Abstract
Previous studies have shown that orphanin FQ/nociceptin (OFQ/N), the endogenous ligand of the opioid receptor-like (ORL-1) receptor, reduces the rewarding and addictive properties of cocaine and other drugs of abuse. In the present study, using the conditioned place preference (CPP) paradigm, as an animal model of drug reward, we assessed whether the rewarding action of acute cocaine would be altered in mice lacking the ORL-1 receptor or in wild type mice treated with J-113397, an ORL-1 receptor antagonist, relative to their saline-treated controls. On day 1, mice were tested for their baseline place preferences, in which each mouse was placed in the neutral chamber of a three-chambered CPP apparatus, allowed to freely explore all the chambers and the amount of time that a mouse spent in each conditioning chamber was recorded for 15 min. On days 2–3, mice received once daily alternate-day saline/cocaine (15 or 30 mg/kg) conditioning for 30 min. On day 4, mice were tested for their postconditioning preferences, as described for day 1. In a subsequent study, the effect of J-113397 (3 mg/kg) on the rewarding action of acute cocaine (15 mg/kg) was also examined in wild type mice. Our results showed that mice lacking the ORL-1 receptor expressed greater CPP than their wild type littermates. Furthermore, the rewarding action of cocaine was enhanced in the presence of J-113397 in wild type mice. Together, the present results suggest that the endogenous OFQ/N/ORL-1 receptor system is involved in the rewarding action of acute cocaine.
Keywords: Cocaine, orphanin FQ/Nociceptin, ORL-1 receptor, knockout mouse, conditioned place preference, Acute reward
Introduction
The opioid receptor-like (ORL-1) receptor is a G-protein-coupled receptor that shows high degree of sequence homology to traditional opioid receptors (for review, see Mogil and Pasternak, 2001). Furthermore, the endogenous ligand of the ORL-1 receptor, known as orphanin FQ (Reinscheid et al., 1995) or nociceptin (Meunier et al., 1995), a heptadecapeptide, also shows some degree of sequence homology to endogenous opioid peptides, and in particular to dynorphin A (Reinscheid et al., 1998). The ORL-1 receptor and its endogenous ligand are widely distributed throughout the central nervous system (CNS) and particularly in brain regions involved in motivational and emotional behaviors (Neal, Jr. et al., 1999b; Neal, Jr. et al., 1999a). Specifically, in situ hybridization and immunohistochemical studies have demonstrated localization of the ORL-1 receptor in the ventral tegmental area (VTA) (Maidment et al., 2002; Norton et al., 2002), where the cell bodies of the mesolimbic dopaminergic reward circuitry originate. Consistent with its localization, behavioral studies have shown that OFQ/N suppresses basal motor activity (Devine et al., 1996; Lutfy et al., 2001), at least in part, through an action in the VTA (Lutfy et al., 2002; Narayanan et al., 2004).
There is a growing body of evidence implicating the OFQ/N/ORL-1 receptor system in the rewarding and addictive properties of drugs of abuse. For example, intracerebroventricular (ICV) OFQ/N administration has been shown to decrease morphine-stimulated extracellular dopamine in the nucleus accumbens (Nuc Acc) in freely behaving rats (Di Giannuario et al., 1999). In parallel with this, ICV administration of OFQ/N has been reported to block the development of morphine-induced CPP in rats (Ciccocioppo et al., 2000; Murphy et al., 1999). OFQ/N has also been demonstrated to attenuate the acquisition of amphetamine-induced CPP (Kotlinska et al., 2003) and expression of cocaine-induced CPP in rats (Kotlinska et al., 2002). Furthermore, ICV administration of OFQ/N has been shown to attenuate the development of cocaine-induced CPP in mice (Sakoori and Murphy, 2004). However, it is not known whether the rewarding action of cocaine could be altered if the ORL-1 receptor is deleted or blocked pharmacologically using an ORL-1 receptor antagonist. Thus, we examined whether cocaine-induced CPP, an animal model of reward (Bardo and Bevins, 2000), would be altered in mice lacking the ORL-1 receptor or affected in wild type mice treated with J-113397, an ORL-1 receptor antagonist (Kawamoto et al., 1999). Our hypothesis was that if OFQ/N decreases cocaine-induced CPP, then deletion or pharmacological antagonism of the ORL-1 receptor would lead to an increase in cocaine-induced CPP.
