Abstract
3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine (METH) are amphetamine analogues with similar persistent neurochemical effects in the mouse which some have described as neurotoxicity. We attempted to identify dose regimens of MDMA and METH with similar effects on behavioral and physiological variables in the mouse, then quantified the effects of these dose regimens on neurochemistry and microglial markers. Four discrete injections of saline, MDMA (10, 20, or 30 mg/kg), or METH (5 or 10 mg/kg) were administered to mice at 2 h intervals. Body weight was quantified immediately before each injection, and 2 h after the last injection, while core temperature and locomotor activity were continuously monitored via radiotelemetry. Mice were sacrificed 72 h after the final injection and brains were rapidly dissected on ice. Dopamine content in various brain regions was quantified via high pressure liquid chromatography (HPLC), and microglial activation was assessed by saturation binding of the peripheral benzodiazepine receptor (PBR) ligand [3H]PK11195. Specific dose regimens of MDMA and METH induced similar reductions in body weight, depletions of dopamine and its metabolites, and similar hyperthermic and locomotor stimulant effects, but only METH activated microglia in striatum. These results suggest that repeated high doses of MDMA and METH that produce hyperthermia, locomotor stereotypy, weight loss and neurochemical depletion are not consistently accompanied by microglial activation. The finding that METH, but not MDMA, induces microglial effects in the striatum consistent with neurotoxicity might imply different mechanisms of toxic action for these two psychostimulants.
The neurotoxic effects of amphetamine analogues were first described by Pletscher and coworkers (1963; 1964) in reports demonstrating sustained depletions of serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in rats previously treated with p-chloroamphetamine (PCA). Characterization of the neurotoxic effects of PCA in the rat was furthered by studies reporting inhibition of 5-HT synthesis and uptake (Sanders-Bush and Sulser, 1970), as well as persistent anatomical changes (Harvey et al., 1975) following a single administration of the drug. Study of the neurotoxic effects of other amphetamine analogues soon followed. In this regard, Gibb and colleagues reported a long-lasting inhibition of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine (DA) synthesis, following repeated high doses of methamphetamine (METH) in the rat, as well as a concomitant depletion of DA and its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) (Gibb et al., 1990). Continued study revealed that the effects of METH are even more pronounced on brain 5-HT systems in the rat. Following administration of large doses of METH, an enormous (greater than 90%) long-lasting inhibition of tryptophan hydroxylase (TPH, the rate-limiting enzyme in 5-HT synthesis), as well as sustained decrease in 5-HT and 5-HIAA content were observed (Gibb et al., 1994).
Studies of the toxic effects of 3,4-methylenedioxymethamphetamine (MDMA) present a somewhat more complex picture, but the effects of this compound on 5-HT systems are generally regarded to be more selective and more potent than those of METH in most species. In rats, MDMA transiently inhibits TPH activity and depletes 5-HT and 5-HIAA after a single large dose, although these effects may ameliorate if dosing is halted for at least 2 weeks (Gibb et al., 1990). These neurochemical effects are specific to 5-HT systems, as MDMA administration has not been shown to alter levels of HVA, 3-methoxy-4-hydroxyphenylethyleneglycol (MHPG, a major metabolite of NE), DA or NE (DeSouza et al., 1990; Ricaurte et al., 2000) in the rat or in primates. In the mouse, the persistent effects of METH and MDMA primarily impact central DA (Colado et al., 2004; Easton and Marsden, 2006), although high and repeated dose regimens of both compounds may also elicit 5-HT decrements (Gibb et al., 1994). Despite these profound and long-lasting effects, only rarely have reactive gliosis and behavioral consequences been demonstrated following MDMA administration to animal species in which this compound has selective 5-HT effects (Kish, 2002; Wang et al., 2004). This has served to maintain a continued debate regarding the appropriateness of the term “neurotoxicity” when applied to the neurochemical changes induced by MDMA (e.g. Grob, 2000; Kalia, 2000).
In this regard, there are at least three morphological criteria that are generally agreed to indicate neural damage: frank cell loss, argyrophilia (silver staining) and reactive gliosis (O'Callaghan and Miller, 1993). With regards to the latter measure, immunocytochemistry techniques have traditionally been used to quantify glial fibrilliary acidic protein (GFAP), the major protein of astrocyte intermediate filaments, in various brain preparations following application of some suspected toxicant (O'Callaghan and Miller, 1993). More recently, binding of the peripheral benzodiazepine receptor (PBR, recently renamed as Translocator protein [18 kDa]) ligand 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide ([3H]PK11195) has been employed as an index of microglial activation. As the name implies, PBR are expressed in the periphery, but are also located centrally in glial cells, primarily in astrocytes and microglia (Park et al., 1996). The functions of this receptor within the CNS are not clear at this time, although PBR have been shown to regulate neurosteroid synthesis (Papadopoulos et al., 2006), mitochondrial function (Casellas et al., 2002) and neuroinflammation in microglial cells (Vinnetti et al., 2006), all of which could play a role in neurotoxicity. Multiple studies have reported that the density of PBR increases concomitantly with glial activation following neural injury induced by various insults, including inflammation, metabolic stress, trauma, transient global forebrain ischemia and chemically-induced brain injury (Casellas et al., 2002). In some cases of CNS injury, microgliosis can occur rapidly after the insult (Stephenson et al., 1995; Streit and Graeber, 1993), leading some to speculate that this early increase in PBR density may be an index of microglial activation which could be used as an antecedent, but indirect, marker of neuronal damage (Benavides et al., 1987; Escubedo et al., 1998). Most germane to the present research, microglial activation has been described as a pharmacologically specific marker for the neurotoxic amphetamines in mice, and may represent one of the earliest components of the neurotoxic process induced by particular dose regimens of these drugs (Thomas et al., 2004).
