Abstract
Human intestinal lamina propria T lymphocytes (LPT), when investigated ex vivo, exhibit functional properties profoundly different from those of peripheral blood T lymphocytes (PBT). One prominent feature represents their enhanced sensitivity to CD2 stimulation when compared to PBT. Given that LPT are hyporesponsive to T cell receptor (TCR)/CD3 stimulation, an alternative activation mode, as mimicked by CD2 triggering in vitro, may be functional in mucosal inflammation in vivo. This study provides insight into signalling events associated with the high CD2 responsiveness of LPT. When compared to PBT, LPT show an increased activation of the phosphoinositide 3/protein kinase B/glycogen synthase kinase 3β (PI3-kinase/AKT/GSK-3β) pathway in response to CD2 stimulation. Evidence is provided that up-regulation of this pathway contributes to the enhanced CD2-induced cytokine production in LPT. Given the importance of TCR-independent stimulation for the initiation of intestinal immune responses analysis of signalling pathways induced by ‘co-stimulatory’ receptors may provide valuable information for therapeutic drug design.
Keywords: cytokine production, lamina propria T lymphocytes, PI3-kinase pathway
Introduction
The intestinal mucosa represents the body's largest interface with its environment − the latter containing a multitude of repeatedly occurring foreign antigens. Under normal conditions lamina propria lymphocytes are reduced in their ability to mount an adaptive immune response: T lymphocytes when isolated from the normal human intestinal lamina propria are poor responders towards antigen receptor triggering, a finding that correlates well with in situ analysis where very few T cells proliferate [1–3]. Furthermore, mucosal myeloid cells express only low levels of co-stimulatory molecules (e.g. CD58, CD80/86) and CD14 [4–6]. Under inflammatory conditions, however, monocytes expressing these co-stimulatory molecules are present in the lamina propria and enable lamina propria T lymphocytes (LPT) to mount immune responses towards luminal antigens [7]. Importantly, LPT from normal tissue respond vigorously to CD2 (receptor for CD58) triggering in vitro compared to autologous peripheral blood T lymphocytes (PBT) [1, 8–10]. CD2 stimulation represents an alternative ‘non-specific’ mode of T cell activation [11, 12], suggesting that T lymphocytes − in addition to antigen-specific immune responses − can also subserve an ‘innate’ immune function, e.g. when homing to mucosal surfaces.
While recent data provide conclusive evidence regarding the mechanisms of how adaptive T cell receptor-driven responses are prevented in the healthy lamina propria [13], so far the molecular basis for the enhanced CD2 responsiveness of LPT has not been elucidated in detail. In this study we analysed the CD2-induced activation of the phosphoinositide 3-kinase/protein kinase B/glycogen synthase kinase 3β (PI3/AKT/GSK-3β) pathway in isolated CD4+ lPT and PBT, and its potential contribution to the high cytokine production in LPT was investigated.
Materials and methods
Reagents
CD2 monoclonal antibodies M1 and M2 were produced in our own laboratory. Mouse monoclonal antibody (mAb) 3PT was kindly provided by Drs S. F. Schlossman and E. L. Reinherz (Dana-Farber Cancer Institute, Boston, USA). Ly294002, Calyculin A as well as phospho-GSK-3β (Ser9), phospho-AKT (Ser473 and Thr308), and AKT-specific antibodies were purchased from Cell Signalling Technology (Danvers, MA, USA). GSK-3β mAb was obtained from BD Bioscience (Heidelberg, Germany).
Tissues/samples
All human studies were approved by the ethics committee of the University of Heidelberg and were performed in accordance with the principles laid down in the Declaration of Helsinki. Informed consent was obtained from the patients. Gut specimens were derived from individuals undergoing resection for localized colon cancer or benign colonic diseases. Colonic mucosa, being microscopically normal, was dissected from the surgical specimen near the resection margin and processed immediately for isolation of lamina propria cells.
Preparation of T lymphocytes
Lamina propria mononuclear cells were isolated according to a modified method of Bull and Bookman [14]. Briefly, the mucosal layer was dissected from the fresh tissue and washed extensively in RPMI-1640 (Invitrogen, Karlsruhe, Germany) and antibiotics. Mucus as well as part of the epithelial cells were removed by carefully scraping the tissue surface with a scalpel. Subsequently, the mucosa was cut into 2–4 mm pieces and incubated in a shaking waterbath at 37°C with 0·7 mM ethylenediamine tetraacetic acid (EDTA) (Sigma, Munich, Germany) in Hanks' balanced salt solution (HBSS) without Ca2+ and Mg2+ for 45 min to remove epithelial cells. This incubation was repeated twice. After extensive washing the tissue was digested in a shaking waterbath at 37°C for 10–12 h by 45 U/ml collagenase (Sigma) and 27 U/ml deoxyribonuclease I (Sigma) in RPMI-1640 containing 2% fetal calf serum (FCS) (Sigma), 2% l-glutamine (Invitrogen) and antibiotics. The resulting cell suspension was separated from undigested tissue by filtration through a 70 μm nylon mesh (Becton Dickinson, Heidelberg, Germany). For further isolation of lamina propria T lymphocytes, the cell suspension was subjected to Percoll (GE Healthcare, Munich, Germany) density gradient centrifugation. CD4+ lPT were purified using anti-CD4+ magnetic beads (Invitrogen). Briefly, lamina propria lymphocytes were incubated with anti-CD4 magnetic beads and separated from unlabelled cells using the MPC-L magnet (Invitrogen). Subsequently, beads and antibody were released from the cells by a polyclonal anti-Fab antibody specific for the CD4 antibody on the Dynabeads (Invitrogen) [purity > 98% as determined by fluorescence activated cell sorter (FACS) analysis]. Peripheral blood was taken during the operation. Peripheral blood CD4+ T cells were obtained by Ficoll-Hypaque (GE Healthcare) density gradient centrifugation and magnetic beads separation as described above. For preparation of CD45RO+ CD4+ PBT lymphocytes were subject to negative magnetic cell separation using CD45RA MicroBeads (Miltenyi) prior to purification of CD4+ T lymphocytes (see above).
