Abstract
Oral tolerance to foods can be regulated by microorganisms in the gut lumen. We hypothesized that pretreatment with avirulent Salmonella typhimurium strains could prevent food allergy in mice. Mice were administered S. typhimurium PhoPc (STPhoPc) or S. typhimurium AroA prior to oral sensitization to β-lactoglobulin in the presence of cholera toxin. An oral antigen challenge after sensitization assessed antigen-induced anaphylaxis. Antigen-specific antibody titres were measured by enzyme-linked immunosorbent assay in the serum and enzyme-linked immunospot (ELISPOT) in the spleen, and cytokine-secreting cells were measured by ELISPOT in the Peyer's patches, lamina propria and epithelium cells. We showed first that S. typhimurium could up-regulate interleukin (IL)-12 and IL-10 secretion by gut T cells. Mice pretreated with STPhoPc had decreased anaphylaxis upon challenge, along with decreased immumoglobulin G1 (IgG1) and IgE antibody titres. Mice having received S. typhimurium AroA had partly decreased anaphylaxis as well as decreased serum IgG1 antibody titres in the serum, and increased serum IgA antibody titres. Antibody titres could be correlated with increased numbers of spleen and Peyer's patches antibody-producing cells. STPhoPc-treated mice showed significantly decreased anaphylaxis when compared with the control mice, while S. typhimurium AroA-pretreated mice had a similar immune response together with increased secretory IgA titres. Our experiments have proved a potential immunomodulatory protective effect by two avirulent S. typhimurium strains.
Keywords: food-hypersensitivity, Salmonella typhimurium, T lymphocytes, tolerance
Introduction
Food-induced anaphylaxis is a severe manifestation of immumoglobulin E (IgE)-mediated food allergy [1]. Avoiding allergy to common food allergens is the goal of primary prevention [2, 3]. Unfortunately, to date no effective preventive measures are available for IgE-mediated food allergy [4].
Animal models of IgE-mediated food allergy have been developed by us and others [5–10], allowing investigation of potential preventive measures. Previously, we have shown in a mouse model sensitized to the common milk allergen β-lactoglobulin (BLG) that tolerance mechanisms were mediated mainly by interleukin (IL)-10-secreting T cells in Peyer's patches, with decreased BLG-specific IgE titres in the serum and increased BLG-specific secretory IgA titres in the faeces [11, 12]. Thus, we were interested to explore preventive interventions targeting the T helper type 1 (Th1)/Th2 balance, or promoting IL-10 secretion in the gut.
The microbiota provides immunological stimuli to the gut according to the type of bacteria present. Among them, various avirulent Salmonella strains are common hosts of the gut. Two strains have been studied extensively in mice, mainly as candidates for immunization against infection by Salmonella. The AroA–S. typhimurium was derived initially from the wild-type strain SL1344 [13], and the avirulant ΔphoP was also obtained from the same strain [14]. Both strains have been characterized mainly in mice models with regard to their immunogenicity and have revealed a strong cross-reactive immune response to S. typhy lipopolysaccharide (LPS) [15]. In a previous study, Dreher et al. have shown polarization of human dendritic cells by two mutant, avirulant, S. typhimurium strains, the S. typhimurium PhoPc (STPhoPc) and the S. typhimurium mutant AroA (STAroA) [16]. In addition to the diminished virulence caused by the gene mutation, both mutants were killed by the cellular host 24 h after infection. Thus, these strains might have a potential for avirulant immunomodulation, but need further characterization for their effect in mice.
In the study reported here, we showed first that these strains have a modulatory potential in mice, and then administered STPhoPc and STAroA to mice prior to oral sensitization with a common food allergen. We observed in STAroA-pretreated mice inhibition of IgE-type sensitization as well as induction of antigen-specific IgA antibodies, while pretreatment with STPhoPc induced a strong inhibition of IgE and IgG1 antibody titres correlated with a significant decrease in severity of antigen-induced anaphylaxis.
Materials and methods
Bacterial strains
Two attenuated S. typhimurium strains were used in these experiments. S. typhimurium AroA is unable to synthesize aromatic amino acids and para-aminobenzoic acids as a result of a mutation of the aroA gene, and STPhoPc bears a mutation in the phoQ gene (phoQ24) leading to deregulation in the virulence genes [17]. Both strains isolated from agar plates were grown at 37°C in liquid Luria–Bertani medium (Difco, Detroit, MI, USA) for 24 h at 37°C. The density of the bacteria was adjusted by optical density measurement at 450 nm, and resupended in 0·2 M NaHCO3 at 2·5 × 109plaque-forming units (PFU)/ml.
Oral administration of S. typhimurium, mice sensitization to BLG and oral challenges
C3H/HeOuJ females were purchased from Charles River (L'Arbresle, France) and were housed at the Animal Facilities of the University of Geneva School of Medicine. Unlike the C3H/HeJ strain, these mice do not bear a spontaneous mutation in the Toll-like receptor 4 gene and are not resistant to endotoxins. Animals were used between 4 and 5 weeks of age and were fed with standard mice pellets without milk proteins.
