Abstract
Restricted growth before birth is associated with impaired insulin secretion but with initially enhanced insulin sensitivity in early postnatal life, which then progresses to insulin resistance and impaired glucose homeostasis by adulthood. This suggests that prenatal restraint impairs insulin secretion, but increases insulin sensitivity, before birth. Poor placental growth and function are major causes of restricted fetal growth in humans. We have therefore investigated the effects of restricted placental growth and function on plasma glucose, α-amino nitrogen and insulin concentrations and glucose- and arginine-stimulated insulin secretion in the fetal sheep at 120 and 140 days gestational age, and on insulin sensitivity, measured by hyperinsulinaemic euglycaemic clamp, at 130 days gestational age. Placental restriction decreased fetal blood pH and oxygen content, and weight in late gestation by ∼20%. Reduced fetal and placental weights and indices of poor placental function, in particular fetal hypoxia and hypoglycaemia, were associated with impaired glucose- and arginine-stimulated insulin secretion, but not with changes in insulin sensitivity in the fetal sheep. We conclude that the impaired insulin secretion capacity reported in children and adults after intrauterine growth restriction, and in the neonatal and young adult sheep which is small at birth, is present in utero and persists. Whether this reflects the actions of the adverse intrauterine environment or changes to intrinsic capacity is unclear, but in utero interventions may be necessary to improve postnatal insulin secretion in the infant who is growth-restricted before birth.
Small size at birth is consistently associated with an increased risk of diabetes and impaired glucose tolerance in later life in humans (Newsome et al. 2003). This appears to be due in part to insulin resistance, as insulin sensitivity is consistently negatively related to birth size in children, young and old adults (Newsome et al. 2003). However, this dysfunction appears to emerge in postnatal life, because whole-body insulin sensitivity is increased in the neonate and infant following intrauterine growth restriction (IUGR) (Bazaes et al. 2003; Mericq et al. 2005), which may contribute to their characteristic neonatal catch-up growth. Whether enhanced insulin sensitivity is present in the IUGR fetus or this develops after birth is not known. The impact of poor fetal growth on insulin secretion postnatally is more controversial, as indirect indices of insulin secretion are not consistently related to birth weight (Newsome et al. 2003). More recently, however, direct evidence has emerged that postnatal insulin secretion is impaired following IUGR. Insulin disposition (insulin secretion relative to sensitivity) was reduced in three studies of 3-year-old children, 4- to 14-year-old children and 19-year-old men, defined as IUGR or small-for-gestational age (SGA) at birth, compared to those of normal birth weight (Li et al. 2001; Jensen et al. 2002; Mericq et al. 2005), although two other studies in prepubertal children and in 25-year-old adults of both sexes found no effect of IUGR or SGA on insulin disposition (Jaquet et al. 2000; Veening et al. 2003).
Other evidence indicates that the capacity for insulin secretion might be impaired from before birth in the IUGR human fetus. One study reported a lower proportion of β-cells in the pancreas of human fetuses with severe IUGR (< 1.5 kg at term gestation) (Van Assche et al. 1977), although this proportion did not differ between control and less-restricted fetuses (< 2.3 kg birth weight) in another study (Béringue et al. 2002). In utero measures of insulin secretion or sensitivity are not available in humans, however, and animal models of fetal growth restriction have therefore been used to characterize the impact of IUGR on insulin secretion and sensitivity, and the underlying mechanisms leading to these. Placental insufficiency, due to poor placental growth and/or function, is a common cause of restricted fetal growth in humans (Bleker et al. 2006). Uterine artery ligation in rats in late gestation (day 18, term ∼day 21) restricts fetal and placental growth, decreases the proportion of β-cells in islets, and reduces amniotic C-peptide levels at day 20 of gestation, indicative of impaired fetal insulin secretion (De Prins & Van Assche, 1982; De Prins et al. 1983). Postnatally, rats born following uterine artery ligation have normal fasting glucose and insulin, but impaired glucose tolerance and signs of impaired insulin sensitivity in the first week of life, and subsequently develop frank diabetes as adults (Simmons et al. 2001). Insulin secretion was also impaired, particularly the first phase insulin response to glucose, from early postnatal life at 1 week of age, prior to development of fasting hyperglycaemia (Simmons et al. 2001). The postnatal outcomes of this late gestation onset of placental restriction in the rat thus differ somewhat from those following chronic IUGR in humans, where insulin sensitivity is enhanced in early postnatal life (Bazaes et al. 2003; Mericq et al. 2005), and insulin resistance develops later (Newsome et al. 2003).
Placental restriction (PR) can be readily induced by surgical removal of the majority of placental implantation sites from the endometrium before pregnancy in the sheep, with similar metabolic, endocrine and growth consequences for the fetus as in human IUGR (Robinson et al. 1979; Robinson et al. 1980; Owens et al. 1989; Owens et al. 1994). The PR sheep fetus is hypoxic and hypoglycaemic (Robinson et al. 1979; Owens et al. 1987, 1989). Plasma insulin concentrations are also reduced in the PR fetus, and correlate with those of glucose (Robinson et al. 1980). Glucose-stimulated insulin secretion has been measured only indirectly to date in this model. A 4 h infusion of glucose into maternal blood, which increased maternal plasma glucose from ∼2.5 to ∼7.5 mm and fetal plasma glucose from ∼0.5–2.1 mm in control pregnancies, increased plasma glucose by only 50% in PR fetuses (Harding, 1982; Harding et al. 1985). This did not increase fetal plasma insulin in control fetuses, nor in four PR fetuses in which insulin was measured, although as plasma was collected for glucose and insulin analyses only at 2 and 4 h after commencement of the glucose infusion, acute insulin secretory responses to changes in glucose would not have been observed in this study (Harding, 1982). The chronic restriction of placental and fetal growth in this experimental model in sheep reduces size at birth and importantly is followed by catch-up growth in the first month of life, which is related in part to their enhanced insulin sensitivity (Gatford et al. 2002; De Blasio et al. 2006, 2007). Placental restriction and small size at birth were associated with increased insulin sensitivity of glucose metabolism, but reduced glucose-stimulated insulin secretion in the young sheep at ∼1 month of age. These effects of PR and small size at birth are followed by fasting hypoinsulinaemia, impaired glucose tolerance and insulin action in the adult, at least in males (De Blasio et al. 2007; Owens et al. 2007). The effects of PR and small size at birth on postnatal insulin secretion and sensitivity in sheep thus follow a similar pattern of postnatal development as in the human following IUGR and small size at birth.
We hypothesized that placental restriction would impair glucose- and arginine-stimulated insulin secretion but enhance insulin sensitivity before birth. We determined the effect of restricted implantation and placental growth on insulin secretion and sensitivity in chronically catheterized, conscious fetal sheep, before and after the late gestation cortisol surge, in control and placentally restricted fetuses. Evidence from studies in fetal horses suggests that this increase in cortisol in late gestation enhances glucose-stimulated insulin secretion, in preparation for the transition to postnatal life (Fowden et al. 2005). Since the late gestation cortisol surge is advanced and amplified in the PR fetus (Phillips et al. 1996), we hypothesized that the effects of PR on insulin secretion might differ with gestational age. In addition, we have assessed the relationships between these functional outcomes (insulin secretion and sensitivity) and markers of the degree of fetal restriction (pH, 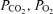 , oxygen content, plasma glucose), fetal adaptations to these (plasma insulin and cortisol abundance), and fetal-placental growth (fetal and placental weight, fetal: placental weight ratio).
, oxygen content, plasma glucose), fetal adaptations to these (plasma insulin and cortisol abundance), and fetal-placental growth (fetal and placental weight, fetal: placental weight ratio).
