Abstract
A highly sensitive and accurate time-resolved immunofluorometric assay (TR-IFMA) has been developed, for the first time, to measure plasma vascular endothelial growth factor (VEGF) in patients with gastric tumours. A monoclonal anti-hVEGF antibody and a biotinylated anti-hVEGF antibody were used to develop a non-competitive ‘sandwich’-type assay. Fluorescence can be measured by a time-resolved fluorometer after binding of europium (Eu)3+-labelled streptavidin to the biotinylated immunoglobulin. Plasma VEGF concentrations were measured by TR-IFMA in 92 healthy controls, in 36 benign stomach disease patients and in 92 gastric cancer patients before surgery. The association between plasma VEGF levels and clinicopathological features was evaluated. A standard curve for VEGF TR-IFMA has been developed with good sensitivity (0·37 pg/ml). Accuracy studies, specificity, parallelism and precision data were determined and all were found to be satisfactory. The validity of the VEGF assay was confirmed by the good correlation between the results obtained by TR-IFMA and commercial enzyme-linked immunosorbent assay (ELISA) (ELISA result = 1·862 + 0·953 (TR-IFMA result), r = 0·944]. The plasma levels of VEGF are higher in gastric cancer patients than in healthy controls. VEGF levels were associated significantly with the presence of distant metastases, as well as invasion depth of the tumour and tumour stage, but not with tumour location, tumour histology, differentiation or the presence of lymph node metastases. At the cut-off of 217·79 pg/ml, the diagnostic sensitivity, specificity and accuracy of the TR-IFMA were 40·2%, 93·7% and 69·9%, respectively. A highly sensitive and reliable TR-IFMA for VEGF has been developed. The determination of plasma VEGF levels may be clinically useful.
Keywords: gastric tumour, plasma, time-resolved immunofluorometric assay, VEGF
Introduction
Solid tumour growth and metastasis is angiogenesis-dependent [1]. It is generally assumed that microvessel formation around a tumour is stimulated by various angiogenic factors secreted by the tumour cells [2]. Among them, vascular endothelial growth factor (VEGF) is considered to be one of the strongest promoters of angiogenesis in gastrointestinal tumours [3]. VEGF is a dimeric, heparin-binding glycoprotein that functions as a potent mitogen of vascular endothelial cells, providing an opportunity for their migration and organization for the neovascularization of micrometastases [4, 5]. VEGF exists in four isoforms resulting from alternative exon splicing of its ribonucleic acid (RNA) transcript [6, 7]. Of these isoforms, only VEGF121 and VEGF165 are secreted in soluble form, with VEGF165 being the predominant soluble isoform. The larger transcripts (VEGF189 and VEGF206) are bound to heparin-containing proteoglycans in the extracellular matrix [8].
Soluble forms of VEGF have been measured in patients with various types of cancer and increased levels of VEGF were demonstrated in most patients with cancers (including gastric cancer) [9–13]. This VEGF elevation in serum is suggested to relate closely to tumour progression and metastasis. Data on the VEGF concentration in blood specimens from patients with gastric tumours and its correlation to clinicopathological features have been reported elsewhere. However, published data on evaluating the diagnostic efficiency for circulating VEGF as a tumour marker in gastric cancer are sparse.
Various immunoassay methods have been developed to measure VEGF in blood, such as radioimmunoassay (RIA) [14, 15] and enzyme-linked immunosorbent assay (ELISA) [16]. Compared with previous techniques, the time-resolved immunofluorometric assays (TR-IFMA) are characterized by lower detection limits and greater specificity, reproducibility and practicability. They are now used widely for routine estimation of tumour markers [17–19]. Recently, we have developed a time-resolved immunofluorometric assay for the determination of cancer antigen (CA) 72-4 in sera of patients with gastric tumours. The assay was more sensitive and specific than conventional immunoradiometric assay (IRMA) and was easy to perform and automate [17].
After europium (Eu) chelates were developed as labels in the 1980s [20, 21], TR-IFMA has been reported as an ideal immunoassay technique. Sensitivity is higher with this technique than with methods based on tritiated tracers or enzyme-conjugated tracers. In addition, TR-IFMA offers an alternative, challenging immunoassays based on 125I labels with the advantage of a stable, non-radioactive tracer [22]. Fluorescence of the Eu3+ tracer is long-lived and allows for the differentiation of the short-lived background fluorescences of biological material, plastics and optics [23]. The highly specific activity of the label increases the sensitivity of immunoassays while minimizing non-specific binding of the labelled bioaffinity molecule. Other favourable features of Eu3+ complexes have been reviewed [24–26].