A murine model of food allergy as published earlier was used in the experiments [6, 8]. Mice were sensitized orally at days 0, 7, 14 and 21 with 20 mg BLG (Sigma, Buchs, Switzerland) in a solution of 0·2 M NaHCO3 containing 10 μg of cholera toxin (List Biological Laboratories, Campbell, CA, USA). Bacterial strains at 109 PFU in 400 μl were given three times by the same route in a single dose every day prior to each oral sensitization (Fig. 1). On day 28, all mice were challenged by intragastric gavage with 100 mg of BLG. Anaphylaxis was graded using a previously published reaction score (0: no reaction to 3: severe reaction) [8], and correlated with the body temperature measured at various times following challenge with the infrared ear thermometer (Braun, Kronberg, Germany). In separate experiments aimed to explore the effect on the immune system of the two S. typhimurium strains, bacteria were given for 3 days as described above but without subsequent sensitization with the food antigen. All experiments were approved by the Animal Studies Ethics Committee and performed in accordance with their guidelines.
Fig. 1.
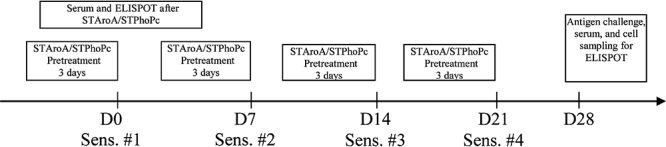
Protocol used for pretreatment with Salmonella typhimurium PhoPc (STPhoPc) and S. typhimurium AroA (STAroA) and sensitization with β-lactoglobulin and cholera toxin. ELISPOT, enzyme-linked immunospot.
Isolation of lymphocytes
Cells from the Peyer's patches, lamina propria (LPL) and intra-epithelial lymphocytes (IEL) were isolated using modified methods as described previously [18]. Briefly, fat was removed, Peyer's patches were excised mechanically and the gut was flushed extensively with Hank's balanced salt solution (HBSS) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 100 μg/ml gentamicin, 15 mM HEPES, 2 mM NaHCO3 and 10% fetal calf serum (FCS) (all from Sigma). Intestinal pieces were opened longitudinally and cut into 5-mm pieces. The tissue was incubated in calcium- and magnesium-free HBSS containing 2 mM ethylenediamine tetraacetic acid (EDTA) and 1 mM dithiothreitol (Sigma) for 30 min at 37°C with magnetic stirring, then vortexed vigorously and filtered through a 70-μm nylon filter. IEL were obtained by filtrating the supernatant through a nylon wool column. The remaining tissue was washed three times with RPMI-1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 100 μg/ml gentamicin, 15 mM HEPES and 10% FCS (cRPMI) (all from Sigma), and intestinal pieces were incubated subsequently with magnetic stirring for 30 min at 37°C in cRPMI, supplemented with 1 μg/ml collagenase D (Roche, Mannheim, Germany). Cells were then separated from tissue debris by purification through a 70-μm nylon filter. This step was repeated once.
Peyer's patches were incubated in calcium- and magnesium-free HBSS containing 2 mM EDTA and 1 mM dithiothreitol for 30 min at 37°C with magnetic stirring, then crushed and filtered through mesh wire screens.
Cell suspensions from Peyer's patches, LPL and epithelium were washed twice and lymphocytes were enriched by discontinuous 30/40% Percoll (Bioscience, Uppsala, Sweden) upon lympholyte M (Cedarlane, Horby, Canada) gradients for 20 min at 600 g at room temperature. Lymphocytes were harvested from the Percoll 30% lympholyte M interface.
Enzyme-linked immunoassay Salmonella- and BLG-specific antibodies in sera and faeces
Sera were obtained on day 28 before challenge from tail bleeding of individual mice. BLG-specific antibody titres in serum were determined by a method adapted from Adel-Patient et al. [19]. Briefly, Maxisorp microtitre plates (Nunc, Roskilde, Denmark) were coated for 18 h at room temperature with 250 ng/well streptavidin (Fluka, Buchs, Switzerland), followed by overnight incubation at 4°C with 10 mg/ml of polyvinylpyrrolidon K25 (Fluka). One microgram of biotinyled BLG was incubated for 3 h at room temperature. Various dilutions of sera were diluted in enzyme-linked immunosorbent assay (ELISA) buffer [phosphate-buffered saline (PBS) 10% horse serum] and incubated for 3 h at 37°C. Corresponding polyclonal goat anti-mouse IgA, IgG1, IgG2a peroxidase-labelled antibodies (Southern Biotechnologies, Birmingham, AL, USA) at 1 : 1000 in ELISA buffer were added for 90 min at 37°C. For IgE measurement we used a monoclonal rat anti-mouse IgE antibody (clone R35-72; BD, San Diego, CA, USA) at 2 μg/ml followed by peroxidase-coupled anti-rat antibody (Caltag, San Francisco, CA, USA) at 1 : 1000. Antibody titres were determined by the addition of an enzyme substrate, ortho-phenylenediamine and H2O2 (Sigma), and absorbance was measured at 490 nm on a plate reader (Molecular Device Corporation, Menlo Park, CA, USA). Results were analysed with soft max software (Molecular Device Corporation) and expressed as arbitrary units, with pooled sera from BLG and alum-immunized mice used as a reference serum to make the titration curve.
