Abstract
Graft-versus-host disease (GVHD) is a major complication of allogeneic bone marrow transplantation. Extracorporeal photochemotherapy (ECP) has been introduced as an alternative treatment for GVHD refractory to conventional immunosuppressive treatment, although its mechanism of action is not yet clear. We investigated, in seven GVHD patients, the effects of ECP on dendritic cell maturation and cytokine production in an in vitro model that could mimic the potential in vivo effect of reinfusion of ECP-treated peripheral blood mononuclear cells. The model was based on co-culture of ECP-treated lymphocytes with monocyte-derived dendritic cells (DCs) of the same patient. We found that the co-culture of ECP-treated lymphocytes with immature DCs reduced CD54, CD40 and CD86 mean fluorescence intensity (MFI) significantly after lipopolysaccharide (LPS) stimulation, without affecting human leucocyte antigen D-related and CD80 MFI. In the same co-culture model, DCs produced increased amounts of interleukin (IL)-10 when co-cultured with ECP-treated lymphocytes and stimulated with LPS, while IL-12 and tumour necrosis factor-α production were not affected. These results suggest that reinfusion of large numbers of autologous apoptotic lymphocytes is significant for the therapeutic outcome of ECP through down-regulation of co-stimulatory molecules on DCs, inducing non-fully mature DCs with a low signal 2 and up-regulation of IL-10, which is an immunosuppressive cytokine.
Keywords: co-stimulatory molecules, dendritic cells, extracorporeal photopheresis, GVHD
Introduction
Graft-versus-host disease (GVHD) is a severe and frequent complication of allogeneic bone marrow transplantation, having high morbidity and mortality [1]. An antigenic mismatch in the human leucocyte antigen (HLA) complex between donor and host, with consequent sensitization of donor T lymphocytes towards tissue antigens of the recipient and cytotoxic destruction of the recipient's organs, seems to be the major aetiopathological factor. GVHD may occur as an acute (aGVHD) or chronic event (cGVHD), the former occurring within 100 days after allogeneic stem cell infusion and the latter after 100 days.
Extracorporeal photochemotherapy (ECP) has been proposed recently as an alternative treatment in patients with aGVHD or cGVHD who do not respond to conventional immunosuppressive treatment or who cannot undergo appropriate immunosuppressive treatment for any reason [2]. ECP consists in 8-methoxypsoralen (8-MOP) treatment and ultraviolet A (UVA) irradiation of the patient's peripheral blood leucocytes obtained by a leucapheresis process. The mechanism of action of ECP has not been elucidated fully: the primary targets of ECP are peripheral blood mononuclear cells (PBMC) isolated by the leukapheresis process, and especially lymphocytes that undergo programmed cell death after this treatment, whereas monocytes are resistant [3, 4].
Previous reports seem to show that ECP modulates dendritic cell (DCs) populations in cGVHD: a decrease in circulating CD80+ and CD123+ DCs and a decrease of DC function was noted after ECP [5]. Moreover, ECP seems to induce a shift from myeloid DCs to plasmacytoid DCs, together with a shift from a predominantly T helper type 1 (Th1) cytokine profile to a Th2 cytokine profile [6].
It is well known that DCs can phagocytose autologous apoptotic cells [7], and this has been proposed as an important mechanism in the induction of tolerance to self-antigens [8, 9]. However, other authors have suggested that mediators released by apoptotic cells can induce DCs maturation [10]. The aim of the current investigation was to study whether and how in GVHD patients the co-culture of monocyte-derived immature dendritic cells and ECP-treated lymphocytes of the same patient affects DC maturation and cytokine production.
Materials and methods
Patients
Blood samples were obtained from seven GVHD patients, four affected by aGVHD and three affected by cGVHD (mean age 49 years, range 32–55). All patients had undergone different immunosuppressive treatments for GVHD (Table 1), but either did not respond properly or developed severe drug-induced side-effects. ECP was therefore tried. All patients gave their informed consent to be enrolled in the study.
Table 1.
