Abstract
Factor H is the major regulatory protein of the alternative pathway of complement activation. Abnormalities in factor H have been associated with renal disease, namely glomerulonephritis with C3 deposition including membranoproliferative glomerulonephritis (MPGN) and the atypical haemolytic uraemic syndrome (aHUS). Furthermore, a common factor H polymorphism has been identified as a risk factor for the development of age-related macular degeneration. These associations suggest that alternative pathway dysregulation is a common feature in the pathogenesis of these conditions. However, with respect to factor H-associated renal disease, it is now clear that distinct molecular defects in the protein underlie the pathogenesis of glomerulonephritis and HUS. In this paper we review the associations between human factor H dysfunction and renal disease and explore how observations in both spontaneous and engineered animal models of factor H dysfunction have contributed to our understanding of the pathogenesis of factor H-related renal disease.
Keywords: complement, Factor H, glomerulonephritis, thrombosis
Biological function of complement factor H
Complement factor H was first described in 1965 [1]. It is an abundant, single chain glycoprotein circulating at a concentration of approximately 400 mg/l. The major source of factor H is the liver, but extra-hepatic synthesis has been demonstrated in a variety of tissues including endothelial cells [2], glomerular mesangial cells [3] and in rodents, podocytes [4]. The protein is composed of 20 short consensus repeat (SCR) domains, also known as complement control protein domains, or Sushi repeats. Each SCR domain is composed of approximately 60 amino acids based on a framework of four conserved cysteine residues.
The principle function of factor H is to down-regulate alternative pathway complement activation, which it achieves in three ways. It acts as an essential co-factor in the factor I-mediated proteolytic conversion of plasma C3b to iC3b [5,6]. iC3b may then be cleaved into further fragments (C3dg and C3c) by factor I using the membrane-bound co-factor CR1 (complement receptor 1, CD35). Factor H is also able to block the formation of the alternative pathway C3 convertases by binding to C3b, thereby inhibiting the interaction between C3b and factor B [7–10]. Factor H also possesses decay acceleration activity, i.e. promotes the dissociation of these C3 convertases once they have formed [8]. It is also able to distinguish between activator and non-activator surfaces in vivo, an effect mediated partly by its ability to bind sialic acid [11–13].
Deletion mutagenesis studies have demonstrated that regulation of fluid-phase C3 activation requires the N-terminal five SCR domains [14–16]. In contrast, targeting of the protein to cell surfaces (surface recognition function) is mediated by C-terminal domains [17]. That the complement regulatory and surface recognition functions reside in distinct locations of the protein is of major importance in the understanding of the molecular pathogenesis of factor H-associated renal disease.
Age-related macular degeneration (AMD) [18–21], atypical haemolytic uraemic syndrome (HUS) (reviewed in [22]) and membranoproliferative glomerulonephritis (MPGN) [20,23–27] are associated with polymorphisms or mutations in the factor H gene. This suggested the existence of a genotype–phenotype relationship [28].
In the first section of this review we will discuss the renal pathology associated with factor H dysfunction and explore its similarities to the renal pathology described in other situations where alternative pathway dysregulation occurs. We start by reviewing the pathology described in individuals with homozygous factor H deficiency.
Human factor H deficiency associated with very low or absent factor H levels
The phenotypic analysis of individuals with inherited complete deficiency of factor H has provided important insights into the biological role of factor H in vivo. However, such cases are extremely rare; in Table 1 we summarize the clinical and laboratory investigations of 33 factor H-deficient individuals from 15 families [23,25–27,29–38]. In all these individuals factor H levels were absent or markedly reduced.
Table 1.
Human factor H deficiency associated with very low or absent factor H levels.
| Family | Onset, sex, race | Clinical features | Complement profile | Notes |
|---|---|---|---|---|
| 1a [W] [38] | 8 months, M, Asian (parents related) | Recurrent HUS | CH50 13 µ/ml (25–45), AP50 4·5 µ/ml (12–26) | Free C3d in plasma using C3 crossed immuno-electrophoresis |
| Bilateral otitis media (Haemophilus influenzae) associated with HUS relapse | C3 0·12 g/l (0·75–1·75) | |||
| Factor B 35% (70–150), properdin 60% (60–130) | ||||
| C5, 6, 7, 8, 9 levels normal | ||||
| Renal biopsy: HUS – see Table 2 | Factor I levels normal | |||
| C3 nephritic factor absent | ||||
| 1b [Z] [38] | 3 years, M, Asian (parents related) | Healthy | CH50 11 µ/ml (25–45), AP50 6 µ/ml (12–26) | Free C3d in plasma using C3 crossed immuno-electrophoresis |
| C3 0·17 g/l (0·75–1·75) | ||||
| Factor B 32% (70–150), properdin 60% (60–130) | ||||
| C5, 6, 7, 8, 9 levels normal | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 2a [case III-3] [23] | 14 months, M, Algerian (parents related) | Recurrent macroscopic haematuria following infections from the age of 14 months | CH50 0% NHP, AP50 0% NHP | Homozygous CYS431SER change affecting SCR7 [26] |
| C3 8% (70–150) | ||||
| Factor B 20% (65–150) | ||||
| Renal biopsy: ‘atypical DDD’ – see Table 2 | C5 15% (60–150) | |||
| C6 45% (60–140) | ||||
| C7 30% (60–110) | ||||
| C8 45% (60–140) | ||||
| C9 30% (70–150) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 2b [case III-5] [23] | 4·5 months, M, Algerian (parents related) | Failure to thrive, macroscopic haematuria, otitis media (Escherichia coli) | CH50 0%, AP50 0% | Residual factor H present as evidenced by bright glomerular staining within mesangium and thickened glomerular capillary walls for factor H on IF studies of second renal biopsy |
| C3 5% (70–150) | ||||
| Factor B 15% (65–150) | ||||
| Recurrent pulmonary sepsis | C5 15% (60–150), C6 35% (60–140), | |||
| C7 35% (60–110), C8 35% (60–140), | ||||
| Renal biopsies: ‘atypical DDD’ – see Table 2 | C9 20% (70–150) | |||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 3a, b, c*[H1, H2, H3] [25] | 3 female siblings | MPGN in all sepsis due to Neisseria meningitidisin two | CH50 < 5 µ/ml, AP50 < 10% in all | Renal biopsy details not reported |
| C3 0·08, 0·12 and 0·07 g/l, respectively,* | ||||
| Factor B 15, 12 and 14% normal, respectively, | ||||
| C5 < 10% normal in all three cases | ||||
| C9 ≤ 10% normal | ||||
| Factor I levels normal – all cases | ||||
| C3 nephritic factor absent – all cases | ||||
| 4a [II-3] [37] | 11 years, F, Italian (parents related) | SLE: psychosis, discoid skin lesions, chronic renal failure | CH50 absent | Heterozygous C2 deficiency |
| C2 15% (75–120), C3 < 1 mg/dl (80–140) | ||||
| Skin biopsy (unaffected skin): positive lupus band test, i.e. IgM, IgA and C3 at dermo–epidermal junction | Factor B < 1 mg/dl (12–28) | |||
| Properdin 47% (70–130) | Factor H and FHL-1 absent in sera [39] | |||
| C5 5% (70–120), C6 52% (59–129), | ||||
| Renal biopsy: lupus nephritis – see Table 2 | C7 38% (60–120), C8 21% (70–130), | Homozygous GLU189stop change affecting SCR3 [39] | ||
| C9 35% (50–140) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 4b [II-4] [37] | ?, M, Italian (parents related) | Healthy | CH50 absent | Factor H and FHL-1 absent in sera [39] |
| C2111% (75–120), C3 < 1 mg/dl (80–140) | ||||
| Factor B < 1 mg/dl (12–28), | Homozygous GLU189stop change affecting SCR3 [39] | |||
| Properdin 55% (70–130) | ||||
| C5 5% (70–120), C6 52% (59–129), | ||||
| C7 24% (60–120), C8 42% (70–130), | ||||
| C9 24% (50–140) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 4c [II-2] [37] | ?, F, Italian (parents related) | Asthma, haematuria | CH50 absent | Heterozygous C2 deficiency |
| C2 44% (75–120), C3 < 1 mg/dl (80–140) | ||||
| Factor B < 1 mg/dl (12–28) | Factor H and FHL-1 absent on Western blotting [39] | |||
| Properdin 39% (70–130) | ||||
| C5 5% (70–120), C6 52% (59–129), | ||||
| C7 38% (60–120), C8 21% (70–130), | Homozygous GLU189stop change affecting SCR3 [39] | |||
| C9 35% (50–140) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 5 [36] | 15 years, F, Danish | Two episodes of meningitis (Neisseria meningitidis) | CH50 < 2% NHP (> 67), AP50 < 38% (> 61) | Parents had 50% normal factor |
| C3 < 2% NHP (> 84) | H levels | |||
| Factor B 6% (>63), properdin 18% (> 70) | C3dg and to lesser extent C3c elevated in plasma | |||
| C5 7% (> 68), C6 30% (> 63), C7 < 0·5% | ||||
| (> 61), C8 39% (> 75), C9 50% (> 53) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 6a [III-2] [35] | 19 years, F, French-Canadian (parents unrelated) | 3 episodes of HUS: the first was preceded by non-bloody diarrhoea, the second had no clear trigger and required dialysis for 9 months, the third was preceded by an upper respiratory tract infection renal biopsy: HUS – see Table 2 | CH50 54% NHP (77–122), AP50 37% (75–125) | Female sibling died at early age from HUS |
| C3 19 mg/dl (72–135) | ||||
| Factor B 17 mg/dl (22–48) | ||||
| Properdin 115% (82–119) | ||||
| C5 40% (67–133) | ||||
| C8100% (67–133) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 6b [III-3] [35] | 24 years, F, French-Canadian (parents unrelated) | Healthy | CH50 51% NHP (77–122), AP50 38% (75–125) | |
| C3 19 mg/dl (72–135) | ||||
| Factor B 14 mg/dl (22–48) | ||||
| Properdin 92% (82–119) | ||||
| C5 50% (67–133) | ||||
| C8 75% (67–133) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 7 [34] | 6 years, M, Sioux Indian | Hypertension and heart failure at 14 months, recurrent haematuria and proteinuria | C3 < 27 mg/dl (88–155) | FHL-1 present in sera |
| Factor B 56 µg/ml (112–300) | ||||
| Renal biopsy: Type III collagen glomerulopathy – see Table 2 | Factor I levels normal | CYS536ARG affecting SCR9 on one allele, CYS959TYR affecting SCR16 on the other resulting in impaired FH secretion [40,41] | ||
| 8 [II-9] [33] | 59 years, F, Dutch, (parents unrelated) | Recurrent furunculosis in childhood, recurrent urinary tract infections, SCLE lesions on face from 30 years age, meninigitis aged 48 years (N. meningitidis serogroup X) | C3 absent using antigenic assay | FHL-1 present in sera |
| C3 haemolytic assay: 2–3% (75–125%) | ||||
| Factor B < 1% (13–22) | Absent 37 and 42 kDa factor H-related proteins | |||
| Properdin 4·5–6 µg/ml (17–27·7) | ||||
| C2 9–13 U/l (18–23) | Reduced C2 levels also noted in some relatives hence possible heterozygous C2 deficiency | |||
| C5 2–42% (76–136) | ||||
| C7 reduced | ||||
| Values represent ranges over 2 years | ||||
| C3 nephritic factor absent | ||||
| 9a, b, c, d, e, f, g, h, i, j [32] | 10 cases: 6 M and 4 F, median age at presentation 2 weeks (range 1–20 weeks) inbred Bedouin kindred from Southern Israel | All presented with HUS defined as acute renal insufficiency and microangiopathic haemolytic anaemia, three had diarrhoea – 10 patients died, one is alive on dialysis | Full complement profile measured in 4 cases: | Homozygous c.3747A > T transversion and 24 base in SCR20 resulting in deletion of the four most C-terminal amino acids [42,43] |