Methods
Animals and surgery
All procedures in this study were approved by the University of Adelaide Animal Experimentation and Ethics Committee, and complied with the Australian code of practice for the care and use of animals for scientific purposes. Placental growth was restricted (PR) by removal of the majority of endometrial caruncles from the uterus of non-pregnant Australian Merino ewes prior to mating as previously described (Robinson et al. 1979). All surgery was performed under aseptic conditions with general anaesthesia induced by an intravenous injection of sodium thiopentone (1.25 g ml−1, Boehringer Ingelheim, NSW, Australia), and maintained by inhalation of 2–4% halothane in oxygen. Ewes were kept under observation for 4–7 days postsurgery before being returned to the field, and a recovery period of at least 10 weeks was allowed prior to mating. Mature adult PR and unoperated control ewes of similar ages entered a timed-mating programme and pregnancies were confirmed by ultrasound at approximately 60 days gestational age (term = d 149). From 90 days gestational age (d 90), pregnant ewes were housed in individual pens in animal holding rooms with a 12 h light–dark cycle, and fed once daily between 09.00 h and 13.00 h with water available ad libitum. Nineteen control and nine PR ewes were fasted overnight prior to surgery at d 108.9 ± 0.5, and at least one experiment was completed in each of 14 control and six PR fetuses from this group. Catheters were inserted into either the carotid artery and jugular vein or a femoral artery and femoral vein of each fetus, as well as into the carotid artery and jugular vein of each ewe, under general anaesthesia and asepsis as described above. Only one fetus was catheterized in multiple-bearing ewes. Antibiotics were given intramuscularly to each ewe prior to surgery (600 mg benzylpenicillin sodium, CSL Ltd, Australia and 80 mg gentamycin as gentamycin sulphate, David Bull Laboratories, Australia), and to each fetus immediately following surgery (300 mg benzylpenicillin each intra-amniotic and i.v.). Analgesia was given to each ewe postsurgery (1 ml flunixin meglumine 50 mg ml−1i.m., Finadyne solution, Schering-Plough Animal Health). Insulin secretion tests in late gestation (d 140) were also performed on an additional seven PR fetuses of the same flock and genotype, which had surgery as described above to insert catheters at d 119.3 ± 0.2 (Edwards et al. 1999). Fetal arterial blood pH, 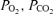 , oxygen saturation and haemoglobin content were measured using an ABL 520 analyser (Radiometer, Copenhagen, Denmark) at 2 day intervals throughout the study and before each experiment. Arterial oxygen content was calculated as previously described (Edwards et al. 1999). Animals recovered for at least 8 days after surgery before experimental protocols commenced. At least one measure of insulin secretion or sensitivity was obtained on 27 fetal sheep (14 control, 13 PR). Complete measures (insulin secretion responses to glucose and arginine at ∼120 and ∼140 days gestational age, insulin sensitivity at ∼130 days gestational age, and postmortem data) were obtained for 13 fetuses (9 control, 4 PR), which remained healthy and with patent catheters throughout the study. Figure 1 shows animal numbers throughout the study.
, oxygen saturation and haemoglobin content were measured using an ABL 520 analyser (Radiometer, Copenhagen, Denmark) at 2 day intervals throughout the study and before each experiment. Arterial oxygen content was calculated as previously described (Edwards et al. 1999). Animals recovered for at least 8 days after surgery before experimental protocols commenced. At least one measure of insulin secretion or sensitivity was obtained on 27 fetal sheep (14 control, 13 PR). Complete measures (insulin secretion responses to glucose and arginine at ∼120 and ∼140 days gestational age, insulin sensitivity at ∼130 days gestational age, and postmortem data) were obtained for 13 fetuses (9 control, 4 PR), which remained healthy and with patent catheters throughout the study. Figure 1 shows animal numbers throughout the study.
Figure 1.
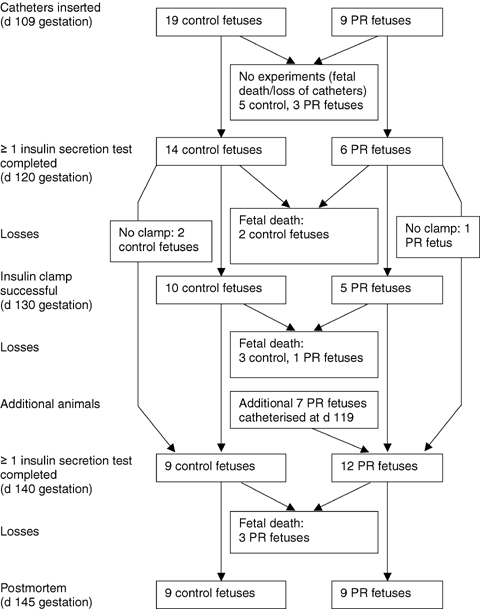
Flow diagram for experiments on fetal sheep
Estimated fetal body weights were calculated at 120, 130 and 140 days gestational age (corresponding to ages at experiments) from the following growth curves, established previously in our laboratory for control and placentally restricted fetuses (Owens et al. 1986): estimated body weight of control fetus (g) = (69.3 × gestational age in days) − 5789; estimated body weight of PR fetus (g) = (63.9 × gestational age in days) − 5940.
In vivo measurement of insulin secretion
Insulin secretion responses to glucose and arginine were measured in fetuses in late gestation at ∼d 120, and at ∼d 140 (Fowden, 1980b). The two secretagogues were administered in random order, with at least 46 h recovery between each test. Blood samples (2 ml in PR fetuses at d 120, and 3.5 ml otherwise) were collected via the fetal arterial catheter 30 and 0 min prior to administration of each secretagogue for measurement of basal plasma insulin and glucose concentrations. Fetuses received a bolus of dextrose (0.5 g (kg estimated body weight)−1 as 25% dextrose, at d 121.3 ± 0.3 and d 139.5 ± 0.5) or l-arginine (100 mg (kg estimated body weight)−1 in 0.9% NaCl, at d 121.6 ± 0.4 and d 138.9 ± 0.5), infused over 30 s via the intravenous catheter, and blood was sampled via the fetal arterial catheter at 5, 15, 30 and 60 min after infusion. Additional samples were taken 15 min before and 45 min after administration of the secretagogue in control fetuses at d 140 only. Blood was centrifuged, and plasma stored at −20°C for later analyses (glucose and insulin before and after each test, and plasma α-amino nitrogen responses to arginine only).
In vivo measurement of insulin sensitivity
The insulin sensitivity of glucose metabolism was measured by hyperinsulinaemic euglycaemic clamp (HEC), at d 129.8 ± 0.2. Fasting blood glucose and plasma insulin were calculated as mean concentrations in fetal arterial blood samples collected 10, 5 (glucose only) and 0 min before starting an infusion of human insulin (Actrapid human insulin, Novo Nordisk A/S, Denmark) into the fetal venous catheter at 0.6 mU insulin (kg estimated body weight)−1 min−1 for 120 min. Dextrose infusion (25% w/v, i.v.) into the fetal venous catheter commenced 15 min later. Blood was sampled (0.2 ml) every 5 min, whole blood glucose concentrations were rapidly analysed using a glucometer (HemoCue AB, Sweden), and the glucose infusion rate was adjusted to maintain euglycaemia. Additional blood samples (2 ml) were collected from the fetal arterial catheter 10 and 0 min before and 60, 75, 90, 105 and 120 min after the insulin infusion started, for measurement of plasma glucose and insulin. Steady-state blood glucose (2nd hour of the clamp) averaged 102.9 ± 1.7% of blood glucose concentrations prior to the clamp. The glucose infusion rate was calculated as mg glucose infused per minute, both in absolute terms, and divided by estimated fetal bodyweight. The insulin sensitivity of glucose metabolism was calculated as the steady state glucose infusion rate (in absolute terms, or per kg estimated body weight), divided by steady state plasma insulin concentration during the second hour of the clamp. The metabolic clearance rate of insulin (MCRinsulin) was calculated by dividing the insulin infusion rate by the increase in plasma insulin from fasting to steady-state conditions.
Autopsy
All surviving ewes and fetuses were killed with an overdose of sodium pentobarbitone (Lyppards, Castle Hill, NSW, Australia) between d 140 and d 145 (mean 142.8 ± 0.4 days), and fetal sheep were delivered by hysterotomy. Fetuses and placentae were weighed and numbers of cotyledons in each placenta were counted. Postmortem data were included only for fetuses with at least one measure of insulin secretion or sensitivity and data for non-catheterized sibling fetuses were not included.
Analysis of cortisol, insulin, glucose and AAN in plasma
Plasma cortisol concentrations were measured by RIA in two samples collected before each IVGTT and before the HEC, and the mean of these two values calculated for each gestational age. Cortisol was measured using a primary rabbit polyclonal antibody raised against human cortisol (ICN Diagnostics), and cortisol-3-(O-carboxymethyl) oximonio-(2-[125I]iodohistamine) (GE Healthcare) as tracer. Before assay, cortisol was extracted from plasma using dichloromethane as previously described (Bocking et al. 1986). Recovery of 125I-labelled tracer was 98.3 ± 0.6% and intra- and interassay CV were 7.8% and 15.6%, respectively, for a QC sample containing 14.0 nmol l−1 total cortisol (n = 3 assays). The minimum detectable cortisol concentration was 1.56 nmol l−1. The anticortisol antisera cross-reacted with 11-deoxycortisol (12.3%), corticosterone (5.5%), cortisone (2.1%), 17β-hydroxyprogesterone (1.0%), progesterone (0.25%) and testosterone (< 0.1%) at 50% displacement on the cortisol standard curve.
