Abstract
Adoptive immunotherapy with in vitro expanded antigen-specific cytotoxic T lymphocytes (CTLs) may be an effective approach to prevent, or even treat, Aspergillus (Asp) infections. Such lines can be generated using monocyte-derived dendritic cells (DC) as antigen-presenting cells (APC) but requires a relatively high volume of starting blood. Here we describe a method that generates Asp-specific CTL responses more efficiently using a protocol of antigen presented on DC followed by Epstein–Barr virus (EBV)-transformed B lymphoblastoid cell lines (BLCL) as APC. Peripheral blood mononuclear cells were stimulated weekly (2–5×) with a complete pool of pentadecapeptides (PPC) spanning the coding region of Asp f16 pulsed onto autologous mature DC. Cultures were split and stimulated subsequently with either PPC-DC or autologous PPC-pulsed BLCL (PPC-BLCL). Lines from the DC/BLCL arm demonstrated Asp f16-specific cytotoxicity earlier and to a higher degree than lines generated with PPC-DC alone. The DC/BLCL-primed lines showed a higher frequency of Asp f16-specific interferon (IFN)-γ producing cells but an identical effector cell phenotype and peptide specificity compared to PPC-DC-only-primed lines. Tumour necrosis factor (TNF)-α, but not IL-10, appeared to play a role in the effectiveness of BLCL as APC. These results demonstrate that BLCL serve as highly effective APC for the stimulation of Asp f16-specific T cell responses and that a culture approach using initial priming with PPC-DC followed by PPC-BLCL may be a more effective method to generate Asp f16-specific T cell lines and requires less starting blood than priming with PPC-DC alone.
Keywords: adoptive immunotherapy, antigen-presenting cells, Aspergillus infection, Epstein–Barr virus-transformed B lymphoblastoid cell lines
Introduction
Aspergillus fumigatus is a ubiquitous and opportunistic fungal pathogen for humans and animals. Invasive pulmonary aspergillosis (IPA), caused mainly by A. fumigatus, is characterized by hyphal invasion and destruction of pulmonary tissue and is a leading cause of morbidity and mortality in recipients of haematopoietic stem cell transplant, solid organ transplant patients [1–3] and patients with human immunodeficiency virus (HIV) infection [4,5]. The overall mortality from untreated IPA is nearly 85%. Even with treatment, mortality is still as high as 50% [6,7]. The high mortality of IPA reflects the severe state of immunosuppression of affected patients and the suboptimal in vivo efficacy of anti-fungal agents against Aspergillus (Asp) species. Therefore, development of additional therapies directed toward restoration of host-immune defence post-transplantation is needed urgently.
T cells are recognized increasingly as important mediators of protection from IPA. Studies in mice [8–11] and in humans [12,13] have shown that a T helper 1 (Th1)/Th2 dysregulation and a switch to a Th2-type immune response during an IPA infection may contribute to a poor outcome. Resistance to infection in a murine model of IPA was associated with a Th1-type response characterized by high levels of tumour necrosis factor (TNF)-α and interleukin (IL)-12 and the presence of interstitial lymphocytes producing interferon (IFN)-γ and IL-2 [11]. Resistance was increased in susceptible mice with predominant Th2 type responses upon local IL-4 or IL-10 neutralization or IL-12 administration [11]. Adoptive transfer of CD4+ splenocytes from mice sensitized to a crude culture extract of A. fumigatus into naive mice was found to prolong survival significantly after a subsequent intravenous challenge with A. fumigatus conidia [9]. In humans, a significant antigen-specific proliferation of IFN-γ-producing T cells has been found in healthy individuals and in patients surviving IPA [14]. All this evidence points to a crucial role of a Th1-type cellular immune response against A. fumigatus for the control of IPA and suggests the possibility of prevention and treatment of IPA by restoring the host immune responses through infusion of A. fumigatus-specific CTLs. A recent study has demonstrated that infusion of donor-derived Asp-specific Th1-type CD4+ clones to patients with haematological malignancies induced a protective high IFN-γ/low IL-10 production phenotype within 3 weeks of infusion, controlled Asp antigenaemia, and helped to clear invasive aspergillosis [15].
The use of dendritic cells (DC) as antigen-presenting cells (APC) has been the most widely explored approach for the establishment of T cell lines and clones specific for human tumour or viral antigens in vitro. In our own studies DC were shown to be efficient in their capacity to induce T cell immunity to A. fumigatus[16,17]. However, DC have several significant drawbacks, including: (i) their relative rarity in peripheral blood [18]; (ii) the fact that they are not homogeneous but represent several populations of functionally disparate cell types [19]; (iii) the difficulty in expanding DC ex vivo from non-stem cell sources [20]; and (iv) the expense and time required for isolating DC directly from blood or generating DC from other cells types, such as monocytes. On the contrary, Epstein–Barr virus (EBV)-transformed B lymphoblastoid cell lines (BLCL) are easy to establish, and have been shown to be effective as APC in generating adenovirus and cytomegalovirus (CMV)-specific T-cell lines [21,22]. As one A. fumigatus antigen, Asp f16, has been shown to stimulate both T and B cell responses from patients with allergic bronchopulmonary aspergillosis (ABPA) [23] and to be associated with potentially protective Th1-type T cell responses [16,17,24], we evaluated BLCL pulsed with a complete pentadecapeptide pool (PPC) spanning the 427-aa coding region of Asp f16 as APC to expand and stimulate Asp f16-specific CTLs in our protocol. We demonstrated that the sequential stimulation with PPC-DC followed by PPC-BLCL resulted in much stronger lytic activity and a higher frequency of IFN-γ-producing Asp f16-specific T cells, but with an identical peptide specificity and effector cell phenotype compared to the use of DC alone as APC.
Materials and methods
Peripheral blood mononuclear cells and human leukocyte antigen typing
Peripheral blood mononuclear cells (PBMCs) from healthy non-mobilized apheresis donors (RD0601, RD0604, RD0308 and RD0309) were collected and studied after written informed consent under research protocols approved by the Medical College of Wisconsin and Froedtert Hospital Investigational Review Boards. PBMCs were isolated by Ficoll-Hypaque (Biochrom, Berlin, Germany) density gradient centrifugation. Sequence-based human leucocyte antigen (HLA) typing was performed by the Immunogenetics Laboratory, Blood Center of South-eastern Wisconsin, Milwaukee, WI, USA.
Generation of EBV-transformed BLCL
BLCL were generated by infection of PBMCs with concentrated supernatant from the EBV-producing cell line B95-8, as described previously [25]. BLCL were maintained in RPMI-1640 complete medium (RPMI-1640 with 2 mM l-glutamine and 25 mM Hepes plus penicillin–streptomycin, 100 U/ml and 100 g/ml, respectively; Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (C-six Diagnostic, Inc., Mequon, WI, USA).
Asp f16 overlapping pentadecapeptides
A complete pool containing all 104 pentadecapeptides, each at 1 μg/ml, with 21 small pools arranged in a matrix (10 vertical and 11 horizontal) consisting of four to 11 peptides at 2 μg/ml, were prepared as reported previously [16,17]. The complete peptide pool was used to generate Asp f16-specific T cell lines, and small pools together with single peptides were used to identify the specificity of the CTLs.
Generation and pulsing of fast-DC
Monocyte-derived fast-DC were prepared from adherent blood monocytes as described previously [26,27]. Fast-DC were CD14– and expressed mature DC surface markers to the same degree as standard-DC, and maintained this phenotype after withdrawing cytokine from the cultures. Fast-DC and standard-DC were equally capable of inducing A. fumigatus and CMV-specific T cell proliferation, as well as priming antigen-specific CTL activity as reported [26]. Asp f16 peptide pulsing of DC was performed prior to maturation by pulsing with Asp f16 peptides for 6 h. Mature, pulsed fast-DC were then used to generate T cell lines.