| C3 12, 0, 14, 0% (45–91) | ||||
| Factor B 18, 14, 57, 118% (36–86) | ||||
| Properdin 24, 54, 46, 90% (39–69) | ||||
| C5 34, 25, 52, 61% (41–103) | ||||
| C9 4, 18, 96, 89% (36–64) | ||||
| Renal biopsy: HUS – see Table 2 | Factor I levels normal | Normal factor H mRNA levels but defective secretion [44] | ||
| 10a [patient 4] [31] | 7 months, M, African | Hypertension, nephrotic syndrome, schistocytes on blood film | During remission: | FHL-1 present in sera |
| CH50 < 10% NHP (70–130) | ||||
| Renal biopsy: HUS | C3 < 3 mg/dl (65–110) | Homozygous TYR899stop change affecting SCR15 [26] | ||
| Factor B < 12 mg/dl (16–40) | ||||
| Long-term weekly FFP infusions maintained remission (Nathanson et al. 2001) | Factor I levels normal | |||
| C3 nephritic factor absent | ||||
| 10b [patient 5] [31] | 11 months, M, African (first cousin of patient 10) | Haemolytic anaemia aged 1 month, HUS aged 11 months, now dialysis-dependent schistocytes on peripheral blood smear | During remission: | 150 kDa factor H protein absent on |
| CH50 12% NHP (70–130) | Western blotting but 42 kDa FHL-1 protein present | |||
| C3 < 3 mg/dl (65–110) | ||||
| Factor B < 12 mg/dl (16–40) | ||||
| Renal biopsy: HUS | Factor I levels normal | Homozygous TYR899stop change affecting SCR15 [26] | ||
| C3 nephritic factor absent | ||||
| 11a [patient 3] [26] | ?, M, Turkish brother of 11b | Presented with MPGN | C3170 mg/l (660–1250) | Homozygous ARG127LEU change affecting SCR2 [26] |
| Clinical details unknown | Factor B 70 mg/l (90–320) | |||
| CH50 < 10% (70–130) | ||||
| 11b [patient 4] [26] | ?, M, Turkish brother of 11a | Presented with MPGN | C3100 mg/l (660–1250) | Homozygous ARG127LEU change affecting SCR2 [26] |
| Clinical details unknown | Factor B 50 mg/l (90–320) | |||
| CH50 < 10% (70–130) | ||||
| 12 [patient 6] [26] | 8 years, F, Caucasian | Presented with nephritic syndrome | C3 < 40 mg/l (660–1250) | Homozygous CYS673TYR change affecting SCR11 [26] |
| MPGN type I | Factor B 17 mg/l (90–320) | |||
| CH50 < 10% (70–130) | ||||
| 13 [29] | 12 months, M, Turkish (parents related) | Periorbital oedema and microscopic haematuria aged 12 months | CH50 < 10% (82–102) | |
| AP50 < 10% (84–150) | ||||
| No red cell fragments on blood film | C3 80 mg/l (825–1140) | |||
| Factor B 44 mg/l (140–200) | ||||
| Renal biopsy: fibrillary glomerulopathy – see Table 2 | C5 97 mg/dl (120–160) | |||
| Properdin 35% (80–120) | ||||
| Factor I levels normal | ||||
| C3 nephritic factor absent | ||||
| 14 [patient 1] [27] | 48 years, M | Nephrotic syndrome aged 48 years | C3 < 0·10 g/l (0·67–1·29) | 42 kDa FHL-1 protein present |
| History of hepatitis B infection in childhood | Factor B 23% (59–154) | |||
| Properdin 24% (54–157) | Homozygous PRO621THR change affecting SCR10 | |||
| C3d 32 mg/l (<7) | ||||
| Renal biopsy MPGN type I – see Table 2 | C5 57% (72–171) | |||
| Subsequent relapse with clinical features of HUS (thrombocytopenia, anaemia and renal failure) | Factor I levels normal | |||
| 15 [30] | 22 days, F, Korean | Generalized oedema, haematuria, proteinuria | Persistently low C3 | CYS1077TRP affecting SCR18 on one allele, GLN1139Stop affecting SCR19 on the other |
| Renal failure, thrombocytopenia, schistocytes on peripheral blood smear | ||||
| Improved with FFP infusions, relapses frequently followed infections | ||||
| Renal biopsy: HUS – see Table 2 |
( ) indicate normal ranges, first [] indicate case descriptors used in original reports, second [] denotes citation, %, % of standard,
ages, clinical details and normal ranges not reported. Mutation nomenclature: initiation methionine is amino acid number one, A of the ATG codon is nucleotide 74. AP50, alternative pathway haemolytic activity; CH50, total complement haemolytic activity; DDD, dense deposit disease; FFP, fresh frozen plasma; FHL, factor H-like protein; HUS, haemolytic uraemic syndrome; HUVEC, human umbilical vascular endothelial cells; NHP, normal human pool, MPGN, membranoproliferative glomerulonephritis; SCLE, subacute cutaneous lupus erythematosus; SCR, short consensus repeat.
That factor H is the major regulator of alternative pathway activation in vivo is evident from the complement profile described in this deficiency state. Typically, complete factor H deficiency is associated with secondary severe depletion of circulating C3 together with reduction in the alternative pathway activation protein, factor B (Table 1). As a consequence of the reduced C3 levels, functional assays of complement haemolytic activity, whether triggered by alternative (AP50) or classical (CH50) pathway activation, are reduced or even absent.
Properdin levels are also usually reduced (cases 1a, 1b, 4a, 4b, 4c, 5, 8, 13, 14, two cases from family 9), although two factor H-deficient individuals from one family had normal values (cases 6a, 6b) as did two of the factor H-deficient individuals from family 9. Where reported, terminal pathway components are usually low. Thus, in the 17 factor H-deficient individuals in whom C5 levels were measured they were reduced in 14 (cases 2a, 2b, 3a, 3b, 3c, 4a, 4b, 4c, 5, 6a, 6b, 8, 9, 13, 14) and normal in only two individuals (cases 1a and 1b).
Plasma factor I levels remain normal in complete factor H deficiency. Notably, this is not the case in the converse situation. Thus, individuals with homozygous factor I deficiency develop secondary reduction in factor H, thought to be the result of binding of factor H to C3b [45].
The majority of factor H-deficient individuals have developed clinical disease, with only three individuals healthy at the time of reporting (cases 1b, 4b, 6b). As a consequence of secondary C3 deficiency there is a relatively high incidence of bacterial infection including Neisserial meningitis (cases 3a, 3b, 5, 8). However, the striking manifestation is that of renal disease. Renal disease has been reported in 28 of the 33 individuals that we have summarized in Table 1.
The renal manifestations include 15 cases of haemolytic uraemic syndrome (HUS, 1a, 6a, 9a–j, 10a, 10b, 15) and nine cases of membranoproliferative glomerulonephritis (MPGN, 3a, 3b, 3c, 11a, 11b, 2a, 2b, 12, 14). Furthermore, one individual was reported as type III collagen glomerulopathy (case 7) and another fibrillary glomerulopathy (case 13). A factor H-deficient individual with systemic lupus erythematosus (SLE) and lupus nephritis (case 4a) has also been reported, although it is important to note that this individual was also thought to have heterozygous C2 deficiency. Interestingly, one individual who presented with nephritic syndrome and renal biopsy showed MPGN type I [27]. However, this patient also had a history of hepatitis B infection, which is associated with MPGN type I independently of factor H dysfunction. Subsequently, the patient developed clinical features of HUS (progressive renal failure with thrombocytopenia and non-immune haemolytic anaemia), but no further renal biopsy was performed. Finally, we include one individual (case 4c) from the same family who was noted to have to have microscopic haematuria, although no renal biopsy was reported.
Clearly, factor H deficiency is associated with a markedly increased incidence of renal disease. However, an obvious question is why is this deficiency state associated with both MPGN and HUS? Before we attempt to answer this we will first define the renal histopathological features of both MPGN and HUS and secondly review the renal histopathological features reported in factor H-deficient individuals.
Renal histopathological features of MPGN and HUS
MPGN (also termed mesangiocapillary glomerulonephritis) refers to the morphological appearance of a glomerular lesion characterized by thickening of the glomerular capillary wall together with hypercellularity in mesangial areas, changes readily identified by light microscopy. As thus defined, the lesion is non-specific, occurring in a diverse range of conditions. These conditions have been placed into three categories, namely (i) associated with deposition of immunoglobulins and/or complement. This includes immune complex-associated MPGN type I, which may be primary or secondary (e.g. SLE, chronic infections including hepatitis C infection and subacute bacterial endocarditis); (ii) chronic thrombotic microangiopathies including late stages of acute HUS and thrombotic thrombocytopenia purpura; and (iii) paraprotein ‘deposition’ diseases which include type I cryoglobulinaemia, immunotactoid glomerulopathy and fibrillary glomerulopathy [46]. In the present context, this classification serves to emphasize the fact that the morphological appearance of MPGN can develop in chronic HUS. Distinguishing the underlying cause of MPGN requires ultrastructural assessment of the glomerulus using electron microscopy together with immunofluorescent studies assessing the presence of immunoglobulin and complement components.
In association with immune-complex diseases, MPGN is characterized typically by the presence of both complement and immunoglobulin within the glomerulus, together with the presence of subendothelial electron-dense deposits along the glomerular basement membrane (GBM). This subtype is referred to as MPGN type I.
Much less commonly, MPGN may be associated with the presence of glomerular C3 deposition in the absence of immunoglobulin. In this situation the electron microscopic appearance of the GBM commonly shows the characteristic changes described as dense deposit disease (DDD). The defining abnormality in DDD is the highly characteristic electron microscopic appearance of the GBM where there is striking transformation of the central part of the GBM (termed the lamina densa) by linear electron-dense material [47]. Electron-dense deposits may also be seen within the tubular basement membrane and mesangium. The light microscopic features of DDD include MPGN [48,49]. This latter observation led to the proposal that DDD should be considered a variant of MPGN [49], and DDD was referred to subsequently as MPGN type II. However, subsequent studies have emphasized the fact that MPGN is only one of the light microscopic features of DDD. For example, mesangial proliferative and necrotizing glomerulonephritis may be seen [50]. Moreover, in a recent study of 69 cases of DDD, an MPGN pattern was seen in only 17 (25%) of the biopsies [51]. The most common pattern was a mesangial proliferative lesion (n = 30, 45%). Crescentic (n = 12, 18%) and acute proliferative and exudative lesions (n = 8, 12%) were also noted. Irrespective of the glomerular morphology, DDD is associated with glomerular C3 deposition with little, if any, immunoglobulin [51]. In view of this, we do not think it is accurate to refer to DDD as a subtype of MPGN and hence will use the term ‘DDD’ in preference to ‘MPGN type II’ further in this review. The pathogenesis and therapy of DDD has recently been reviewed comprehensively [52].
A further subtype of idiopathic MPGN was described by Strife and colleagues [53]. In their report of seven individuals with MPGN they described GBM ultrastructural changes that were consistent with neither DDD nor MPGN type I. These biopsies demonstrated contiguous subepithelial and subendothelial deposits associated with basement membrane disruption. Granular deposition of C3 and properdin was present in these biopsies. Unlike DDD, immunoglobulins and Clq were variably present. This variant was termed MPGN type III.
