Abstract
Multiple sclerosis (MS) ameliorates typically during pregnancy but after the delivery the relapse rate often increases. Our study was conducted to understand the immunoregulatory mechanisms accompanying this phenomenon. MS patients were followed-up prospectively during pregnancy and 6 months postpartum, with immunological characterization of the peripheral blood. Groups of age- and parity-matched healthy pregnant women, and age- and sex-matched non-pregnant women and non-pregnant MS patients were studied as controls. In our patient cohort, the annualized relapse rate was 1·0 ± 1·0 relapses/woman/year (mean ± standard deviation) during the year before pregnancy, but dropped to 0·2 ± 0·9 during the third trimester (P = 0·02). After the delivery the relapse rate increased again to 1·4 ± 1·9 (1–3 months postpartum versus third trimester P = 0·003). While percentages of peripheral blood CD3, CD4, CD8 and CD19 immune cell subsets were unchanged over the observation period, reduced disease activity during the last trimester was associated with a significant increase in the percentage of circulating CD56bright natural killer (NK) cells. Simultaneously, the proportion of circulating CD56dim NK cells was clearly reduced. No alteration was noted in CD4+ CD25high forkhead box P3+ regulatory T cells. Production of interferon-gamma by peripheral blood lymphocytes was down-regulated significantly during pregnancy in comparison to the postpartum period, resulting in an increased T helper type 2 (Th2) : Th1 ratio during pregnancy. In conclusion, pregnant state in MS patients is characterized by an increase in the percentage of CD56bright NK cells and by enhanced Th2 type cytokine secretion. Our findings suggest a potential role for CD56bright regulatory NK cells in the control of autoimmune inflammation during pregnancy in MS.
Keywords: autoimmunity, immunoregulation, multiple sclerosis, NK cell, pregnancy
Introduction
Pregnancy is a known modulator of disease activity in multiple sclerosis (MS). During late pregnancy the relapse rate is 70% lower when compared to the time before pregnancy, but after the delivery aggravation of the disease is commonly encountered [1]. This is also a frequently observed phenomenon in other T helper type 1 (Th1) autoimmune diseases [2–4]. The reasons for this pattern of disease activity lie presumably in the physiological alterations taking place in the mother's immune and endocrinological system during pregnancy, but the molecular details of the protective mechanisms responsible for amelioration of MS in late pregancy remain largely elusive.
Pregnancy induces alterations in the maternal immune system in order to protect the semi-allogeneic fetus from an immunological attack by the mother's immune system [5]. To establish and maintain a successful pregnancy, selective immune tolerance and immunomodulation is required. No general immunosuppression takes place in the maternal immune system; on the contrary, there is an increased proinflammatory burden derived from the innate immune system − especially during the third trimester [6]. The immunomodulatory mechanisms contributing to the control of harmful autoimmune activity are thus directed probably towards the adaptive immune system [7]. Immunoregulatory factors specific for pregnancy include pregnancy-specific serum proteins and tolerance-promoting signalling molecules such as alpha-fetoprotein, human leucocyte antigen (HLA)-G, CD200, Fas-ligand, co-inhibitory B7-molecules and indoleamine 2,3-dioxygenase [8–10]. These factors probably support quiescence of autoimmune diseases during pregnancy. In addition, placenta-derived hormones, oestrogens and progesterone can directly affect the function of the immune cells such as CD4+ and CD8+ T cells, NK cells and macrophages, which express oestrogen receptors. The assumption that pregnancy hormones are involved critically in controlling MS activity is advocated by a recent study in which oestriol applied therapeutically proved beneficial for MS patients [11]. Furthermore, oestrogens also delay the onset of experimental autoimmune encephalomyelitis (EAE) [12,13]. Other putative beneficial mechanisms in the control of autoimmune diseases during pregnancy include a shift from a prevailing Th1 response to a Th2-type response [14,15] and an increase in the number of functional regulatory T cells (Treg) [16,17].
To date, the majority of studies addressing immunological changes during pregnancy explored changes in early pregnancy, while immunological profiling during the third trimester of pregnancy − the clinically most relevant period in terms of autoimmune disease amelioration − is largely unexplored [18–21]. The objective of our prospective study was to characterize potential regulatory elements altering MS activity during pregnancy and postpartum.
Materials and methods
Study subjects
A total of 42 pregnant relapsing–remitting MS patients (RRMS) were recruited to the study during a period of 1·5 years in 10 different neurology centres in Finland. The majority of the patients (88%) joined the study during the first trimester of pregnancy, and 93% (n = 39) of patients were followed until 6 months postpartum. A group of freshly diagnosed, non-pregnant, non-treated RRMS patients was included for comparison (n = 18). The patient characteristics are shown in Table 1. Neurological examinations were performed at gestational weeks 10–12 and at month 6 postpartum and included the assessment of disability by the Expanded Status Scale (EDSS) [22]. From each individual, a total of seven serial whole blood samples were drawn at given time-points during and after pregnancy. Non-pregnant MS patients matched well to pregnant MS patients, especially concerning EDSS at initiation and relapse rate before pregnancy (Table 1). In addition to MS patients, control groups of pregnant healthy women (n = 25, age 30·3 ± 3·9 years) and healthy non-pregnant women (n = 18, age 29·7 ± 5·2 years) were studied. Only one blood sample was drawn from non-pregnant MS patients and healthy controls. Only Caucasian women were included in the study. The study was approved by the ethical committee of the Turku University Hospital and written informed consent was obtained from all subjects.