It has been revealed that VEGF is produced and secreted from megakaryocytes and platelets associated with blood coagulation [27], so serum samples may not be suitable for the measurement of circulating VEGF levels, because the clot formation in the collecting process of serum induces platelet activation and subsequent abundant cytokine release into sera [28]. Given the above problems, we have been using ethylenediamine tetraacetic acid (EDTA)-treated plasma but not serum for the measurement of circulating VEGF.
The purpose of our study was to evaluate a rapid and convenient fluorometric method based on time-resolved fluorescence of an Eu3+ chelate to analyse VEGF and to compare it with the established ELISA method. We evaluated plasma VEGF levels in healthy controls and in gastric cancer patients. We then correlated these levels with clinicopathological features.
Materials and methods
Chemicals
Monoclonal anti-human VEGF antibody and biotinylated anti-human VEGF antibody were obtained from R&D Systems, Inc., (Minneapolis, MN, USA). N1-[p-isothiocyanatobenzyl]-diethylene-triamine-N1,N2,N3,N4-tetraacetate chelated with Eu3+ (DTTA–Eu3+) was purchased from Tianjin Radio-Medical Institute (Tianjin, China). Bovine serum albumin (BSA) and streptavidin were purchased from Sigma-Aldrich Co., St Louis, MO, USA. Buffers were as described previously [17]. Other chemicals used were of analytical grade.
VEGF standards
Recombinant human VEGF 165 antigen were products of R&D Systems, Inc. The preparation was reconstituted according to the manufacturer's recommendations and diluted in 10 mmol/l phosphate-buffered saline (PBS) (pH 7·4) containing 2·0% BSA and 0·04% NaN3. This step resulted in the desired standard concentrations of 5, 50, 250, 500 and 1000 pg/ml.
Patients and specimens
We examined 92 adults with newly diagnosed and histologically confirmed gastric adenocarcinomas (40 women and 52 men aged 48–87 years) and 36 adults (15 women and 21 men aged 48–88 years) with benign stomach disease (stomach ulcers in 10 patients and gastritis in 26). Patients who had received chemotherapy, radiotherapy or blood transfusion before surgery were excluded from the study. Eighty-one cases were of primary gastric cancer, whereas in 11 patients the tumour had developed in the gastric remnant many years after resectional surgery for benign gastroduodenal ulcer disease. Tumour staging was based on clinical information, radiological reports (chest radiography, abdominal ultrasonography and computerized tomography), operative findings and pathology reports, with reports of staging made in accordance with the TNM staging system for gastric cancer [29]. Tumours were classified histologically as intestinal or diffuse according to their Lauren type [30].
Control subjects consisted of 92 age- and sex-matched healthy volunteers [median age 57 (range 43–87) years; 52 men and 40 women]. The absence of disease was confirmed by clinical history, physical examination and routine laboratory tests including liver and renal function tests. Shanghai JiaoTong University School of Medicine Ethics Committee approved the project and written informed consent was obtained from all patients and controls before their inclusion.
Blood samples were collected before the gastric cancers were treated. None of the patients had received chemotherapy or radiation therapy before this collection. Venous blood samples were collected into EDTA-treated sterile tubes and centrifuged to obtain plasma samples, which were stored at −80°C until they were assayed.
Purification and labelling of streptavidin (SA)
Before SA was labelled with Eu3+, columns prepacked with gel-filtration medium (Sephadex G-25M; GE Healthcare, Hong Kong, China) were used for desalting and buffer exchange. We dissolved 1 mg of purified SA in 0·5 ml of 50 mmol/l sodium carbonate buffer (pH 9·8) containing 0·9% NaCl. This solution was added to a glass vial containing 0·5 mg of DTTA–Eu3+ and then incubated overnight at 4°C. Labelled SA was separated from excess free label by gel filtration by using a 1·5 × 50 cm beaded column (Sepharose CL-6B; GE Healthcare) and the elution buffer. The elution was monitored at 280 nm with the UV monitor [EM-1 Econo ultraviolet (UV) monitor, Bio-Rad, Hercules, CA, USA] and 8·2 ml of labelled SA was collected. The concentration of SA in the purified conjugate solution was determined using the Beer–Lambert method, in which the UV absorbed by the DTTA–Eu3+ was subtracted. The concentration of Eu3+ was measured against Eu3+ calibrators in the fluorometer after fluorescence enhancement. The mean number of Eu3+ ions coupled to one SA molecule was 6·3, as calculated from the molar concentrations of Eu3+ and SA. Labelled SA could be stored at 4°C for at least 1 year without any loss of immunoreactivity.