For the measurement of Salmonella-specific antibodies, Maxisorp microtitre plates (Nunc) were coated with 100 μl/well of 1 μg/ml sonicated bacteria in PBS and incubated overnight at room temperature. Wells were blocked with polyvinylpyrrolidon K25 for 2 h at 37°C. Detection of specific antibody titres was performed using the same method as BLG-specific ELISA.
Cytokine production after Salmonella- and BLG-specific antibody production measured by enzyme-linked immunospot
Twenty-four hours after the first set of Salmonella administration, mice were killed and cytokine production was quantified on mononuclear cells isolated from gastrointestinal-associated lymphoid tissue. Briefly, MultiScreen 96-well nitrocellulose plates (Millipore Corporation, Billerica, MA, USA) were coated overnight at 4°C with 5 μg/ml in PBS of either monoclonal antibodies against mouse IL-10 (clone JES5-2A5) (BD, San Diego, CA, USA) or mouse IL-12 (provided from kit) (R&D Systems, Abingdon, Oxon, UK). Plates were blocked for 2 h at 37°C with culture medium. 1 × 105, 2 × 105 and 4 × 105 cells/well in Dubelcco's modified Eagle's medium supplemented with 10% non-essential amino acid, 10 mM sodium-pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 100 μg/ml gentamicin, 15 mM HEPES, 2 × 10−5 M 2-mercapto-ethanol, 1 μg/ml polymixin B and 10% FCS were incubated in triplicate for 5 days at 37°C in 5% CO2. Following washing, either biotin-labelled anti-mouse IL-10 (clone SXC-1) (BD) or IL-12 (R&D) at 1 μg/ml were added for overnight incubation at 4°C, followed by peroxidase–streptavidin (Sigma) at 1 : 1000 for 2 h at 37°C. After washing, spots were visualized upon addition of the chromogenic substrate 3-amino-9-ethylcarbazone and H2O2 (Sigma), counted automatically with the KS enzyme-linked immunospot (ELISPOT) version 4.2.1 software (Zeiss, Halbermoos, Germany). Results were expressed as cell-forming units (CFU) per 106 cells. For BLG-specific antibody production, the mice were killed on day 28 and spleens removed. For measurement of BLG-specific IgG1, IgG2a and IgA antibodies, ELISPOT plates (Millipore) were coated with streptavidin overnight at 37°C, followed by addition of 1 μg of biotinylated BLG for 3 h. Lymphoblasts were resuspended as 1 × 106 and 2 × 106 cells in Iscove's modified Dulbecco's medium supplemented with penicillin, streptomycin, glutamine, 100 μg/ml gentamicin, polymixin B and 5% FCS for 24 h at 37°C, followed by overnight incubation at 4°C with anti-IgA, anti-IgG1 and anti-IgG2a antibodies (Southern Biotechnology). Spot measurement was similar to the procedure outlined above. All cytokine procedures were optimized initially for in vitro cytokine measurements.
Statistics analysis
Data were expressed as mean ± standard error of the mean unless stated otherwise. Statistical significance between groups was analysed by using the Wilcoxon signed-rank test for non-parametric unpaired data. Anaphylaxis scores (reactors versus non-reactors) were analysed by Fisher's exact test. All experiments were repeated at least twice for validation.
Results
Salmonella-induced cytokine production in the gut
Either STPhoPc or STAroA were administered orally by gavage for three consecutive days to naive mice; Peyer's patches, LPL or epithelial cells were then collected and cytokine production was assessed by ELISPOT. None of the two strains induced an IL-12 secretion in the Peyer's patches (Fig. 2a). However, the number of IL-12-producing cells in the LPL was doubled after exposure to both strains (naive: 245·5 ± 47·3 CFU, STPhoPc: 440·0 ± 46·1 CFU, STAroA: 476·0 ± 113·1 CFU, P < 0·01) and increased in epithelial cells only after exposure to STAroA (naive: 628·3 ± 98·1 CFU, STAroA: 963·3 ± 80·6 CFU). Peyer's patches cells were activated for IL-10 after STAroA (naive: 388·2 ± 225·6 CFU, STAroA: 801·9 ± 355·5 CFU, P < 0·05), and to some extent in LPL after STPhoPc and STAroA, but the strongest increase in IL-10 production was seen in IEL for STPhoPc (naive: 1177·8 ± 273·6 CFU, STPhoPc: 2420·0 ± 732·6 CFU, P < 0·05) (Fig. 2b). Significant differences in IL-4 production were not observed (results not shown). These results show a clear immunological effect for both strains with specific activation of cells of the immune system in various compartments of the gut. IL-12 is activated clearly by both strains in the LPL as well as in the epithelium. However, only cells from the LPL produced significantly higher amounts of IL-12 after Salmonella than in naive mice, with no difference between the strains. Interestingly, IL-10 secretion was activated in particular by STPhoPc in the LPL as well as in the epithelium, and to a higher extent than by STAroA. Remarkably, in the epithelium IL-10 titres were twice as higher after STPhoPc than after naive mice or STAroA, suggesting that STPhoPc might induce a potential tolerogenic effect through IL-10 in the epithelial compartment.