Materials and Methods
Subjects
Male mice were housed 2–4 per cage with free access to food and water in a temperature- and humidity-controlled room on a 12-h light/12-h dark cycle. All experiments were conducted in accordance with the ethical guidelines of the National Institute of Health and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences (Pomona, California, USA). All observations were made during the light cycle.
Drugs
Cocaine hydrochloride was obtained from Sigma (St. Louis, MO, USA). J-113397 was generously supplied by the NIDA Drug Supply (RTI International, Research Triangle Park, North Carolina, USA). All doses of the drug used in this study are for the salt form of the drug and were prepared in normal saline and administered in a volume of 10mL/kg in mice.
Experimental Procedures
Dose-response relationship of cocaine-induced conditioned place preference (CPP) in mice
We first determined whether a single alternate-day saline/cocaine or cocaine/saline conditioning would induce CPP in C57BL/6J mice (12–16 weeks old). The description of the CPP apparatus is provided elsewhere (Marquez et al., 2006). In brief, the CPP apparatus was a three-chambered compartment: a central smaller gray chamber and two conditioning chambers distinguishable by visual (decorated with 1-inch black and white horizontal or vertical stripes) and olfactory (almond or orange scent) cues. The CPP paradigm was carried out over a four-day period. On day 1, mice were tested for basal place preferences toward the CPP conditioning chambers. On this day, each mouse was placed in the central neutral chamber of the CPP apparatus and allowed to explore all three chambers for 15 min. The amount of time that the mouse spent in each chamber was recorded and used for analysis of the preconditioning preference (Day 1). Mice were then divided into three groups to receive a single alternate-day saline/saline or saline/cocaine or cocaine/saline conditioning as shown below:
| Day 2 | Day 3 | |
|---|---|---|
| Group 1 | Saline | Saline |
| Group 2 | Saline (or Cocaine15) | Cocaine15 (or Saline) |
| Group 3 | Saline (or Cocaine30) | Cocaine30 (or Saline) |
As shown above, mice were treated with either saline or cocaine (15 or 30 mg/kg) on day 2 and confined to the vehicle-paired chamber (if they received saline) or drug-paired chamber (if they received cocaine) for 30 min. On day 3, mice were treated with the alternate treatment (saline or cocaine) and confined to the opposite conditioning chamber for 30 min. The assignment of the treatments (saline or cocaine) to the conditioning chambers was balanced. On day 4, each mouse was tested for postconditioning place preference as described for preconditioning place preference (day 1).
The role of the ORL-1 receptor in the rewarding action of acute cocaine
Mice lacking the ORL-1 receptor (Nishi et al., 1997), crossed on C57BL/6J strain for 6–8 generations, and their wild type littermates were tested for basal place preferences toward the conditioning chambers on day 1, as described above. On days 2 and 3, mice received cocaine (15 or 30 mg/kg) or saline conditioning sessions. On day 4, mice were tested for their postconditioning place preferences. The assignment of the treatments (saline or cocaine) to the conditioning chambers was balanced, as described above. To further confirm the results obtained in the ORL-1 knockout mice, in a subsequent study, the effect of J-113397, an ORL-1 receptor antagonist (Kawamoto et al., 1999), on cocaine-induced CPP was determined in wild type mice. Mice were tested for baseline place preference on day 1. On day 2, mice were treated with saline or J-113397 (3 mg/kg, s.c.), followed 15 min later by an injection of saline or cocaine (15 mg/kg, i.p.), and confined to the conditioning chambers for 30 min. The choice of the dose of J-113397 was based on our previous results (Lutfy et al., 2003). On the following conditioning day (day 3), mice treated with saline on day 2 were injected with saline or J-113397 and those treated with J-113397 on that day received saline followed by saline or cocaine and confined to the opposite conditioning chamber for 30 min leading to four groups: saline-saline, saline-cocaine, J-113397-saline and J-113397-cocaine. On day 4, mice were tested for postconditioning preference, as described above.
Data Analysis
Data are presented as means (±SEM) and analyzed using a two-way analysis of variance (ANOVA) followed by the Least Square of Means to reveal significant differences between different treatments/genotypes. A CPP response was considered to be present if the mice spent significantly greater time in the drug-paired chamber as compared to their saline-paired chamber. A p<0.05 was considered statistically significant.