Coating of polystyrene microtitre strips
Coating of polystyrene microtitre strips (Nunc, Roskilde, Denmark) was performed by a modification of our previously described methods [17]. Concentrated monoclonal anti-human VEGF antibody (1 mg/ml) was diluted to 5 μg/ml in coating buffer. We added 200 μl of the diluted antibodies per well and incubated them overnight at 4°C. The strips were aspirated and washed four times with washing buffer. Then, 200 μl of blocking buffer was dispensed into each well, and the strips were incubated for 4 h at room temperature. After we removed the solution from the wells, the strips were stored at 4–8°C in a dry sealed bag. No loss of immunoreactivity was observed during storage for 4 weeks at 37°C.
Assay protocol of TR-IFMA
Two-step assay procedures were used. In brief, 50 μl of standards or plasma samples were incubated at room temperature for 30 min in monoclonal anti-human VEGF antibody-coated wells with 100 μl of assay buffer containing the diluted biotinylated anti-human VEGF antibody (1 : 1000). Subsequently the assay wells were aspirated and washed six times with the washing buffer. We added 200 μl of Eu3+-labelled SA to every well and then mixed them gently for 20 min at room temperature. The bound Eu3+-label was then dissociated from the surface with 200 μl of enhancement solution and the resulting fluorescent chelate solution was subjected to single photon counting with a time-resolved fluorometer (Auto DELFIA 1235; Wallac, Turku, Finland).
Assay protocol of ELISA
VEGF kits for correlation studies were purchased from R&D Systems, Inc. (Quantikine, catalogue no. DVE00, Minneapolis, MN, USA).The assay was performed according to the instructions enclosed in the kits. In brief, 100 μl of assay diluent buffer, standards, controls or samples were dispensed into each well and then incubated at room temperature for 2 h. The contents of each well were discarded and washed with washing buffer and incubated subsequently with 200 μl of VEGF conjugate. After incubation at 35°C for another 2 h, the contents of each well were then discarded and washed as before. The colour reaction was performed with 200 μl of substrate solution at room temperature for 15 min and the absorbance was read in the ELx800 reader (Bio-Tek Instruments, Winooski, VT, USA) at 450 nm.
Assessment of assay performance
We assessed the performance of TR-IFMA by evaluating its calibration curve, detection limit, analytical recovery, specificity, precision and its dilution linearity.
Statistical analysis
Statistical analysis was performed using the SAS 6·12 statistical software package. All data are presented as median value (interquartile range, Q3–Q1), with non-parametric analyses being employed to assess differences. The Kruskal–Wallis analysis of variance and the rank sum test (Wilcoxon) were used to evaluate differences between multiple groups, unpaired observations, respectively. Correlations were evaluated using the Spearman's rank test. Significance was presumed at P < 0·05.
Results
Calibration curve and detection limit
Figure 1 shows a typical standard curve (log–log plot) for VEGF TR-IFMA. The sensitivity [±2 standard deviations (s.d.)] of the assay, as calculated from 12 replicates of the zero standard, was about 0·37 pg/ml. The calibration curve was linear over the whole measurement range (0·37–1000 pg/ml), and no high-dose hook effect was observed up to 5 ng/ml. The precision profile of the assay was determined from 15 replicates; the coefficients of variation at the concentration standards of 5, 25, 50, 250 and 1000 pg/ml were all < 6·0%.
Fig. 1.
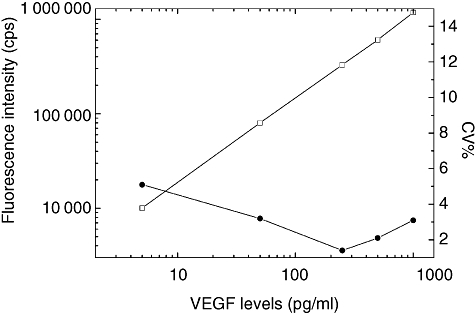
Typical standard curve (□) and precision profile (•) for vascular endothelial growth factor (VEGF) time-resolved immunofluorometric assay (TR-IFMA). Each point on the standard curve is the mean of 12 duplicate measurements. The zero standard (background) resulted in a mean 1865 counts per second (cps). Background fluorescence was subtracted from all measurements.
