Abstract
We aimed to delineate factors operating at the interface of macrophage–mycobacterium interaction which could determine the fate of a ‘subclinical’ infection in healthy people of a tuberculosis-endemic region. Ten study subjects (blood donors) were classified as ‘high’ or ‘low’ responders based on the ability of their monocyte-derived macrophages to restrict or promote an infection with Mycobacterium tuberculosis. Bacterial multiplication between days 4 and 8 in high responder macrophages was significantly lower (P < 0·02) than low responders. All donor sera were positive for antibodies against cell-membrane antigens of M. tuberculosis and bacilli opsonized with heat-inactivated sera were coated with IgG. In low responder macrophages, multiplication of opsonized bacilli was significantly less (P < 0·04) than that of unopsonized bacilli. The levels of tumour necrosis factor (TNF)-α and interleukin (IL)-12 produced by infected high responder macrophages was significantly higher (P < 0·05) than low responders. However, infection with opsonized bacilli enhanced the production of IL-12 in low responders to its level in high responders. The antibody level against membrane antigens was also significantly higher (P < 0·05) in high responders, although the antigens recognized by two categories of sera were not remarkably different. Production of certain other cytokines (IL-1β, IL-4, IL-6 and IL-10) or reactive oxygen species (H2O2 and NO) by macrophages of high and low responders did not differ significantly. The study highlights the heterogeneity of Indian subjects with respect to their capability in handling subclinical infection with M. tuberculosis and the prominent role that TNF-α, opsonizing antibodies and, to a certain extent, IL-12 may play in containing it.
Keywords: cytokines, macrophages, opsonizing antibodies, subclinical infection, tuberculosis
Introduction
A third of the world's population is considered to be infected with Mycobacterium tuberculosis and nearly 2 million people die every year from tuberculosis (TB) [1]. The success of M. tuberculosis as a pathogen for humankind is largely dependent upon its capacity to avoid destruction by host cells, particularly the macrophages. The outcome of infection thus varies from a ‘latent’ infection with no clinical symptoms to the disseminated disease [2]. The immune response towards the pathogen is complex and immune parameters that confer protection against TB are not yet elucidated fully. The cytokine tumour necrosis factor (TNF)-α has been implicated in protection against TB in the early stages of the disease [3, 4]. In one study [5], no cytokines were as effective as TNF-α in the killing of intracellular M. tuberculosis. Blocking TNF-α has been shown to allow the disease to emerge from latency [6]. Individuals with deficiency in interleukin (IL)-12 or interferon (IFN)-γ receptors have also been found to be highly susceptible to mycobacterial infection [7, 8] and immunotherapy with IFN-γ has been reported to be effective against multi-drug-resistant TB [9]. Apart from cytokines, a role for reactive nitrogen/oxygen intermediates has also been emphasized for intracellular killing of M. tuberculosis[10], particularly in the mouse model of TB [11].
A number of studies have highlighted the role of opsonizing antibodies in protection against TB in the mouse model. In the study by Armstrong and Hart [12], although prior opsonization of M. tuberculosis with anti-mycobacterium antibodies promoted phagosome–lysosme fusion in infected macrophages, it did not augment intracellular killing of the bacilli. Subsequent studies have, however, demonstrated that opsonization with antibodies of certain immunoglobulin (Ig) isotypes significantly improves containment of the infection. Opsonization with monoclonal IgG1/G3 antibodies against M. tuberculosis cell surface antigen arabinomannan has been reported to enhance significantly the survival of mice following challenge with infection [13, 14]. Similarly, opsonization of M. bovis with a monoclonal IgG2 antibody against the surface antigen MPB83 also increased the survival of mice upon challenge [15]. Williams et al. [16] have reported that intranasal inoculation of monoclonal IgA antibody against the cell surface antigen α-crystallin protects mice against an intranasal infection challenge. In the subsequent study [17], addition of IFN-γ prior to infecting the macrophages with opsonized bacilli produced a synergistic increase in nitric oxide and TNF-α production, along with a two- to threefold decrease in bacterial counts.
Almost the entire population of a TB-endemic country such as India, which accounts for nearly a fourth of the global burden of the disease, is considered to be infected with M. tuberculosis. In a recent study, enumeration of IFN-γ-secreting T cells reactive with peptides derived from pathogen-specific antigens early secreted antigenic target 6 (ESAT-6) and culture filtrate protein (CFP-10) suggested a more than 80% prevalence of TB infection in urban India [18]. Besides exposure to the pathogen, certain host factors may also contribute to the susceptibility of a population to the infection. For example, individuals of certain ethnic origins have low neutrophil counts [19] and it has been shown recently by Martineau et al. [20] that risk of infection with M. tuberculosis in contacts of TB patients (measured in terms of ESAT-6/CFP-10-reactive T cells) is associated inversely and independently with their blood neutrophil counts.