Stimulation of T lymphocytes
CD4+ PBT and CD4+ lPT were cultured in the presence of soluble CD2 mAb M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml) for the indicated time-periods in the absence of TCR/CD3 stimulation. No cytokines were added.
Gene expression analysis
PBT (5 × 105) (or CD4+ PBT) and LPT (or CD4+ LPT) were collected in 300 μl lysis buffer from the MagnaPure mRNA Isolation Kit I (Roche Diagnostics, Mannheim, Germany) and mRNA was isolated with the MagnaPure-LC device using the mRNA-I standard protocol. The elution volume was set to 50 μl. An aliquot of 8·2 μl RNA was reverse transcribed using avian myeloblastosis virus reverse transcriptase (AMV-RT) and oligo-(dT) as primer (first-strand cDNA synthesis kit, Roche Diagnostics) according to the manufacturer's protocol in a thermocycler. After termination of the cDNA synthesis, the reaction mix was diluted to a final volume of 500 μl and stored at −20°C until polymerase chain reaction (PCR) analysis. Primer sets optimized for the LightCycler (RAS, Mannheim, Germany) were developed and provided by SEARCH-LC GmbH (Heidelberg, Germany). The PCR was performed with the LightCycler FastStart DNA Syber Green I kit (RAS) according to the protocol provided in the parameter-specific kits. To control for specificity of the amplification products, a melting curve analysis was performed. No amplification of unspecific products was observed. The number of transcripts was calculated from a standard curve, obtained by plotting known input concentrations of four different plasmids at log dilutions to the PCR-cycle number (CP) at which the detected fluorescence intensity reaches a fixed value. This approach reduced variations dramatically due to handling errors over several logarithmic dilution steps. To correct for differences in the content of mRNA, the calculated transcript numbers were normalized according to the expression of the housekeeping gene cyclophilin B. Values were thus given as transcripts per 1000 transcripts of CPB.
Measurement of interleukin (IL)-2 production
Cells (2 × 105/well) were cultured in round-bottomed microtitre plates (Nunc, Wiesbaden, Germany) in RPMI-1640 supplemented with 10% FCS, 2% l-glutamine and antibiotics as well as reagents, in a total volume of 200 μl/well. After 60 h, the supernatants were harvested, cleared by centrifugation and frozen at −20°C until assayed. The IL-2 content of the supernatants was determined by a cytokine-specific enzyme-linked immunosorbent assay (ELISA) (BD Bioscience, Heidelberg, Germany). The tests were performed according to the manufacturer's instructions.
Preparation of cell extracts and Western blot analysis
Whole cell extracts of CD4+ PB-T and CD4+ LP-T were prepared as described previously [15]. Briefly, cells were resuspended in 40 mM Tris (pH 8·0), 60 mM sodium-PPi, 10 mM EDTA, 100 nM Calyculin A and lysed by addition of an equal volume of 10% sodium dodecyl sulphate (SDS) and boiling for 20 min. Cell extracts were resolved by 12% SDS-polyacrylamide gel electrophoresis (PAGE), transferred onto a polyvinylidene difluoride (PVDF) membrane (Pall, Dreieich, Germany) and stained with the appropriate antibody. Following incubation with horseradish peroxidase-conjugated goat anti-mouse (dilution : 10 000; Dianova, Hamburg, Germany) or goat anti-rabbit (dilution 1 : 2000; Cell Signalling Technology, Danvers, MA, USA) immunoglobulin (IgG) the signal was visualized by enhanced chemiluminescence (GE Healthcare, Munich, Germany). Densitometric analysis was performed using a scanner (GS-800; Biorad, Munich, Germany) and Quantity One Software (Biorad). The phosphorylation index was determined as follows: phospho-AKT or phospho-GSK-3β levels were normalized based on total AKT and GSK-3β levels, respectively. Phospho-AKT and phospho-GSK-3β levels after 1 h of CD2 stimulation were set to 1. Phosphorylation levels at all other conditions were calculated as a fraction/multiple of 1.
Statistical analysis
Where indicated, data are presented as the mean ± standard deviation/standard error of the mean or as box plots. The probability of differences was assessed using Student's paired t-test. P-values < 0·05 were considered as significant.