HUS refers to the triad of renal failure, thrombocytopenia and non-immune microangiopathic haemolytic anaemia. The latter is demonstrated usually by the finding of red cell fragments (schistocytes) on peripheral blood smears. The acute renal lesion in HUS is thrombotic microangiopathy (TMA). Thrombotic microangiopathy may affect glomeruli, arterioles and arteries. In acute disease glomeruli show capillary loop thrombosis often with accumulation of fragmented red blood cells within capillary lumens. Ultrastructurally, there is damage to the endothelium with lifting of the endothelium from the basement membrane and the accumulation of flocculent material, sometimes containing fibrin and fragmented red blood cells, beneath the endothelium. Arterioles show thrombosis and fibrin, with their walls and arteries, may show marked loose myxoid expansion of the intima. With chronic injury the glomeruli develop laminated subendothelial thickening of the basement membrane.
In summary, the characteristic renal lesion associated with acute HUS is thrombotic microangiopathy. MPGN is a non-specific glomerular lesion. Clues to the aetiology of MPGN derive from a combination of immunofluorescence and ultrastructural studies. Hence, in DDD there is characteristic deposition of glomerular C3 without immunoglobulin together with electron-dense deposits within the central part of the GBM. In contrast, in MPGN associated with immune-complex disease, both immunoglobulin and C3 are present together with subendothelial electron-dense GBM deposits. An MPGN pattern associated with chronic thrombotic microangiopathy is associated with detachment of the endothelium from the GBM and the presence of subendothelial laminated thickening of the GBM without electron-dense deposits.
Renal biopsy findings in homozygous human factor H deficiency
We have summarized the reported renal biopsy findings in homozygous factor H-deficient humans in Table 2. In four reports of HUS and factor H deficiency renal biopsy findings were reported (Table 2). These biopsies showed convincing ultrastructural evidence of thrombotic microangiopathy, and included the presence of subendothelial deposits of flocculent material (cases 1a, 6a). Notably, there was no evidence of GBM electron-dense deposits and the clinical data indicated the presence of red cell fragments in all of these patients.
Table 2.
Renal biopsy findings in individuals with human factor H deficiency associated with very low or absent factor H levels.
| Case | Light microscopy | Immunofluorescence | Electron microscopy | Diagnosis |
|---|---|---|---|---|
| (a) Haemolytic uraemic syndrome | ||||
| 1a [W] [38] | Diffuse mesangial and endothelial cell proliferation and swelling | n.a. | Capillary wall thickening due to subendothelial deposits of floccular material | HUS* |
| Few capillary loops contained hyaline thrombi large vessels normal | ||||
| Interstitium normal, tubules showed minor vacuolation only | Deposits of floccular material also seen in mesangium | |||
| 6a [III-2] [35] | Diffuse endothelial swelling with normal cellularity of glomerular tuft | Focal and segmental deposits of fibrinogen on glomerulus and afferent arteriole | Granular, fluffy and fibrillary subendothelial deposits with marked thickening of GBM | HUS* |
| Necrotizing lesion affecting afferent arteriole | Discrete C3 deposits along capillary walls IgA, IgM, C1q, C3 deposits along afferent arteriolar walls | Mild mesangial matrix expansion but no deposits | ||
| Tubulointerstitium normal | ||||
| 9a–j [32] | Four kidney biopsies from 3 patients and 2 autopsy specimens were studied and reported in summary form: | |||
| Early stages of disease significant changes in arterioles and minor changes in glomeruli were noted | C3 staining noted on glomerular capillary walls while staining for IgG, IgA, IgM and fibrinogen was negative | Swollen endothelial cells narrowing lumen of interlobular arterioles | HUS* | |
| Arteriolar changes included: ‘onion skin’ changes, luminal stenosis and oedema, intimal thickening | Arteriolar walls stained intensely for actin | |||
| Arteriolar walls stained intensely for actin | ||||
| Glomerular changes included: endothelial swelling, mesangial proliferation and increased matrix formation. Very few capillary fibrin thrombi with platelet clumping were seen | ||||
| Second biopsy in patient 4, aged 6 months: | ||||
| Advanced fibrotic change in glomeruli and tubulointerstitium | ||||
| 15 [30] | Marked mesangial and endothelial cell proliferation and mesangial matrix with fibrillar appearance | Peripheral and mesangial staining for C3 and fibrinogen | Subendothelial widening and mesangial interposition without electron-dense deposit | HUS* |
| Double-contoured glomerular capillary loops observed focally | No staining for type III collagen | Some fibrils in mesangium | ||
| (b) Membranoproliferative glomerulonephritis | ||||
| 2a [case III-3] [23] | Diffuse capillary wall thickening with double contours and | Abundant, bright granular deposits of | Diffuse, irregular thickening of the BM of the capillary loops and mesangial stalks due to presence of electron-dense, homogeneous argyrophilic deposits, located mainly within the internal part of the GBM | Atypical DDD |
| Increased mesangial matrix | C3 in capillary walls and mesangial stalk | |||
| Arteries normal | Rare, segmental linear deposits were observed in the GBM | |||
| Interstitium normal | ||||
| Few tubules showed thickened basement membranes | Deposits only contained C3 | |||
| No deposits seen in tubular BM or within arteries | Mesangial interpositioning (mesangial cell cytoplasm in capillary walls) and focal effacement of foot processes | |||
| Mesangial matrix increased and contained electron-dense, homogeneous argyrophilic deposits | ||||
| Interstitium normal | ||||
| 2b [case III-5] [23] | Biopsy one (aged 6 months): | |||
| Mesangial cell and matrix increase | Scattered, bright and granular C3 deposits in mesangial areas and diffuse linear C3 appearance in capillary walls | n.a. | Atypical DDD | |
| Swollen endothelial cells | ||||
| Frequent neutrophils and monocytes in capillary lumens | ||||
| Occasional capillary wall double contours | ||||
| Arteries contained swollen endothelial cells | ||||
| Biopsy two (aged 2 years and 4 months): | ||||
| Mesangial cell and matrix increase | Abundant, bright and granular C3 deposits in mesangial stalks and capillary walls | Irregular thickening of the BM of the capillary loops and mesangial stalks due to presence of electron-dense, homogeneous argyrophilic deposits, intramembranous in location with occasional enlargement of the lamina rara interna. Occasional effacement of foot processes | ||
| Swollen endothelial cells | ||||
| Capillary walls diffusely thickened with occasional double contours | Few segmental linear C3 deposits along basement membranes | |||
| Epithelial cells hypertrophied | ||||
| Moderate interstitial fibrosis | No C3 staining in tubular BM or arteries | |||
| Arteries contained swollen endothelial cells | ||||
| Mesangial matrix increased and contained electron-dense, homogeneous argyrophilic deposits | ||||
| C5b-9 present on mesangial stalks, thickened capillary walls and tubular BM | No deposits were seen in vascular, tubular or Bowman's capsule BM | |||
| Positive staining with rabbit anti-human factor H antibody detected on mesangial stalks and thickened capillary walls | Monocytes and neutrophils seen in capillary loops and between BM and endothelial cells | |||
| 14 [patient 1] [27] | MPGN with duplication of GBM | C3 deposits in capillary basement membrane and mesangium | Subendothelial GBM deposits | MPGN type I (see also text) |
| Endothelial swelling and few thrombi noted in glomerular capillaries | ||||
| Global sclerosis in many glomeruli | ||||
| (c) Other | ||||
| 7 [34] | Biopsy one (aged 13 months): | |||
| Diffuse thickening of capillary loops with double contours | Trace mesangial and GBM staining for C3, C4, IgG, IgM, properdin and fibrinogen | Striking subendothelial widening by lucent granular and irregular membrane-like material | Type III collagen glomerulopathy (see also text) | |
| Glomerular endothelial proliferation and mesangial hypercellularity | ||||
| Occasional arterioles with reduplication of elastic lamina | Mesangial interpositioning (mesangial cell cytoplasm in capillary walls) | |||
| Biopsy two (aged 2 years): | ||||
| Diffuse thickening of capillary loops with double contours | Trace mesangial and GBM staining for C3, C4, IgG, IgM, properdin and fibrinogen | Subendothelial lucent space containing fibrillar material not organized into discrete deposits | ||
| Glomerular endothelial proliferation and mesangial hypercellularity | ||||
| Occasional medial hypertrophy in afferent arterioles and interlobular arteries | ||||
| Focal tubular atrophy | ||||
| Biopsy three (aged 5 years): | ||||
| Diffuse thickening of capillary loops with double contours | Trace mesangial staining for C1q, C4, | Subendothelial and mesangial deposition of flocculent material not organized into discrete deposits | ||
| Glomerular endothelial proliferation and mesangial hypercellularity and increased matrix | IgG and IgM | |||
| Arteriosclerosis of all small vessels | Segmental C3 deposition in capillary loops | Mesangial interpositioning (mesangial cell cytoplasm in capillary walls) and hypercellularity | ||
| Focal tubular atrophy | Diffuse mesangial and capillary loop staining for type III collagen | Collagen fibrils in mesangium and capillary loops | ||
| 13 [29] | Biopsy one (aged 17 months): | |||
| Severe lobulated glomeruli with mesangial hypercellularity; two glomeruli globally sclerotic. Diffuse GBM thickening and double contour appearance | Granular staining of GBM for C3 | Fibrillary glomerulopathy (see also text) | ||
| Biopsy two (aged 2·5 years): | ||||
| Glomeruli showed mesangial hypercellularity, capillary obliteration, GBM thickening and duplication; seven glomeruli globally sclerotic | Severe granular staining of GBM and mesangium for C3 | GBM thickening and lamellation but no electron-dense deposits | ||
| Staining for type III collagen not performed | Mesangium and GBM showed thin, non-amyloid fibrils | |||
| Mesangial hypercellularity and increased matrix | ||||
| Epithelial foot processes and capillary lumens were obliterated | ||||
| 4a [II-3] [37] | Diffuse mesangial and endothelial proliferation, 6/25 glomeruli showed crescents | Granular deposition of IgG and C3 in mesangium | n.a. | Lupus nephritis† (also see text) |
| Tubules and arterioles normal | ||||
First parentheses [] indicate case descriptors used in original reports, second parentheses [] denote citation.
Blood film showed numerous shistocytes,
patient with co-existent heterozygous C2 deficiency who presented with clinical (malar rash, arthralgia) and laboratory features (anti-nuclear antibodies) consistent with diagnosis of systemic lupus erythematosus. BM, basement membrane; DDD, dense deposit disease; GBM, glomerular basement membrane; HUS, haemolytic uraemic syndrome; n.a.: not available.
Conversely, in the cases reported by Levy et al. (cases 2a, b) the renal biopsies showed changes that had some features of DDD [23]. These included intramembranous electron-dense GBM changes together with glomerular C3 staining in the absence of immunoglobulin (case 2b). However, there were a number of pathological features that were not typical of DDD. First, electron-dense deposits were not seen in the basement membrane of Bowman's capsule or the tubular basement membranes which may be seen in DDD, albeit infrequently [51]. Secondly, deposits were also seen along the subendothelial part of the GBM (lamina rara interna, cases 2a and b). While subepithelial deposits have been noted in DDD in addition to the intramembranous change, subendothelial deposits appear to be unusual [48]. Thirdly, the GBM deposits stained directly for C3. This is not seen typically in DDD where, using immunofluorescence techniques, C3 staining appeared to localize along the margins of the intramembranous deposits giving rise to the appearance of ‘rail-road tracks’[54]. Similarly, deposits within the mesangium stained for C3 circumferentially, resulting in the appearance of mesangial ‘rings’ on immunofluorescent staining. A similar staining pattern for both C3 and C9 has been noted in DDD with immuno-electron microscopic techniques using peroxidase-conjugated antibodies [55]. However, in one case of DDD the deposits did stain directly for C9 and, to a lesser extent, C3 with immuno-electron microscopy using gold-conjugated antibodies (Dr Jill Moss, personal communication).