Table 1.
Patient demographics.
| Characteristics | Pregnant MS patients | Non-pregnant MS patients |
|---|---|---|
| n* (total) | 42 | 18 |
| Age (year)† | 29·5 ± 3·8 | 35·7 ± 10·4 |
| Duration‡ (year) | 4·5 ± 3·6 (range 0·5–13) | 1·6 ± 3·0 years (range 0·5–12) |
| Number of relapses§ | 4·3 ± 2·7 (range 1–12) | 2·6 ± 1·0 |
| EDSS¶ at initiation | 1·5 ± 1·0 | 1·6 ± 1·3 |
| EDSS at 6 months PP†† | 1·67 ± 1·07 | n.a.‡‡ |
| DMT§§ (n) | ||
| No DMT | 18 | 18 |
| Avonex | 7 | 0 |
| Betaferon | 6 | 0 |
| Copaxone | 5 | 0 |
| Rebif 22 | 6 | 0 |
| DMT during 1–6 months PP (n) | 6 (initiation 2–5 months PP) | n.a. |
| Breastfeeding (n) | 42 (duration range 4 week−6 months) | n.a. |
Number of patients
age at onset of study, years, mean ± standard deviation
duration of disease at onset of study
total number of relapses experienced before the study onset
Expanded Disability Status Scale (EDSS), for pregnant patients at 10–12 gestational weeks, for non-pregnant patients, at the time of sampling
postpartum (PP)
not applicable (n.a.)
disease-modifying therapy (DMT) before pregnancy. MS: multiple sclerosis.
Preparation and flow cytometric analysis of peripheral blood lymphocytes (PBL)
At each visit 20 ml of heparinized blood was drawn for the isolation of PBL. Cells were isolated by density gradient centrifugation using Ficoll-HypaqueTM PLUS (Amersham Biosciences, Uppsala, Sweden) within either 4 h of sample drawing (patients in Turku) or the following day. Shipping did not reduce the cell viability significantly, and no effect was noted on the expression of the surface molecules studied (data not shown). Immunofluorescence (IF) staining was performed as described previously [23]. For certain staining procedures [stainings for CD56+ cells and CD4+ CD25high forkhead box P3 (FoxP3)+ cells] viably frozen cells were used, after controlling that freeze–thaw does not significantly alter the expression level of the molecules of interest. Detection of Foxp3+ CD4+ CD25+ natural regulatory cells was detected using phycoerythrin (PE) anti-human FoxP3 staining kit (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. Briefly, for flow cytometry analysis, PBL were first stained with surface antibodies (anti-CD4 and anti-CD25), fixed, permeabilized and then stained with anti-human Foxp3 (PCH101; eBioscience). When frozen cells were stained, the full series of samples from a given patient was recovered from the freezer and the staining procedure performed in one experiment to confirm that the alterations observed in the expression of the molecules of interest were not caused by potential day-to-day variation in the staining procedure.
Monoclonal antibodies
PE-conjugated anti-CD3 (clone HIT3a), anti-CD56, anti-CD16 (clone B73·1), anti-CD19, fluorescein isothiocyanate (FITC)-conjugated anti-CD4, FITC-conjugated anti-CD25 (clone M-A251) and peridinin chlorophyll protein (PerCP)-conjugated anti-CD4 (clone SK3) were from BD Biosciences. PE-conjugated anti-human Foxp3 antibody (clone PCH101) and its isotype control antibody were purchased from eBioscience. Hybridoma cell lines producing monoclonal antibodies against CD8 (CRL8014) and CD3 (CRL8001) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). PE-conjugated anti-mouse-CD4 (Caltag Laboratories, Burlingame, CA, USA) and mouse immunoglobulin 1 (IgG1) antibody against chicken T cells (3G6; used as hybridoma supernatant) were used as negative controls.
Measurement of interferon (IFN)-γ- and interleukin (IL)-4-producing PBL
For the detection of IFN-γ- and IL-4-producing (respectively, Th1- and Th2-type) lymphocytes, 0·5–1 × 106 cells from 10 individual MS patients and 10 controls isolated at two different time-points were stimulated for 5 h with 5 ng/ml phorbol myristate acetate (PMA) (Sigma-Aldrich, St Louis, MO, USA) and 500 ng/ml ionomycin (Calbiochem, San Diego, CA, USA) at a density of 2 × 106 cells/ml to induce maximal cytokine production. A proportion of the cells were kept as an unstimulated control population. After 2 h of stimulation, brefeldin A (10 μg/ml) was added and incubation was continued for an additional 3 h. After stimulation, the cells were washed twice with buffer [0·5% bovine serum albumin (BSA) and 0·01% azide in phosphate-buffered saline (PBS)], fixed with 4% paraformaldehyde for 15 min and permeabilized with 0·5% saponin in PBS (pH 7) containing 0·5% BSA and 0·01% azide. Finally, intracellular cytokine staining was performed with anti-IFN-γ–FITC or with anti-IL4–PE (Caltag Laboratories, Burlingame, CA, USA) for 20 min. The cells were assayed using a fluorescence cytometer (FACScan, Becton Dickinson Biosciences, San Jose, CA, USA). In total, 30 000 events were collected from each tube. Lymphocytes were gated according to side- and forward-scatter properties (Fig. 1a) and analysed using the CellQuest software (BD Biosciences, San Jose, CA, USA) to determine the percentages of positive cells. Double staining with IFN-γ–FITC and CD3–PE was performed in order to demonstrate that the cells producing intracellular IFN-γ were T cells (Fig. 1b).
