Abstract
Several chronic diseases are characterized by inflammation, T cell recruitment and tissue remodelling. We hypothesized that activated T cells may stimulate remodelling of extracellular matrix (ECM) in vitro. Total T cells (CD3+) as well as CD4+ and CD8+ subsets were isolated from peripheral blood and stimulated, after which conditioned media (CM) were obtained. CM was added to human lung fibroblasts in three-dimensional collagen gels and the area of gels was measured daily. Hydroxyproline was determined as a measure of collagen degradation in the gels. Matrix metalloproteinase (MMP) activity in the culture media was analysed by gelatine zymography. Cytokine secretion of stimulated CD4+ and CD8+ T cells was analysed. CD3+ CM augmented collagen gel contraction in a time- and dose-dependent manner (P < 0·0001). CD4+ T cell CM was more potent than CD8+ T cell CM (P < 0·001). CD3+ CM and CD4+ T cell CM, but not CD8+ T cell CM, stimulated fibroblast-mediated collagen degradation and MMP-9 activity. A broad-spectrum MMP-inhibitor added to the culture system inhibited both gel contraction and MMP activity. Activated CD4+ T cells secreted significantly more tumour necrosis factor (TNF) and interleukin (IL)-6 compared to CD8+ T cells. CD3+ CM from patients with chronic obstructive pulmonary disease stimulated fibroblast-mediated collagen gel contraction to the same magnitude as CD3+ CM from healthy controls. In conclusion, activated CD4+ T cells can stimulate fibroblast-mediated degradation of ECM in vitro. This could be a mechanism by which activated T cells stimulate degradation of lung tissue leading to pulmonary emphysema.
Keywords: cell interactions, chronic obstructive pulmonary disease (COPD), matrix metalloproteinases (MMPs)/collagenases, T cells
Introduction
Several chronic inflammatory lung diseases such as rheumatoid arthritis, idiopathic pulmonary fibrosis, asthma, sarcoidosis and chronic obstructive pulmonary disease (COPD) are characterized by T cell recruitment and remodelling of the extracellular matrix (ECM). Whereas T helper type 2 (Th2) cells are important for the progression of fibrosis, interferon (IFN)-γ-producing Th1 cells has the opposite effect by inducing collagen degradation [1].
In COPD, activated T cells, in particular of the CD8+ subset, are accumulated in both the central and peripheral airways but their role in the pathophysiology of the disease has not yet been clarified [2–5]. It is suggested that the T cells in COPD are preferentially of Th1 and T cytotoxic type 1 (Tc-1) phenotypes [4,6]. Recent reports indicate that CD4+ T cells might also be of importance, especially in severe COPD [7–10].
In emphysema, the lung parenchyma and its elasticity is destroyed. It is believed that an imbalance between proteases and anti-proteases is involved in this destructive process [11]. There is an increased activity of matrix metalloproteinases (MMPs), in particular MMP-2 and MMP-9 in emphysematous lung tissue [12,13]. In addition, alveolar macrophages from patients with emphysema transcribe and release elevated levels of MMP-9 compared to healthy controls [13]. Moreover, guinea pigs exposed to smoke develop emphysema and show increased levels of MMP-9 in the lungs [14]. When these animals are treated with a broad-spectrum MMP-inhibitor the emphysematous changes can be attenuated.
Given that activated T cells are present in the tissue in different chronic inflammatory diseases, including COPD, we hypothesized that these cells have the capacity to stimulate fibroblast-mediated tissue degradation in vitro. To test this hypothesis we separated human T cells, stimulated them and used the conditioned media in a functional in vitro assay for tissue remodelling, the three-dimensional collagen gel contraction assay [15].
Materials and methods
Subjects
Peripheral blood was obtained from 18 healthy, non-smoking individuals between the ages of 26 and 60. These blood donors were not taking any medications on a regular basis. For the experiment reported in the last paragraph of the Results section, peripheral blood was obtained from an additional five COPD patients and five age-matched healthy individuals (Table 1). The COPD patients were all ex-smokers, whereas the control group were never smokers. Informed consent was obtained from each subject. The study was approved by the Regional Ethical Review Board, Stockholm, Sweden.
Table 1.
Characteristics of chronic obstructive pulmonary disease (COPD) patients and controls (see Results, last section).
| COPD | Controls | |
|---|---|---|
| Subjects (n) | 5 | 5 |
| Age (years) | 65 (60–77) | 59 (56–68) |
| Male/female | 1/4 | 2/3 |
| Smoking history (pack-years) | 34 (24–62) | 0 |
| FVC % pred | 81 (57–97) | 111 (89–129) |
| FEV1 % pred | 54 (23–63) | 106 (87–142) |
| FEV1/FVC % | 42 (25–61) | 77 (74–87) |
Data presented as n or median (range), % pred, percentage predicted; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Collagen type I
Type I collagen (rat-tail tendon collagen: RTTC) was extracted according to a previously published method [16].
Cell cultures
Human fetal lung fibroblasts (HFL-1) were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). The cells were cultured as described previously [16]. The fibroblasts were passaged twice a week and were used between the 15th and 20th passages. Confluent fibroblasts were trypsinized [trypsin–ethylenediamine tetraacetic acid (EDTA); Sigma-Aldrich, St. Louis, MO, USA; 0·05% trypsin 0·53 mM EDTA], resuspended in Dulbecco's modified essential medium (DMEM) without serum and used for collagen gel cultures.
