Abstract
The nucleus accumbens is involved in the modulation of motivated behaviour by reward-associated sensory information. However, little is known about the specific nature of the nucleus accumbens' contribution to generating movement. We investigated motor encoding by nucleus accumbens neurons in rats performing a delayed response task that allowed us to dissociate the effects of sensory and motor events on firing. In a subset of neurons, firing in the delay period preceding movement was highly selective; this selectivity was tightly correlated with the direction of the subsequent movement, but not with the sensory properties of the instructive cue. Direction selectivity in this population of neurons developed over the course of the delay period, with the strongest selectivity apparent just prior to movement onset. Selectivity was also apparent in nucleus accumbens neurons during movement, such that firing showed a tight correlation with movement direction, but not the instructive cue presented nor the spatial destination of the movement. These results are consistent with the hypothesis that a subpopulation of nucleus accumbens neurons contributes to the selection and execution of specific motivated behaviours.
Reward-predictive cues have potent effects upon behaviour; animals that can exploit these cues to guide behaviour greatly increase the likelihood of obtaining the rewards necessary for survival and reproductive success. The nucleus accumbens (NAcc) is a critical element of the brain circuit that mediates motivated behaviours, including those guided by reward predictive cues. The NAcc receives convergent glutamatergic input from the prefrontal cortex, hippocampus and amygdala, as well as a dense dopaminergic input from the ventral tegmental area (Brog et al. 1993; Wright & Groenewegen, 1995). Each of these areas is implicated in encoding aspects of reward-related information. The NAcc, in turn, projects to brain regions associated with generation of motor behaviours, such as the pedunculopontine tegmentum and the ventral pallidum (Groenewegen & Russchen, 1984; Heimer et al. 1991). This anatomical arrangement is one basis of the influential hypothesis that the NAcc serves as a ‘limbic–motor interface’, in which information about reward, context and drive is integrated to guide motivated behaviour (Mogenson et al. 1980).
How does the NAcc contribute to the transformation of cue-related sensory information into motor commands for reward-directed movement? Models of striatal function propose a role for this brain region in facilitating the selection of one behaviour from among competing alternatives (Pennartz et al. 1994; Redgrave et al. 1999; Hikosaka et al. 2006; Nicola, 2007). Key to testing this idea is determining how firing in NAcc neurons encodes movement parameters (e.g. timing and direction of movement), particularly during periods of movement planning when behavioural selection is likely to take place. Previous electrophysiological studies of NAcc responses to reward-predictive cues, though numerous, have chiefly focused on reward-related encoding. NAcc firing in response to cues is modulated by association with a reward, the identity and magnitude of a reward, and the valence (aversive or appetitive) of an associated reinforcer (Hollerman et al. 1998; Carelli & Ijames, 2001; Hassani et al. 2001; Cromwell & Schultz, 2003; Setlow et al. 2003; Nicola et al. 2004; Peoples et al. 2004; Roitman et al. 2005; Wilson & Bowman, 2005). These and other studies demonstrate that NAcc firing importantly encodes information related to anticipated outcomes. However, few studies to date have focused explicitly on elucidating motor encoding in the firing of NAcc neurons. Understanding how the NAcc encodes motor information is important for understanding normal appetitive behaviour as well as disorders of motivation such as addiction.
In the present study, we used electrophysiological recording techniques to investigate NAcc movement encoding in rats during performance of a delayed response task. Importantly, behavioural responding was temporally separated from cue presentation in this task, allowing neural activity related to these two distinct processes to be distinguished. Our aims were (1) to determine how NAcc neurons encode motor preparatory signals prior to movement execution; and (2) to elucidate movement correlates of NAcc firing during execution of reward-related behaviours.
Our results show that neural activity in the NAcc, both prior to and during movement execution, encodes movement variables with a surprising degree of specificity. This result suggests that subsets of NAcc neurons encode information sufficient to facilitate the selection and implementation of specific behaviours.
Methods
Experimental subjects
All procedures used were approved by the Ernest Gallo Clinic and Research Center Animal Care and Use Committee. Male Long–Evans rats (n = 9; Charles River Laboratories, Wilmington, MA, USA) were used for electrophysiological recordings. Throughout the experimental period, rats were food restricted to maintain 90%ad libitum body weight. Data from a subset of these rats (n = 6) describing the response properties of a single class of NAcc neurons that possess long-lasting inhibitions of firing were published previously (Taha & Fields, 2006). These firing patterns are not analysed further in the present study.
Behavioural paradigm and surgical procedures
Rats were trained in a delayed response task in an operant chamber (42 × 53 × 40 cm) equipped with an audio stimulus generator, house lights, and a central nosepoke hole flanked by identical reward receptacles (Medical Associates, Georgia, VT, USA). A continuous white noise cue signalled to the animal that a trial could be initiated at any time. Trials were initiated by performance of a sustained nosepoke. At nosepoke onset, the white noise cue was terminated. One of two instructive tones (3 kHz or 7 kHz at 90 dB, chosen randomly) was delivered at short latency after the initiation of the nosepoke (200–350 ms latency, randomized), cueing the animal to respond to either the left or the right reward receptacle for delivery of a sucrose reward. Tone duration was randomly varied (950–1550 ms). Importantly, rats were required to withhold responding until tone offset. Tone-response direction pairings remained constant for each rat throughout training and recording sessions, but were counterbalanced across rats. Successful completion of each trial required that the nosepoke be maintained for the duration of the tone presentation, and that the subsequent response be directed to the appropriate reward receptacle. Rats typically executed short-latency, rapid movements from the central nosepoke to the reward receptacles (average latency ±s.d. to reward receptacle = 390 ± 98 ms). Successful trials resulted in delivery of 100 μl of 10% sucrose into the instructed reward receptacle. During a 4 s baseline interval immediately after reward receptacle exit following sucrose delivery, new trials could not be initiated. Rats had to abstain from nosepoke responses and reward receptacle entry during this interval; if either of these behaviours occurred, the baseline interval was extended until a 4 s interval occurred without any recorded behavioural responses. This ensured that reward-related behaviours were absent during the baseline interval. House lights were briefly extinguished (0.25 s), and the white noise cue was again delivered to signal the start of the next trial.
Termination of the nosepoke prior to tone offset constituted an error, as did responding to the non-instructed reward receptacle. Errors of either kind terminated the ongoing trial, and were immediately signalled by extinguishing the house lights for 10 s, during which time sucrose rewards could not be earned, nor could additional trials be initiated. Rats were trained in the delayed response task until they completed > 50 successful trials in two consecutive behavioural sessions. Average training time was approximately 4 months.
After behavioural training, rats were stereotaxically implanted bilaterally with eight-wire electrode arrays (NB Laboratories, Denison, TX, USA) directed at the NAcc (anterioposterior, 1.2–1.7; mediolateral, 0.7–1.5; dorsoventral, 7.2–7.5). Arrays were composed of blunt-cut stainless steel 50 μm wires arranged in a 2 × 4 array. Anaesthesia was induced and maintained with 3% isofluorane (Baxter, Deerfield, IL, USA). Respiratory rate and toe pinch reflex were monitored to ensure adequate anaesthesia was maintained during surgery. Rats were treated postoperatively with 0.5 mg kg−1 medetomidine HCl (Domitor; Pfizer, Exton, PA, USA) via intraperitoneal injection for analgesia.
Single unit recording and discrimination
Neural activity was recorded in the nucleus accumbens during 2 h sessions while rats performed the delayed response task. Rats typically completed between 50 and 100 trials in each experimental session. Neural signals were recorded with a unity-gain head stage amplifier, amplified 10 000-fold and captured digitally using commercial hardware and software (Plexon Instruments, Dallas, TX, USA). Discrimination of individual units was performed off-line using principal component analysis of waveform shape to discriminate between different units simultaneously recorded on a single wire. Single units were identified by constancy of waveform shape, autocorrelogram and interspike interval. In many cases, single units persisted for multiple recording sessions. In these cases, data from these sessions were pooled in analysing the unit's response properties.