Fig. 2.
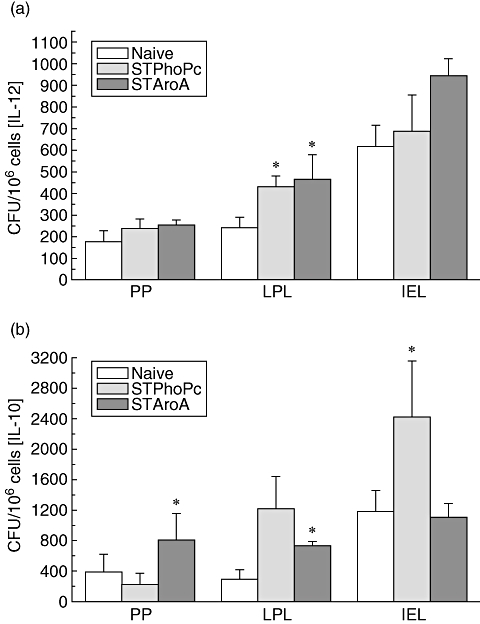
Cytokine-secreting cell count in Peyer's patches (PP), lamina propria (LPL) and epithelial lymphocytes (IEL) by enzyme-linked immunospot for interleukin (IL)-12 (a) and IL-10 (b) after Salmonella typhimurium PhoPc (STPhoPc) and S. typhimurium AroA (STAroA) as well as naive mice. Pooled results from three separate experiments are presented as means ± standard error of the mean. *P < 0·05 when compared with naïve mice.
Salmonella-induced serum-specific antibody
Next, we were interested to characterize Salmonella-specific IgG1 and IgG2 antibody titres after either oral STPhoPc or STAroA administration. IgG1 titres reflecting a Th2-type stimulation were highest after STAroA administration (63 483 ± 8611 AU), but also increased after STPhoPc (42 342 ± 5839 AU) (Fig. 3a). IgG2a titres were increased similarly after both STPhoPc (177 311 ± 119 572 AU) and STAroA administration (190 789 ± 29 743 AU), suggesting a Th1-type stimulation by both strains (Fig. 3b). However, the increase in IgA titres was highest after STPhoPc administration (18 739 ± 7538 AU) (Fig. 3c). Overall, STPhoPc or STAroA induced a mixed strain-specific antibody production in the gut. IgE antibodies were also measured, but could not be detected. Very high titres of all three antibodies measured could be observed after administration of either STPhoPC or STAroA. Titres could not allow clear discrimination of a differential effect with either Salmonella strain. Antibody production in Peyer's patches cells and LPL cells were also assayed by ELISPOT, with similar results to spleen cells.
Fig. 3.
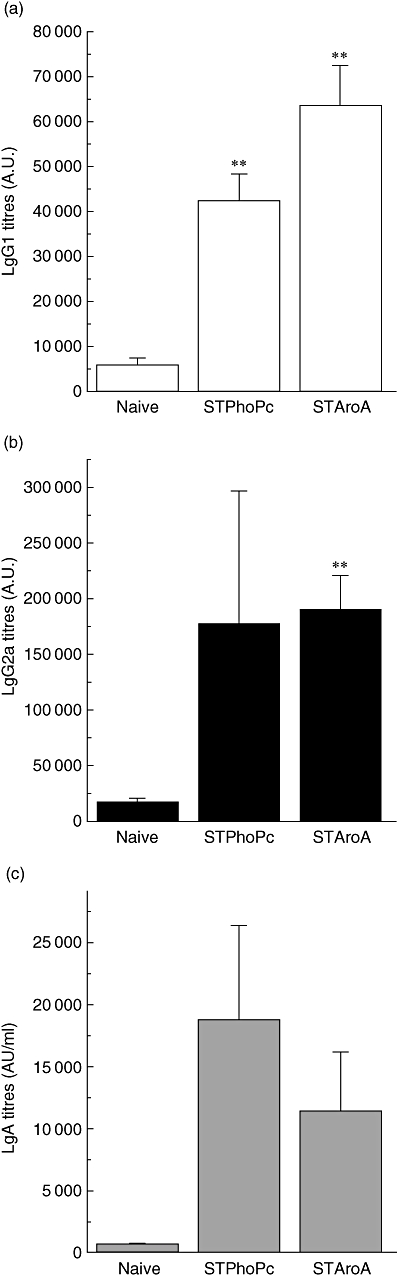
Serum Salmonella-specific immunoglobulin G1 (IgG1) (a), IgG2a (b) and IgA (c) titres after S. typhimurium PhoPc (STPhoPc) and S. typhimurium AroA (STAroA) as well in naive mice. Pooled results from three separate experiments are presented as means ± standard error of the mean. **P < 0·005 when compared with naive mice.
