Abstract
Dendritic cells (DCs) can acquire unique features or phenotypes in different tissue microenvironments and decide whether immunity or tolerance develops. DCs observed within the decidua have been implicated in pregnancy maintenance. However, the precise distribution of decidual DC subsets and their phenotypic characteristics are largely unknown. Using flow cytometry, we identified three DC subsets in normal human first-trimester decidua: BDCA-1+ CD19- CD14- myeloid DC type 1 (MDC1), BDCA-3+ CD14- myeloid DC type 2 (MDC2) and BDCA-2+ CD123+ plasmacytoid DC (PDC). The percentage of MDC1 to mononuclear cells in the decidua was similar to that in the peripheral blood controls. The percentage of MDC2 in the decidua was significantly higher than that in the peripheral blood controls, whereas the percentage of PDC was significantly lower. Both MDC1 and MDC2 subsets expressed human leucocyte antigen D-related, CD86 and CD80 at low levels, suggesting a characteristic of immature myeloid DCs. Immunoglobulin-like transcript 3, suggested to be involved in immune tolerance induction, was also expressed on decidual MDC1 and MDC2 subsets. In addition, as gestational age increased from 6 to 9 weeks, the numbers of MDC1 decreased but MDC2 increased significantly. This is the first study to demonstrate the presence of three previously unidentified BDCA-1+, BDCA-3+ and BDCA-2+ DC subsets in human decidua, these decidual DCs might play important role in the maintenance of pregnancy.
Keywords: BDCA, decidua, dendritic cells, ILT3, pregnancy
Introduction
For a successful human pregnancy, the balance between defensive immunity against pathogens and tolerance of its fetus at the site of contact between mother and child, which is the decidua, is of crucial importance. Serving as an immunologically privileged tissue, the decidua and its components, especially decidual leucocytes, play essential functions in pregnancy maintenance [1]. The decidual leucocyte population has been a centre of interest for the understanding of the mechanism that might control maternal immune responses in successful pregnancy [2–5]. During the first trimester of pregnancy, the human decidua is rich in leucocytes which make up 10–15% of all decidual cells. This leucocyte population is composed of 70% uterine natural killer cells, 10% T cells and 20% major histocompatibility complex (MHC) class II-positive antigen-presenting cells (APCs), which are thought to be mainly macrophages [3, 4].
Interestingly, the human decidua was described recently to harbour dendritic cells (DCs), which were considered as suitable candidates for mediating the necessary balance of maternal immune responses to fetal allograft in maternal–fetal interaction [5–9]. DCs are specialist APCs that originate from the bone marrow and play critical roles in the initiation and direction of immune responses [10, 11]. Accumulating evidence suggests that DCs in situ can induce antigen-specific unresponsiveness or tolerance in central lymphoid organs and in the periphery [12]. Most importantly, different DCs subsets may play a prominent role in dictating the quantity and quality of immune responses [13]. The presence of DCs in the decidua has pointed to a critical role of DCs at the fetal–maternal interface [14]. Recently, it has been demonstrated that the immature myeloid DC subpopulation in human decidua might induce immune tolerance [7] or promote a T helper type 2 (Th2)-dominant state [8], leading to maintenance of pregnancy. In addition, the CD83+ DC subpopulation has been identified in the decidua, indicating the presence of immunostimulatory mature DC subsets [5, 6].
In the past, the investigation of DC subsets in the human decidua has been hampered by the lack of specific markers identifying DCs directly and by the scarcity of DCs. Several groups have reported the presence of certain DC subsets at the fetal–maternal interface; however, the precise distributional and phenotypic characteristics of DC subsets in the human decidua are still poorly understood. DCs represent only 1–2% of circulating peripheral blood mononuclear cells (PBMC) and approximately 1·0% of all cells in the isolates of all decidual cells [7]. Methods for the detection and isolation of DCs are based commonly on a multitude of immunophenotypic criteria, such as the absence of certain leucocyte lineage (Lin)-specific markers (e.g. CD3, CD14, CD19, CD16 and CD56) and the presence of human leucocyte antigen D-related (HLA-DR), CD4 or CD33. Two distinct lineages of DC subsets, myeloid DC (MDC) and plasmacytoid DC (PDC), have been characterized in humans. MDC are characterized as Lin– HLA-DR+ CD11cbright and also express myeloid markers (CD13 and CD33) as well as Fc receptors [CD32, CD64 (FcγRI) and FcεRI] with monocytoid morphology in appearance. PDC are characterized as Lin- HLA-DR+ CD123bright, expressing neither myeloid lineage markers nor Fc receptors [15].