For the population of single units, the range of experimental sessions across which single unit response were pooled ranged from a minimum of one to maximum of seven sessions. There was no minimum spike rate required for inclusion in the analyses.
Analysing neural response properties
Two kinds of neural firing were of principal interest in our analysis. First, we identified neurons with increased/decreased neural activity during delay (termed D+ and D– firing patterns, respectively) or movement (M+ and M−) intervals, relative to firing during a baseline period. Second, we identified neurons in which firing rate was dependent on trial contingency for the instructive cue and/or response direction – firing rates in these neurons differed for distinct cues/response directions. We refer to these as selective responses. Note that these categories are not mutually exclusive. For example, a neuron could be both M+, showing an overall increase in firing during movement, and also show movement selectivity, in which firing during one cue/response direction was significantly different from that occurring for the other cue/response direction. Statistical analyses related to each of these firing patterns are discussed in turn below.
Identifying delay and movement excitations and inhibitions
Delay and movement responsive neurons were identified by comparing firing during these behavioural intervals with firing during a baseline period, the 2 s interval prior to the onset of the white noise cue (the start signal that indicated a new trial could be initiated). The delay period consisted of the duration of the nosepoke interval, and the subsequent movement period began with nosepoke exit, and terminated with reward receptacle entry. A stringent criterion for significance (P < 0.01, t test) was used to identify delay and movement responsive neurons, to ensure that only well defined excitations and inhibitions were considered in this analysis.
Identifying selective firing and quantifying selectivity
Neurons with selective firing were identified by comparing firing rates occurring during trials in which the rat responded to the right reward receptacle to those in which it responded to the left reward receptacle. Only correct trials – those in which movement occurred in the direction instructed by the cue – were included in this analysis, which was applied to identify selectivity occurring both in the delay period and the subsequent movement period. Neurons in which firing rate differed significantly (P < 0.01, t test) between the two trial types were identified as having selective firing.
Visual inspection of firing patterns suggested that in many neurons, delay selectivity was not present early in the delay interval, but emerged during later stages of the delay period, just prior to movement onset. Therefore, we analysed delay selectivity over both early (400 ms following cue onset) and late (last 400 ms of the delay prior to movement) portions of the delay period. Neurons that possessed selective firing in either of these intervals were included in the pool of neurons identified as having delay selective firing. Selectivity during these two periods was statistically analysed using Wilcoxon's signed rank test comparing the magnitude of selectivity (see selectivity index, below) occurring during these two intervals.
To quantify the magnitude of selective firing, we used the following selectivity index (SI): SI = (PcuePmovement−NcueNmovement)/(PcuePmovement+NcueNmovement). P indi-cates the firing rate for the preferred cue/movement direction, which by definition was the cue/movement direction associated with the higher firing rate. N indicates the firing rate for the non-preferred cue/movement direction. This index ranged from a minimum value of 0 (no difference in firing rates on preferred and non-preferred trials) to a maximum value of 1 (no firing occurred during non-preferred trials).
Dissociating the role of instructive cues and response direction in selective firing
To dissociate the roles of instructive cues and response direction on firing, we analysed error trials in which the rat moved in the non-instructed direction. RM ANOVA on ranks was used to statistically compare firing across different trial contingencies for the entire population of neurons with selective firing. Statistics were performed on raw firing rates. In Figs 4 and 7, data shown were normalized to the firing rate occurring during preferred cue/response trials (i.e. successful trials in which firing was elevated) to emphasize the change from baseline selectivity. This analysis was used both for neurons with delay and movement selective firing.
Figure 4.
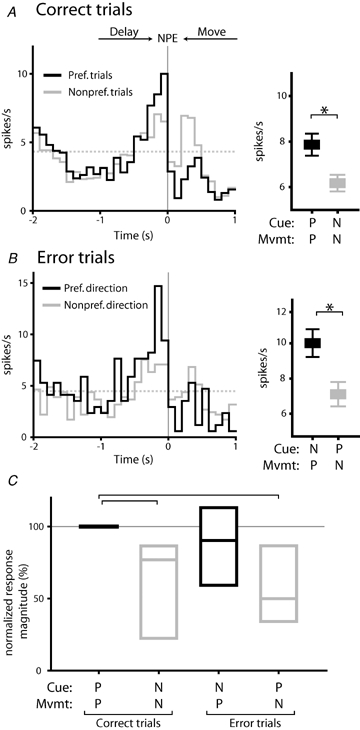
Delay selective firing predicts subsequent response direction A, an example neuron with direction selective firing occurring during the delay period. Histograms are constructed around nosepoke exit (NPE: synchronous with movement onset), and show correct trials in which presentation of the preferred cue was followed by movement to the preferred reward receptacle (black line, histogram) and trials in which presentation of the non-preferred cue was followed by movement to the non-preferred reward receptacle (grey line). Note that correct task performance occurred in both preferred and non-preferred trial types. Broken grey line indicates baseline firing rate. Mean firing rates occurring over the 400 ms preceding the nosepoke exit for these two trial conditions are shown in the panel at right (P, preferred; N, non-preferred). Firing rates were significantly different for the two trial types (P < 0.01, t test). B, error trials from the same neuron depicted in A. Peak firing rates were higher when the non-preferred cue was followed by movement in the preferred response direction (black line, histogram). Mean firing rates were significantly higher when the preferred response direction occurred, relative to trials in which the preferred cue was presented (right panel; P < 0.05). C, for the population of delay selective neurons, selectivity was correlated with the forthcoming direction of movement, rather than the instructive cue presented (overall P < 0.001, RM ANOVA on ranks). Firing rates for each neuron were normalized to that occurring during PcuePmvmt trials. Spike rates were significantly lower during correct and error trials in which movement in the non-preferred direction occurred (P < 0.05 relative to PcuePmvmt, Dunn's post hoc test) but not for error trials in which movement in the preferred direction took place (P > 0.05). Box plots show median, 1st and 3rd quartile values.
Figure 7.
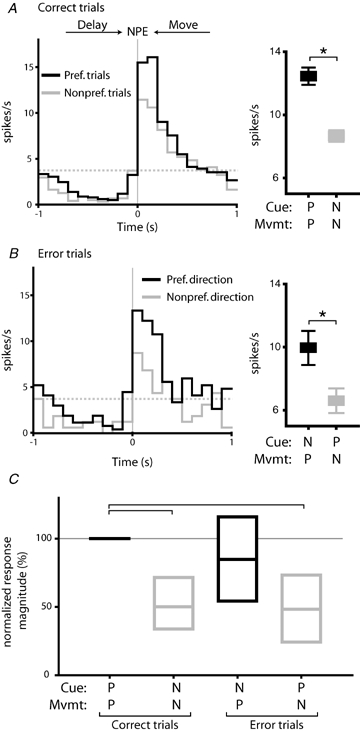
Movement selective firing is closely related to response direction A, an example neuron with movement direction selectivity. The histogram shows that firing rate in this neuron was elevated during trials in which the preferred cue and response direction occurred (black line), relative to trials in which the non-preferred cue and response direction occurred (grey line). This difference was significant (right panel; P < 0.05, t test). Histogram is constructed around nosepoke exit (NPE; synchronous with movement onset). Broken grey line indicates baseline firing. B, firing rates in the same neuron shown in A during error trials. Peak firing rates were higher in trials in which the preferred response direction occurred (black line, histogram) relative to those in which the preferred cue (grey line) was presented. Differences in mean firing rate were significant (right panel; P < 0.05). C, firing rates in movement selective neurons were significantly higher when the preferred response direction occurred for correct and error trials (overall effect of trial type, P < 0.001, RM ANOVA on ranks). Firing was significantly lower in both correct and error trials in which the non-preferred movement direction occurred (P < 0.05, Dunn's post hoc test).