Immunosuppressive treatment of seven graft-versus-host disease (GVHD) patients undergoing extracorporeal photochemotherapy and enrolled in the study.
| Patients | Sex | GVHD | Treatment | Number of ECP cycles performed when enrolled |
|---|---|---|---|---|
| 1 | F | Chronic | Tacrolymus, mophetil mycophenolate | 3rd cycle |
| 2 | F | Acute | Prednisone, cyclosporine | 2nd cycle |
| 3 | M | Acute | Cyclosporine | 3rd cycle |
| 4 | F | Chronic | Prednisone | 2nd cycle |
| 5 | M | Acute | Prednisone | 2nd cycle |
| 6 | M | Chronic | Cyclosporine, prednisone | 7th cycle |
| 7 | M | Acute | Cyclosporine, prednisone | 2nd cycle |
ECP: extracorporeal photochemotherapy.
Photopheresis
ECP cycles were performed using a UVAR apparatus (Therakos Inc., West Chester, PA, USA). Treatment involved harvesting leucocytes using a collect and elutriation six-cycle apheresis system. Liquid 8-MOP, 100 000 ng, was injected directly into the buffy coat bag which was then exposed to UVA (2 J/cm2) for 90 min. The treated cells were then reinfused immediately. This process was repeated on the following day, patients returning every week in the first 3 months. The frequency of treatment was then modified according to clinical response.
Generation of DCs
PBMC were obtained from GVHD patients' heparinized peripheral blood samples immediately before ECP and were isolated by density gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) and washed twice in phosphate-buffered saline (PBS). Monocytes were isolated from PBMC by positive selection with anti-CD14-conjugated microbeads (Miltenyi Biotech Gmbh, Bergish Gladbach, Germany). To induce DC differentiation, monocytes were cultured in 24-well plates (Costar, Cambridge, MA, USA) at 5 × 105 cells/ml in RPMI-1640 medium supplemented with heat-inactivated fetal calf serum (FCS) (10%), l-glutamine (2 mM), penicillin–streptomycin (100 U/ml, 100 ug/ml), non-essential amino acids, sodium pyruvate, interleukin (IL)-4 (10 ng/ml; R&D Systems, Minneapolis, MN, USA) and granulocyte–macrophage colony-stimulating factor (GM-CSF) (50 ng/ml; R&D Systems) at 37°C in 5% CO2 for 7 days. On day 3 fresh medium was added to the cultures.
Co-culture of immature DCs with ECP-treated lymphocytes
PBMC were obtained from the treated buffy coat bag (BCB) just prior to reinfusion by density gradient centrifugation over Ficoll-Hypaque. Lymphocytes were isolated by negative selection with anti-CD14-conjugated microbeads (Miltenyi Biotech Gmbh). Cells were counted and vitality was assessed by Trypan blue exclusion. Co-culture of immature monocyte-derived DCs and ECP-treated lymphocytes at a ratio of 1 : 5, together with lipopolysaccharide (LPS) (1 ug/ml) for 48 h was performed. Then supernatants were collected and stored at −80°C until IL-10, IL-12p70 and tumour necrosis factor (TNF)-α were measured by enzyme-linked immunosorbent assay (ELISA).
Flow cytometric analysis
Dendritic cell phenotypic analysis was performed on day 9 with regard to mature monocyte-derived dendritic cells in the presence and absence of ECP-treated lymphocytes.
The cells were washed once with PBS and stained for 20 min on ice with conjugated antibodies in PBS with 0·1% sodium azide and 0·5% FCS. Control samples included cells alone and isotype control. Then cells were washed again and resuspended in PBS with 0·5% bovine serum albumin (BSA). The cells were acquired by flow cytometry (PAS, Partec, Munster, Germany) and at least 10 000 events gated on dendritic cells were analysed. The expression levels of antigens were expressed as mean fluorescence intensity (MFI). The following mouse anti-human monoclonal antibodies were used: CD86-phycoerythrin (PE), CD40-fluorescein isothiocyanate (FITC), CD80-FITC, HLA-DR–FITC and CD54-PE (all from Pharmingen).
Detection of cytokine production by ELISA
Microtitre plates (Corning Easy Wash; Celbio, Milan, Italy) were coated overnight with unconjugated anti-cytokine antibody (Pharmingen) at 2 ug/ml in 0·1 M Na2HPO4 pH 9 buffer and blocked with PBS/Tween. A biotin-labelled anti-cytokine antibody at 1 μg/ml in PBS/10% FCS was used. The plates were developed using avidin–horseradish peroxidase (HRP) (Vector, Burlingame, CA, USA) and 2,2 azino-bis substrate (Sigma, Milano, Italy) with a lower limit of detection of 15·6 pg/ml.