Analytical recovery
We assessed the analytical recovery of TR-IFMA by adding three amounts of VEGF to give final exogenous concentrations of 50·0, 250·0 and 500·0 pg/ml to five plasma samples from different patients. The endogenous VEGF concentrations were 64·46, 125·2, 217·69, 330·58 and 878·9 pg/ml; 50 μl of each of these exogenous VEGF was spiked into 950 μl of plasma samples for a spiking ratio of 1 : 19, leaving the plasma matrix of the spiked sample relatively intact. To calculate expected values, 95% of the unspiked value was added to 5% of the spiking solution concentration. We calculated the percentage recovery after we subtracted the concentrations of endogenous VEGF from the experimentally determined amounts. Recoveries ranged from 95% to 115% (Table 1).
Table 1.
Analytical recovery of vascular endothelial growth factor (VEGF) added to plasma samples.
| Amount of VEGF (pg/ml) | ||
|---|---|---|
| Added, expected | Observed | Recovery (%)* |
| 50·0 | ||
| 63·74 | 61·09 | 96 |
| 121·44 | 139·26 | 115 |
| 209·31 | 209·71 | 100 |
| 316·55 | 309·38 | 98 |
| 837·46 | 836·37 | 100 |
| 250·0 | ||
| 63·74 | 70·99 | 96 |
| 121·44 | 132·05 | 100 |
| 209·31 | 208·88 | 95 |
| 316·55 | 327·61 | 100 |
| 837·46 | 826·32 | 98 |
| 500·0 | ||
| 86·24 | 83·41 | 97 |
| 143·94 | 145·39 | 101 |
| 231·81 | 227·61 | 98 |
| 339·05 | 339·50 | 100 |
| 859·96 | 860·36 | 100 |
Mean ± standard deviation = 99·60% ± 4·64, n = 15.
Specificity of VEGF TR-IFMA
This VEGF TR-IFMA recognizes both natural and recombinant human VEGF165 and shows no cross-reactivity with any of the cytokines tested [e.g. VEGF-C, VEGF-D, human angiogenin, interleukin (IL)-1, IL-2, IL-3, IL-4, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, TNF-β] (data not shown).
Precision
Within-run precision studies were conducted by assaying human sera in three concentrations 10 times each. We assessed between-run precision by analysing control samples in 10 successive runs and evaluated day-to-day precision by testing the control samples on 10 consecutive days. All coefficients of variation were well below 5% (Table 2).
Table 2.
Precision of TR-IFMA for vascular endothelial growth factor (VEGF).
| VEGF level, mean ± s.d. (U/ml) | Coefficient of variation (%) |
|---|---|
| Within run (n = 10) | |
| 19·92 ± 0·76 | 3·8 |
| 83·39 ± 0·90 | 1·1 |
| 380·45 ± 11·62 | 3·1 |
| Between run (n = 10) | |
| 19·28 ± 0·94 | 4·9 |
| 88·53 ± 1·47 | 1·7 |
| 387·45 ± 11·04 | 2·9 |
| Day to day (n = 10) | |
| 19·21 ± 0·46 | 2·4 |
| 85·5 ± 1·08 | 1·3 |
| 386·24 ± 17·16 | 4·4 |
SD: standard deviation.
Dilution
Table 3 shows the results of our evaluation of the dilution linearity of TR-IFMA when we used samples diluted serially with assay buffer. Expected values were derived from initial concentrations of VEGF in the undiluted samples. Correlating the results obtained from TR-IFMA with the expected concentrations, we found that the dilution curves were linear over the whole range of concentrations. Expected and measured values were well correlated.
Table 3.
Dilution linearity of samples with high vascular endothelial growth factor (VEGF) levels.*
| Sample 1 | Sample 2 | Sample 3 | ||||
|---|---|---|---|---|---|---|
| Dilution factor | Expected | Observed | Expected | Observed | Expected | Observed |
| None | n.a. | 25·66 | n.a. | 240·45 | n.a. | 677·30 |
| ×2 | 12·83 | 13·01 | 120·23 | 121·62 | 338·65 | 330·60 |
| ×4 | 6·42 | 6·41 | 60·11 | 60·45 | 169·33 | 165·34 |
| ×8 | 3·21 | 2·31 | 30·06 | 32·81 | 84·66 | 86·09 |
| ×16 | 1·60 | 1·80 | 15·03 | 14·11 | 42·33 | 36·04 |
| ×32 | 0·80 | 1·77 | 7·51 | 6·89 | 21·17 | 22·34 |
We conducted a regression analysis of expected (x) versus measured (y) values (in units per millilitre). Respective slopes, intercepts and r-values were 0·98187, 0·17814 and 0·99063 for sample 1; 1·01366, −0·04837 and 0·99956 for sample 2; and 0·97813, −0·27582 and 0·99967 for sample 3; n.a.: not applicable.