Generation of Asp f16-specific T cell lines
Non-adherent PBMCs were co-cultured with irradiated (25 Gy), autologous, mature, Asp f16 complete peptide pool-pulsed fast-DC (PPC-DC) at a 1 : 10 ratio (stimulators : responders) in RPMI-1640 complete medium supplemented with 10% pooled human serum (PHS) (GTI Diagnostics, Waukesha, WI, USA) (10% PHS). IL-2 at 1000 IU/ml was used throughout the whole stimulation process to expand T cells [28]. IL-12 at 500 pg/ml (R&D Systems, Inc.,Minneapolis, MI, USA) was added to the culture medium only for the first round of stimulation to promote the development of Th1-type immune responses [29,30]. Cells were restimulated weekly with PPC-DC, then the cultures were split and stimulated subsequently either with PPC-DC or with irradiated (75 Gy), autologous PPC-BLCL weekly at a 1 : 4 ratio. The cultures were fed every third day with half-fresh media.
Enzyme-linked immunospot (ELISPOT) assay
ELISPOT assays were used to detect Asp f16-specific T cells that secrete IFN-γ according to the instruction manual (BD Biosciences, San Diego, CA, USA) with some modifications. Briefly, 96-well ELISPOT plates were coated with capture antibodies to IFN-γ overnight at 4°C, washed once with 10% PHS and then blocked with the same medium for 2 h. Cells were removed from the cultures, washed and resuspended at 106/ml in 10% PHS and 100 μl was added to the ELISPOT plate wells in duplicate as responder cells. Autologous mature DC were pulsed with either Asp f16 peptides (either the complete pool, small pools or single peptides) for 2 h and then used as stimulator cells in the ELISPOT assay. Peptide pulsed-DC (100 μl) were added to the wells containing responder cells. The plates were incubated for 24 h at 37°C in a humidified atmosphere with 5% CO2. T cells with phorbol myristate acetate (PMA) (5 ng/ml) plus ionomycin (500 ng/ml) (Sigma, St Louis, MO, USA) were used as a positive control. T cells plus non-pulsed DC (NP-DC) were the negative control. Background controls included NP-DC, PPC-DC and T cells. Following overnight incubation, the plates were developed according to the manufacturer's instructions. Coloured spots were counted by ImmunoSpot TM Series 1 Analyser and analysed by supporting ImmunoSpot TM software (Cellular Technology, Cleveland, OH, USA).
Chromium release assays
The cytotoxicity of each CTL line was analysed using a standard 4 h 51Chromium release assay at a range of effector : target (E : T) ratios as described previously [16,17]. Target cells included 51Chromium-labelled non-pulsed autologous BLCL (NP-BLCL) and Asp f16 peptide complete pool pulsed autologous BLCL (PPC-BLCL). Specific lysis was calculated as: %specific lysis = [experimental counts per minute (cpm) − spontaneous cpm)/(maximum cpm − spontaneous cpm].
CD4+ and CD8+ T cell population enrichment
After the seventh or eighth round of stimulation of cultures from donors RD0601 and RD0604, CD4+ and CD8+ T cell populations from each of the T cell lines were enriched by depleting either CD4+ or CD8+ T cells using Dynabeads CD8 or Dynabeads CD4 (Invitrogen, Dynal AS, Oslo, Norway), according to the manufacturer's instructions.
Flow cytometric analysis of cell surface markers on T cell lines, DC and BLCL and intracellular cytokine production within T cells
Cell surface staining was performed as described previously [16,17]. The antibodies used included: fluorescein isothiocyanate (FITC), phycoerythrin (PE) or peridinin chlorophyll (PerCP)-conjugated CD3, CD4, CD8, CD14, CD19, CD25, CD40, CD45, CD56, CD80, CD83, CD86 and anti-HLA-DR (all from BD Biosciences, San Jose, CA, USA). The cells were fixed with 2% paraformaldehyde before acquisition on a FACSCaliburTM cytometer (BD Immunocytometry Systems, San Jose, CA, USA). List mode data were analysed using FloJo version 8 (Treestar, Ashland, OR, USA). For intracellular staining, intracellular IFN-γ production of T cells was determined using FastImmune Intracellular Cytokine Detection Kits (BD Biosciences). Effectors incubated with either staphylococcal enterotoxin B superantigen (SEB; Sigma) or no antigens were considered as positive and negative controls, respectively, for the assay.
Cytokine detection
Matured DC and BLCL were pulsed with Asp f16 PPC for 2 h, then incubated at 37°C, 5% CO2 for 24 h before collecting supernatants. Th1 and Th2 cytokines were measured by Bio-Plex cytokine assay (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions.
Hyphae killing assay
Hyphae killing was determined by using a tetrazolium dye XTT, as described previously [16,17,31]. The percentage of fungal cell damage was defined by the following equation: [1 − (A450 of hyphae incubated with CTL-A450 of CTL alone)/(A450 of hyphae live)] × 100.
Antibody blocking experiments
Asp f16-primed T cells were split into four parts and restimulated subsequently with either Asp f16 PPC-DC, PPC-BLCL or PPC-BLCL in the presence of goat anti-recombinant human TNF-α neutralizing antibodies (0·1 μg/ml) (R&D Systems) or with PPC-BLCL in the presence of goat anti-recombinant human IL-10 neutralizing antibodies (1 μg/ml) (R&D Systems). The concentration of recombinant human TNF-α and recombinant human IL-10 (rhIL-10) neutralizing antibodies was determined based upon the company's data sheet, at which 0·25 ng/ml rhTNF-α and 5 ng/ml of rhIL-10 could be 100% neutralized within 24 h and 48 h, respectively. After 1 week of incubation, the lytic activity of all the T cell lines was assessed by chromium release assay.
Statistics
Statistical analysis was performed with the paired t-test using the excel program to compare between two or more groups, respectively. P-values of < 0·05, < 0·01 and < 0·001 were considered statistically significant, highly significant and very highly significant, respectively.
Results
CTL activity in cultures primed only with PPC-DC compared to switching to PPC-BLCL
We have reported previously that Asp f16 CTL responses could be induced after the third round of stimulation using Asp f16 PPC-DC [16,17]. Later we found that the PBMCs from two donors, RD0601 and RD0604, failed to develop CTL responses even after five rounds of weekly stimulation using PPC-DC as APC. To determine if BLCL used as APC might induce a T cell response, the lines were split into two parts and stimulated subsequently with either PPC-DC or with autologous PPC-BLCL. CTL activity was assessed by 51Cr-release assay and compared. As shown in Fig. 1, with continued PPC-DC priming CTL activity by the line from donor RD0601 was not detected until after the seventh priming with 3·6%-, 15·6%- and 29·1%-specific lysis after the sixth, seventh and eighth primings (E : T ratio = 50 : 1), respectively; in contrast, lytic activity of the cells switched to PPC-BLCL as APC increased to 15·2%, 32·1% and 49·0% (P < 0·001), respectively, with little or no EBV-specific killing (data not shown). Similarly, the line primed only with PPC-DC from donor RD0604 had CTL activity of 10·8%, 19·6% and 22·3% after six, seven and eight primings, respectively, with PPC-DC versus 31·6%-, 35·8%- and 40·1%-specific lysis (P < 0·0001), respectively, using PP-BLCL. These results demonstrate that BLCL can serve as APC for the presentation of Asp f16 and stimulate more potent cytolytic activity than lines primed only with DC as APC.
Fig. 1.