Statistical analysis
Wilcoxon's test for non-parametric paired samples was used for statistical analysis. P < 0·05 was considered significant.
Results
Phenotypic characteristics of LPS-stimulated DCs incubated with ECP-treated lymphocytes for 48 h
When GVHD patients' immature DCs were stimulated with LPS for 48 h, MFI of HLA-DR, CD40, CD80, CD83, CD86 and CD54 was increased (data not shown). When ECP-treated lymphocytes were added in a 5 : 1 ratio together with LPS to DCs of the same patients, HLA-DR and CD80 MFI on DCs were not modified in comparison with LPS-treated DCs (Figs 1 and 2). On the contrary, CD54, CD40 and CD86 MFI were reduced significantly on DCs co-cultured with ECP-treated lymphocytes (Figs 1 and 2). When DCs were co-cultured with untreated lymphocytes of the same patient together with LPS, no modification of MFI of co-stimulatory molecules was found (Fig. 1).
Fig. 1.
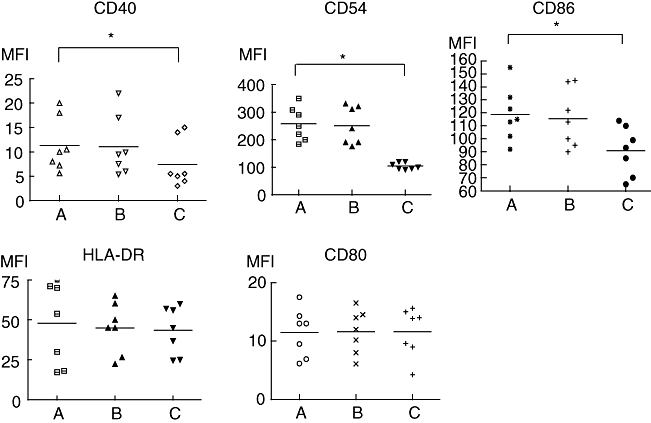
The addition of extracorporeal photochemotherapy (ECP)-treated lymphocytes together with lipopolysaccharide (LPS) down-regulates CD40, CD54 and CD86 expression on dendritic cells (DCs). Monocyte-derived immature DCs were stimulated with LPS (1 μg/ml) for 48 h (a); untreated isolated lymphocytes of the same patient were added in a ratio of 5 : 1 together with LPS to monocyte-derived immature DCs for 48 h (b); ECP-treated isolated lymphocytes of the same patient were added in a ratio of 5 : 1 together with LPS to monocyte-derived immature DCs (c). Individual symbols represent individual experiments; bars show the median. *P < 0·05, n = 6.
Fig. 2.
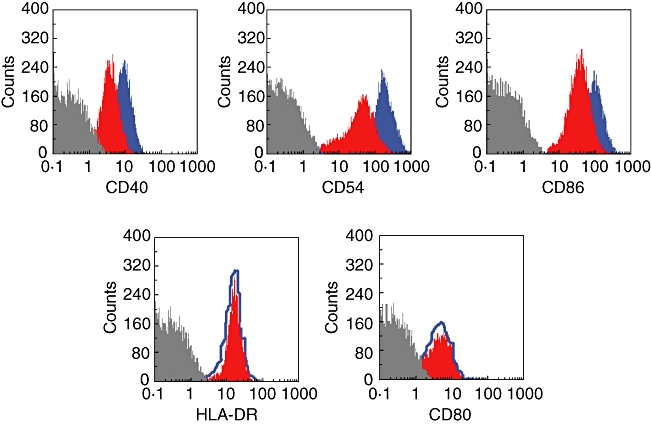
The addition of extracorporeal photochemotherapy (ECP)-treated lymphocytes together with lipopolysaccharide (LPS) down-regulates CD40, CD54 and CD86 expression on dendritic cells (DCs), without affecting human leucocyte antigen D-related (HLA-DR) or CD80. A representative experiment is shown. Flow cytometric plots show the fluorescence intensity of the DC markers with LPS (blue graphs or line) or with LPS and ECP-treated lymphocytes (red graphs). Grey plots represent the isotype control.