Results
A single alternate-day saline/cocaine (30 mg/kg) conditioning induced CPP in C57BL/6J mice
Figure 1 illustrates preference of mice towards the vehicle-paired (open bars) and drug-paired (black bars) chambers on the preconditioning day (Day 1) and postconditioning day (Day 4). A two-factor ANOVA (conditioning chambers and doses of cocaine) of the postconditioning data (Day 4) revealed a significant interaction between conditioning chamber and dose (F2,36 = 4.69; p<0.02). Further analysis of data revealed a significant increase in the amount of time spent in the drug-paired over the vehicle-paired chamber in mice conditioned with the high dose cocaine (30 mg/kg). This result indicates that a single alternate-day saline/cocaine (30 mg/kg) conditioning session induces a significant CPP.
Fig. 1. High dose cocaine (30 mg/kg) induced CPP following a single conditioning.
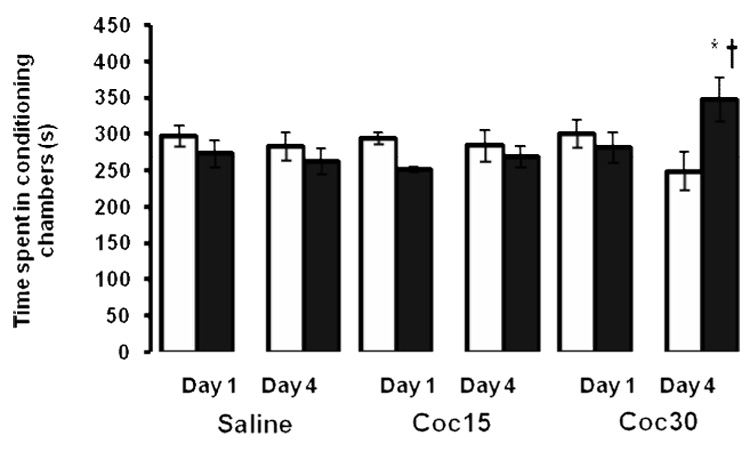
Mice were tested for baseline place preference toward the vehicle-paired (open bars) and drug-paired (closed bars) chambers on day 1. On days 2 and 3, mice were treated with either saline or cocaine (15 or 30 mg/kg) and confined to the conditioning chambers for 30 min. On day 4, mice were tested for postconditioning preference. The data are mean (±SEM) of 6–8 mice /group. *p<0.05 compared to its respective vehicle-paired chamber; †p<0.05 compared to drug-paired chamber of saline and cocaine (15 mg/kg) groups.
CPP induced by a single cocaine conditioning was enhanced in mice lacking the ORL-1 receptor
Our dose-response study showed that a single alternate-day saline/cocaine (30 mg/kg), as compared to saline/saline, conditioning session produced a significant CPP in C57BL/6J mice (Fig. 1). Thus, we first used this paradigm to determine whether the rewarding action of acute cocaine (30 mg/kg) would be altered in mice lacking the ORL-1 receptor (Fig. 2). A two-factor ANOVA (conditioning chambers and genotype) revealed a significant interaction between conditioning chamber and genotype (F1,36 = 9.40; p<0.03). Further analysis of data revealed a significant increase in the amount of time spent in the drug-paired as compared to vehicle-paired chamber for both wild type and mutant mice (p<0.05), showing that both genotypes expressed CPP. However, the magnitude of this response was significantly greater in ORL-1 receptor knockout mice as compared to their wild type littermates (p<0.05). We next assessed whether the rewarding action of a lower dose of cocaine (15 mg/kg) would be altered in mice lacking the ORL-1 receptor as compared to their wild type littermates (Fig. 3). Consistent with our dose-response study in C57BL/6J mice (Fig. 1), conditioning with the lower dose of cocaine (15 mg/kg) failed to induce CPP in wild type mice (p<0.05; compare vehicle-paired chamber versus drug-paired chamber). In contrast, the same conditioning paradigm elicited a significant CPP in ORL-1 receptor knockout mice, which was evidenced as a significant increase in the amount of time spent in the drug-paired versus the vehicle-paired chamber in mutant mice (p<0.05).
Fig. 2. The rewarding action of acute cocaine was enhanced in mice lacking the ORL-1 receptor.