Dissociating sensory and motor variables in driving onset/offset of delay responsive neurons
A two step process was used to determine if onset/offset of neural responses were better correlated with motor or sensory events. First, histograms (100 ms time bins) were constructed around each of the relevant events (for onset responses. These events were the nosepoke onset and cue onset; for offset responses, these were cue offset and nosepoke exit), and smoothed with a boxcar filter (filter width of 500 ms). For each histogram, we then calculated the derivative of the firing rate as a function of time. This curve was smoothed (boxcar averaging, 700 ms filter) and from this smoothed curve, we extracted the absolute value of the maximal rate of change (in units of spikes s−2) in firing for each event, and the time (relative to the event) at which this occurred. Statistical comparison of maximal changes in firing rate allowed identification of the event associated with the most rapid and robust change in spike frequency. Absolute values of this measure were used to facilitate comparison of onset and offset responses, as the sign of the rate of change was opposite for these responses. Timing information allowed us to determine if the change in firing preceded or followed the particular event around which the histogram was constructed. Smoothing used in this analysis was necessary to limit noise introduced by typically low NAcc spike rates. Statistical comparisons were performed using Wilcoxon's signed rank test.
Some neurons showed delay modulations that were very long-lasting. This was particularly true of inhibitions, which not infrequently persisted through the period of reward consumption. Offset responses of these neurons were not included in this analysis if there was no clear change in firing occurring near the time of delay offset.
Analysing movement responses and response latency to reward receptacle
Spike rates in M+ and M− neurons were compared during error trials to determine if trial outcome (success or failure) affected firing in these neurons. In addition, we analysed firing rates for each of these trial types as a function of latency to reach the reward receptacle (binned into latencies less than and greater than 500 ms) following nosepoke termination. Two-way RM ANOVA was used to analyse firing, with trial type and response latency taken as independent factors. Statistics were performed on raw spike rates. In Fig. 6, data shown were normalized to firing rate occurring during successful, short latency (< 500 ms) trials to emphasize the relative magnitude of changes in spike frequency.
Figure 6.
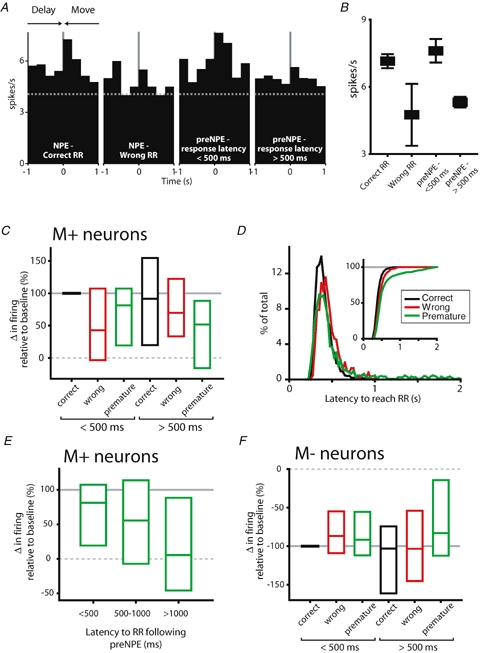
Movement responses are contingent on trial type and response latency A, firing in an example M+ neuron, with histograms constructed around nosepoke exit in each panel. From left to right, nosepoke exits (NPE) shown occur during: correct trials; error trials in which movement to the wrong reward receptacle occurred; error trials in which the delay was prematurely terminated but was followed by a short latency (< 500 ms) response at the reward receptacle; and premature movement error trials (premature NPE, preNPE) in which response latency to the reward receptacle was long (> 500 ms). In this neuron, an excitatory response occurred during correct trials and during prematurely terminated trials which were followed by short latency locomotion to the reward receptacle (1st and 3rd panels, respectively). This excitatory response was absent when movement to the wrong reward receptacle occurred, or when latency to reward receptacle entry was slow after premature delay termination (2nd and 4th panels, respectively). B, mean firing rate for the example neuron shown in A for each trial type. Mean firing rates were significantly different (P < 0.05, 1-way ANOVA). C, normalized response magnitude for the population of M+ neurons. Firing rates showed a dependence on trial type and response latency (main effect of trial type, P < 0.01; main effect of response latency; P < 0.05, 2-way RM ANOVA). Firing during wrong reward receptacle and premature movement errors was significantly lower than that occurring during correct trials (P < 0.05, Holm–Sidak post hoc test). Firing was also significantly attenuated during long latency reward receptacle responses relative to short latency responses (P < 0.05). Median change in firing rate (relative to baseline firing) and first and third quartile responses are represented in bar graphs. Trial types shown are divided into those in which short latency (< 500 ms) and long latency (> 500 ms) responses to the reward receptacle occurred. D, distribution of response latencies to reach the reward receptacle for each trial type. For all trial types, response latency was typically rapid (median response latencies: correct trials, 370 ms; wrong RR trials, 420 ms; premature movement errors, 402 ms). However, premature movement error trials showed a bimodal distribution of response latencies, with a subset of responses occurring between 1 and 2 s following nosepoke exit, clearly visible in the cumulative frequency distribution (inset graph). E, M+ firing rates decreased monotonically with increasing response latency during premature movement errors (P < 0.05, RM ANOVA on ranks). F, M− firing rates were dependent upon trial type (main effect of trial type, P < 0.05, 2 way RM ANOVA). Post hoc tests showed firing during both error types was significantly attenuated relative to correct trials (P < 0.05, Holm–Sidak post hoc test). For M− neurons, there was no dependence of firing rate on latency to reach the reward receptacle.
Dissociating movement direction from destination in movement selective neurons
We analysed firing occurring during spontaneous approach to reward receptacles to determine if selective firing was preserved under these conditions. Spontaneous reward receptacle approach was defined to include any reward receptacle entry that occurred more than 2 s after any nosepoke exit; this interval was sufficiently lengthy to exclude both correct and error trials, as reward receptacle approach for these trials invariably occurred within 2 s of nosepoke exit. The last 400 ms prior to reward receptacle entry was used during calculation of firing rate for spontaneous reward receptacle approach – this interval approximates the mean latency for movement to the reward receptacle during correct trial performance (391 ms). Selectivity indices were calculated for each type of reward receptacle approach, and statistically compared using Wilcoxon's signed rank test.
Histology
Rats were deeply anaesthetized with pentobarbital (300 mg kg−1) and recording sites were marked by passing positive current (10 μA, 20 s) through each recording electrode. Rats were perfused with a solution of 10% formaldehyde and 3% potassium ferricyanide to mark sites of iron deposition. Brains slices were cut at 40 μm, and recording sites located under a light microscope and recorded on atlas figures adapted from Paxinos & Watson (1997). In 7 of the 9 rats, recording sites for each unit were mapped onto recording sites to determine core versus shell distribution of firing patterns (186 units mapped of 218 total units recorded). In 2 of the 9 rats, left versus right brain hemispheres were not clearly marked during the histology process, and so core/shell positions could not be assigned to these electrode locations.
Results
Behaviour and recording sites
In the delayed response task (Fig. 1A and B) rats completed 60 ± 5% (mean ±s.e.m.) of all trials correctly (Fig. 1C). Delay length averaged 1.3 ± 0.3 s (mean ±s.d.) in correct trials. Two kinds of errors resulted in termination of the ongoing trial and a brief time out period. The first of these, which comprised the majority of the errors committed (27 ± 5% of all trials), was a failure to maintain the nosepoke for the duration of the instructive cue. Following correctly maintained nosepokes, movement errors to the incorrect reward receptacle were less common (13 ± 2% of all trials). There was no directional response bias during correct or error trials (Fig. 1D: P > 0.05, comparing responses to the left and right reward receptacles, t test).
Figure 1.