In the present experiments, we studied several different doses of METH and MDMA across identical injection regimens in the mouse, as previous research has determined that both METH and MDMA have the capacity to deplete DA and 5-HT, to increase striatal GFAP expression, and to cause fiber and terminal degeneration as revealed by silver staining to a similar extent in this species (O'Callaghan and Miller, 1994). Thus, in the mouse, any observed differences between the effects of METH and MDMA on PBR binding potential are less likely to be due to different neurochemical effects across compounds (Sprague et al., 1998). The goal was to employ effect scaling (e.g. Wang et al., 2004) to equate dose regimens of METH and MDMA which may differ along pharmacokinetic parameters based on the assumption that drug doses which induce comparable effects (behavioral, physiological, neurochemical, etc.) may be considered equivalent, and therefore directly compared. These studies thus represent the first such attempt to compare the neurotoxic effects of METH and MDMA at equivalent doses, since previous reports (e.g., Pubill et al., 2003) employed only single dose regimens of each compound with no corresponding measures of dose equivalence.
Experimental Procedures
Animals
Male NIH Swiss mice (Harlan Sprague Dawley Inc., Indianapolis, IN) weighing approximately 20-30 g were housed 12 animals per 44.5 × 22.3 × 12.7 cm Plexiglas cage in a temperature-controlled room at the University of Michigan that was maintained at an ambient temperature of 22±2°C at 45-50% humidity. Lights were set to a 12-h light/dark cycle. Animals were fed Lab Diet rodent chow (Laboratory Rodent Diet #5001, PMI Feeds, Inc., St. Louis, MO) and water ad libitum until immediately before testing. Animals were not used in experiments until at least 2 days after arrival in the laboratory, and were tested in groups of at least 6. Each animal received only a single dose regimen of saline, METH or MDMA, and was sacrificed (see below) immediately after use. All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health. Experimental protocols were approved by the Animal Care and Use Committee at the University of Michigan.
Core temperature and locomotor activity experiments
Following appropriate anesthetization with ketamine (100 mg/kg, intraperitoneal [ip]) and xylazine (10 mg/kg, ip), the abdominal area of each mouse was shaved and sanitized with iodine swabs. A rostral-caudal cut approximately 1.5 cm in length was made with skin scissors, providing access to the intraperitoneal cavity. A cylindrical glass-encapsulated radiotelemetry probe (model ER-4000 E-Mitter, Mini Mitter, Bend, OR, USA) was then inserted, and the incision was closed using absorbable 5-0 chromic gut suture material. Surgeries were carried out at least 7 days before initiation of the METH or MDMA drug regimens, allowing time for incisions to heal and for mice to recover normal body weights. Following surgery, all implanted mice were individually housed in 15.24 × 25.40 × 12.70 cm Plexiglas mouse cages for the duration of all temperature and locomotor activity experiments. Implanted transmitters produced activity- and temperature-modulated signals which were sent to a receiver (model ER-4000 Receiver, Mini Mitter Co., Inc.) underneath each mouse cage; motor stereotypy is not obtained from telemetry data, but its presence or absence was noted by trained observers. On experimental days, mice were weighed, marked, and returned to their individual cages. METH and MDMA doses were then calculated and prepared for injection. Animals were subsequently removed from their cage and injected with various doses of METH, MDMA or equivolume saline and returned to their cage, while temperature and locomotor activity data were collected at regular intervals and processed simultaneously by the Vital View data acquisition system (Mini Mitter Co., Inc.) for 24 hours. Body weight was thus quantified immediately before each injection and 2 h after the last injection.
Brain dissection and tissue preparation
Seventy two hours after termination of an injection regimen, mice were euthanized by cervical dislocation and decapitation. This time point was previously demonstrated to yield maximal neurochemical depletion and microglial activation as assessed by [3H]PK11195 binding in rats administered METH (Escubedo et al., 1998; Pubill et al., 2004). Brains were rapidly removed on ice, and dissected to hippocampus, cortex, striatum, cerebellum, thalamus, hypothalamus and brainstem regions using blunt curved microforceps. Tissue samples were immediately placed into cryovials on dry ice, then stored at –70° until assay. Although only cortical and striatal samples were analyzed for markers of neurotoxicity, samples from all dissected regions were used to establish the basal binding potential of [3H]PK11195 in the mouse (see Table 1).
Table 1.
Equilibrium dissociation constants (KD, in nM) and maximal binding (Bmax, in fmol/mg protein) of [3H]PK11195 in various regions of the mouse brain, as determined by full saturation binding analysis. Data represent the mean of six samples ± SEM. See the Methods section for more details.
| Region | KD (nM) | Bmax (fmol / mg) |
|---|---|---|
| Hippocampus | 1.87 ± 0.31 | 1597.67 ± 91.30 |
| Cortex | 1.43 ± 0.14 | 1089.00 ± 31.61 |
| Striatum | 1.28 ± 0.46 | 1059.13 ± 298.58 |
| Cerebellum | 1.83 ± 0.56 | 1609.67 ± 138.99 |
| Thalamus | 0.78 ± 0.08 | 623.87 ± 24.06 |
| Hypothalamus | 1.35 ± 0.21 | 725.00 ± 99.37 |
| Brainstem | 1.17 ± 0.08 | 1054.13 ± 38.88 |
Quantification of dopamine and metabolites
For tissue determinations, each sample was transferred to 400 μl ice-cold 0.1 N perchloric acid containing N-methyl-5-HT and 3,4-dihydroxybenzylamine as internal standards (for indoleamines and catecholamines, respectively). The tissue was sonicated in this solution and centrifuged at 23,000 × g for 20 min at 4° C. A portion of the supernatant (50 μl) was removed and analyzed by high pressure liquid chromatography (HPLC) to determine the concentration of DOPAC, HVA, and DA. The column employed was from Bioanalytical Systems (BAS: West Lafayette, IN: Phase II ODS-3mM, 100 mm × 3.2 mm). The on-line degassed mobile phase consisted of an 8% solution of acetonitrile containing 0.6% tetrahydrofuran, 0.1% diethylamine, 0.025 mM ethylenediaminetetra-acetic acid, 2.3 mM 1-octane-sulfonic acid, 30 mM sodium citrate and 13.7 mM sodium dihydrogen phosphate (final pH 3.1), and was delivered at 600 ml/min. Tissue pellets were saved for protein determination using the DC Protein Assay (BioRad, Hercules, CA) with bovine serum albumin as the protein standard. In this system, optical densities are automatically converted into mg units derived from a standard curve. All protein values are based upon the mean of three replicates. Chromatograms were recorded using a DA-5 data acquisition analog to digital interface module coupled to an LC-4C electrochemical detector (BAS). Post-separation signals were derived from a 2 mm glassy-carbon working electrode whose potential was set at 600 mV versus a Ag/Ag Cl reference. Peak height quantification involved dividing the peak height of the unknown by that of the internal standard, and referring this ratio to external standards. Samples from all animals were processed in parallel on the same day for each brain region.