Recently, Dzionek et al. [16] identified three blood dendritic cell antigens (BDCA): BDCA-1 (CD1c), BDCA-2 (CD303) and BDCA-3 (CD141), which were found to be markers for distinct subsets of DCs in human peripheral blood. Based on analyses of monoclonal antibodies (MoAbs) directed against BDCA, freshly isolated blood DCs can be divided into three subsets: two myeloid subsets–myeloid type 1 (MDC1) and myeloid type 2 (MDC2), and a plasmacytoid DC (PDC) subpopulation. The use of the new BDCA-1, BDCA-2 and BDCA-3 MoAbs provides an unique opportunity to detect, enumerate and isolate BDC populations precisely from whole blood or peripheral tissues directly without apparent functional perturbation [16, 17]. This will be a valuable aid for determining the presence of DCs and their phenotypes in human decidua. The aim of this study was to identify and characterize DCs in the cell isolates obtained from normal human first-trimester decidua with the recently developed BDCA markers. Whether the number of these DC subsets changes with gestational age was also investigated.
Materials and methods
Tissue and blood samples
The first-trimester decidua (n = 44) at 6–9 weeks of gestational age were obtained from clinically normal pregnancies, which were terminated at the Obstetrics and Gynaecology Department of Qilu Hospital. The total decidual tissue was placed immediately into ice-cold RPMI-1640 and kept for no more than 30 min before subsequent cell isolation. Among these induced abortion cases, 26 peripheral blood samples were collected simultaneously from the same women. Informed consent was obtained from all subjects, and this investigation was approved by the Shandong University Human Investigation Committee.
Decidual and peripheral blood mononuclear cell preparations
For isolation of decidual cells, a non-enzymatic method was used as described previously [7, 18]. Briefly, the decidual tissue was dissected thoroughly, free of products of conception and blood clots, washed twice in cold RPMI-1640 and minced finely into fragments of ∼1 mm3. The chopped tissue was ground in a small volume of cold RPMI-1640 with a 20-ml syringe plunger, diluted with more cold RPMI-1640 until the undissociated tissue pieces had settled. The above procedure was repeated several times and the supernatants were collected. To minimize activation of DCs during the isolation procedure, the whole operation was performed on ice. The cell suspension obtained by this method was passed through a series of stainless steel wire mesh sieves from 125-μm to 74-μm, and washed once in ice-cold phosphate-buffered saline (PBS). The decidual mononuclear cells were then isolated by density gradient centrifugation over a standard Ficoll-Hypaque (1·077, Pharmacia) and washed twice in cold PBS. PBMC were isolated by the standard Ficoll-Hypaque method. All the mononuclear cell suspensions obtained above were labelled immediately for subsequent flow cytometric analysis.
Flow cytometry
Freshly isolated mononuclear cells were stained by direct immunofluorescence for three-colour flow cytometry. The MoAbs used in this study are listed in Table 1. For each cell surface marker, corresponding isotype-matched control antibody conjugated with the same fluorescent dye (all from eBioscience, San Diego, CA, USA) were also used. Aliquots of 2× to 5 × 106 cells were resuspended in 80 μl of PBS containing 0·5% bovine serum albumin (BSA) and 20 μl of FcR blocking reagent (Miltenyi-Biotec, Bergisch Gladbach, Germany). MoAbs were added according to the manufacturer's protocol and incubated with the cells for 20 min in the dark at 4°C. After incubation, the cells were washed once in PBS, resuspended in 600 μl of PBS and then analysed with a fluorescence activated cell sorter (FACS) Calibur™ flow cytometer using CELLQuest software™ (BD Biosciences, San Jose, CA, USA). A total of 100 000 events per sample were collected.
Table 1.