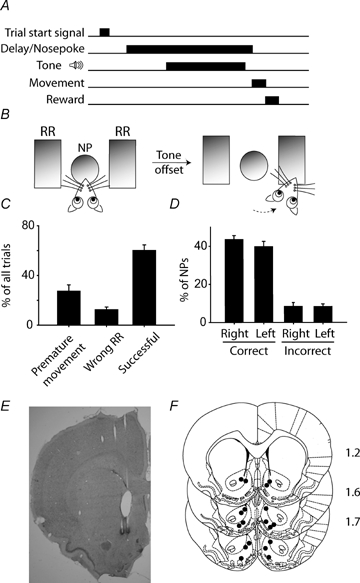
Behavioural paradigm and task performance A and B, a nosepoke (NP) after the start signal initiated the delay period. After a short variable interval, one of two instructive tones was delivered, signalling the required response direction (left or right). Rats were required to maintain the nosepoke until tone offset, which served as a trigger signal for movement to the reward receptacle (RR). Correct trials, in which the nosepoke was maintained for the duration of the tone presentation and the subsequent movement was directed to the instructed reward receptacle, resulted in delivery of a sucrose reward. C, movement prior to tone offset (27 ± 5% of all trials) or movement to the wrong reward receptacle (13 ± 2%) constituted error trials, and sucrose was withheld. In the remaining 60 ± 5% of trials, the NP was maintained until tone offset and movement was made to the correct reward receptacle, resulting in sucrose delivery. D, analysing only trials in which the delay was successfully maintained, there was no direction bias for subsequent movement to the reward receptacle (P > 0.05, comparing percentage correct trials for left versus right, t test). E, a representative section showing electrode placements in the NAcc. F, all recording sites were confined to the nucleus accumbens, spanning the core and shell regions. Anteroposterior distance relative to bregma is shown to the right of each section in millimetres.
All recording locations were confined to the NAcc, spanning the core and shell subregions (Fig. 1E and F). There were no differences in the core versus shell distribution of most firing patterns (all P > 0.05, comparing core versus shell distribution of each firing pattern to overall distribution of all units recorded, χ2 test). Neurons that fired selectively during movement were encountered in the shell at elevated frequencies (P < 0.05). However, this was a weak association which was not apparent when core/shell distributions were analysed within rats (all P > 0.05). Because we did not selectively target core or shell subregions, many recording sites were very close to the border region, potentially obscuring subregion-specific responses.
Neural responses
We recorded a total of 218 NAcc neurons from nine rats in the delayed response task. NAcc responses were heterogeneous, with some neurons responding to every component of the delayed response task. We focus in this analysis on modulations occurring specifically during the delay and movement periods, the intervals in which presumptive movement planning and execution, respectively, took place. Many neurons in our sample showed responses during these intervals. Inhibitions of neural activity were particularly common, during both the delay (D–, 91/218 neurons; 42%) and movement intervals (M−, 76/218; 35%). Excitatory responses occurring during the delay (D+, 41/218; 19%) and movement interval (M+, 47/218; 22%) were also encountered. Note that response categories were mutually exclusive within a single behavioural interval, but not across successive intervals. For example, D− neurons often also showed M− firing patterns.
The onset of changes in firing rate during the delay period was typically gradual, whether the change was an increase or decrease in spike frequency. Representative examples are shown in Fig. 2. Firing rates changed progressively over a short period near the beginning of the delay period, usually starting before the nosepoke that initiated the delay. Delay excitations (D+, Fig. 2A) and inhibitions (D−, Fig. 2B and C) were often maintained for the duration of the delay period. In D+ neurons, firing rate usually increased over the course of the delay period, with peak firing occurring near movement onset (Fig. 2A).
Figure 2.
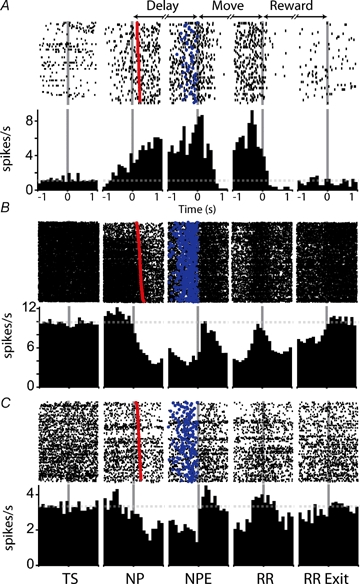
Firing patterns recorded in the nucleus accumbens during the delayed response task Three example neurons are shown in raster plots and perievent histograms. Firing patterns shown include delay excitation (A), delay inhibitions (B and C) and movement excitations (A and C). Histograms and rasters were constructed around the following behavioural events: TS, trial start; NP, nosepoke (delay start); NPE, nosepoke exit (delay termination); RR, reward receptacle entry; RR Exit, reward receptacle exit. In this and other histograms, broken grey lines indicate baseline firing rate for each neuron. Time (in seconds) is represented on the x-axis of each histogram; each panel extends from −1 to +1 relative to the behavioural event around which the histogram was constructed. Trials shown in rasters are rank ordered by latency from the nosepoke to cue onset (red symbols). Blue symbols indicate the nosepoke exit time for each trial. Time bins of 100 ms were used in constructing histograms. Only successful trials are represented in the histograms; the number of behavioural trials contributing to each histogram was 54 (A), 535 (B), and 213 (C) trials.
In contrast to the gradual onset of neural modulations, the offset of firing initiated during the delay period typically occurred abruptly near the time of movement onset. In some neurons, the change in firing occurring at delay offset was a return to baseline firing rate (Fig. 2A and B); in other neurons, a reversal in the direction of the neural response observed during the delay period occurred, as in the example shown in Fig. 2C, in which the transition from the delay to movement period was accompanied by a rapid shift from inhibition to excitation.
Delay onset and offset responses are closely associated with movement
Cue and movement events occurred in close temporal proximity during the delay. At delay onset, cue delivery followed nosepoke initiation at short latency; at the end of the delay period, cue offset was followed by movement. Thus, changes in firing rate occurring at delay onset and offset could be related either to cue or to movement events. To distinguish between these possibilities, we constructed perievent histograms around onset (nosepoke and cue onset) and offset (cue offset and nosepoke exit) events (Fig. 3A and B). Comparison of these histograms allowed us to identify the event most closely correlated with the change in firing, as changes in spike frequency were rapid and maximal in the histogram constructed around the event giving rise to the change in firing rate.
Figure 3.