The biopsy findings reported in case 14 were consistent with MPGN type I, with the presence of subendothelial GBM deposits noted in the setting of MPGN [27]. However, as noted above, this individual also had a history of hepatitis B infection which in itself is associated with this type of renal lesion.
The biopsy findings reported in the individual with ‘type III collagen glomerulopathy’ are, in our view, consistent with primary thrombotic microangiopathy with subsequent accumulation of collagen [34]. The first and second biopsies showed ultrastructural features in keeping with endothelial injury, i.e. subendothelial widening by granular material, together with the absence of GBM deposits. The final biopsy, where type III collagen glomerular staining was demonstrated, showed flocculent material in both the mesangium and the subendothelial space together with light microscopic features of MPGN. It seems likely that the changes on this final biopsy represent a chronic healing phase of HUS. Similarly, the changes in the biopsy of the individual with ‘fibrillary glomerulopathy’ are difficult to interpret from the published figures, but could represent a variant of chronic HUS [29].
In summary, homozygous factor H deficiency is associated with two distinct renal pathologies, namely thrombotic microangiopathy and glomerulonephritis with C3 deposition. This suggests that alternative pathway dysregulation is important in the pathogenesis of these two renal diseases. We now review, under separate headings, the additional evidence from human and animal studies that support this.
Alternative pathway dysregulation and MPGN − human studies
There are several distinct situations in which an association between alternative pathway dysregulation and the development of MPGN has been reported.
MPGN and C3 nephritic factor
Uncontrolled alternative pathway C3 activation is also associated with C3 nephritic factor (C3NeF). Spitzer first reported the presence of a serum ‘factor’ responsible for the breakdown of C3 in a patient with MPGN [56]. C3NeF is now known to be an IgG autoantibody directed against a neo-epitope on the alternative pathway C3 convertase [57]. The binding of C3NeF to the C3 convertase renders it resistant to inactivation by factor H. The consequent uncontrolled alternative pathway activation results in increased plasma C3 activation with reduction in C3 levels and elevation of circulating C3 fragments, predominantly C3d. The clinical associations of the C3NeF include MPGN. It is detected in approximately 30% of patients with MPGN type I, but this association rises to 70% for DDD [58,59]. It is also found in a significant proportion of patients with partial lipodystrophy (PLD), many of whom develop DDD [60]. These observations provided circumstantial evidence to support the hypothesis that uncontrolled plasma C3 activation is important in the pathogenesis of DDD. However, there are lines of evidence that challenge this. Thus, C3NeF may be detected in individuals without any evidence of ill health [61], may be absent in up to one-third of individuals with DDD [62] and does not appear to correlate with the clinical course of the nephritis [58,59]. Plasma C3 levels do not correlate with the clinical course of the associated MPGN [49] and have no predicative value in identifying recurrence of DDD in renal transplants [63,64]. However, perhaps the strongest challenge, and certainly the most counterintuitive, derives from sequential histological studies of DDD recurrence in renal transplant kidneys [65]. It has been known for many years that DDD recurs almost invariably in renal transplants [48,66,67]. However, in this study the evolution of the pathology was assessed by studying sequential renal biopsies of transplanted kidneys [65]. Eleven patients with an original diagnosis of DDD underwent renal transplantation. In nine patients the disease recurred in the transplant kidney and in three of these patients the initial post-transplant biopsies showed evidence of capillary wall electron-dense deposits in the absence of significant capillary wall staining for C3. In two of these patients further renal biopsies were performed which demonstrated progression of the capillary wall electron-dense deposits. However, in only one patient could significant capillary wall C3 deposition be detected. The authors concluded that the morphological change in the GBM in DDD precedes the appearance of glomerular C3, implying that the activation of C3 in DDD represents either an epiphenomenon or occurs subsequent to the development of the GBM deposits [65].
Association between DDD and dysfunctional factor H protein
DDD has been reported in a family in which a mutant factor H molecule was identified [24]. In this consanguineous Turkish family, two individuals developed biopsy-proven DDD which was associated with both the presence of C3NeF and a homozygous factor H mutation. The mutation resulted in the deletion of a single lysine residue within SCR4 of the protein (Lys224del). Antigenic levels of factor H within plasma were normal in the affected individuals, but both C3 and factor B levels were reduced. Furthermore, increased levels of C3d, a C3 breakdown product, were also noted. Functional studies demonstrated that the mutant protein had impaired C3 regulatory capacity in plasma while the surface recognition domains of the mutant protein were normal. In contrast to the atypical features noted in the renal biopsies of the factor H-deficient individuals reported by Levy et al.[23], the renal biopsies in this family were consistent with DDD, all showing thickening of the GBM by light microscopy, C3c deposition along the GBM and segmental osmiophilic transformation of the lamina densa [24]. The mother of the affected individuals, who was heterozygous for the factor H mutation, was also C3NeF-positive. However, she had not developed renal disease, suggesting that the presence of the C3NeF alone was insufficient for renal disease to develop.
MPGN and an autoantibody to factor H
An individual with MPGN who had a circulating 46 kDa λ light chain dimer that inhibited factor H has also been described [68]. The patient presented at age 57 years with renal impairment, haematuria and proteinuria. Renal biopsy showed mesangial hypercellularity, mesangial matrix expansion and thickening of peripheral capillary loops with GBM ‘splitting’ evident on silver stains. Immunofluorescence using anti-C3c anti-sera showed coarse granular C3 deposits within the mesangium and capillary walls. A similar appearance, although with weaker intensity, was seen using anti-sera specific for iC3b. Using anti-C3dg anti-sera extensive linear staining was noted along the capillary walls. Staining for properdin, C5, C7 and C9 was similar in distribution to that for C3. Factor H was detected in a linear staining pattern along the basement membrane, but not as coarse granular deposits. Immunoglobulins were not detected. Electron microscopic analysis showed subendothelial and intramembranous electron-dense GBM deposits. It is notable that the presence of subendothelial GBM deposits in addition to intramembranous changes are similar to the ultrastructural changes reported in the factor H-deficient individuals described as atypical DDD (cases 2a, b) [23]. The patient also had low levels of C3 and factor B and incubation of the patient's sera with normal human sera resulted in rapid conversion of C3. Factor H and I levels were normal. The cause of this conversion was not C3NeF but an immunoglobulin λ light chain dimer. It has now been shown that this abnormal protein binds to SCR3 of factor H, thereby blocking one of the C3b interaction sites on the factor H molecule [69]. It appears, therefore, that functional blocking of factor H by this light chain dimer had initiated the development of MPGN in this individual.
Familial dysfunctional C3 molecules and MPGN
MPGN has also been reported in families in which dysfunctional C3 molecules are present [70,71]. These C3 molecules are able to bind factor B and generate a functional alternative pathway C3 convertase. However, the C3 molecules have reduced affinity for factor H and hence the generated C3 convertase is resistant to physiological inhibition by factor H. The defect was reversed partially by the addition of normal C3 but not with the addition of C3 isolated from the affected individuals. The net result is that this inherited defect results in alternative pathway C3 dysregulation.
In the ‘P’ family, five individuals were identified with low serum C3 levels and modest reductions in factor B levels [71]. Two of these individuals developed renal disease. One developed haematuria and proteinuria at the age of 10 years. Renal biopsy, at the age of 12 years, showed both subendothelial and subepithelial electron-dense GBM deposits. C3 was present along the capillary walls in association with properdin. This biopsy description is thus consistent with MPGN type III [53]. Although the aetiology of this histological pattern is not known, the description of this variant in this family, together with the observation that in the original reports all patients showed reduced plasma C3 with normal C4 levels at some stage, suggests that MPGN type III is one of the renal injury patterns that may occur in C3 dysregulation states. The second affected sibling presented with proteinuria and recurrent respiratory tract infections at the age of 6 years. Renal biopsy showed glomerular hypercellularity and immunofluorescence demonstrated mesangial staining for C3, C5 and properdin but no immunoglobulin. Subepithelial electron-dense GBM deposits were present on electron microscopy. In all five cases factor H and factor I levels were normal and C3 nephritic factor was not detected.
In the other family, termed the ‘D’ family, a mother developed haematuria during pregnancy and her son developed haematuria at the age of 3·5 months [70]. Both had C3 and factor B levels of 50% normal, but normal levels of factor H and I and no C3 nephritic factor. The mother's renal biopsy showed mesangial hypercellularity and matrix expansion. Immunofluorescence staining showed glomerular deposition of C3, C5, C6, C8 and properdin but no immunoglobulin. Numerous intramembranous and subendothelial GBM electron-dense deposits were seen. Again, this biopsy has similar ultrastructural GBM changes to those seen in the factor H-deficient individuals with atypical DDD (cases 2a, b) [23] and in the individual with MPGN in association with an anti-factor H antibody [68]. Similar findings were seen in the son's renal biopsy, except that subepithelial GBM electron-dense deposits were also seen.
Glomerulonephritis C3 with and without MPGN
Recently, a report of 19 patients with a distinct renal lesion described as glomerulonephritis C3 has been published [72]. This glomerular lesion was characterized histologically by the presence of isolated deposits of C3 without immunoglobulins. This lesion was then subdivided further into biopsies in which there was evidence of MPGN (referred to as glomerulonephritis C3 with MPGN, n = 13) and those where there was no evidence of MPGN (glomerulonephritis C3 without MPGN, n = 6). Interestingly, the incidence of C3 dysregulation (assessed by the presence of C3NeF and/or low plasma C3 levels) was higher in the patients with MPGN compared to those without. In contrast, although the numbers were small, the incidence of a mutation in a complement regulatory protein, including factor H, was higher in the group without MPGN. Electron microscopic analysis was available in only one individual from each subgroup. However, in neither case did it show evidence of intramembranous deposits. In these two cases mesangial and epimembranous deposits were noted in the absence of GBM deposits. Thus, glomerulonephritis C3 with or without MPGN appears distinct from DDD, MPGN type I and MPGN type III.
Summary
These data provide strong evidence that alternative pathway dysregulation due to factor H dysfunction is associated with glomerulonephritis. The most universal feature in this setting is the presence of glomerular deposition of C3 in the absence of immunoglobulin. The C3 deposits are found commonly in the mesangium and on the capillary wall. The morphological response of the glomerulus is predominantly that of MPGN, but this is not invariable. At the ultrastructural level the C3 deposition may be associated with the presence of mesangial and subendothelial deposits of electron-dense material or may show the characteristic electron-dense transformation of the basement membrane known as DDD. In some cases there may also be subepithelial deposits. Situations in which factor H dysfunction is present, i.e. deficiency, anti-factor H antibody and ‘resistant’ C3 convertases result in GBM ultrastructural changes that have similarities with, but are not typical of, DDD which we refer to as atypical DDD (Table 3). In this respect it is of interest that the classical features of DDD were clearly present in the family with a circulating mutant factor H protein together with C3NeF [24]. The situation is complicated further by the observation that C3NeF may be associated with a lesion distinct from DDD, namely glomerulonephritis C3 [72]. It might be better to classify all the lesions we have described here as C3 glomerulopathy and then subclassify them further depending on the morphological pattern of glomerular change and whether or not there is electron-dense transformation of the basement membrane (Fig. 1).
Table 3.
Atypical versus typical DDD
| Typical DDD | Atypical DDD | |
|---|---|---|
| Examples | DDD associated with C3NeF | Human, porcine and murine factor H deficiency |
| GBM pathology | Defined by linear intramembranous electron-dense deposits | Intramembranous electron-dense deposits variable |
| Subendothelial deposits uncommon | Subendothelial deposits reported | |
| Tubular and Bowman's capsule basement membrane deposits | Reported | Not reported |
| Staining of GBM deposits | Direct staining not demonstrated | GBM deposits stain directly for C3 |
| Margins of both GBM deposits (‘rail-road tracks’) and mesangial deposits (‘rings’) stain for C3 | ||
| Temporal relationship of glomerular C3 staining and GBM deposits | GBM deposits have developed in transplant kidneys prior to appearance of glomerular C3 staining | GBM C3 staining precedes appearance of GBM deposits in factor H-deficient pig and mouse strain |
Fig. 1.