Epidemiological data suggest that only about 10% of individuals infected with M. tuberculosis run a lifetime risk of developing TB [2, 21]. The infection remains dormant or latent in a majority of individuals who are apparently able to mount a protective immune response. Are there factors discernable at the level of macrophage–mycobacterium interaction which could determine the course of natural infection in a TB endemic situation? This study was aimed at addressing this question. The study subjects were healthy, bacille Calmette–Guérin (BCG)-vaccinated, north Indian blood donors who were exposed environmentally to M. tuberculosis. They could be categorized as ‘relatively resistant’ (high responders) or ‘relatively susceptible’ (low responders) based on the ability of their monocyte-derived macrophages to restrict or promote an intracellular infection with M. tuberculosis. We sought correlations with certain cytokines and reactive nitrogen/oxygen species produced by infected macrophages and also monitored the effect of opsonization of bacilli with anti-mycobacterium antibodies present in the donor sera.
Materials and methods
Study subjects
Ten healthy laboratory workers (male, aged 25–35 years) volunteered as blood donors. All were BCG-vaccinated and, being residents of a TB endemic region (north India), were considered as environmentally exposed to M. tuberculosis.
Sera
Serum samples were collected and stored in aliquots at −80°C. Heat-inactivated sera (to inactivate complement components) were prepared by keeping an aliquot at 56°C for 1 h in a waterbath.
M. tuberculosis
Three-week-old cultures of M. tuberculosis H37Rv (ATCC no. 25618) on Lowenstein–Jensen (L-J) medium were harvested and washed with Middlebrook (MB) 7H9 broth by way of suspension and centrifugation. A 100-mg bacterial pellet was transferred to each tube containing 5 ml MB7H9 broth (with 0·5% Tween 80). One tube was subjected to 10 cycles of sonication (3 × 10 s pulses/cycle) in a bath sonicator (Misonix, Inc., Farmingdale, NY, USA) in order to obtain single-cell suspensions. After each cycle, the tube was centrifuged (480 g) to settle the clumps and the optical densities (OD) of the suspension was read at 580 nm. A plateau in increasing OD was attained after the sixth cycle, indicating the highest availability of free bacilli. The remaining tubes were thus subjected to six sonication cycles, centrifuged and their suspensions pooled. The final OD was adjusted to 0·6 with MB7H9 broth containing 15% glycerol. A 1-ml aliquot of this suspension contained an average of 2·5 × 108 bacilli, determined by counting colony forming units (CFU) after culture on L-J medium for 3 weeks. All aliquots were stored at −80°C.
M. tuberculosis antigens
Bacilli harvested from L-J medium were processed as described previously [22] for preparation of subcellular fractions. In brief, cells suspended in phosphate-buffered saline (PBS) were probe-sonicated and centrifuged initially at 23 000 g to remove unbroken cells and cell-wall debris, and later at 150 000 g to obtain the cell membrane (sediment) and cytosol (supernatant). Protein was estimated by the modified Lowry's method [23]. Antigens were sterilized by either autoclaving (in the case of membrane) or filtration through a 0·22 μM membrane (in case of cytosol), and stored at −80°C in aliquots.
T cell proliferation assay
Peripheral blood mononuclear cells (PBMCs) were isolated from 10 ml citrated blood by centrifugation over Ficoll-isopaque. Washed cells were suspended (2 × 106 cells/ml) in culture medium (RPMI-1640) containing 10% heat-inactivated pooled normal human serum and dispensed in 96-well culture plates (0·1 ml/well). Mitogen (PHA-P) or antigens were added in triplicate wells (10 μl/well) to provide the following concentrations per ml culture (determined as optimal based on previous experiments): phytohaemagglutinin (PHA), 2 μg; M. tuberculosis cytosol, 25 μg and membrane, 2·5 μg. The cultures (final volume 0·2 ml/well) were incubated for 6 days in a CO2 incubator and pulsed with [3H]-thymidine (1 μCi/well) for the final 18 h before harvesting and counting in a liquid scintillation counter. Results were expressed as stimulation index (SI) [SI = mean counts per minute (cpm) with antigen or mitogen ÷ mean cpm with medium alone]. An SI value of ≥ 3 was considered a positive response.
Prior to pulsing (on day 5), 50 μl culture supernatant was removed from each well and stored at −80°C for estimation of IFN-γ using the method described below.
Detection of anti-mycobacterium antibodies in sera
Enzyme-linked immunosorbent assay (ELISA)
M. tuberculosis cytosol or membrane antigens (10 μg protein/ml in 0·05 M carbonate buffer, pH 9·5) were coated (50 μl/well, overnight, 4°C) in ELISA plates. After washing with Tris-buffered saline (0·05 M Tris, 0·1 M NaCl, pH 7·4) containing 0·05% Tween 20 (TBS-T), the plates were incubated (2 h at 37°C) with ‘blocking’ solution (2% skimmed milk powder dissolved in TBS-T, 100 μl/well). This solution was removed later and test sera (diluted 1 : 100 in 1% milk-TBS-T) were added to antigen-coated as well as buffer-coated wells (50 μl/well, in duplicate) and incubated for 2 h at 37°C. Plates were washed with TBS-T and incubated with 50 μl/well peroxidase-conjugated affinity purified anti-human immunoglobulin (Sigma, St. Louis, MO, USA; diluted 1 : 5000 in 1% milk-TBS-T) for 2 h at 37°C. Plates were washed finally with TBS-T and the substrate solution (0·04% o-phenylene diamine + 0·03% H2O2 in 0·05 M citrate phosphate buffer, pH 5) was added (50 μl/well) and incubated in the dark for 20 min. Reaction was stopped by adding 7% H2SO4 (50 μl/well). OD were read at 492 nm on a plate reader. Difference in mean OD between antigen-coated and buffer-coated wells was recorded for each test serum.