Concentrations of insulin in plasma were analysed by radioimmunoassay using a commercially available kit (Phadeseph Insulin RIA, Pharmacia, Upsala, Sweden). The intra-assay CV for the insulin assay was 3.5% and the interassay CV was 12.5% for an ovine QC containing 9.7 mU insulin l−1 (n = 10 assays). Concentrations of glucose in plasma were quantitatively determined (COBAS MIRA) by enzymatic analysis (Glucose HK assay kit, Roche Diagnostics, NSW, Australia). Plasma α-amino nitrogen (AAN) concentrations were measured by colourimetric assay (Evans et al. 1993).
Statistics
Effects of placental restriction on outcomes were analysed by one-way ANOVA. Fetal blood gas data were analysed by repeated measures ANOVA, for effects of placental restriction (between factor) and gestational age (within factor), and measures taken at surgery and during the next four days of postsurgery recovery, and from hypoxic fetuses that died or were killed within 24 h were excluded. Additionally, for animals with glucose or arginine tests at both ages, data were analysed by repeated measures ANOVA for effects of placental restriction (between factor) and gestational age (within factor). In order to test the a priori hypothesis that an environment which restricted fetal growth would impair insulin sensitivity and/or secretion, relationships between measures of the severity of placental or fetal restriction (fetal environment: fetal plasma 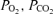 , oxygen content and glucose concentration at the time of experiments; fetal endocrine responses to environment: insulin and cortisol concentrations prior to experiments; fetal and placental growth: fetal and placental weights at autopsy) and measures of insulin sensitivity and secretion were analysed by one-sided Pearson correlation.
, oxygen content and glucose concentration at the time of experiments; fetal endocrine responses to environment: insulin and cortisol concentrations prior to experiments; fetal and placental growth: fetal and placental weights at autopsy) and measures of insulin sensitivity and secretion were analysed by one-sided Pearson correlation.
Results
Placental and fetal growth
Overall, placental restriction reduced the number of cotyledons in the placenta by 69% (P < 0.001), and tended to reduce placental weight (−32%, P = 0.096, Table 1). PR also tended to reduce fetal weight (−20%, P = 0.079) at postmortem (Table 1). Actual fetal weights at 143 days gestational age in surviving control and PR fetuses were 9% and 25% higher, respectively, than estimated weights calculated from the equations shown above.
Table 1.
Effects of placental restriction on placental and fetal growth
| All fetuses | Singletons only | |||
|---|---|---|---|---|
| Control | Placentally restricted | Control | Placentally restricted | |
| Number of animals | 9 | 9 | 9 | 6 |
| Placental weight (g) | 433 ± 47 | 293 ± 63* | 433 ± 47 | 267 ± 54* |
| Cotyledons in placenta | 59 ± 5 | 19 ± 4*** | 59 ± 5 | 19 ± 6*** |
| Fetal:placental weight | 11.3 ± 1.1 | 15.1 ± 1.9* | 11.3 ± 1.1 | 16.6 ± 1.8* |
| Fetal weight (g) | 4508 ± 269 | 3598 ± 405* | 4508 ± 269 | 4006 ± 464 |
Fetuses were killed at 142.8 ± 0.4 days of gestation (term = 149 days). Postmortem data are only included for fetuses with at least one measure of insulin secretion or sensitivity and do not include non-catheterized siblings for litters with more than one fetus. Data are presented as means ±s.e.m. Differences between control and placentally restricted fetuses overall or in singleton fetuses only are indicated as follows:
P < 0.1
P < 0.05
P < 0.001. The cohort of fetuses studied included two twin PR fetuses (from separate litters) and one triplet PR fetus.
Fetal blood parameters
PR decreased fetal arterial blood pH (Fig. 2A, P = 0.008), but pH did not change with gestational age, nor did the effects of PR vary with age (P > 0.15 for each). Fetal arterial blood 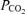 was not altered by PR (P = 0.166) and did not change with gestational age (P = 0.20) (Fig. 2B). PR decreased the oxygen content of fetal arterial blood (P < 0.001), which decreased with advancing gestational age (P < 0.001), and changed similarly with age in PR and control fetuses (P = 0.11) (Fig. 2C).
was not altered by PR (P = 0.166) and did not change with gestational age (P = 0.20) (Fig. 2B). PR decreased the oxygen content of fetal arterial blood (P < 0.001), which decreased with advancing gestational age (P < 0.001), and changed similarly with age in PR and control fetuses (P = 0.11) (Fig. 2C).
Figure 2.
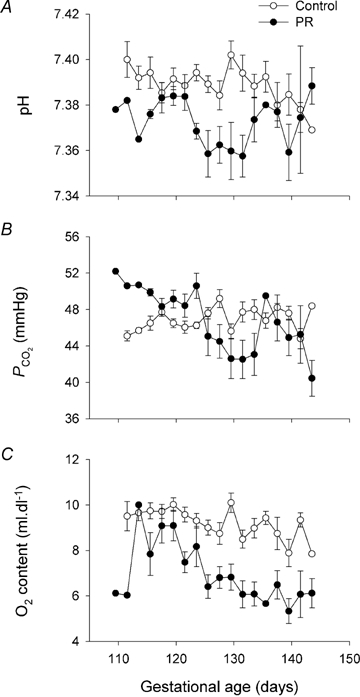
Placental restriction and fetal arterial blood parameters Blood pH (A), 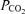 (B) and oxygen content (C) in control (○) and PR fetuses (•). Data are presented as means ±s.e.m., and were calculated from measures made at two-day intervals for all animals with at least one in vivo experiment, excluding measures taken on hypoxic fetuses immediately prior to killing. Where blood parameters on a single animal were measured twice during the 2 days, data from the earlier time point were included in calculations.
(B) and oxygen content (C) in control (○) and PR fetuses (•). Data are presented as means ±s.e.m., and were calculated from measures made at two-day intervals for all animals with at least one in vivo experiment, excluding measures taken on hypoxic fetuses immediately prior to killing. Where blood parameters on a single animal were measured twice during the 2 days, data from the earlier time point were included in calculations.
Overall, fetal plasma cortisol increased with gestational age (P < 0.001), and tended to change differently with gestational age in control and PR fetuses (P = 0.066), although plasma cortisol did not differ between treatments at any single gestational age (Fig. 3). In the nine control and five PR fetuses for which data were available at all three gestational ages, plasma cortisol increased with gestational age (P < 0.001, linear contrast, P = 0.018, quadratic contrast) and changed differently with gestational age in control and PR fetuses (P = 0.025, quadratic contrast), although plasma cortisol did not differ between treatments at any single gestational age (P > 0.2 for all).
Figure 3.
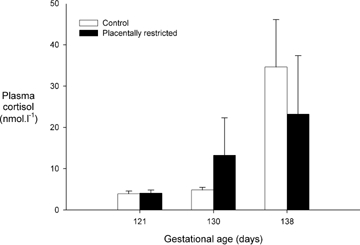
Placental restriction and fetal plasma cortisol Fetal plasma cortisol concentrations in control (open bars) and PR fetuses (filled bars). Data are presented as means ±s.e.m. Plasma cortisol was calculated as the mean of concentrations in two pre-experiment plasma samples for each animal and age. Cortisol was measured in plasma collected prior to glucose tolerance tests at 121 days mean gestational age (n = 11 control, 6 PR fetuses), prior to glucose tolerance tests at 139 days mean gestational age (n = 10 control, 6 PR fetuses), and before the hyperinsulinaemic euglycaemic clamp at 130 days mean gestational age (n = 9 control, 5 PR fetuses).
Glucose-stimulated insulin secretion – effects of placental and fetal growth restriction
At 121 days gestation, PR did not alter blood oxygen content, plasma glucose and insulin concentrations immediately prior to the experiment, or glucose AUC after glucose administration (Fig. 4, Table 2). PR tended to decrease insulin AUC (P = 0.057) and insulin secretion corrected for the glucose AUC (P = 0.088) at 121 days gestation (Table 2). At 139 days gestation, PR did not alter plasma glucose and insulin concentrations immediately prior to the experiment, or their responses to glucose administration, but tended to decrease blood oxygen content (P = 0.060, Fig. 4, Table 2).
Figure 4.