Antibodies used for flow cytometry.
| Antibody | Fluorochrome | Clone | Species | Isotype | Source |
|---|---|---|---|---|---|
| BDCA-1 | FITC | AD5–8E7 | Mouse | IgG2α | Miltenyi Biotec, Bergisch Gladbach, Germany |
| BDCA-2 | FITC | AC144 | Mouse | IgG1 | Miltenyi Biotec |
| BDCA-3 | FITC | AD5–14H12 | Mouse | IgG1 | Miltenyi Biotec |
| CD14 | PE | M5E2 | Mouse | IgG2α | BD Pharmingen™, San Diego, CA, USA |
| CD19 | PE | SJ25C1 | Mouse | IgG1 | Becton-Dickinson, San Jose, CA, USA |
| CD123 | PE | 9F5 | Mouse | IgG1 | Becton-Dickinson |
| HLA-DR | PE-Cy5 | G46-6(L243) | Mouse | IgG2αk | BD Pharmingen™ |
| CD80 | PE-Cy5 | 2D10·4 | Mouse | IgG1 | eBioscience, San Diego, CA, USA |
| CD86 | PE-Cy5 | IT2·2 | Mouse | IgG2b | eBioscience |
| ILT-3 | PE-Cy5 | ZM3·8 | Mouse | IgG1 | Beckman Coulter, Marseille, France |
FITC: fluorescence activated cell sorter; HLA-DR: human leucocyte antigen D-related; ILT3: immunoglobulin-like transcript 3; PE: phycoerythrin.
Statistical analysis
The distributional and phenotypic differences were analysed by Mann–Whitney U-test and paired t-test, respectively. One-way analysis of variance followed by Neuman–Keuls analysis as a post-hoc test was used to assess the differences in the percentages of DC subsets by gestational age. A P-value < 0·05 was considered to be statistically significant. All analysis was performed using spss version 11·5 for Windows (SPSS, Chicago, IL, USA).
Results
BDCA-1+, BDCA-3+ and BDCA-2+ DC subsets in decidua
Flow cytometry was used to analyse the cell preparations freshly isolated by the non-enzymatic method. As shown in Fig. 1a, the R1 gate was drawn based on scatter signals to exclude cell debris and dead cells. The events falling in the R1 gate were then analysed in terms of staining for BDCA-1, CD14, CD19, BDCA-3, CD14, BDCA-2 and CD123. BDCA-1+ CD14– CD19– cells were characterized as MDC1 (Fig. 1b), BDCA-3+ CD14– cells were counted as MDC2 (Fig. 1c) and BDCA2+ CD123+ cells were identified as PDC (Fig. 1d). The percentage of MDC1 to mononuclear cells in the decidua was similar to that in the peripheral blood controls (median, 0·20%; range, 0·10–0·33% versus median, 0·27%; range 0·19–0·33%; P = 0·055) (Fig. 2a). The percentage of MDC2 in the decidua was significantly higher than that in the peripheral blood (median, 0·53%; range, 0·29–0·63% versus median, 0·13%; range, 0·10–0·19%; P < 0·001) (Fig. 2b), whereas the percentage of PDC in the decidua was significantly lower than that in the peripheral blood (median, 0·11%; range, 0·09–0·14% versus median, 0·32%; range, 0·26–0·36%; P < 0·001) (Fig. 2c).
Fig. 1.
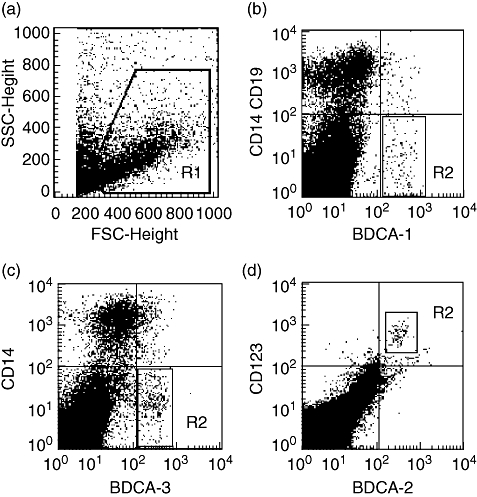
Gating strategy used to identify BDCA-1+, BDCA-3+ and BDCA-2+ dendritic cell (DC) subsets in decidua by flow cytometry. (a) The R1 gate was drawn on the basis of forward and side scatter (FSC and SSC) to exclude cell debris and dead cells. (b) The events falling in the R1 gate were analysed in terms of staining for BDCA-1, CD14 and CD19, and BDCA-1+CD14-CD19- cells were characterized as myeloid DC type 1 (MDC1). (c) The R1-gated events were then analysed in terms of staining for BDCA-3 and CD14, and BDCA-3+ CD14– cells were characterized as myeloid DC type 2 (MDC2). (d) The R1-gated events were then analysed in terms of staining for BDCA-2 and CD123, and BDCA-2+ CD123+ cells were characterized as plasmacytoid DC (PDC). Representative stainings from one sample are shown.
Fig. 2.