Monocyte-derived DCs produced increased amounts of IL-10 in response to LPS when co-cultured with ECP-treated lymphocytes, whereas IL-12 production was not affected
When peripheral blood monocyte-derived immature DCs were co-cultured with ECP-treated lymphocytes and stimulated with LPS, IL-10 production was increased significantly in comparison with LPS-stimulated DCs alone and DCs co-cultured with untreated lymphocytes of the same subject (Fig. 3). In the same in vitro model, IL-12p70 and TNF-α production were not affected by the co-culture either with untreated or ECP-treated lymphocytes (Fig. 3).
Fig. 3.

Peripheral blood monocyte-derived dendritic cells (DCs) co-cultured with extracorporeal photochemotherapy (ECP)-treated lymphocytes produced more interleukin (IL)-10, whereas IL-12 and tumour necrosis factor-α production were not affected. Monocyte-derived immature DCs were unstimulated (a); monocyte-derived immature DCs were stimulated with lipopolysaccharide (LPS) (1 μg/ml) for 48 h (b); untreated isolated lymphocytes of the same patient were added in a ratio of 5 : 1 together with LPS to monocyte-derived immature DCs for 48 h (c); ECP-treated isolated lymphocytes of the same patient were added in a ratio of 5 : 1 together with LPS to monocyte-derived immature DCs (d). Cytokine production in the supernatants was evaluated by enzyme-linked immunosorbent assay. *P < 0·05, n = 6.
Discussion
ECP is a Food and Drug Administration (FDA)-approved therapy used in pathological situations with suspected involvement of circulating pathogenic T cells as GVHD, autoimmune diseases or rejection in organ transplantation [11–15]. In spite of its 20-year-old clinical use, mechanisms explaining the efficacy of ECP are not understood fully, but they are probably related to an immunomodulatory effect. The enigma of ECP therapy is how the damage to a small proportion of the total circulating leucocytes induces a distant response in untreated cells, because less than 10% of peripheral blood leucocytes are exposed to 8-MOP and UVA. The most well-known mechanism of action is the induction of a massive apoptosis of lymphocytes involving both the Fas/FasL system and the Bcl-2 protein family, and including malignant cutaneous T cell lymphoma (CTCL) cells [3, 16]. Monocytes appear to be resistant to apoptotic effects induced by ECP [4, 17, 18] and are activated releasing cytokines [19, 20]. Other authors have suggested that monocytes can differentiate into immature DCs, after overnight incubation of the ECP-treated mixture [21].
Previous reports appear to show that ECP modulates DC populations: in cGVHD a decrease in circulating CD80+ and CD123+ DCs and a decrease of DC function was noted after ECP [5], together with a shift from myeloid DCs to plasmacytoid DCs and a shift from a Th1 cytokine profile to a Th2 cytokine profile [6]; however, these findings are not generally accepted and the effect of ECP on in vivo DC homeostasis still remains unclear.
Several investigations demonstrated clearly that immature DCs engulf and degrade apoptotic cells avidly [22]. The phagocytosis of autologous apoptotic cells has been proposed as an important mechanism in the induction of tolerance to self-antigens because engulfment of these apoptotic cells by dendritic cells would occur in the absence of any additional signal of maturation, hence avoiding the induction of an autoimmune response. However,considerable controversy still surrounds the consequences of interaction between DCs and dying cells: in a human model Sauter et al. have shown that immature dendritic cells phagocyte apoptotic cells quickly, but the uptake of apoptotic cells does not inhibit the secretion of inflammatory cytokines when dendritic cells are stimulated with LPS [23]. Newton et al. found that immature dendritic cells rarely phagocyte autologous apoptotic T lymphocytes and this process does not affect dendritic cell surface phenotype nor their ability to elicit a secondary immune response [24]. On the contrary, in a mouse model, other authors have reported that the co-culture of apoptotic cells with immature dendritic cells impairs the expression of some surface markers after addition of LPS [25].