Fig. 1.
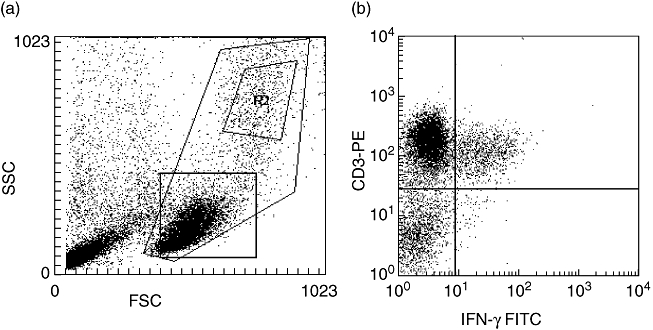
Representative staining for flow cytometry experiments. (a) Shown is a representative forward-scatter/side-scatter plot. R2 shows the monocyte gate (of CD14-positive monocytes). The lower square shows the lymphocyte gate. (b) Shown is a dot plot of the cells in the lymphocyte gate after phorbol myristate acetate and ionomycin treatment and double staining with interferon (IFN)-γ–fluorescein isothiocyanate and CD3–phycoerythrin. All IFN-γ-producing cells were CD3+ T cells.
Statistical analysis
The relapse rates per patient per year during each 3-month period during pregnancy and postpartum were compared to the relapse rate during the year before pregnancy by analysis of variance (anova) for repeated measurements. The proportions of positive cells at different time-points within the MS patient-groups were also compared with anova for repeated measurements. Analyses were performed separately for all measured parameters. Statistical analyses were performed using the SAS System for Windows, release 9·1 (SAS Institute Inc., Cary, NC, USA). Two-sided P-values less than 0·05 were considered as statistically significant.
Results
MS disease activity during and after pregnancy
Annualized relapse rates before, during and after pregnancy are shown in Fig. 2a. In accordance with the literature, relapse rates in our study cohort were lowered significantly during the third trimester of pregnancy and increased significantly after delivery. The mean EDSS of pregnant MS patients was not altered during the follow-up (1·5 ± 1·3 at early pregnancy versus 1·4 ± 0·9 6 months postpartum; mean ± standard deviation).
Fig. 2.
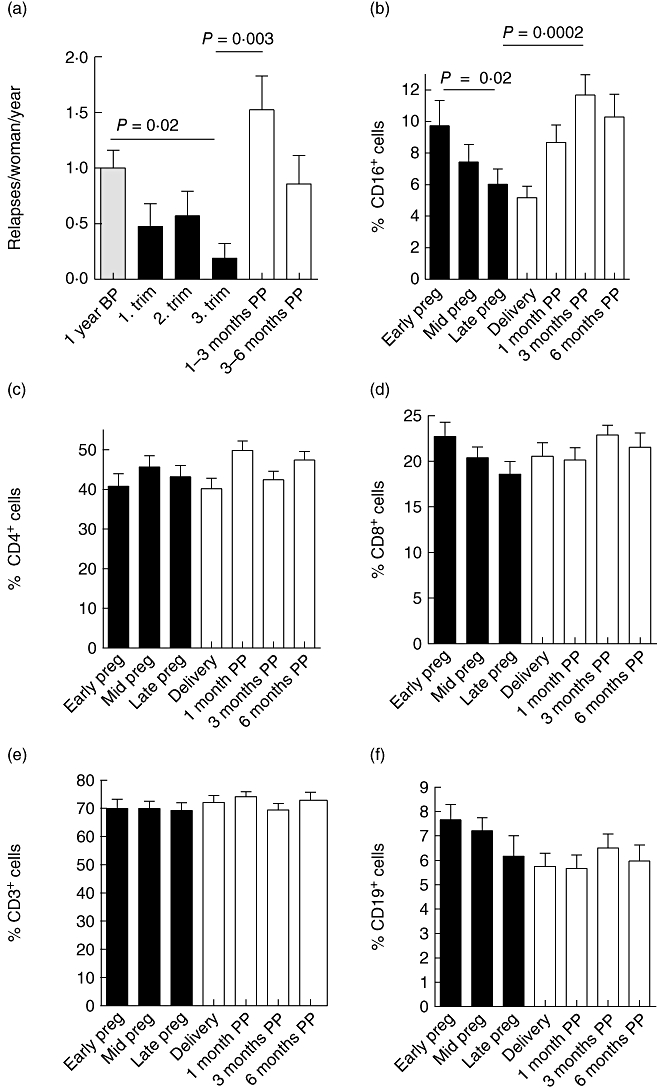
(a) Annualized relapse rates of 42 pregnant women with multiple sclerosis (MS) before, during and after pregnancy [relapses/woman/year; mean ± standard error of the mean (s.e.m.)]. Year before pregnancy (1 year BP) 1·0 ± 0·2; first trimester 0·5 ± 0·2; third trimester 0·2 ± 0·1; 1–3 months postpartum (mo PP) 1·5 ± 0·3; 3–6 months postpartum 0·9 ± 0·3. (b–f) The mean percentages ± s.e.m. of CD16+ natural killer (NK) cells (b), CD4+ T cells (c), CD8+ T cells (d), CD3+ T cells (e), CD19+ B cells (f) (n = 21–35). Blood samples were drawn at weeks 10–12, 26–28 and 35–37 of pregnancy (early pregnancy, mid-pregnancy and late pregnancy, black bars), at the time of delivery, and 1, 3 and 6 months postpartum (mo pp, white bars). At each time-point peripheral blood mononuclear cells were freshly isolated, immunofluorescence stained and analysed by fluorescence activated cell sorter as described in Material and methods. (b) Significant alteration (P < 0·0001) was observed in the proportion of CD16+ cells: 9·7 ± 1·6% CD16+ cells in early pregnancy versus 6·0 ± 0·95% CD16+ cells in late pregnancy; P = 0·02; 11·7 ± 1·3% CD16+ cells at 3 months postpartum; 3 months postpartum versus late pregnancy P = 0·0002. (c–f) No significant alteration was observed in the proportions of CD4+, CD8+ and CD3+ T cells or CD19+ B cells. Statistical analysis was performed using analysis of variance for repeated measurements with the Tukey–Kramer post-test.
Reduction of CD16+ NK cells during pregnancy and increase after the delivery