T cell conditioned media
Freshly collected blood was placed into sodium heparin tubes and mononuclear cells were separated by density gradient centrifugation (Ficoll-PaqueTM PLUS; Amersham Biosciences, Uppsala, Sweden) at 381 g at room temperature for 20 min. T cells were stained with CD3-fluorescein isothiocyanate (FITC) or CD4-phycoerythrin (PE)Cy5 or CD8-PE monoclonal antibodies (DakoCytomation, Glostrup, Denmark) for 20 min in the dark at +4°C. The stained cells were then isolated by cell sorting in a MoFlo high-speed cellsorter (DakoCytomation). The purity was always >93%. CD4+ and CD8+ T cells were also separated by negative isolation kits (Invitrogen, Carlsbad, CA, USA). Conditioned media (CM) was obtained from T cells (CD3+, CD4+ or CD8+) cultured at a concentration of 1·0 × 106/ml in serum-free DMEM. The T cells were activated by anti-CD3/anti-CD28-coated magnetic beads in a ratio of 1·2 : 1 bead/cell (Dynal Biotech, Oslo, Norway) and incubated (+37°C, 5% CO2) in 96-well plates for 3 days. The cells were removed by centrifugation and CM was stored at −80°C until use.
Collagen gel contraction assay
Collagen gels were prepared as described previously using 3 × 105 fibroblasts (HFL-1)/ml gel solution [16]. After gelation the collagen gels were released from the surface of the culture wells using a sterile spatula and transferred into 60 mm tissue culture dishes (Falcon, BD Franklin Lakes, NJ, USA) containing 5 ml of serum-free DMEM. The T cell CM was added to DMEM at a concentration of 1/5, unless indicated otherwise. In another set of experiments CD3+ CM (at a concentration of 1/10) from COPD patients were compared to healthy controls. In some experiments the broad-spectrum MMP-inhibitor GM6001 (Calbiochem, Darmstadt, Germany) was added, together with CD3+ CM, in a final concentration of 10 μM to the medium in which the gels were floated. The floating gels were incubated at 37°C, 5% CO2 for 4 days.
In all the experiments, the area of the collagen gels was measured using an image analyser system (Leica Microsystems AG, Wetzlar, Germany). Using the previously described method [16], the area of each gel could be measured while sterility was preserved.
Measurement of cell proliferation
In order to estimate cell numbers in the gels, the DNA content was assayed fluorometrically with Hoechst dye 33258 (Sigma-Aldrich, St Louis, MO, USA) using a modification of a previously published method [17].
Gelatine zymography
The medium in which the collagen gels were floated was collected and concentrated 25–40 × with Amicon Ultra-4 filter [5000 molecular weight cut-off (MWCO); Millipore, Carrigtwohill, Ireland], by centrifugation at 7500 g for 30 min. The concentrated medium was diluted with an equal amount of 2× sample buffer (0·125 M Tris HCl pH 6·8, 4% (w/v) sodium dodecyl sulphate (SDS), 20% (v/v) glycerol, 0·04% (w/v) bromophenol blue) and subjected to SDS-polyacrylamide gel electrophoresis (PAGE) under non-reducing conditions in 6% acrylamide gels containing 2 mg/ml gelatine. After separation of MMPs by electrophoresis (MiniVE complete; Amersham-Biosciences), gels were washed by gentle shaking for 2 × 30 min at room temperature in 2·5% (v/v) Triton-X 100. The gels were then incubated in metalloproteinase buffer (20 mM glycine, 10 mM CaCl2, 1 μM ZnCl2, pH 8·3) for 48 h at 37°C, and stained subsequently with 0·1% Coomassie blue. Zones of proteolysis appeared as clear bands against a blue background. Supernatants from HT1080 cells were used as positive controls [18].
RNA extraction and cDNA synthesis
HFL-1 cells were seeded in DMEM, supplemented with 10% fetal calf serum (FCS) in a 12-well tissue culture plate until 80% confluence and starved overnight. The cells were then stimulated with CD3+ CM for 24 h and total RNA was extracted using the RNeasy extraction kit (Qiagen Inc., Valencia, CA, USA), according to the manufacturer's instructions. The RNA was reverse-transcribed from 1 to 2 μg RNA in a total volume of 20 μl using 20 μM random hexamer primers, 10 mM deoxyribonucleoside triphosphate (dNTP), 40 U RNasin (Pharmacia Biotech, Uppsala, Sweden) and 200 U SuperScriptTM RNase H– reverse transcriptase (Invitrogen) according to the manufacturer's instructions.
Semiquantification of MMP-9
The basal and stimulated MMP-9 mRNA expression in HFL-1 was quantified using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Three μl of cDNA were amplified by real-time polymerase chain reaction (PCR) with 1 ×TaqMan universal PCR master mix (Applied Biosystems). As primers and probes, the Assay on Demand Kit from Applied Biosystems was used for quantification of MMP-9 (Hs00234579_m1). Ribosomal protein large P0 (RPLP0) was used as a housekeeping gene to normalize for RNA loading. All TaqMan assays were cDNA-specific. Each sample was analysed in duplicate using the ABI prism 7000 (Applied Biosystems).