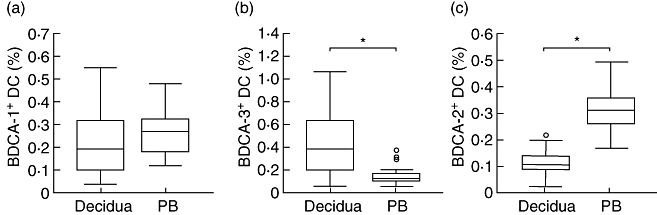
BDCA+ dendritic cell (DC) subsets percentages in decidua (n = 44) and peripheral blood (PB) controls (n = 26). Percentages of (a) BDCA-1+, (b) BDCA-3+ and (c) BDCA-2+ DC subsets to decidual mononuclear cells in decidua and the corresponding DC subpopulations to peripheral blood mononuclear cells in peripheral blood controls were analysed by flow cytometry. Results are presented in box-and-whisker plots, which demonstrate the median (horizontal lines within the boxes), the quartiles (boxes), the smallest and greatest values (whiskers outside boxes) and the outliers (small circles) in the distribution. *P < 0·05.
Phenotypes of BDCA-1+ and BDCA-3+ DC subsets
To characterize further the phenotypes of fresh decidual DCs, flow cytometric analysis was performed with a panel of MoAbs including HLA-DR, CD80, CD86 and immunoglobulin-like transcript 3 (ILT3). Based on the gating strategy shown in Fig. 1, the events falling in R2 gate were then analysed in terms of staining for these MoAbs. As informative markers involved in antigen presentation and T cell co-stimulation, HLA-DR, CD86 and CD80 were observed to be expressed on the MDC1 subset from decidua at low levels, which were similar to that from peripheral blood (P = 0·389, P = 0·250 and P = 0·192, respectively) (Fig. 3a, b). The expression of HLA-DR and CD80 on MDC2 subset in the decidua was significantly lower than that in the peripheral blood (P = 0·005 and P = 0·002, respectively), while the expression of CD86 on the MDC2 subset did not differ significantly in decidua compared to peripheral blood (P = 0·053) (Fig. 3a, b). In addition, as one of the most important markers involved in immune tolerance induction, ILT3 (also known as CD85K, LIR5) was shown to be expressed consistently on MDC1 and MDC2 subsets [median MFI 51·50; range 39·06–62·93 (n = 17), median MFI 42·01; range 37·19–52·08 (n = 15), respectively] (Fig. 3c).
Fig. 3.
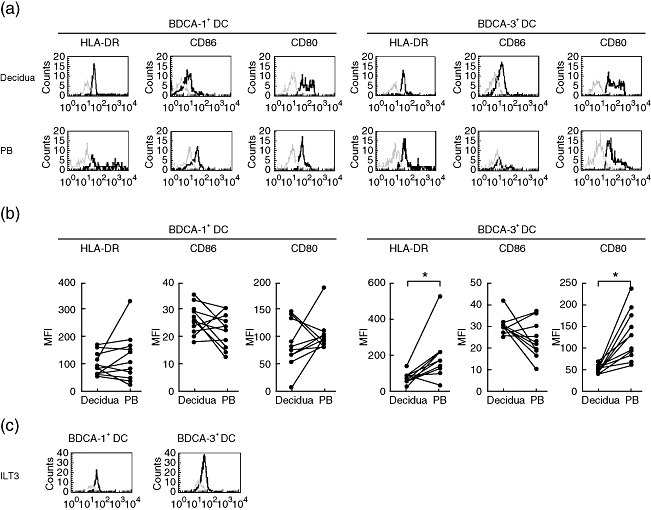
Expression of humal leucocyte antigen D-related (HLA-DR), CD86 and CD80 on BDCA-1+ dendritic cell (DC) and BDCA-3+ DC subsets. (a) Expression of these antigens on BDCA-1+ DC and BDCA-3+ DC subsets from decidua as well as from peripheral blood (PB) controls were analysed. Representative stainings from one sample are shown. (b) The geometric mean fluorescence intensity of these antigens on BDCA-1+ DC (n = 11) and BDCA-3+ DC (n = 11) subsets from decidua were compared with PB controls. Dots linked with a line represent samples from the same individual. (c) Expression of immunoglobulin-like transcript 3 on BDCA-1+ DC and BDCA-3+ DC subsets from decidua were analysed. Representative stainings from one sample are shown.The bold lines show cells staining with the indicated markers, and the faint lines show cells staining with isotype matched controls. *P < 0·05.