Immunophenotyping of peripheral blood cells was performed by flow cytometric assessment. Analysing major immune cells subsets (CD16, CD3, CD4, CD8, CD19) we found a significant reduction (of 38%) in the percentage of CD16+ NK cells from first to third trimester in MS patients (Fig. 2b). After the delivery the proportion of CD16+ cells increased again (Fig. 2b). Six months after the delivery the proportion of CD16+ NK cells was still higher than during the third trimester (10·3 ± 1·4% versus 6·0 ± 0·95%, P = 0·004). A similar reduction and increase was observed in healthy pregnant controls (Table 2). The percentage of CD16+ cells was diminished markedly both in MS patients and in controls during the third trimester of pregnancy when compared to a time of no pregnancy (P < 0·05; anova with Tukey's post-test; Table 2). Proportions of CD3+, CD4+ and CD8+ T cells or CD19+ B cells were not altered significantly in the course of pregnancy of MS patients (Fig. 2c–f) or of healthy control subjects (not shown).
Table 2.
Percentages (mean ± standard error of the mean) of CD16+ lymphocyte subpopulations in the peripheral blood of multiple sclerosis (MS) patients and healthy controls during a non-pregnant state, during pregnancy and 6 months postpartum.
| Non-pregnant* | Early pregnancy† | Late pregnancy‡ | 6 months PP§ | P value¶ | |
|---|---|---|---|---|---|
| MS patients (n) | 12,4 ± 1·6 (18) | 9·7 ± 1·6 (23) | 6·0 ± 1·0†† (27) | 10·3 ± 1·4 (17) | 0·01 |
| Healthy controls (n) | 11·4 ± 1·0 (18) | 8·7 ± 1·4 (23) | 6·3 ± 0·7†† (25) | 12·7 ± 2·2 (15) | 0·002 |
Non-pregnant subjects
sampling at 10–12 gestational weeks
sampling at 35–37 gestational weeks
sampling 6 months postpartum (PP)
overall P, analysis of variance.
Percentage of CD16+ cells in non-pregnant versus late pregnant MS patients P < 0·05 and in non-pregnant healthy subjects versus late pregnant healthy subjects P < 0·05, Tukey's post-test.
Proportion of CD56bright NK cells increases during pregnancy
After this first survey we performed a more detailed analysis of NK cell subpopulations in a subgroup of patients (n = 12). Using double stainings of lymphocytes to identify subgroups of NK cells during pregnancy and postpartum, we found a reduction in the proportion of CD56dim CD3– NK cells in the course of pregnancy (Fig. 3b), while at the same time the percentage of CD56bright NK cells, a subpopulation of NK cells with putative regulatory function [24], increased (Fig. 3d). During late pregnancy the proportion of circulating CD56bright NK cells was higher than postpartum, both in relation to all PBL (Fig. 3c) and in relation to all CD56+ CD3– NK cells (Fig. 3d). The analysed subgroup of patients was representative for the study cohort, as seven of 12 patients experienced one to two relapses during the 6 months postpartum and none of 12 patients had a relapse during the third trimester. The observed changes seem to be related to pregnancy and not related specifically to MS, as NK cell subtyping in pregnant healthy controls was comparable to that observed in MS patients (data not shown). The modulation of CD56 expression is most probably an indirect effect of pregnancy, as in vitro treatment of lymphocytes with high concentrations of oestrogen had no direct effect on the expression levels of CD56 expression (data not shown).