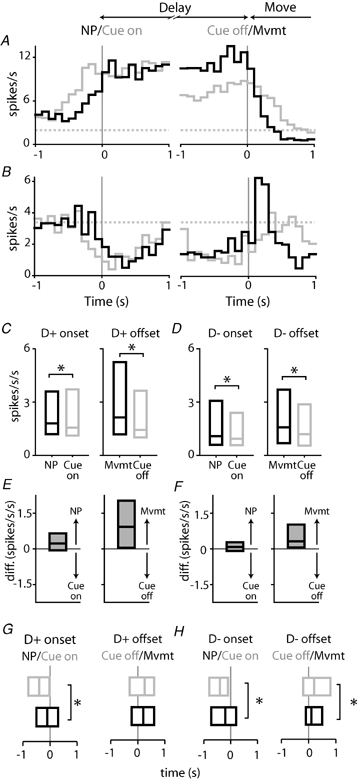
Changes in firing rate occurring near delay onset and offset are closely tied to movement, rather than the instructive cue A, a single example neuron, showing perievent histograms constructed around onset responses (left panel) occurring at nosepoke (NP: black line) and cue delivery (Cue on: grey line); and offset responses (right panel) occurring at movement onset (Mvmt: black line; identical with delay termination) and cue termination (Cue off: grey line). For this neuron, the excitatory response occurring near delay onset (left panel) was similar for nosepoke and cue onset. However, the excitatory response preceded cue onset, indicating that firing was not driven by cue delivery. The offset of this D+ firing pattern is more closely associated with movement onset than cue offset; the rate of change in firing is larger and more rapid in the histogram constructed around movement. B, onset and offset histograms for a D− M+ neuron. Conventions are identical to those described in A above. Note that the change in the rate of firing at delay offset (right panel) in this neuron was large, relative to delay onset; and that the offset response was more closely associated with movement onset than cue offset. These patterns were typical of the entire population of neurons. C, absolute rate of change in firing for the population of D+ onset (left panel) and offset (right panel) responses. Box plots depict median and 1st and 3rd quartile values. For onset responses, changes in firing occurring at nosepoke onset were larger than those occurring at cue onset (P < 0.05, Wilcoxon's signed rank test). Similarly, offset responses were larger at the time of movement initiation relative to cue offset (P < 0.05). All units are in spikes s−2. D, average absolute rate of change in firing D− onset and offset responses. Figure conventions are identical to those in C above. At both delay onset (left panel) and offset (right panel), changes in firing rate were larger during nosepoke events (both P < 0.05). E and F, data shown in C and D are regraphed as differences in the rate of change of firing, comparing movement and cue parameters. For each graph, values > 0 indicate higher values for movement parameters (nosepoke and movement onset) relative to cue parameters (cue onset and cue offset). Note that for all graphs shown in E and F, median values are positive, indicating higher rates of change in firing associated with movement parameters. G, for D+ neurons, delay onset responses occurred just prior to nosepoke onset (black bar), but significantly earlier relative to cue onset (grey bar) (left panel; P < 0.05). Delay offset responses occurred shortly after cue offset (grey bar) and movement initiation (black bar) (right panel; P > 0.05). Bars show median, 1st and 3rd quartile values of the time at which the change in firing occurred relative to the event of interest. H, for D− neurons, onset and offset responses (left and right panels, respectively) corresponded closely to nosepoke events, rather than cue onset/offset (P < 0.05 for both comparisons). Conventions are identical to those in E.
At delay onset, the rate of change in firing was similar in histograms constructed around nosepoke and cue onset (Fig. 3A and B, left panels). However, the onset of these changes typically preceded both nosepoke and cue onset, indicating that these firing patterns could not have been initiated by cue delivery (though possibly related to anticipation of cue onset). Differences in histograms constructed around cue offset and movement onset were more striking. In most neurons, changes in firing rate occurring near delay offset were closely tied to movement onset, rather than the termination of cue delivery (Fig. 3A and B, right panels). This was true of neurons showing both D+ and D− firing patterns.
We quantified the changes in firing associated with cue and motor events (see Methods) to allow statistical comparisons. For the population of both D+ and D− firing patterns, delay onset and offset responses were larger during motor events – nosepoke initiation at delay onset (Fig. 3C–F, left panels) and the nosepoke exit with which movement was initiated at delay offset (Fig. 3C–F, right panels; for all comparisons P < 0.05, Wilcoxon's signed rank test).
The timing of these responses also suggested a close association with motor events. Changes in firing for D+ and D− firing patterns at delay onset occurred just prior to nosepoke onset, but occurred significantly earlier than the corresponding cue onset (Fig. 3E and F, left panels; P < 0.05). At delay offset a similar pattern occurred, with maximal changes in firing occurring just after movement initiation and significantly later relative to cue offset (Fig. 3E and F, right panels, P < 0.05 for D− only). These data suggest a close association between the onset of movement and the timing and amplitude of changes in spike frequency occurring during the delay period.
Delay selective firing predicts subsequent movement direction
A subset of neurons (14/218; 6%) showed delay selective responses, in which firing rates during the delay period differed as a function of tone-response direction pairings. The example neuron shown in Fig. 4A and B illustrates this firing pattern. The firing rate in this neuron was greatest in trials in which the preferred cue-response direction occurred (black line, histogram), and significantly lower when the non-preferred cue-response pairing occurred (grey line, histogram; right panel, P < 0.05, t test). Note that both trial types reflect correct performance of the delayed response task – the term ‘preferred’ is used only to indicate the cue and response direction associated with the higher firing rate.
As was the case for delay onset/offset responses, selectivity could be associated either with sensory cues, reflecting the cue delivered on a particular trial, or with motor preparatory processing, encoding related to movement from the nosepoke to the reward receptacle. To dissociate the effects of these variables on neural firing rates, we examined firing occurring during error trials in which responses were directed to the non-instructed reward receptacle; these error trials allowed us to dissociate the impact of preferred (and non-preferred) cue and movement variables on firing rates. In the example neuron shown, firing rates varied as a function of response direction, rather than instructive cue (Fig. 4B). Neural activity was robust when the non-preferred cue was followed by movement in the preferred direction and significantly lower when the preferred cue was followed by the non-preferred response direction (P < 0.05).
For the population of delay selective neurons, firing rates differed significantly depending on trial type (Fig. 4C; overall P < 0.001, RM ANOVA on ranks). Relative to successful trials in which movement in the preferred direction occurred (PcuePmvmt), firing rates were significantly lower for both correct and error trials in which the non-preferred movement direction occurred (NcueNmvmt and PcueNmvmt, respectively; P < 0.05, Dunn's post hoc test). Firing rates during error trials in which the preferred movement direction occurred (NcuePmvmt) did not differ from firing rates during PcuePmvmt trials (P > 0.05). Together, these data suggest that response direction was the critical variable in giving rise to delay selectivity in this population of NAcc neurons.
Delay selective neurons more often showed higher firing rates for the ipsilateral movement direction (8/14 ipsilateral, 6/14 contralateral) but this difference was not significant (P > 0.05; z-test).
An interesting feature of selectivity in these neurons was that the degree of selective firing was not constant over the delay, but rather developed over the course of the delay period, peaking just prior to movement onset. The example neuron shown in Fig. 4A and B possessed robust selectivity near the time of delay offset; however, selectivity in this neuron was not apparent immediately after cue presentation (Fig. 5A). For the population of delay selective neurons, selectivity was significantly larger late in the delay period relative to early in the delay period (Fig. 5B; P = 0.01, Wilcoxon's signed rank test). Together with the association of delay onset and offset responses with motor events, and the predominance of selectivity for response direction, these data suggest that delay responses predominantly encode aspects of planned movements, rather than the sensory features of the instructive cue.
Figure 5.
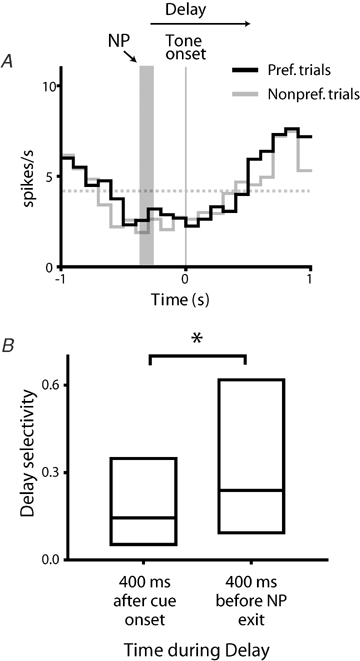
Selective firing increases over the delay period A, firing for the same example neuron shown in Fig. 4A and B is shown in histograms constructed around instructive tone onset. Histograms depict firing occurring during the preferred (black line) and non-preferred (grey line) trial types. Firing rates occurring immediately after tone presentation were very similar for both trial types, indicating an absence of selective firing early in the delay period. The vertical grey bar shows the approximate time of the nosepoke (NP), which preceded tone onset by a variable interval. Gray broken line indicates baseline firing. B, selectivity occurring during the early and late portions of the delay interval (first 400 ms following cue presentation and last 400 ms of the delay period, respectively) differed significantly (P = 0.01, Wilcoxon's signed rank test); selectivity late in the delay was substantially higher.
