Abstract
Persistent T cell activation is a common finding in anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated systemic vasculitis (AAV) patients. Because imatinib, a selective inhibitor of the ABL, ARG, PDGFR and c-KIT tyrosine kinases, inhibits T cell activation, this study was conducted to evaluate the potential use of imatinib for the treatment AAV patients refractory to conventional therapy. In particular, we investigated the inhibition of T cell activation by this drug and its efficacy on activated T cells from anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated systemic vasculitides (AASV) patients. T cell stimulation has been induced by anti-CD3/anti-CD28 antibodies or by phorbol myristate acetate (PMA)/ionomycin. T cell proliferation was analysed by tritiumthymidine incorporation. Cell cycle progression was determined by propidium iodide staining using fluorescence activated cell sorter (FACS) analysis and by RNAse protection assay (RPA). Cytokine levels were assessed by enzyme-linked immunosorbent assay. T cell proliferation was inhibited significantly by imatinib, due most probably to cell cycle arrest in the G1-phase. This was paralleled by inhibition in the expression of cyclin-dependent kinases 1 and 2 mRNA. The expression of CD25 in naive and memory T cells was decreased significantly by imatinib in activated T cells. Similarly, conversion from naive to memory T cells after T cell activation was impaired by imatinib. Imatinib did not influence interleukin-2 and tumour necrosis factor-α production but increased interferon-γ production. These observed effects of imatinib were similar in T cells from AASV patients and from healthy individuals. Imatinib might be an alternative therapeutical option for AASV patients refractory to conventional therapy.
Keywords: ANCA-associated vasculitis, imatinib, T-cell
Introduction
Anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated systemic vasculitides (AASV) are characterized by necrotizing inflammation of small blood vessels. According to the Chapel Hill nomenclature, AASV encompass Wegener's granulomatosis (WG), microscopic polyangiitis (MPA) and Churg Strauss syndrome. Over the past decade a number of in vitro studies and experimental animal models [1–3] have suggested a direct role for ANCA in the pathogenesis of AASV [1, 4]. Nevertheless, clinical studies suggest that T cells similarly play a significant role in the onset or perpetuation of this disease, because T cell depleting or suppressing treatment modalities induce remission successfully in AASV patients [5–9]. Activated T cells are found both in peripheral blood and in granulomatous lesions of WG [10] patients. Furthermore, an increased T cell reactivity towards PR-3 has been demonstrated in a number of studies [11–14]. It seems that disease activity in WG patients is associated with elevated T cell activation markers in serum and on peripheral blood lymphocytes; however, this has not been demonstrated convincingly in all studies [15–17].
Imatinib (IM) mesylate is a potent inhibitor of a defined class of tyrosine kinases (TKs), i.e. ABL, ARG, PDGFR and c-KIT. Clinically, IM is a highly effective treatment option for malignancies that are characterized by constitutive up-regulation of these TKs, e.g. chronic myeloid leukaemia and gastrointestinal stromal tumours. TKs of the ABL/ARG family are also involved in T cell receptor (TCR) signalling [18] and hence in downstream events of T cell activation, e.g. proliferation and cytokine production. Consequently, IM inhibits T cell activation and proliferation as has been demonstrated recently by Seggewis et al. [19].
Although thus far IM has not been used clinically in the treatment of autoimmune disorders, there are experimental data that suggest a potential benefit for IM in the treatment of these disorders [20]. Paniagua et al. [20] were able to demonstrate that IM inhibits collagen induced arthritis effectively in mice and blocks PDGFR-mediated signalling in synovial fibroblasts obtained from rheumatoid arthritis patients.
Due to the fact that the standard treatment protocol consisting of corticosteroids and cyclophosphamide for AASV is associated with a high treatment-related morbidity and mortality [21], new treatment modalities are warranted. Especially in frequently relapsing patients, in patients who are refractory to standard therapy and in patients suffering from side effects of cyclophosphamide, there is an unmet need for refining therapeutic options. As we have demonstrated previously that T-suppressing agents, e.g. 15-deoxyspergualin [15, 16], are highly effective in such patients, the rationale for the present study was to investigate whether IM might also be an alternative treatment option for these patients. Thus we tested the efficacy of this drug to inhibit T cell activation in vitro, using peripheral blood lymphocytes obtained from healthy individuals and AASV patients. In addition, we assessed the mechanisms by which this inhibition was mediated.
Methods
Patients
Patients (n = 7) with histologically and serologically proven ANCA associated autoimmune disease (WG, n = 6 and MPA, n = 1 according to the Chapel Hill nomenclature) were investigated. All patients were ANCA-positive by enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence (PR3-ANCA, n = 6, MPO-ANCA, n = 1). The mean age of the studied patients was 68 ± 11 years. Treatment consisted of corticosteroids (n = 4), azathioprine (n = 1), mycophenolate mofetil (n = 2), rituximab (n = 1) and 15-deoxyspergualin (n = 2). Pooled buffy coats from healthy individuals were used as controls. The study was approved by the institutional ethics committee and all patients gave informed consent.