Fig. 3.
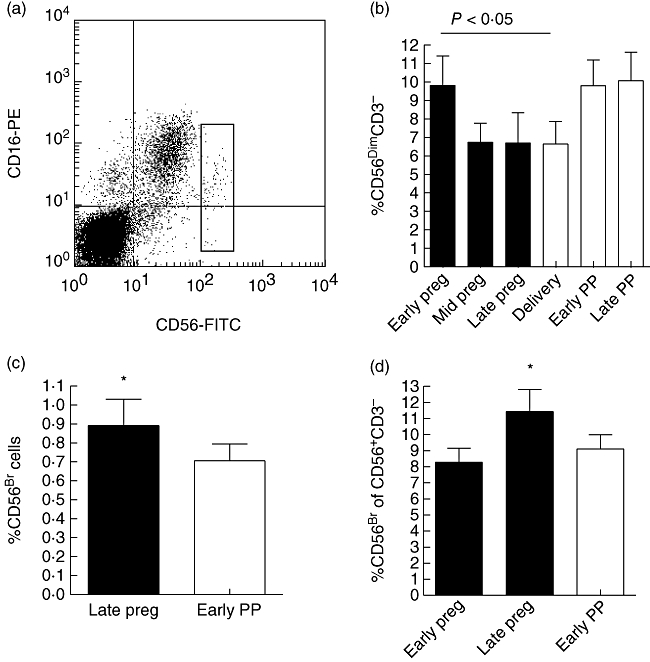
CD56-expression on lymphocytes of multiple sclerosis (MS) patients during and after pregnancy (PP, postpartum). (a) CD56CD16 double staining of peripheral blood lymphocytes (PBL) from a non-pregnant MS patient. Gated cells in the dot plot represent the CD56bright natural killer (NK) subset. (b–d) Viably frozen lymphocytes from 12 MS patients were double-stained using anti-CD56 and anti-CD3 antibodies. Proportion of CD56bright cells and CD56dim cells was calculated and the results are given as mean percentage of positive cells ± standard error of the mean (s.e.m.). (b) The overall alteration in the mean percentage of CD56dimCD3– cells in serial samples was significant; overall P = 0·02 (repeated-measures analysis of variance; black bars = pregnancy, white bars = postpartum). Percentage of CD56dim cells of all PBL ± s.e.m.: early pregnancy 9·8 ± 1·6; mid-pregnancy 6·7 ± 1·0; late pregnancy 6·7 ± 1·6; delivery 6·6 ± 1·2; early PP 9·8 ± 1·4; late PP 10·1 ± 1·5. Early pregnancy versus delivery P < 0·05, Bonferroni's multiple comparison post-test. (c, d) Proportion of CD56bright cells is significantly higher during the third trimester than at other time-points in comparison to either all PBL (c) or to all CD56+ cells (d). (c) Percentage of CD56bright cells of all PBL ± s.e.m.: late pregnancy 0·89 ± 0·14; early PP 0·70 ± 0·09; P = 0·0319, paired Student's t-test. (d) Percentage of CD56bright cells of all CD56+ CD3– NK cells ± s.e.m.: early pregnancy 8·28 ± 0·88; late pregnancy 11·45 ± 1·36; early PP 9·11 ± 0·88; overall P = 0·0179; repeated-measures analysis of variance, early pregnancy versus late pregnancy and late pregnancy versus early PP, P < 0·05; Bonferroni's multiple comparison post-test.
No alteration in Treg cells during pregnancy versus postpartum
Regulatory T cell populations have been demonstrated to be critically involved in the control of autoimmunity. To further elucidate the potential immunoregulatory mechanisms operative during pregnancy, we analysed percentages of the major population of naturally occurring regulatory T cells, the CD4+ CD25high FoxP3+ Treg cells during pregnancy and postpartum. Frequency of this population showed no alteration in relation to pregnancy/postpartum (Fig. 4a, b).
Fig. 4.
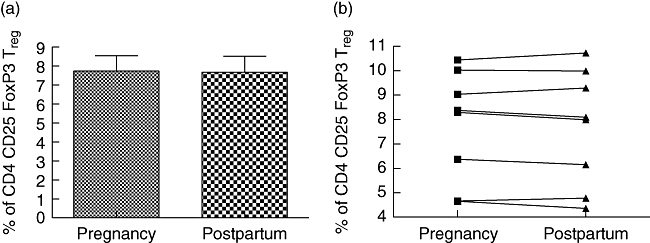
The frequency of CD4+ CD25high forkhead box P3 (Foxp3)+ regulatory T cells in peripheral blood of multiple sclerosis patients shown during late pregnancy and early postpartum stage (i.e. at gestational weeks 35–37 and 4–5 weeks postpartum; n = 8). (a) Peripheral blood lymphocytes from patients were stained for cell surface expression of CD4 and CD25. The stained cells were fixed and stained intracellularly for FoxP3. For analysis, the peripheral blood lymphocytes were gated on CD4+ lymphocytes (based on forward and side light-scatter and CD4 staining) and analysed for CD25high and FoxP3 expression. (b) Each symbol represents an individual patient. No alteration in the proportion of CD4+ CD25high Foxp3+ regulatory T cells was observed.