Hydroxyproline assay
Hydroxyproline was quantified as a measure of collagen content in the gels using a modification of a previously described method [19]. Briefly, gels were centrifuged at 11 500 g for 10 min to eliminate fluid and then heated at 67°C for 10 min to dissolve the collagen. Twenty microlitres of the supernatant was mixed gently with 20 μl of 3·3 N sodium hydroxide followed by autoclaving at 121°C for 15 min. Chloramine T reagent (450 μl/sample) was then applied and samples were oxidized at room temperature for 20 min. Following this, samples were gently mixed with 500 μl of freshly prepared Ehrlich's reagent and developed for 20 min at 65°C. Absorbance of each sample was measured at 550 nm.
Measurement of cytokines
CD4+ and CD8+ T cells were stimulated for 3 days. Concentrations of interleukin (IL)-1β, IL-4, IL-6, IL-8, IL-10, interferon (IFN)-γ and tumour necrosis factor (TNF) in the CM were determined using cytometric bead array (CBA) flex set (BD, Franklin Lakes, NJ, USA). Thirty microlitres of all samples were prepared as described in the protocol and the cytokines were detected within the range 10–2500 pg/ml in a FACSCantoTM II flow cytometer (BD). The data were analysed with FCAP Array Software version 1·01 (BD).
Statistical analysis
Results are presented as median with lower and upper quartile values (p25–p75) unless stated otherwise. The Mann–Whitney U-test was used when two groups were compared. When more than two groups were evaluated, analysis of variance (anova) followed by Tukey's multiple comparison tests were performed. P-values < 0·05 were considered significant. Statistical comparisons and graphs were performed by use of GraphPad Prism version 4·03.
Results
T cell conditioned media stimulate fibroblast-mediated collagen gel contraction
Fibroblast-populated collagen gels incubated with CD3+ CM from healthy individuals contracted more during the 4-day culture period compared to control gels (Fig. 1a). Increasing concentrations of CD3+ CM were added to the culture system showing that the augmentation of fibroblast-mediated collagen gel contraction was both time- and concentration-dependent (Fig. 1b). The increased gel contraction by CD3+ CM was not seen earlier than 2 days of culture. The experiment was repeated on multiple occasions with a final concentration of 1/5 CD3+ CM (Table 2) and median (p25–p75) of these 10 experiments at day 4 as the percentage of initial gel area was 3·3 (1·2–14·6)% (CD3+ CM) versus 38·2 (31·9–43·2)% (control). Although the magnitude of gel contraction varied in individual experiments, pooled data showed a significant (P < 0·0001) difference in contraction between CD3+ CM-stimulated and control gels.
Fig. 1.
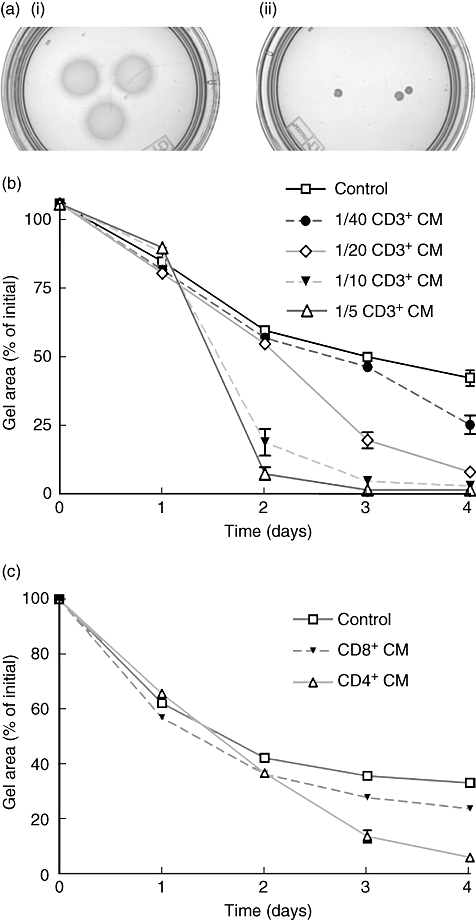
T cell conditioned media (CM) stimulate fibroblast-mediated gel contraction. Fibroblasts [3 × 105 human fetal lung fibroblasts (HFL)] were cast into collagen gels in a 24-well culture plate. After gelation the gels were released into a 60 mm tissue culture dish containing serum-free Dulbecco's modified essential medium (DMEM) with or without T cell CM. The area of the floating gels was measured daily with an image analyser. (a) Photograph of collagen gels after 4 days of culture. (i) Control gels with fibroblasts. (ii) Gels with fibroblasts stimulated with 1/5 CD3+ CM. (b) Time- and concentration-dependent augmentation of fibroblast-mediated collagen gel contraction by various concentrations of CD3+ CM (1/40, 1/20, 1/10 and 1/5). Vertical axis: the percentage of initial gel area. Horizontal axis: time in days. Data are presented as mean of triplicate gels ± standard error of the mean (s.e.m.) from one representative experiment. (c) CD4+ and CD8+ T cell stimulation of fibroblast-mediated collagen gel contraction. CD4+ and CD8+ T cell CM were diluted 1/5 in serum-free DMEM. Data presented as in (b).
Table 2.