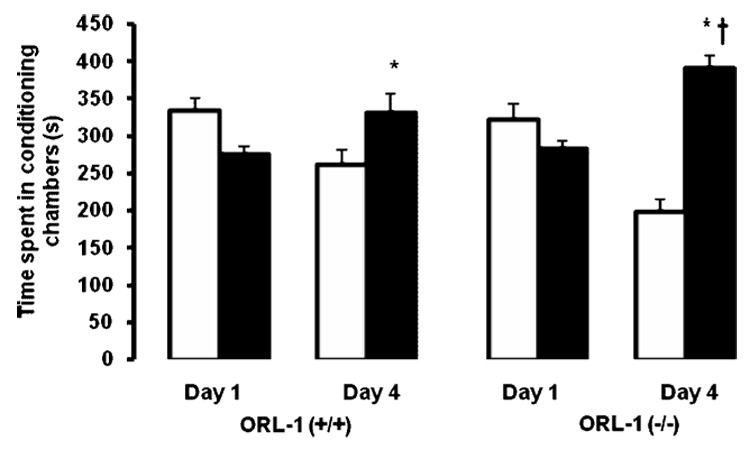
Mice were tested for preconditioning preference on day 1, received a single alternate-day saline/cocaine (30 mg/kg) or cocaine/saline conditioning session on days 2 and 3 and then tested for postconditioning preference on day 4. The amount of time spent in the vehicle-paired (open bars) and drug-paired (closed bars) chamber was recorded for 15 min on each test day (Days 1 and 4). The data are mean (±SEM) of 10 mice /genotype. *p<0.05 compared to their respective vehicle-paired chamber; †p<0.05 compared to drug-paired chamber in wild type littermates.
Fig. 3. Mice lacking the ORL-1 receptor expressed CPP following conditioning with a lower dose of cocaine (15 mg/kg).
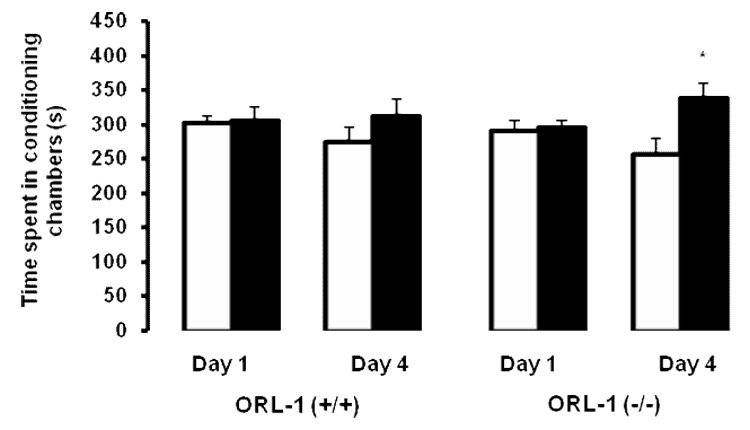
Mice were treated as described under the legend for figure 2 except the dose of cocaine was 15 mg/kg in this experiment. The data are mean (±SEM) of 10 mice/genotype. *p<0.05 compared to its respective vehicle-paired chamber.
The rewarding action of acute cocaine was increased in the presence of J-113397, an ORL-1 receptor antagonist
Given the complexity of the knockout mice, we also determined whether the rewarding action of acute cocaine would be altered in mice injected with J-113397 (3 mg/kg) prior to cocaine conditioning (Fig. 4). In the absence of cocaine, J-113397 alone, failed to induce any CPP in wild type mice (data not shown). Once again, consistent with our dose-response study (Fig. 1) and the result of CPP with the low dose cocaine in wild type mice (Fig. 3), conditioning with cocaine (15 mg/kg) failed to induce CPP in wild type mice, which was evidenced as no significant difference in the amount of time spent in the drug-paired versus vehicle-paired conditioning chamber (p>0.05). However, pretreatment with J-113397 prior to the cocaine conditioning session significantly increased the amount of time spent in the drug-paired as compared to vehicle-paired chamber (p<0.05), showing that cocaine induced CPP in the presence of J-113397. This result confirms our result obtained in mice lacking the ORL-1 receptor, showing the importance of the endogenous OFQ/N/ORL-1 receptor system in the rewarding action of acute cocaine.
Fig. 4. The rewarding action of cocaine was enhanced in the presence of J-113397, an ORL-1 receptor antagonist.