[3H]PK11195 binding
Assay of PBR binding was conducted after the method of Pubill et al. (2004), with some modifications. Specific brain regions from individual mice were pooled together within a given treatment and briefly thawed at room temperature before homogenizing on ice with a Brinkmann Polytron homogenizer set at ‘2’ along with 20 ml of a homogenizing buffer (5 mM TRIS-HCl, 329 mM sucrose, 4.5 μg/μl aprotonin, 0.1 mM phenylmethylsulphonylfluoride, 1mM sodium orthovanidate, pH 7.4) containing 5 mM TRIS-HCl and 320 mM sucrose. Samples were subsequently centrifuged at 15,000 RPM for 30 minutes at 4° C. After centrifugation, the resulting pellets were washed once by resuspension via a Dounce glass homogenizer in fresh homogenizing buffer. After the sample was centrifuged a second time at 15,000 RPM for 30 minutes at 4° C, the pellet was again resuspended in a holding buffer consisting of 50 mM TRISHCl, 120 mM NaCl, and 5 mM KCl. The protein content of each pooled sample region was determined by the Bradford Protein Assay (Bradford 1976). Thus prepared, samples were frozen and stored at -80° C until used in the PBR binding assay. Three replicate aliquots were drawn from all pooled tissue homogenates just prior to binding.
As mouse data collected using this assay had not been previously published at the time these studies were conducted, equilibrium binding assays for basal levels of PBR expression in specific brain regions of interest were initially determined using saline control animals. In order to increase precision, a six point curve for saturation binding was generated using GraphPad Prism and the following concentrations of [3H]PK11195 (specific activity: 85Ci/mmol) (PerkinElmer Life Sciences, Boston MA): 10nM, 5 nM, 2.5 nM, 1.25 nM, 0.625 nM, 0.313 nM. The final volume of each assay was 100 μl, which in addition to [3H]PK11195 also included 20 μl of TRIS-HCl holding buffer and 100 μg of protein. 10 μM of Ro5-4864 (4'-chlorodiazepam) (Sigma, St. Louis, MO) was used to determine the extent of non-specific binding. Basal [3H]PK11195 binding potentials for each region of interest are thus presented in Table 1.
Equilibrium binding assays for PBR expression in animals receiving drug treatment were performed using a single [3H]PK11195 concentration of 8nM. The binding of [3H]PK11195 at 10nM in the saline control samples was used as a basis for comparison between the control and treatment groups, as both concentrations were well within the saturation range of the saline control Scatchard plots. In all binding experiments, samples were incubated for 120 minutes at 0-4° C. In addition, all glassware that came in contact with [3H]PK11195 was siliconized by Sigmacote (Sigma, St. Louis, MO) to minimize loss of [3H]PK11195 via non-specific binding to experimental surfaces. After incubation, samples were filtered under vacuum using a Brandel cell harvester through glass fiber filters (Schleicher and Schuell #32, Keene, NH) that were presoaked in 0.5% polyethyleneimine. Tubes were rapidly washed 3 times in succession with ice cold TRISHCl holding buffer. Finally, the radioactivity on the filters was counted by liquid scintillation in 4 ml of EcoLume scintillation mixture (ICN, Aurora, OH).
Data analysis
Locomotor activity and core temperature data were compared to a single saline control group by two-way repeated measures ANOVA (with Bonferroni adjustment) and analyzed post-hoc after the method of Dunnett. Body weights, as well as data from neurochemistry and PBR binding experiments, were analyzed by one-way repeated measures ANOVA (with Bonferroni adjustment) and analyzed post-hoc using Tukey's HSD test. In the event that normality was violated, groups were compared using a Kruskal-Wallis one way ANOVA on ranks, and all pairwise multiple comparisons were performed using Dunn's method. All statistical tests were performed using commercially available software, and significance was judged at P<0.05. The P values for Dunnett's and Dunn's tests are unavailable from our software, which simply reports whether or not P values are less than the critical value of 0.05.
Drugs
Racemic methamphetamine and racemic MDMA were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC.) Both drugs were weighed as salts, dissolved in physiological saline, and injected intraperitoneally in a final volume of 1.0 ml/100g. [3H]PK11195 (specific activity 88.5 Ci/mmol) was purchased from Perkin-Elmer Life Science (Boston, MA), and Ro5-4864 was purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were obtained from standard commercial sources. MDMA and METH were administered every two hours, for four total injections. This dosing regimen has previously been shown to induce persistent neurochemical depletions and terminal degenerations in the mouse (O'Callaghan and Miller 1994; Miller and O'Callaghan 1995).