Immunoblotting
M. tuberculosis membrane proteins were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (12·5% gel, using a broad sample well) and electroblotted onto nitrocellulose paper which was later cut into strips. Individual strips were blocked for 2 h at room temperature (RT) with 3% skimmed milk powder in TBS-T and, after removing the blocking solution, incubated with donor sera (diluted 1 : 25 in 1% milk-TBS-T). After washing with TBS-T, the strips were incubated (2 h, RT) with peroxidase-conjugated affinity purified anti-human immunoglobulin (Sigma; diluted 1 : 1000 in 1% milk-TBS-T). One strip was incubated with the monoclonal antibody ML09 (diluted 1 : 500) which binds to the mycobacterial lipoarabinomannan [24] (a gift from Dr J. Ivnayi, Guy's Hospital, London, UK). This strip was probed with peroxidase-conjugated affinity purified anti-mouse immunoglobulin (Sigma; diluted 1 : 1000). Colour was developed using the substrate (0·05% 4-chloronaphthol + 0·02% H2O2 in methanol-TBS). The strip containing electroblotted molecular weight markers was stained with amido black.
Assay for intracellular multiplication of M. tuberculosis
Live and live-opsonized M. tuberculosis
Aliquots of M. tuberculosis were thawed, dispensed in Eppendorf tubes (100 μl/tube) and centrifuged (9000 g × 10 min). The supernatant was replaced with 100 μl RPMI medium in one set of tubes, and 100 μl of heat-inactivated donor sera in the second set of tubes. The two tube sets thus contained live and live-opsonized bacilli. After thorough mixing, the tubes were incubated (37°C, 1 h) and centrifuged. The settled bacilli were washed and suspended in 100 μl RPMI medium.
To determine whether the bacilli were actually opsonized with IgG class of antibodies, samples of live and opsonized bacteria were applied as smears on glass coverslips. Dried, methanol-fixed bacilli were covered with blocking solution [2% bovine serum albumin (BSA) in PBS containing 0·05% Tween 20] and incubated for 2 at 37°C. After removing the blocking solution, smears were covered with peroxidase conjugated affinity purified goat anti-human IgG (Fc specific) antibody (Sigma) diluted 1 : 1000 in blocking solution and incubated for 2 at 37°C. The PBS-washed bacteria were incubated further (5 min) with peroxidase substrate [metal enhanced 3′,3′-diaminobenzidine (DAB); Pierce, Rockford, IL, USA], washed, dried, mounted on a glass slide and visualized under oil immersion lens of a microscope.
Macrophages
PBMCs (prepared as above) were dispensed in 48-well culture plates (106 cells/well) and incubated overnight (5% CO2, 37°C). The wells were washed thoroughly with RPMI medium to remove non-adherent cells. Ten to 12% of the cells became adhered as determined by counting the non-adherent cells. Adherent cells were allowed to differentiate into macrophages by incubating for 5 days in RPMI medium containing 10% heat-inactivated pooled normal human serum. On day 5, the medium was replaced with serum-free RPMI. When characterized by non-specific esterase staining, >98% of the differentiated cells were esterase positive.
Infection
Aliquots of live and live-opsonized bacilli were passed 20 times through a 25-guage needle to make single-cell suspensions. Bacilli were counted using a Petroff–Hausser chamber (Hausser Scientific, Horsham, PA, USA) and diluted to 2·5 × 106 bacilli/ml. Macrophages (approximately 105 cells/well) from each donor were processed as follows: one well was kept as control and three wells each were infected with live and live-opsonized bacilli at a multiplicity of infection (MoI) of 5 : 1 (bacteria : macrophage). To allow phagocytosis, the cultures were kept in a CO2 incubator for 3 h. Medium containing free bacilli was removed and macrophages were washed thoroughly with serum-free RPMI medium. Finally, 1 ml medium containing 5% autologous serum was added to each well. Immediately (at zero hour) medium from a set of three wells (uninfected control, live and opsonized bacilli) was collected, filtered (0·22 μM) and stored (−80°C) for cytokine estimations. The wells containing live and opsonized bacilli were processed for CFU determination. Collection of culture supernatants and CFU determinations were also performed in the same manner on days 4 and 8.
CFU determination
Infected macrophages were lysed with 0·1% saponin in water (200 μl/well). Complete lysis of cells, as monitored under phase contrast microscope, occurred in 10 min. The lysates were mixed thoroughly and 20 μl of their 1 : 100 dilutions (in MB7H9 broth) were inoculated on L-J medium. Colonies were counted after 3 weeks and results expressed as CFU/well.