Isolation of peripheral blood mononuclear cells (PBMC) and T cells
PBMC were isolated from buffy coats or heparinized blood by gradient centrifugation using Ficoll-Hypaque (Amersham Biosciences, Freiburg, Germany). T cells were isolated from PBMC by negative selection (Miltenyi Biotec, Bergisch-Gladbach, Germany). Overall purity of the isolated T cells was above 95%.
Proliferation, T cell activation and cytokine production
PBMC or purified T cells were seeded (105 cells/well) in high-binding 96-well flat-bottomed plates (Greiner Bio-One, Frickenhausen, Germany) coated with anti-CD3 (clone UCHT-1) and anti-CD28 (clone 37407·11, both 1 μg/ml, R&D Systems, Wiesbaden, Germany). T cell stimulation via second messengers was performed by supplementing the medium with 50 ng/ml phorbol myristate acetate (PMA) and 1 μg/ml ionomycin (both from Sigma-Aldrich, St Louis, MO, USA). IM (kindly provided by Novartis, Basle, Switzerland) was added to the cultures in different concentrations (0–10 μM). Cells were cultured for 3 days in Iscove's modified Dulbecco's medium containing 10% fetal calf serum (FCS) (both from PAN Biotech, Aidenbach, Germany) and 1% penicillin/streptomycin (Sigma-Aldrich). T cell proliferation was assessed by thymidine incorporation using 1 μCi of [3H]-thymidine (Amersham, Freiburg, Germany), added during the final 16 h of the culturing period. [3H]-thymidine incorporation was measured by scintillation counting in a liquid scintillation counter (LS 6500; Beckman Coulter, Krefeld, Germany). To investigate intracellular interleukin (IL)-2 expression, 1 × 106 PBMC were stimulated for 4 h with PMA/ionomycin (50 ng/ml and 1 μg/ml, respectively) and hereafter first incubated for 30 min with 10 μl of fluorescein isothiocyanate (FITC)-conjugated CD45RO, peridinin chlorophyll protein (PerCP)-conjugated CD8 and allophycocyanin (APC)-conjugated CD4 monoclonal antibodies. After the cells were washed twice with phosphate-buffered saline (PBS), the cell membrane was permeabilized by adding 600 μl of 1/10 diluted perm-wash buffer (BD Bioscience, Heidelberg Germany). Subsequently, phycoerythrin (PE)-conjugated anti-IL-2 monoclonal antibody was added for 30 min at 4°C. Cells were washed extensively and resuspended in 300 μl of PBS. Analysis of IL-2 expression was performed by fluorescence activated cell sorter (FACS) as described below. Expression of interferon (IFN)-γ and tumour necrosis factor (TNF)-α in supernatants of activated T cells was assessed by ELISA according to the manufacturer's instructions (R&D).
Flow cytometry
Antigen expression on T lymphocyte subsets was determined by triple immunofluorescence staining using directly conjugated antibodies. To this end, PBMC were incubated for 30 min at 4°C with saturating amounts (10 μl) of conjugated monoclonal antibodies directed against CD4, CD45RO and CD25 (all from BD Biosciences). The antibodies were either conjugated to FITC, R-phycoerythrin (RPE), PercP or APC depending on the combination of specific antibodies used. The cells were washed twice to remove unbound antibodies and were finally resuspended in 300 μl of Cell Wash (BD Biosciences). Three-colour analysis was performed on a FACSCalibur flowcytometer (BD Biosciences) and data were analysed using WinMDI version 2·8 software.
Cell cycle progression was assessed by propidium iodide staining. CD4+ T cells, 106, were stimulated with PMA (50 ng/ml)/ionomycin (1 μg/ml) in the presence or absence of 10 μM IM. On days 1, 3 and 5, cells were harvested and centrifuged for 5 min at 300 g. Pellets were washed twice with PBS and stained with 500 μl propidium iodide solution (50 μg/ml in PBS; Sigma-Aldrich). Apoptotic cells were defined as cells with DNA content below the G1 peak. The number of cells in the G1 or S + G2 phase was calculated by histogram analysis. The non-apoptotic cells were gated and set at 100%. The G1 phase was identified as the population of cells with the lowest propidium iodide signal within the gated cells. Using a marker in the WinMDI software, the percentage of cells in the G1 phase could be calculated. As the S and G2 phase could not be defined clearly in the histogram, the S + G2 phase was assessed as a whole and expressed as a percentage of gated cells.
RNase protection assay
Total RNA was isolated from PMA (50 ng/ml)/ionomycin (1 μg/ml) stimulated (72 h) CD4+ T cells using TRIzol Reagent (Invitrogen, Karlsruhe, Germany). Cells were incubated in the presence or absence of 10 μM of IM. RNA was precipitated with isopropanol, washed with 70% ethanol and subjected to RNase protection assays using the BD RiboQuantTM multiprobe sets (BD Biosciences). RNase protection assays were carried out according to the manufacturer's instructions. Gels were dried and subjected to autoradiography. RNA transcripts were identified by appropriate length of the protective fragments. In each of the template sets housekeeping genes L32 and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) were included as internal controls.