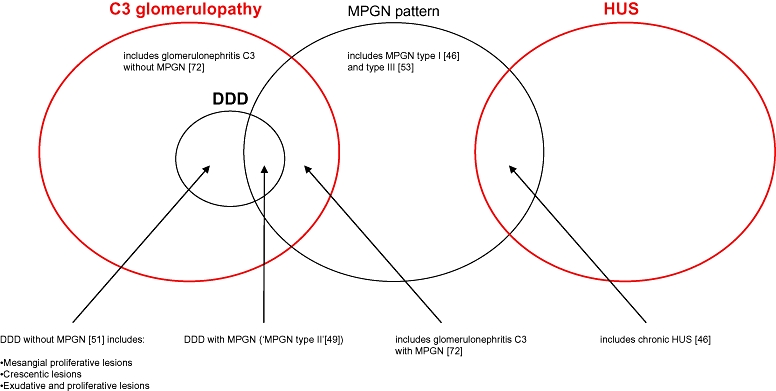
The spectrum of renal pathology associated with alternative pathway dysregulation. Factor H dysfunction is associated with haemolytic uraemic syndrome and glomerulonephritis with deposition of C3. The pathological manifestations of glomerulonephritis with deposition of C3 are heterogeneous and can be encompassed by the term C3 glomerulopathy. Numbers in parentheses refer to references.
Despite this complexity, data from animal studies, which we discuss next, have led to important mechanistic insights into the pathogenesis of renal disease associated with factor H dysfunction.
Alternative pathway dysregulation and MPGN − animal studies
Early animal studies
Following the description of the strong association between C3NeF and DDD, attempts to induce this lesion in both rabbits and mice were performed by inducing chronic C3 activation in the hope of mimicking the action of C3NeF [73,74]. However, despite achieving prolonged periods of hypocomplementaemia these strategies were unsuccessful. Verroust and colleagues could not induce glomerular damage in rabbits by repeated injections of alternative pathway activators such as zymosan [74]. Prolonged hypocomplementaemia was achieved in mice by repeated injections of cobra venom factor (CVF) over a period of 3 months [73]. The mice were thymectomized neonatally, irradiated lethally and bone marrow reconstituted in order to prevent antibody formation to and neutralization of the effects of CVF. Despite achieving serum C3 levels of < 10% of wild-type control levels no evidence of renal damage was seen, as assessed by both light microscopy and at the ultrastructural level using electron microscopy. No deposition of C3 was observed within the glomeruli of the CVF-treated mice. Within the limitations of these experimental models, the observation that induction of chronic C3 activation in animals did not result in spontaneous renal injury argued against a pathogenic role for C3NeF in DDD. There remains no animal model of C3NeF associated DDD. However, clear evidence that spontaneous renal injury developed in the setting of C3 dysregulation subsequently became evident from studies of a factor H-deficient pig strain and gene-targeted factor H-deficient mice.
Porcine factor H deficiency
Lethal MPGN has been described in a strain of pigs [75,76]. Affected piglets appeared healthy at birth but soon developed a rapidly progressive glomerulonephritis with a median survival of 37 days. All affected piglets were deficient in factor H [77,78] due to a mutation in SCR20 which prevents secretion of the mutant protein [79]. The disease was 100% penetrant in homozygous factor H-deficient pigs, while no heterozygous carriers developed disease.
The renal lesion in affected piglets was characterized by MPGN [75,76]. Positive glomerular staining for C3 and the membrane attack complex (MAC) in association with vitronectin was present, while immunoglobulin and factor B staining was negative. Ultrastructural changes included subendothelial and intramembranous electron-dense deposits. In older lesions subepithelial and mesangial electron-dense deposits were also present [76]. Unlike human DDD, electron-dense deposits were not seen in the basement membranes of either tubules or Bowman's capsule [76]. Furthermore, immune electron microscopic studies showed that the GBM deposits stained strongly for both C3 and MAC. Notably, no pathology was evident in any of the other tissues examined (synovium, lungs, spleen, choroid plexus and ciliary body).
The chronology of the glomerulonephritis has been documented extensively [76]. Massive glomerular deposits of C3 and the MAC were present in factor H-deficient fetuses before the onset of mesangial hypercellularity or GBM deposits. In fetuses, the C3 and MAC staining was initially linear, along capillary walls, without mesangial staining. However, mesangial staining became evident from 1 week and, after 3 weeks, abundant and granular mesangial C3 staining developed with increasingly coarse linear capillary staining. The earliest light microscopic changes were evident in piglets aged 4 days and showed mild mesangial hypercellularity and expansion together with increased glomerular neutrophil numbers. By 3 weeks these changes were marked and mesangial interpositioning was evident from 4 weeks. GBM electron-dense deposits developed initially on the subendothelial side of the lamina densa (earliest changes evident at 4 days) and progressed gradually to occupy more of the GBM, including the subepithelial side. After the age of 4 weeks laminar disintegration of the dense deposits created the effect of intramembranous lucencies with double contours.
Finally, it was stated that a renal transplant experiment where a normal pig kidney was transplanted into a factor H-deficient pig resulted in appearance of complement deposits in the transplant kidney within 24 h without the appearance of GBM deposits [76]. This contrasts with the human study of DDD, where GBM deposits appeared in the absence of complement deposition [65].
The factor H-deficient pig strain has now been eradicated [80], but its elegant characterization has provided important insights into the sequential pathological changes that occur in MPGN associated with factor H deficiency.
Murine factor H deficiency
Further insights into the pathogenesis of MPGN associated with factor H deficiency have derived from studies in factor H-deficient mice [81]. These animals were developed by targeted gene deletion of exon 3 of the murine factor H gene in embryonic stem cells. Similar to homozygous factor H-deficient humans, these animals have extremely low levels of plasma C3 as a result of uncontrolled alternative pathway activation [81].
Spontaneous renal disease in factor H-deficient mice
At 8 months all the homozygous factor H-deficient mice had developed light microscopic features of MPGN [81]. Hence, like homozygous factor H-deficient pigs, MPGN in factor-deficient mice was fully penetrant. Similarly, no evidence of spontaneous renal disease was evident in heterozygous factor H-deficient mice. However, in contrast to the uniform lethality seen in the factor H-deficient pigs, mortality in murine factor H deficiency was 23% [81].
Although the MPGN in the factor H-deficient mice had many similarities with human DDD, particularly the presence of hypocomplementaemia, deposition of C3 and electron-dense material along the GBM there are, like factor H-deficient pigs, some important differences. The GBM electron-dense deposits in the factor H-deficient mice are located predominantly in a subendothelial location and linear transformation of the laminar dense is not seen. Deposits were seen infrequently in the mesangium and did not develop in the basement membrane of the tubules and Bowman's capsule. Similar to factor H-deficient pigs and humans, the GBM deposits stain directly for C3 and C9 but not immunoglobulin using immune electron microscopy [81].
Sequential analysis of the renal histopathology in these mice showed an identical evolution to that described in the factor H-deficient pigs. Hence, glomerular C3 deposition preceded the appearance of GBM-dense deposits which, in turn, preceded the histological light microscopic appearances of MPGN. Glomerular C3 deposition was evident as early as the first week of life, a time when both the light and electron microscopic appearance of the glomerulus was normal [81].
Novel mechanistic insights that derived from the factor H-deficient mouse model include the observation that MPGN in this animal was totally dependent on C3 activation. Hence, preventing C3 activation (achieved by crossing factor H-deficient mice with factor B-deficient animals) completely prevented the development of MPGN [81]. Furthermore, preventing C5 activation (achieved by crossing factor H-deficient mice with C5-deficient animals) did not prevent the development of the GBM deposits (Fig. 2) [82]. However, the inability to activate C5 was associated with a reduction in glomerular cellularity, serum creatinine levels and mortality [82]. This suggests that while it would not be expected to prevent the development of GBM deposits, chronic inhibition of C5 activation in humans with MPGN associated with factor H dysfunction may be beneficial by reducing the glomerular inflammatory response. Whether this would also be applicable to factor H-sufficient individuals with DDD and C3NeF is not clear.
Fig. 2.
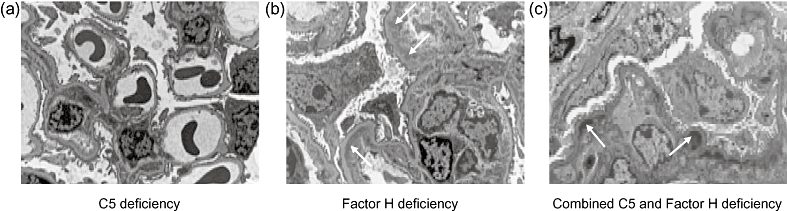
Electron micrographs of glomeruli from 12-month-old (a) C5-deficient mice, (b) factor H-deficient mice and (c) mice with combined deficiency of H and C5. The ultrastructural appearances of the glomeruli in the C5-deficient mice are normal. In contrast, in both the factor H-deficient and combined factor H and C5-deficient animals, subendothelial electron-dense glomerular basement membrane deposits are present (arrows indicate examples).
A novel observation has come recently from studies of animals with combined deficiency of both factor I and factor H [83]. Factor I-deficient mice develop uncontrolled C3 activation through the alternative pathway together with renal abnormalities characterized by mesangial C3 deposits with nodular mesangial expansion, but importantly no evidence of GBM abnormalities. Human factor I deficiency is associated similarly with uncontrolled C3 activation through the alternative pathway and renal disease is uncommon and no association with DDD has been described [84–87]. Remarkably, factor H-deficient mice with deficiency of factor I do not develop MPGN. In these animals the glomerular lesion is identical to that seen in mice deficient in factor I alone (Fig. 3). Administration of a source of factor I to mice with combined deficiency of H and I resulted in the appearance of glomerular C3 staining in a pattern identical to that seen in mice with deficiency of factor H alone. These data show that in the setting of factor H deficiency, factor I is required for both GBM C3 deposition and the morphological changes of MPGN to develop. Furthermore, renal transplant studies demonstrated that the GBM C3 in factor H-deficient animals is derived principally from the circulation. In the absence of factor I uncontrolled generation of C3b occurs, but further metabolism of C3b cannot occur. In contrast, in factor H deficiency C3b metabolites are seen. Taken together these animal data suggest that plasma C3b targets the mesangium while plasma C3b metabolites target the GBM. The implication, therefore, is that in situations where GBM C3 is present, strategies that sequester C3b metabolites in the circulation will be therapeutically beneficial.
Fig. 3.
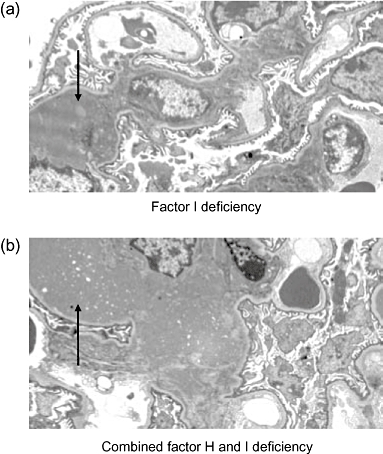
Electron micrographs of glomeruli from 8-month-old (a) factor I-deficient and (b) mice with combined deficient of H and I. The ultrastructural appearances of the glomerular basement membrane are normal in both cases. In contrast, in both groups of mice the glomeruli contained mesangial electron-dense material corresponding to areas of nodular mesangial expansion seen by light microscopy (arrows indicate examples).