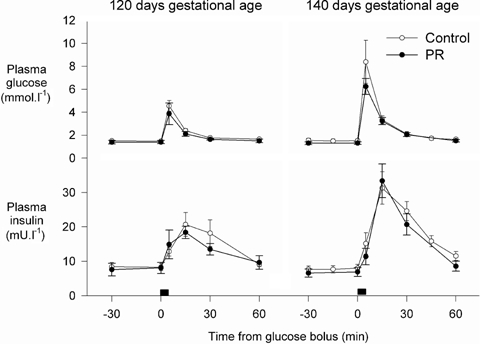
Plasma glucose and insulin concentrations in control and PR fetuses during glucose secretion tests at 121 and 139 days gestational age
Table 2.
Effects of placental restriction on glucose-stimulated insulin secretion
| Control | Placentally restricted | |
|---|---|---|
| 121 days gestation | ||
| Number of animals | 14 | 5 |
| Blood O2 content (ml dl−1) | 9.69 ± 0.30 | 8.72 ± 0.86 |
| Plasma glucose (mmol l−1) | 1.50 ± 0.08 | 1.39 ± 0.15 |
| Plasma insulin (mU l−1) | 8.4 ± 1.1 | 7.5 ± 2.1 |
| Plasma insulin:glucose (mU mmol−1) | 5.72 ± 0.82 | 4.99 ± 1.04 |
| Glucose AUC (mmol min l−1) | 58.3 ± 4.3 | 55.3 ± 10.6 |
| Insulin AUC (mU min l−1) | 500 ± 66 | 293 ± 76# |
| Insulin secretion (mU mmol−1) | 9.0 ± 1.2 | 5.6 ± 1.5# |
| 139 days gestation | ||
| Number of animals | 9 | 9 |
| Blood O2 content (ml dl−1) | 8.82 ± 0.65 | 6.75 ± 0.79# |
| Plasma glucose (mmol l−1) | 1.52 ± 0.16 | 1.30 ± 0.11 |
| Plasma insulin (mU l−1) | 7.8 ± 0.9 | 6.7 ± 1.2 |
| Plasma insulin: glucose (mU mmol−1) | 5.20 ± 0.47 | 4.96 ± 0.70 |
| Glucose AUC (mmol min l−1) | 92.5 ± 17.9 | 82.8 ± 15.9 |
| Insulin AUC (mU min l−1) | 773 ± 98 | 704 ± 106 |
| Insulin secretion (mU mmol−1) | 10.2 ± 1.9 | 9.9 ± 1.6 |
Effects of PR are indicated as follows:
P < 0.1. Insulin data were log transformed prior to statistical analysis.
At 121 days gestational age, plasma glucose correlated negatively with fetal plasma oxygen content and positively with placental weight (Table 3). Plasma insulin correlated negatively with fetal  , and positively with fetal and placental weight, and the plasma insulin: glucose ratio was also positively correlated with fetal and placental weight (Table 3). Glucose tolerance (measured as area under the glucose profile) was worst in fetuses with poor fetal-placental growth (Table 3). Insulin area under the curve did not correlate with measures of the fetal environment, fetal endocrine responses to the environment or fetal-placental growth, but relative glucose-stimulated insulin secretion (i.e. corrected for the glucose AUC) was positively correlated with fetal blood oxygenation and plasma insulin (Table 3).
, and positively with fetal and placental weight, and the plasma insulin: glucose ratio was also positively correlated with fetal and placental weight (Table 3). Glucose tolerance (measured as area under the glucose profile) was worst in fetuses with poor fetal-placental growth (Table 3). Insulin area under the curve did not correlate with measures of the fetal environment, fetal endocrine responses to the environment or fetal-placental growth, but relative glucose-stimulated insulin secretion (i.e. corrected for the glucose AUC) was positively correlated with fetal blood oxygenation and plasma insulin (Table 3).
Table 3.
Fetal environment and growth and glucose-stimulated insulin secretion
| Fetal environmental measure immediately before experiment | Fetal endocrine responses to environment | Fetal and placental growth | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O2 content | Plasma glucose | Plasma insulin | Plasma cortisol | Fetal weight | Placental weight | Fetal: placental weight | |||
| 121 days gestation | |||||||||
| Plasma glucose | −0.334 | NS | −0.442 | — | 0.347 | NS | NS | 0.424 | NS |
| 0.075 | 0.025 | 0.073 | 0.074 | ||||||
| 20 | 20 | 19 | 13 | ||||||
| Plasma insulin | −0.484 | NS | NS | 0.347 | — | NS | 0.716 | 0.598 | −0.427 |
| 0.018 | 0.073 | 0.003 | 0.015 | 0.073 | |||||
| 19 | 19 | 13 | 13 | 13 | |||||
| Plasma insulin:glucose | −0.370 | NS | NS | — | — | NS | 0.704 | 0.514 | NS |
| 0.059 | 0.004 | 0.036 | |||||||
| 19 | 13 | 13 | |||||||
| Glucose AUC | NS | −0.302 | NS | −0.306 | −0.342 | NS | −0.805 | −0.439 | NS |
| 0.098 | 0.094 | 0.076 | < 0.001 | 0.067 | |||||
| 20 | 20 | 19 | 13 | 13 | |||||
| Insulin AUC | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Insulin secretion | −0.381 | 0.393 | 0.459 | NS | 0.410 | NS | 0.405 | NS | NS |
| 0.054 | 0.048 | 0.024 | 0.041 | 0.085 | |||||
| 19 | 19 | 19 | 19 | 13 | |||||
| 139 days gestation | |||||||||
| Plasma glucose | NS | NS | NS | — | 0.730 | NS | NS | 0.452 | −0.449 |
| < 0.001 | 0.039 | 0.040 | |||||||
| 17 | 16 | 16 | |||||||
| Plasma insulin | NS | 0.438 | 0.386 | 0.730 | — | NS | 0.536 | 0.640 | −0.453 |
| 0.039 | 0.063 | < 0.001 | 0.016 | 0.004 | 0.039 | ||||
| 17 | 17 | 17 | 16 | 16 | 16 | ||||
| Plasma insulin: glucose | −0.404 | 0.463 | 0.459 | — | — | NS | 0.620 | 0.490 | NS |
| 0.054 | 0.031 | 0.032 | 0.005 | 0.027 | |||||
| 17 | 17 | 17 | 16 | 16 | |||||
| Glucose AUC | NS | −0.558 | −0.427 | NS | NS | NS | −0.639 | NS | NS |
| 0.010 | 0.044 | 0.004 | |||||||
| 17 | 17 | 16 | |||||||
| Insulin AUC | −0.422 | NS | NS | NS | NS | NS | NS | NS | NS |
| 0.046 | |||||||||
| 17 | |||||||||
| Insulin secretion | NS | 0.648 | 0.608 | NS | NS | NS | 0.600 | NS | NS |
| 0.002 | 0.005 | 0.007 | |||||||
| 17 | 17 | 16 | |||||||
Data are shown as r, p, n for each correlation.
At 139 days gestational age, plasma glucose correlated positively with placental weight, and plasma insulin correlated positively with fetal oxygenation, plasma glucose, and fetal and placental weight (Table 3). Glucose tolerance was poorest in fetuses with low oxygenation and weight (Table 3). Insulin area under the curve correlated negatively with fetal  , but not with other measures of the fetal environment, fetal response or fetal-placental growth (Table 3). Relative glucose-stimulated insulin secretion correlated positively with fetal oxygen status and fetal weight (Table 3). No outcome measures at 121 or 139 days gestational age were correlated with fetal blood pH (P > 0.1 for all).
, but not with other measures of the fetal environment, fetal response or fetal-placental growth (Table 3). Relative glucose-stimulated insulin secretion correlated positively with fetal oxygen status and fetal weight (Table 3). No outcome measures at 121 or 139 days gestational age were correlated with fetal blood pH (P > 0.1 for all).
Glucose-stimulated insulin secretion – effects of gestational age
Glucose-stimulated insulin secretion was measured in nine control and five PR fetuses at both ∼120 and ∼140 days gestation. In these fetuses, plasma glucose, insulin and insulin: glucose ratios were similar at both gestational ages and not affected by PR (P > 0.3 for all). The AUC glucose was greater at 140 than at 120 days gestation (P = 0.019), but was not altered by PR (P > 0.5 for all). AUC insulin after glucose administration was greater at 140 than at 120 days gestation (P = 0.006) and was reduced by PR (P = 0.031), and similarly at both gestational ages (P = 0.172). Relative glucose-stimulated insulin secretion was not altered by placental restriction (P = 0.155) nor by gestational age (P = 0.153).