S. typhimurium strains modulate antigen-specific antibody response
Antigen-specific antibody titres were measured in the serum prior to the oral test challenge. BLG-specific IgE titres were increased after oral sensitization in the control group, as observed previously [11], indicating an IgE-type sensitization (Fig. 4a). IgE titres in the STPhoPc-pretreated mice and to a lesser extent in the STAroA group were lower than in the control group (control 8·06 ± 2·6 × 103 AU/ml, STPhoPc 2·8 ± 2·6 × 103 AU/ml, STAroA 6·5 ± 3·7 × 103 AU/ml, P < 0·05 for STPhoPc). Induction of the Th2-type isotype IgG1 was also decreased by STAroA (control 3·9 ± 1·7 × 106 AU/ml, STAroA 0·7 ± 0·3 × 106 AU/ml, P < 0·05) and,similarly to IgE, to a higher extent with STPhoPc (0·3 ± 0·1 × 106 AU/ml, P < 0·005) (Fig. 4b). The Th1-type isotype antibodies IgG2a titres were similar in the control group and in the pretreated groups (Fig. 4c). In addition, STAroA induced a significant increase in IgA antibody titres (2·2 ± 0·9 × 105, P < 0·05 when compared with control mice: 0·5 ± 1·1 × 105 AU/ml) (Fig. 4d). Clearly, the most striking effect on antibodies after preventive administration of either Salmonella strain is the strong inhibition of food-specific IgE antibody by STPhoPc. As IgE is involved in mast cell degranulation in both mice and humans, this provides indirect evidence of potential protection from allergy to the specific food antigen. Similarly, IgG1 could be inhibited strongly by STPhoPc, but also by STAroA. Food challenges in pretreated mice performed thereafter could help to discriminate the specific clinical implication for either antibody type.
Fig. 4.
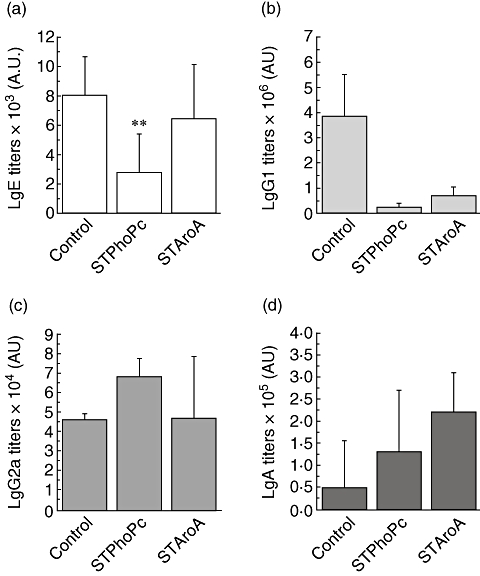
Serum β-lactoglobin-specific immunoglobulin E (IgE) (a), IgG1 (b), IgG2a (c) and IgA antibody (d) titres in control mice and mice pretreated with Salmonella typhimurium PhoPc (STPhoPc) and S. typhimurium AroA (STAroA). Results from a representative experiment are shown. Results presented as means ± standard error of the mean. *P < 0·05, **P < 0·005 when compared with controls.
Antibody production by spleen cells correlate with antibody levels in the serum
The mice were killed 4 days after the fourth sensitization, and spleen cells were isolated. The number of cells producing BLG-specific IgG1, IgG2a and IgA antibodies was counted by ELISPOT. A high number of IgG1-producing cells were induced by BLG in the control group (85·7 ± 13·1 CFU), while the number was much lower in the STPhoPc (56 ± 6·6 CFU, P < 0·05) and in particular in the STAroA group (33 ± 2·8 CFU, P < 0·005) (Fig. 5). In contrast, the number of IgA-secreting cells was increased strongly in the STPhoPc (18·3 ± 5 CFU, P < 0·005) and in the STAroA group (32·7 ± 4·2 CFU, P < 0·005) when compared with the control group (4·2 ± 2·2 CFU). In correlation with the increase of IgG2a antibody titres in the STPhoPc group, the number of IgG2a-producing cells in the spleen was highest in this group (24·7 ± 8·5 CFU, P < 0·05). These data correlate well with the antibody titres found in the serum, and suggest that spleen cells contribute to the production of BLG-specific antibodies found in the serum. We were unable to detect IgE-producing cells because of the low number of antibody-secreting cells and the limit of detection of the method.
Fig. 5.