Number of changes of different DC subsets in decidua with gestational age
In order to investigate further whether the numbers of different DC subsets in decidua change with gestational age, we analysed the percentages of DCs at different gestational ages. We found that the percentage of MDC1 at 8 weeks' gestation was significantly lower than that at 6 and 7 weeks' gestation (all P-values < 0·05, Fig. 4a). In addition, a significantly lower percentage of MDC1 was observed at 9 weeks' gestation compared to that at 6 and 7 weeks' gestation (all P-values < 0·05, Fig. 4a). Conversely, the percentage of MDC2 at 8 and 9 weeks' gestation was significantly higher than that at 6 and 7 weeks' gestation (all P-values < 0·05, Fig. 4b). This finding suggested that as gestational age increased from 6 to 9 weeks, the numbers of MDC1 decreased but MDC2 increased significantly, whereas no significant differences were observed between different weeks of pregnancy with regard to the percentage of PDC in decidua (P > 0·05, Fig. 4c).
Fig. 4.
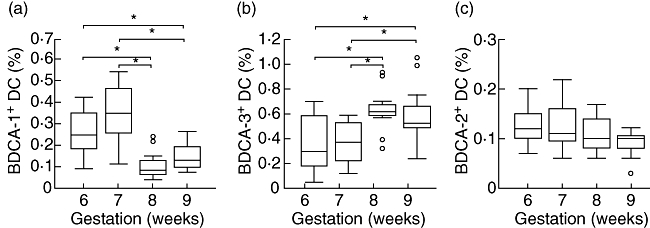
Number changes of decidual BDCA-1+, BDCA-3+ and BDCA-2+ dendritic cell (DC) subsets with gestational age. As gestational age increased from 6 to 9 weeks, the numbers of (a) BDCA-1+ DC subsets decreased, (b) but BDCA-3+ DC subsets increased significantly. There were no significant differences of BDCA-2+ DC subsets with increasing gestational age (for each weeks of gestation n = 11). Results are presented in box-and-whisker plots, which demonstrate the median (horizontal lines within the boxes), the quartiles (boxes), the smallest and greatest values (whiskers outside boxes) and the outliers (small circles) in the distribution. *P < 0·05.
Discussion
In this study, by using the appropriate gating strategy and selecting recently developed DCs specific surface markers we identified three BDCA+ DC subsets from normal human first-trimester decidua: MDC1 (BDCA-1+ CD19- CD14-), MDC2 (BDCA-3+ CD14-) and PDC (BDCA-2+ CD123+). To our knowledge, this is the first study to demonstrate the presence of three previously unidentified DC subsets in the human decidua. One could argue that BDCA-1+ cells are considered falsely as DCs, because BDCA-1 is also expressed on a minor subset of human B lymphocytes, and some BDCA-1+ cells in blood are weakly positive for CD14. However, by excluding CD19+ B cells and CD14+ monocytes from analysis, one can conclude that BDCA-1+ CD19- CD14- cells are true MDC1. In addition to MDC2, BDCA-3 is also expressed at low levels on CD14+ monocytes in peripheral blood, so CD14+ monocytes were excluded from this analysis and BDCA-3+ CD14- cells could be counted as true MDC2, whereas BDCA-2 is expressed exclusively on PDC and co-expression of BDCA-2 and CD123 is referred to straightforwardly as PDC.
We found that MDC1 accounted for ∼0·20% of mononuclear cells in the isolates of all first-trimester decidual cells, and the proportion was similar to that of the peripheral blood. In contrast, two previous studies [7, 8] reported that the percentage of Lin- HLA-DR+ CD11c+ myeloid DCs in the decidua was ∼1%, which was significantly higher than that in peripheral blood. The discrepancies may be explained by different methods were used for DC evaluation. In the two previous studies, the DCs in decidua were analysed based on a negative selection system. In the present study, we used anti-BDCA-1 MoAb to identify the populations of decidual DCs.
The detailed functions of MDCs in decidua are largely unknown; the most interesting and attractive function of decidual DCs may be of sampling and presenting trophoblast antigens to T lymphocytes, and inducing certain forms of immunity responsible for the maintenance of pregnancy [7]. DCs can acquire unique features or phenotypes in the mucosal tissues microenvironment to regulate specific local immune functions. The DCs observed within the decidua, a highly specialized mucous membrane, have been proposed as the ‘gatekeepers’ of the decidua, inducing tolerance under normal physiological conditions [19]. Thus, the subpopulation, the maturation state and the nature of the decidual DCs may be critical in T cell allorecognition of trophoblast antigens. It has been reported that the function of DCs can be characterized partly by the dynamic regulation of co-stimulatory molecules (CD86, CD80) and of HLA-DR [20, 21]. We found that decidual MDC1 subset expressed HLA-DR, CD86 and CD80 at low levels, which is consistent with previous reports of the immature characteristics of decidual MDC subset [7]. Until recently, immature DCs (iDCs) were believed to control peripheral tolerance by inducing the differentiation of human T regulatory (Treg) cells [22, 23].