Results
Effects on body weight
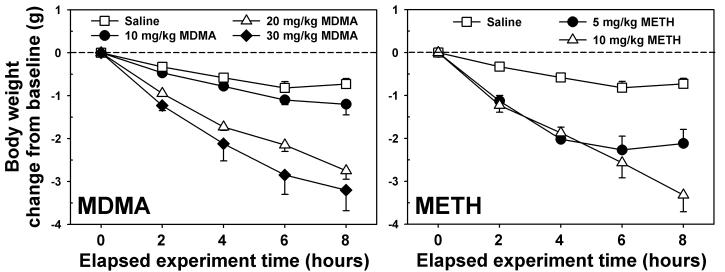
Mice injected with saline every two hours for four total injections lost approximately 0.5 g over the 8 hr observation period (open squares, Figure 1.) Administration of MDMA induced a dose- and time-dependent weight loss over the treatment interval, with mice in the 20 (open triangles, Figure 1, left panel) and 30 mg/kg/inj (closed diamonds, Figure 1, left panel) groups losing approximately 3 g of body weight by 2 h after the final injection. Statistical testing revealed a main effect of dose (F=17.82, p<0.001) and time (F=121.63, p<0.001), and the interaction between these two factors was also significant (F=10.49, p<0.001). Post-hoc analysis indicated that mice treated with 20 or 30 mg/kg MDMA differed from those treated with 10 mg/kg MDMA and their saline controls at all time points after the second injection. Treatment with METH also induced a dose- and time-dependent weight loss over the treatment interval. Statistical testing revealed a main effect of dose (F=12.16, p<0.001) and time (F=80.64, p<0.001), and the interaction between these two factors was also significant (F=8.19, p<0.001). Mice receiving 5 mg/kg/inj METH (closed circles, Figure 1, right panel) shed approximately 2 g of body weight by the last injection, and maintained reduced weights over the subsequent 2 h. In contrast, mice administered 10 mg/kg/inj METH (open triangles, Figure 1, right panel) lost approximately 3 g of body weight by 2 h after the final injection. Post-hoc analysis indicates that mice treated with 5 or 10 mg/kg METH differed from their saline controls at all time points after the second injection, and also that the two METH groups differed significantly from each other at the last time point measured.
Figure 1.
Effects of repeated administration of saline, MDMA (left) or METH (right) on body weight over 8 hours. Mice weighed immediately prior to each injection (administered at times 0, 2, 4, and 6 hours), then again 2 hours after the final injection. Abscissae: Time elapsed since the first injection. Ordinates: Change in initial body weight, expressed in grams. For graphical clarity, statistical differences are described in the Results section. Error bars represent ± SEM.
Effects on locomotor activity
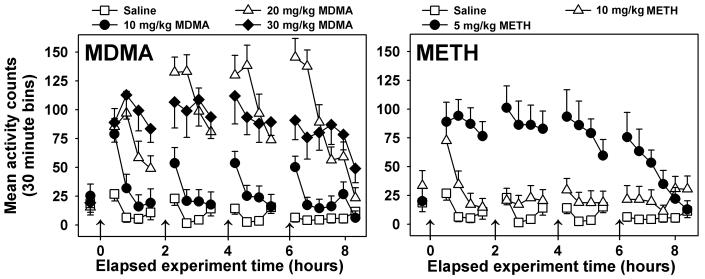
Locomotor activity was greatest in the 30 min periods immediately following saline injections (open squares, Figure 2), but no orderly changes were observed at later time points. Statistical testing confirmed main effects of injection condition (F=15.33, P<0.001), time (F=24.98, P<0.001), and a significant interaction of these two factors (F= 5.37, P<0.001). Post-hoc tests indicated that mice administered 20 or 30 mg/kg MDMA, or 5 mg/kg METH were significantly more active than saline-injected controls over the treatment duration (P<0.05 in all cases). Mice repeatedly injected with 10 mg/kg/inj MDMA (closed circles, Figure 2, left panel) exhibited increased locomotor activity in the 30 min periods following each injection, but activity was not different from saline control animals at other timepoints. In contrast, injection of 20 mg/kg MDMA (open triangles, Figure 2, left panel) profoundly increased locomotor activity at all but the last timepoint measured. The highest dose of MDMA administered (closed diamonds, Figure 2, left panel) induced locomotor stereotypy (visually noted), decreasing ambulatory activity as compared to the 20 mg/kg MDMA group. Nevertheless, mice repeatedly administered 30 mg/kg MDMA exhibited more locomotor activity than did saline control for all timepoints measured. Injection of 5 mg/kg METH (closed circles, Figure 2, right panel) profoundly increased locomotor activity at all but the last two timepoints measured. However, 10 mg/kg METH (open triangles, Figure 2, right panel) induced locomotor stereotypy (visually noted), decreasing ambulatory activity as compared to the 5 mg/kg METH group.
Figure 2.
Effects of repeated administration of saline, MDMA (left) or METH (right) on locomotor activity. Abscissae: Time elapsed since the first injection. Arrows indicate times of drug or saline injections. Ordinates: Activity counts, measured via radiotelemetry every 5 minutes, and presented as 30 minute averages. For graphical clarity, statistical differences are described in the Results section. Error bars represent ± SEM.