Th2 : Th1 ratio is increased during late pregnancy versus postpartum
To study whether the observed reduction in the MS disease activity is associated with a reduction in the production of Th1-type cytokines, we measured the intracytoplasmic IFN-γ and IL-4 production in the PBL both during a late pregnant and early postpartum stage. In MS patients, the percentage of IFN-γ-secreting cells within the PBL pool was significantly lower during pregnancy than postpartum (Fig. 5a). The difference in the IL-4-producing cells was less pronounced (Fig. 5c). This resulted in a Th2 : Th1 ratio of 0·36 during pregnancy and 0·23 after the delivery. In healthy pregnant controls no alteration was observed in IFN-γ-producing cells (Fig. 5b), but the percentage of IL-4-producing cells was significantly higher during pregnancy than postpartum (Fig. 5d). Thus, in controls the Th2 : Th2 ratio was also higher during pregnancy than postpartum (0·32 versus 0·13).
Fig. 5.
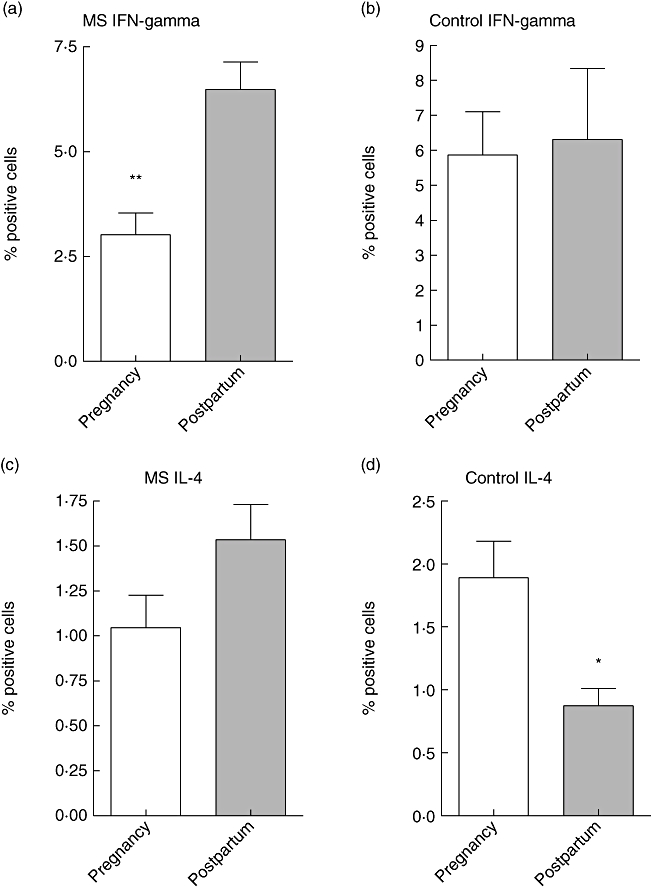
Intracellular detection of interferon (IFN)-γ and interleukin (IL)-4 was performed as described in Materials and methods. Briefly, peripheral blood lymphocytes (PBL) were isolated from multiple sclerosis patients and from healthy controls during late pregnancy (gestational weeks 35–37; white bars) and early postpartum (4–5 weeks postpartum; grey bars). After in vitro stimulation of PBL with phorbol myristate acetate, the cells were permeabilized and stained with anti-IFN-γ and anti-IL-4 monoclonal antibodies. Detection of positive cells was performed using fluorescence activated cell sorter, and shown are the percentages of positive cells ± standard error of the mean.
Discussion
The aim of this paper was identification of immunomodulatory factors potentially contributing to reduced MS disease activity during pregnancy. By studying the largest number so far of pregnancy-related samples from MS patients, we have identified CD56bright NK cells as good candidates for regulators of MS disease activity during pregnancy, as their proportion is increased during pregnancy simultaneously with cessation of disease activity. When relapse rate increased after the delivery, the proportion of CD56bright NK cells was diminished. Functional studies addressing the role of CD56bright NK cells in the pathogenesis of autoimmune diseases have shown that CD56bright NK cells possess regulatory capacity beneficial for the control of autoimmune diseases; they are able to limit the survival of activated T cells [24]. The immunoregulatory function of CD56bright NK cells is promoted by daclizumab, a drug currently under investigation for the treatment of MS [24–26]. Interestingly, IFN-β therapy of MS patients leads to decreased CD56dim NK cell numbers [27] and an increased proportion of circulating CD56bright NK cells [28].