Comparison of TR-IFMA and ELISA
The samples from patients with gastric tumour were tested concurrently with TR-IFMA and ELISA. We observed linear relativity between TR-IFMA and ELISA results when the assays were performed in duplicate at the same time (Fig. 2). The linear regression equation was as follows: ELISA result = 1·862 + 0·953 (TR-IFMA result). The correlation coefficient r was 0·944. Mean VEGF concentrations determined with the two methods were in acceptable agreement.
Fig. 2.
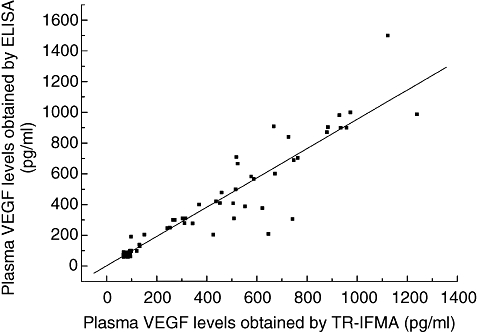
Correlation between vascular endothelial growth factor (VEGF) levels in the plasma of patients with gastric tumours determined with time-resolved immunofluorometric assay (TR-IFMA) and enzyme-linked immunosorbent assay (r = 0·944, n = 92).
Distribution of plasma VEGF concentrations
Plasma VEGF levels were detectable in all control subjects. The data were tested and were found to be distributed normally. Their median VEGF level was 77·09 (35·09) pg/ml. There was no significant difference in VEGF levels between males and females [75·26 (38·91) pg/ml versus 77·53 (34·65) pg/ml, respectively; P = 0·592]. No correlation was found between VEGF levels and age (r = −0·17; P = 0·321).
The data obtained from patients with gastric cancer and benign stomach disease were found to be non-normally distributed. Preoperative plasma VEGF levels [95·00 (429·44) pg/ml] in patients with gastric cancer were significantly higher than those in controls (P = 0·001). There was no significant difference between males and females regarding VEGF levels [92·77 (408·33) pg/ml versus 98·05 (422·76) pg/ml, respectively; P = 0·265], nor there was any correlation between VEGF levels and age (r = −0·14; P = 0·171). The relationships between VEGF levels and clinicopathological variables are shown in Table 4. There was a significant correlation between VEGF levels and disease stage with higher VEGF levels detected as the disease stage increased (P = 0·036). Patients with distant metastases had significantly higher VEGF levels when compared with those without metastatic disease (P = 0·009). Although the relationship between plasma VEGF levels and the invasion depth of the tumour was not statistically significant by Wilcoxon's rank sum test (P = 0·0564), further analysis using the Kruskal–Wallis analysis of variance test showed that patients with tumours penetrating the serosa (T3–4) had significantly higher VEGF levels when compared to those with tumours limited to the gastric wall (T1–2) [95·9 (226·0) pg/ml versus 254·69 (546·2) pg/ml, respectively; P = 0·041]. There were no significant associations between VEGF levels and tumour location (antrum, body or cardia), differentiation (well, moderate or poor), primary versus gastric stump cancer, intestinal versus diffuse histology or the presence of lymph node metastases.
Table 4.
Relationship between plasma vascular endothelial growth factor (VEGF) levels and pathological variables in gastric patients.
| No. | Plasma VEGF (pg/ml)* | Significance | |
|---|---|---|---|
| Tumour location | |||
| Gastric stump | 8 | 179·49 (706·04) | P = 0·5513† |
| Body | 33 | 132·34 (499·0) | |
| Cardia | 10 | 175·4 (331·1) | |
| Antrum | 41 | 86·81 (432·37) | P = 0·5569‡ |
| Tumour histology | |||
| Diffuse | 26 | 94·07 (545·55) | |
| Intestinal | 66 | 95·0 (381·6) | P = 0·7028† |
| Differentiation | |||
| Poorly | 40 | 91·09 (318·72) | |
| Moderate | 38 | 114·80 (378·08) | |
| Well | 14 | 307·4 (464·78) | P = 0·1528‡ |
| Tumour class | |||
| T1 | 20 | 126·31 (286·53) | |
| T2 | 17 | 87·1 (54·86) | |
| T3 | 32 | 204·2 (558·83) | |
| T4 | 23 | 94·6 (446·41) | P = 0·0564,† 0·041‡ |
| TNM stage | |||
| I | 19 | 87·1 (166·44) | |
| II | 5 | 94·2 (13·11) | |
| III | 44 | 93·7 (380·53) | |
| IV | 24 | 336·25 (733·41) | P = 0·036‡ |
| Lymph node metastases | |||
| Absent | 25 | 314·6 (493·05) | |
| Present | 67 | 93·3 (349·26) | P = 0·1488† |
| Distant metastases | |||
| Absent | 87 | 94·6 (359·7) | |
| Present | 5 | 882·9 (207·82) | P = 0·009† |
Values are median (interquartile range).