Arginine-stimulated insulin secretion – effects of placental and fetal growth restriction
At 122 days gestation, PR did not alter plasma AAN or insulin concentrations immediately prior to the experiment, nor the AAN AUC, insulin AUC and insulin secretion corrected for the AAN stimulus after arginine administration, but did reduce blood oxygen content (P = 0.035, Fig. 5, Table 4). At 140 days gestation, PR reduced plasma insulin (P = 0.030) and the plasma insulin: AAN ratio (P = 0.032), did not change plasma AAN concentrations immediately prior to the experiment, and tended to decrease blood oxygen content (P = 0.058, Fig. 5, Table 4). PR tended to increase the AUC for AAN (P = 0.058), did not change the AUC for insulin, and tended to decrease relative arginine-stimulated insulin secretion (i.e. corrected for the AAN AUC) at 140 days gestation (P = 0.072, Table 4).
Figure 5.
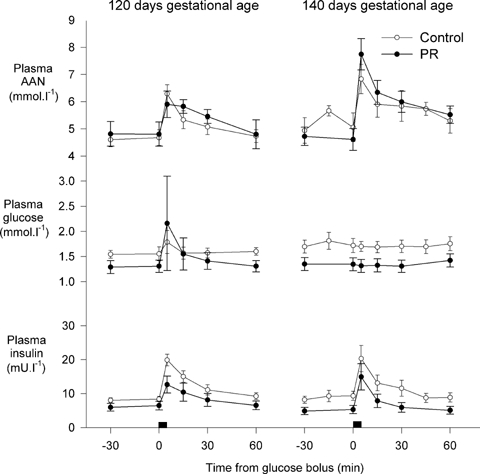
Plasma α-amino nitrogen, glucose and insulin in control and PR fetuses during arginine secretion tests at 122 and 140 days gestational age
Table 4.
Effects of placental restriction on arginine-stimulated insulin secretion
| Control | Placentally restricted | |
|---|---|---|
| 122 days gestation | ||
| Number of animals | 14 | 6 |
| Blood O2 content (ml dl−1) | 9.91 ± 0.25 | 8.51 ± 0.75* |
| Plasma α-amino nitrogen (meq.l−1) | 4.64 ± 0.26 | 4.81 ± 0.45 |
| Plasma insulin (mU l−1) | 8.2 ± 0.7 | 6.2 ± 1.2 |
| Plasma insulin:α-amino nitrogen (mU.meq−1) | 1.88 ± 0.21 | 1.28 ± 0.23 |
| α-Amino nitrogen AUC (meq min l−1) | 34.1 ± 3.6 | 42.8 ± 8.5 |
| Insulin AUC (mU min l−1) | 253 ± 32 | 172 ± 53 |
| Insulin secretion (mU meq−1) | 8.58 ± 1.54 | 6.07 ± 3.07 |
| 140 days gestation | ||
| Number of animals | 9 | 10 |
| Blood O2 content (ml dl−1) | 8.42 ± 0.71 | 6.39 ± 0.70# |
| Plasma α-amino nitrogen (meq l−1) | 4.98 ± 0.49 | 4.67 ± 0.37 |
| Plasma insulin (mU l−1) | 8.9 ± 1.2 | 5.1 ± 1.0* |
| Plasma insulin:α-amino nitrogen (mU meq−1) | 2.11 ± 0.57 | 1.04 ± 0.17* |
| α-Amino nitrogen AUC (meq min l−1) | 53.8 ± 3.5 | 88.4 ± 14.8# |
| Insulin AUC (mU min l−1) | 230 ± 59 | 154 ± 57 |
| Insulin secretion (mU meq−1) | 4.61 ± 1.18 | 2.57 ± 1.05# |
Effects of PR are indicated as follows:
P < 0.1
P < 0.05. Insulin data were log transformed prior to statistical analysis.
At 122 days gestational age, plasma AAN correlated positively with fetal blood 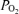 and weight (Table 5). Plasma insulin and plasma insulin: AAN ratio positively correlated with plasma glucose and measures of fetal-placental growth (Table 5). The area under the AAN profile correlated negatively with fetal weight, and the area under the insulin profile correlated positively with plasma glucose and insulin concentrations (Table 5). Relative arginine-stimulated insulin secretion correlated negatively with fetal blood
and weight (Table 5). Plasma insulin and plasma insulin: AAN ratio positively correlated with plasma glucose and measures of fetal-placental growth (Table 5). The area under the AAN profile correlated negatively with fetal weight, and the area under the insulin profile correlated positively with plasma glucose and insulin concentrations (Table 5). Relative arginine-stimulated insulin secretion correlated negatively with fetal blood 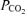 and positively correlated with plasma insulin (Table 5).
and positively correlated with plasma insulin (Table 5).
Table 5.
Fetal environment and growth and arginine-stimulated insulin secretion
| Fetal environmental measure immediately before experiment | Fetal endocrine responses to environment | Fetal and placental growth | |||||||
|---|---|---|---|---|---|---|---|---|---|
| O2 content | Plasma glucose | Plasma insulin | Plasma cortisol | Fetal weight | Placental weight | Fetal: placental weight | |||
| 122 days gestation | |||||||||
| Plasma AAN | NS | 0.424 | NS | NS | NS | −0.375 | 0.496 | NS | NS |
| 0.031 | 0.069 | 0.043 | |||||||
| 20 | 17 | 13 | |||||||
| Plasma insulin | NS | NS | NS | 0.457 | — | NS | 0.691 | 0.673 | −0.514 |
| 0.021 | 0.004 | 0.006 | 0.036 | ||||||
| 20 | 13 | 13 | 13 | ||||||
| Plasma insulin:AAN | NS | NS | NS | 0.440 | — | NS | 0.435 | 0.709 | − 0.612 |
| 0.026 | 0.069 | 0.003 | 0.013 | ||||||
| 20 | 13 | 13 | 13 | ||||||
| AAN AUC | NS | −0.372 | −0.349 | NS | −0.315 | NS | −0.537 | −0.495 | 0.519 |
| 0.059 | 0.072 | 0.095 | 0.036 | 0.051 | 0.042 | ||||
| 19 | 19 | 19 | 12 | 12 | 12 | ||||
| Insulin AUC | −0.302 | NS | NS | 0.390 | 0.556 | NS | NS | 0.423 | NS |
| 0.098 | 0.044 | 0.005 | 0.075 | ||||||
| 20 | 20 | 20 | 13 | ||||||
| Insulin secretion | −0.394 | NS | NS | 0.340 | 0.523 | NS | NS | 0.431 | − 0.484 |
| 0.048 | 0.077 | 0.011 | 0.081 | 0.056 | |||||
| 19 | 19 | 19 | 12 | 12 | |||||
| 140 days gestation | |||||||||
| Plasma AAN | NS | 0.650 | 0.650 | NS | 0.406 | NS | 0.570 | NS | NS |
| 0.002 | 0.002 | 0.047 | 0.013 | ||||||
| 18 | 17 | 18 | 15 | ||||||
| Plasma insulin | NS | 0.650 | 0.650 | NS | 0.406 | NS | 0.570 | NS | NS |
| 0.002 | 0.002 | 0.047 | 0.013 | ||||||
| 18 | 17 | 18 | 15 | ||||||
| Plasma insulin: AAN | NS | 0.630 | 0.543 | 0.699 | — | NS | 0.562 | NS | NS |
| 0.002 | 0.010 | < 0.001 | 0.012 | ||||||
| 19 | 18 | 19 | 16 | ||||||
| AAN AUC | NS | NS | NS | 0.656 | — | NS | NS | 0.442 | −0.351 |
| 0.002 | 0.049 | 0.100 | |||||||
| 18 | 15 | 15 | |||||||
| Insulin AUC | NS | −0.612 | −0.414 | −0.619 | −0.617 | NS | −0.806 | NS | NS |
| 0.003 | 0.049 | 0.003 | 0.003 | < 0.001 | |||||
| 18 | 17 | 18 | 18 | 15 | |||||
| 0.396 | NS | NS | 0.720 | 0.590 | −0.452 | NS | NS | NS | |
| 0.047 | < 0.001 | 0.004 | 0.052 | ||||||
| 19 | 19 | 19 | 14 | ||||||
| Insulin secretion | NS | NS | NS | 0.787 | 0.631 | NS | 0.454 | 0.375 | NS |
| < 0.001 | 0.002 | 0.045 | 0.084 | ||||||
| 18 | 18 | 15 | 15 | ||||||
Data are shown as r, p, n for each correlation.