Endothelial cells
Human umbilical vein endothelial cells (HUVEC) were isolated from fresh umbilical cords as described previously [22]. The cells were seeded in T25 flasks (Greiner, Frickenhausen, Germany) coated with gelatin (1%) and cultured in endothelial cell growth medium (EGM) (Promocell, Heidelberg, Germany). Confluent monolayers were passaged by Trypsin/ethylenediamine tetraacetic acid (EDTA) (Sigma-Aldrich). Characterization of endothelial cells was performed on the basis of a positive uptake of acetylated low-density lipoprotein, Factor VIII-related antigen and platelet/endothelial cell adhesion molecule 1 (PECAM) (CD31) expression, and a negative staining for alpha smooth muscle actin.
Statistical analysis
All data are given as means ± standard deviation (s.d.). Differences in continuous variables were compared by means of Wilcoxon test. A two-sided P < 0·05 was considered to indicate statistical significance.
Results
Influence of IM on T cell proliferation
We first tested whether IM influences T cell proliferation of purified CD4+ T cells obtained from healthy individuals. The T cells were stimulated by a combination of anti-CD3 and anti-CD28 monoclonal antibodies or by PMA/ionomycin. Three days after stimulation with either plate-bound anti-CD3/anti-CD28 or PMA/ionomycin, IM inhibited T cell proliferation significantly as shown in Fig. 1.
Fig. 1.
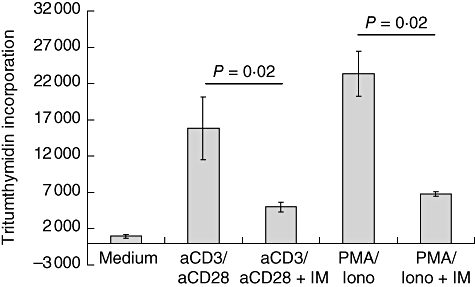
Imatinib (IM) inhibits T cell proliferation. Peripheral blood mononuclear cells were stimulated for 3 days with either plate-bound anti-CD3/anti-CD28 or with phorbol myristate acetate/ionomycin. Cells were cultured in the presence or absence of IM (10 μM) T cell proliferation was assessed by [3H]-thymidine In all experiments and for all conditions six wells of a 96-well plate were used. The results of a representative experiment (n = 5) is depicted and data are expressed as mean [3H]-thymidine incorporation ± standard deviation.
Inhibition in T cell proliferation was associated with cell cycle arrest, as evidenced by an increased proportion of T cells in the G1-phase. In addition, there was a slight increase in DNA fragmentation over time in IM-treated T cells, suggesting the occurrence of apoptosis (Fig. 2a, b). RNase protection assays revealed inhibition of cyclin-dependent kinases (cdk) 1 and 2 mRNA expression in IM (10 μM)-treated T cells (Fig. 3).
Fig. 2.
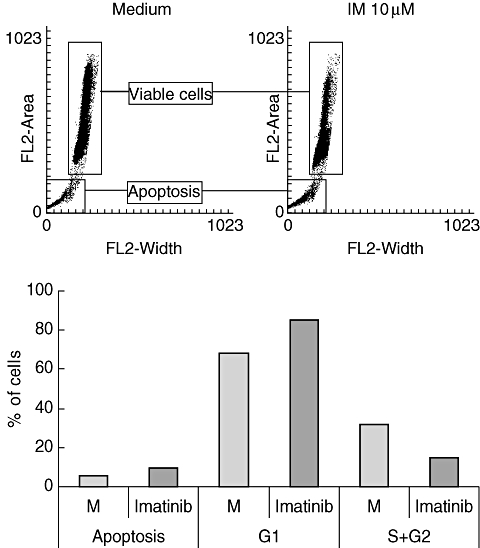
Influence of imatinib (IM) on cell cycle progression. Isolated T cells were stimulated with phorbol myristate acetate/ionomycin as described in Fig. 1. Cell cycle progression was assessed by propidium iodide and analysed by fluorescence activated cell sorter analysis. Results of a representative experiment (n = 3) are depicted as histogram (a). In (b) results are expressed as mean percentage of apoptotic cells or as mean percentage of cells in the G1 or S + G2 phase.
Fig. 3.
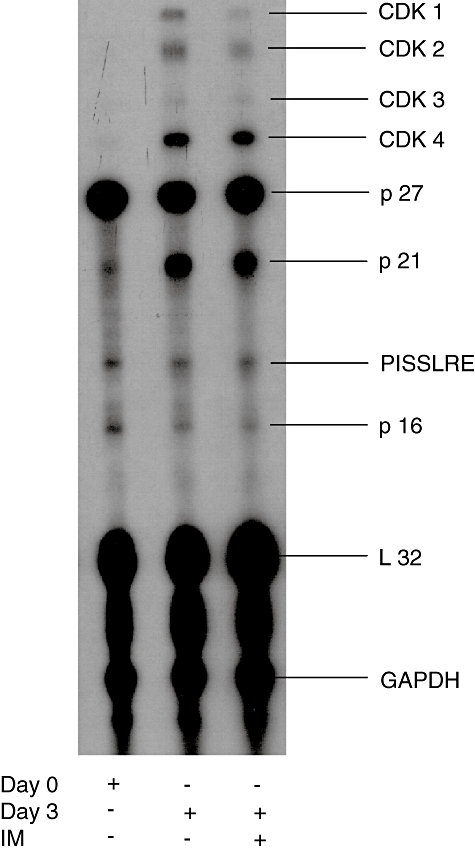
Influence of imatinib (IM) on CDK mRNA expression. Purified CD4+ T cells were stimulated for 3 days with phorbol myristate acetate/ionomycin in the presence or absence of 10 μM IM. Total RNA was isolated and subjected to RNAse protection assay as described in Materials and methods. Total RNA, isolated from unstimulated CD4+ T cells, was included in each experiment. The results of a representative experiment (n = 3) is depicted.