Experimentally triggered renal disease in factor H-deficient mice
Homozygous factor H-deficient mice have been shown to have an increased susceptibility to renal injury following the induction of experimental nephritis [81,82]. This exaggerated response was evident in young animals prior to the development of light microscopic changes of MPGN, suggesting that it is the complement dysregulation rather than pre-existing renal disease that renders these animals susceptible to nephrotoxic agents. Experimental heterologous nephrotoxic nephritis develops following the intravenous injection of heterologous (derived from sheep) anti-mouse GBM antibody. Binding of the heterologous antibody along the GBM results typically in a transient glomerular neutrophil influx in wild-type animals which resolves within 24 h. However, in homozygous factor H-deficient animals increased glomerular neutrophils remained present at 24 h and were still detectable 6 days after administration of the nephrotoxic antibody [82]. This was also associated with evidence of increased glomerular C3 deposition indicating that, despite profound fluid-phase consumption of complement, factor H-deficient mice retained the ability to deposit further C3 within the kidney following the binding of antibody along the GBM. This ‘additional’ C3 deposition may derive from local C3 production, as it is known that human glomerular epithelial cells and mesangial cells synthesize C3 [88,89].
The abnormal response to heterologous nephrotoxic nephritis was absent in factor H-deficient mice with deficiency of C5 but persisted in animals with combined deficiency of factor H and C6 [82]. This demonstrated that it was the production of C5a not the formation of the MAC that was responsible for enhanced glomerular neutrophil response in this model. Furthermore, administration of an anti-mouse C5 antibody to factor H-deficient mice prior to the induction of heterologous nephrotoxic nephritis prevented both glomerular neutrophil accumulation and proteinuria [82]. These data indicate that, in the setting of factor H dysfunction, (i) the threshold for renal injury is lowered significantly following exposure to a complement-activating nephrotoxic insult; and (ii) C5a production is a major mediator of renal injury in this setting.
What is the relevance of these observations to human DDD? It is known that patients with DDD may remain stable clinically for long periods. However, relapses associated with acute inflammatory changes within the kidney often result in significant decline in renal function or threaten transplant kidney survival. Increased glomerular neutrophil numbers, often with crescent formation, can occur in human DDD [49,51,62] and in DDD lesions developing in transplant kidneys in the absence of transplant-related pathology [67]. Moreover, during rapidly progressive disease all glomerular deposits, including paramesangial deposits, react strongly with anti-C5 antibodies [90], suggesting C5 activation is a particularly important component of renal injury during acute inflammatory episodes. These observations suggest that anti-C5 therapy could be effective in preserving renal function during disease flares in human DDD.
Summary
The factor H-deficient mouse has demonstrated that MPGN associated with factor H deficiency is absolutely dependent on both the ability to activate C3 and to cleave C3b. There also appears to be an important role for C5 activation in the development of glomerular inflammation in this type of MPGN. Studies using experimental models of nephritis in these animals have shown that factor H dysfunction is associated with an increased susceptibility to complement-activating nephrotoxic insults. In these scenarios C5 activation appears to play a major role in mediating glomerular injury.
What is clear from the published reports in both the factor H-deficient pig and mouse is that neither animal developed HUS. In contrast, as discussed above, there are at least 15 reports in the literature of human factor H deficiency and HUS. It has now become evident that factor H-associated HUS is associated much more commonly with heterozygous mutations in the protein that result in normal plasma levels of both factor H and C3 [22]. Insights from these human studies had direct relevance on the development of a murine model of factor H-associated HUS and are therefore discussed next.
Alternative pathway dysregulation and HUS − human studies
There is now overwhelming evidence that atypical HUS is associated with defective regulation of the alternative pathway of complement activation. Atypical HUS (also termed non-diarrhoea-associated HUS) refers to HUS that is not related to infection with verocytotoxin-producing Escherichia coli or Shigella bacterial strains (diarrhoea-associated HUS). In atypical HUS mutations have been identified in factor H [42,91–94], factor I [95] and membrane co-factor protein (MCP, CD46) [96,97]. Furthermore, ‘gain-of-function’ mutations in the alternative pathway activation protein, factor B, have also been associated with atypical HUS [98]. Thus, impaired regulation of the alternative pathway is central to the pathogenesis of atypical HUS.
A detailed overview of the genetics of factor H-associated aHUS has been reported in a separate section of this review series [28]. In summary, such mutations are typically heterozygous and associated frequently with normal antigenic levels of both C3 and factor H [22]. Importantly, these mutations tend to cluster at the C-terminus of the protein, predominantly within SCR domains 16–20. In vitro studies have demonstrated that such mutant proteins display impaired binding to heparin, surface-bound C3b and endothelium, which is critical for regulation of complement activation on cellular surfaces [99–101]. These were key observations because it had been described previously that the plasma complement regulatory activity of factor H (decay-accelerating and factor I cofactor activities) were localized to the opposite end of the protein, within the N-terminal four SCR domains [22]. It therefore appeared that, in the majority of cases, factor H-associated HUS mutations impaired specifically the surface recognition functions of the protein, leaving plasma C3 regulatory function intact. This was supported by the frequent finding that these mutations were associated with normal plasma C3 levels in contrast to low C3 levels which typically accompany factor H-associated MPGN. This, together with the observations that complete absence of this protein in pigs and mice led to the development of MPGN and not HUS, led to the hypothesis that atypical HUS required not only defective regulation of complement activation along the renal endothelium but also some degree of plasma C3 regulation. This hypothesis was tested recently in mice, which we discuss next.
Alternative pathway dysregulation and HUS − animal studies
Factor H-deficient mice expressing mutant factor H protein and HUS
To establish that a combination of plasma C3 regulation and defective C3 regulation along the renal endothelium was required for factor H-associated HUS to develop, factor H-deficient mice that expressed a mutant murine factor H protein, functionally equivalent to those described in humans, was generated [102]. This mutant mouse factor H protein lacked the carboxyl-terminal five SCR domains (denoted as FHΔ16-20). Analogous to atypical HUS-associated factor H mutant proteins, FHΔ16-20 demonstrated impaired binding to heparin and endothelial cells [human umbilical vascular endothelial cells (HUVEC)] but retained normal factor I co-factor activity in vitro. Factor H-deficient mice expressing FHΔ16-20 at approximately 30% of pooled wild-type mouse levels (denoted as Cfh–/–FHΔ16-20high) had significantly increased plasma C3 levels compared to unmanipulated factor H-deficient animals demonstrating that the FHΔ16-20 protein was able to regulate plasma C3 activation in vivo.
Supporting the original hypothesis was the demonstration that these animals developed spontaneous thrombotic microangiopathy. The renal histopathology in these animals was characteristic of HUS [102]. Ultrastructural abnormalities did not show any evidence of GBM deposits. Thus the introduction of the FHΔ16-20 mutant protein had resulted in not only the development of HUS but also the prevention of MPGN. In other words, the phenotype of the factor H-deficient animals had switched from that of MPGN to HUS.
While this supported the observation that plasma C3 regulation was required for HUS to develop the question remained: to what degree? In a separate line of transgenic animals the FHΔ16-20 protein was found to be circulating at very low levels (approximately 2% of wild-type levels) and, as would be expected, the degree of plasma C3 regulation was significantly lower than that seen in the Cfh–/–FHΔ16-20high animals although greater than that seen in the factor H-deficient animals [102]. In a cohort of 8-month-old Cfh–/–FHΔ16-20low animals no evidence of HUS developed. Renal histology in these Cfh–/–FHΔ16-20low animals showed less prominent capillary wall C3 staining and less frequent subendothelial GBM deposits compared to factor H-deficient mice. Thus, it appeared that the expression of even very low levels of the FHΔ16-20 protein was sufficient to ameliorate the MPGN phenotype but insufficient to allow HUS to develop.
In summary, the similarities between the surface recognition domains of murine and human factor H [103] allowed the generation of a mutant mouse factor H protein that functionally mimicked HUS-associated human factor H mutations. Factor H-deficient mice expressing the mutant protein spontaneously developed HUS, not MPGN, providing the first in vivo proof of principle evidence that factor H mutations specifically impairing surface recognition result in spontaneous HUS.
Heterozygous factor H-deficient mice expressing mutant factor H protein and HUS
It is important to note that the majority of HUS-associated mutations in complement genes are found normally in heterozygosis in HUS patients. In this respect, it is notable that heterozygous factor H-deficient mice expressing the FHΔ16-20 protein (denoted as Cfh+/–FHΔ16-20high) did not develop HUS spontaneously. Similarly, unmanipulated heterozygous factor H-deficient mice also do not develop spontaneous renal disease. However, in humans HUS-associated factor H mutations show incomplete penetrance. Furthermore, most episodes of HUS in these individuals are preceded by some identifiable environmental trigger. Infection, immunosuppressive drugs, cancer therapies, oral contraceptive agents and pregnancy or postpartum period are all factors that may trigger aHUS in individuals carrying CFH mutations in heterozygosis and the syndrome has developed in the native kidney of live-related kidney donors who had previously unidentified FH mutations [104,105]. It is also clear that in many individuals multiple genetic triggers are also required for HUS to develop [106,107]. Therefore, both heterozygous factor H-deficient mice and Cfh+/–FHΔ16-20 animals may only develop aHUS following an additional insult, either genetic, or environmental, or both. Alternatively it could, of course, be possible that interspecies differences in the regulation of C3 on cell surfaces by factor H and other complement regulators contributes to the apparent resistance of Cfh+/–FHΔ16-20 mice to aHUS.
Unanswered questions
While there is no doubt that there has been significant progress in understanding many aspects of factor H-associated renal disease, certain key questions remain unanswered. Perhaps the most obvious is why the renal endothelium is specifically targeted in individuals with aHUS-associated factor H mutations? Currently there is no clear explanation for this. Recently, a genomic deletion affecting two members of the factor H-related protein family has been associated with HUS [108]. The biological role, if any, of factor H-related proteins remains unclear. This deletion is associated with the presence of anti-factor H antibodies [109] and these antibodies appear to affect the recognition functions of factor H [109,110]. However, how this deletion predisposes to the formation of anti-factor H antibodies is unknown.
We have also elucidated the pathological disparities in the literature between human DDD associated with C3NeF and the glomerular lesions described in association with factor H deficiency (Table 3). Particularly problematic is the observation that in human DDD, GBM lesions are reported to precede the appearance of glomerular C3 staining. Moreover, direct staining of GBM deposits for C3, while demonstrated in factor H-deficient humans, pigs and mice, is not typical in human DDD. The importance of these pathological distinctions is not clear.
Finally, while data from the factor H-deficient and factor H-transgenic mice indicate that some degree of plasma C3 regulation is required for atypical HUS to develop, it is evident that some factor H-deficient humans with very low plasma C3 levels develop HUS and not MPGN (Table 1). This suggests that additional factors may influence the phenotypic outcome in human factor H deficiency.
Concluding remarks
We have reviewed the renal pathology associated with factor H dysfunction. Both HUS and glomerulonephritis with C3 deposition have been associated with abnormal factor H function, suggesting that alternative pathway dysregulation is important in the pathogenesis of both lesions.
The pathological features of glomerulonephritis associated with C3 deposition are heterogeneous and might be described most clearly by the term C3 glomerulopathy. Distinct defects within the factor H protein that impair surface recognition activity appear to underlie the majority of factor H-associated human HUS. In contrast, MPGN is associated with factor H deficiency states. Animal models mimicking these factor H defects have provided us with important insights into the molecular pathogenesis of these conditions and provide tools in which therapeutic strategies can be explored.
Acknowledgments
MCP is a Wellcome Trust Research Fellow. We also acknowledge support from KIDNEEDS.