At 140 days gestational age, plasma AAN correlated positively with fetal blood oxygen status, plasma insulin concentration and fetal weight (Table 5). Plasma insulin also correlated positively with fetal oxygen status, plasma glucose and fetal weight, whilst the plasma insulin: AAN ratio correlated positively with plasma glucose and placental weight (Table 5). The area under the AAN profile correlated negatively with fetal blood oxygen status, plasma glucose and insulin concentrations and fetal weight. The area under the insulin profile following administration of arginine correlated positively with fetal blood 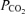 , plasma glucose and insulin concentrations (Table 5). Relative arginine-stimulated insulin secretion correlated positively with plasma glucose and insulin concentrations and with fetal weight (Table 5). No outcome measures at 122 or 140 days gestational age were correlated with fetal blood pH (P > 0.1 for all).
, plasma glucose and insulin concentrations (Table 5). Relative arginine-stimulated insulin secretion correlated positively with plasma glucose and insulin concentrations and with fetal weight (Table 5). No outcome measures at 122 or 140 days gestational age were correlated with fetal blood pH (P > 0.1 for all).
Arginine-stimulated insulin secretion – effects of gestational age
Arginine-stimulated insulin secretion was measured in eight control and five PR fetuses at ∼120 and ∼140 days gestation. PR and gestational age did not alter plasma AAN and insulin concentrations or the ratio of plasma insulin: AAN concentrations (P > 0.1 for all). The area under the AAN profile was greater at 140 than at 120 days gestation (P = 0.009) but not altered by PR (P > 0.2). PR did not alter AUC insulin after arginine administration, nor did this vary with gestational age (P > 0.2 for each). PR also did not alter relative arginine-stimulated insulin secretion (P = 0.15), which tended to decrease between 120 and 140 days gestational age (P = 0.075), and the effect of gestational age was similar in both groups (P > 0.9).
Insulin sensitivity – effects of placental and fetal growth restriction
Blood glucose prior to the HEC (1.91 ± 0.06 mmol l−1) and steady-state blood glucose (1.97 ± 0.07 mmol l−1) during the HEC did not differ between the 10 control and five PR fetuses studied at 130 days gestational age (P > 0.7 for each). PR did not alter (P > 0.2 for each) plasma glucose (1.13 ± 0.09 mmol l−1), the ratio of plasma insulin: plasma glucose (4.98 ± 0.09 mU mmol−1), preclamp (5.6 ± 0.6 mU l−1) or steady-state (17.7 ± 2.7 mU l−1) plasma insulin concentrations, the metabolic clearance rate of insulin (82 ± 15 ml (kg estimated body weight) −1 min−1). PR also did not change the steady-state glucose infusion rate either calculated as total glucose infused per fetus (4.45 ± 1.30 mg min−1), or calculated on a per kg estimated body weight basis (1.52 ± 0.41 mg (kg estimated body weight)−1 min−1), and hence did not change insulin sensitivity in the fetal sheep either calculated per fetus (0.328 ± 0.075 mg l mU−1 min−1) or calculated on a per kg estimated body weight basis (0.107 ± 0.026 mg l mU−1 (kg estimated body weight)−1 min−1) (P > 0.3 for each).
Plasma insulin and the ratio of plasma insulin: glucose prior to the HEC correlated positively with fetal weight (insulin: r = 0.691, P = 0.009, n = 11; insulin:glucose: r = 0.597, P = 0.026, n = 11) and plasma glucose and insulin concentrations also correlated positively with placental weight (glucose: r = 0.648, P = 0.016, n = 11; insulin: r = 0.627, P = 0.020, n = 11). Plasma glucose and insulin did not correlate with fetal blood gas status, but correlated positively with each other (r = 0.614, P = 0.007, n = 15). Steady-state glucose infusion rate was not related to indices of the fetal environment, endocrine responses or fetal-placental growth (P > 0.1 for each). The metabolic clearance rate of insulin correlated positively with fetal blood pH (r = 0.642, P = 0.009, n = 13) and oxygen content (r = 0.575, P = 0.020, n = 13), and negatively with glucose (r =−0.446, P = 0.048, n = 15) and placental weight (r =−0.546, P = 0.041, n = 11). Insulin sensitivity was not correlated with fetal blood gases, and correlated negatively with plasma insulin (r =−0.596, P = 0.010, n = 15).
Discussion
Low fetal and placental weights and indices of poor placental function, particularly fetal hypoxia and hypoglycaemia, were associated with impaired glucose- and arginine-stimulated insulin secretion, but not with altered insulin sensitivity in the fetal sheep. By administering glucose directly to the fetus and sampling fetal blood before and after glucose administration, we were able to measure glucose-stimulated insulin secretion in fetal control and PR sheep, in contrast to previous studies in this model where glucose was administered to the mother (Harding, 1982; Harding et al. 1985). We have now shown that impaired placental growth and function, a known major cause of IUGR in humans, impairs glucose-stimulated insulin secretion and/or action on glucose metabolism from fetal life, to postnatal life, in the neonate (De Blasio et al. 2007) and through to adulthood (Owens et al. 2007) in the sheep. This is the first time that impaired glucose-stimulated insulin secretion before birth has been shown to precede impaired postnatal glucose-stimulated insulin secretion induced by fetal growth restriction in the one experimental model. In contrast, fetal whole-body insulin sensitivity, whether calculated on a per fetus basis or corrected for estimated fetal weight, did not differ between control and PR fetuses, and was not predicted by fetal or placental growth or function.
Placental restriction and fetal growth
In the present study, surgical restriction of placental growth only reduced fetal weight by ∼20% at postmortem. Both control and PR singleton fetuses were heavier than predicted at postmortem, by 9 and 25%, respectively. To some extent this may reflect a survivor effect, particularly in PR sheep, with larger fetuses more likely to survive to postmortem. Because of the death of severely restricted fetuses in late gestation, we would expect that mean fetal weight at experiments was relatively lower in PR fetuses than at postmortem. Nevertheless, the degree of placental restriction induced in this cohort did result in hypoxia and reduce fetal blood pH, indicative of impaired placental function. Interestingly, other studies in which restriction of fetal growth, and by implication restriction of fetal metabolism, was more severe, did not find differences in fetal blood pH between control and growth-restricted fetuses (Harding et al. 1985; Limesand et al. 2006). We also saw some evidence of accelerated maturational increases in fetal plasma cortisol in the PR fetus, although these were less marked than reported previously in a more restricted cohort in which PR decreased fetal weight by ∼33% (Phillips et al. 1996).
Placental restriction and insulin secretion
Fetal glucose- and arginine-stimulated insulin secretion were related strongly to fetal and placental size and placental function, but did not differ between control and PR fetuses, although the latter tended to have lower stimulated insulin secretion at some ages. Because the AUC insulin is divided by AUC glucose or AUC AAN (i.e. corrected for the secretagogue stimulus), our calculations of stimulated insulin secretion are independent of fetal weight. This lack of difference in stimulated insulin secretion between the control and PR groups contrasts with the ∼70% reductions in insulin AUC following glucose or arginine stimulation reported for IUGR fetal sheep by Limesand et al. (2006). The more severe effects of IUGR on stimulated insulin secretion in the latter study may reflect the more severe impairment of fetal (−56%) and placental growth (−60%) and placental function (plasma glucose −48%; blood 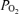 −38%) induced by chronic maternal exposure to elevated ambient temperatures from day 39 to day 96 of gestation (term ∼150 days), compared to the 20% reduction in fetal weight observed in the present study. Similarly, lack of effect of PR on basal fetal plasma glucose in the present study, in contrast to previous reports of a 30–40% reduction in plasma glucose, may reflect the differing severities of growth restriction in the two cohorts (20%versus 38–48% reduction in fetal body weight) (Robinson et al. 1979; Owens et al. 1989). In the present study, we also did not stratify PR fetuses based on fetal weight as well as restriction, as was done elsewhere (Robinson et al. 1979), and our results are consistent with the finding that fetal plasma glucose, from 130 days gestational age onwards, was not reduced in PR fetuses that were within the normal weight range (Robinson et al. 1979).
−38%) induced by chronic maternal exposure to elevated ambient temperatures from day 39 to day 96 of gestation (term ∼150 days), compared to the 20% reduction in fetal weight observed in the present study. Similarly, lack of effect of PR on basal fetal plasma glucose in the present study, in contrast to previous reports of a 30–40% reduction in plasma glucose, may reflect the differing severities of growth restriction in the two cohorts (20%versus 38–48% reduction in fetal body weight) (Robinson et al. 1979; Owens et al. 1989). In the present study, we also did not stratify PR fetuses based on fetal weight as well as restriction, as was done elsewhere (Robinson et al. 1979), and our results are consistent with the finding that fetal plasma glucose, from 130 days gestational age onwards, was not reduced in PR fetuses that were within the normal weight range (Robinson et al. 1979).