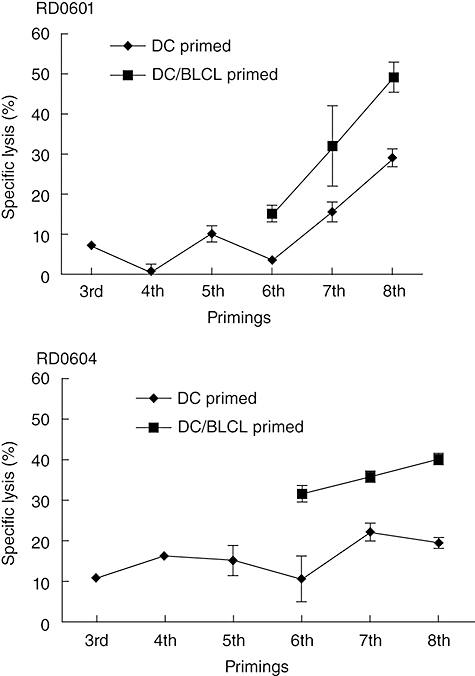
Aspergillus (Asp) f16-specific cytotoxic lymphocyte (CTL) activity in lines primed with complete peptide pool pulsed pentadecapeptides-dendritic cells (PPC-DC) compared to lines primed with PPC-DC followed by PPC-B lymphoblastoid cell lines (BLCL). Non-adherent peripheral blood mononuclear cells from donor RD0601 or RD0604 were primed with Asp PPC-DC for five rounds weekly, then split into two parts and primed subsequently with either PPC-DC (denoted as DC-primed) or with PPC-BLCL (denoted as DC/BLCL primed) weekly. CTL activity to PPC-pulsed and non-pulsed BLCL targets was tested 1 week following each round of stimulation. Shown are the mean and standard deviation of the percentage of specific lysis by triplicate wells of effector cells from the two donors against PPC pulsed autologous BLCL (effector : target ratio = 50 : 1). Lysis of non-pulsed targets was consistently below 10% (not shown).
Frequency of IFN-γ-producing T cells and peptide specificity
IFN-γ-producing cells in the lines from both donors were enumerated by ELISPOT after the sixth, seventh and eighth primings. Responses to the complete Asp f16 peptide pool were determined first. As shown in Fig. 2a, spot-forming cells (SFCs) per 105 cells in lines switched to PPC-BLCL as APC (DC/BLCL) were 1·6–10 times higher than those in the DC-only-primed lines from both donors (P < 0·05), indicating a more potent Asp f16-specific response in DC/BLCL-primed lines.
Fig. 2.
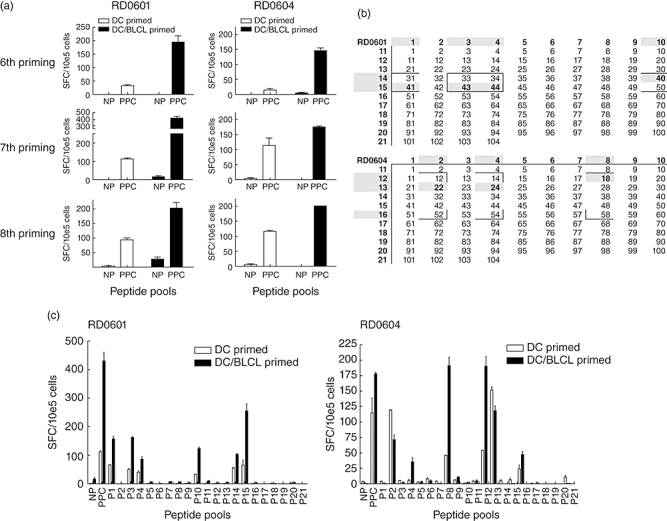
Peptide specificity and frequency of peptide reactive cells in dendritic cells (DC)-primed versus DC/B lymphoblastoid cell lines (BLCL)-primed lines. The frequency of interferon (IFN)-γ producing T cells responding to the complete pool of Aspergillus (Asp) f16 peptides [complete peptide pool pulsed pentadecapeptides (PPC)] was tested by enzyme-linked immunospot (ELISPOT) 1 week following the sixth, seventh and eighth primings for DC-primed lines and for DC followed by BLCL-primed lines (DC/BLCL) from donors RD0601 and RD0604. The results are expressed as the mean ± standard deviation (s.d.) number of spot-forming cells (SFC) per 105 cells in replicate wells tested after addition of PPC-pulsed DC or non-pulsed (NP) DC (a). DC-primed and DC/BLCL-primed lines from both donors were tested after the seventh or eighth priming by IFN-γ ELISPOT for reactivity to 21 small pools of Asp f16 peptides. Results are expressed as mean ± s.d. of SPC per 105 cells plated in replicate wells (b). Based on the results of the small pool screening, candidate single peptides (boxed areas within the matrix) as well as the immediately flanking peptides were pulsed onto autologous DC and the DC-primed and DC/BLCL-primed lines were tested after the seventh or eighth priming by IFN-γ ELISPOT. Shaded areas indicate IFN-γ production significantly above background for the small pools (outer numbers) and for the single peptides (inner numbers) recognized by the T cell lines. For technical reasons candidate peptides 52, 54 and 58 for donor RD0604 were not tested. Similar to the small pool screening shown (b), the number of SPC/105 were higher in DC/BLCL-primed lines although the pattern of SP recognition was identical (c).
Next, we went on to identify the specificity of the T cell lines after the seventh or eighth priming. ELISPOT assay using Asp small pool pulsed DC as stimulators demonstrated that small pools P1, P3, P4, P10, P14, P15 and P2, P4, P8, P12, P13 and P16 stimulated the T cell lines from donor RD0601 and RD0604, respectively (Fig. 2b). DC-only-primed lines and DC/BLCL-primed cell lines from each donor showed a similar pattern of small peptide pool recognition. In most cases, the DC/BLCL-primed line showed a higher frequency of IFN-γ-producing cells in response to the small pools (SFCs of DC/BLCL-primed in comparison with SFCs of DC-primed: RD0601, P < 0·05 or P < 0·01 for P1, P3, P4 and P10, except for P14 and P15; RD0604, P16, P < 0·05; P2, P4, P8, P12 and P13, not statistically significant). Based on small pool reactivity, the cell lines were tested by ELISPOT using DC pulsed with candidate single peptides (Fig. 2c). The results showed that both lines from donor RD0601 recognized a sequence shared by the single peptides SP40 and SP41 (HTYTIDWTKDA position 161–171) and a second sequence shared by SP43 and SP44 (TWSIDGAVVRT position 173–182). The T cell lines from donor RD0604 recognized minimally four different single pentadecapeptides, three of which were confirmed. These included SP18 (GAEFTVAKQGDAPTI position 69–83), SP22 (TDFYFFFGKAEVVMK position 85–99) and SP24 (KAEVVMKAAPGTGVV position 93–108). Due to technical reasons the candidate peptides contained in small pool P16 were not tested, but based on the frequency of IFN-γ-producing cells from the small pool screening, the most likely candidate is SP58 (RGTVHHVRQVRPYRE position 229–243). Similar to the small pool screening, there was an identical pattern of single peptide recognition, but in general a higher frequency of T cells recognizing the individual peptides in the lines switched to BLCL as APC (not shown). The HLA restriction of the CTLs was tested using partially matched single peptide-pulsed BLCL, as described previously [16,17]. For both donors, lines primed only with PPC-DC and those switched to PPC-BLCL were found to share the same HLA class II restriction (data not shown).
Phenotype analysis of the Asp f16-specific lines
The phenotype of the cell lines was determined by flow cytometry. The lines were tested after the fifth priming to the eighth priming and were shown to be predominately CD3+ T cells, with a mixture of CD4+ and CD8+ T cells (Table 1). For donor RD0604, the percentage of CD4+ T cells in PPC-DC-primed lines was increased from around 35% after the fifth priming to more than 95% after the eighth priming, while the phenotype of the cells switched to PPC-BLCL increased from around 67% CD4+ T cells after the sixth priming to 99% after the eighth priming. Whereas the CD4+ T cell content of the PPC-DC-primed lines from donor RD0601 remained constant at approximately 87%−95%, the fraction switched to PPC-BLCL decreased from 94·7% CD4+ at week 6 to 76·0% CD4+ at week 8, with a corresponding rise in CD8+ from 12·3% to 21·7%. In general, the percentage of CD8+ T cells in the DC/BLCL-primed lines was higher than that in cell lines primed exclusively with DC.
Table 1.