Our study suggests that CD56bright NK cells may play a pivotal role in balancing inflammation. NK cells have been implicated in the regulation of autoimmunity via interaction with cells of the adaptive immune system [29,30]. In MS, depression of cytotoxic NK cell activity has been shown to precede clinical relapses, whereas the proportion of CX3CR1+ (CD56dim) NK cells was noted to be increased during relapses [31,32]. NK cells of MS patients actively suppress potentially pathogenic autoimmune T cells that are responsible for the inflammatory reaction in the central nervous system (CNS); this may help to maintain clinical remission in some patients [33]. NK cells inhibit T cell proliferation via up-regulation of the cell cycle inhibitor, p21 [34]. Moreover, animal studies suggest a protective role for NK cells in EAE, the model for MS [35–38]. Mouse NK cells do not express CD56, but the different mouse NK cell subsets can be distinguished based on the expression of CD127 (or IL7Ra) [39]. Mouse CD127+ NK cells correspond to human CD56bright NK cells. In mouse, the CD127+ NK cells originate from the thymus, and migrate to peripheral lymphoid organs to regulate T cell functions. The CD127dim NK cells originate from the bone marrow and these cells, thought to correspond to the human CD56dim cells, are more efficient at killing target cells, and reside in the spleen and liver. Interestingly, polymorphisms in the IL-7Ra (or CD127) were identified recently as a genetic risk factor for multiple sclerosis [40,41].
CD4+ CD25high regulatory T cells and T helper cells producing Th2-type cytokines are among other cell types that have the potential to control autoimmune activity in MS [42]. According to our study, secretion of Th2 cytokines is enhanced during late pregnancy, but no alteration could be noted in the percentage of CD4+ CD25high FoxP3+ Treg cells when compared to time postpartum, unlike that suggested by some previous work [16,17]. This is in line with the observation that the function of the CD4+ CD25high regulatory T cells of MS patients may be altered, leading to breakdown of immunological self-tolerance despite unchanged proportion of Treg cells compared to healthy controls [42,43].
NK cells do not have to recognize major histocompatibility complex restricted (auto)antigens to exert their function, which makes CD56bright NK cells good candidates of regulators of autoimmune activity during pregnancy. Our future aim is to clarify the mechanisms of how CD56bright NK cells control MS disease during pregnancy. Learning how to re-establish and maintain a balance between autoreactive and immunoregulatory cells probably has important implications for the management of MS.
Acknowledgments
The participation of all MS patients and control subjects to the study is greatly acknowledged. We are grateful for the investigators and study nurses for their active participation in the project. Ms Suvi Hyvönen and Ms Sari Mäki are acknowledged for excellent technical assistance and Dr Tero Vahlberg for help with the statistical analyses. The work of H. W. is supported by the Deutsche Forschungsgemeinschafte (DFG 1722/3-2, SFB) and the IZKF Würzburg (48-0-0). L. A. has obtained research grants from the Finnish Medical Foundation, Finnish Neurological Foundation and Finnish MS Foundation.
APPENDIX
Finnish Multiple Sclerosis and Pregnancy Study Group researchers and sites (number of patients shown in parenthesis): Dr Laura Airas and Dr Saara Väisänen, Department of Neurology, MSc Maija Saraste, MediCity Research Laboratory and Dr Anna Alanen, Department of Obgyn, University of Turku (12); Dr Markus Färkkilä and Dr Juha Multanen, Department of Neurology and Dr Risto Kaaja, Department of Obgyn, University of Helsinki (12); Dr Mauri Reunanen and Dr Anne Remes, Department of Neurology and Dr Tytti Raudaskoski, Department of Obgyn, University of Oulu (three); Dr Tuula Pirttilä, Department of Neurology and Dr Maritta Räsänen, Department of Obgyn, University of Kuopio (three); Dr Irina Elovaara, Department of Neurology and Dr Jukka Uotila, Department of Obgyn, University of Tampere (three); Dr Mikko Kuoppamäki, Department of Neurology and Dr Laila Närhinen, Department of Obgyn, Satakunta Central Hospital, Pori (two); Dr Peter Baumann Department of Neurology and Dr Riitta Aliharmi, Department of Obgyn, Rovaniemi Central Hospital (one); Dr Seppo Tuisku, Department of Neurology, Keski-Pohjanmaa Central Hospital, Kokkola (one); Dr Juha Huhtakangas, Department of Neurology, Kainuu Central Hospital, Kajaani (one); Dr Annikki Salmivaara, Department of Neurology, Päijät-Häme Central Hospital, Lahti (one); Dr Laura Kupila, Department of Neurology, Rauma District Hospital (one); Dr Merja Kaislakoski, Department of Neurology, Joensuu Central Hospital (one); Dr Taneli Sarasoja, Department of Neurology, Central Hospital of Central Finland, Jyväskylä (one).