Results
CD2-induced phosphorylation of AKT and GSK-3β is enhanced in LPT compared to PBT
The PI3-kinase pathway is involved in cytokine gene transcription [e.g. IL-2, interferon (IFN)-γ] in response to co-stimulatory signals such as those delivered through the CD28 receptor [16, 17]. Here, we have analysed the phosphorylation/activation state of two major downstream mediators of PI3-kinase, namely the serine/threonine kinases AKT and GSK-3β [18, 19], in both resting and CD2-activated CD4+ PBT as well as CD4+ LPT, the latter isolated from healthy human mucosal tissue. While AKT activity is induced following phosphorylation at Ser473 and Thr308 [20–22], the constitutively active GSK-3β can be inactivated functionally by AKT-dependent phosphorylation at Ser9 [23, 24]. As shown in Fig. 1a, resting LPT show a higher degree of phosphorylation of GSK-3β at Ser9 than PBT (average increase 1·6 ± 0·4-fold; n = 4). Treatment of LPT with the PI3-kinase inhibitor LY294002 [25] at a maximal inhibitory concentration of 10 μM reduces significantly the constitutively enhanced GSK-3β phosporylation, suggesting that the latter results at least in part from basal in vivo activity of PI3-kinase.
Fig. 1.
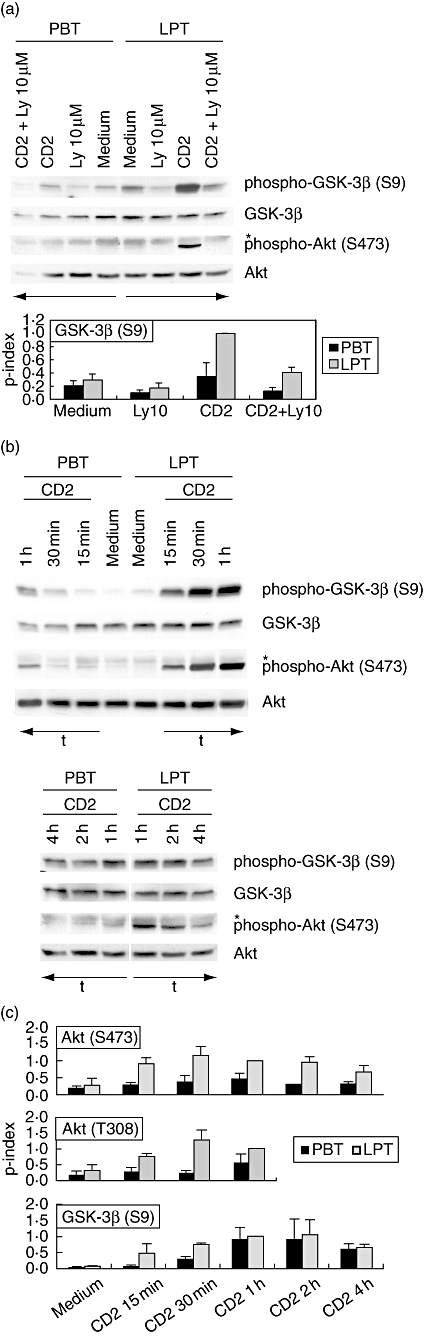
Enhanced CD2-induced phosphorylation of protein kinase B (AKT) and glycogen synthase kinase 3β (GSK-3β) in lamina propria T lymphocytes (LPT). (a) CD4+ peripheral blood T lymphocytes (PBT) and LPT were cultured in the presence or absence of CD2 monoclonal antibody (mAb) M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml) for 15 min. Ly294002 was added 30 min prior to stimulation. The phosphorylation state of GSK-3β (Ser9) and AKT (Ser473) was determined by immunoblotting of whole cell extracts. Results are representative of at least three independent experiments. *Non-specific band. The phosphorylation index of GSK-3β (Ser9) (lower panel) was determined as described in Materials and methods. Data are expressed as mean ± standard deviation (s.d.) (n = 3). (b) CD4+ PBT and LPT were cultured in the presence or absence of CD2 mAb M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml) for the indicated time-periods. The phosphorylation state of GSK-3β (Ser9) and AKT (Ser473) was determined by immunoblotting of whole cell extracts. Results are representative of at least three independent experiments. *Non-specific band. (c) Densitometric determination of the phosphorylation index of AKT (Ser473 and Thr308; upper panels) and GSK-3β (Ser9; lower panel) in CD4+ PBT and LPT in response to CD2 activation. Data are expressed as mean ± s.d. (n = 2–8).