Cytokine assays
Estimations of IFN-γ in culture supernatants of PBMCs, and TNF-α, IL-1β, IL-4, IL-6, IL-10 and IL-12 in culture supernatants of macrophages, were performed by using ELISA kits (BD OptEIA; BD Biosciences, San Diego, CA, USA) following the supplier's protocols.
Assays for nitric oxide and hydrogen peroxide
NO was measured as nitrite by the modified Greiss method [25] using a kit (Sigma), following the supplier's protocol. H2O2 was estimated by the method of Pick and Mizel [26], based on horseradish peroxidase (HRP)-mediated oxidation of phenol red by H2O2. In brief, the substrate (2·8 μM phenol red and 0·285 units of HRP/ml) was mixed with standard (H2O2) or test (culture supernatant) samples in a 96-well plate. The reaction was stopped by adding 0·1 M NaOH and ODs taken at 620 nm. H2O2 in the samples was determined using a standard curve.
Statistical analyses
Significance of differences was determined by Student's t-tests (paired/unpaired) and relationship between variables was determined by computing correlation coefficients (r). For both t and r values, P < 0·05 was considered significant.
Results
Determination of exposure of the donors to M. tuberculosis
All donors showed a positive T cell proliferative response (SI > 5) to M. tuberculosis antigens. Moreover, as depicted in Fig. 1a, the response to cytosol (CS) antigens [SI 38·23 ± 9·50, mean ± standard error of the mean (s.e.m.)] was significantly higher (P < 0·02) than that to cell membrane (CM) antigens (SI 16·16 ± 4·88). Production of IFN-γ by the activated T cells served as another indicator of exposure of the donors. Once again, the response toCS (92·99 ± 22·89 pg/ml) was higher than that to CM (45·61 ± 24·46 pg/ml), although this difference was not statistically significant (Fig. 1b). The basal level of IFN-γ (in absence of activation by antigen) was 14·95 ± 3·15 pg/ml.
Fig. 1.
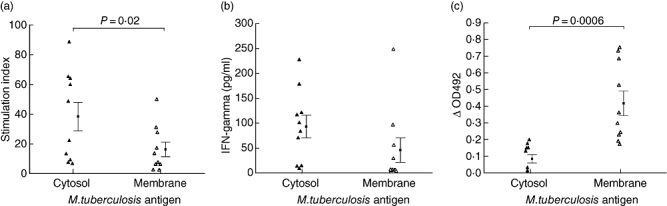
Determination of exposure of the donors (n = 10) to Mycobacterium tuberculosis: (a) T cell proliferation, (b) interferon-γ production and (c) serum antibody levels against cytosol and membrane antigens of the pathogen. Statistically significant differences were noted with respect to (a) and (c).
The presence of anti-M. tuberculosis antibodies in the donor sera also served as an indicator of their exposure. All sera were antibody positive (Fig. 1c). However, in contrast to the T cell responses, antibody levels against CS (OD 0·08 ± 0·02) were significantly lower (P < 0·001) than those against CM antigens (OD 0·41 ± 0·07). Heat-inactivation of the sera did not alter the antibody levels significantly.
Classification of donors and effect of opsonization on intracellular multiplication of M. tuberculosis
Live M. tuberculosis, opsonized with heat-inactivated donor sera could be stained with anti-human IgG (Fc-specific), suggesting the coating of the bacteria with IgG class of antibodies. Unopsonized bacilli could not be stained in this manner.
The ability of the donor macrophages to restrict or promote an infection with live or live-opsonized M. tuberculosis was determined by counting viable bacilli (CFU) on days 0, 4 and 8 post-infection. The difference in CFU between days 0 and 4 was not significant. However, day 8 counts (mean CFU/well ± s.e.m.) for both types of infection (17 340 ± 3374 for live and 18 330 ± 3113 for opsonized bacilli) were significantly higher (P < 0·02) than the corresponding day 4 counts (7700 ± 2011 for live and 8880 ± 1512 for opsonized bacilli). This observation was used as the basis for classifying donors as ‘low’ (relatively susceptible) or ‘high’ (relatively resistant) responders.
Macrophages from low responders allowed more than threefold (5·35 ± 1·26), and those from high responders more than twofold (1·44 ± 0·19) multiplication of live bacilli between days 4 and 8 (Fig. 2). This difference was statistically significant (P < 0·02). Moreover, in low responders, the intracellular multiplication of live-opsonized bacilli was significantly lower (P < 0·04) than that of live bacilli, although the lowered levels were still significantly higher (P < 0·02) than those of high responders (Fig. 2).
Fig. 2.