Effect of IM on T cell activation markers
To test whether the expression of T cell activation markers following T cell stimulation were also affected by IM, we assessed the proportion of naive (CD54RO–) and memory (CD45RO+) T cells and the expression of CD25 on both subsets. While 3 days after stimulation with anti-CD3/anti-CD28 the proportion of CD45RO+ T cells was clearly increased in untreated cells, this was inhibited significantly by IM (proportion of CD45RO+ T cells: 96·1% versus 74% for untreated and IM-treated cells, P < 0·05). Similarly, the up-regulation of CD25 on these cells was inhibited by IM (overall CD25 expression: 47·8% compared to 7·8% for untreated and IM treated cells, respectively, P < 0·05) (Fig. 4).
Fig. 4.
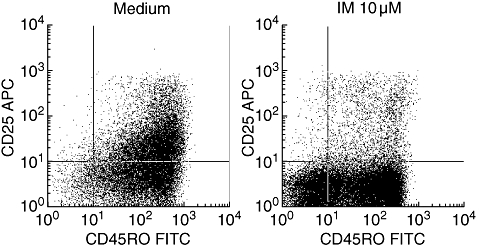
Influence of imatinib (IM) on T cell activation markers. Peripheral blood mononuclear cells were stimulated for 3 days with plate-bound anti-CD3/anti-CD28. The cells were cultured in the presence or absence of 10 μM IM. On day 3, the cells were stained for CD4, CD45RO and CD25 and analysed by fluorescence activated cell sorter analysis. CD4+ T cells were gated and displayed as dot-blots for the expression of CD25 and CD45RO. Note that in the presence of IM conversion from CD45RO– cells to CD45RO+ cells is inhibited and that the expression of CD25 in both subsets is decreased. The results of a representative experiment (n = 3) are depicted.
Effect of IM on cytokine production
Due to the finding that IM inhibited T cell proliferation and the up-regulation of T cell activation markers we determined the influence of IM on IL-2 production. Cells were stimulated with PMA/ionomycin for 4 h and intracellular IL-2 was assessed thereafter. IM did not influence IL-2 production in these cells as depicted in Fig. 5a. We also tested the influence of IM on TNF-α and IFN-γ production in supernatants of stimulated T cells. Although TNF-α production was not influenced by IM (data not shown), the production of IFN-γ was up-regulated significantly by IM in PMA/ionomycin or anti-CD3/anti-CD28-stimulated PBMC and T cells (Fig. 5b).
Fig. 5.
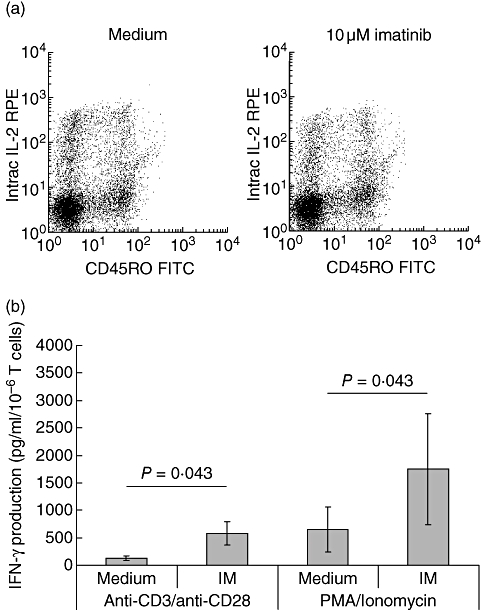
Influence of imatinib (IM) on cytokine production. (a) Peripheral blood mononuclear cells were stimulated for 4 h with phorbol myristate acetate (PMA)/ionomycin and stained for interleukin-2 in CD4+CD45RO– and CD4+CD45RO+ cells. (b) Purified CD4+ T cells were stimulated for 7 days with either anti-CD3/anti-CD28 or PMA/ionomycin. The cells were cultured in the presence or absence of 10 μM IM. On day 7, supernatants were harvested and interferon (IFN)-γ production was assessed by enzyme-linked immunosorbent assay. In (b) a total of four different experiments were performed. The results are expressed as mean IFN-γ production (pg/ml/106 cells) ± standard deviation.
Activation of endothelial cells by T cell supernatants
To study if the increased IFN-γ production was of functional relevance we tested the ability of supernatants obtained from untreated and IM-treated PBMC to induce human leucocyte antigen D-related (HLA-DR) expression on cultured endothelial cells. Although IFN-γ production was higher in the supernatant of IM-treated PBMC, the induction of HLA-DR on endothelial cells was modest and did not differ from supernatants from untreated PBMC (Fig. 6a). Interestingly, endothelial cells stimulated with supernatants of IM-treated PBMC showed a diminished expression of vascular cell adhesion molecule (VCAM)-1, compared to endothelial cells stimulated with supernatants of untreated PBMC. This was not observed for intercellular adhesion molecule (ICAM)-1 expression (Fig. 6a). The diminished expression of VCAM-1 was due probably to carry-over of IM, as IM directly inhibited VCAM-1 expression in TNF-α-stimulated endothelial cells (Fig. 6b).