In response to CD2 stimulation, both AKT (Ser473) and GSK-3β (Ser9) phosphorylation increase strongly in LPT (Fig. 1a,b): 15 min after initiation of CD2 activation AKT and GSK-3β phosphorylation are clearly detectable in LPT [AKT (Ser473)]: average increase of 8·3 ± 2·2 above basal levels; n = 3; GSK-3β (Ser9): average increase of 3·8 ± 1·5 above basal levels; n = 5) but only marginally increased in PBT [AKT (Ser473)]: average increase of 2 ± 0·4 above basal levels; n = 3; GSK-3β (Ser9): average increase of 1·5 ± 0·3 above basal levels; n = 5). Treatment with Ly294002 (10 μM) completely inhibits CD2-induced AKT phosphorylation at Ser473 in both LPT and PBT (time-point 15 min; Fig. 1a) and 1 h (data not shown). This confirms that in both cell populations AKT activity is controlled predominantly by the PI3-kinase pathway. Unlike AKT phosphorylation, Ly294002 treatment (10 μM) inhibits CD2-induced GSK-3β phosphorylation only partially at 15 min of stimulation (74 ± 11% inhibition; n = 3; Fig. 1a) and 1 h (data not shown). Kinetic analysis reveals that at all time-points tested during a 4-h stimulation period, phosphorylation levels of AKT at Ser473 remain significantly higher in LPT when compared to PBT (Fig. 1b,c). Peak activation levels during this time-period exist 30 min to 1 h after CD2 engagement in both cell populations. Similarly, AKT phosphorylation at Thr308 (although only weakly detectable under the experimental conditions employed) is enhanced in LPT when compared to PBT at 15 min to 1 h of CD2 stimulation (2 h and 4 h not tested). CD2-induced GSK-3β phosphorylation in LPT also exceeds that in PBT after a stimulation period of 15 and 30 min (Fig. 1b,c). However, at later time-points, similar GSK-3β phosphorylation levels are observed in the two cell populations.
Up-regulation of PI3-kinase pathway activation contributes to high CD2-induced cytokine/CD40L gene expression in LPT
Activation of freshly isolated CD4+ LPT from healthy colonic mucosa by a mitogenic combination of CD2 mAbs induces significantly higher IL-2 gene expression (Fig. 2a, left panel) and secretion (Fig. 2a, right panel) than by autologous CD4+ PBT. Similar to IL-2, CD2-induced expression of the genes encoding granulocye–macrophage colony-stimulating factor (GM-CSF), tumour necrosis factor (TNF)-α, IFN-γ and CD40L is also enhanced (Fig. 2b; shown is the ratio of mRNA expression of CD4+ LPT/CD4+ PBT after CD2 stimulation). The enhanced expression of all genes analysed in LPT and PBT, respectively, is not due to different kinetics in the two cell populations. Figure 2c shows data for IL-2 and CD40L responses that are representative for all other cytokines tested. While activation of the PI3-kinase pathway through CD2 stimulation [26] has been studied with regard to up-regulation of integrin activation [27, 28] and cytoskeletal reorganization [29], its involvement in CD2-mediated cytokine production has not been demonstrated as yet. As shown in Fig. 3a, Ly294002 treatment inhibits significantly CD2-induced IL-2, IFN-γ and GM-CSF gene expression in both PBT and LPT in a dose-dependent manner, indicating that the PI3-kinase pathway contributes to optimal CD2-induced IL-2/IFN-γ/GM-CSF gene transcription. Similar results were obtained for TNF-α and CD40L (data not shown). Inhibition of CD2-induced PI3-kinase activity by Ly294002 (0·5–1 μM) in LPT to levels induced in PBT (comparable AKT phosphorylation; Fig. 3b) results in a significant decrease in IL-2 (19 ± 12%−40 ± 9%; n = 3–6), IFN-γ (39 ± 4%−44 ± 14%; n = 3–6) and GM-CSF (21 ± 9%−45 ± 18%; n = 3–6) gene expression (Fig. 3a) as well as TNF-α (16 ± 7%−26 ± 9%; n = 3–6) and CD40L (17 ± 5–27 ± 11%; n = 3–6) gene expression (data not shown) in LPT. This indicates that the up-regulation of the PI3-kinase pathway contributes to the enhanced CD2-induced cytokine and CD40L gene expression in LPT when compared to PBT.
Fig. 2.
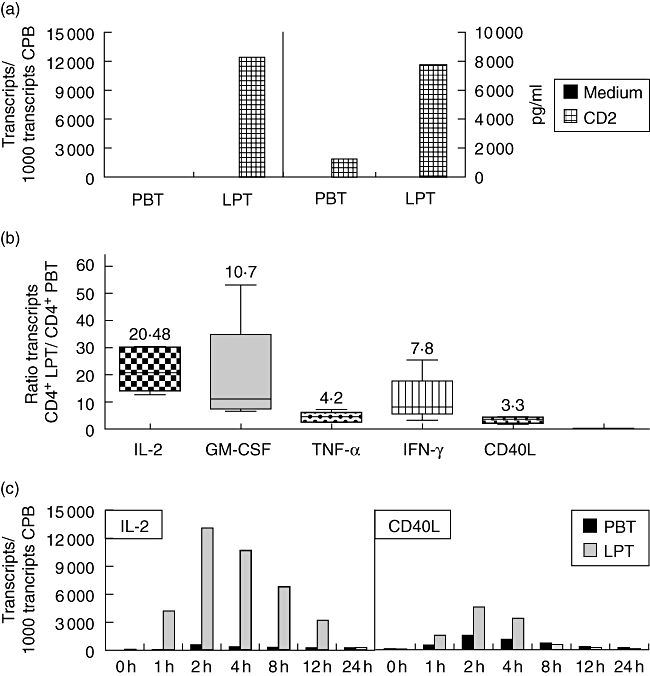
Enhanced cytokine/CD40L production by CD2-activated lamina propria T lymphocytes (LPT). Isolated peripheral blood T lymphocytes (PBT) and LPT were activated by CD2 monoclonal antibody (mAb) M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml). (a) After 4 h, interleukin (IL)-2 mRNA expression was determined by quantitative reverse transcribed polymerase chain reaction. Culture supernatant was harvested after 24 h, and the IL-2 content was determined by enzyme-linked immunosorbent assay (detection limit 16 pg/ml). Results are representative of at least three independent experiments. (b) Ratio cytokine (and CD40L) mRNA expression of CD4+ LPT/CD4+ PBT after CD2 stimulation for 4 h (box plot diagrams showing median, 25th and 75th percentile, minimum and maximum; n = 5). (c) Time–course of CD2-induced IL-2 and CD40L mRNA expression in PBT and LPT. Results are representative of three independent experiments.