The percentage (%) of CD3+, CD4+ and CD8+ T cells in lines from donors RD0601 and RD0604.
| RD0601 | RD0604 | ||||||
|---|---|---|---|---|---|---|---|
| Priming | Cell lines | CD3+ | CD4+ | CD8+ | CD3+ | CD4+ | CD8+ |
| Fifth | DC primed | 88·9 | 98·5 | 14·0 | 97·6 | 34·5 | 60·0 |
| Sixth | DC primed | 99·0 | 94·3 | 10·9 | 97·7 | 75·0 | 29·1 |
| DC/BLCL primed | 99·2 | 94·7 | 12·3 | 96·7 | 67·1 | 35·0 | |
| Seventh | DC primed | 95·2 | 87·2 | 13·8 | 99·7 | 93·5 | 11·0 |
| DC/BLCL primed | 98·8 | 85·4 | 20·9 | 99·9 | 82·5 | 19·1 | |
| Eighth | DC primed | 98·0 | 90·6 | 13·3 | 99·9 | 95·2 | 6·5 |
| DC/BLCL primed | 90·0 | 76·0 | 21·7 | 96·2 | 98·8 | 5·0 | |
Immunophenotype was performed by flow cytometry following the indicated number of primings using dendritic cells (DC) alone or DC followed by B lymphoblastoid cell lines (BLCL) as antigen-presenting cells (APC) (DC/BLCL). The results are the percentage of antigen-positive cells in the cultures. Fifth, sixth, seventh and eighth denote weekly stimulation with Asp f16.
Asp f16 responding cell phenotype
In order to identify the subset of T cells responding to the Asp f16 peptides, intracellular IFN-γ production was assessed by flow cytometric analysis. Gates were first set on CD4+ or CD8+ cells and then assessed for co-expression of IFN-γ and CD69. We observed that IFN-γ was produced mainly by CD4+ T cells in lines from both donors, and consistent with the ELISPOT data a higher percentage of CD4+ cells from the DC/BLCL-primed cultures produced IFN-γ than the DC-primed cultures, although there was a small amount of IFN-γ produced by CD8+ cells (Fig. 3a). These results indicated that the CTL responses from both donors might be mediated mainly or exclusively by CD4+ T cells. To confirm this postulation, CD4+ and CD8+ T cells were enriched in each of the T cell lines by negative depletion and the lytic activity was determined. As shown in Fig. 3b, the CD8-depleted population contained most, if not all, of the CTL activity compared with the original T cell line. A significant reduction in CTL activity between the unseparated and the CD8 depleted fractions was seen only in the DC/BLCL-primed RD0601 culture (P < 0·05). In contrast, little or no lytic activity was detected in the CD4-depleted populations (P < 0·01), confirming that cytotoxic activity was mediated mainly by CD4+ T cells in both of the T cell lines.
Fig. 3.
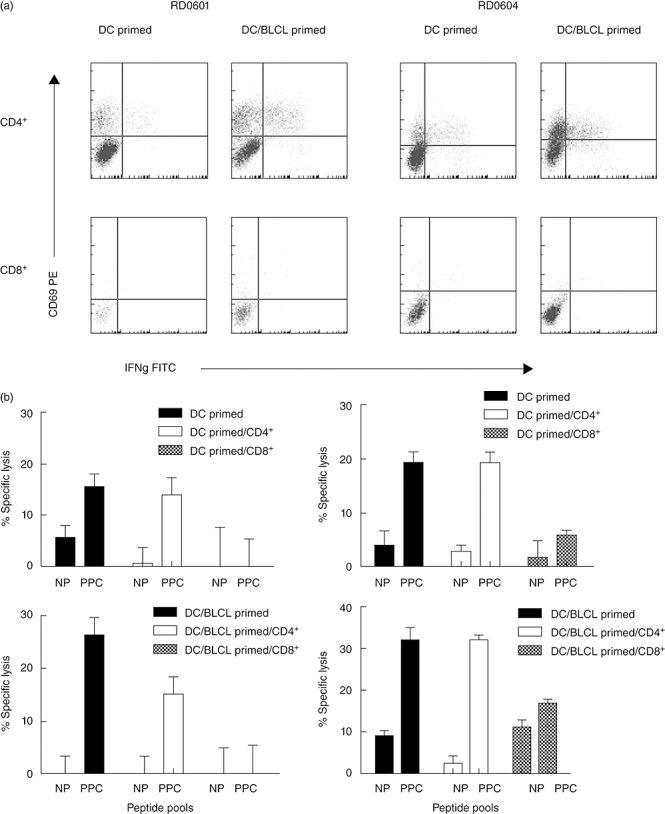
Identification of the T cell subset producing interferon (IFN)-γ and mediating cytotoxic lymphocyte (CTL) activity in response to Aspergillus f16 complete peptide pool pulsed pentadecapeptides (PPC). IFN-γ-producing cells stimulated by Aspergillus complete peptide pool were detected by intracellular staining after gating on CD4+ and CD8+ T cells from dendritic cells (DC)-primed lines and DC/B lymphoblastoid cell lines (DC/BLCL)-primed lines from donors RD0601 and RD0604. Shown are the dot plots of CD69 (y axis) versus IFN-γ (x axis) from the activated CD4+ or CD8+ T cells. Little or no IFN-γ was detected in non-stimulated lines (not shown) (a). CTL assays were performed using unseparated (solid bars), CD4+-enriched (open bars) and CD8+-enriched (hatched bars) cells from the DC-primed lines and DC/BLCL-primed lines from donors RD0601 and RD0604. CD4+ and CD8+ T cells were enriched by negative selection. Shown are the percentage of specific lysis of non-pulsed (NP) BLCL and Aspergillus complete peptide pool pulsed (PPC) BLCL targets by the separated CD4+ and CD8+ T cells (effector : target ratio = 50 : 1) (b).
Direct killing of Asp hyphae by Asp f16-specific T cell lines
To assess if the T cell lines primed with PPC-BLCL have the ability to kill Asp hyphae directly, as reported previously for DC-primed lines [16,17], Asp hyphae germinated from conidia after overnight culture were co-incubated with the Asp f16-specific CTLs. As shown in Fig. 4, for both donors DC/BLCL-primed lines as well as DC-primed lines could cause hyphae damage directly. For donor RD0601, the DC/BLCL-primed line resulted in somewhat greater hyphae killing (59·5% versus 42·3%, P < 0·05), while the reverse was true for the lines from donor RD0604, where greater killing was mediated by the DC-only-primed line (85·6% versus 57·2%, P < 0·001). These results suggest that Asp f16-specific CTLs were involved in the direct killing of Asp, but that the killing was not related directly to the degree to which peptide-pulsed targets were lysed in 51Cr release assays.
Fig. 4.
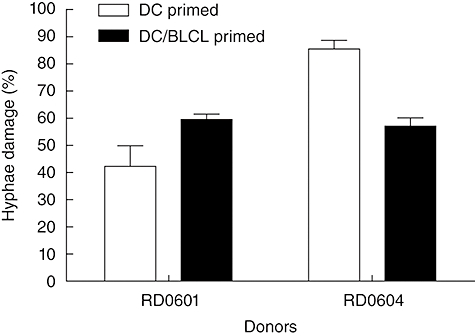
Effect of the Aspergillus f16-specific T cell lines on Aspergillus fumigatus hyphae. Cells from dendritic cells (DC)-primed or DC/B lymphoblastoid cell lines (BLCL)-primed cultures were incubated with A. fumigatus hyphae for 2 h and hyphae damage was assessed using a tetrazolium dye XTT-formazan assay. Results are the mean ± standard deviation of two experiments (each performed in triplicate) of the percentage of A. fumigatus hyphae damage calculated according to the formula in Materials and methods.
Surface antigen expression and cytokine secretion analysis of Asp PPC-DC and PPC-BLCL
We have demonstrated the use of PPC-BLCL as APC results in Asp f16-specific T cell lines with identical peptide specificity but with more potent cytolytic activity and a higher frequency of IFN-γ-producing T cells than T cell lines primed exclusively with PPC-DC. We then addressed the question of what causes this response difference. It is known that co-stimulatory molecules and cytokines constitute the second signal required for efficient activation of antigen-specific T cells. Therefore, we determined and compared the levels of co-stimulatory molecule expression and the cytokine secretion profiles of the PPC-pulsed DC and BLCL used in our studies. As expected, CD40, CD45, CD80, CD83, CD86 and HLA class II-DR were all up-regulated on Asp peptide-pulsed, mature DC from the two donors (Fig. 5a). The expression profile of surface co-stimulatory molecules on PPC-BLCL highly resembled the expression profile of DC, except that there was little or no expression of CD83 (Fig. 5a). Supernatants from the overnight culture were used to test the cytokine secretion profile of the DC and BLCL. Of note, BLCL produced higher levels of IL-10 and TNF-α than DC (Fig. 5b). The low expression levels of CD83 and high levels of IL-10 and TNF-α may contribute to the stronger T cell responses induced after switching to BLCL priming.