Movement responses are related to latency to reach the reward receptacle
Neurons showed both M+ and M− responses during locomotion to reward receptacles. Many of these responses showed a dependence on trial type. Figure 6A shows an example of an M+ firing pattern, in which firing was elevated during movement to the reward receptacle. Firing was lower for this neuron during error trials in which the rat moved to the wrong reward receptacle, suggesting a dependence on trial outcome. However, firing during premature movement errors was not uniformly lower – surprisingly, this firing was dependent upon the animals' latency to reach the reward receptacle. When this latency was short (< 500 ms), excitation was present in the neuron. For long latencies, however (> 500 ms), excitation was absent (Fig. 6B; overall effect of trial type, P < 0.05, 1-way ANOVA).
To determine if these two parameters of trial outcome (success or failure) and latency to reach the reward receptacle affected firing in the population of M+ neurons, we analysed firing rates for each of these trial types as a function of latency, dividing trials into those followed by short (< 500 ms) or long (> 500 ms) latencies to reach the reward receptacle. Data from this analysis are shown in Fig. 6C, where firing rates were normalized to the increase from baseline occurring during correct trials with short response latencies.
Overall, firing in the population of M+ neurons showed a dependence on both trial outcome and latency to reach the reward receptacle. Firing rates were highest during correct task performance and significantly lower for error trials, and higher for trials in which locomotion to the reward receptacle was fast relative to those in which it was slow (main effect of trial type, P < 0.01; main effect of latency, P < 0.05, 2-way RM ANOVA). Firing rates were significantly lower for slow relative to fast response latencies, and lower for both types of error trials relative to correct trials (P < 0.05 for all comparisons, Holm–Sidak post hoc test).
To further investigate the dependence of firing rate on response latency, we performed an additional analysis on trials in which premature movement occurred. Unlike other trial types, there was a pronounced bimodal distribution in the response latencies occurring during premature movement trials (Fig. 6D). For correct and wrong reward receptacle trials, reward receptacle entry always occurred within 1 s of nosepoke exit. However, for premature movement errors, response latencies ranged as high as 2 s, a difference readily apparent in the graph of cumulative response frequency (Fig. 6D, inset). To take advantage of this larger spread of response latencies, we therefore analysed M+ responses during premature movement trials as a function of latency to reach the reward receptacle, binning trials into < 500, 500–1000 and > 1000 ms groups. Firing rate showed a strong monotonic decrease with increasing response latency in this analysis (Fig. 2E; P < 0.05, RM ANOVA on ranks), with spike rates very similar to baseline firing at the longest response latencies (significantly lower than spike rates occurring during trials with short response latencies; P < 0.05, Dunn's post hoc test). These analyses demonstrate that M+ firing reflects both trial outcome (success or failure) and movement parameters (latency to reach the reward receptacle).
For M− neurons, differences in firing occurring during trial types were smaller than those observed for M+ neurons, but still significant (Fig. 6F; main effect of trial type P < 0.05, 2-way RM ANOVA). Firing in M− neurons was significantly attenuated during both types of error trials (P < 0.05, Holm–Sidak post hoc test). Unlike M+ neurons, there was no dependence on response latency.
Movement selective firing is closely associated with response direction
A subset of neurons possessed selective firing during the movement period (32/218, 15% of all neurons). As can be seen in the example neuron in Fig. 7A, firing rates differed depending on the cue/response direction contingency (right panel; P < 0.05, paired t test). As for delay selectivity, we analysed movement selectivity by analysing error trials, allowing us to dissociate motor events and sensory cues in determining the source of movement selectivity. In error trials, firing was most robust in this neuron when the preferred response direction occurred, rather than the preferred cue (Fig. 7B; preferred versus non-preferred response direction; P < 0.05).
Response direction was the critical determinant of selective firing in the population of movement selective neurons (Fig. 7C; P < 0.001, RM ANOVA on ranks). Firing rates were higher when the preferred movement direction was executed, rather than the preferred cue presented. Movement in the non-preferred response direction resulted in lower firing rates for both correct and error trials (P < 0.05 for both PcueNmvmt and NcueNmvmt relative to PcuePmvmt trials, Dunn's post hoc test). Thus for both movement and delay selective neurons, firing rates were driven by movement direction, rather than the sensory or reward predicting properties of instructive cues. As for D selective neurons, a majority of M selective neurons had higher firing rates for the ipsilateral response direction (18/32 ipsilateral, 14/32 contralateral), but this difference was not significant (P > 0.05, z-test).
Movement selectivity encodes response direction, not goal of movement
In the delayed response task, rats started each trial at the central nosepoke port. Because of this invariant starting point, the movement direction required to reach a particular reward receptacle never changed during task performance. Thus apparent selectivity for a response direction could instead reflect encoding related to the goal of the movement – the reward receptacle itself, rather than the movement direction needed to reach it. To distinguish between these possibilities, we analysed firing occurring during spontaneous approaches to the reward receptacle. These included all reward receptacle approaches that occurred outside of the delayed response task. These spontaneous approaches were typically initiated after grooming bouts occurring away from the nosepoke port, and locomotion to the reward receptacle involved a variety of locomotor trajectories.
The representative neuron shown in Fig. 8 possessed robust selectivity during correct task performance. For this neuron, significantly higher firing rates occurred during movement to the left reward receptacle (Fig. 8A; P < 0.05, t test). However, during spontaneous reward receptacle approach, firing rates remained close to baseline levels and selectivity was not apparent (Fig. 8B, P > 0.05).
Figure 8.
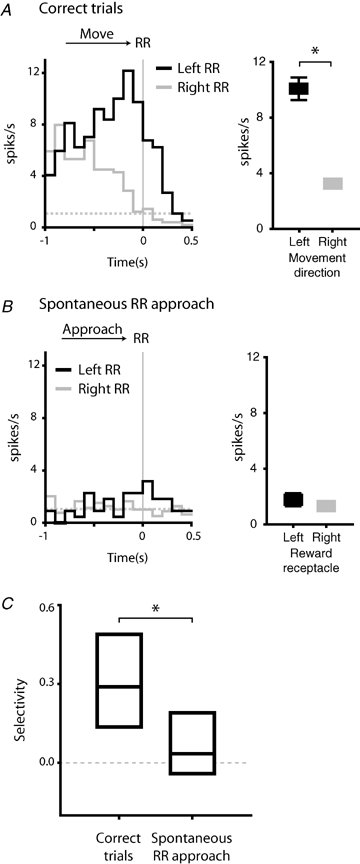
Movement selective responses encode movement direction, rather than movement destination A, movement selectivity in an example neuron. This neuron fired more robustly during movement to the left reward receptacle (left panel; black line) relative to rightward movement (grey line). The difference in firing was significant (right panel; P < 0.05, comparing left versus right movement, t test). Histograms are constructed around reward receptacle entry (RR). Values shown indicate mean firing rates in the 400 ms preceding reward receptacle entry for each movement direction. Broken grey line indicates baseline firing rate. B, selective firing in the example neuron shown in A was much reduced during spontaneous approach to the reward receptacles (P > 0.05). Firing was near baseline spike rate during spontaneous approach to both the left (black line) and right (grey line) reward receptacles. C, selective firing was significantly higher during correct task performance, relative to spontaneous reward receptacle approach (P < 0.001, Wilcoxon's signed rank test). Bar chart shows median, first and third quartile values.
For the population as a whole, selectivity was present only during correct task performance, and significantly reduced during spontaneous reward receptacle approach (Fig. 8C; P < 0.001, Wilcoxon's signed rank test). This suggests that the specific movements executed during task performance are encoded by selective firing, rather than the spatial goal of those movements.
Discussion
In the present experiment, we analysed cue and movement related firing in the NAcc of rats performing a delayed response task for sucrose reward. Our data suggest that NAcc neurons contribute both to motor preparatory processing occurring prior to movement onset, and to movement execution. In both delay and movement periods, firing in NAcc neurons was, to an unexpected degree, influenced by movement parameters. We discuss the firing patterns we recorded during delay and movement periods and the implications of these results below.