Effects on core temperature
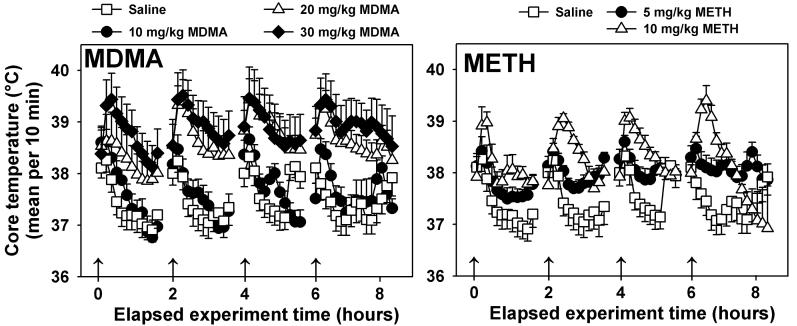
Following saline injection (open squares, Figure 3, both middle panels), brief mild elevations in core temperature were observed in all mice; saline-treated animals recovered normal core temperatures (approximately 37°C) within approximately 20 min post-injection. Statistical testing confirmed main effects of injection condition (F=3.42, P=0.017), time (F=14.77, P<0.05), and a significant interaction of these two factors (F= 3.86, P<0.05). Mice repeatedly administered 10 mg/kg MDMA (closed circles, Figure 3, middle left panel) exhibited temperature responses similar to those of saline-treated mice, but marked hyperthermic responses were observed in mice treated with 20 (open triangles, Figure 3, middle left panel) or 30 mg/kg/inj MDMA (closed diamonds, Figure 3, middle left panel). For both of these groups, temperatures approximately 2.5°C higher than saline control were measured following injection, core temperatures remained elevated above those exhibited by saline controls over the 8 h observation period, and post-hoc testing indicated that both the 20 mg/kg MDMA and 30 mg/kg MDMA dose regimen differed significantly from saline controls (P<0.05). A similar pattern of results was obtained in METH-treated mice. Mice administered 5 mg/kg/inj METH (closed circles, Figure 3, middle right panel) did not express initial hyperthermias following injection, but displayed elevated core temperatures as compared to saline control animals due to the lack of a return to basal temperatures over the treatment regimen. In contrast, mice receiving 10 mg/kg/inj METH (open triangles, Figure 3, middle right panel) exhibited initial transient hyperthermias (approximately 2°C) following each injection, but recovered basal core temperatures by 2 h after the last METH administration. Post-hoc testing detected a significant difference between mice repeatedly injected with 10 mg/kg METH, but not 5 mg/kg METH, as compared to saline controls (P<0.05).
Figure 3.
Effects of repeated administration of saline, MDMA (left) or METH (right) on body temperature. Abscissae: Time elapsed since the first injection. Arrows indicate times of drug or saline injections. Ordinates: Core temperature, measured via radiotelemetry every 5 minutes, and expressed as 10 minute averages. For graphical clarity, statistical differences are described in the Results section. Error bars represent ± SEM.
Effects on dopamine and metabolites
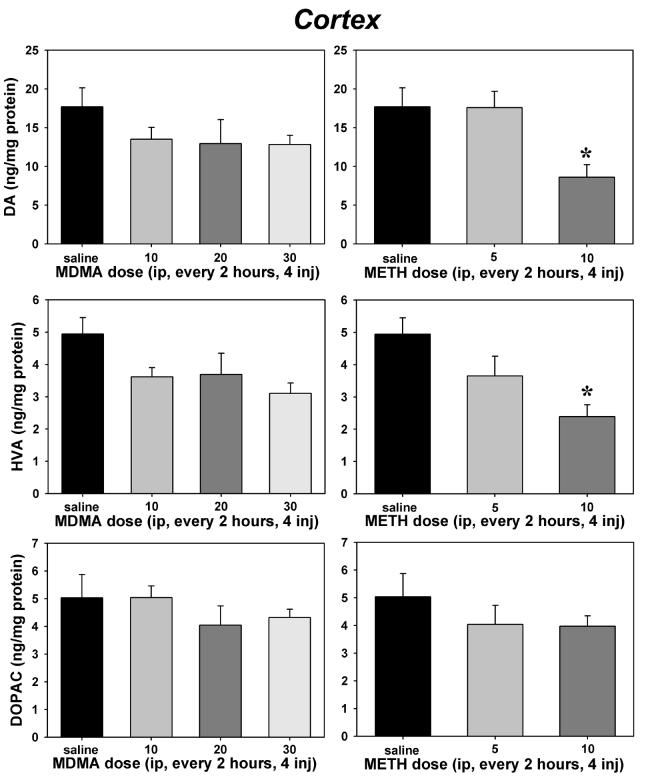
Within the cortex, there was an overall effect of treatment on DA (F=2.90, P=0.031, Figure 4, top panels) and HVA concentrations (F=3.31, P=0.017, Figure 4, middle panels), but DOPAC (Figure 4, bottom panels) was not significantly altered. Repeated injection of 10 mg/kg METH produced a significant depletion of both DA (P=0.035) and HVA (P=0.006) as compared to saline controls, but MDMA had no significant effects on cortical concentrations of DA or its primary acidic metabolites at any dose tested.
Figure 4.
Effects of repeated administration of saline, MDMA (left) or METH (right) on cortical tissue concentrations of dopamine (top panels), homovanillic acid (middle panels) and 3,4-dihydroxyphenylacetic acid (bottom panels). Abscissae: Dose administered every 2 hours for 4 total injections. Ordinates: Tissue concentrations expressed in ng/mg protein. Asterisks indicate significant differences from saline controls following ANOVA and Tukey's HSD post-hoc tests. Error bars represent ± SEM.