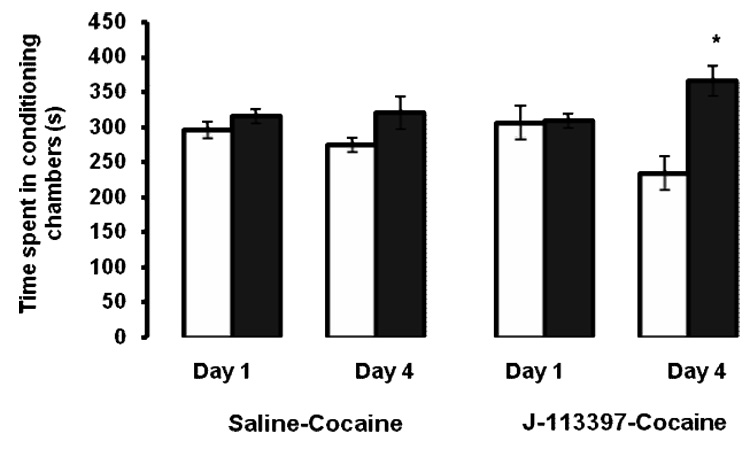
Mice were tested for preconditioning preference on day 1, received a single alternate/day saline/cocaine (15 mg/kg) or cocaine/saline conditioning session in the presence or absence of J-113397 on days 2 and 3 and then tested for postconditioning preference on day 4, in which the amount of time spent in the vehicle-paired (open bars) and drug-paired (closed bars) chamber was recorded for 15 min. The data are mean (±SEM) of 7–9 mice /group. *p<0.05 compared to its vehicle-paired chamber.
Discussion
Ample evidence suggests that OFQ/N, the endogenous ligand of the ORL-1 receptor, acts to negatively modulate the function of the mesolimbic dopaminergic reward circuitry (Lutfy et al., 2001; Lutfy et al., 2002; Maidment et al., 2002; Murphy and Maidment, 1999; Narayanan et al., 2004; Norton et al., 2002; Zheng et al., 2002) and to attenuate the rewarding and addictive properties of drugs of abuse, such as morphine and cocaine (Ciccocioppo et al., 2000; Di Giannuario et al., 1999; Kotlinska et al., 2002; Kotlinska et al., 2003; Lutfy et al., 2001; Lutfy et al., 2002; Murphy et al., 1999; Sakoori and Murphy, 2004). In the present study, we tested the hypothesis that the endogenous OFQ/N/ORL-1 receptor system functions to reduce the rewarding action of acute cocaine.
In order to test our hypothesis, we first established a paradigm to represent the rewarding action of acute cocaine. Our results explicitly demonstrated that a single conditioning can induce CPP in mice and this response is dependent on the dose of cocaine. We then used this paradigm and determined whether the rewarding action of acute cocaine would be altered in mice lacking the ORL-1 receptor. We observed that a single conditioning session with cocaine (30 mg/kg) induced CPP in wild type mice, which was greater in ORL-1 receptor knockout mice, indicating that the endogenous OFQ/N/ORL-1 receptor system functions to weaken processes leading to acquisition of cocaine CPP, thereby shifting the dose-response curve of cocaine to the right in wild type mice. In order to test this possibility, we then assessed whether the rewarding action of a lower dose of cocaine (15 mg/kg) would be altered in ORL-1 receptor knockout mice as compared to their wild type littermates. As expected, conditioning with this dose of cocaine failed to induce CPP in wild type mice. In contrast, a significant CPP was observed in ORL-1 knockout mice, suggesting that the endogenous OFQ/N/ORL-1 receptor system serves as a negative modulator of the rewarding action of acute cocaine.
In order to provide further support for this notion, we tested whether the acute rewarding action of cocaine (15 mg/kg) would be enhanced by J-113397, an ORL-1 receptor antagonist (Kawamoto et al., 1999). Mice pretreated with J-113397, as compared to saline-treated controls, displayed a robust CPP, emphasizing the modulatory role of the endogenous OFQ/N/ORL-1 receptor system on cocaine reward. A previous report showed CPP following repeated administration of J-113397 in both wild type and ORL-1 receptor knockout mice (Koizumi et al., 2004). However, in our case, a single alternate-day conditioning with J-113397 failed to induce any CPP (data not shown). Thus, the enhanced CPP observed in the present study is due to a direct or indirect interaction of cocaine with the endogenous OFQ/N/ORL-1 receptor system which requires further investigation. Presently, it is not fully understood how lack of the ORL-1 receptor or its blockade by J-113397 has led to the enhanced cocaine-induced CPP. Previous studies have demonstrated that the VTA could be one of the brain regions where OFQ/N could exert its modulatory actions (Lutfy et al., 2002; Maidment et al., 2002; Murphy and Maidment, 1999; Norton et al., 2002; Zheng et al., 2002). Thus, we speculate that cocaine conditioning may cause the release of OFQ/N in the VTA or elsewhere along the mesolimbic reward circuitry, a response that can lead to reduction in the strength of the conditioned response in wild type mice. However, this endogenous regulatory mechanism is absent or blocked, thereby leading to enhanced CPP in ORL-1 receptor knockout mice or in mice treated with J-113397. Indeed, our preliminary data showed that cocaine altered the level of endogenous OFQ/N in the midbrain and other limbic structures in rats (Narayanan et al., 2000). However, further studies are needed to determine whether cocaine could cause the release of endogenous OFQ/N in brain regions implicated in cocaine reward and addiction.