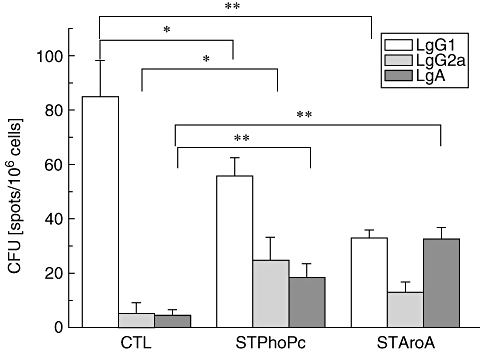
β-lactoglobin-specific immunoglobulin 1 (IgG1), IgG2a and IgA antibody secreting cells in the spleen of control mice (CTL) and mice pretreated with Salmonella typhimurium PhoPc (STPhoPc) or S. typhimurium AroA (STAroA) counted by enzyme-linked immunospot. Combined results from two independent representative experiments are shown. Results presented as means ± standard deviation. *P < 0·05, **P < 0·005.
Oral challenge to BLG
The mice were challenged with BLG on day 28, following four sensitization procedures. In the control (non-pretreated) group, 14 of 20 mice reacted with a score significant for anaphylaxis (scores 2 and 3) (Table 1). The mice pretreated with either STPhoPc or STAroA were also challenged orally with BLG in order to test a potential effect on anaphylaxis. Only eight of 22 reacted significantly in the STPhoPc-treated group showing significantly decreased anaphylaxis scores in these mice (P = 0·036), while 13 of 22 mice reacted in the STAroA group, representing partial protection, although without statistical significance. The measure of body temperature correlated with the clinical scores, as a drop in temperature (i.e. a higher differential value from baseline shown by a lower point on the curve) was observed only in mice with anaphylaxis, i.e. mainly in the control group and to some extent in the STAroA-pretreated group of mice (Fig. 6). Challenges correlated clearly with the results observed above, as only STPhoPc-pretreated mice were protected against food allergy. In these mice high titres of IL-10 were found in the epithelium, as well as strong inhibition of food-specific IgE antibody production. In STAroA-pretreated mice, reduced food-specific IgG1 titres were measured and this might have contributed partly to reduced symptoms of anaphylaxis.
Table 1.
Anaphylactic reactions after oral antigen challenge assessed by symptom scores.
| Anaphylaxis scores at 30 min (no. of mice) | ||||||
|---|---|---|---|---|---|---|
| Groups of mice | Score 0 (non-reactors) | Score 1 (non-reactors) | Total non-reactors (score 0 and 1) | Score 2 (reactors) | Score 3 (reactors) | Total reactors (score 2 and 3) |
| Control mice | 1 | 5 | 6 | 6 | 8 | 14 |
| STPhoPc pretreated | 7 | 7 | 14 | 5 | 3 | 8 |
| STAroA pretreated | 1 | 8 | 9 | 5 | 8 | 13 |
P = 0·036 between control and Salmonella typhimurium PhoPc (STPhoPc); P = not significant between control and S. typhimurium AroA (STAroA). Studies were performed in groups of seven animals and pooled for analysis.
Fig. 6.
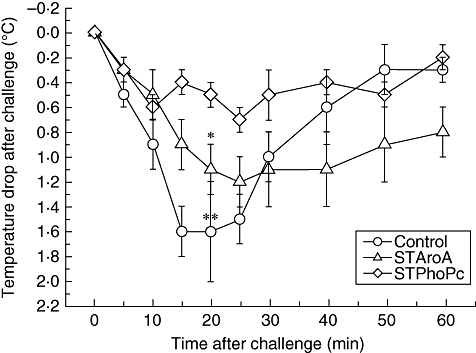
Temperature drop after β-lactoglobulin challenges at day 28 in control mice sensitized to β-lactoglobulin, mice pretreated with Salmonella typhimurium PhoPc (STPhoPc) or S. typhimurium AroA (STAroA). Pooled results from three separate experiments are presented as means ± standard deviation. **P < 0·001, *P < 0·05 when compared with control.
Discussion
Two avirulent S. typhimurium strains were administered orally to mice prior to allergic sensitization to common food antigens. Both strains induced a strain-specific immune response in the gut. When administered prior to sensitization with a food antigen, STPhoPc strongly inhibited food-specific IgG1 and IgE antibody production, correlating with a significant decrease in antigen-induced anaphylaxis, while STAroA inhibited IgG1 strongly and to some extent IgE production, but without a clear preventive effect on anaphylaxis.