Neural firing during the delay period
We characterized several novel features of neural activity in NAcc neurons occurring during a delay period prior to movement initiation. Firing occurring during this delay period was characterized by a tight correspondence with movement parameters, namely the timing and direction of reward-directed actions. In particular, the offset of changes in delay period firing occurred very close to the time at which movement to the reward receptacle was initiated. For the subset of neurons that showed selective firing during the delay period, this selectivity anticipated the subsequent direction of movement, rather than the sensory properties of the instructive cue presented. Finally, this selectivity, while weak early in the delay, increased in magnitude during the late periods of the delay just prior to movement initiation.
Previous studies of NAcc responses to conditioned cues in the rodent have demonstrated firing correlates to a host of reward parameters. NAcc neurons encode the identity of a predicted reinforcer (Carelli & Ijames, 2001), the rewarding or aversive nature of a reinforcer (Setlow et al. 2003; Roitman et al. 2005; Wilson & Bowman, 2005) as well as the availability of reward (Nicola et al. 2004; Peoples et al. 2004). Few studies in rodents, however, have dissociated cue presentation from the ensuing behavioural response, making it difficult to unambiguously determine the most relevant parameter encoded by changes in neural activity – the predictive cue (and reward information associated with the cue), motor parameters (both planning and execution), or both, could contribute. In the present behavioural task, temporal separation of cue presentation and movement onset allowed us to examine the relative contribution of these factors separately. Somewhat surprisingly, we found that NAcc encoding during this delay period strongly reflected motor variables in all measures examined – timing of the onset and offset of firing, selective firing, and the timing of selectivity itself.
The patterns of delay period firing we describe here are similar to those previously reported in studies of the primate striatum (Apicella et al. 1992; Schultz et al. 1992, 1993), in which many neurons possessed movement related firing prior to the onset of movement. In go/no-go tasks, for instance, subsets of neurons showed premovement firing that was specific for the instructed response – a large subset of neurons fired selectively prior to ‘go’ responses, while a smaller group showed selective firing during ‘no-go’ trials (Hollerman et al. 1998). Similar to our present results, when tasks requiring a directional behavioural response were employed, direction-selective firing was often encountered (Hassani et al. 2001). The results we report here extend these findings by demonstrating that in the rat, this encoding in the NAcc reflects selectivity for the movement direction itself, rather than the target location or stimulus properties of the instructive cue.
Previous studies have shown a strong dependence of cue-related firing on predicted reward properties; our results raise the possibility that this encoding occurs in movement coordinates, such that expectation of reward facilitates implementation of the specific behaviour necessary to obtain it. An influence of anticipated reward value on movement encoding is a necessary step in the transformation of goals into behaviour. Reward predictive-cues have powerful and consistent effects on behaviour: reward anticipation decreases response latencies and increases response speeds (Bowman et al. 1996; Bowman & Brown, 1998; Hauber et al. 2000). Elegant studies of firing in the oculomotor striatum have demonstrated widespread effects of reward anticipation on firing in neurons that have a powerful modulatory role on initiating and executing eye movements (Hikosaka et al. 2006). Reward anticipatory firing in subsets of these neurons is correlated with increased probability of a saccade in the rewarded direction, and increased speed of the saccade (Lauwereyns et al. 2002; Itoh et al. 2003). These experiments elucidate some of the mechanisms through which reward encoding in striatal circuits facilitates execution of motivated behaviours. In the present study, we intentionally did not vary reward value; all successful trials resulted in an identical volume of sucrose delivery. Thus the precise manner in which NAcc neurons integrate reward and movement encoding remains unknown. Future studies in which both reward value and required response direction are systematically varied will be required to determine if single neurons in the NAcc jointly encode commands for movement and anticipated reward.
Though the present results show that the NAcc encodes information related to movement planning, the contribution of this neural activity to generating behaviour is not clear. Lesions studies demonstrate that NAcc firing importantly contributes to behavioural performance in delayed response tasks similar to the one employed in the present experiments. Thus, excitotoxic lesions of the ventral striatum increase premature responding in a sustained nosepoke task (Bowman & Brown, 1998), and lesions of the NAcc core increase premature responses in a serial reaction time task (Christakou et al. 2004).
NAcc delay period firing could either promote or inhibit planned movements. Premovement firing has been described in many brain regions (Kurata & Wise, 1988; Alexander & Crutcher, 1990), and has been suggested to provide a ‘readiness’ signal that speeds or facilitates some aspect of the subsequent behavioural response. In the case of the NAcc, however, it is also possible that premovement firing serves to inhibit forthcoming movement, ensuring that movement is delayed until the appropriate moment. This latter possibility is supported by lesion and pharmacology literature showing that NAcc inactivation promotes behavioural activation in many experimental paradigms (Reading & Dunnett, 1995; Bowman & Brown, 1998; Basso & Kelley, 1999; Di Ciano et al. 2001; Christakou et al. 2004; Yun et al. 2004a). Such inhibition could take place through indirect, rather than direct, projections to motor output nuclei. It is interesting in this regard to note that in recordings from the primate striatum, ‘no-go’ selective responses were much more commonly encountered when ‘no-go’ trials were rewarded, relative to paradigms in which they were unrewarded (Apicella et al. 1992; Schultz & Romo, 1992). Presumably, greater response suppression is required when movement must be withheld to obtain a reward (as opposed to no reward), and increased firing in striatal circuits that suppress movement is recruited under these conditions.
Neural firing during movement
Similar to delay responses, NAcc firing patterns recorded during locomotion to the reward receptacle encoded correlates of movement. The amplitude of movement responses was contingent upon both the trial outcome (changes in firing were larger during successful trials relative to error trials) and, for M+ responses, the latency with which movement to the reward receptacle took place (responses were larger during short-latency movements). A population of neurons showed selectivity during the movement period, and this selectivity reflected the direction of movement, rather than the instructive cue presented, or the spatial goal of the movement – selective firing was absent during movements to the reward receptacles that occurred outside the delayed response task and originated at locations other than the nosepoke hole.
Pharmacological studies have established a key role for the NAcc in generating locomotion (Mogenson et al. 1980; Tzschentke & Schmidt, 2000). Previous electrophysiological studies of NAcc function, though not focusing explicitly on locomotion, have demonstrated that a large subset of NAcc neurons possess firing related to ongoing movement (Chang et al. 1994; Yun et al. 2004b; Taha & Fields, 2005; Day et al. 2006; Wan & Peoples, 2006). For many neurons, performance of an operant response alone, in the absence of any reinforcer delivery, is sufficient to elicit firing (Peoples et al. 1997). Our results provide further evidence of an important role for the NAcc in generating locomotor behaviours, demonstrating that a subset of NAcc neurons – those with M+ firing patterns – possess firing rates that correlated with the time required to move from the nosepoke to the reward receptacle. Though these electrophysiological findings are correlative, they suggest that these neurons are involved in regulating reward-directed locomotor behaviours, perhaps by speeding approach when reward is anticipated. A previous study in primates supports such a role for dorsal striatal neuron in regulating the velocity of reward-directed saccades (Itoh et al. 2003).
In the present work, the amplitude of both M+ and M− responses depended upon trial outcome – these responses were larger during correct trial performance, and attenuated during error trials. The source of this difference in firing rate is unclear, but one possibility is that movements employed during locomotion to the reward receptacle were subtly different during correct and error trials. Alternatively, it is possible that reward expectation might be diminished during error trials, and differences in firing rate could reflect differing reward expectancies during correct and error trials. This interpretation is consistent with previous studies demonstrating a role for reward expectancy in modulating movement related NAcc firing patterns (Cromwell & Schultz, 2003).