Fig. 3.
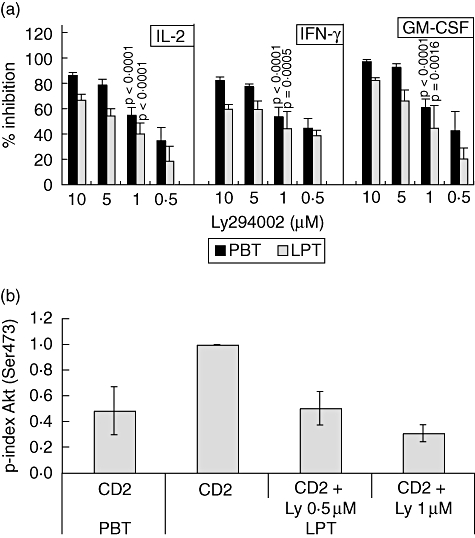
Inhibition of cytokine/CD40L gene expression by Ly294002. (a) CD4+ peripheral blood T lymphocytes (PBT) and CD4+ LPT were cultured in the presence or absence of CD2 monoclonal antibody (mAb) M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml) for 4 h. Ly294002 was added 30 min prior to stimulation. Interleukin (IL)-2, interferon (IFN)-γ and granulocyte–macrophage colony-stimulating factor (GM-CSF) mRNA expression was determined by quantitative reverse transcribed polymerase chain reaction. Relative inhibition (in percentage) of cytokine/CD40L gene expression by Ly294002 is shown. Data are expressed as mean ± standard deviation (n = 3–6). The effectiveness of cytokine/CD40L inhibition was tested statistically for 1 μM Ly294002 (n = 6). (b) CD2-induced AKT phosphorylation in CD4+ PBT and lamina propria T lymphocytes (LPT) in the presence or absence of Ly294002 was quantified by densitometry (n = 3–4).
Up-regulation of the PI3-kinase pathway in CD2-activated LPT is unrelated to their CD45RO+ phenotype
Differential CD2-induced PI3-kinase pathway activation and cytokine production by PBT and LPT could, perhaps, result from the differing composition of T cell subsets in the two compartments: whereas the T cell population in the intestinal lamina propria consists almost exclusively of CD45RO+ T cells (∼90%) [30, 31], the blood population contains approximately equal numbers of CD45RA+ and CD45RO+ T cells [32, 33]. To address this possibility, we compared phosphorylation levels of AKT and GSK-3β as well as cytokine production in CD2-activated CD45RO+ CD4+ PBT with total CD4+ PBT. As shown in Fig. 4a, both AKT (Ser473) and GSK-3β (Ser9) phosphorylation are similar in both cell populations following CD2 stimulation. Note that basal GSK-3β phosphorylation is increased in CD45RO+ PBT when compared to CD4+ PBT (average increase 1·5 ± 0·6-fold; n = 3) to a similar extent as in LPT when compared to PBT (average increase 1·6 ± 0·4-fold; see above). CD2-triggered IL-2 secretion is not enhanced in CD45RO+ CD4+ PBT in comparison to total CD4+ PBT (Fig. 4b). A 1·7–2-fold increased induction of cytokine/CD40L gene expression can be observed in CD45RO+ CD4+ PBT (Table 1); however, this increase is lower than that observed in LPT when compared to PBT, in particular with regard to IL-2, GM-CSF and IFN-γ.
Fig. 4.