Strategies for immunomodulation of the immune response to foods have been studied using various microorganisms. These approaches were driven mainly by clinical and experimental observations suggesting that the infectious environment can influence allergy [20, 21]. In a similar mouse model of food allergy to that used in our studies, Bashir et al. demonstrated that administration of the helminth Heligmosomoides polygirus prevents antigen-specific IgE and IgG1 antibody secretion [22]. They could also demonstrate a role for IL-10, as IL-10-blocking antibodies abrogated the protective effect provided by the helminthic infection. Furthermore, mice pretreated with the helminth were protected partially from antigen-induced anaphylaxis after oral challenge. Similar approaches using other microorganisms were explored by others. Shida et al. injected intraperitoneally a heat-killed Lactobacillus casei strain into ovalbumin-transgenic mice fed ovalbumin for sensitization [23]. They observed a reduction of antigen-specific IgG1 and IgE antibody production, as well as a protective effect of L. casei in pretreated mice. Their experimental protocol, however, differs from ours as the microorganisms were not administered into the gut and no adjuvant was used in the sensitization phase. Other investigators explored the role of bacterial microorganisms for treatment. Li et al. followed a subcutaneous immunotherapy protocol by injecting heat-killed Listeria monocytogenes with a mixture of recombinant peanut allergens into mice previously sensitized orally to peanuts [24]. Treated mice displayed a significant diminution of serum peanut-specific IgE and a raise of IgG2a titres, suggesting a Th1-type effect. In addition, anaphylaxis scores were lower in treated mice using peanut challenge. Interestingly, there was a Th1-type shift in cytokines measured in splenocyte culture supernatants (increased interferon-γ, decreased IL-4, IL-13 and IL-5), but no changes in IL-10 levels. More recently, we observed a clear preventive effect on food-induced anaphylaxis after preventive administration of a Lactococcus lactis transfected to secrete murine IL-10 (LL-rmIL10) directly in the gut of pretreated mice [25]. Similarly to others, this study [23] also showed a potential immunomodulatory effect of non-transfected probiotics on food-specific sensitization in mice.
In the study reported here, we hypothesized that avirulant Salmonella paratyphi strains might have a potential for protective immune modulation in a mouse model of food allergy. The mouse strain used (C3H/HOuJ, different from the C3H/HeJ mice) does not bear the LPS response locus (mutation in Toll-like receptor 4 gene, Tlr4Lps–d) making C3H/HeJ mice endotoxin-resistant. Furthermore, we avoided using C3H/HeJ (Tlr4Lps–d) mice as they are highly susceptible to infection by Gram-negative bacteria such as Salmonella enterica. In experiments reported here with C3H/HOuJ mice, the PhoPc Salmonella strain generated specifically IL-10 secretion by IEL and IL-12 by LPL. Pretreatment with STPhoPc decreased food-specific IgG1 and IgE antibody production significantly. This immunomodulatory shift after STPhoPc correlated with a significant decrease of antigen-induced anaphylaxis, similar to the one observed with other microorganisms in previously published reports [24, 25]. STAroA-pretreated mice showed a different pattern with strongly decreased antigen-specific IgG1 and partly decreased IgE antibody titres in the serum. In addition, STAroA did not activate IL-10 secretion by gut T cells. Interestingly, the specific imprint of the strains tested here had a major influence on the clinical outcome after oral antigen challenge, as inhibition of anaphylaxis was only partial in STAroA and much more effective after pretreatment with STPhoPc. Our impression is that the increased IL-10 induction in IEL by STPhoPc is essential for clinical tolerance, as IL-10 in IEL was similar after STAroA and in control mice. Mice pretreated with STAroA had specifically higher titres of the Th1 cytokine IL-12, as well as increased IgG1 antibody titres. In other reports, STAroA have been inducing consistently a Th1-type skewing [26, 27], as in our study. However, it remains unclear by which mechanisms STAroA and STPhoPc induce a different response in our mouse model of food allergy. It can be suspected that besides a skewing of the T helper response, other mechanisms such as activation of Toll-like receptors may be involved, suggesting further extensive studies. Take together, our results suggest that a Th1-type activation might be partially protective, in particular by reducing Th2-type antibody production, but that a clinical effect is modulated mainly by increased IL-10 in lymphoid structures associated with the gut, as shown earlier [11]. These results go together with the IL-10-driven protective effect by helminths [22].
The results presented above were from a mouse model of food allergy with an IgE-type sensitization and food-induced anaphylaxis similar to that in humans. However, variations in the immune response might appear in humans, and until validation in humans further studies are needed. Nevertheless, we believe that potential applications for Salmonella in allergy prevention are real, as a recent publication reports a similar effect in ovalbumin-sensitized mice pretreated with the attenuated Salmonella strain SL7207 [28], as well as a lower incidence of allergic rhinitis, conjunctivitis and asthma in children infected naturally with Salmonella during infancy [29].
Acknowledgments
The study was funded by Grants 3200-107752 and 3200-065203 from the Swiss National Science Foundation, the Helmut Horten Foundation and the Gertrude von Meissner Foundation. We also wish to thank Menno Kok for providing the bacterial strains tested here.