Fig. 6.
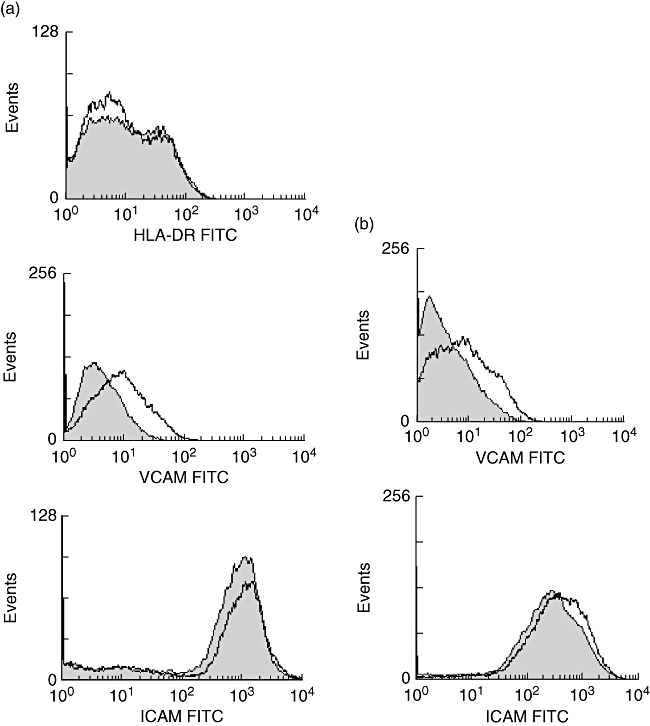
Induction of human leucocyte antigen D-related (HLA-DR) and adhesion molecules on endothelial cells. (a) Phorbol myristate acetate (PMA)/ionomycin-stimulated peripheral blood mononuclear cells were cultured for 3 days in the presence or absence of 10 μM imatinib (IM). Hereafter the supernatants were collected and added to cultured human umbilical vein endothelial cells (HUVEC) for 48 h. The expression of HLA-DR, vascular cell adhesion molecule (VCAM) and intercellular adhesion molecule (ICAM) was assessed by fluorescence activated cell sorter (FACS) analysis in HUVEC incubated with supernatants of IM/PMA/ionomycin-treated T cells (filled histogram) and in HUVEC incubated with supernatants of PMA-ionomycin-treated T cells (open histogram). (b) HUVEC were stimulated for 3 days with 10 ng/ml of tumour necrosis factor-α. The cells were cultured in the presence or absence of 10 μM IM. Hereafter, the surface expression of ICAM and VCAM was determined by FACS analysis. The filled histogram represents antigen expression in the absence of IM, the open histogram represents antigen expression in the presence of IM. In (a) and (b) the results of a representative experiment (n = 3) are depicted.
Does IM also affect T cell activation in PBMC isolated from AASV patients?
T cell activation and proliferation as well as cytokine production were determined in seven patients with proven AASV. As in healthy individuals, IM significantly inhibited both T cell receptor and second messenger-mediated T cell proliferation, as shown in Fig. 7a. Because the proportion of memory T cells was already high in the majority of the studied AASV patients, the influence of IM on skewing of the naive to the memory phenotype could not be studied. Although at baseline, CD25 expression in the AASV patient group was higher in comparison to healthy controls, IM was able to inhibit further up-regulation of CD25 significantly by anti-CD3/anti-CD28 (Fig. 7b). IFN-γ production by anti-CD3/anti-CD28 or PMA/ionomycin-stimulated PBMC was similar to healthy controls also up-regulated in AASV patients (Fig. 7c).
Fig. 7.
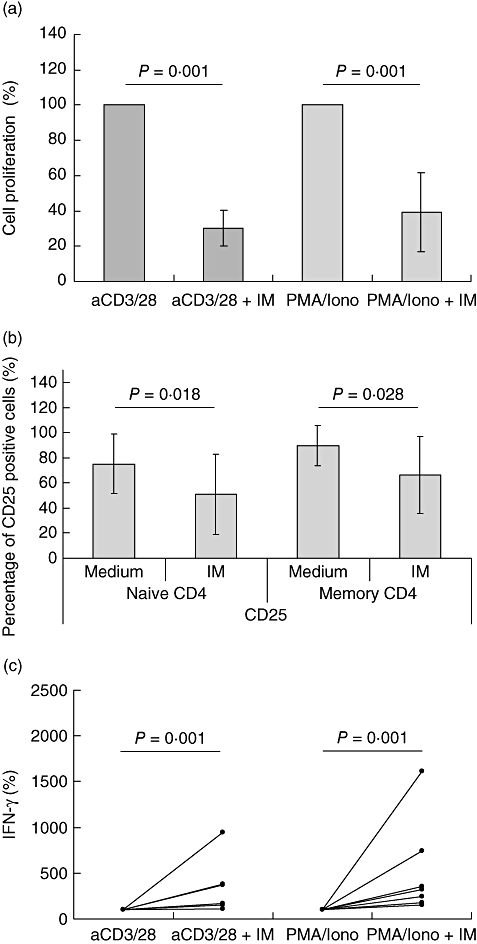
Influence of imatinib (IM) on T cell activation with peripheral blood mononuclear cells (PBMC) of anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated systemic vasculitides (AASV) patients. (a) PBMC obtained from seven AASV patients were stimulated for 3 days with either plate-bound anti-CD3/anti-CD28 or phorbol myristate acetate/ionomycin. The cells were cultured in the presence or absence of 10 μM IM. T cell proliferation was assessed by [3H]-thymidine incorporation. T cell proliferation in the absence of IM was set at 100%. The results are expressed as mean percentage of cell proliferation ± standard deviation (s.d.). (b) PBMC were stimulated as in (a), and stained for CD4, CD45RO and CD25 on day 3. The expression of CD25 in the naive and memory CD4 cells was assessed and expressed as mean percentage of CD25-positive cells ± s.d. for each of the subsets. (c) PBMC were stimulated as in (a) and supernatants were collected on day 3.