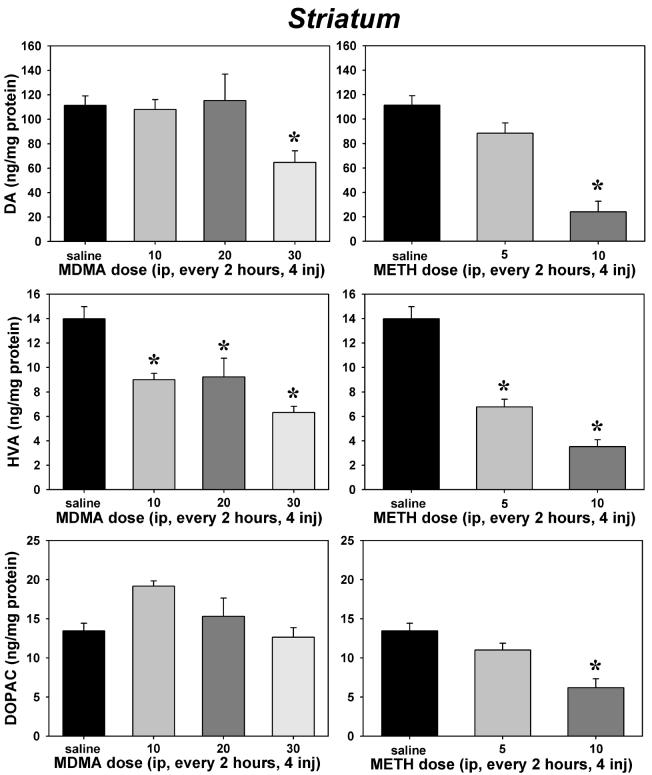
The impact of MDMA and METH on striatal neurochemistry was generally more pronounced than that observed in the cortex. A main effect of treatment was found on DA (H=24.63, P<0.001 [using a Kruskal-Wallis one way ANOVA on ranks due to a lack of normality for these data], Figure 5, top panels), HVA (F=19.27, P<0.001, Figure 5, middle panels) and DOPAC concentrations (F=12.62, P<0.001, Figure 5, bottom panels). Post-hoc tests indicated that only the highest doses of MDMA (P<0.05) and METH (P<0.05) significantly depleted striatal DA. HVA seemed most sensitive to repeated dosing, as repeated 10 mg/kg MDMA (P=0.002), 20 mg/kg MDMA (P=0.005), 30 mg/kg MDMA (P<0.001), 5 mg/kg METH (P<0.001) and 10 mg/kg METH (P<0.001) regimens all induced significant reductions in this metabolite. Although no effects of MDMA on striatal DOPAC were observed, 10 mg/kg METH did significantly (P=0.003) reduce tissue concentrations of this metabolite.
Figure 5.
Effects of repeated saline, MDMA (left) or METH (right) on striatal tissue concentrations of dopamine (top panels), homovanillic acid (middle panels) and 3,4-dihydroxyphenylacetic acid (bottom panels). Abscissae: Dose administered every 2 hours for 4 total injections. Ordinates: Tissue concentrations expressed in ng/mg protein. Asterisks indicate significant differences from saline controls following ANOVA and Tukey's HSD post-hoc tests. Error bars represent ± SEM.
Effects on [3H]PK11195 binding
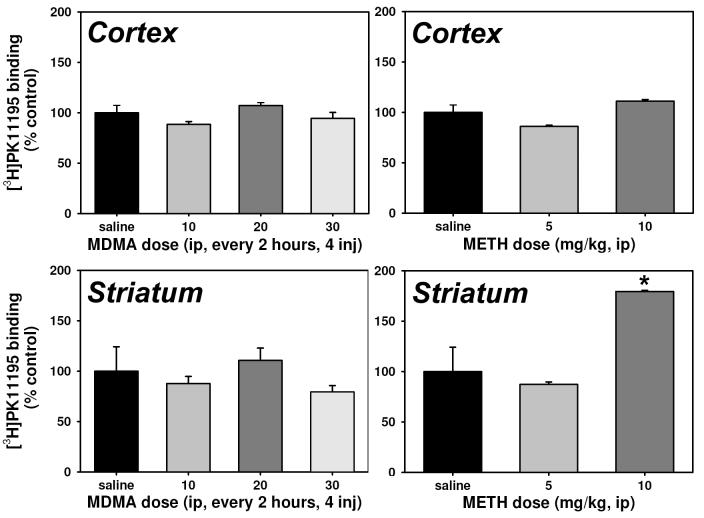
No dose regimen of MDMA or METH significantly increased [3H]PK11195 binding within the cortex over that quantified in saline-treated controls (Figure 6, upper panels). Similarly, within striatal homogenates (Figure 6, lower panels), all three MDMA dose regimens were without effect on [3H]PK11195 binding. Repeated injection of 10 mg/kg METH (P<0.001), but not 5 mg/kg METH, significantly increased binding at striatal PBR, although the overall ANOVA was not significant (P>0.05).
Figure 6.
Effects of repeated administration of saline, MDMA (left) or METH (right) on cortical (top) and striatal (bottom) peripheral benzodiazepine receptor expression. Abscissae: Dose administered every 2 hours for 4 total injections. Ordinates: Peripheral benzodiazepine receptor binding (Bmax), expressed as a percent of saline control values. Asterisks indicate significant differences from saline controls following ANOVA and Tukey's HSD post-hoc tests. Error bars represent ± SEM.
Discussion
Recently, some researchers have proposed that effect scaling (e.g. Wang et al., 2004) may be a particularly useful method for equating dose regimens across drugs which may differ along pharmacokinetic parameters. The principle behind effect scaling is that drug doses which induce identical effects (behavioral, physiological, neurochemical, etc.) across drugs may be considered equivalent, and therefore directly compared, even though these doses will likely differ on a mg/kg basis. This paradigm may have particular resonance with behavioral pharmacologists, as it is similar in many ways to the common practice of comparing “behaviorally equivalent” doses across drugs.
A previous report by Pubill and associates (2003) utilized ex vivo [3H]PK11195 binding in the rat brain to compare and contrast the neurotoxic effects of METH and MDMA. In that study, increased PBR binding was noted in the striatum (39%) and cortex (32%) following METH administration, but not following MDMA administration, leading the authors to conclude that “there are differences between the brain responses to the neurotoxicity induced by METH and MDMA as far as glial activation is concerned” (Pubill et al., 2003). Based upon the data reported, however, it seems difficult to determine whether this conclusion truly represents a real difference between the neurotoxic potentials of MDMA and METH, or simply reflects the methodological peculiarities of this particular study. In this regard, two points seem especially relevant: the specific dosing regimens employed, and the species in which these effects were studied. Pubill and associates (2003) reported data from a single regimen of each drug: 10 mg/kg METH subcutaneously (sc) every 2 hours for 4 total doses, and 20 mg/kg MDMA sc twice per day (at 7 hour intervals) for 4 days. Thus, METH-exposed animals were dosed much more frequently than were MDMA-exposed animals, potentially leading to very different pharmacokinetics across these two drug regimens. Additionally, as noted in the Introduction, the neurochemical effects of METH are distributed across dopaminergic and serotonergic systems in the rat (Gibb et al., 1990; Gibb et al., 1994), while MDMA exerts selective serotonergic effects in this species (Gibb et al., 1990; DeSouza et al., 1990). Thus, it may be the case that [3H]PK11195 binding in the rat is simply more sensitive to dopaminergic effects than to serotonergic effects.