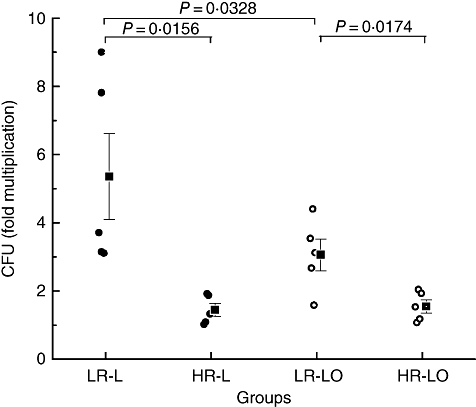
Fold multiplication [from day 4 to day 8) of Mycobacterium tuberculosis in macrophages of low (LR) and high responders (HR), five donors each]. Multiplication of live or live-opsonized bacilli in low responders (LR-L/LR-LO) was significantly higher than the corresponding values in high responders (HR-L/HR-LO). Within the LR group, multiplication of live-opsonized bacilli was significantly lower than that of live bacilli.
Immunological parameters differentiating high and low responders
Anti-M. tuberculosis antibodies in the sera
Antibodies in the donor sera were directed primarily against cell membrane antigens of M. tuberculosis (Fig. 1c). We observed further (Fig. 3a) that high responders had a significantly higher (P < 0·05) level of anti-CM antibodies (OD 0·53 ± 0·11) than the low responders (OD 0·23 ± 0·03).
Fig. 3.
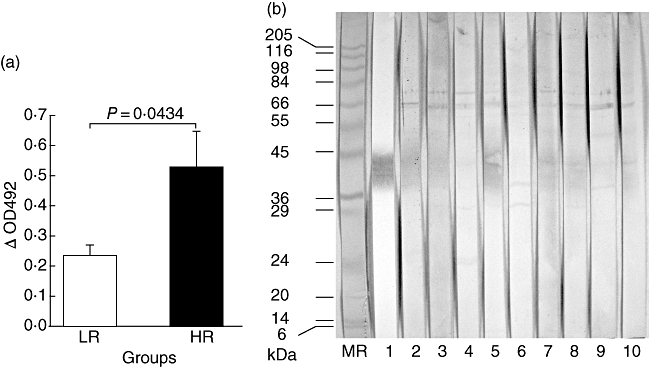
(a) Antibody levels (mean OD ± standard error of the mean) against Mycobacterium tuberculosis membrane antigens in low responders (LR) were significantly lower than high responders (HR). (b) Immunoblotting of membrane antigens with (i) monoclonal antibody ML09 (lane 1) shows presence of lipoarabinomannan and (ii) donor sera (lanes 2–6, HR; 7–10, LR) shows antigens which were recognized commonly by most sera (result with one LR serum is not available). The strip (MR) containing electroblotted molecular mass markers (kDa) was stained with amido black.
Immunoblotting of CM with individual sera revealed that the antigens recognized by high and low responders did not differ remarkably (Fig. 3b). The characteristic diffused band of the cell envelope-associated antigen-lipoarabinomannan (LAM) was stained prominently by the anti-LAM monoclonal antibody ML09. The corresponding band was stained less prominently and to a variable extent by the donor sera. In addition, two major bands of approximately 65 kDa (a doublet) and 75 kDa were also recognized by all sera. The other prominent bands, stained by some of the sera, were of approximately 30 and 38 kDa.
Release of cytokines by infected macrophages
The concentration of TNF-α (Fig. 4a) in culture supernatants of high responder macrophages infected with live bacilli (395·3 ± 94·3 pg/ml) or live-opsonized bacilli (337·3 ± 51·33 pg/ml) was significantly higher (P < 0·05) than the corresponding values for low responders (136·1 ± 49·8 pg/ml for live, and 93·54 ± 34·73 pg/ml for opsonized bacilli).
Fig. 4.
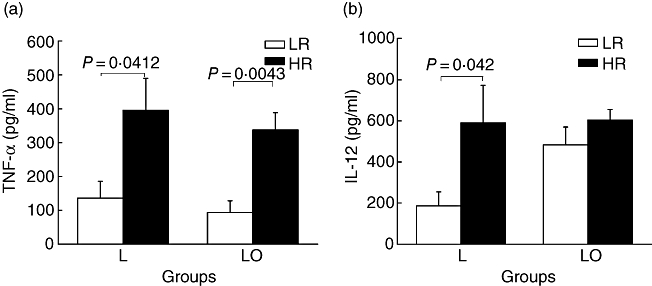
Tumour necrosis factor (TNF)-α (a) and interleukin (IL)-12 (b) levels in day 8 culture supernatants of infected macrophages. TNF-α levels of high responder (HR) macrophages infected with live (L) or live-opsonized (LO) Mycobacterium tuberculosis were significantly higher than corresponding values for low responders (LR). Interleukin (IL)-12 level of HR macrophages infected with L bacilli were also significantly higher than corresponding values for LR. However, following infection with LO bacilli, there was an increase in IL-12 levels of LR macrophages.
IL-12 produced by high responder macrophages (Fig. 4b) in response to live bacilli (590·5 ± 189·9 pg/ml) was also significantly higher (P < 0·05) than the corresponding value for low responders (186·8 ± 67·8 pg/ml). However, upon infection with opsonized bacilli, its production by low responders was raised to a level (483 ± 87·12 pg/ml), equivalent to that of the high responders (604 ± 189·1 pg/ml).
