Abstract
We hypothesized that inspiratory muscle training (IMT) would attenuate the sympathetically mediated heart rate (HR) and mean arterial pressure (MAP) increases normally observed during fatiguing inspiratory muscle work. An experimental group (Exp, n = 8) performed IMT 6 days per week for 5 weeks at 50% of maximal inspiratory pressure (MIP), while a control group (Sham, n = 8) performed IMT at 10% MIP. Pre- and post-training, subjects underwent a eucapnic resistive breathing task (RBT) (breathing frequency = 15 breaths min−1, duty cycle = 0.70) while HR and MAP were continuously monitored. Following IMT, MIP increased significantly (P < 0.05) in the Exp group (−125 ± 10 to −146 ± 12 cmH2O; mean ±s.e.m.) but not in the Sham group (−141 ± 11 to −148 ± 11 cmH2O). Prior to IMT, the RBT resulted in significant increases in HR (Sham: 59 ± 2 to 83 ± 4 beats min−1; Exp: 62 ± 3 to 83 ± 4 beats min−1) and MAP (Sham: 88 ± 2 to 106 ± 3 mmHg; Exp: 84 ± 1 to 99 ± 3 mmHg) in both groups relative to rest. Following IMT, the Sham group observed similar HR and MAP responses to the RBT while the Exp group failed to increase HR and MAP to the same extent as before (HR: 59 ± 3 to 74 ± 2 beats min−1; MAP: 84 ± 1 to 89 ± 2 mmHg). This attenuated cardiovascular response suggests a blunted sympatho-excitation to resistive inspiratory work. We attribute our findings to a reduced activity of chemosensitive afferents within the inspiratory muscles and may provide a mechanism for some of the whole-body exercise endurance improvements associated with IMT.
Fatiguing work of the inspiratory muscles is associated with significant neural and cardiovascular consequences. Induction of inspiratory muscle fatigue in healthy humans by means of voluntary resistive inspiration has been shown to result in time-dependent increases in muscle sympathetic nerve activity, heart rate (HR) and mean arterial pressure (MAP) (St Croix et al. 2000), and a gradual reduction in arterial blood flow to the resting limb (Sheel et al. 2001). Furthermore, research in the anaesthetized rat has shown fatiguing contractions of the diaphragm to be responsible for an increase in the activity of type IV afferent fibres associated with increased sympathetic outflow (Hill, 2000). Collectively, these findings suggest the existence of a sympathetically mediated metaboreflex, or chemoreflex, that originates from fatigued inspiratory muscles. There is also evidence to suggest that the inspiratory muscle metaboreflex becomes active with whole-body exercise. Leg blood flow during high-intensity exercise has been shown to be inversely related to the work of breathing, and the consequent changes in leg vascular resistance have been shown to be directly related to the degree of noradrenaline (norepinephrine) spillover (Harms et al. 1997). More recently it has been demonstrated that a transient infusion of lactic acid into the phrenic circulation reduces limb blood flow and raises MAP in resting or exercising canines (Rodman et al. 2003). During prolonged intense whole-body exercise in healthy humans, inspiratory muscle fatigue can occur (Johnson et al. 1993) and the associated metaboreflex response may therefore limit exercise performance under such conditions.
Inspiratory muscle training (IMT) has been associated with significant improvements in whole-body exercise performance but the mechanism(s) behind this phenomenon have not been made clear. Previous authors have documented increased cycling endurance (Spengler et al. 1999; Stuessi et al. 2001) whereas others have not observed any consistent improvement in exercise performance (Fairbarn et al. 1991; Sonetti et al. 2001; Guenette et al. 2006). IMT has been associated with favourable changes in exercising lactate levels (Spengler et al. 1999), diaphragm thickness (Downey et al. 2006), and inspiratory muscle fatigue resistance (Boutellier et al. 1992; Boutellier & Piwko, 1992) and strength (Sonetti et al. 2001). Based on the above brief summary, we asked if the cardiovascular effects of the inspiratory muscle metaboreflex could be attenuated with IMT. Specifically, we hypothesized that following IMT, subjects would exhibit an attenuated HR and MAP response during a resistive breathing task.
Methods
Subjects
All experimental procedures and protocols were approved by the Clinical Research Ethics Board of the University of British Columbia and conformed to the Declaration of Helsinki. Sixteen men provided informed written consent prior to beginning the study. Subjects were young (25.8 ± 0.8 years), of normal mass (76.9 ± 2.6 kg) and height (179.0 ± 2.1 cm) and had a healthy range for body mass index (23.9 ± 0.6 kg m−2). All subjects were free from cardiovascular, pulmonary and neurological disease. For screening purposes, all subjects performed pulmonary function testing using a portable spirometer (Spirolab II, Medical International Research, Rome, Italy). All subjects had a normal forced vital capacity (5.4 ± 0.3 l) and forced expired volume in 1 s (4.5 ± 0.2 l) (Knudson et al. 1983).