References
- 1.Moneret-Vautrin DA, Morisset M, Flabbee J, Beaudouin E, Kanny G. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy. 2005;60:443–51. doi: 10.1111/j.1398-9995.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen LK. In search of a new paradigm: mechanisms of sensitization and elicitation of food allergy. Allergy. 2005;60:549–58. doi: 10.1111/j.1398-9995.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 3.Arshad SH. Food allergen avoidance in primary prevention of food allergy. Allergy. 2001;56(Suppl. 67):113–16. doi: 10.1034/j.1398-9995.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- 4.Eigenmann PA. Future therapeutic options in food allergy. Allergy. 2003;58:1217–23. doi: 10.1046/j.1398-9995.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 5.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein antigen and cholera toxin. J Immunol. 1994;153:647–57. [PubMed] [Google Scholar]
- 6.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow's milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–14. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 7.Li XM, Serebrisky D, Lee SY, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 8.Frossard CP, Hauser C, Eigenmann PA. Oral carrageenan induces antigen-dependent oral tolerance: prevention of anaphylaxis and induction of lymphocyte anergy in a murine model of food allergy. Pediatr Res. 2001;49:417–22. doi: 10.1203/00006450-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Teuber SS, Del Val G, Morigasaki S, et al. The atopic dog as a model of peanut and tree nut food allergy. J Allergy Clin Immunol. 2002;110:921–7. doi: 10.1067/mai.2002.130056. [DOI] [PubMed] [Google Scholar]
- 10.Adel-Patient K, Ah-Leung S, Creminon C, et al. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin Exp Allergy. 2005;35:539–46. doi: 10.1111/j.1365-2222.2005.02225.x. [DOI] [PubMed] [Google Scholar]
- 11.Frossard CP, Tropia L, Hauser C, Eigenmann PA. Lymphocytes in Peyer's patches regulate clinical tolerance in a murine model of food allergy. J Allergy Clin Immunol. 2004;113:958–64. doi: 10.1016/j.jaci.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Frossard CP, Hauser C, Eigenmann PA. Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J Allergy Clin Immunol. 2004;114:377–82. doi: 10.1016/j.jaci.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Stocker BA, Hoiseth SK, Smith BP. Aromatic-dependent ‘Salmonella sp.’ as live vaccine in mice and calves. Dev Biol Stand. 1983;53:47–54. [PubMed] [Google Scholar]
- 14.Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–73. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hormaeche CE, Joysey HS, Desilva L, Izhar M, Stocker BA. Immunity conferred by Aro-Salmonella live vaccines. Microb Pathog. 1991;10:149–58. doi: 10.1016/0882-4010(91)90075-l. [DOI] [PubMed] [Google Scholar]
- 16.Dreher D, Kok M, Cochard L, et al. Genetic background of attenuated Salmonella typhimurium has profound influence on infection and cytokine patterns in human dendritic cells. J Leuk Biol. 2001;64:583–9. [PubMed] [Google Scholar]
- 17.Miller SI, Mekalanos JJ. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–90. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefrancois L. Carbohydrate differentiation antigens of murine T cells: expression on intestinal lymphocytes and intestinal epithelium. J Immunol. 1987;138:3375–84. [PubMed] [Google Scholar]
- 19.Adel-Patient K, Creminon C, Bernard H, et al. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J Immunol Methods. 2000;235:21–32. doi: 10.1016/s0022-1759(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 20.Strachan DP, Taylor EM, Carpenter RG. Family structure, neonatal infection, and hay fever in adolescence. Arch Dis Child. 1996;74:422–6. doi: 10.1136/adc.74.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 22.Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284–92. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- 23.Shida K, Takahashi R, Iwadate E, et al. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin Exp Allergy. 2002;32:563–70. doi: 10.1046/j.0954-7894.2002.01354.x. [DOI] [PubMed] [Google Scholar]
- 24.Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003;170:3289–95. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- 25.Frossard CP, Steidler L, Eigenmann PA. Oral administration of an IL-10-secreting Lactococcus lactis strain prevents food-induced IgE sensitization. J Allergy Clin Immunol. 2007;119:952–9. doi: 10.1016/j.jaci.2006.12.615. [DOI] [PubMed] [Google Scholar]
- 26.Lillard JW, Boyaka PN, Singh S, McGhee JR. Salmonella-mediated mucosal cell-mediated immunity. Cell Mol Biol (Noisy-le-Grand) 2001;47:1115–20. [PubMed] [Google Scholar]
- 27.Raupach B, Kaufmann SH. Bacterial virulence, proinflammatory cytokines and host immunity: how to choose the appropriate Salmonella vaccine strain? Microbes Infect. 2001;3:1261–9. doi: 10.1016/s1286-4579(01)01486-1. [DOI] [PubMed] [Google Scholar]
- 28.Wu CJ, Chen LC, Kuo ML. Attenuated Salmonella typhimurium reduces ovalbumin-induced airway inflammation and T-helper type 2 responses in mice. Clin Exp Immunol. 2006;145:116–22. doi: 10.1111/j.1365-2249.2006.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelosi U, Porcedda G, Tiddia F, et al. The inverse association of salmonellosis in infancy with allergic rhinoconjunctivitis and asthma at school-age: a longitudinal study. Allergy. 2005;60:626–30. doi: 10.1111/j.1398-9995.2005.00747.x. [DOI] [PubMed] [Google Scholar]