CD3+ conditioned media (CM) stimulate fibroblast-mediated collagen gel contraction.
| Exp. | Fibroblasts | Fibroblasts + CD3+ CM* |
|---|---|---|
| 1 | 36·3 ± 0·9 | 4·5 ± 0·1 |
| 2 | 35·1 ± 1·3 | 5·7 + 1·1 |
| 3 | 30·5 ± 0·7 | 9·4 ± 0·5 |
| 4 | 40·8 ± 0·7 | 30·9 ± 1·3 |
| 5 | 40·8 ± 0·7 | 1·5 ± 0·04 |
| 6 | 45·5 ± 0·2 | 19·7 ± 4·9 |
| 7 | 51·3 ± 0·1 | 2·0 ± 0·3 |
| 8 | 40·0 ± 2·7 | 1·4 ± 0·2 |
| 9 | 24·9 ± 0·2 | 1·0 ± 0·1 |
| 10 | 33·2 ± 0·4 | 0·6 ± 0·1 |
| Median | 38·2 | 3·3*** |
| (p25–p75) | (31·9–43·2) | (1·2–14·6) |
Values are percentage of initial gel area as mean ± standard error of the mean of triplicate gels from 10 individual experiments at day 4. Median (p25–p75) of all experiments is presented.
CD3+ CM was diluted to 1/5 in serum free Dulbecco's modified essential medium.
P < 0·0001 versus fibroblasts alone (Mann–Whitney).
CD4+ and CD8+ T cells isolated through cell sorting (n = 4) or by negative isolation with beads (n = 4) were stimulated for 3 days as described above. CD4+ T cell CM and CD8+ T cell CM were added separately to the media in which the gels were floated (Fig. 1c). Pooled data from these eight separate experiments confirmed that CD4+ T cell CM stimulated fibroblast-mediated collagen gel contraction significantly more than both CD8+ T cell CM and control; 5·9 (3·5–6·4)% (CD4+) of initial gel area after 4 days of culture versus 36·1 (29·6–45·8)% (CD8+) and 37·1 (34·6–45·8)% (control). Data were analysed with one-way anova followed by Tukey's multiple comparison test, P < 0·001 for both CD4+versus CD8+ and CD4+versus control. There was no significant difference in the ability of CD8+ T cell CM to stimulate contraction compared to control.
Cell proliferation
Fibroblast-populated collagen gels stimulated with CD3+ CM had slightly more DNA than control gels [optical density (OD)]-value: 1083 (959–1155) versus 832 (726–981); data from four sets of experiments in triplicate). However, the difference was not statistically significant (P = 0·11).
Gelatine zymography
Control cultures with fibroblasts alone contained both pro- and active forms of MMP-2, but no MMP-9 (Fig. 2a). When fibroblasts in gel cultures were stimulated with CD3+ CM, additional bands of active MMP-9 were detected. CD3+ CM alone did not contain any detectable MMP-2 or MMP-9 activity (data not shown). Gel cultures stimulated with CD4+ and CD8+ T cell CM from four different donors were compared (Fig. 2b). Active MMP-9 was detected only in CD4+ T cell CM-stimulated culture media (Fig. 2c). This difference was statistically significant (P < 0·05).
Fig. 2.
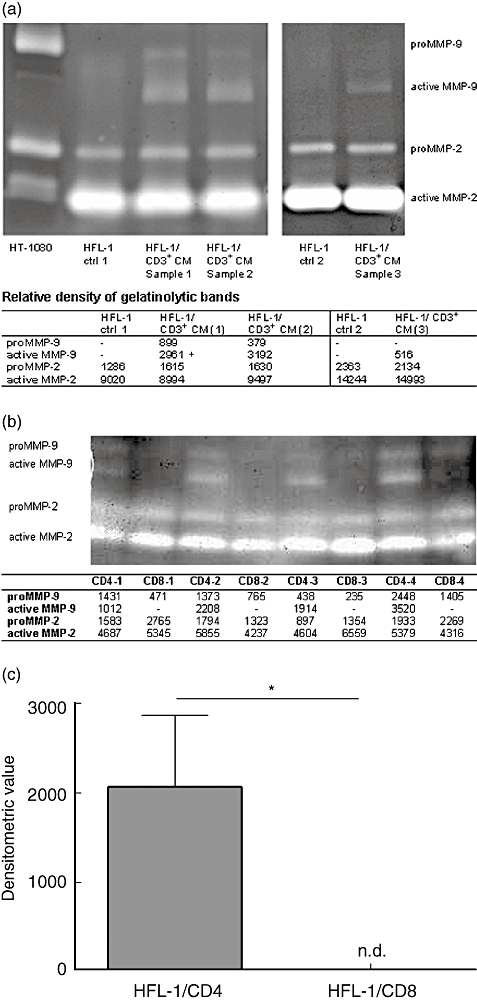
T cell conditioned media (CM) stimulate fibroblasts to release active matrix metalloproteinase (MMP)-9 in three-dimensional cultures. Media from gel cultures were collected and concentrated. Proteins were separated by electrophoresis on an acrylamide gel containing gelatine. MMP-2 and MMP-9 activity were detected after proteolysis. Densitometry was used to determine the intensity of MMP-9 activity. (a) MMP-2 and MMP-9 activity in media stimulated with CD3+ CM (lanes 3, 4 and 6) from three separate experiments were compared to control samples [human fetal lung fibroblasts (HFL) cultured in Dulbecco's modified essential medium (DMEM) alone, lanes 2 and 5]. The first lane contained supernatants from HT1080 cells (positive control). (b) MMP-2 and MMP-9 activity after stimulation with CD4+ T cell CM and CD8+ T cells CM separately from four different experiments (CD4-1/CD8-1, CD4-2/CD8-2, CD4-3/CD8-3 and CD4-4/CD8-4). (c) The relative MMP-9 activity as densitometric value in media from gel cultures stimulated with CD4+ and CD8+ T cell CM (P < 0·05, Mann–Whitney, n = 4), n.d. = not detected.