In the mouse, however, MDMA and METH have previously been shown to deplete both DA and 5-HT, increase GFAP expression within the striatum, and induce fiber and terminal degeneration (O'Callaghan and Miller, 1994) to a similar degree. In the present studies, only METH altered striatal [3H]PK11195 binding, a pattern of results which is reasonably congruent with that of Pubill and colleagues (2003). Importantly, the present findings can not easily be explained by invoking differential sensitivities to the neurochemical effects of these two amphetamine analogues. Indeed, repeated administration of 10 mg/kg METH (which induced significant microglial activation in the striatum) and 30 mg/kg MDMA (which did not) produced nearly equivalent decreases in body weight, elicited locomotor stereotypy, produced transient hyperthermias, and similarly depleted DA and HVA within the striatum. Thus, according to principles of effect scaling, these dose regimens should be reasonably comparable. The finding that the behavioral and physiological effect of these particular dose regimens were accompanied by increased [3H]PK11195 binding only following METH administration may imply important differences in the toxic mechanisms of these two drugs.
Glial activation is an indirect marker for neurotoxicity, and may be particularly sensitive to insults induced by the amphetamines (Escubedo et al., 1998; Thomas et al., 2004). Binding sites for [3H]PK11195 are expressed on astrocytes and microglia, and the density of these sites increases following toxicity accompanied by glial activation (Venetti et al., 2006). Neurotoxicological studies have shown that specific dose regimens of METH result in the degeneration of striatal dopaminergic fibers accompanied by increased [3H]PK11195 in the brains of rats (Escubedo et al., 1998; Pubill et al., 2003), while some dose regimens of MDMA can also induce these effects in mice (Chipana et al., 2006). Previous studies (e.g., Bowyer et al., 1992; Malberg and Seiden, 1998) have demonstrated that the degree of MDMA- or METH-induced neurodegeneration observed in rodents is correlated with the degree of hyperthermia produced by these drug regimens. In the present studies, both MDMA and METH elicited hyperthermic effects to a similar extent. Thus, increased temperature seems to be a necessary, but not sufficient, condition for the development of amphetamine-induced neurotoxicity in the mouse.
In contrast to the present results, Thomas and colleagues (2004) reported significant MDMA-induced microglial activation in mouse striatum. This discrepancy is difficult to explain, since they used a dose regimen (20 mg/kg MDMA, every 2 h for four total injections) which was also employed in these studies. Nevertheless, their subjects were female C57BL/6 mice, while ours were male NIH Swiss mice. We are not aware of any studies explicitly designed to test for gender or strain differences in response to MDMA administration in murine subjects, but some reports from the human (Milani et al., 2004; Verheyden et al., 2002; Liechti et al., 2001) and rodent (Palenicek et al., 2005) literature do suggest that females are more sensitive to MDMA than are males. A second difference between these studies concerns the amount of time which elapsed between drug administration and tissue harvest. Thomas and associates (2004) sacrificed their animals 48 h after the last injection, while our subjects were euthanized 72 h after the last injection. Another possible explanation for these divergent results could be due to use of a different microglial marker (staining fixed brain sections with HRP-conjugated isolectin B4 [ILB4]) by Thomas and coworkers (2004).
In summary, the data presented in this report demonstrate dose-related effects of MDMA and METH on body weight, locomotor activity, and core temperature in the mouse. Dose regimens of MDMA and METH which induced similar reductions in body weight, locomotor stereotypy, and hyperthermia tended to deplete striatal DA and its primary acidic metabolites, but only METH altered cortical neurochemistry. Importantly, increased binding of [3H]PK11195 (presumably to PBR) in the striatum – the endpoint most unambiguously associated with neurotoxicity – was produced by METH administration, but not by MDMA administration. These findings are in agreement with previous observations of differential effects of MDMA and METH on neurotoxicity (Pubill et al., 2003) and serve to extend those findings by outlining behavioral and physiological effects across multiple dose regimens administered according to the same injection schedule. Although the neurotoxicity profile of MDMA would appear to be less intense than that elicited by METH in the present experiments, the fact that MDMA can induce significant behavioral, physiological, and neurochemical effects without profound neurotoxicity highlights the fact that risks such as hyperthermia are associated with even acute use of MDMA.
Acknowledgements
The authors thank Mary J. Clark for expert technical assistance on the in vitro portions of this project. These studies supported by USPHS Grants DA04087, DA09161 and DA05923.