Discussion
In the present study, we investigated the influence of IM on T cell activation in vitro. We were able to show that IM inhibited both anti-CD3/anti-CD28 and second messenger-mediated T cell proliferation in association with a cell cycle arrest in the G1-phase, concomitantly with down-regulation of CDK 1 and 2 mRNA expression. IM inhibited skewing of naive towards memory T cells and up-regulation of CD25 in activated T cells. In stimulated T cells, production of IFN-γ was up-regulated by IM, while no influence was found on TNF-α and IL-2 production. TNF-α-mediated up-regulation of VCAM-1, but not of ICAM, on endothelial cells was inhibited by IM. Concentrations of IFN-γ measured in the supernatants of activated PBMC did not exceed 3 ng/ml. This might explain why these supernatants did not induce HLA-DR expression on endothelial cells to a large extent, because we use routinely 20 ng/ml of recombinant IFN-γ for a strong induction of HLA-DR (data not shown). Although the highest concentration of IFN-γ was measured in supernatants of IM-treated PBMC, these supernatants did not induce higher HLA-DR expression on endothelial cells.
Our data are compatible with previous published data of Seggewiss et al. [19], showing an inhibitory effect of IM on T cell activation. In their study, a reduced tyrosine phosphorylation of the zeta chain-associated protein kinase 70 kDa (ZAP70) and linker for T cell activation (LAT) was present, suggesting the lymphocyte-specific protein tyrosine kinase (LCK) as a probable target for IM. Furthermore, as our data reveal that IM inhibits second messenger-mediated T cell proliferation, kinases downstream of LCK might represent additional IM targets.
It must be stressed that we did not investigate whether IM influenced antigen-specific T cell activation in this study. Although we cannot exclude formally that IM will behave differently in this regard, this seems highly unlikely as PMA/ionomycin treatment activates a second messenger cascade that is similar to that described for antigen-specific T cell activation. Also, inhibition of T cell activation occurred both in PBMC obtained from healthy controls and patients.
Lucas et al. [23] studied the expression of cyclin-dependent kinases (CDK) 1, 2 and 4 in stimulated T cells and found, similar to our results, that none of these genes was expressed in resting T cells but only after T cell activation. While CDK4 was not influenced by IM, the expression of CDK1 and CDK2 was clearly inhibited. These kinases are involved in G1 to S and G2 to M transition of the cell cycle [23], thus explaining why T cell proliferation was arrested in the presence of IM. It must be pointed out that CDK activation is not only regulated by de novo expression. Inhibition of CDK1 activity by Tyr-15 phosphorylation regulates entry directly into mitosis and plays a pivotal role in cell cycle control [24]. Although we did not assess whether IM influences CDK1 phosphorylation, Yu et al. [25] reported that treatment of K562 cells with the combination of IM and a mitogen activated protein kinase (MAPK) inhibitor resulted in diminished CDK1 phosphorylation, which was associated with promotion of apoptosis in these cells [26].
It remains to be addressed whether IM is a good candidate drug for the treatment of vasculitis patients refractory to conventional therapy. Clearly, in vitro IM also inhibits T cell activation in cells from vasculitis patients. As T cells in these patients seem to be persistently activated [27] and T cell-suppressing therapies have shown their benefit in vasculitis [5–7], it is tempting to speculate that IM would indeed be a good alternative. However, it must be stressed that IM also increased IFN-γ production in activated T cells. While initiation of WG seems to be associated with an aberrant T helper 1 (Th1)-type response, as disease progresses a Th2-type becomes more apparent [28]. Thus, an increase in IFN-γ production might be a disadvantage in the early phase of vasculitis, but could also prevent skewing towards Th2 when vascultis becomes more generalized [29, 30]. Recent studies have also indicated that IFN-γ might protect against autoimmune diseases such as experimental autoimmune uveitis and scleritis by inhibition of Th17 cells [31]. Similar findings for autoimmune arthritis in mice have been described by Hirota et al. [32]. To our knowledge, however, an aberrant IL-17 response in AASV patients has not been reported thus far.