Inhibition of gel contraction and MMP activity by GM6001
GM6001 was capable of inhibiting both CD3+ CM-stimulated and -unstimulated fibroblast-mediated collagen gel contraction (Fig. 3). Control gels cultured with GM6001 had a significantly inhibited gel-contraction compared to control gels without GM6001 [72·4 (69·3–78·3)% of initial gel area (median, minimum–maximum) versus 40·8 (30·5–41·4)%], P < 0·01. Gels stimulated with CD3+ CM was inhibited even more by GM6001 [81·9 (74·0–89·0)% versus 9·4 (1·5–31)%], P < 0·001. In addition, when the MMP-inhibitor GM6001 was added to the culture system, both MMP-2 and MMP-9 activities were decreased when measured with gelatine zymography (data not shown).
Fig. 3.
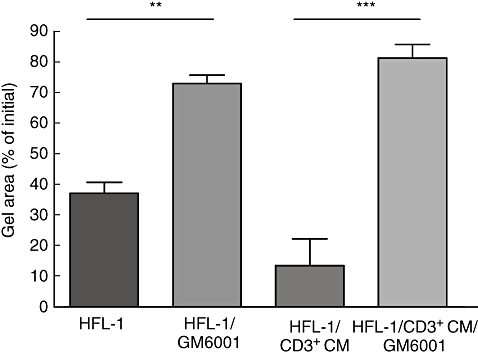
Matrix metalloproteinase (MMP) inhibitor GM6001 attenuates CD3+ conditioned media (CM) mediated collagen gel contraction. Fibroblasts [human fetal lung fibroblasts (HFL)-1] were cast in collagen gels and released into serum-free Dulbecco's modified essential medium (DMEM) with or without CD3+ CM and the broad-spectrum MMP-inhibitor GM6001. After 4 days of culture, the area of the gels was measured with an image analyser. Vertical axis: percentage of initial gel area. Horizontal axis: condition. Data shown as mean ± standard error of the mean of triplicate gels from three separate experiments. (**P < 0·01, ***P < 0·001, analysis of variance followed by Tukey's).
Fibroblast MMP-9 mRNA expression
Stimulation of fibroblasts with CD3+ CM from two healthy individuals resulted in a 2·4- and a 9·6-fold increase of MMP-9 expression, respectively, compared to control. The corresponding cycle threshold (Ct) values were 39·1 for the control and 37·7 and 35·9 for the CD3+ CM-stimulated samples. In an experiment with CD3+ CM from a third individual MMP-9 mRNA expression was undetectable in the control sample (after 45 PCR cycles). However, the reverse transcription (RT)–PCR Ct value of the CD3+ CM-treated sample was 38·6. The corresponding Ct values of the housekeeping gene (RPLP0) in all samples were in the range of 22·5–22·8, showing that the increase was not an artefact.
Collagen degradation
Hydroxyproline was measured in order to estimate collagen content in the gels. The amount of hydroxyproline was significantly lower in gels stimulated with CD3+ CM compared to control gels (Fig. 4a). Data from four pooled experiments of triplicates at day 4 showed the decrease of collagen as 10·4 (3·6–14·7) μg hydroxyproline when CD3+ CM was added versus 20·9 (19·1–25) μg in the control, P < 0·05. Gels stimulated with CD4+ T cell CM had significantly lower collagen content compared to gels stimulated with CD8+ T cell CM. Median hydroxyproline content in CD4+ T cell CM-stimulated gels was 29 (13–49)% of control (HFL-1) compared to CD8+ T cell CM-stimulated gels; 116 (102–137)%, P < 0·05 (Fig. 4b).
Fig. 4.
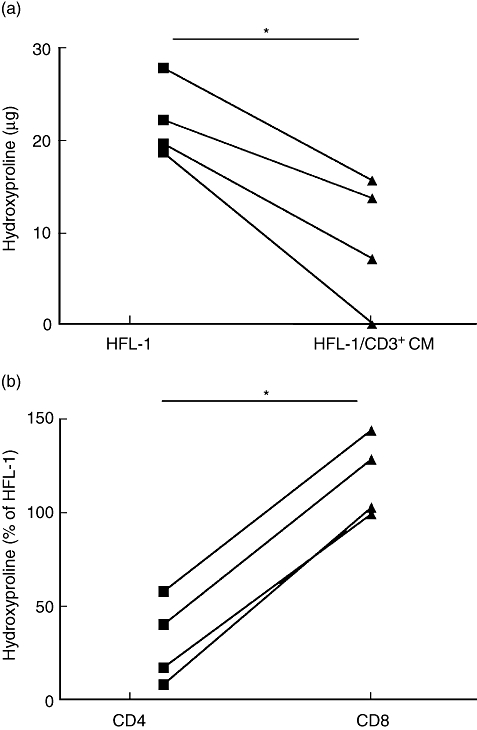
T cell conditioned media (CM) stimulate collagen degradation. Collagen gels from day 4 were prepared and assayed for hydroxyproline as a measure of collagen content. (a) Hydroxyproline content in triplicates of gels from four separate experiments with control gels [human fetal lung fibroblasts (HFL)-1] and gels incubated with CD3+ CM. Vertical axis: μg hydroxyproline. Horizontal axis: samples (*P < 0·05, Mann–Whitney). (b) Hydroxyproline content in triplicate gels stimulated with CD4+ T cell CM and CD8+ T cell CM from four separate experiments. Vertical axis: hydroxyproline (% of control, HFL-1). Horizontal axis: samples (*P < 0·05, Mann–Whitney).