Rank sum test (Wilcoxon).
Kruskal–Wallis analysis of variance.
Plasma VEGF levels [95·165 (43·565) pg/ml] in patients with benign stomach disease (stomach ulcers and gastritis) were significantly higher than those in controls (P = 0·04). Compared with the levels in gastric cancers, plasma VEGF levels in patients with benign stomach disease were significantly lower (P = 0·03).
The cut-off was set at the 95th percentile of the 92 control subjects, i.e. 217·79 pg/ml for TR-IFMA. Sensitivity was calculated as the proportion of patients gastric cancer with VEGF concentrations equal to or higher than each decision threshold, and (1 – specificity) was calculated as the proportion of control subjects with VEGF concentrations higher than the same threshold. With the cut-off of 217·79 pg/ml, the sensitivity and specificity, respectively, were 40·2% and 93·7%. Figure 3 shows the receiving operating characteristic curves (ROC) for the TR-IFMA results. The areas under the curve were 0·699 (95% confidence interval, 0·629–0·769) for TR-IFMA.
Fig. 3.
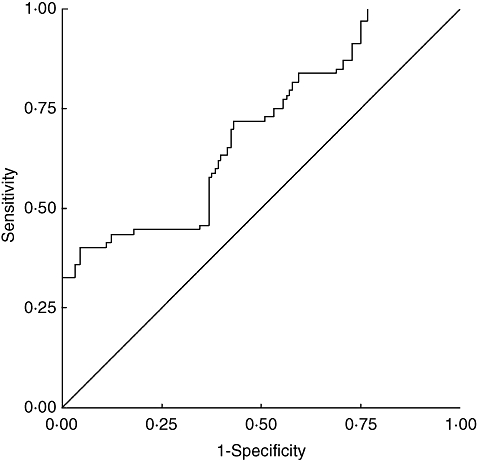
Receiver operating characteristic curve for time-resolved immunofluorometric assay (TR-IFMA) results in patients with gastric cancer, patients with benign stomach disease, and control subjects.
Discussion
We have developed a sensitive non-competitive ‘sandwich’-type time-resolved immunofluorometric assay for determining VEGF in plasma with use of a streptavidin–biotin detection system. A monoclonal ‘capture’ antibody is immobilized in polystyrene microtitre strips. The detection antibody is a biotinylated affinity-purified antibody, and streptavidin labelled with the Eu3+ chelate is used as the fluorescent label. The resulting complex that forms, monoclonal antibody-VEGF-polyclonal antibody–biotin–streptavidin–DTTA–Eu3+, is quantified on the well by excitation with a nitrogen laser beam; the specific delayed fluorescence is monitored at about 615 nm. The use of the well-known streptavidin–biotin system in the assay design has a number of advantages: (i) it is a universal detection system in both sandwich and competitive immunoassays; (ii) the labelling of streptavidin with the Eu3+ chelate is very easy and efficient, without any loss of biological activity; (iii) labelled streptavidin and biotinylated antibodies are very stable reagents; and (iv) amplification is introduced.
The performance characteristics of TR-IFMA are in many respects equivalent to or better than those reported for ELISAs [18]. Its analytical sensitivity was about 0·37 pg/ml, compared with about 4–6 pg/ml for currently available ELISAs. Several factors contributed to the high sensitivity of TR-IFMA, notably the rejection of optical background with measurements in the time-resolved mode and the use of high concentrations of monospecific antibodies. The validity of TR-IFMA for plasma VEGF was confirmed by good correlation between the results obtained by TR-IFMA and those by ELISA. Cross-reactivity data with major interfering cytokines have been shown to be highly specific for the detection of the main VEGF isoform, namely the VEGF165 isoform. Analytical recovery studies, parallelism (dilution of plasma samples) and precision data determined for TR-IFMA were found to be satisfactory. Assay time is shortened by 50 min (04·25 h for ELISA). The assay reagents were used in an instrument so that the assay could be performed on an automated platform. To our knowledge, this is the first report of the use of TR-IFMA to the measurement of plasma VEGF levels.