Fig. 5.
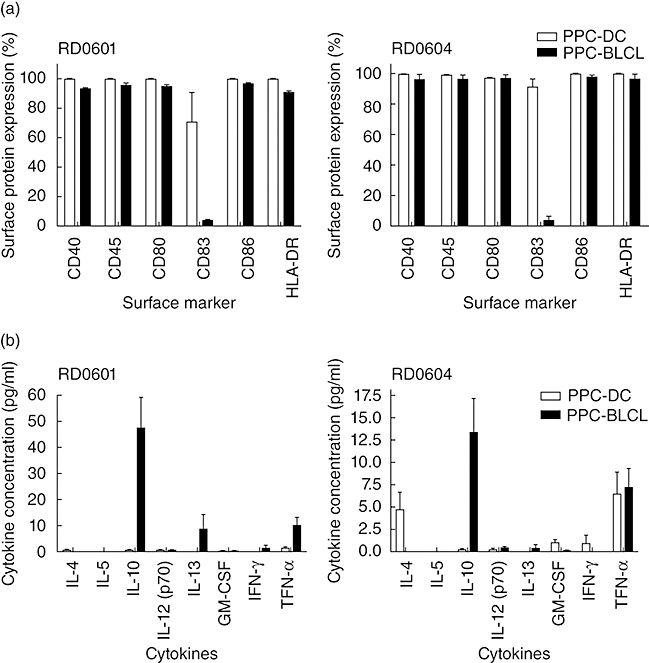
Surface antigen expression on Aspergillus (Asp) f16 complete peptide pool pulsed pentadecapeptides-pulsed mature dendritic cells (PPC-DC) and PPC-B lymphoblastoid cell lines (BLCL) and cytokine secretions of the pulsed DC and BLCL. Mature DC and Epstein–Barr virus (EBV)-transformed BLCL from donor RD0601 and RD0604 were pulsed with Aspergillus fumigatus complete peptide pool for 2 h, and then incubated at 37°C overnight. The next day, cells were collected by centrifugation, stained with conjugated antibodies and analysed by flow cytometry. Supernatants were saved for cytokine detection later by Bio-Plex cytokine assay (Bio-Rad). Mature DC (open bars) or BLCL (solid bars) were pulsed with Asp f16 A. fumigatus complete peptide pool overnight then stained for cell surface antigens. The results are shown as the percentage of cells expressing the indicated antigen. Data represents mean ± standard deviation (s.d.) of two experiments (a). Supernatants from the PPC-pulsed DC (open bars) or BLCL (solid bars) were tested for cytokine production by Bio-Plex cytokine assay. Cytokine concentrations (pg/ml) are mean ± s.d. of three independent tests (each performes in duplicate) (b).
Role of TNF-α and IL-10 in induction of Asp f16 responses
TNF-α is a Th1-type cytokine that activates immune response, whereas IL-10 is an inhibitory cytokine for T cells. We therefore hypothesized that the addition of neutralizing antibody to IL-10 might increase the induction of T cell responses by PPC-BLCL while neutralizing TNF-α would weaken T cell responses. To address this issue, fifth-round primed T cells from donor RD0601 were restimulated with PPC-DC, PPC-BLCL, PPC-BLCL in the presence of TNF-α neutralizing antibodies, or PPC-BLCL in the presence of IL-10 neutralizing antibodies. After 1 week of incubation, the lytic activity of the T cell lines was assessed. As expected, using PPC-BLCL as APC stimulated stronger cytotoxic activity than PPC-DC (Fig. 6). The ability of the PPC-BLCL APC to induce CTL activity in the DC/BLCL-primed line was abrogated in the presence of neutralizing TNF-α antibodies, whereas there was no effect on CTL activity of neutralizing IL-10 antibodies (Fig. 6). These data suggest that the increased TNF-α produced by PPC-BLCL was involved in their ability to serve as more potent APC, and that IL-10 did not interfere with this effect, at least in the present study.
Fig. 6.
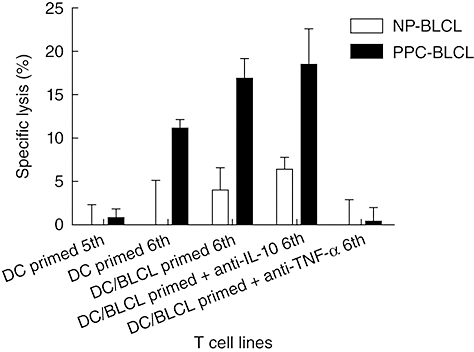
Analysis of the effects of interleukin (IL)-10 and tumour necrosis factor (TNF)-α on the induction of cytotoxic lymphocyte (CTL) responses. T cells from donor RD0601 after fifth-round priming were restimulated with Aspergillus f16 complete peptide pool pulsed pentadecapeptides-dendritic cells (PPC-DC), PPC-B lymphoblastoid cell lines (BLCL), PPC-BLCL in the presence of TNF-α neutralizing antibodies and PPC-BLCL in the presence of IL-10 neutralizing antibodies separately. After 1 week of incubation, the cytolytic activity of all the T cell lines was assessed by chromium release assay. NP-BLCL: non-pulsed BLCL, PPC-BLCL: Asp f16 peptide complete pool pulsed BLCL. Shown are the percentages of specific lysis of BLCL by the lines (effector : target ratio = 50 : 1).
Effect of earlier priming with PPC-BLCL
PBMC from additional donors were tested to determine the effect of switching to BLCL APC earlier in the course of priming and the effect on lines from donors who responded to fewer rounds of stimulation using PPC-DC. Two experiments using cells from donors that have been well characterized and reported previously [16,17] are shown in Table 2. Asp f16-specific T cell lines from donor RD0308 were raised using PPC-DC alone, PPC-BLCL alone or after two primings with PPC-DC, before switching to PPC-BLCL as APC. Asp-specific CTL activity was detected after the second priming with PPC-DC that increased with subsequent primings. Lines initiated with PPC-BLCL showed strong EBV-specific CTL activity as soon as 1 week after priming, and although slightly greater lysis towards PPC-BLCL targets was seen by week 2, strong lytic activity to the non-pulsed BLCL persisted. However, the line that was pulsed twice with PPC-DC followed by PPC-BLCL as APC showed a similar degree of Asp f16-specific lysis as the line primed with PPC-DC alone with little or no lysis of non-pulsed BLCL targets (Table 2). PBMCs from donor RD0309 required five rounds of priming with PPC-DC alone to exhibit Asp f16-specific CTL activity, but required only four rounds of stimulation when switched to PPC-BLCL after the second priming with PPC-DC. EBV-specific CTL activity was present, but did not dominate the cultures when preceded with minimally two rounds of stimulation with PPC-DC (Table 2).
Table 2.