References
- 1.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339:285–91. doi: 10.1056/NEJM199807303390501. Pregnancy Multiple Sclerosis Group. [DOI] [PubMed] [Google Scholar]
- 2.Spector TD, Da Silva JA. Pregnancy and rheumatoid arthritis: an overview. Am J Reprod Immunol. 1992;28:222–5. doi: 10.1111/j.1600-0897.1992.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 3.Rabiah PK, Vitale AT. Noninfectious uveitis and pregnancy. Am J Ophthalmol. 2003;136:91–8. doi: 10.1016/s0002-9394(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 4.Muller AF, Berghout A. Consequences of autoimmune thyroiditis before, during and after pregnancy. Minerva Endocrinol. 2003;28:247–54. [PubMed] [Google Scholar]
- 5.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. Reproductive immunology 2003: reassessing the Th1/Th2 paradigm? Immunol Lett. 2004;92:207–14. doi: 10.1016/j.imlet.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21:3352–7. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- 8.Irony-Tur-Sinai M, Grigoriadis N, Lourbopoulos A, Pinto-Maaravi F, Abramsky O, Brenner T. Amelioration of autoimmune neuroinflammation by recombinant human alpha-fetoprotein. Exp Neurol. 2006;198:136–44. doi: 10.1016/j.expneurol.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Clark DA. Tolerance signaling molecules. Chem Immunol Allergy. 2005;89:36–48. doi: 10.1159/000087911. [DOI] [PubMed] [Google Scholar]
- 10.Bebo BF, Dveksler GS. Evidence that pregnancy specific glycoproteins regulate T-cell function and inflammatory autoimmune disease during pregnancy. Curr Drug Targets Inflamm Allergy. 2005;4:231–7. doi: 10.2174/1568010053586255. [DOI] [PubMed] [Google Scholar]
- 11.Sicotte NL, Liva SM, Klutch R, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52:421–8. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52:1230–8. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 13.Polanczyk MJ, Jones RE, Subramanian S, et al. T lymphocytes do not directly mediate the protective effect of estrogen on experimental autoimmune encephalomyelitis. Am J Pathol. 2004;165:2069–77. doi: 10.1016/S0002-9440(10)63257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore W, Arias M, Stroud N, Stek A, McCarthy KA, Correale J. Preliminary studies of cytokine secretion patterns associated with pregnancy in MS patients. J Neurol Sci. 2004;224:69–76. doi: 10.1016/j.jns.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Ramon S, Navarro AJ, Aristimuno C, et al. Pregnancy-induced expansion of regulatory T-lymphocytes may mediate protection to multiple sclerosis activity. Immunol Lett. 2005;96:195–201. doi: 10.1016/j.imlet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruse N, Greif M, Moriabadi NF, Marx L, Toyka KV, Rieckmann P. Variations in cytokine mRNA expression during normal human pregnancy. Clin Exp Immunol. 2000;119:317–22. doi: 10.1046/j.1365-2249.2000.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzi M, Vigano A, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1 : Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhard G, Noll A, Schlebusch H, Mallmann P, Ruecker AV. Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. Biochem Biophys Res Commun. 1998;245:933–8. doi: 10.1006/bbrc.1998.8549. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 23.Airas L, Salmi M, Jalkanen S. Lymphocyte–vascular adhesion protein-2 is a novel 70-kDa molecule involved in lymphocyte adhesion to vascular endothelium. J Immunol. 1993;151:4228–38. [PubMed] [Google Scholar]
- 24.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56 (bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci USA. 2004;101:8705–8. doi: 10.1073/pnas.0402653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–9. doi: 10.1212/01.wnl.0000267662.41734.1f. [DOI] [PubMed] [Google Scholar]
- 27.Perini P, Wadhwa M, Buttarello M, et al. Effect of IFNbeta and anti-IFNbeta antibodies on NK cells in multiple sclerosis patients. J Neuroimmunol. 2000;105:91–5. doi: 10.1016/s0165-5728(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 28.Saraste M, Irjala H, Airas L. Expansion of CD56bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol Sci. 2007;28:121–6. doi: 10.1007/s10072-007-0803-3. [DOI] [PubMed] [Google Scholar]
- 29.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 30.Flodstrom M, Shi FD, Sarvetnick N, Ljunggren HG. The natural killer cell − friend or foe in autoimmune disease? Scand J Immunol. 2002;55:432–41. doi: 10.1046/j.1365-3083.2002.01084.x. [DOI] [PubMed] [Google Scholar]
- 31.Kastrukoff LF, Lau A, Wee R, Zecchini D, White R, Paty DW. Clinical relapses of multiple sclerosis are associated with ‘novel’ valleys in natural killer cell functional activity. J Neuroimmunol. 2003;145:103–14. doi: 10.1016/j.jneuroim.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Infante-Duarte C, Weber A, Kratzschmar J, et al. Frequency of blood CX3CR1-positive natural killer cells correlates with disease activity in multiple sclerosis patients. FASEB J. 2005;19:1902–4. doi: 10.1096/fj.05-3832fje. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Aranami T, Endoh M, Miyake S, Yamamura T. The regulatory role of natural killer cells in multiple sclerosis. Brain. 2004;127:1917–27. doi: 10.1093/brain/awh219. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi PP, Roberts PC, Wolf NA, Swanborg RH. NK cells inhibit T cell proliferation via p21-mediated cell cycle arrest. J Immunol. 2005;174:4590–7. doi: 10.4049/jimmunol.174.8.4590. [DOI] [PubMed] [Google Scholar]
- 35.Huang D, Shi FD, Jung S, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–87. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto Y, Kohyama K, Aikawa Y, et al. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–8. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 38.Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;163:24–30. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–24. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 40.Lundmark F, Duvefelt K, Iacobaeus E, et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat Genet. 2007;39:1108–13. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 41.International Multiple Sclerosis Genetic Consortium. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–62. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 42.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147:412–8. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