VEGF levels in our study are lower than those in serum samples reported by others [31–33]. Recently, VEGF has been shown to be induced and released from activated platelets in vitro [14, 15]. During clot formation, platelets are activated and many cytokines are released. However, EDTA-Na added into the drawn venous blood acts as a chelating agent of divalent cations and suppresses platelet activation and aggregation. Several studies have reported that plasma VEGF concentration was lower than the matched serum VEGF concentration. The serum VEGF concentration was not related to any clinicopathological factors. It has been recommended that plasma rather than serum should be considered suitable for the measurement of circulating VEGF. The plasma VEGF level is the most sensitive marker of the status of gastric cancer [32–34].
This study shows a marked difference between preoperative VEGF concentrations in gastric cancer patients when compared with age- and sex-matched controls, with a significant association between these levels and tumour stage, the invasion depth of the tumour and the presence of distant metastases. These results suggest that plasma VEGF levels may be a useful diagnostic marker for tumour progression, especially metastasis. Our findings are in agreement with previous reports [29–32]. Elevated plasma VEGF levels were defined as being greater than the 95th percentile value in the healthy control group in accordance with the recommendations of Werther et al. [35]. The cut-off for TR-IFMA was 217·79 pg/ml. The present cut-off is probably not optimal. Final adjustment will require measurements from several hundreds of healthy individuals and careful analysis of age-dependent variations in the background values. The areas under the curve were 0·699 for (Fig. 3), showing that the diagnostic accuracy of TR-IFMA was not excellent. One possibility could be that elevated VEGF levels in plasma samples were also seen in patients with widespread infectious or inflammatory disease. Higher plasma VEGF levels than in healthy volunteers were detected in patients with benign stomach disease (stomach ulcers and gastritis).
In conclusion, the novel time-resolved immunofluorometric assay described here provides a rapid and sensitive method for the measurement of plasma VEGF. This study has demonstrated higher VEGF levels in gastric cancer patients when compared with normal controls. These levels correlate with tumour stage and the presence of distant metastases. The data obtained for specificity, sensitivity, parallelism, accuracy and imprecision revealed reliable performance of VEGF TR-IFMA for routine use.
Acknowledgments
We thank the staff of the Departments of Surgery and Gastroenterology at our hospital for providing the patients' clinical data. This study was supported financially by National Natural Science Foundation of China (project 30470497) and Program for Outstanding Medical Academic Leader (project LJ06002).
References
- 1.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–4. [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211–18. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- 6.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–14. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 7.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- 8.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–7. [PubMed] [Google Scholar]
- 9.Forster Y, Meye A, Albrecht S, Schwenzer B. Tissue factor and tumor: clinical and laboratory aspects. Clin Chim Acta. 2006;364:12–21. doi: 10.1016/j.cca.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Adams J, Carder PJ, Downey S, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000;60:2898–05. [PubMed] [Google Scholar]
- 11.Davies MM, Jonas SK, Kaur S, Allen-Mersh TG. Plasma vascular endothelial but not fibroblast growth factor levels correlate with colorectal liver metastasis vascularity and volume. Br J Cancer. 2000;82:1004–8. doi: 10.1054/bjoc.1999.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wynendaele W, Derua R, Hoylaerts MF, et al. Vascular endothelial growth factor measured in platelet poor plasma allows optimal separation between cancer patients and volunteers: a key to study an angiogenic marker in vivo? Ann Oncol. 1999;10:965–71. doi: 10.1023/a:1008377921886. [DOI] [PubMed] [Google Scholar]
- 13.Ranieri G, Coviello M, Chiriatti A, et al. Vascular endothelial growth factor assessment in different blood fractions of gastrointestinal cancer patients and healthy controls. Oncol Rep. 2004;11:435–9. [PubMed] [Google Scholar]