Predictors of insulin secretion and glucose tolerance
Fetal plasma glucose was positively correlated with placental, rather than fetal weight, consistent with reduced fetal nutrient availability in fetuses with poor placental growth and function. Fetal plasma insulin was consistently positively correlated with plasma glucose, as well as with fetal and placental weight, but also not with fetal blood gases. The results of the present study suggest that glucose supply is the main determinant of basal insulin secretion, and the lack of anabolic stimulus from insulin may contribute to the growth restriction of PR fetuses. Glucose area under the curve was negatively related to fetal weight, and at 140 days gestational age was also negatively related to fetal oxygenation. This probably reflects smaller or less functional placentae, which impair fetal growth from mid-gestation and limit fetal oxygenation as gestation progresses, and which have decreased placental capacity for glucose clearance from the fetus back to the mother (Owens et al. 1989). Glucose-stimulated insulin secretion was positively related to fetal weight and to oxygenation at 140 days gestation, and less strongly positively related to oxygenation earlier in gestation. Development of a relationship between glucose-stimulated insulin secretion and oxygen status with ageing might reflect a threshold effect for oxygen, since blood oxygen content fell overall, and particularly in PR fetuses, across this time. Impaired insulin secretion, measured as an elevated proinsulin: insulin ratio, was similarly associated with low fetal weight and poor oxygenation in human fetuses (Verhaeghe et al. 2005), although this was measured in a basal, rather than glucose-stimulated, state. A smaller in vivo study found an absence of glucose-stimulated insulin secretion and slower return of fetal glucose to basal concentrations following administration of a glucose bolus in severely IUGR human fetuses compared to controls in late gestation (Nicolini et al. 1990), consistent with impaired glucose-stimulated insulin secretion in IUGR across species.
Mechanisms for impaired insulin secretion in the growth-restricted fetus
In vitro and in vivo studies in sheep have identified some potential mechanisms by which an adverse fetal environment may compromise insulin secretion. Chronic restrictions of nutrient supply in IUGR fetal sheep due to poor placental growth and function in the high-temperature model of PR in sheep reduce β-cell replication and consequently β-cell mass, in association with decreased pancreatic insulin content and gene expression, which reduces the capacity for insulin production (Limesand et al. 2005). In vitro glucose-stimulated oxidation, insulin biosynthesis and insulin content of isolated islets are also reduced in this model of PR (Limesand et al. 2006). The latter two mechanisms would contribute to impaired insulin secretion in response to either secretagogue, whilst the response to glucose would be further reduced by the impairment in glucose metabolism. Acute hypoxia in fetal sheep impairs glucose-stimulated insulin secretion in vivo and activates the sympathoadrenal axis, whilst insulin secretory responses to glucose can be restored by administration of the α2-adrenergic blocker idazoxan, implicating elevated sympathoadrenal tone as a mechanism for hypoxia-induced reductions in insulin secretion, at least in response to acute hypoxia (Jackson et al. 1993; Jackson et al. 2000). Similarly, PR increases plasma noradrenaline and adrenaline concentrations in the fetal sheep in late gestation (Simonetta et al. 1997), and these are inversely related to oxygenation of fetal blood. This elevation in circulating catecholamines might be expected to impair stimulated insulin secretion, since infusion of adrenaline (1 mg min−1) abolished in vivo insulin responses to glucose and arginine in normal fetal sheep (Fowden, 1980a). PR advances and amplifies the late gestation cortisol surge in the fetal sheep, without changing circulating ACTH (Phillips et al. 1996), and we saw evidence of a similar effect of PR on cortisol in the present study. Although several studies in the rat have implicated chronically increased in utero exposure to glucocorticoids as an underlying mechanism programming impaired postnatal glucose homeostasis, insulin secretion and insulin sensitivity (Lindsay et al. 1996; Nyirenda et al. 1998; Gesina et al. 2004), plasma cortisol concentrations were generally not related to outcomes in the fetal sheep in the present study.
Gestational age and insulin secretion
In fetuses studied at both 120 and 140 days of gestation, advancing gestational age was associated with increased AUC insulin following glucose administration, but no change in basal secretion, nor in relative glucose-stimulated insulin secretion. This increase in glucose AUC, and hence the secretion stimulus probably reflects maturational changes in glucose clearance. The magnitude of the acute increase in insulin concentrations after glucose administration also increased with ageing, suggesting an improving capacity for rapid insulin release over this period. This contrasts with results of previous studies where no change in initial insulin secretion response to glucose was found with maturation from 119 to 142 days gestational age (Fowden, 1980b), although because the same amount of glucose was administered at each age, the dose per unit weight would have decreased with age in the latter study, whereas we adjusted the glucose dose for predicted fetal weight in the present study. Another study using a hyperglycaemic clamp approach in the fetal sheep did find increasing insulin secretion in response to hyperglycaemia with advancing gestational age, such that glucose-stimulated insulin secretion at ∼50% of term was only 20% of that near term (Aldoretta et al. 1998). Between ∼120 and ∼140 days gestational age in the present study, the area under the curve for AAN also increased, reflecting decreasing capacity for arginine clearance. The insulin AUC and the acute increase in insulin concentrations following arginine administration did not increase over this period in the present study, leading to a fall in relative arginine-stimulated insulin secretion (lower secretion per unit of arginine). In contrast, Aldoretta et al. (1998) reported a fivefold increase in the initial insulin increase following an arginine bolus between ∼50% of term and near-term in fetal sheep, although this response was not adjusted for the change in arginine achieved. Together, these studies suggest that the effectiveness of arginine as an insulin secretagogue may decrease between day 120 and 140 of gestation, whilst an increase in the capacity for rapid and total insulin secretion allows glucose-stimulated insulin secretion to be maintained despite slower glucose clearance.
Placental restriction and insulin sensitivity
Insulin sensitivity did not differ in control and placentally restricted fetuses. Interestingly, Limesand et al. (2006) reported similar glucose disposal per unit fetal weight in control and IUGR fetuses during a hyperglycaemic clamp despite lower levels of insulin in the IUGR group, which might reflect increased insulin sensitivity as well as impaired insulin secretion in the IUGR fetal sheep, and possibly group differences in placental glucose uptake. Although under basal conditions, umbilical loss of fetal glucose to the placenta and mother in late gestation is reduced from about 40% to about 25% by PR (Owens et al. 1989), insulin does not change placental uptake of glucose under clamp conditions (DiGiacomo & Hay, 1990), so that the glucose infusion rate required to maintain euglycaemia in the present study reflects uptake of glucose by the fetus rather than the placenta. Insulin sensitivity on a per kilogram basis may, however, have been overestimated in the PR fetus because the glucose infusion rate was calculated relative to estimated fetal body weight, and the ratio of actual to estimated body weights at postmortem was higher in PR (25%) than control (9%) fetuses, at least in the subset of fetuses that survived to postmortem. However, insulin sensitivity calculated from total glucose infusion rate per fetus was also not reduced by PR, implying that restriction of placental and fetal growth did not impair insulin sensitivity. Consistent with this, insulin sensitivity was not related to measures of nutrient and oxygen availability.
Conclusions
Poor placental growth and function impair insulin secretory capacity before birth in the sheep. We have previously reported that this impaired insulin secretion, relative to sensitivity, persists postnatally in the placentally restricted sheep, consistent with impaired postnatal insulin secretion reported following IUGR in humans. Together, this implies that interventions before birth may be required to ameliorate the effects of impaired placental growth and function, and possibly other causes of IUGR, on insulin secretion. In contrast, the changes in postnatal insulin sensitivity observed after placental restriction in sheep and IUGR in humans, i.e. neonatal hypersensitivity followed by a switch to insulin resistance in later life, are not preceded by changes in insulin sensitivity before birth.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia. These studies were initiated during Abigail Fowden's visit to the University of Adelaide, sponsored as a Faculty of Health Sciences Visiting Professor. We thank Damian Adams and Linda Mundy for their skilled research assistance, and Sanita Grover for assistance with cortisol assays.