Reports on increased IFN-γ production during IM treatment are conflicting. While Aswald et al. have reported on an increased frequency of IFN-γ-producing T cells in IM-treated chronic myeloid leukemia patients [33], Gao et al. found a decreased production of Th1 cytokines in activated CD4+ T cells [34]. In addition, Borg et al. have reported on an increased IFN-γ production by natural killer (NK) cells in IM-treated patients with gastrointestinal stromal tumours [35].
Because activation of PDGFR plays an important role in the pathophysiology of crescent formation [36, 37], and because IM inhibits PDGFR-mediated signalling [20], a beneficial effect of IM on vasculitis patients might well be beyond its effects on T cell activation. Biopsy findings displayed the de novo expression of VCAM-1 in the glomerular tuft, particularly in PR3-ANCA-positive patients [38]. Because neutrophil VLA-4/VCAM−1 interaction might contribute to neutrophil infiltration, the finding that IM inhibits TNF-α mediated up-regulation of VCAM-1 provides a further rationale for implementation of IM in vasculitis treatment.
In conclusion, our data demonstrate that in vitro IM is highly effective in inhibiting T cell activation. In consideration of persistent T cell activation in AASV patients, the role of PDGF in crescent formation and the requirement of adhesion molecules for neutrophil infiltration, we therefore postulate that IM would be a promising drug for the treatment of AASV patients refractory to conventional therapy.
References
- 1.Falk R, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–19. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rarok A, Limburg PC, Kallenberg CG. Neutrophil-activating potential of antineutrophil cytoplasm autoantibodies. J Leukoc Biol. 2003;74:3–15. doi: 10.1189/jlb.1202611. [DOI] [PubMed] [Google Scholar]
- 3.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:919–21. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schreiber A, Luft FC, Kettritz R. Membrane proteinase 3 expression and ANCA-induced neutrophil activation. Kidney Int. 2004;65:2172–83. doi: 10.1111/j.1523-1755.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 5.Lockwood C, Thiru S, Isaacs JD, Hale G, Waldmann H. Long-term remission of intractable systemic vasculitis with monoclonal antibody therapy. Lancet. 1993;341:1620–2. doi: 10.1016/0140-6736(93)90759-a. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt W, Hagen EC, Neumann I, Nowack R, Flores-Suarez LF, van der Woude FJ European Vasculitis Study Group. Treatment of refractory Wegener's granulomatosis with antithymocyte globulin (ATG): an open study in 15 patients. Kidney Int. 2004;65:1440–8. doi: 10.1111/j.1523-1755.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt W, Birck R, Heinzel PA, et al. Prolonged treatment of refractory Wegener's granulomatosis with 15-deoxyspergualin: an open study in seven patients. Nephrol Dial Transplant. 2005;20:1083–92. doi: 10.1093/ndt/gfh763. [DOI] [PubMed] [Google Scholar]
- 8.Birck R, Warnatz K, Lorenz HM, et al. 15-Deoxyspergualin in patients with refractory ANCA-associated systemic vasculitis: a six-month open-label trial to evaluate safety and efficacy. J Am Soc Nephrol. 2003;14:440–7. doi: 10.1097/01.asn.0000048716.42876.14. [DOI] [PubMed] [Google Scholar]
- 9.Kalsch A, Schmitt WH, Breedijk A, et al. In vivo effects of cyclic administration of 15-deoxyspergualin on leucocyte function in patients with Wegener's granulomatosis. Clin Exp Immunol. 2006;146:455–62. doi: 10.1111/j.1365-2249.2006.03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham M, Huang XR, Dowling JP, Tipping PG, Holdsworth SR. Prominence of cell-mediated immunity effectors in ‘pauci-immune’ glomerulonephritis. J Am Soc Nephrol. 1999;10:499–506. doi: 10.1681/ASN.V103499. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer E, Stegeman CA, Hiutema MG, Limburg PC, Kallenberg CG. T cell reactivity to proteinase 3 and myeloperoxidase in patients with Wegener's granulomatosis (WG) Clin Exp Immunol. 1994;98:448–53. doi: 10.1111/j.1365-2249.1994.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith M, Coulthart A, Pusey CD. T cell responses to myeloperoxidase (MPO) and proteinase 3 (PR3) in patients with systemic vasculitis. Clin Exp Immunol. 1996;103:253–8. doi: 10.1046/j.1365-2249.1996.d01-629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King W, Brooks CJ, Holder R, Hughes P, Adu D, Savage CO. T lymphocyte responses to anti-neutrophil cytoplasmic autoantibody (ANCA) antigens are present in patients with ANCA-associated systemic vasculitis and persist during disease remission. Clin Exp Immunol. 1998;112:539–46. doi: 10.1046/j.1365-2249.1998.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popa E, Franssen CF, Limburg PC, Huitema MG, Kallenberg CG, Tervaert JW. In vitro cytokine production and proliferation of T cells from patients with anti-proteinase 3- and antimyeloperoxidase-associated vasculitis, in response to proteinase 3 and myeloperoxidase. Arthritis Rheum. 2002;46:1894–904. doi: 10.1002/art.10384. [DOI] [PubMed] [Google Scholar]