- 14.Anthony FW, Evans PW, Wheeler T, Wood PJ. Variation in detection of VEGF in maternal serum by immunoassay and the possible influence of binding proteins. Ann Clin Biochem. 1997;34:276–80. doi: 10.1177/000456329703400309. [DOI] [PubMed] [Google Scholar]
- 15.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus. Circulation. 2000;102:185–90. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez CR, Fei DT, Keyt B, Baly DL. A sensitive fluorometric enzyme-linked immunosorbent assay that measures vascular endothelial growth factor 165 in human plasma. J Immunol Methods. 1998;219:45–55. doi: 10.1016/s0022-1759(98)00131-8. [DOI] [PubMed] [Google Scholar]
- 17.Sheng SL, Wang Q, Huang G. Development of time-resolved immunofluorometric assays for CA 72-4 and application in sera of patients with gastric tumors. Clin Chim Acta. 2007;380:106–11. doi: 10.1016/j.cca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Leinonen J, Zhang WM, Finne P, Stenman UH. Dual-label immunoassay for simultaneous measurement of prostate-specific antigen (PSA)-alpha1-antichymotrypsin complex together with free or total PSA. Clin Chem. 2003;49:97–103. doi: 10.1373/49.1.97. [DOI] [PubMed] [Google Scholar]
- 19.Christopoulos TK, Lianidou ES, Diamandis EP. Ultrasensitive time-resolved fluorescence method for alpha-fetoprotein. Clin Chem. 1990;36(8 Pt 1):1497–02. [PubMed] [Google Scholar]
- 20.Siitari H, Hemmilä I, Soini E, Lövgren T, Koistinen V. Detection of hepatitis B surface antigen using time-resolved fluoroimmunoassay. Nature. 1983;301:258–60. doi: 10.1038/301258a0. [DOI] [PubMed] [Google Scholar]
- 21.Hemmilä I, Dakubu S, Mukkala VM, Siitari H, Lövgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984;137:335–43. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- 22.Fiet J, Giton F, Fidaa I, Valleix A, Galons H, Raynaud JP. Development of a highly sensitive and specific new testosterone time-resolved fluoroimmunoassay in human serum. Steroids. 2004;69:461. doi: 10.1016/j.steroids.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Soini E, Kojola H. Time-resolved fluorometer for lanthanide chelates − a new generation of nonisotopic immunoassays. Clin Chem. 1983;29:65–8. [PubMed] [Google Scholar]
- 24.Diamandis EP. Immunoassays with time-resolved fluorescence spectroscopy. Principles and applications. Clin Biochem. 1988;21:139–50. doi: 10.1016/0009-9120(88)90001-x. [Review]. [DOI] [PubMed] [Google Scholar]
- 25.Khosravi MJ, Diamandis EP. Immunofluorometry of chorio-gonadotropin by time–resolved fluorescence spectroscopy, with a new europium chelate as label. Clin Chem. 1987;33:1994–9. [PubMed] [Google Scholar]
- 26.Dickson EF, Pollak A, Diamandis EP. Ultrasensitive bioanalytical assays using time-resolved fluorescence detection. Pharmacol Ther. 1995;66:207–35. doi: 10.1016/0163-7258(94)00078-h. [Review]. [DOI] [PubMed] [Google Scholar]
- 27.Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA. 1997;94:663–8. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verheul HM, Hoekman K, Luykx-de Bakker S, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187–90. [PubMed] [Google Scholar]
- 29.Kennedy BJ. The unified international gastric cancer staging classification system. Scand J Gastroenterol. 1987;22(Suppl. 133):11–13. [Google Scholar]
- 30.Lauren P. The two histological main types of gastric carcinoma. Difffuse and so-called intestinal type carcinoma. An attempt at a histoclinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 31.Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg. 2002;236:37–42. doi: 10.1097/00000658-200207000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta M, Konno H, Tanaka T, et al. The significance of circulating vascular endothelial growth factor (VEGF) protein in gastric cancer. Cancer Lett. 2003;192:215–25. doi: 10.1016/0304-3835(02)00681-x. [DOI] [PubMed] [Google Scholar]
- 33.Hyodo I, Doi T, Endo H, et al. Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer. 1998;34:2041–5. doi: 10.1016/s0959-8049(98)00282-2. [DOI] [PubMed] [Google Scholar]
- 34.Huang SP, Wu MS, Wang HP, Yang CS, Kuo ML, Lin JT. Correlation between serum levels of interleukin-6 and vascular endothelial growth factor in gastric carcinoma. J Gastroenterol Hepatol. 2002;17:1165–9. doi: 10.1046/j.1440-1746.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- 35.Werther K, Christensen IJ, Brunner N, Nielsen HJ. Soluble vascular endothelial growth factor levels in patients with primary colorectal carcinoma. Eur J Surg Oncol. 2000;26:657–62. doi: 10.1053/ejso.2000.0977. [DOI] [PubMed] [Google Scholar]