References
- Aldoretta PW, Carver TD, Hay WW. Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate. 1998;73:375–386. doi: 10.1159/000014000. [DOI] [PubMed] [Google Scholar]
- Bazaes RA, Salazar TE, Pittaluga E, Pena V, Alegria A, Iniguez G, Ong KK, Dunger DB, Mericq MV. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics. 2003;111:804–809. doi: 10.1542/peds.111.4.804. [DOI] [PubMed] [Google Scholar]
- Béringue F, Blondeau B, Castellotti MC, Bréant B, Czernichow P, Polak M. Endocrine pancreas development in growth-retarded human fetuses. Diabetes. 2002;51:385–391. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- Bleker OP, Buimer M, van der Post JAM, van der Veen F. Ted (G. J.) Kloosterman: On intrauterine growth. the significance of prenatal care. studies on birth weight, placental weight and placental index. Placenta. 2006;27:1052–1054. doi: 10.1016/j.placenta.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bocking AD, McMillen IC, Harding R, Thorburn GD. Effect of reduced uterine blood flow on fetal and maternal cortisol. J Dev Physiol. 1986;8:237–245. [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth and adiposity in the young lamb. Endocrinology. 2007;148:1350–1358. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction alters circulating thyroid hormone in the young lamb postnatally. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1016–R1024. doi: 10.1152/ajpregu.00103.2006. [DOI] [PubMed] [Google Scholar]
- De Prins FA, Van Assche FA. Intrauterine growth retardation and development of endocrine pancreas in the experimental rat. Biol Neonate. 1982;41:16–21. doi: 10.1159/000241511. [DOI] [PubMed] [Google Scholar]
- De Prins FA, Van Assche FA, Milner RDG. C-peptide levels in amniotic fluid in experimental fetal growth retardation. Biol Neonate. 1983;43:181–185. doi: 10.1159/000241626. [DOI] [PubMed] [Google Scholar]
- DiGiacomo JE, Hay WW. Placental-fetal glucose exchange and placental glucose consumption in pregnant sheep. Am J Physiol Endocrinol Metab. 1990;258:E360–E367. doi: 10.1152/ajpendo.1990.258.2.E360. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Simonetta G, Owens JA, Robinson JS, McMillen IC. Restriction of placental and fetal growth in sheep alters fetal blood pressure responses to angiotensin II and captopril. J Physiol. 1999;515:897–904. doi: 10.1111/j.1469-7793.1999.897ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PC, Ffolliott-Powell FM, Harding JE. A colorimetric assay for amino nitrogen in small volumes of blood: reaction with β-napthoquinone sulphonate. Anal Biochem. 1993;208:334–337. doi: 10.1006/abio.1993.1056. [DOI] [PubMed] [Google Scholar]
- Fowden AL. Effects of adrenaline and amino acids on the release of insulin in the sheep fetus. J Endocrinol. 1980a;87:113–121. doi: 10.1677/joe.0.0870113. [DOI] [PubMed] [Google Scholar]
- Fowden AL. Effects of arginine and glucose on the release of insulin in the sheep fetus. J Endocrinol. 1980b;85:121–129. doi: 10.1677/joe.0.0850121. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Gardner DS, Ousey JC, Giussani DA, Forhead AJ. Maturation of pancreatic β-cell function in the fetal horse during late gestation. J Endocrinol. 2005;186:467–473. doi: 10.1677/joe.1.06176. [DOI] [PubMed] [Google Scholar]
- Gatford KL, Clarke IJ, De Blasio MJ, McMillen IC, Robinson JS, Owens JA. Perinatal growth and plasma GH profiles in adolescent and adult sheep. J Endocrinol. 2002;173:151–159. doi: 10.1677/joe.0.1730151. [DOI] [PubMed] [Google Scholar]
- Gesina E, Tronche F, Herrera P, Duchene B, Tales W, Czernichow P, Breant B. Dissecting the role of glucocorticoids on pancreas development. Diabetes. 2004;53:2322–2329. doi: 10.2337/diabetes.53.9.2322. [DOI] [PubMed] [Google Scholar]
- Harding JE. Oxford University: 1982. The placenta and the growth of the foetus. PhD thesis. [Google Scholar]
- Harding JE, Jones CT, Robinson JS. Studies on experimental growth restriction in sheep. The effects of a small placenta in restricting transport to and growth of the fetus. J Dev Physiol. 1985;7:427–442. [PubMed] [Google Scholar]
- Jackson BT, Cohn HE, Morrison SH, Baker RM, Piasecki GJ. Hypoxia-induced sympathetic inhibition of the fetal plasma insulin response to hyperglycemia. Diabetes. 1993;42:1621–1625. doi: 10.2337/diab.42.11.1621. [DOI] [PubMed] [Google Scholar]
- Jackson BT, Piasecki GJ, Cohn HE, Cohen WR. Control of fetal insulin secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2179–R2188. doi: 10.1152/ajpregu.2000.279.6.R2179. [DOI] [PubMed] [Google Scholar]
- Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 2000;85:1401–1406. doi: 10.1210/jcem.85.4.6544. [DOI] [PubMed] [Google Scholar]
- Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA. Early differential defects of insulin secretion and action in 19-year-old Caucasian men who had low birth weight. Diabetes. 2002;51:1271–1280. doi: 10.2337/diabetes.51.4.1271. [DOI] [PubMed] [Google Scholar]
- Li C, Johnson MS, Goran MI. Effects of low birth weight on insulin resistance syndrome in Caucasian and African-American children. Diabetes Care. 2001;24:2035–2042. doi: 10.2337/diacare.24.12.2035. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–R1305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147:1488–1497. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11β-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996;39:1299–1305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- Newsome CA, Shiell AW, Fall CHD, Phillips DIW, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism? A systemic review. Diabet Med. 2003;20:339–348. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- Nicolini U, Hubinot C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–430. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Effect of restriction of placental growth on umbilical and uterine blood flows. Am J Physiol Regul Integr Comp Physiol. 1986;250:R427–R434. doi: 10.1152/ajpregu.1986.250.3.R427. [DOI] [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Effect of restriction of placental growth on oxygen delivery to and consumption by the pregnant uterus and fetus. J Dev Physiol. 1987;9:137–150. [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Glucose metabolism in pregnant sheep when placental growth is restricted. Am J Physiol Regul Integr Comp Physiol. 1989;257:R350–R357. doi: 10.1152/ajpregu.1989.257.2.R350. [DOI] [PubMed] [Google Scholar]
- Owens JA, Kind KL, Carbone F, Robinson JS, Owens PC. Circulating insulin-like growth factors-I and -II and substrates in fetal sheep following restriction of placental growth. J Endocrinol. 1994;140:5–13. doi: 10.1677/joe.0.1400005. [DOI] [PubMed] [Google Scholar]
- Owens JA, Thavaneswaran P, De Blasio MJ, McMillen IC, Robinson JS, Gatford KL. Sex-specific effects of placental restriction on components of the metabolic syndrome in young adult sheep. Am J Physiol Endocrinol Metabl. 2007;292:E1879–E1889. doi: 10.1152/ajpendo.00706.2006. [DOI] [PubMed] [Google Scholar]
- Phillips ID, Simonetta G, Owens JA, Robinson JS, Clarke IJ, McMillen C. Placental restriction alters the functional development of the pituitary-adrenal axis in the sheep fetus during late gestation. Pediatr Res. 1996;40:861–866. doi: 10.1203/00006450-199612000-00014. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Hart IC, Kingston EJ, Jones CT, Thorburn GD. Studies on the growth of the fetal sheep. The effects of reduction of placental size on hormone concentrations in fetal plasma. J Dev Physiol. 1980;2:239–248. [PubMed] [Google Scholar]
- Robinson JS, Kingston EJ, Jones CT, Thorburn GD. Studies on experimental growth retardation in sheep. The effect of removal of endometrial caruncles on fetal size and metabolism. J Dev Physiol. 1979;1:379–398. [PubMed] [Google Scholar]
- Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Simonetta G, Rourke AK, Owens JA, Robinson JS, McMillen IC. Impact of placental restriction on the development of the sympathoadrenal system. Pediatr Res. 1997;42:805–811. doi: 10.1203/00006450-199712000-00015. [DOI] [PubMed] [Google Scholar]
- Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–753. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- Veening MA, van Weissenbruch MM, Heine RJ, Delemarre-van de Waal HA. β-cell capacity and insulin sensitivity in prepubertal children born small for gestational age. Influence of body size during childhood. Diabetes. 2003;52:1756–1760. doi: 10.2337/diabetes.52.7.1756. [DOI] [PubMed] [Google Scholar]
- Verhaeghe J, van Bree R, van Herck E, Coopmans W. Exogenous corticosteroids and in utero oxygenation modulate indices of fetal insulin secretion. J Clin Endocrinol Metab. 2005;90:3449–3453. doi: 10.1210/jc.2004-2512. [DOI] [PubMed] [Google Scholar]