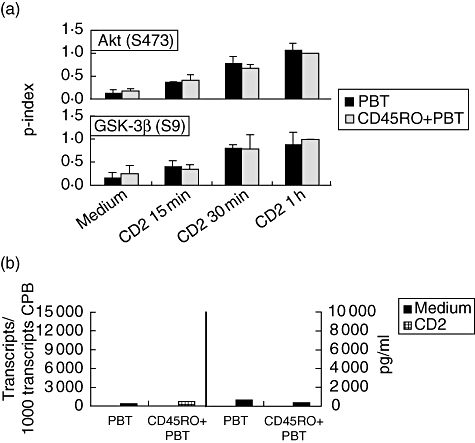
Protein kinase B glycogen synthase kinase 3β (AKT/GSK-3β) phosphorylation and cytokine/CD40L gene expression in CD2-activated CD45RO+ CD4+ peripheral blood T lymphocytes (PBT) and total CD4+ PBT. (a) CD4+ PBT and CD45RO+ PBT were cultured in the presence or absence of CD2 monoclonal antibody (mAb) M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml) for the indicated time-periods. The phosphorylation state of GSK-3β (Ser9) and AKT (Ser473) was determined by immunoblotting of whole cell extracts. The phosphorylation index of AKT (Ser473 and Thr308; upper panels) and GSK-3β (Ser9; lower panel) was determined as described in Materials and methods. Data are expressed as mean ± standard deviation (n = 3). (b) Isolated CD4+ PBT and CD45RO+ PBT were activated by CD2 mAb M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml). After 4 h, IL-2 mRNA expression was determined by quantitative reverse transcribed polymerase chain reaction. Culture supernatant was harvested after 24 h and the IL-2 content was determined by enzyme-linked immunosorbent assay (detection limit 16 pg/ml). Results are representative of three independent experiments.
Table 1.
Ratio normalized gene transcripts CD2-activated RO+ peripheral blood T lymphocytes (PBT)/PBT and lamina propria T lymphocytes (LPT/PBT).
| RO+CD4+PBT/CD4+PBT* | CD4+LPT/CD4+PBT* | |
|---|---|---|
| IL-2 | 1·7 ± 0·3 | 21·6 ± 3·6 |
| GM-CSF | 2·0 ± 0·6 | 18·8 ± 8·7 |
| TNF-α | 1·7 ± 0·3 | 4·1 ± 0·9 |
| IFN-γ | 1·8 ± 0·3 | 10·7 ± 3·8 |
| CD40L | 1·9 ± 0·3 | 3·0 ± 0·5 |
Cells were activated by CD2 monoclonal antibpdy M1 (1 μg/ml), M2 (1 μg/ml) and 3PT (0·33 μg/ml). After 4 h, cytokine/CD40L mRNA expression was determined by quantitative reverse transcribed polymerase chain reaction. Normalization was performed as described in Materials and methods. Ratios are expressed as mean ± standard error of the mean (n = 3–5). GM-CSF: granulocyte–macrophage colony-stimulating factor; IL: interleukin; IFN: interferon; TNF: tumour necrosis factor.
Discussion
The body's outer surfaces are exposed permanently to foreign antigens yet contain vast numbers of immunocompetent cells. This is particularly true for the intestinal mucosa, where large numbers of T lymphocytes and myeloid cells of the lamina propria are in close proximity to an enormous load of microbial and nutritional antigens. Adaptive immune responses in this microenvironment would lead to immunization and clonal expansion of antigen reactive T cells with the result of chronic inflammation. Importantly, adaptive T cell responses are controlled tightly in the human lamina propria by a number of mechanisms, including production of inhibitory cytokines such as IL-10 [34] and TGF-β by epithelial cells [35], cysteine metabolism [13] and low expression of co-stimulatory molecules on myeloid cells [4]. Accordingly, the differentiation state of LPT is clearly distinct from that of PBT. One prominent feature of LPT is their inability to proliferate in response to T cell receptor/CD3 directed stimuli in vitro [1, 36]. However, T cells of the intestinal lamina propria do not represent an anergic T cell population, e.g. due to chronic antigen exposure: unlike T lymphocytes that have been ‘tolerized’ through antigen receptor engagement [11], LPT respond vigorously when stimulated through the CD2 receptor [1, 8–10], or in the context of autologous monocytes from circulating blood, or in the presence of 2-ME [4, 13]. It is therefore tempting to speculate that an alternative mode of activation that can be mimicked by CD2-mediated stimulation enables T lymphocytes to fulfil an ‘innate’ immune function when homing to the intestinal mucosa.
As yet, few studies have addressed the molecular mechanisms underlying this enhanced CD2 responsiveness. In this regard, increased thioredoxin production has been shown to contribute to the high CD2-inducible cytokine production in LPT [37]. Furthermore, differential usage of promoter elements regulating IFN-γ expression in PBT and LPT has been demonstrated [38]. In the present report we provide evidence for an enhanced up-regulation of the PI3-kinase pathway in CD4+ LPT when compared to CD4+ PBT following CD2 stimulation. When LPT from healthy colonic mucosa and autologous PBT, respectively, are activated through CD2, in vitro phosphorylation of the serine/threonine kinase AKT at Ser473 is enhanced significantly in LPT as opposed to PBT. Furthermore, inhibitory Ser9 phosphorylation of GSK-3β, a direct target of AKT, is also increased in LPT when compared to PBT, at least during the initial period of CD2 stimulation. The PI3-kinase inhibitor Ly294002 completely blocks CD2-induced AKT phosphorylation and markedly inhibits GSK-3β phosphorylation in LPT, indicating that these phosphorylation events are controlled solely (AKT) or significantly (GSK-3β) by the PI3-kinase pathway. The enhancement of Ly294002-sensitive AKT/GSK-3β phosphorylation in CD2-stimulated LPT therefore reflects an up-regulation of the PI3-kinase pathway in this cell population in comparison to PBT.