- 15.Marinaki S, Kälsch AI, Grimminger P, et al. Persistent T-cell activation and clinical correlation in ANCA-associated vasculitis. Nephrol Dial Transplant. 2006;21:1825–32. doi: 10.1093/ndt/gfl097. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Hansen H, Tatsis E, Csernok E, Lemke H, Gross WL. High plasma levels of the soluble form of CD30 activation molecule reflect disease activity in patients with Wegener's granulomatosis. Am J Med. 1997;102:517–23. doi: 10.1016/s0002-9343(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt W, Heesen C, Csernok E, Rautmann A, Gross WL. Elevated serum levels of soluble interleukin-2 receptor in patients with Wegener's granulomatosis. Association with disease activity. Arthritis Rheum. 1992;35:1088–96. doi: 10.1002/art.1780350914. [DOI] [PubMed] [Google Scholar]
- 18.Wange R. TCR signaling: another Abl-bodied kinase joins the cascade. Curr Biol. 2004;14:R562–4. doi: 10.1016/j.cub.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Seggewiss R, Lore K, Greiner E, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–9. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 20.Paniagua R, Sharpe O, Ho PP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116:2633–43. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman G, Kerr GS, Leavitt RY, et al. Wegener's granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe E, Nachman RL, Becker CG. Culture of human endothelial cellsderived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas J, Szepesi A, Domenico J, Tordai A, Terada N, Gelfand EW. Differential regulation of the synthesis and activity of the major cyclin-dependent kinases, p34cdc2, 33cdk2, and p34cdk4, during cell cycle entry and progression in normal human T lymphocytes. J Cell Physiol. 1995;165:406–16. doi: 10.1002/jcp.1041650222. [DOI] [PubMed] [Google Scholar]
- 24.Welburn J, Tucker JA, Johnson T, et al. How tyrosine 15 phosphorylation inhibits the activity of cyclin-dependent kinase 2-cyclin A. J Biol Chem. 2007;282:3173–81. doi: 10.1074/jbc.M609151200. [DOI] [PubMed] [Google Scholar]
- 25.Yu C, Krystal G, Varticovksi L, et al. Pharmacologic mitogen-activated protein/extracellular signal-regulated kinase kinase/mitogen-activated protein kinase inhibitors interact synergistically with STI571 to induce apoptosis in Bcr/Abl-expressing human leukemia cells. Cancer Res. 2002;62:188–99. [PubMed] [Google Scholar]
- 26.Shimizu T, O'Connor PM, Kohn KW, Pommier Y. Unscheduled activation of cyclin B1/Cdc2 kinase in human promyelocytic leukemia cell line HL60 cells undergoing apoptosis induced by DNA damage. Cancer Res. 1995;55:228–31. [PubMed] [Google Scholar]
- 27.Marinaki S, Neumann I, Kälsch AI, et al. Abnormalities of CD4 T cell subpopulations in ANCA-associated vasculitis. Clin Exp Immunol. 2005;140:181–91. doi: 10.1111/j.1365-2249.2005.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamprecht P. Off balance: T-cells in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides. Clin Exp Immunol. 2005;141:201–10. doi: 10.1111/j.1365-2249.2005.02808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller A, Trabandt A, Gloeckner-Hofmann K, et al. Localized Wegener's granulomatosis: predominance of CD26 and IFN-gamma expression. J Pathol. 2000;192:113–20. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH656>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 30.Sanders J, Stegeman CA, Kallenberg CG. The Th1 and Th2 paradigm in ANCA-associated vasculitis. Kidney Blood Press Res. 2003;26:215–20. doi: 10.1159/000072987. [DOI] [PubMed] [Google Scholar]
- 31.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–18. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 32.Hirota K, Hashimoto M, Yoshitomi H, et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–7. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aswald J, Lipton JH, Aswald S, Messner HA. Increased IFN-gamma synthesis by T cells from patients on imatinib therapy for chronic myeloid leukemia. Cytokines Cell Mol Ther. 2002;7:143–9. doi: 10.1080/13684730210002319. [DOI] [PubMed] [Google Scholar]
- 34.Gao H, Lee BN, Talpaz M, et al. Imatinib mesylate suppresses cytokine synthesis by activated CD4 T cells of patients with chronic myelogenous leukemia. Leukemia. 2005;19:1905–11. doi: 10.1038/sj.leu.2403933. [DOI] [PubMed] [Google Scholar]
- 35.Borg C, Terme M, Taïeb J, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–88. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fellstrom B, Klareskog L, Heldin CH, et al. Platelet-derived growth factor receptors in the kidney − upregulated expression in inflammation. Kidney Int. 1989;36:1099–102. doi: 10.1038/ki.1989.306. [DOI] [PubMed] [Google Scholar]
- 37.Fujigaki Y, Sun DF, Fujimoto T, et al. Mechanisms and kinetics of Bowman's epithelial–myofibroblast transdifferentiation in the formation of glomerular crescents. Nephron. 2002;92:203–12. doi: 10.1159/000064469. [DOI] [PubMed] [Google Scholar]
- 38.Arrizabalaga P, Solé M, Iglesias C, Escaramís G, Ascaso C. Renal expression of ICAM-1 and VCAM-1 in ANCA-associated glomerulonephritis − are there differences among serologic subgroups? Clin Nephrol. 2006;65:79–86. doi: 10.5414/cnp65079. [DOI] [PubMed] [Google Scholar]