Effect of antigen-presenting cells (APC) source on Aspergillus f16-specific cytotoxic lymphocyte (CTL) activity.
| RD0308 | RD0309 | |||||||
|---|---|---|---|---|---|---|---|---|
| Week | Target | PPC-DC | PPC-BLCL | 2DC/BLCL | PPC-DC | 2DC/BLCL | 3DC/BLCL | 4DC/BLCL |
| 1 | NP-BLCL | NT | 40·8 ± 3·9 | NT | ||||
| PPC-BLCL | NT | 40·6 ± 0·8 | NT | |||||
| 2 | NP-BLCL | 4·1 ± 0·8 | 26·9 ± 2·3 | 13·4 ± 1·3 | ||||
| PPC-BLCL | 14·2 ± 2·8 | 35·3 ± 3·9 | 15·2 ± 6·0 | |||||
| 3 | NP-BLCL | 10·6 ± 1·5 | 34·9 ± 1·1 | 7·6 ± 3·9 | 4·5 ± 1·6 | 10·2 ± 5·8 | ||
| PPC-BLCL | 34·7 ± 2·8 | 42·9 ± 1·0 | 42·0 ± 1·9 | 8·2 ± 4·5 | 11·8 ± 1·8 | |||
| 4 | NP-BLCL | 9·6 ± 1·2 | 37·5 ± 1·8 | 4·6 ± 2·6 | 1·6 ± 0·5 | 25·0 ± 1·3 | 10·5 ± 2·7 | |
| PPC-BLCL | 46·6 ± 1·7 | 46·7 ± 2·0 | 37·2 ± 1·4 | 10·5 ± 0·5 | 46·6 ± 2·9 | 19·7 ± 0·9 | ||
| 5 | NP-BLCL | 6·0 ± 0·4 | NT | NT | 4·8 ± 3·9 | 20·3 ± 6·7 | 23·8 ± 5·3 | 10·7 ± 1·5 |
| PPC-BLCL | 47·8 ± 0·7 | NT | NT | 46·8 ± 6·4 | 60·8 ± 2·8 | 50·6 ± 1·5 | 43·9 ± 4·3 | |
Peripheral blood mononuclear cells from donors RD0308 and RD0309 were primed weekly with Asp f16 complete peptide pool pulsed pentadecapeptides-pulsed dendritic cells (PPC-DC), B lymphoblastoid cell lines (BLCL) or with PPC-BLCL after the indicated number of primings with PPC-DC. CTL activity was tested 1 week after the indicated priming and is shown as the %specific lysis ± standard deviation of triplicate wells at an effector : target ratio of 50 : 1. Targets were non-pulsed (NP)-BLCL or Asp f16 PPC-BLCL. NT, not tested.
A PPC-DC-primed line from donor RD0308 has been reported previously as recognizing a single class II HLA-DRB1*0301-restricted peptide (WSIDGAVVR, position 174–182) [17]. The PPC-DC-primed line in this study and the line switched to PPC-BLCL after the second priming had identical peptide specificity and a similar percentage of CD4+ T cells (> 94%) when tested after the fourth priming. Similarly, both DC-primed and DC/BLCL-primed lines from donor RD0309 recognized the same single HLA class I-restricted peptide (LPLCSAQTW position 14–22), as was identified previously in an earlier study [16]. Both the DC-primed and the DC/BLCL-primed lines from RD0309 were a mixture of CD4+ and CD8+ cells after the fifth priming; however, the DC/BLCL-primed line showed a higher percentage of CD8+ T cells (85% CD3+CD8+versus 50% in the DC-only-primed line), possibly reflecting concurrent expansion of a population of EBV-specific T cells in the line expanded with BLCL.
Lines generated from two other donors primed two to three times with PPC-DC have also shown Asp f16-specific CTL activity with low to moderate EBV-specific killing within 1 or 2 weeks after being switched to PPC-BLCL as APC (not shown). However, EBV-specific CTL activity did dominate even after three rounds of PPC-DC priming in one donor with a particularly high frequency of EBV-specific T cells, indicating that, for some donors, the number of stimulations with PPC-DC may need to be increased before switching to BLCL as APC (not shown). These data indicate that, for most donors, switching to PPC-BLCL as APC after two to three primings with PPC-DC results in T cell lines recognizing predominately Asp f16, with low to moderate EBV-specific reactivity.
Discussion
The development of reproducibly effective methods for generating antigen-specific CTLs is necessary for the clinical application of adoptive immunotherapy. For this purpose, monocyte-derived DC have been studied extensively as APC in expanding antigen-specific CTL precursors [16,17,28,32–34]. Although DC are proved to be the most potent APC of the immune system, their use to expand CTL lines to clinically feasible numbers is limited by the volume of blood required for the DC preparations, especially for weak antigens that may require repetitive priming to generate responses. Thus, alternative sources of autologous APC, such as EBV-transformed BLCL, have been considered and studied [35].
BLCL are morphologically, phenotypically and functionally reminiscent of DC after activation [36], and can be established readily from a small amount of blood. They have good antigen processing and presenting functions and have been used effectively in generating T cell lines with specificity for EBV [37]. Most adults have encountered EBV and harbour the virus in a latent form that reactivates periodically, resulting in repeated stimulation of the immune system. Reactivation of EBV is controlled by EBV-specific CTLs, which constitute on average 1·3% (range 0·1–3·8%) of circulating CD8+ T cells [38]. The combined DC/BLCL stimulation protocol was proposed first by Lucas et al. [21], and studied further by Sili et al. [35] in an attempt to generate CMV pp65-specific CTL on a large scale. Although these experiments did not compare directly lines primed with DC alone, they demonstrated success in generating lines with predominately CD8+ and CD4+ CMV pp65 specificity after two rounds of stimulation with DC prior to use of pp65 transduced-BLCL as APC [21,22,35]. However, similar to EBV, CMV-specific T cells constitute a relatively high percentage of circulating T cells in seropositive individuals [39]. Therefore, a potential concern with using BLCL as APC for the generation of Asp f16-specific responses was that under the same conditions, the competition between the less numerous Asp f16-specific and EBV-specific T cell clones would result in the domination of one specificity and the loss of the other.
To overcome the CTL expansion limitations imposed by the lack of sufficient DC and to utilize the characteristics of DC to prime naive and memory T cell responses in donors who responded poorly to PPC-DC-only priming, we explored the use of a combined DC/BLCL stimulation protocol. PBMCs were stimulated initially with PPC-DC followed by two to three rounds of stimulation with PPC-BLCL, and the lytic activity and specificity of the resulted lines was compared. We found that the combined DC/BLCL stimulation protocol was highly effective in generating Asp f16-specific CTL lines in a shorter period of time, with more potent CTL activity and a higher frequency of IFN-γ-producing T cells, but with the same specificity as lines primed with DC only. We have demonstrated that, as predicted, if lines are initiated using PPC-BLCL as APC, EBV-specific activity dominates and obscures any Asp-specific component. However, for most donors two to three primings with DC prior to use of BLCL as APC resulted in lines that predominately recognize Asp f16, with low to moderate reactivity to EBV antigens (Table 2). A greater number of primings with DC, as was performed on cells from donors RD0601 and RD0604, resulted in even less EBV-specific CTL activity after switching to BLCL stimulators. For our purposes, lines with both EBV and Asp-specific T cells are desirable, because the risk factors for Asp infection also make patients more susceptible to EBV reactivation. However, the optimal time during line expansion to utilize BLCL as APC may vary depending upon the starting frequency of EBV-specific precursors for a given donor, as evidenced by a line from one donor (of seven tested) in which EBV specificity predominated even after three stimulations with PPC-DC (not shown). In this situation, as well as for donors who respond poorly to Asp f16 presented on DC, five or more primings with DC may be more effective in enriching Asp f16-specific precursors that can be expanded further with antigen-pulsed BLCL.
Compared to lines generated with DC alone, the combined DC/BLCL protocol generated Asp f16-specific CTL responses that were detectable approximately 1 week sooner (three of four donors) and with higher cytotoxicity activity. For two of the four lines studied, Asp f16-specific CTL activity was mediated exclusively by CD4+ T cells, one line contained both CD4+ and CD8+ effectors and one line contained only CD8+ effectors, demonstrating that BLCL are effective in expanding Asp f16-specific CTL of both subsets. Antigen-specific CD4+ CTLs have been described in tumour, bacterial and viral infections [40–46]. As reported previously [40,41,43,46], CD4+ CTLs, like CD8+ CTLs, can use a perforin–granzyme mechanism to lyse a variety of targets, suggesting that CD4+ CTLs also play an important role in protection against bacterial and viral infection and tumour progression. Although the mechanisms through which Asp f16-specific CTLs protect against fungal infection are still not understood fully, the hyphae killing activity demonstrated in the present study, and in our previous studies that included CD4+ and CD8+ effectors [16,17], suggests that both subsets are involved in the protection against fungal infection and shows that this activity is maintained in lines switched to BLCL as APC.