Further investigations will address the signalling events causing the enhanced CD2-induced AKT phosphorylation in LPT. In particular, it will be examined whether the latter is due to inactivation of the lipid phosphatase PTEN by thioredoxin [39], a thiol disulphide oxidoreductase highly expressed in LPT [37]. It also remains to be analysed as to whether the residual Ser9 phosphorylation of GSK-3β in the presence of saturating concentrations of LY294002 is related to the kinetics of reversible phosphorylation in the PI3-kinase pathway and/or, alternatively, up-regulation of other signalling pathways in LPT such as those regulating protein kinase C, protein kinase A, p90rsk kinase and/or extracellular-regulated kinase (ERK)activity. These kinases have all been shown to phosphorylate GSK-3β directly at Ser9 − or at least prime it for subsequent phosphorylation [24, 40]. Differential regulation of the above-mentioned signalling events in PBT versus LPT may also account for the discrepancy between similar GSK-3β phosphorylation but lower AKT phosphorylation in PBT in comparison to LPT at 1–4 h of CD2 stimulation.
The role of the PI3-kinase pathway for CD2-induced expression of the IL-2, TNF-α and CD40L gene expression is evident from the fact that these responses are reduced significantly in the presence of LY294002 in both cell populations. That up-regulation of PI3-kinase pathway activation in LPT indeed contributes to the high cytokine/CD40L expression in response to CD2 stimulation is demonstrated by the fact that inhibition of this pathway to levels induced in PBT clearly reduces cytokine/CD40L gene expression in LPT. Downstream mechanisms by which PI3-kinase activation contributes to up-regulation of gene expression may involve AKT-dependent nuclear factor kappa B (NF-κB) activation [41, 42] or nuclear retention of nuclear factor of activated T cells (NFAT) due to GSK-3β inhibition [43].
Apart from regulating cytokine gene expression, the PI3-kinase/AKT pathway is involved in the control of cellular metabolism and promotes cell survival and cell cycle progression [44–48]. Up-regulation of this pathway in LPT may lead, therefore, to enhanced cell cycling. Interestingly, a recent report demonstrated that the cell-doubling time following CD2 activation of LPT is markedly shorter than that of PBT. This was associated with increased Rb phosphorylation [49]. Note that Rb phosphorylation is influenced negatively by inhibition of PI3-kinase in T lymphocytes [44].
The reasons for the altered signalling state in activated LPT remain unknown. It cannot be attributed to the high proportion of CD45RO+ T lymphocytes within the LPT population, as the CD2-induced PI3-kinase pathway activation is not up-regulated in CD45RO+ T lymphocytes compared to total T lymphocytes. Moreover, CD2 expression levels are similar in both PBT and LPT. We have also carefully excluded that any of the functional and molecular features that we have described to be characteristic for LPT are related to the preparation procedure.
Importantly, protective effects of PI3-kinase inhibitors have been demonstrated recently in two experimental animal models of chronic inflammation [50, 51]. It may therefore be interesting to investigate whether targeting the PI3-kinase pathway would be a useful therapeutic strategy to control inflammation in the human intestine in the case of inflammatory bowel disease.
Acknowledgments
We are grateful to Dr Johannes Winter and his team (Department of Surgery, St Vincentius Hospital, Speyer) for providing colon specimens. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 405, project B6/A4).
References
- 1.Pirzer UC, Schurmann G, Post S, Betzler M, Meuer SC. Differential responsiveness to CD3-Ti versus CD2-dependent activation of human intestinal T lymphocytes. Eur J Immunol. 1990;20:2339–42. doi: 10.1002/eji.1830201025. [DOI] [PubMed] [Google Scholar]
- 2.Qiao L, Schurmann G, Betzler M, Meuer SC. Functional properties of human lamina propria T lymphocytes assessed with mitogenic monoclonal antibodies. Immunol Res. 1991;10:218–25. doi: 10.1007/BF02919696. [DOI] [PubMed] [Google Scholar]
- 3.Autschbach F, Schurmann G, Qiao L, Merz H, Wallich R, Meuer SC. Cytokine messenger RNA expression and proliferation status of intestinal mononuclear cells in noninflamed gut and Crohn's disease. Virchows Arch. 1995;426:51–60. doi: 10.1007/BF00194698. [DOI] [PubMed] [Google Scholar]
- 4.Qiao L, Braunstein J, Golling M, et al. Differential regulation of human T cell responsiveness by mucosal versus blood monocytes. Eur J Immunol. 1996;26:922–7. doi: 10.1002/eji.1830260430. [DOI] [PubMed] [Google Scholar]
- 5.Rogler G, Hausmann M, Vogl D, et al. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205–15. doi: 10.1046/j.1365-2249.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith PD, Smythies LE, Mosteller-Barnum M, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–6. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 7.Rogler G, Hausmann M, Spottl T, et al. T-cell co-stimulatory molecules are upregulated on intestinal macrophages from inflammatory bowel disease mucosa. Eur J Gastroenterol Hepatol. 1999;11:1105–11. doi: 10.1097/00042737-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Targan SR, Deem RL, Liu M, Wang S, Nel A. Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J Immunol. 1995;154:664–75. [PubMed] [Google Scholar]
- 9.Boirivant M, Fuss I, Fiocchi C, Klein JS, Strong SA, Strober W. Hypoproliferative human lamina propria T cells retain the capacity to secrete lymphokines when stimulated via CD2/CD28 pathways. Proc Assoc Am Physicians. 1996;108:55–67. [PubMed] [Google Scholar]