Cytokine secretion
CD4+ (n = 4) and CD8+ T cells (n = 4) were obtained by negative isolation with beads.
Concentrations of cytokines secreted by activated CD4+ and CD8+ T cells were measured after 3 days of stimulation. IL-1β could not be detected and there were no significant differences in the IL-8, IL-10 and IFN-γ secretion (Table 3). However, IL-6 and TNF was increased significantly in supernatants from CD4+ T cells in comparison to CD8+ T cells (P < 0·05 for both). The increased IL-4 secretion from stimulated CD4+ T cells should be interpreted with caution, as the values were in the lower part or below the detection limit on the standard curve.
Table 3.
Cytokine concentrations in conditioned media (CM) from activated CD4+ and CD8+ T cells.
| Cytokine | CD4 | CD8 |
|---|---|---|
| IL-1β | Not detected | Not detected |
| IL-4 | 16 (12–18)* | 6 (3–9) |
| IL-6 | 269 (110–424)* | 9 (1–36) |
| IL-8 | 26 (9–111) | 6 (3–37) |
| IL-10 | 24 (8–53) | 7 (1–9) |
| IFN-γ | 857 (176–1895) | 347 (54–2757) |
| TNF | 2880 (1507–4781)* | 496 (131–1462) |
Values are in pg/ml, median (range).
P < 0·05 (Mann–Whitney). IFN: interferon; IL: interleukin; TNF: tumour necrosis factor.
The effect of CD3+ conditioned media from COPD patients on fibroblast-mediated collagen gel contraction
CD3+ CM collected from five COPD patients and five matched controls were added separately to the gel culture media in which the gels were floated. Both CD3+ CM from COPD patients and controls stimulated fibroblast-mediated collagen gel contraction. However, there was no difference over time between these groups. Median at day 4 of CD3+ CM from the COPD group: 14·4 (3·4–19·6)% of initial gel area versus the healthy group: 7·9 (3·3–13·8)%, P = 0·55 (Mann–Whitney).
Discussion
In the current study, we demonstrate that activated human T cells can stimulate fibroblast-mediated contraction of collagen gels. We found that conditioned media (CM) from CD4+ T cells was more potent than CD8+ T cell CM in mediating this effect. In the three-dimensional culture system, a degradation of collagen was observed together with an increased MMP-9 expression and activity. A broad-spectrum MMP-inhibitor added to the culture system inhibited both gel contraction and MMP activity. Finally, we found that activated CD4+ T cells secrete more IL-6 and TNF compared to activated CD8+ T cells.
The three-dimensional collagen gel contraction assay used in this study, described originally by Bell et al. [15], is used as a method for studying fibroblast-mediated remodelling in vitro. Even though this culture system is a simplification of in vivo conditions, the system has been used to elucidate effects of potential fibrotic mediators [16,20–22]. The method allows studies of mediators affecting fibroblasts cultured in a three-dimensional manner and also provides an opportunity to study cell–cell interactions. In the present study we did not culture T cells and fibroblasts together in order to exclude any possible allogeneic reaction between the two cell types.
It has been demonstrated previously that the number of CD8+ T cells in the small airways in COPD correlates with lung function impairment [2,3]. CD8+ T cells have the ability to induce cell death through granzymes, perforins and Fas-induced apoptosis and it has been suggested that CD8+ T cells are associated with apoptosis of alveolar epithelial cells in emphysema [23]. Other studies show that both CD4+ and CD8+ T cell numbers increase in peripheral airways in severe COPD [24,25]. Aoshiba et al. suggested that lymphocyte subsets are distributed differently, depending on the severity of emphysema, which can vary in different sites of the lung [7]. They showed that CD4+ T cells are more abundant in severe emphysematous lesions, whereas CD8+ T cells are found preferentially in mild emphysematous tissue. Our finding that CD4+ T cell CM stimulate fibroblasts to increased protease activity and remodelling in vitro supports these previous observations and suggests that this subtype might be of importance in severe COPD, where tissue degradation and emphysema are predominant findings.