In summary, we have used a combination of pharmacologic and knockout mouse strategies to demonstrate that cocaine-induced CPP was enhanced in the presence of J-113397, an ORL-1 receptor antagonist, as well as in mice lacking the ORL-1 receptor. Together, the current results suggest that the endogenous OFQ/N/ORL-1 receptor system is critically involved in the rewarding action of cocaine and may represent a potential target for the development of drugs for pharmacotherapy of cocaine addiction.
Acknowledgments
The authors wish to thank Dr. Arbi Nazarian for his suggestions and comments. We also express our gratitude to Dr. Hiroshi Takeshima for generous supply of the ORL-1 receptor heterozygous breeding pairs. The present study was supported in part by the NIDA Grant DA016682.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 2.Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- 3.Devine DP, Taylor L, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- 4.Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, et al. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397) J Med Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi M, Sakoori K, Midorikawa N, Murphy NP. The NOP (ORL1) receptor antagonist Compound B stimulates mesolimbic dopamine release and is rewarding in mice by a non-NOP-receptor-mediated mechanism. Br J Pharmacol. 2004;143:53–62. doi: 10.1038/sj.bjp.0705906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotlinska J, Rafalski P, Biala G, Dylag T, Rolka K, Silberring J. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur J Pharmacol. 2003;474:233–239. doi: 10.1016/s0014-2999(03)02081-8. [DOI] [PubMed] [Google Scholar]
- 8.Kotlinska J, Wichmann J, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol. 2002;13:229–235. doi: 10.1097/00008877-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, et al. Buprenorphine-induced antinociception is mediated by muopioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23:10331–10337. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maidment NT, Chen Y, Tan AM, Murphy NP, Leslie FM. Rat ventral midbrain dopamine neurons express the orphanin FQ/nociceptin receptor ORL-1. Neuroreport. 2002;13:1137–1140. doi: 10.1097/00001756-200207020-00013. [DOI] [PubMed] [Google Scholar]
- 13.Marquez P, Baliram R, Gajawada N, Friedman TC, Lutfy K. Differential involvement of enkephalins in analgesic tolerance, locomotor sensitization, and conditioned place preference induced by morphine. Behav Neurosci. 2006;120:10–15. doi: 10.1037/0735-7044.120.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 15.Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- 16.Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- 17.Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- 18.Narayanan S, Lam H, Carroll FI, Lutfy K. Orphanin FQ/nociceptin suppresses motor activity through an action along the mesoaccumbens axis in rats. J Psychiatry Neurosci. 2004;29:116–123. [PMC free article] [PubMed] [Google Scholar]
- 19.Narayanan S, Lam H, Maidment NT, Lutfy K. Abs, 31stINRC. Washington, USA: 2000. Cocaine sensitization increases the level of orphanin FQ-immunoreactivity in the rat hypothalamus. [Google Scholar]
- 20.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999a;412:563–605. [PubMed] [Google Scholar]
- 21.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- 22.Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, et al. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton CS, Neal CR, Kumar S, Akil H, Watson SJ. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J Comp Neurol. 2002;444:358–368. doi: 10.1002/cne.10154. [DOI] [PubMed] [Google Scholar]
- 24.Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem. 1998;273:1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- 25.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 26.Sakoori K, Murphy NP. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 2004;172:129–136. doi: 10.1007/s00213-003-1643-3. [DOI] [PubMed] [Google Scholar]
- 27.Zheng F, Grandy DK, Johnson SW. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br J Pharmacol. 2002;136:1065–1071. doi: 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]