- 10.Braunstein J, Qiao L, Autschbach F, Schurmann G, Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997;41:215–20. doi: 10.1136/gut.41.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meuer SC, Hussey RE, Fabbi M, et al. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984;36:897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- 12.Hunig T, Tiefenthaler G, Meyer zum Buschenfelde KH, Meuer SC. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987;326:298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- 13.Sido B, Braunstein J, Breitkreutz R, Herfarth C, Meuer SC. Thiol-mediated redox regulation of intestinal lamina propria T lymphocytes. J Exp Med. 2000;192:907–12. doi: 10.1084/jem.192.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bull DM, Bookman MA. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977;59:966–74. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loh C, Carew JA, Kim J, Hogan PG, Rao A. T-cell receptor stimulation elicits an early phase of activation and a later phase of deactivation of the transcription factor NFAT1. Mol Cell Biol. 1996;16:3945–54. doi: 10.1128/mcb.16.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Lockhart M, Marin E, Graf B, et al. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J Immunol. 2004;173:7120–4. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 18.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 19.Wymann MP, Marone R. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol. 2005;17:141–9. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 21.Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003;546:108–12. doi: 10.1016/s0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 22.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–7. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 24.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 26.Ward SG, Ley SC, MacPhee C, Cantrell DA. Regulation of D-3 phosphoinositides during T cell activation via the T cell antigen receptor/CD3 complex and CD2 antigens. Eur J Immunol. 1992;22:45–9. doi: 10.1002/eji.1830220108. [DOI] [PubMed] [Google Scholar]
- 27.van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811–13. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 28.Kivens WJ, Hunt SW, Mobley JL, et al. Identification of a proline-rich sequence in the CD2 cytoplasmic domain critical for regulation of integrin-mediated adhesion and activation of phosphoinositide 3-kinase. Mol Cell Biol. 1998;18:5291–307. doi: 10.1128/mcb.18.9.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KH, Meuer SC, Samstag Y. Cofilin: a missing link between T cell co-stimulation and rearrangement of the actin cytoskeleton. Eur J Immunol. 2000;30:892–9. doi: 10.1002/1521-4141(200003)30:3<892::AID-IMMU892>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.James SP, Fiocchi C, Graeff AS, Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology. 1986;91:1483–9. [PubMed] [Google Scholar]
- 31.Schieferdecker HL, Ullrich R, Weiss-Breckwoldt AN, et al. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990;144:2541–9. [PubMed] [Google Scholar]
- 32.Smith SH, Brown MH, Rowe D, Callard RE, Beverley PC. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986;58:63–70. [PMC free article] [PubMed] [Google Scholar]
- 33.Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–8. [PubMed] [Google Scholar]
- 34.Autschbach F, Braunstein J, Helmke B, et al. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121–30. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao L, Schurmann G, Betzler M, Meuer SC. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology. 1991;101:1529–36. doi: 10.1016/0016-5085(91)90388-2. [DOI] [PubMed] [Google Scholar]
- 37.Sido B, Giese T, Autschbach F, Lasitschka F, Braunstein J, Meuer SC. Potential role of thioredoxin in immune responses in intestinal lamina propria T lymphocytes. Eur J Immunol. 2005;35:408–17. doi: 10.1002/eji.200424500. [DOI] [PubMed] [Google Scholar]
- 38.Gonsky R, Deem RL, Bream JH, Lee DH, Young HA, Targan SR. Mucosa-specific targets for regulation of IFN-gamma expression: lamina propria T cells use different cis-elements than peripheral blood T cells to regulate transactivation of IFN-gamma expression. J Immunol. 2000;164:1399–407. doi: 10.4049/jimmunol.164.3.1399. [DOI] [PubMed] [Google Scholar]
- 39.Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN's tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–33. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Ding Q, Xia W, Liu JC, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–70. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–4. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 42.Jones RG, Parsons M, Bonnard M, et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X (L) levels in vivo. J Exp Med. 2000;191:1721–34. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–4. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- 44.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–89. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 45.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 46.Chen WS, Xu PZ, Gottlob K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–8. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–77. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 48.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT − a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Sturm A, Itoh J, Jacobberger JW, Fiocchi C. p53 negatively regulates intestinal immunity by delaying mucosal T cell cycling. J Clin Invest. 2002;109:1481–92. doi: 10.1172/JCI14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barber DF, Bartolome A, Hernandez C, et al. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–5. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 51.Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–43. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]