We explored further the relationship between bacterial multiplication within the donor macrophages and levels of TNF-α or IL-12 produced by them. As shown in Fig. 5, statistically significant inverse correlations were observed between TNF-α levels and the multiplication of live as well as opsonized bacilli. An inverse, although statistically insignificant, correlation (r =−0·5651, P = 0·07) was also noted between levels of IL-12 and multiplication of the live bacilli.
Fig. 5.
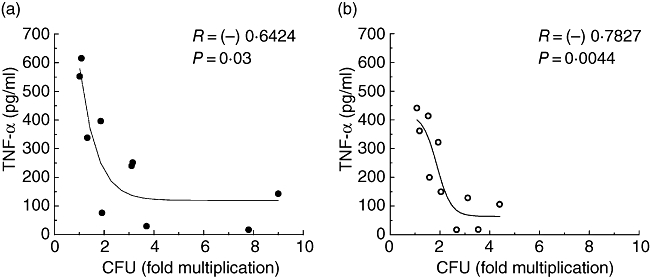
Relationship between tumour necrosis factor-α levels and bacterial multiplication in the donor macrophages (n = 10). Statistically significant inverse correlations were seen with respect to infection with live bacilli (a) as well as live-opsonized bacilli (b). Regression lines (non-linear) are shown.
Among the other cytokines studied, production of IL-10 by high responder macrophages infected with live or opsonized bacilli (593·6 ± 76·1 pg/ml and 555·8 ± 65·4 pg/ ml) was higher than the corresponding values for low responders (285·3 ± 30·9 pg/ml and 279·4 ± 79·2 pg/ml). However, these differences were not statistically significant. Statistically significant differences (with respect to high and low responders) were also not seen in the levels of IL-1β, IL-4 and IL-6 (data not shown).
Release of reactive oxygen and nitrogen species by infected macrophages
Macrophages infected with live mycobacteria produced NO and H2O2 in the range of 22·88 ± 2·04 μM and 14·46 ± 8·08 μM, respectively. However, the levels did not differ between high and low responders. There was also no difference in levels with respect to infection with live or opsonized bacilli.
Discussion
The study was aimed at delineating the factors operating at the interface of innate and adaptive immunity (macrophage–mycobacterium interaction) which could determine the course of a ‘subclinical’ infection in apparently healthy people living in a TB endemic area. The T cell as well as antibody response of the donors towards M. tuberculosis antigens suggested their exposure to the mycobacterium. Tools for a more definitive diagnosis of the subclinical infection, particularly in an endemic situation such as India, are not currently available. Chest radiography is considered a non-specific as well as a grossly insensitive technique which frequently falls short of detecting even the cases of pulmonary TB [27, 28]. The utility of the purified protein derivative (PPD) skin test is also compromised by its lack of specificity, which leads to false-positive results in people who are BCG-vaccinated or exposed environmentally to other mycobacteria [28]. The pathogen-specific laboratory assays, on the other hand, are unlikely to show adequate sensitivity for detecting a subclinical infection. For example, sensitivity of the ELISPOT assay which detects IFN-γ produced by ESAT-6- and CFP-10-specific T lymphocytes [18] could be as low as 60%, even in the culture-positive TB patients [29].
We considered that the efficiency of clearance of M. tuberculosis by macrophages (in terms of CFU counts) could be used as a robust criterion to differentiate the subjects who had the capability to restrict the infection (high responders) and those who did not (low responders). After infection, the mean survival time of macrophages was 9–10 days. Therefore, CFU counts were taken on days 0, 4 and 8. The difference in intracellular multiplication of the pathogen between days 4 and 8 in high and low responders was statistically significant, despite the limitation of number of blood donors. This difference should also be viewed in the light of the fact that all donors were BCG-vaccinated. Results of a major field trial, however, have shown that the BCG vaccine falls short of protecting the Indian population against TB [30].
All donors were positive for antibodies against cell-envelope (membrane) antigens of M. tuberculosis and heat-inactivation of sera did not reduce the antibody levels. Considering antibody rather than complement as the key factor distinguishing TB exposed and unexposed populations, we decided to evaluate the effect of opsonization with antibody-rich, complement-depleted (-inactivated) sera on the uptake and survival of pathogen in donor macrophages. The effect of opsonization with complement on uptake and survival of M. tuberculosis (H37Ra) in the macrophages of healthy unexposed subjects has already been reported [31]. In that study, the authors had noted an increase in uptake of bacteria through complement receptors following opsonization with increasing concentrations of sera and a decrease in uptake following heat-inactivation of the sera.