Our finding that T cells stimulate fibroblast-mediated MMP-9 activity and collagen degradation is in line with the concept that emphysema is due to an imbalance between proteases and anti-proteases [26]. We also demonstrated that CD3+ CM stimulated fibroblasts to express MMP-9 mRNA. In COPD, there is most probably an interplay between several cell types and mediators that contribute to remodelling and degradation of lung tissue. For instance, it has been shown that macrophages and neutrophils are recruited to the lungs of these patients and that they produce a variety of MMPs, such as MMP-1, MMP-2, MMP-8 and MMP-9, that might be of importance in the development of emphysema [27,28]. Fibroblast-mediated collagen gel contraction could be inhibited by a broad-spectrum MMP-inhibitor. The inhibition of basal contraction by GM6001 may be due to inhibition of the constitutive release of MMP-2 whereas the greater inhibition seen with CD3+ CM is, plausibly, an inhibition of both constitutive MMP-2 as well as induced MMP-9 and other MMPs. This strengthens our hypothesis that MMPs are important in fibroblast-mediated remodelling induced by T cell CM.
Contraction of fibroblast-populated collagen gels is dependent upon the number of cells in the gel [17]. To investigate whether cell proliferation during the culture period could explain the increased contractility, total DNA content in the gels was determined. The number of cells measured as DNA content was slightly higher in gels stimulated with CD3+ CM. However, this difference was not statistically significant and this small increase in cell numbers could most probably not explain the pronounced CD3+ CM-induced augmentation of fibroblast-mediated contraction.
The stimulatory effect of CD3+ CM and CD4+ T cell CM in the fibroblast-mediated collagen gel contraction assay was never observed earlier than 2 days of culture. This is in agreement with the results of Distler et al. who showed that microparticles derived from activated or apoptotic T cells can induce different types of fibroblasts to the release of mediators, including MMP-9 [29], and that such a response from the fibroblasts was time-dependent, with a strong MMP activity induction only after 36 h.
Activated T cells have been shown to inhibit collagen production by dermal fibroblasts through TNF and IFN-γ activity [30,31]. We measured concentrations of cytokines in the CD4+ and CD8+ T cell supernatants and found significantly more CD4+ T cell-secreted IL-6 and TNF. The higher concentrations of IL-6 could be important for sustaining the inflammation as this is a proinflammatory cytokine. It has also been shown that IL-6 per se can stimulate fibroblast-mediated collagen degradation through MMP activity [32]. A previous study has shown that both TNF and IFN-γ inhibit collagen gel contraction when added alone or together [33]. However, a functional study of cytokine interaction with fibroblasts showed that when TNF was cultured together with growth factors such as platelet-derived growth factor (PDGF) or basic fibroblast growth factor (bFGF), fibroblasts were stimulated to MMP-9 up-regulation [34]. This was not observed by growth factors or TNF alone. This suggests that cytokines, in particular TNF, in the CD4+ T cell supernatants can induce MMP-9 secretion and simulate fibroblast-mediated gel contraction when either T cell produced growth factors or autocrine growth factors produced by fibroblasts themselves are present. The previous findings of T cell-mediated inhibition of collagen production [30,31], together with our present demonstration of T cell-induced protease activity, suggest a potent role for T cells in tissue remodelling. A decrease in collagen synthesis combined with increased production of tissue degrading enzymes most certainly results in tissue degradation. However, in this study only a few cytokines were investigated. Further studies of the mechanisms involved and the mediators secreted by activated T cells that stimulate fibroblast-mediated tissue remodelling in vitro need to be performed to understand this process fully.
CD3+ CM from COPD patients were also compared to CD3+ CM from age-matched healthy individuals. However, we were not able to detect any difference in their ability to stimulate fibroblast-mediated collagen gel contraction. The reason that T cells from healthy controls behaved in the same manner as T cells from COPD patients may be due to the fact that the cells from both groups were stimulated extensively and had reached a considerable activity. T cells are accumulated in the lung in COPD and are activated in vivo[4,5]. Therefore, we believe that our approach using in vitro-activated peripheral blood T cells may mimic lung T cells in an inflammatory condition. Furthermore, COPD is an extremely heterogeneous disease. Our patients were not included based on their extent of emphysema or systemic involvements. This could hamper a potential effect of T cells from subsets of COPD patients in various biological assays.
In conclusion, we have demonstrated that activated CD4+ T cells can stimulate fibroblasts to degradation of extracellular matrix in vitro. Our data demonstrate that this is due, at least partially, to fibroblast-induced MMP activity and may be a mechanism by which activated T cells stimulate degradation of lung tissue leading to pulmonary emphysema.
Acknowledgments
We would like to thank Margitha Dahl, Gunnel de Forest and Helene Blomqvist for technical assistance. The study was supported by grants from the Swedish Heart Lung Foundation, the Cancer and Allergy Foundation, the Swedish Foundation for Health Care Sciences and Allergy Research, Karolinska Institutet and Hesselman's Foundation, the Torsten and Ragnar Söderberg Foundation, the Swedish Research Council and the Stockholm County Council.