Abbreviations
- 5-HIA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- ANOVA
analysis of variance
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- GFAP
glial fibrilliary acidic protein
- HPLC
high pressure liquid chromatography
- HVA
homovanillic acid
- IP
intraperitoneal
- MDMA
3,4-methylenedioxymethamphetamine
- METH
methamphetamine
- MHPG
3-methoxy-4-hydroxyphenylethyleneglycol
- NE
norepinephrine
- PBR
peripheral benzodiazepine receptor
- PCA
p-chloroamphetamine
- PK11195
1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide
- Ro5-4864
4'-chlorodiazepam
- TPH
tryptophan hydroxylase
Footnotes
Section Editor: Dr. Yoland Smith, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Sonnewald U, Tan KH. Astrocyte modulation of neurotoxic injury. Brain Pathol. 2002;12:475–481. doi: 10.1111/j.1750-3639.2002.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J, Fage D, Carter C, Scatton B. Peripheral type benzodiazepine binding sites are a sensitive indirect index of neuronal damage. Brain Res. 1987;421(12):167–172. doi: 10.1016/0006-8993(87)91287-x. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260(2):817–824. [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camins A, Gabriel C, Aguirre L, Sureda FX, Pubill D, Pallàs M, Escubedo E, Camarasa J. U-83836E prevents kainic acid-induced neuronal damage. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;357:413–418. doi: 10.1007/pl00005187. [DOI] [PubMed] [Google Scholar]
- Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochemistry International. 2002;40:475–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chipana C, Camarasa J, Pubill D, Escubedo E. Protection against MDMA-induced dopaminergic neurotoxicity in mice by methyllycaconitine: involvement of nicotinic receptors. Neuropharmacology. 2006;51(4):885–895. doi: 10.1016/j.neuropharm.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Colado MI, O'Shea E, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology. 2004;173:249–263. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- DeSouza EB, Battaglia G, Insel TR. Neurotoxic effects of MDMA on brain serotonin neurons: evidence from neurochemical and radioligand binding studies. Ann New York Academy of Sciences. 1990;600:682–698. doi: 10.1111/j.1749-6632.1990.tb16918.x. [DOI] [PubMed] [Google Scholar]
- Easton N, Marsden CA. Ecstasy: are animal data consistent between species and can they translate to humans? J Psychopharmacol. 2006;20(2):194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jiménez A, Pubill D, Pallàs M, Camins A, Camarasa J. Migrogliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain Res. 1998;814:120–126. doi: 10.1016/s0006-8993(98)01065-8. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Johnson M, Stone D, Hanson GR. MDMA: Historical Perspectives. Ann New York Academy of Sciences. 1990;600:601–612. doi: 10.1111/j.1749-6632.1990.tb16913.x. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Hanson GR, Johnson M. Neurochemical mechanisms of toxicity. In: Cho AK, Segal DS, editors. Amphetamine and its analogues: psychopharmacology, toxicity, and abuse. Academic Press Inc.; San Diego: 1994. [Google Scholar]
- Grob CS. Deconstructing ecstasy: the politics of MDMA research. Addict Res. 2000;8:549–588. [Google Scholar]
- Harvey JA, McMaster SE, Yunger LM. p-Chloroamphetamine: Selective neurotoxic action in brain. Science. 1975;187:841–843. doi: 10.1126/science.47181. [DOI] [PubMed] [Google Scholar]
- Kalia M. Do validated biological measures of neurotoxicity really support the claim that MDMA is neurotoxic to man? Neuropsychobiology. 2000;42:45. [Google Scholar]
- Kish SJ. How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol Biochem Beh. 2002;71:845–855. doi: 10.1016/s0091-3057(01)00708-0. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology. 2001;154(2):161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18(13):5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani RM, Parrott AC, Turner JJ, Fox HC. Gender differences in self-reported anxiety, depression, and somatization among ecstasy/MDMA polydrug users, alcohol/tobacco users, and nondrug users. Addict Behav. 2004;29(5):965–971. doi: 10.1016/j.addbeh.2004.02.044. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol. 1995;11(13):177–92. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- Nakamura Y. Regulating factors for microglial activation. Biol Pharm Bul. 2002;25(8):945–953. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Quantification of reactive gliosis as an approach to neurotoxicity assessment. NIDA Res Monogr. 1993;136:188–212. [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270(2):741–751. [PubMed] [Google Scholar]
- Palenicek T, Votava M, Bubenikova V, Horacek J. Increased sensitivity to the acute effects of MDMA (“ecstasy”) in female rats. Physiol Behav. 2005;86(4):546–553. doi: 10.1016/j.physbeh.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Park CH, Carboni E, Wood PL, Gee KW. Characterization of peripheral benzodiazepine type sites in a cultured murine BV-2 microglial cell line. Glia. 1996;16(1):65–70. doi: 10.1002/(SICI)1098-1136(199601)16:1<65::AID-GLIA7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pletscher A, Burkard WP, Bruderer H, Gey KF. Decrease of cerebral 5-hydroxytryptamine and 5-hydroxyindoleacetic acid by arylalkylamine. Life Sci. 1963;11:828–833. doi: 10.1016/0024-3205(63)90094-8. [DOI] [PubMed] [Google Scholar]
- Pletscher A, Bartholini G, Bruderer H, Burkard WP, Gey KF. Chlorinated arylalkylamines affecting the cerebral metabolism of 5-hydroxytryptamine. J Pharmacol Exp Ther. 1964;145:344–350. [PubMed] [Google Scholar]
- Pubill D, Canudas AM, Pallàs M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn-Schmiedeberg's Arch Pharmacol. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- Rao VLR, Bowen KK, Rao AM, Dempsey RJ. Up-regulation of the peripheral-type benzodiazepine receptor expression and [3H]PK11195 binding in gerbil hippocampus after transient forebrain ischemia. J Neurosci Res. 2001;64:493–500. doi: 10.1002/jnr.1101. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, McCann UD. (+/−)-Methylenedioxymethamphetamine (MDMA, ‘ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology. 2000;42:5–10. doi: 10.1159/000026664. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Sulser F. P-chloroamphetamine: in vivo investigations on the mechanism of action of the selective depletion of cerebral serotonin. J Pharmacol Exp Ther. 1970;175(2):419–426. [PubMed] [Google Scholar]
- Sprague JE, Everman SL, Nichols DE. An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology. 1998;19(3):427–441. [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci. 1995;15(7 Pt 2):5263–5274. doi: 10.1523/JNEUROSCI.15-07-05263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB. Heterogeneity of microglial and perivascular cell populations: insights gained from the facial nucleus paradigm. Glia. 1993;7(1):68–74. doi: 10.1002/glia.440070112. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367(3):349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Verheyden SL, Hadfield J, Calin T, Curran HV. Sub-acute effects of MDMA (+/−3,4-methylenedioxymethamphetamine, “ecstasy”) on mood: evidence of gender differences. Psychopharmacology. 2002;161(1):23–31. doi: 10.1007/s00213-001-0995-9. [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80(6):308–22. doi: 10.1016/j.pneurobio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53(4):240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]