Upon opsonization with sera from high or low responders, the bacilli became coated with IgG class of antibodies. The high responder macrophages were able to restrict bacterial multiplication to a minimal level with or without opsonization. However, in low responders the multiplication of opsonized bacilli was significantly less than that of the unopsonized bacilli. This observation is supported by previous reports showing enhanced clearance of M. tuberculosis in mice that were infected with bacilli oposonized with the IgG class of antibodies to cell surface antigens [13–15]. Recently, Trivedi et al. [32] have also shown that phagosomes containing particles opsonized with IgG mature rapidly into phagolysosomes and the IgG effect was independent of other particle-associated antigens or serum factors. None the less, despite the restriction imposed by opsonization, bacterial multiplication in low responder macrophages was still significantly higher than that in the high responders. This indicates that opsonization alone cannot bridge the gap between the two groups. Armstrong and Hart [12] had shown an improvement in phagosome–lysosme fusion by opsonization of M. tuberculosis with an immune serum, although it did not lead to an enhancement in intracellular killing of the bacilli. However, their experiments were performed on mouse macrophages, and a heterologous (rabbit) anti-serum to BCG antigens was used for opsonization.
We observed a statistically significant inverse relationship between levels of TNF-α and intracellular multiplication of live or live-opsonized M. tuberculosis. Further, upon infection with live bacilli, the high responder macrophages produced significantly higher levels of TNF-α than the low responders and opsonization did not alter these levels significantly in either group. The role of TNF-α in protection against TB has been well documented. TNF-α blockers used in the therapy of rheumatoid arthritis have been found to be associated with the development of TB in such patients, apparently through activation of a latent or subclinical infection [6, 33]. This can be considered as the most direct evidence for a protective role of TNF-α against TB.
The high responder macrophages also produced significantly higher levels of IL-12 than the low responders upon infection with the unopsonized bacilli. Opsonization, however, enhanced the production of IL-12 in low responders to a level equivalent to that of the high responders. This enhancement coincided with an improved intracellular killing of opsonized bacilli by low responders, although it is not clear what role IL-12 could have in this context. Apparently, IL-12 is a regulatory cytokine which connects the innate and adaptive immune response to mycobacteria and exerts its protective effects mainly through the induction of IFN-γ[34]. Individuals with a deficiency in IL-12 receptors are also highly susceptible to mycobacterial infections [7, 35].
Accumulating data indicate the importance of humoral immunity in the defence against a number of intracellular bacteria including M. tuberculosis[36]. We observed a significantly higher level of anti-M. tuberculosis membrane antibodies in high responders than low responders. However, we could not distinguish the two groups on the basis of antigens recognized by their sera. It would be interesting to see if the recognized antigens (in view of the similarities in molecular masses) share identity with HSP60, HSP70, Ag85 (30 kDa) and PstS (38 kDa), all of which have been shown previously to be important antigens associated with the cell envelope of M. tuberculosis[37, 38]. The presence of antibody to Ag85 in the sera of TB patients has been associated with a good prognosis [39]. Because mycobacterial LAM is an important immunomodulator [40], and as opsonization with LAM-specific monoclonal antibodies have been reported to protect mice against M. tuberculosis infection [14], we were keen to see if the donor sera also recognized LAM. While the cell membrane contained significant amounts of LAM, as evident from staining with anti-LAM monoclonal antibody, corresponding staining with individual sera was somewhat weak. The identity of seroreactive antigens, when available, could throw more light on the nature of the ‘protective’ opsonizing antibodies.
We did not observe any difference between high and low responders with respect to production of NO and H2O2 by infected macrophages. With the limited information available in the literature, there is no direct evidence about the role of reactive oxygen intermediates (ROIs) in the killing of M. tuberculosis within human macrophages [41]. Although the presence of an inducible NO synthase in alveolar macrophages from TB patients has been demonstrated [42], the significance of NO production in human infection remains controversial [10].
In conclusion, our results indicate that: (i) the healthy, subclinically infected population of a TB endemic region can be classified as ‘high’ or ‘low’ responders depending on the efficiency of their macrophages in containing an intracellular infection with M. tuberculosis; (ii) opsonization of bacilli with the natural anti-mycobacterium antibodies present in the sera has some beneficial consequences, particularly for the low responders; and (iii) TNF-α and, to some extent, IL-12 can be considered as important mediators of protection against activation of the subclinical infection.
Acknowledgments
We are grateful to Dr C. M. Gupta (Director, CDRI, Lucknow) for his encouragement and support. Excellent technical assistance was provided by Mr A. S. Verma and Mr Shyam Singh. A. N. G. is grateful to CSIR (India) for a Senior Research Fellowship. This paper is CDRI communication no. 7280.