References
- 1.Nilsson UR, Muller-Eberhard HJ. Isolation of b1F-globulin from human serum, and its characterisation as the fifth component of complement. J Exp Med. 1965;122:277–90. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwaeble W, Schwaiger H, Brooimans RA, et al. Human complement factor H. Tissue specificity in the expression of three different mRNA species. Eur J Biochem. 1991;198:399–404. doi: 10.1111/j.1432-1033.1991.tb16028.x. [DOI] [PubMed] [Google Scholar]
- 3.van den Dobbelsteen ME, Verhasselt V, Kaashoek JG, et al. Regulation of C3 and factor H synthesis of human glomerular mesangial cells by IL-1 and interferon-gamma. Clin Exp Immunol. 1994;95:173–80. doi: 10.1111/j.1365-2249.1994.tb06033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander JJ, Quigg RJ. The simple design of complement factor H: looks can be deceiving. Mol Immunol. 2007;44:123–32. doi: 10.1016/j.molimm.2006.07.287. [DOI] [PubMed] [Google Scholar]
- 5.Harrison RA, Lachmann PJ. The physiological breakdown of the third component of human complement. Mol Immunol. 1980;17:9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- 6.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977;146:257–70. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad DH, Carlo JR, Ruddy S. Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding. J Exp Med. 1978;147:1792–805. doi: 10.1084/jem.147.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiler JM, Daha MR, Austen KF, Fearon DT. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci USA. 1976;73:3268–72. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pangburn MK, Muller-Eberhard HJ. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci USA. 1978;75:2416–20. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaki K, Iida K, Okubo M, Inai S. Reaction mechanisms of beta1H globulin. Int Arch Allergy Appl Immunol. 1978;57:221–32. doi: 10.1159/000232106. [DOI] [PubMed] [Google Scholar]
- 11.Pangburn MK, Morrison DC, Schreiber RD, Muller-Eberhard HJ. Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol. 1980;124:977–82. [PubMed] [Google Scholar]
- 12.Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay − dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci USA. 1978;75:1971–5. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazatchkine MD, Fearon DT, Austen KF. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979;122:75–81. [PubMed] [Google Scholar]
- 14.Kuhn S, Zipfel PF. Mapping of the domains required for decay acceleration activity of the human factor H-like protein 1 and factor H. Eur J Immunol. 1996;26:2383–7. doi: 10.1002/eji.1830261017. [DOI] [PubMed] [Google Scholar]
- 15.Gordon DL, Kaufman RM, Blackmore TK, Kwong J, Lublin DM. Identification of complement regulatory domains in human factor H. J Immunol. 1995;155:348–56. [PubMed] [Google Scholar]
- 16.Sharma AK, Pangburn MK. Identification of three physically and functionally distinct binding sites for C3b in human complement factor H by deletion mutagenesis. Proc Natl Acad Sci USA. 1996;93:10996–1001. doi: 10.1073/pnas.93.20.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pangburn MK. Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacology. 2000;49:149–57. doi: 10.1016/s0162-3109(00)80300-8. [DOI] [PubMed] [Google Scholar]
- 18.Edwards AO, Ritter R, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 19.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 20.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–67. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Levy M, Halbwachs-Mecarelli L, Gubler MC, et al. H deficiency in two brothers with atypical dense intramembranous deposit disease. Kidney Int. 1986;30:949–56. doi: 10.1038/ki.1986.278. [DOI] [PubMed] [Google Scholar]
- 24.Licht C, Heinen S, Jozsi M, et al. Deletion of Lys224 in regulatory domain 4 of factor H reveals a novel pathomechanism for dense deposit disease (MPGN II) Kidney Int. 2006;70:42–50. doi: 10.1038/sj.ki.5000269. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Larrea C, Dieguez MA, Enguix A, Dominguez O, Marin B, Gomez E. A familial deficiency of complement factor H. Biochem Soc Trans. 1987;15:648–9. [Google Scholar]
- 26.Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, et al. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15:787–95. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- 27.Vaziri-Sani F, Holmberg L, Sjoholm AG, et al. Phenotypic expression of factor H mutations in patients with atypical hemolytic uremic syndrome. Kidney Int. 2006;69:981–8. doi: 10.1038/sj.ki.5000155. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez de Cordoba S, Goicoechea de Jorge E. Translational mini-review series on complement factor H: genetics and disease associations of the human complement factor H. Clin Exp Immunol. 2008;151:1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bircan Z, Toprak D, Kilicaslan I, et al. Factor H deficiency and fibrillary glomerulopathy. Nephrol Dial Transplant. 2004;19:727–30. doi: 10.1093/ndt/gfg605. [DOI] [PubMed] [Google Scholar]
- 30.Cho HY, Lee BS, Moon KC, Ha IS, Cheong HI, Choi Y. Complete factor H deficiency-associated atypical hemolytic uremic syndrome in a neonate. Pediatr Nephrol. 2007;22:874–80. doi: 10.1007/s00467-007-0438-x. [DOI] [PubMed] [Google Scholar]
- 31.Rougier N, Kazatchkine MD, Rougier JP, et al. Human complement factor H deficiency associated with hemolytic uremic syndrome. J Am Soc Nephrol. 1998;9:2318–26. doi: 10.1681/ASN.V9122318. [DOI] [PubMed] [Google Scholar]
- 32.Ohali M, Shalev H, Schlesinger M, et al. Hypocomplementemic autosomal recessive hemolytic uremic syndrome with decreased factor H. Pediatr Nephrol. 1998;12:619–24. doi: 10.1007/s004670050515. [DOI] [PubMed] [Google Scholar]
- 33.Fijen CA, Kuijper EJ, Te Bulte M, et al. Heterozygous and homozygous factor H deficiency states in a Dutch family. Clin Exp Immunol. 1996;105:511–16. doi: 10.1046/j.1365-2249.1996.d01-777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt BA, Wyatt RJ, Burke BA, Simonton SC, Kashtan CE. Inherited factor H deficiency and collagen type III glomerulopathy. Pediatr Nephrol. 1995;9:11–15. doi: 10.1007/BF00858956. [DOI] [PubMed] [Google Scholar]
- 35.Pichette V, Querin S, Schurch W, Brun G, Lehner-Netsch G, Delage JM. Familial hemolytic–uremic syndrome and homozygous factor H deficiency. Am J Kidney Dis. 1994;24:936–41. doi: 10.1016/s0272-6386(12)81065-1. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen HE, Christensen KC, Koch C, Thomsen BS, Heegaard NH, Tranum-Jensen J. Hereditary, complete deficiency of complement factor H associated with recurrent meningococcal disease. Scand J Immunol. 1989;30:711–18. doi: 10.1111/j.1365-3083.1989.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 37.Brai M, Misiano G, Maringhini S, Cutaja I, Hauptmann G. Combined homozygous factor H and heterozygous C2 deficiency in an Italian family. J Clin Immunol. 1988;8:50–6. doi: 10.1007/BF00915156. [DOI] [PubMed] [Google Scholar]
- 38.Thompson RA, Winterborn MH. Hypocomplementaemia due to a genetic deficiency of beta 1H globulin. Clin Exp Immunol. 1981;46:110–19. [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Corral P, Bellavia D, Amico L, Brai M, Rodriguez de Cordoba S. Molecular basis for factor H and FHL-1 deficiency in an Italian family. Immunogenetics. 2000;51:366–9. doi: 10.1007/s002510050631. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt BZ, Fowler NL, Hidvegi T, Perlmutter DH, Colten HR. Disruption of disulfide bonds is responsible for impaired secretion in human complement factor H deficiency. J Biol Chem. 1999;274:11782–8. doi: 10.1074/jbc.274.17.11782. [DOI] [PubMed] [Google Scholar]
- 41.Ault BH, Schmidt BZ, Fowler NL, et al. Human factor H deficiency. Mutations in framework cysteine residues and block in H protein secretion and intracellular catabolism. J Biol Chem. 1997;272:25168–75. doi: 10.1074/jbc.272.40.25168. [DOI] [PubMed] [Google Scholar]
- 42.Caprioli J, Bettinaglio P, Zipfel PF, et al. The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J Am Soc Nephrol. 2001;12:297–307. doi: 10.1681/ASN.V122297. [DOI] [PubMed] [Google Scholar]
- 43.Buddles MR, Donne RL, Richards A, Goodship J, Goodship TH. Complement factor H gene mutation associated with autosomal recessive atypical hemolytic uremic syndrome. Am J Hum Genet. 2000;66:1721–2. doi: 10.1086/302877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying L, Katz Y, Schlesinger M, et al. Complement factor H gene mutation associated with autosomal recessive atypical hemolytic uremic syndrome. Am J Hum Genet. 1999;65:1538–46. doi: 10.1086/302673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naked GM, Florido MP, Ferreira de Paula P, Vinet AM, Inostroza JS, Isaac L. Deficiency of human complement factor I associated with lowered factor H. Clin Immunol. 2000;96:162–7. doi: 10.1006/clim.2000.4878. [DOI] [PubMed] [Google Scholar]
- 46.Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47:643–56. doi: 10.1038/ki.1995.82. [DOI] [PubMed] [Google Scholar]
- 47.Berger J, Galle P. Alteration singuliere des membranes basales du rein. J Urol Nephrol. 1962;68:116–22. [PubMed] [Google Scholar]
- 48.Droz D, Zanetti M, Noel LH, Leibowitch J. Dense deposits disease. Nephron. 1977;19:1–11. doi: 10.1159/000180859. [DOI] [PubMed] [Google Scholar]
- 49.Habib R, Gubler MC, Loirat C, Maiz HB, Levy M. Dense deposit disease: a variant of membranoproliferative glomerulonephritis. Kidney Int. 1975;7:204–15. doi: 10.1038/ki.1975.32. [DOI] [PubMed] [Google Scholar]
- 50.Sibley RK, Kim Y. Dense intramembranous deposit disease: new pathologic features. Kidney Int. 1984;25:660–70. doi: 10.1038/ki.1984.71. [DOI] [PubMed] [Google Scholar]
- 51.Walker PD, Ferrario F, Joh K, Bonsib SM. Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol. 2007;20:605–16. doi: 10.1038/modpathol.3800773. [DOI] [PubMed] [Google Scholar]
- 52.Smith RJ, Alexander J, Barlow PN, et al. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18:2447–56. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strife CF, McEnery PT, McAdams AJ, West CD. Membranoproliferative glomerulonephritis with disruption of the glomerular basement membrane. Clin Nephrol. 1977;7:65–72. [PubMed] [Google Scholar]
- 54.Kim Y, Vernier RL, Fish AJ, Michael AF. Immunofluorescence studies of dense deposit disease. The presence of railroad tracks and mesangial rings. Lab Invest. 1979;40:474–80. [PubMed] [Google Scholar]
- 55.Falk RJ, Sisson SP, Dalmasso AP, Kim Y, Michael AF, Vernier RL. Ultrastructural localization of the membrane attack complex of complement in human renal tissues. Am J Kidney Dis. 1987;9:121–8. doi: 10.1016/s0272-6386(87)80089-6. [DOI] [PubMed] [Google Scholar]
- 56.Spitzer RE, Vallota EH, Forristal J, et al. Serum C′3 lytic system in patients with glomerulonephritis. Science. 1969;164:436–7. doi: 10.1126/science.164.3878.436. [DOI] [PubMed] [Google Scholar]
- 57.Daha MR, Fearon DT, Austen KF. C3 Nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976;116:1–7. [PubMed] [Google Scholar]
- 58.Cameron JS, Turner DR, Heaton J, et al. Idiopathic mesangiocapillary glomerulonephritis. Comparison of types I and II in children and adults and long-term prognosis. Am J Med. 1983;74:175–92. doi: 10.1016/0002-9343(83)90606-x. [DOI] [PubMed] [Google Scholar]