General procedures
Mouth pressure (Pm) was measured with a pressure transducer (Model MP45–36-871, Validyne, Northridge CA, USA). The end-tidal partial pressure of CO2 (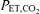 ) was determined using a gas analyser (CD-3A, AEI Technologies, Pittsburgh, PA, USA). Inspiratory flow was determined using a pneumotachograph (Hans-Rudolph 3813, Kansas City, MO, USA). Each of these three devices was calibrated prior to every test. From the flow signal, tidal volume (VT) and breathing frequency (fb) values were determined and minute ventilation (
) was determined using a gas analyser (CD-3A, AEI Technologies, Pittsburgh, PA, USA). Inspiratory flow was determined using a pneumotachograph (Hans-Rudolph 3813, Kansas City, MO, USA). Each of these three devices was calibrated prior to every test. From the flow signal, tidal volume (VT) and breathing frequency (fb) values were determined and minute ventilation (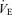 ) was calculated as the product of fb and VT. Offline integration of the Pm signal allowed for the calculation of inspiratory muscle force development (fb×∫Pm). Arterial blood pressure and HR were monitored continuously at the finger on a beat-by-beat basis with the use of a finger arterial plethysmograph (Finometer, FMS, Finapres Medical Systems BV, Arnhem, the Netherlands). Systolic and diastolic blood pressures were measured and MAP was calculated as 1/3 pulse pressure + diastolic pressure. Blood pressure was also determined every minute with the use of an automated blood pressure cuff (BPM-100, VSM MedTech Ltd, Vancouver, Canada). The average blood pressure values obtained from the automatic cuff over the resting eupnoeic period were used to correct the arterial plethysmograph values for each subject. All signals were sampled at 100 Hz using an analog-to-digital converter (PowerLab/16SP model ML795, ADI, Colorado Springs, CO, USA) and stored on a computer for subsequent analyses.
) was calculated as the product of fb and VT. Offline integration of the Pm signal allowed for the calculation of inspiratory muscle force development (fb×∫Pm). Arterial blood pressure and HR were monitored continuously at the finger on a beat-by-beat basis with the use of a finger arterial plethysmograph (Finometer, FMS, Finapres Medical Systems BV, Arnhem, the Netherlands). Systolic and diastolic blood pressures were measured and MAP was calculated as 1/3 pulse pressure + diastolic pressure. Blood pressure was also determined every minute with the use of an automated blood pressure cuff (BPM-100, VSM MedTech Ltd, Vancouver, Canada). The average blood pressure values obtained from the automatic cuff over the resting eupnoeic period were used to correct the arterial plethysmograph values for each subject. All signals were sampled at 100 Hz using an analog-to-digital converter (PowerLab/16SP model ML795, ADI, Colorado Springs, CO, USA) and stored on a computer for subsequent analyses.
Experimental protocol
Subjects were assigned to an experimental (Exp) or a control (Sham) IMT group. The Exp group trained at 50% of MIP, 6 days a week, for 5 weeks. The Sham subjects performed IMT at 10% of MIP, 6 days a week, for 5 weeks. Subjects were blinded to their assigned treatment group. Prior to the commencement of the training program, subjects underwent familiarization testing in order to minimize any potential learning effect. On the experimental days (pre- and post-IMT) subjects underwent a resisted breathing task (see below) designed to elicit the respiratory muscle metaboreflex (Sheel et al. 2001). MIP and maximal expiratory pressure (MEP) tests were also performed on these days prior to the resisted breathing task. Measurements of MEP were made to ensure the expiratory muscles remained untrained.
Respiratory muscle strength testing
MIP and MEP tests required subjects to perform a maximal volitional inspiratory or expiratory effort through a mouthpiece attached to an occluded three-way stopcock (2100 series, Hans Rudolph, Kansas City, MO, USA). A small pinhole within the stopcock served to prevent glottic closure and minimize the recruitement of the buccal muscles. The MIP and MEP tests were performed in a seated position and conformed to standarized procedures (ATS, 2002). Inspiratory manoeuvers were initiated from residual volume and expiratory manoeuvers were initiated from total lung capacity. Three acceptable MIP and MEP values were each averaged and the resulting means were taken as the test values.