References
- 1.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–40. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 2.Bloom BR, Murray CJ. Tuberculosis: commentary on a reemergent killer. Science. 1992;257:1055–64. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa T, Uchida H, Kusumoto Y, et al. Increase in tumor necrosis factor alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991;59:3021–5. doi: 10.1128/iai.59.9.3021-3025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olobo JO, Geletu M, Demissie A, et al. Circulating TNF-alpha, TGF-beta, and IL-10 in tuberculosis patients and healthy contacts. Scand J Immunol. 2001;53:85–91. doi: 10.1046/j.1365-3083.2001.00844.x. [DOI] [PubMed] [Google Scholar]
- 5.Denis M, Gregg EO, Ghandirian E. Cytokine modulation of Mycobacterium tuberculosis growth in human macrophages. Int J Immunopharmacol. 1990;12:721–7. doi: 10.1016/0192-0561(90)90034-k. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Reino JJ, Carmona L, Valverde VR, et al. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–7. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 7.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 8.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 9.Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349:1513–15. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 10.Bekker LG, Freeman S, Murray PJ, et al. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol. 2001;166:6728–34. doi: 10.4049/jimmunol.166.11.6728. [DOI] [PubMed] [Google Scholar]
- 11.Scanga CA, Mohan VP, Tanaka K, et al. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect Immun. 2001;69:7711–17. doi: 10.1128/IAI.69.12.7711-7717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong JA, Hart PD. Phagosome–lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teitelbaum R, Glatman-Freedman A, Chen B, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–93. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamasur B, Haile M, Pawlowski A, et al. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–8. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers MA, Gavier-Widen D, Hewinson RG. Antibody bound to the surface antigen MPB83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol Med Microbiol. 2004;41:93–100. doi: 10.1016/j.femsim.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Williams A, Reljic R, Naylor I, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111:328–33. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reljic R, Clark SO, Williams A, et al. Intranasal IFN gamma extends passive IgA antibody protection of mice against Mycobacterium tuberculosis lung infection. Clin Exp Immunol. 2006;143:467–73. doi: 10.1111/j.1365-2249.2006.03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalvani A, Nagvenkar P, Udwadia Z, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–77. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 19.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. 1999;133:15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]
- 20.Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–94. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 22.Mehrotra J, Mittal A, Rastogi AK, et al. Antigenic definition of plasma membrane proteins of bacillus Calmette–Guerin: predominant activation of human T cells by low-molecular-mass integral proteins. Scand J Immunol. 1999;50:411–19. doi: 10.1046/j.1365-3083.1999.00616.x. [DOI] [PubMed] [Google Scholar]
- 23.Markwell MA, Haas SM, Bieber LL, et al. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–10. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 24.Ivanyi J, Sinha S, Aston R, et al. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983;52:528–36. [PMC free article] [PubMed] [Google Scholar]
- 25.Griess P. Bemerkungen zu der abhandlung der H. H. Weselsky und Benedikt ‘Ueber einige azoverbindungen’[Over the azo reactions] Chem Ber. 1879;12:426.. [Google Scholar]
- 26.Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46:211–26. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 27.Marciniuk DD, McNab BD, Martin WT, Hoeppner VH. Detection of pulmonary tuberculosis in patients with a normal chest radiograph. Chest. 1999;115:445–52. doi: 10.1378/chest.115.2.445. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 29.Dewan PK, Grinsdale J, Kawamura LM. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin Infect Dis. 2007;44:69–73. doi: 10.1086/509928. [DOI] [PubMed] [Google Scholar]
- 30.Narayanan PR. Influence of sex, age and nontuberculous infection at intake on the efficacy of BCG: re-analysis of 15-year data from a double-blind randomized control trial in South India. Indian J Med Res. 2006;123:119–24. [PubMed] [Google Scholar]
- 31.Hirsch CS, Ellner JJ, Russell DG, et al. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol. 1994;152:743–53. [PubMed] [Google Scholar]
- 32.Trivedi V, Zhang SC, Castoreno AB, et al. Immunoglobulin G signaling activates lysosome/phagosome docking. Proc Natl Acad Sci USA. 2006;103:18226–31. doi: 10.1073/pnas.0609182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 35.Jouanguy E, Doffinger R, Dupuis S, et al. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–51. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 36.Guirado E, Amat I, Gil O, et al. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–9. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Gu S, Chen J, Dobos KM, et al. Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Mol Cell Proteomics. 2003;2:1284–96. doi: 10.1074/mcp.M300060-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Sinha S, Kosalai K, Arora S, et al. Immunogenic membrane-associated proteins of Mycobacterium tuberculosis revealed by proteomics. Microbiology. 2005;151:2411–19. doi: 10.1099/mic.0.27799-0. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Rodriguez C, Estrada-Chavez C, Garcia-Vigil J, et al. An IgG antibody response to the antigen 85 complex is associated with good outcome in Mexican Totonaca Indians with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2002;6:706–12. [PubMed] [Google Scholar]
- 40.Hmama Z, Sendide K, Talal A, et al. Quantitative analysis of phagolysosome fusion in intact cells: inhibition by mycobacterial lipoarabinomannan and rescue by an 1-alpha, 2,5-dihydroxyvitamin D3-phosphoinositide 3-kinase pathway. J Cell Sci. 2004;117:2131–40. doi: 10.1242/jcs.01072. [DOI] [PubMed] [Google Scholar]
- 41.Lau YL, Chan GC, Ha SY, et al. The role of phagocytic respiratory burst in host defense against Mycobacterium tuberculosis. Clin Infect Dis. 1998;26:226–7. doi: 10.1086/517036. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]