Consistent with the more potent CTL activity, the combined DC/BLCL protocol induced a higher frequency of IFN-γ-producing T cells than priming with DC alone. IFN-γ is the signature cytokine of the Th1 subset of CD4+ T cells and is produced by natural killer (NK) cells, CD4+ T cells and CD8+ T cells. IFN-γ has many important functions [47]. First, IFN-γ activates macrophages to kill phagocytosed microbes; secondly, IFN-γ stimulates expression of class I and class II MHC molecules and co-stimulatory molecules on APC; thirdly, IFN-γ promotes the differentiation of naive CD4+ T cells to the Th1 subset and inhibits the proliferation of Th2 cells, and fourthly, IFN-γ activates neutrophils and stimulates the cytolytic activity of NK cells. By promoting Th1 cell differentiation and prohibiting Th2 cell proliferation, IFN-γ can polarize the T cell development and expansion towards a Th1 T cell response. As Th1-type cellular immune responses play a crucial role in protecting against Asp infection [8–14], the higher frequency of Asp f16-specific Th1-type cells in lines generated with the combined DC/BLCL protocol may improve the immunotherapeutic potential of this approach to the prevention or treatment of Asp infection, especially in immunocompromised patients.
Co-stimulatory molecules and cytokines have been well known to be the second signals required for the efficient activation of antigen-specific T cells. Thus, we determined and compared the expression levels of co-stimulatory molecules and cytokine secretion profiles of DC and BLCL. All the antigens assessed were expressed at high levels on DC from donors used in these experiments, which confirms their identity as mature DC. BLCL demonstrated similar levels of expression of the co-stimulatory molecules as DC except for CD83, which showed little if any expression on BLCL. CD83 has been known for a decade to be the best marker for mature DC, but its functions remain largely unknown. Previous data demonstrated that soluble CD83 played immunosuppressive roles both in vivo and in vitro, such as the inhibition of maturation of DC and DC-mediated T cell stimulation [48]. On the other hand, evidence also showed that CD83 is required for CD4+ T cell generation in CD83-deficient mice [49], and that CD83 could augment the CD80-dependent proliferation of antigen-specific CD8+ T cells [50]. Thus, while CD83 may play a role in the activation of T cell responses, it does not appear to be required and further studies are needed to elucidate its precise function.
Cytokine gene expression and cytokine production by BLCL have been carried out previously. One group of investigators found that each of 16 BLCL tested expressed high levels of TNF-α, TNF-β and transforming growth factor (TGF)-β mRNA, while IL-10 transcripts were detected in most BLCL but at a lower level [51]. Another group reported that 89% of the BLCL tested produced measurable amounts of IL-10, ranging from 13 pg/ml to 3·7 ng/ml, while 32% of the BLCL produced TNF-α[52]. Interleukin-10, TNF-α, as well as IL-6 produced by most BLCL, may serve in autocrine loops that contribute to the maintenance of the lymphoblastoid phenotype [51,52]. In agreement with previously reported data, the cytokine secretion profile comparison of BLCL and DC showed that BLCL produced much higher levels of IL-10 and somewhat higher levels of TNF-α compared with DC. Blocking experiments showed that neutralizing anti-Hu-IL-10 did not affect significantly the cytolytic activity of the T cells primed with BLCL as APC. The failure of blocking IL-10 to increase CTL activity might be due to a level of IL-10 produced by BLCL in the present study, which might be insufficient to exert inhibitory effects on T cell activation. Alternatively, IL-10 may only play a role in the BLCL autocrine loops. In contrast, the addition of anti-Hu-TNF-α abrogated the ability of BLCL APC to induce Asp f16-specific lytic activity of the T cells, suggesting that TNF-α is involved in the process of T cell activation.
In conclusion, our study demonstrates the feasibility of an approach by which Asp f16-specific CD4+ and CD8+ T cell lines are generated by sequential primings with antigen-pulsed DC followed by priming and expansion using antigen-pulsed BLCL. Asp-specific reactivity in such lines is detected earlier, cytotoxic potential is higher and the lines contain a higher frequency of antigen-specific IFN-γ-producing T cells than lines generated using DC alone. This protocol will be of practical value not only for adoptive immunotherapy of Asp infection, but also for the expansion of T cells to other known viral or tumour antigens for which the generation of DC can be a limiting factor.
Acknowledgments
This work was supported in part by awards from the Medical College of Wisconsin Cancer Center, the Boynton Fund of the Greater Milwaukee Foundation, the Rebecca Slye Fund and the North-west Mutual Liberty Foundation. The authors have no potential financial conflict of interest.
References
- 1.Wiederhold NP, Lewis RE, Kontoyiannis DP. Invasive aspergillosis in patients with hematologic malignancies. Pharmacotherapy. 2003;23:1592–610. doi: 10.1592/phco.23.15.1592.31965. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Patterson T, Denning D. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect Dis Clin North Am. 2002;16:875–94. doi: 10.1016/s0891-5520(02)00035-1. vi. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 4.Addrizzo-Harris DJ, Harkin TJ, McGuinness G, Naidich DP, Rom WN. Pulmonary aspergilloma and AIDS. A comparison of HIV-infected and HIV-negative individuals. Chest. 1997;111:612–18. doi: 10.1378/chest.111.3.612. [DOI] [PubMed] [Google Scholar]
- 5.Denning DW, Follansbee SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:654–62. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 6.Denning DW. Diagnosis and management of invasive aspergillosis. Curr Clin Top Infect Dis. 1996;16:277–99. [PubMed] [Google Scholar]
- 7.Mahfouz T, Anaissie E. Prevention of fungal infections in the immunocompromised host. Curr Opin Invest Drugs. 2003;4:974–90. [PubMed] [Google Scholar]
- 8.Cenci E, Mencacci A, Casagrande A, Mosci P, Bistoni F, Romani L. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J Infect Dis. 2001;184:610–17. doi: 10.1086/322793. [DOI] [PubMed] [Google Scholar]
- 9.Cenci E, Mencacci A, Bacci A, Bistoni F, Kurup VP, Romani L. T cell vaccination in mice with invasive pulmonary aspergillosis. J Immunol. 2000;165:381–8. doi: 10.4049/jimmunol.165.1.381. [DOI] [PubMed] [Google Scholar]
- 10.Cenci E, Mencacci A, Del Sero G, et al. Interleukin-4 causes susceptibility to invasive pulmonary aspergillosis through suppression of protective type I responses. J Infect Dis. 1999;180:1957–68. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 11.Cenci E, Mencacci A, Fe d'Ostiani C, et al. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J Infect Dis. 1998;178:1750–60. doi: 10.1086/314493. [DOI] [PubMed] [Google Scholar]
- 12.Wolach B, Eliakim A, Gottesman G, Yellin A. Pulmonary aspergillosis in a child with hyperimmunoglobulin E syndrome. Clin Infect Dis. 1998;26:204–5. doi: 10.1086/516254. [DOI] [PubMed] [Google Scholar]
- 13.Roilides E, Dimitriadou A, Kadiltsoglou I, et al. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–9. [PubMed] [Google Scholar]
- 14.Hebart H, Bollinger C, Fisch P, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100:4521–8. doi: 10.1182/blood-2002-01-0265. [DOI] [PubMed] [Google Scholar]
- 15.Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106:4397–406. doi: 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of cytotoxic T cell responses directed to human leucocyte antigen Class I restricted epitopes from the Aspergillus f16 allergen. Clin Exp Immunol. 2005;140:81–91. doi: 10.1111/j.1365-2249.2005.02738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of Th1 T cell responses directed to a HLA Class II restricted epitope from the Aspergillus f16 allergen. Clin Exp Immunol. 2005;139:257–67. doi: 10.1111/j.1365-2249.2005.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Witmer-Pack M, Inaba K. Dendritic cells: antigen presentation, accessory function and clinical relevance. Adv Exp Med Biol. 1993;329:1–9. doi: 10.1007/978-1-4615-2930-9_1. [DOI] [PubMed] [Google Scholar]