ILT3 is a novel cell surface molecule of the immunoglobulin superfamily, which is expressed selectively by professional APCs such as monocytes, macrophages and DCs. Similar to other members of the ILT family (ILT2 and ILT4), ILT3 contains putative immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which recruit inhibitory phosphatases and transduce a negative signal into the cell [24, 25]. Most importantly, it has been shown that high expression of ILT3 and ILT4 is crucial to the tolerogenic capacity acquired by DCs, and is a general feature of tolerogenic DCs [26, 27]. We demonstrated that ILT3 was expressed on this immature MDC1 subset, suggesting a tolerogenic characteristic of this subpopulation. Our result is consistent with a previous report that DCs can be rendered tolerogenic by CD8+ CD28– alloantigen-specific T suppressor (Ts) cells by inducing the up-regulation of ILT3 and ILT4 and down-regulation of co-stimulatory molecules on DCs [28]. Such tolerogenic ILT3high ILT4high DCs anergize alloreactive CD4+ CD45RO+ CD25+ T cells converting them into Treg cells which, in turn, continue the cascade of suppression by tolerizing other DCs [26, 27]. Increasing evidence suggests that DCs and T cells interaction at the fetal–maternal interface might play a key role in pregnancy tolerance.
MDC2 is a novel subset of MDC and is similar to MDC1 with respect to phenotype, morphology, endocytic capacity and maturation requirements. However, there are some striking differences; for example, MDC2 does not express the Fc receptors, and the lack of Fc receptor expression indicates that MDC2, unlike MDC1, does not have the capacity of immunoglobulin (Ig)-mediated antigen uptake [16]. A very interesting new finding in this study is that the presence of the MDC2 subset in human decidua and this subset constituted a significant part of the decidual DC population. Similar findings were reported by Demedts et al. [29] in lung resection specimens and Tsoumakidou et al. [30] in human bronchoalveolar lavage fluid. In contrast, it has been shown that MDC2 account for only approximately 0·4% of human PBMC and 3% of peripheral blood DC population. One possible explanation for the increased numbers of MDC2 is that the local environment at the fetal–maternal interface is responsible for accumulation of these cells in the decidua. In keeping with our data of the expression of ILT3 on MDC2, Velten et al. [31] recently identified an ILT2highI LT3high BDCA-3high subset of interleukin (IL)-10-induced DCs with reduced allostimulatory capacity in vitro, suggesting a feature of tolerogenic DCs. It is possible that recruitment of MDC2 in the decidua might be related to a reduced capacity to recognize trophoblast antigens. This is an intriguing hypothesis that might be worthy of further investigation.
There have been some conflicting data in the literature concerning the presence of PDC in human decidua. We identified BDCA-2+ CD123+ PDC consistently in decidua, which has confirmed a previous report [8] that human decidua harbour Lin– HLA–DR+ CD123+ PDC, although Gardner et al. [7] could not detect this PDC subset in decidual tissue. The discrepancies between these results could be related to the scarcity of PDC in decidua or the use of different methods to identify the PDC subpopulation. PDC after appropriate activation can induce T cell differentiation into Th2 cells [32], and have been implicated in performing a tolerogenic function [33, 34]. Moreover, there is evidence that the percentage of IL-12-producing PDC in decidua was significantly lower than those in peripheral blood, and this decreased IL-12 production could facilitate optimal Th2 response development in decidua [8]. It is possible that PDC at the fetal–maternal interface might contribute to the maintenance of pregnancy.