- 59.Schena FP, Pertosa G, Stanziale P, Vox E, Pecoraro C, Andreucci VE. Biological significance of the C3 nephritic factor in membranoproliferative glomerulonephritis. Clin Nephrol. 1982;18:240–6. [PubMed] [Google Scholar]
- 60.Sissons JG, West RJ, Fallows J, et al. The complement abnormalities of lipodystrophy. N Engl J Med. 1976;294:461–5. doi: 10.1056/NEJM197602262940902. [DOI] [PubMed] [Google Scholar]
- 61.Gewurz AT, Imherr SM, Strauss S, Gewurz H, Mold C. C3 nephritic factor and hypocomplementaemia in a clinically healthy individual. Clin Exp Immunol. 1983;54:253–8. [PMC free article] [PubMed] [Google Scholar]
- 62.Vargas R, Thomson KJ, Wilson D, et al. Mesangiocapillary glomerulonephritis with dense ‘deposits’ in the basement membranes of the kidney. Clin Nephrol. 1976;5:73–82. [PubMed] [Google Scholar]
- 63.Leibowitch J, Halbwachs L, Wattel S, Gaillard MH, Droz D. Recurrence of dense deposits in transplanted kidney: II. Serum complement and nephritic factor profiles. Kidney Int. 1979;15:396–403. doi: 10.1038/ki.1979.51. [DOI] [PubMed] [Google Scholar]
- 64.Braun MC, Stablein DM, Hamiwka LA, Bell L, Bartosh SM, Strife CF. Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: the North American Pediatric Renal Transplant Cooperative Study experience. J Am Soc Nephrol. 2005;16:2225–33. doi: 10.1681/ASN.2005020175. [DOI] [PubMed] [Google Scholar]
- 65.Droz D, Nabarra B, Noel LH, Leibowitch J, Crosnier J. Recurrence of dense deposits in transplanted kidneys: I. Sequential survey of the lesions. Kidney Int. 1979;15:386–95. doi: 10.1038/ki.1979.50. [DOI] [PubMed] [Google Scholar]
- 66.Galle P, Hinglais N, Crosnier J. Recurrence of an original glomerular lesion in three renal allografts. Transplant Proc. 1971;3:368–70. [PubMed] [Google Scholar]
- 67.Andresdottir MB, Assmann KJ, Hoitsma AJ, Koene RA, Wetzels JF. Renal transplantation in patients with dense deposit disease: morphological characteristics of recurrent disease and clinical outcome. Nephrol Dial Transplant. 1999;14:1723–31. doi: 10.1093/ndt/14.7.1723. [DOI] [PubMed] [Google Scholar]
- 68.Meri S, Koistinen V, Miettinen A, Tornroth T, Seppala IJ. Activation of the alternative pathway of complement by monoclonal lambda light chains in membranoproliferative glomerulonephritis. J Exp Med. 1992;175:939–50. doi: 10.1084/jem.175.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jokiranta TS, Solomon A, Pangburn MK, Zipfel PF, Meri S. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol. 1999;163:4590–6. [PubMed] [Google Scholar]
- 70.Linshaw MA, Stapleton FB, Cuppage FE, et al. Hypocomplementemic glomerulonephritis in an infant and mother. Evidence for an abnormal form of C3. Am J Nephrol. 1987;7:470–7. doi: 10.1159/000167525. [DOI] [PubMed] [Google Scholar]
- 71.Marder HK, Coleman TH, Forristal J, Beischel L, West CD. An inherited defect in the C3 convertase, C3b,Bb, associated with glomerulonephritis. Kidney Int. 1983;23:749–58. doi: 10.1038/ki.1983.89. [DOI] [PubMed] [Google Scholar]
- 72.Servais A, Fremeaux-Bacchi V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet. 2007;44:193–9. doi: 10.1136/jmg.2006.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simpson IJ, Moran J, Evans DJ, Peters DK. Prolonged complement activation in mice. Kidney Int. 1978;13:467–71. doi: 10.1038/ki.1978.69. [DOI] [PubMed] [Google Scholar]
- 74.Verroust PJ, Wilson CB, Dixon FJ. Lack of nephritogenicity of systemic activation of the alternate complement pathway. Kidney Int. 1974;6:157–69. doi: 10.1038/ki.1974.94. [DOI] [PubMed] [Google Scholar]
- 75.Jansen JH, Hogasen K, Mollnes TE. Extensive complement activation in hereditary porcine membranoproliferative glomerulonephritis type II (porcine dense deposit disease) Am J Pathol. 1993;143:1356–65. [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen JH, Hogasen K, Harboe M, Hovig T. In situ complement activation in porcine membranoproliferative glomerulonephritis type II. Kidney Int. 1998;53:331–49. doi: 10.1046/j.1523-1755.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- 77.Hogasen K, Jansen JH, Mollnes TE, Hovdenes J, Harboe M. Hereditary porcine membranoproliferative glomerulonephritis type II is caused by factor H deficiency. J Clin Invest. 1995;95:1054–61. doi: 10.1172/JCI117751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jansen JH, Hogasen K, Grondahl AM. Porcine membranoproliferative glomerulonephritis type II: an autosomal recessive deficiency of factor H. Vet Rec. 1995;137:240–4. doi: 10.1136/vr.137.10.240. [DOI] [PubMed] [Google Scholar]
- 79.Hegasy GA, Manuelian T, Hogasen K, Jansen JH, Zipfel PF. The molecular basis for hereditary porcine membranoproliferative glomerulonephritis type II: point mutations in the factor H coding sequence block protein secretion. Am J Pathol. 2002;161:2027–34. doi: 10.1016/S0002-9440(10)64481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hogasen K, Jansen JH, Harboe M. Eradication of porcine factor H deficiency in Norway. Vet Rec. 1997;140:392–5. doi: 10.1136/vr.140.15.392. [DOI] [PubMed] [Google Scholar]
- 81.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–8. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 82.Pickering MC, Warren J, Rose KL, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA. 2006;103:9649–54. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. Mol Immunol. 2007;44:3924. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vyse TJ, Spath PJ, Davies KA, et al. Hereditary complement factor I deficiency. Q J Med. 1994;87:385–401. [PubMed] [Google Scholar]
- 85.Amadei N, Baracho GV, Nudelman V, Bastos W, Florido MP, Isaac L. Inherited complete factor I deficiency associated with systemic lupus erythematosus, higher susceptibility to infection and low levels of factor H. Scand J Immunol. 2001;53:615–21. doi: 10.1046/j.1365-3083.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 86.Genel F, Sjoholm AG, Skattum L, Truedsson L. Complement factor I deficiency associated with recurrent infections, vasculitis and immune complex glomerulonephritis. Scand J Infect Dis. 2005;37:615–18. doi: 10.1080/00365540510034536. [DOI] [PubMed] [Google Scholar]
- 87.Sadallah S, Gudat F, Laissue JA, Spath PJ, Schifferli JA. Glomerulonephritis in a patient with complement factor I deficiency. Am J Kidney Dis. 1999;33:1153–7. doi: 10.1016/S0272-6386(99)70155-1. [DOI] [PubMed] [Google Scholar]
- 88.Sacks SH, Zhou W, Pani A, Campbell RD, Martin J. Complement C3 gene expression and regulation in human glomerular epithelial cells. Immunology. 1993;79:348–54. [PMC free article] [PubMed] [Google Scholar]
- 89.Sacks S, Zhou W, Campbell RD, Martin J. C3 and C4 gene expression and interferon-gamma-mediated regulation in human glomerular mesangial cells. Clin Exp Immunol. 1993;93:411–17. doi: 10.1111/j.1365-2249.1993.tb08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.West CD, Witte DP, McAdams AJ. Composition of nephritic factor-generated glomerular deposits in membranoproliferative glomerulonephritis type 2. Am J Kidney Dis. 2001;37:1120–30. doi: 10.1053/ajkd.2001.24511. [DOI] [PubMed] [Google Scholar]
- 91.Neumann HP, Salzmann M, Bohnert-Iwan B, et al. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J Med Genet. 2003;40:676–81. doi: 10.1136/jmg.40.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Warwicker P, Goodship TH, Donne RL, et al. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53:836–44. doi: 10.1111/j.1523-1755.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- 93.Richards A, Buddles MR, Donne RL, et al. Factor H mutations in hemolytic uremic syndrome cluster in exons 18–20, a domain important for host cell recognition. Am J Hum Genet. 2001;68:485–90. doi: 10.1086/318203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perez-Caballero D, Gonzalez-Rubio C, Gallardo ME, et al. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am J Hum Genet. 2001;68:478–84. doi: 10.1086/318201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J, et al. Complement factor I: a susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet. 2004;41:84–8. doi: 10.1136/jmg.2004.019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noris M, Brioschi S, Caprioli J, et al. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet. 2003;362:1542–7. doi: 10.1016/S0140-6736(03)14742-3. [DOI] [PubMed] [Google Scholar]
- 97.Richards A, Kemp EJ, Liszewski MK, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2003;100:12966–71. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, et al. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci USA. 2007;104:240–5. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manuelian T, Hellwage J, Meri S, et al. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111:1181–90. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanchez-Corral P, Perez-Caballero D, Huarte O, et al. Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am J Hum Genet. 2002;71:1285–95. doi: 10.1086/344515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanchez-Corral P, Gonzalez-Rubio C, Rodriguez de Cordoba S, Lopez-Trascasa M. Functional analysis in serum from atypical hemolytic uremic syndrome patients reveals impaired protection of host cells associated with mutations in factor H. Mol Immunol. 2004;41:81–4. doi: 10.1016/j.molimm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 102.Pickering MC, de Jorge EG, Martinez-Barricarte R, et al. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 2007;204:1249–56. doi: 10.1084/jem.20070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng ZZ, Hellwage J, Seeberger H, Zipfel PF, Meri S, Jokiranta TS. Comparison of surface recognition and C3b binding properties of mouse and human complement factor H. Mol Immunol. 2006;43:972–9. doi: 10.1016/j.molimm.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Caprioli J, Noris M, Brioschi S, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–79. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Donne RL, Abbs I, Barany P, et al. Recurrence of hemolytic uremic syndrome after live related renal transplantation associated with subsequent de novo disease in the donor. Am J Kidney Dis. 2002;40:E22. doi: 10.1053/ajkd.2002.36938. [DOI] [PubMed] [Google Scholar]
- 106.Esparza-Gordillo J, Goicoechea de Jorge E, Buil A, et al. Predisposition to atypical hemolytic uremic syndrome involves the concurrence of different susceptibility alleles in the regulators of complement activation gene cluster in 1q32. Hum Mol Genet. 2005;14:703–12. doi: 10.1093/hmg/ddi066. [DOI] [PubMed] [Google Scholar]
- 107.Esparza-Gordillo J, Jorge EG, Garrido CA, et al. Insights into hemolytic uremic syndrome: segregation of three independent predisposition factors in a large, multiple affected pedigree. Mol Immunol. 2006;43:1769–75. doi: 10.1016/j.molimm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Zipfel PF, Edey M, Heinen S, et al. Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet. 2007;43:e41. doi: 10.1371/journal.pgen.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jozsi M, Strobel S, Zipfel SLH, et al. Factor H autoantibodies in atypical hemolytic uraemic syndrome correlate with CFHR1/CFHR3 deficiency and affect recognition functions. Mol Immunol. 2007;44:3924. [Google Scholar]
- 110.Jozsi M, Strobel S, Dahse HM, et al. Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood. 2007;110:1516–18. doi: 10.1182/blood-2007-02-071472. [DOI] [PubMed] [Google Scholar]