References
- 1.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Shaughnessy TC, Ansari TW, Barnes NC, et al. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–7. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 3.Saetta M, Di Stefano A, Turato G, et al. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–6. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 4.Grumelli S, Corry DB, Song LZ, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Stefano A, Caramori G, Capelli A, et al. STAT4 activation in smokers and patients with chronic obstructive pulmonary disease. Eur Respir J. 2004;24:78–85. doi: 10.1183/09031936.04.00080303. [DOI] [PubMed] [Google Scholar]
- 6.Di Stefano A, Caramori G, Ricciardolo FL, et al. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–67. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 7.Aoshiba K, Koinuma M, Yokohori N, et al. Differences in the distribution of CD4+ and CD8+ T cells in emphysematous lungs. Respiration. 2004;71:184–90. doi: 10.1159/000076682. [DOI] [PubMed] [Google Scholar]
- 8.Di Stefano A, Capelli A, Lusuardi M, et al. Decreased T lymphocyte infiltration in bronchial biopsies of subjects with severe chronic obstructive pulmonary disease. Clin Exp Allergy. 2001;31:893–902. doi: 10.1046/j.1365-2222.2001.01098.x. [DOI] [PubMed] [Google Scholar]
- 9.Cosio MG, Majo J. Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest. 2002;121:160S–165S. doi: 10.1378/chest.121.5_suppl.160s. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan AK, Simonian PL, Falta MT, et al. Oligoclonal CD4+ T cells in the lungs of patients with severe emphysema. Am J Respir Crit Care Med. 2005;172:590–6. doi: 10.1164/rccm.200410-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foronjy R, D'Armiento J. The role of collagenase in emphysema. Respir Res. 2001;2:348–52. doi: 10.1186/rr85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106:1081–93. doi: 10.1172/JCI10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlay GA, O'Driscoll LR, Russell KJ, et al. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med. 1997;156:240–7. doi: 10.1164/ajrccm.156.1.9612018. [DOI] [PubMed] [Google Scholar]
- 14.Selman M, Cisneros-Lira J, Gaxiola M, et al. Matrix metalloproteinases inhibition attenuates tobacco smoke-induced emphysema in guinea pigs. Chest. 2003;123:1633–41. doi: 10.1378/chest.123.5.1633. [DOI] [PubMed] [Google Scholar]
- 15.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA. 1979;76:1274–8. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredriksson K, Liu XD, Lundahl J, et al. Red blood cells increase secretion of matrix metalloproteinases from human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol. 2006;290:L326–33. doi: 10.1152/ajplung.00057.2005. [DOI] [PubMed] [Google Scholar]
- 17.Zagai U, Fredriksson K, Rennard SI, et al. Platelets stimulate fibroblast-mediated contraction of collagen gels. Respir Res. 2003;4:13. doi: 10.1186/1465-9921-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Ivanoff A, Klominek J. Expression and activity of matrix metalloproteases in human malignant mesothelioma cell lines. Int J Cancer. 2001;91:638–43. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1102>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Liu X, Skold CM, et al. Collaborative interactions between neutrophil elastase and metalloproteinases in extracellular matrix degradation in three-dimensional collagen gels. Respir Res. 2001;2:300–5. doi: 10.1186/rr73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukamizu H, Grinnell F. Spatial organization of extracellular matrix and fibroblast activity: effects of serum, transforming growth factor beta, and fibronectin. Exp Cell Res. 1990;190:276–82. doi: 10.1016/0014-4827(90)90197-i. [DOI] [PubMed] [Google Scholar]
- 22.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majo J, Ghezzo H, Cosio MG. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J. 2001;17:946–53. doi: 10.1183/09031936.01.17509460. [DOI] [PubMed] [Google Scholar]
- 24.Turato G, Zuin R, Miniati M, et al. Airway inflammation in severe chronic obstructive pulmonary disease: relationship with lung function and radiologic emphysema. Am J Respir Crit Care Med. 2002;166:105–10. doi: 10.1164/rccm.2111084. [DOI] [PubMed] [Google Scholar]
- 25.Retamales I, Elliott WM, Meshi B, et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am J Respir Crit Care Med. 2001;164:469–73. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- 26.Tetley TD. New perspectives on basic mechanisms in lung disease. 6. Proteinase imbalance: its role in lung disease. Thorax. 1993;48:560–5. doi: 10.1136/thx.48.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segura-Valdez L, Pardo A, Gaxiola M, et al. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest. 2000;117:684–94. doi: 10.1378/chest.117.3.684. [DOI] [PubMed] [Google Scholar]
- 28.Vernooy JH, Lindeman JH, Jacobs JA, et al. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest. 2004;126:1802–10. doi: 10.1378/chest.126.6.1802. [DOI] [PubMed] [Google Scholar]
- 29.Distler JH, Jungel A, Huber LC, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA. 2005;102:2892–7. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chizzolini C, Parel Y, De Luca C, et al. Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor alpha. Arthritis Rheum. 2003;48:2593–604. doi: 10.1002/art.11129. [DOI] [PubMed] [Google Scholar]
- 31.Chizzolini C, Rezzonico R, Ribbens C, et al. Inhibition of type I collagen production by dermal fibroblasts upon contact with activated T cells: different sensitivity to inhibition between systemic sclerosis and control fibroblasts. Arthritis Rheum. 1998;41:2039–47. doi: 10.1002/1529-0131(199811)41:11<2039::AID-ART20>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Wisithphrom K, Windsor LJ. The effects of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and transforming growth factor-beta1 on pulp fibroblast mediated collagen degradation. J Endod. 2006;32:853–61. doi: 10.1016/j.joen.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Zhu YK, Liu XD, Skold MC, et al. Cytokine inhibition of fibroblast-induced gel contraction is mediated by PGE(2) and NO acting through separate parallel pathways. Am J Respir Cell Mol Biol. 2001;25:245–53. doi: 10.1165/ajrcmb.25.2.4383. [DOI] [PubMed] [Google Scholar]
- 34.Bond M, Fabunmi RP, Baker AH, et al. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]