We set up a co-culture model that has been used previously in our experiments on ECP: this in vitro model tries to reproduce what happens in vivo when we infuse a great amount of apoptotic lymphocytes into the patient. In this co-culture model we have shown previously that the co-culture of ECP-treated lymphocytes with peripheral blood monocytes enhances monocyte production of IL-10, a well-known immunosuppressive cytokine [26]. Previous authors have shown that immature DCs of children with transplant rejection are able to phagocyte PBMC treated with ECP and that these DCs acquire a tolerogenic phenotype, up-regulating HLA-DR expression but not CD83 [27]. Similarly in GVHD patients, ECP-treated lymphocytes are recognized and phagocytosed by ECP-treated myeloid DCs which are still viable after treatment but show an immature phenotype. This interaction does not lead to any maturation-associated changes [28]. We used a similar in vitro model in which ECP-treated lymphocytes of GVHD patients were added to monocyte-derived immature DCs and DCs were stimulated with LPS immediately after this addition. Because the ability of immature DCs to phagocytose ECP-treated cells has already been shown in transplanted patients [27], it is likely that in our model immature DCs also phagocyte ECP-treated lymphocytes quickly, and that this phagocytosis affects DC maturation. In fact, co-culture with ECP-treated lymphocytes reduces the ability of these cells to up-regulate the co-stimulatory molecules CD40, CD86 and CD54 expression in response to LPS. Therefore co-culture partially inhibits DCs maturation: it is likely that these non-fully mature DCs with a low signal 2 have a poor capacity to stimulate T cell response or may even induce anergy [29, 30]. In another model, the co-culture of immature DCs with early apoptotic Jurkat T cells (ECP-treated lymphocytes may also be considered early apoptotic cells) in the absence of LPS induces a non-fully mature phenotype with a low expression of CD40 and major histocompatibility complex class II complex (MHC) and a reduced capacity to stimulate allogeneic T cell proliferation [31]. Concerning ECP, in another in vitro model, co-incubation of monocytes with ECP-treated CD14 cells also induces DCs with a tolerogenic phenotype and function, and in particular a reduced capacity to induce T cell proliferation [32].
We also found that in our co-culture model of ECP-treated lymphocytes and immature DCs IL-10 production by DCs in response to LPS was increased. In our patients spontaneous IL-10 production by monocyte-derived DCs was not detectable: other authors reported that in cGVHD ECP-treated myeloid DCs produce spontaneously enhanced amounts of IL-10 during the first 2 days of culture [28], but the different data in comparison with ours are due probably to the fact that these cells underwent ECP. IL-10 is an immunosuppressive and anti-inflammatory cytokine which converts immature DCs into tolerizing antigen-presenting cells [33]. IL-10-treated DCs induce alloantigen or peptide-specific T regulatory cells suppressing proliferation of syngeneic T cells in a dose-dependent, antigen-dependent and cell-to-cell contact dependent manner [34, 35]: recent data have shown that ECP increases the percentage of CD4+ CD25+ suppressive T cells in lung and kidney transplanted patients [27, 36, 37], and this could be due to the increased production of IL-10 by immature DCs. Other authors have found that DCs produce less IL-12 and more TNF-α in response to LPS after ingestion of apoptotic cells and this may contribute to tolerance [31], whereas in our co-culture model we could not find any difference with regard to IL-12 and TNF-α production: this may be due to the different time of stimulation with LPS or the different time of incubation with apoptotic cells.
It is important to underline that our co-culture in vitro model tries to mimic the in vivo situation; however, it may not reflect exactly what happens in the ECP-treated patients, because monocyte-derived immature DCs are very similar to myeloid DCs but do not behave exactly as myeloid DCs, especially with regard to their ability to stimulate T cells [38]. Moreover, in our model we investigated only the effect of ECP-treated lymphocytes on DCs, whereas the ECP-treated bag also contains other cells such as monocytes which, after exposure to 8-methoxypsoralen and ultraviolet A light, affect DC phenotype [32].
In conclusion, our results suggest that reinfusion of large numbers of autologous apoptotic lymphocytes is significant for the therapeutic outcome of ECP in cGVHD patients through down-regulation of co-stimulatory molecules on DCs, inducing non-fully mature DCs with a low signal 2 and up-regulation of IL-10, which is an immunosuppressive cytokine. Further experiments will be needed to evaluate whether, in GVHD patients, co-culture of monocyte-derived DCs with ECP-treated lymphocytes also affects their T cell stimulatory capacity and/or induces T regulatory cells.
