]
- 29.Bruunsgaard H, Pedersen AN, Schroll M, Skinhøj P, Pedersen BK. Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp Gerontol. 2001;37:127–36. doi: 10.1016/s0531-5565(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 30.Bruunsgaard H, Pedersen AN, Schroll M, Skinhøj P, Pedersen BK. Proliferative responses of blood mononuclear cells (BMNC) in a cohort of elderly humans: role of lymphocyte phenotype and cytokine production. Clin Exp Immunol. 2000;119:433–40. doi: 10.1046/j.1365-2249.2000.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 32.Trikha M, Corringham R, Klein B, Rossi J-F. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–65. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Tang R, Gao B, et al. Functional variants in the promoter region of chitinase 3-like 1 (CHI3L1) and susceptibility to schizophrenia. Am J Hum Genet. 2007;80:12–18. doi: 10.1086/510438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruit A, Grutters JC, Ruven HJT, van Moorsel CCM, van den Bosch JMM. A CHI3L1 gene polymorphism is associated with serum levels of YKL-40, a novel sarcoidosis marker. Respir Med. 2007;101:1563–71. doi: 10.1016/j.rmed.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Dupuis J, Larson MG, Vasan RS, et al. Genome scan of systemic biomarkers of vascular inflammation in the Framingham Hearth Study: evidence for susceptibility loci on 1q. Atherosclerosis. 2005;182:307–14. doi: 10.1016/j.atherosclerosis.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Johansen JS, Hvolris J, Hansen M, Backer V, Lorenzen I, Price PA. Serum YKL-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br J Rheumatol. 1996;35:553–9. doi: 10.1093/rheumatology/35.6.553. [DOI] [PubMed] [Google Scholar]
- 37.Cohen HJ, Pieper CF, Harris TB, Rao KMK, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol Med Sci. 1997;52A:M201–8. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 38.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;980:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 39.Belge K-U, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 40.Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S. Serum YKL-40 levels in patients with coronary artery disease. Coron Artery Dis. 2007;18:391–6. doi: 10.1097/MCA.0b013e328241d991. [DOI] [PubMed] [Google Scholar]
- 41.Millis AJT, Hoyle M, Reich E, Mann DM. Isolation and characterization of a Mr = 38 000 protein from differentiating smooth muscle cells. J Biol Chem. 1985;260:3754–61. [PubMed] [Google Scholar]
- 42.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJT. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168–73. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 43.Junker N, Johansen JS, Hansen LT, Lund EL, Kristjansen PEG. Regulation of YKL-40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005;96:183–90. doi: 10.1111/j.1349-7006.2005.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun. 2001;285:926–31. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 45.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase-and protein kinase B-mediated signalling pathways. Biochem J. 2002;365:119–26. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J. 2004;380:651–9. doi: 10.1042/BJ20040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem. 2005;280:41213–21. doi: 10.1074/jbc.M510146200. [DOI] [PubMed] [Google Scholar]