Selective firing during reward receptacle approach (Fig. 8) was apparent during task performance, but not during spontaneous receptacle approach. While the goal of both both types of movement was the same – the reward receptacle – the locomotion required to reach this goal differed. During task performance, movements invariably started from the central nosepoke port. This constrained the locomotor paths possible to reach the goal, and ensured that for a given direction of movement, the movement executed to the reward receptacle on each trial was very similar. In contrast, spontaneous approaches could be initiated from any point in the cage and included a variety of approach trajectories. Thus, the occurrence of selective firing in the former, but not the latter, scenario, suggests that this selectivity was related to the movement, rather than the destination of the movement. Other factors, such as reward expectancy, may also have differed during task performance versus spontaneous approach. However, reward expectancy is unlikely to have contributed to selective firing occurring during task performance. Firing during correct task performance differed during movements to the left and right reward receptacles, despite the fact that identical reward volumes delivered to these receptacles would give rise to identical reward expectancy for both directions of movement. Thus reward expectancy is unlikely to have contributed significantly to the selective firing.
We did not find significant lateralization for contra- or ipsilateral movement in direction selective NAcc neurons. This finding contrasts with the pronounced lateralization of encoding apparent in studies of the dorsal striatum, where the majority of neurons typically fire selectively for movement into the contralateral hemifield (Lauwereyns et al. 2002). Lesions studies provide additional evidence that the connections of the ventral striatum to motor effectors are less lateralized than those of the dorsal striatum. Unilateral 6-hydroxydopamine lesions of the NAcc had only subtle lateralization effects on responding in a visual discrimination task – rats with these lesions showed elevated contralateral responding only when the response requirement was made more eccentric (Carli et al. 1989). Unilateral lesions of the dorsal striatum, in contrast, showed a consistent bias toward ipsilateral responding under all response requirements.
General implications for NAcc function
An important hypothesis of function in striatal circuits is that they facilitate action selection (Pennartz et al. 1994; Redgrave et al. 1999; Nicola, 2007). Selection of a specific action has been proposed to occur through a mechanism of mutual inhibition, in which robust firing in neurons encoding the ‘winning’ behaviour not only promotes initiation of that behaviour through effects on downstream motor circuits, but also inhibits firing in other populations of striatal neurons encoding competing actions. This model requires representation of specific and competing behaviours in distinct subsets of NAcc neurons. Our data show that this selectivity for one of a pair of competing movements is encoded by NAcc neurons during preparation for and execution of motivated behaviours. During delay and movement intervals, distinct subsets of neurons encoded competing motor information (i.e. left versus right). This encoding could serve as the substrate for a behavioural selection mechanism. Further studies are needed to determine if inhibitory interactions occur between subsets of NAcc neurons encoding competing behavioural responses.
NAcc movement encoding: instructive or permissive?
Our finding of movement selective firing in NAcc neurons does not eliminate a broader contribution of the NAcc in gating the initiation of motivated behaviour in general, acting as a simple ‘go’ signal without specifying the nature of the behaviour to be executed. In a previous paper (Taha & Fields, 2006) we argued that a distinct subset of neurons, which show long-lasting inhibitions during the delayed-response task, have a firing pattern consistent with such a role. There is clear evidence from the behavioural literature that neurons in the NAcc shell gate feeding behaviour (reviewed in Kelley, 2004). Pharmacological inhibition of firing in the NAcc causes a robust feeding response, likely through disinhibition of neurons in target regions (Stratford & Kelley, 1997, 1999). Some behavioural evidence suggests that a gating function for NAcc shell neurons may extend beyond consummatory behaviours to the appetitive behaviours that precede them. Inhibition of the NAcc shell increases lever-pressing in a reinstatement paradigm (McFarland et al. 2004) and inhibition of the core-shell border increases responding in a discriminative stimulus paradigm (Yun et al. 2004a).
Considering the present data together with our previous study, we propose that subsets of NAcc neurons contribute to motivated behaviour in two distinct ways. Neurons with long-lasting inhibitions gate appetitive and consummatory behaviours, acting in a permissive fashion. In contrast, other neurons, such as those showing movement selective firing, facilitate planning and execution of specific reward-directed actions.
Acknowledgments
The authors gratefully acknowledge S. L. Borgland, P. Newton and F. Ambroggi for insightful comments on the manuscript, and V. Kharazia and R. Van for histological assistance. This work was supported by funds provided by the State of California, the Wheeler Center for the Neurobiology of Addiction, and the Department of Defense (HLF); and NARSAD (SAT).
References
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. doi: 10.1152/jn.1992.68.3.945. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABAA receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- Bowman EM, Brown VJ. Effects of excitotoxic lesions of the rat ventral striatum on the perception of reward cost. Exp Brain Res. 1998;123:439–448. doi: 10.1007/s002210050588. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the ‘accumbens’ part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carli M, Jones GH, Robbins TW. Effects of unilateral dorsal and ventral striatal dopamine depletion on visual neglect in the rat: a neural and behavioural analysis. Neuroscience. 1989;29:309–327. doi: 10.1016/0306-4522(89)90059-6. [DOI] [PubMed] [Google Scholar]
- Chang JY, Sawyer SF, Lee RS, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci. 1994;14:1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical–ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, Carelli RM. Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci. 2006;23:1341–1351. doi: 10.1111/j.1460-9568.2006.04654.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol. 2001;85:2477–2489. doi: 10.1152/jn.2001.85.6.2477. [DOI] [PubMed] [Google Scholar]
- Hauber W, Bohn I, Giertler C. NMDA, but not dopamine D2, receptors in the rat nucleus accumbens are involved in guidance of instrumental behavior by stimuli predicting reward magnitude. J Neurosci. 2000;20:6282–6288. doi: 10.1523/JNEUROSCI.20-16-06282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol. 1998;80:947–963. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- Itoh H, Nakahara H, Hikosaka O, Kawagoe R, Takikawa Y, Aihara K. Correlation of primate caudate neural activity and saccade parameters in reward-oriented behavior. J Neurophysiol. 2003;89:1774–1783. doi: 10.1152/jn.00630.2002. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kurata K, Wise SP. Premotor and supplementary motor cortex in rhesus monkeys: neuronal activity during externally- and internally-instructed motor tasks. Exp Brain Res. 1988;72:237–248. doi: 10.1007/BF00250247. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes Da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Lynch KG, Lesnock J, Gangadhar N. Accumbal neural responses during the initiation and maintenance of intravenous cocaine self-administration. J Neurophysiol. 2004;91:314–323. doi: 10.1152/jn.00638.2003. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Uzwiak AJ, Gee F, West MO. Operant behavior during sessions of intravenous cocaine infusion is necessary and sufficient for phasic firing of single nucleus accumbens neurons. Brain Res. 1997;757:280–284. doi: 10.1016/s0006-8993(97)00299-0. [DOI] [PubMed] [Google Scholar]
- Reading PJ, Dunnett SB. Embryonic striatal grafts reverse the disinhibitory effects of ibotenic acid lesions of the ventral striatum. Exp Brain Res. 1995;105:76–86. doi: 10.1007/BF00242184. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T, Romo R, Scarnati E. Reward-related activity in the monkey striatum and substantia nigra. Prog Brain Res. 1993;99:227–235. doi: 10.1016/s0079-6123(08)61349-7. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Romo R. Role of primate basal ganglia and frontal cortex in the internal generation of movements. I. Preparatory activity in the anterior striatum. Exp Brain Res. 1992;91:363–384. doi: 10.1007/BF00227834. [DOI] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38:625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci. 2005;25:1193–1202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–142. [PubMed] [Google Scholar]
- Wan X, Peoples LL. Firing patterns of accumbal neurons during a pavlovian-conditioned approach task. J Neurophysiol. 2006;96:652–660. doi: 10.1152/jn.00068.2006. [DOI] [PubMed] [Google Scholar]
- Wilson DI, Bowman EM. Rat nucleus accumbens neurons predominantly respond to the outcome-related properties of conditioned stimuli rather than their behavioral-switching properties. J Neurophysiol. 2005;94:49–61. doi: 10.1152/jn.01332.2004. [DOI] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004a;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004b;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