References
- 1.Klingebiel T, Schlegel PG. GVHD: overview on pathophysiology, incidence, clinical and biological features. Bone Marrow Transplant. 1998;21:S45–9. [PubMed] [Google Scholar]
- 2.Lazarus HM, Rowe JM. New and experimental therapies for treating graft-versus-host disease. Blood Rev. 1995;9:117–33. doi: 10.1016/s0268-960x(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 3.Bladon J, Taylor PC. Extracorporeal photopheresis in cutaneous T-cell lymphoma and graft-versus-host disease induces both immediate and progressive apoptotic processes. Br J Haematol. 2002;146:59–68. doi: 10.1046/j.1365-2133.2002.04560.x. [DOI] [PubMed] [Google Scholar]
- 4.Tambur AR, Ortegel JW, Morales A, Klingemann H, Gebel HM, Tharp MD. Extracorporeal photopheresis induces lymphocyte but not monocyte apoptosis. Transplant Proc. 2000;32:747–8. doi: 10.1016/s0041-1345(00)00966-0. [DOI] [PubMed] [Google Scholar]
- 5.Foss FM, Gorgun G, Miller KB. Extracorporeal photopheresis in chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29:719–25. doi: 10.1038/sj.bmt.1703529. [DOI] [PubMed] [Google Scholar]
- 6.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–7. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman TK, Meidenbauer N, Dworacki G, Kanaya H, Whiteside TL. Generation of tumor-specific T lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res. 2000;60:3542–9. [PubMed] [Google Scholar]
- 8.Heath WR, Kurts C, Miller JFAP, Carbone FR. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J Exp Med. 1998;187:1549–53. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class-I restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 11.McKenna KE, Whittaker S, Rhodes LE, et al. British Photodermatology Group & UK Skin Lymphoma Group. Evidence-based practice of photopheresis 1987–2001: a report of a workshop of the British Photodermatology Group and UK Skin Lymphoma Group. Br J Dermatol. 2006;154:7–20. doi: 10.1111/j.1365-2133.2005.06857.x. [DOI] [PubMed] [Google Scholar]
- 12.Heshmati F, Andreu G for the French Group of Extracorporeal Photochemotherapy. Extracorporeal photochemotherapy: a historical perspective. Tranfus Apher Sci. 2003;28:25–34. doi: 10.1016/S1473-0502(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 13.Greinix HT, Socie G, Bacialugo A, et al. Assessing the potential role of photopheresis in hematopoietic stem cell transplant. Bone Marrow Transplant. 2006;38:265–73. doi: 10.1038/sj.bmt.1705440. [DOI] [PubMed] [Google Scholar]
- 14.Duvic M, Chiao N, Talpur R. Extracorporeal photopheresis for the treatment of cutaneous T-cell lymphoma. J Cutan Med Surg. 2003;7:3–7. doi: 10.1007/s10227-003-5001-1. [DOI] [PubMed] [Google Scholar]
- 15.Marshall SR. Technology insight: ECP for the treatment of GVHD- can we offer selective immune control without generalized immunosuppression? Nat Clin Pract Oncol. 2006;3:302–14. doi: 10.1038/ncponc0511. [DOI] [PubMed] [Google Scholar]
- 16.Di Renzo M, Rubegni P, Sbano P, et al. ECP-treated lymphocytes of chronic graft-versus-host disease patients undergo apoptosis which involves both the Fas/FasL system and the BCL-2 protein family. Arch Dermatol Res. 2003;295:175–82. doi: 10.1007/s00403-003-0415-6. [DOI] [PubMed] [Google Scholar]
- 17.Wolnicka-Glubisz A, Rijnkels JM, Sarna T, Beijersbergen van Henegouwen GM. Apoptosis in leukocytes induced by UVA in the presence of 8-methoxypsoralen, chrolpromazine or 4,6,4′-trimethylangelicin. J Photochem Photobiol B. 2002;68:65–72. doi: 10.1016/s1011-1344(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 18.Bladon J, Taylor PC. Treatment of cutaneous T cell lymphoma with extracorporeal photopheresis induces Fas-ligand expression on treated T cells, but does not suppress the expression of costimulatory molecules on monocytes. J Photochem Photobiol B. 2003;69:129–38. doi: 10.1016/s1011-1344(02)00414-1. [DOI] [PubMed] [Google Scholar]