Decidual DCs probably function to co-ordinate the temporal and spatial immunological shifts necessary for the implantation and progression of pregnancy [19]. We observed that the numbers of MDC1 decreased but MDC2 increased significantly as gestational age increased from 6 to 9 weeks. This finding is in keeping with the suggestion that frequency and subset of decidual DCs were finely tuned in a timely manner during pregnancy and decidualization, as the numbers of immunostimulatory CD83+ DC subsets in the decidua of normal pregnancies decreased significantly with increasing gestational age (7–14 weeks) [5, 35, 36]. In addition, it has been documented that a number of immune and endocrine signals involved in the biology of pregnancy ultimately determine the maturation, differentiation and activation status of DCs. In turn, these changes are responsible for the dual capacity of DCs to trigger an immune response, which is associated with fetal rejection, or to promote cell tolerance at the fetal–maternal interface. Interestingly, many of the signals involving rendering DCs tolerogenic have been shown to be in abundance at the fetal–maternal interface, especially in early gestation. These include Treg cells, Ts cells, transforming growth factor-β, IL-10, HLA-G and 1,25(OH)2D3 [37–42]. For example, the expression of ILT3 on MDC has been shown to be up-regulated by the local cellular and molecular network [42]. The role of these potent DC subsets for immunoregulation of human pregnancy awaits further studies. It is tempting to speculate upon the gene-expressing profiles of the purified decidual DCs for understanding the detailed functions they may perform in the maintenance of pregnancy.
Several DC subsets consisting of CD83+ DCs and Lin- HLA-DR+ CD11c+ myeloid DCs with differing functional and phenotypic characteristics have been identified in human decidua. It is most important to remember that the enzymic digestion method after a period of in vitro culture used in these studies might induce phenotypical and functional changes in DCs. DCs are extremely sensitive to such in vitro manipulations, so the characteristics of the isolated DCs may not reflect the true in vivo situation. In order to avoid the possible effect of enzymatic treatment on the fluorescence intensity of surface antigens, a rapid mechanical method was used in the present study [7, 18]. However, one potential problem with the non-enzymatic method of extraction used is that DCs may be more likely to remain clumped or adherent to other cell types, and hence under-represented in the gated populations studied. However, Gardner et al. [7] compared these two methods for decidual leucocyte preparations and found that the mean total yield of cells and phenotypes of DCs was similar for each of the dissociation methods. In addition, one of the difficulties with analysing the data is that the DCs represent such a small subpopulation of the total decidual leucocytes that results are inevitably very variable. Further work will be required to enrich BDCA+ DC subsets by depletion of T cells, monocytes and natural killer (NK) cells so that most of these problems might be avoided.
In conclusion, we have demonstrated the presence of the three previously unidentified BDCA+ DC subsets in normal human first-trimester decidua: BDCA-1+ CD19- CD14- MDC1, BDCA-3+ CD14- MDC2 and BDCA-2+ CD123+ PDC. The distributional and phenotypic characteristics of these decidual DC subsets may be relevant to the immune tolerance necessary for the maintenance of pregnancy. Therefore, this study provides a basis for further research of the roles of DCs in immunoregulation of human pregnancy at the fetal–maternal interface.
Acknowledgments
This work was supported partly by National Natural Science Foundation of China to Beihua Kong (no. 30571953) and to Kun Song (no. 30700897) and Science and Technology Financial Special Foundation from Shandong Province to Beihua Kong (no. SDSP2005-0410-06).
References
- 1.Loke YW, King A, Burrows TD. Decidua in human implantation. Hum Reprod. 1995;10(Suppl. 2):14–21. doi: 10.1093/humrep/10.suppl_2.14. [DOI] [PubMed] [Google Scholar]
- 2.Billingham RE. Transplantation immunity and the maternal-fetal relation. New Engl J Med. 1964;270:667–72. doi: 10.1056/NEJM196403262701306. [DOI] [PubMed] [Google Scholar]
- 3.Mincheva-Nilsson L, Baranov V, Yeung MM, Hammarstrom S, Hammarstrom ML. Immunomorphologic studies of human decidua-associated lymphoid cells in normal early pregnancy. J Immunol. 1994;152:2020–32. [PubMed] [Google Scholar]
- 4.Loke YW, King A. Human implantation. Cell biology and immunology. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 5.Askelund K, Liddell HS, Zanderigo AM, et al. CD83(+) dendritic cells in the decidua of women with recurrent miscarriage and normal pregnancy. Placenta. 2004;25:140–5. doi: 10.1016/S0143-4004(03)00182-6. [DOI] [PubMed] [Google Scholar]
- 6.Kammerer U, Schoppet M, McLellan AD, et al. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol. 2000;157:159–69. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod. 2003;69:1438–46. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki S, Tsuda H, Sakai M, et al. Predominance of Th2-promoting dendritic cells in early human pregnancy decidua. J Leukoc Biol. 2003;74:514–22. doi: 10.1189/jlb.1102566. [DOI] [PubMed] [Google Scholar]