- 19.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 20.Ardeshna KM, Pizzey AR, Thomas NS, Orr S, Linch DC, Devereux S. Monocyte-derived dendritic cells do not proliferate and are not susceptible to retroviral transduction. Br J Haematol. 2000;108:817–24. doi: 10.1046/j.1365-2141.2000.01956.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun Q, Pollok KE, Burton RL, et al. Simultaneous ex vivo expansion of cytomegalovirus and Epstein–Barr virus-specific cytotoxic T lymphocytes using B-lymphoblastoid cell lines expressing cytomegalovirus pp65. Blood. 1999;94:3242–50. [PubMed] [Google Scholar]
- 22.Sun Q, Burton RL, Dai LJ, Britt WJ, Lucas KG. B lymphoblastoid cell lines as efficient APC to elicit CD8+ T cell responses against a cytomegalovirus antigen. J Immunol. 2000;165:4105–11. doi: 10.4049/jimmunol.165.7.4105. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee B, Kurup VP, Greenberger PA, Johnson BD, Fink JN. Cloning and expression of Aspergillus fumigatus allergen Asp f16 mediating both humoral and cell-mediated immunity in allergic bronchopulmonary aspergillosis (ABPA) Clin Exp Allergy. 2001;31:761–70. doi: 10.1046/j.1365-2222.2001.01076.x. [DOI] [PubMed] [Google Scholar]
- 24.Bozza S, Gaziano R, Lipford GB, et al. Vaccination of mice against invasive aspergillosis with recombinant Aspergillus proteins and CpG oligodeoxynucleotides as adjuvants. Microbes Infect. 2002;4:1281–90. doi: 10.1016/s1286-4579(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 25.Keever-Taylor CA, Behn B, Konings S, Orentas R, Davies B, Margolis D. Suppression of EBV release from irradiated B lymphoblastoid cell-lines: superior activity of ganciclovir compared with acyclovir. Cytotherapy. 2003;5:323–35. doi: 10.1080/14653240310002243. [DOI] [PubMed] [Google Scholar]
- 26.Ramadan G, Konings S, Kurup VP, Keever-Taylor CA. Generation of Aspergillus- and CMV-specific T-cell responses using autologous fast DC. Cytotherapy. 2004;6:223–34. doi: 10.1080/14653240410006040. [DOI] [PubMed] [Google Scholar]
- 27.Dauer M, Obermaier B, Herten J, et al. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–76. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 28.Montes M, Rufer N, Appay V, et al. Optimum in vitro expansion of human antigen-specific CD8 T cells for adoptive transfer therapy. Clin Exp Immunol. 2005;142:292–302. doi: 10.1111/j.1365-2249.2005.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emtage PC, Clarke D, Gonzalo-Daganzo R, Junghans RP. Generating potent Th1/Tc1 T cell adoptive immunotherapy doses using human IL-12. Harnessing the immunomodulatory potential of IL-12 without the in vivo-associated toxicity. J Immunother. 2003;26:97–106. doi: 10.1097/00002371-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Xu S, Koski GK, Faries M, et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171:2251–61. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 31.Meshulam T, Levitz SM, Christin L, Diamond RD. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanil ide (XTT) J Infect Dis. 1995;172:1153–6. doi: 10.1093/infdis/172.4.1153. [DOI] [PubMed] [Google Scholar]
- 32.Regn S, Raffegerst S, Chen X, Schendel D, Kolb HJ, Roskrow M. Ex vivo generation of cytotoxic T lymphocytes specific for one or two distinct viruses for the prophylaxis of patients receiving an allogeneic bone marrow transplant. Bone Marrow Transplant. 2001;27:53–64. doi: 10.1038/sj.bmt.1702752. [DOI] [PubMed] [Google Scholar]
- 33.Foster AE, Bradstock KF, Sili U, Marangolo M, Rooney CM, Gottlieb DJ. A comparison of gene transfer and antigen-loaded dendritic cells for the generation of CD4+ and CD8+ cytomegalovirus-specific T cells in HLA-A2+ and HLA-A2- donors. Biol Blood Marrow Transplant. 2004;10:761–71. doi: 10.1016/j.bbmt.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Van den Bosch GA, Ponsaerts P, Nijs G, et al. Ex vivo induction of viral antigen-specific CD8 T cell responses using mRNA-electroporated CD40-activated B cells. Clin Exp Immunol. 2005;139:458–67. doi: 10.1111/j.1365-2249.2005.02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sili U, Huls MH, Davis AR, et al. Large-scale expansion of dendritic cell-primed polyclonal human cytotoxic T-lymphocyte lines using lymphoblastoid cell lines for adoptive immunotherapy. J Immunother. 2003;26:241–56. doi: 10.1097/00002371-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Schultze JL, Grabbe S, von Bergwelt-Baildon MS. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004;25:659–64. doi: 10.1016/j.it.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Rooney CM, Aguilar LK, Huls MH, Brenner MK, Heslop HE. Adoptive immunotherapy of EBV-associated malignancies with EBV-specific cytotoxic T-cell lines. Curr Top Microbiol Immunol. 2001;258:221–9. doi: 10.1007/978-3-642-56515-1_14. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Lemas VM, Flinn IW, Krone C, Ambinder RF. Application of the ELISPOT assay to the characterization of CD8(+) responses to Epstein–Barr virus antigens. Blood. 2000;95:241–8. [PubMed] [Google Scholar]
- 39.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewinsohn DM, Bement TT, Xu J, et al. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–9. [PubMed] [Google Scholar]
- 41.Rivoltini L, Radrizzani M, Accornero P, et al. Human melanoma-reactive CD4+ and CD8+ CTL clones resist Fas ligand-induced apoptosis and use Fas/Fas ligand-independent mechanisms for tumor killing. J Immunol. 1998;161:1220–30. [PubMed] [Google Scholar]
- 42.Kayagaki N, Yamaguchi N, Nakayama M, et al. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–47. [PubMed] [Google Scholar]
- 43.Yasukawa M, Ohminami H, Yakushijin Y, et al. Fas-independent cytotoxicity mediated by human CD4+ CTL directed against herpes simplex virus-infected cells. J Immunol. 1999;162:6100–6. [PubMed] [Google Scholar]
- 44.Gagnon SJ, Ennis FA, Rothman AL. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J Virol. 1999;73:3623–9. doi: 10.1128/jvi.73.5.3623-3629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munz C, Bickham KL, Subklewe M, et al. Human CD4(+) T lymphocytes consistently respond to the latent Epstein–Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–60. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khanolkar A, Yagita H, Cannon MJ. Preferential utilization of the perforin/granzyme pathway for lysis of Epstein–Barr virus-transformed lymphoblastoid cells by virus-specific CD4+ T cells. Virology. 2001;287:79–88. doi: 10.1006/viro.2001.1020. [DOI] [PubMed] [Google Scholar]
- 47.Abbas AKL, Pober JS. Cellular and molecular immunology. 4. Philadelphia: W. B. Saunders Company; 2000. [Google Scholar]
- 48.Fujimoto Y, Tedder TF. CD83: a regulatory molecule of the immune system with great potential for therapeutic application. J Med Dent Sci. 2006;53:85–91. [PubMed] [Google Scholar]
- 49.Fujimoto Y, Tu L, Miller AS, et al. CD83 expression influences CD4+ T cell development in the thymus. Cell. 2002;108:755–67. doi: 10.1016/s0092-8674(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 50.Hirano N, Butler MO, Xia Z, et al. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107:1528–36. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rochford R, Cannon MJ, Sabbe RE, et al. Common and idiosyncratic patterns of cytokine gene expression by Epstein–Barr virus transformed human B cell lines. Viral Immunol. 1997;10:183–95. doi: 10.1089/vim.1997.10.183. [DOI] [PubMed] [Google Scholar]
- 52.Wroblewski JM, Copple A, Batson LP, Landers CD, Yannelli JR. Cell surface phenotyping and cytokine production of Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines (LCLs) J Immunol Methods. 2002;264:19–28. doi: 10.1016/s0022-1759(01)00565-8. [DOI] [PubMed] [Google Scholar]