- 19.Craciun LI, Stordeur P, Schandene L, et al. Increased production of interleukin-10 and interleukin-1 receptor antagonist after extracorporeal photochemotherapy in chronic graft-versus-host disease. Transplantation. 2002;74:995–1000. doi: 10.1097/00007890-200210150-00017. [DOI] [PubMed] [Google Scholar]
- 20.Vowels BR, Cassin M, Boufal MH, Walsh LJ, Rook AH. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-alpha by monocytes: implication for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol. 1992;98:686–92. doi: 10.1111/1523-1747.ep12499907. [DOI] [PubMed] [Google Scholar]
- 21.Berger CL, Hanlon D, Kanada D, Girardi M, Edelson RL. Transimmunization, a novel approach for tumor immunotherapy. Transfus Apheresis Sci. 2002;26:205–16. doi: 10.1016/s1473-0502(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 22.Xu W, Roos A, Daha MR, van Kooten C. Dendritic cell and macrophage subsets in the handling of dying cells. Immunobiology. 2006;211:567–75. doi: 10.1016/j.imbio.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells but not primary tissue cells or apoptotic cells induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–33. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton PJ, Weller IVD, Katz DR, Chain BM. Autologous apoptotic T cells interact with dendritic cells but do not affect their surface phenotype or their ability to induce recall immune responses. Clin Exp Immunol. 2003;133:50–8. doi: 10.1046/j.1365-2249.2003.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi M, Kobayashi Y. Cytokine production in association with phagocytosis of apoptotic cells by immature dendritic cells. Cellular Immunol. 2003;226:105–15. doi: 10.1016/j.cellimm.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Di Renzo M, Rubegni P, Pasqui AL, et al. Extracorporeal photopheresis affects IL10 and IL12 monocyte production in chronic graft-versus-host-disease patients. Br J Dermatol. 2005;153:59–65. doi: 10.1111/j.1365-2133.2005.06482.x. [DOI] [PubMed] [Google Scholar]
- 27.Lamioni A, Parisi F, Isacchi G, et al. The immunological effects of extracorporeal photopheresis unravelled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79:846–50. doi: 10.1097/01.tp.0000157278.02848.c7. [DOI] [PubMed] [Google Scholar]
- 28.Spisek R, Gazova Z, Bartunkova J. Maturation state of dendritic cells during the extracorporeal photopheresis and its relevance for the treatment of chronic graft-versus-host disease. Transfusion. 2006;46:55–65. doi: 10.1111/j.1537-2995.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 29.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 30.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86-) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic celsl of early or late phases. Immunology. 2004;173:189–96. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 32.Legitimo A, Consolini R, Failli A, et al. In vitro treatment of monocytes with 8-methoxypsoralen and ultraviolet A light induces dendritic cells with a tolerogenic phenotype. Clin Exp Immunol. 2007;148:564–72. doi: 10.1111/j.1365-2249.2007.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 34.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinbrinck K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10 treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 36.Lamioni A, Carsetti R, Legato A, et al. Induction of regulatory T cells after prophylactic treatment with photopheresis in renal transplant recipients. Transplantation. 2007;83:1393–6. doi: 10.1097/01.tp.0000261635.30578.d8. [DOI] [PubMed] [Google Scholar]
- 37.Meloni F, Cascina A, Miserere S, Perotti C, Vitulo P, Fietta AM. Peripheral CD4(+)CD25(+) TREG cell counts and the response to extracorporeal photopheresis in lung transplant recipients. Transplant Proc. 2007;39:213–7. doi: 10.1016/j.transproceed.2006.10.227. [DOI] [PubMed] [Google Scholar]
- 38.Osugi Y, Vuckovic S, Hart DNJ. Myeloid blood CD11c+ dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100:2858–66. doi: 10.1182/blood.V100.8.2858. [DOI] [PubMed] [Google Scholar]