- 9.Rieger L, Honig A, Sutterlin M, et al. Antigen-presenting cells in human endometrium during the menstrual cycle compared to early pregnancy. J Soc Gynecol Invest. 2004;11:488–93. doi: 10.1016/j.jsgi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Hart DNJ. Dendritic cells: unique leucocyte populationswhich control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 12.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 13.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–16. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 14.Juretic K, Strbo N, Crncic TB, Laskarin G, Rukavina D. An insight into the dendritic cells at the maternal–fetal interface. Am J Reprod Immunol. 2004;52:350–5. doi: 10.1111/j.1600-0897.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 15.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 17.Narbutt J, Lesiak A, Zak-Prelich M, et al. The distribution of peripheral blood dendritic cells assayed by a new panel of anti-BDCA monoclonal antibodies in healthy representatives of the polish population. Cell Mol Biol Lett. 2004;9:497–509. [PubMed] [Google Scholar]
- 18.Coates PT, Barratt-Boyes SM, Zhang L, et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–21. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 19.Blois SM, Kammerer U, Alba Soto C, et al. Dendritic cells: key to fetal tolerance? Biol Reprod. 2007;77:590–8. doi: 10.1095/biolreprod.107.060632. [DOI] [PubMed] [Google Scholar]
- 20.McLellan AD, Starling GC, Williams LA, Hock BD, Hart DN. Activation of human peripheral blood dendritic cells induces the CD86 co-stimulatory molecule. Eur J Immunol. 1995;25:2064–8. doi: 10.1002/eji.1830250739. [DOI] [PubMed] [Google Scholar]
- 21.Nijman HW, Kleijmeer MJ, Ossevoort MA, et al. Antigen capture and major histocompatibility class II compartments of freshly isolated and cultured human blood dendritic cells. J Exp Med. 1995;182:163–74. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 24.Cella M, Dohring C, Samaridis J, et al. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med. 1997;185:1743–51. doi: 10.1084/jem.185.10.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–9. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 26.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 27.Manavalan JS, Rossi PC, Vlad G, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–58. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 28.Kim-Schulze S, Scotto L, Vlad G, et al. Recombinant Ig-like transcript 3-Fc modulates T cell responses via induction of Th anergy and differentiation of CD8+ T suppressor cells. J Immunol. 2006;176:2790–8. doi: 10.4049/jimmunol.176.5.2790. [DOI] [PubMed] [Google Scholar]
- 29.Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol. 2005;32:177–84. doi: 10.1165/rcmb.2004-0279OC. [DOI] [PubMed] [Google Scholar]
- 30.Tsoumakidou M, Tzanakis N, Papadaki HA, Koutala H, Siafakas NM. Isolation of myeloid and plasmacytoid dendritic cells from human bronchoalveolar lavage fluid. Immunol Cell Biol. 2006;84:267–73. doi: 10.1111/j.1440-1711.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 31.Velten FW, Duperrier K, Bohlender J, Metharom P, Goerdt S. A gene signature of inhibitory MHC receptors identifies a BDCA3(+) subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro. Eur J Immunol. 2004;34:2800–11. doi: 10.1002/eji.200324732. [DOI] [PubMed] [Google Scholar]
- 32.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 33.Kuwana M, Kaburaki J, Wright TM, Kawakami Y, Ikeda Y. Induction of antigen-specific human CD4(+) T cell anergy by peripheral blood DC2 precursors. Eur J Immunol. 2001;31:2547–57. doi: 10.1002/1521-4141(200109)31:9<2547::aid-immu2547>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 34.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi-Tehrani M. Kinetics of murine decidual dendritic cells. Reproduction. 2007;133:275–83. doi: 10.1530/rep.1.01232. [DOI] [PubMed] [Google Scholar]
- 36.Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod. 2004;70:1018–23. doi: 10.1095/biolreprod.103.022640. [DOI] [PubMed] [Google Scholar]
- 37.Heikkinen J, Möttönen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136:373–8. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilburgs T, Roelen DL, van der Mast BJ, et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(Suppl. A):S47–53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–42. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 40.Zehnder D, Evans KN, Kilby MD, et al. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am J Pathol. 2002;161:105–14. doi: 10.1016/s0002-9440(10)64162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans KN, Nguyen L, Chan J, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75:816–22. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 42.Penna G, Roncari A, Amuchastegui S, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–7. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]