Resistive breathing task
Subjects refrained from caffeine, exercise, alcohol and food for 12 h prior to each RBT. All RBTs took place with subjects in a semi-recumbant position. Each trial began with 8 min of eupnoea in order to ensure stable baseline cardiorespiratory parameters. The RBT trials were designed to increase the metabolic requirements and compromise diaphragm perfusion by combining a prolonged breath duty cycle and high force output. Subjects inspired (custom-made Y valve) against an added resistive load to a target mouth pressure of 60% MIP by following a tracing of Pm on a computer monitor to a pre-set target for each inspiration. Throughout the trial, subjects maintained a fb of 15 breaths min−1 and a prolonged duty cycle (TI/TTOT= 0.7) by listening to a computer-generated audio signal with distinct inspiratory and expiratory tones. This protocol has previously been shown to reduce diaphragmatic blood flow in anaesthetized dogs (Bellemare et al. 1983) and predict the onset of diaphragm fatigue in humans (Bellemare & Grassino, 1982a, b; Sheel et al. 2001, 2002). The maximal Pm of each breath was continously displayed for the subjects on a computer monitor. Subjects were instructed to isolate the diaphragm during inspiration and minimize accessory breathing muscle recruitment. Subjects were verbally encouraged to maintain proper timing, breathing technique and Pm as needed. Termination of the RBTs was based upon the presence of a plateau in the rise in MAP. The RBTpost was performed at the same absolute intensity and duration as the RBTpre. Each RBT was followed by a minute of non-resistive recovery. Inspired CO2 was increased as needed to maintain 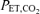 at eucapnoeic levels.
at eucapnoeic levels.
Inspiratory muscle training
Following the pre-test, subjects were assigned a respiratory muscle trainer (Powerlung ‘Sport’, Vacumed, Ventura, CA, USA) set at a resistance corresponding to 50% (Exp) or 10% (Sham) of their seated baseline MIP. Subjects in the Exp group were encouraged to inspire briskly whereas subjects in the Sham group inspired normally. To ensure the subjects did not experience expiratory resistance, subjects removed the device during expiration. In addition, subjects were encouraged to practice slow and relaxed expirations, each lasting 4–6 s. Subjects were instructed to perform IMT sessions once a day, 6 days a week, for 5 weeks. Each IMT session involved three sets of 75 breaths with a 5 min rest between sets. The training protocol used by the Exp group in this study was based closely upon an IMT regimen previously shown to elicit significant improvements in MIP (Sonetti et al. 2001). Subjects reported to the laboratory for supervised training sessions once a week, during which time seated MIP and MEP values were re-assessed, and the resistance on the trainers was adjusted to maintain the same relative workload. Subjects kept daily logs of their IMT activity indicating the date and time of their training sessions.
Data and statistical analysis
Spirometry values, descriptive characteristics, and baseline MIP and MEP values were compared between the Sham and Exp groups using independent t tests. A dependent t test was used to assess for possible differences in fb×∫Pm between RBTpre and RBTpost. MIP and MEP values for each group were compared from pre- to post-training using one-tailed paired t tests. For the analysis of the RBT data, mean values were calculated for each minute of the RBTs of each subject. A single mean value was calculated for both the entire 8 min resting period and the first minute of recovery. Due to variation in the duration of RBTs between subjects, select points (eupnoea, minutes 1–3, the final minute and first minute of recovery) were used for statistical comparisons. For each RBT, repeated measures ANOVA were performed to detect significant changes from eupnoeic values in those variables measured. Tukey's post hoc test was used when differences were discovered. To compare the HR and MAP data pre- to post- for each time point in each group, one-tailed dependent t tests with Bonferroni corrections were performed. An alpha level of 0.05 was used for all tests of significance. All data are expressed as means ±s.e.m. All statistics were performed with the use of commercial statistical software (STATISTICA, Version 6.1, Statsoft Inc., Tulsa, OK, USA).
Results
Baseline measurements and subject compliance
Subjects in the Sham and Exp groups were similar for lung function measures and physical characteristics (P > 0.05). During eupnoeic breathing there were no systematic trends for any measured respiratory variables (Tables 1 and 2, P > 0.05). The within-subject coefficients of variation for resting HR and MAP were in excellent agreement with previously reported values (Sheel et al. 2001). The average resting coefficients of variation for HR over the 2 days was ±4.1% and ±2.4% for the Sham and Exp groups, respectively. The resting coefficients of variation for MAP were similarly low for the Sham (±2.2%) and Exp (±1.9%) groups. Based on the self-report training logs, subjects in the Sham group maintained a mean IMT compliance of 88% over the course of the 5 weeks. Exp subjects reported 97% compliance; however, this was not statistically different from that of the Sham subjects (P > 0.05). The trend for a lower training compliance in the Sham group was driven primarily by one subject who exhibited a compliance of only 40%. With data from this subject removed, the training compliance of the Sham group was greater than 95%.
Table 1.
Group mean data for the Sham group during resistive breathing
| VT (l) | fb (breaths min−1) |
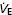 (l min−1) (l min−1) |
TI/TTOT | Peak Pm (cmH2O) | VT/TI(l s−1) |
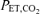 (mmHg) (mmHg) |
SBP (mmHg) | DBP (mmHg) | |
|---|---|---|---|---|---|---|---|---|---|
| Pre | |||||||||
| Eupnoea | 0.768 | 14.10 | 9.2 | 0.343 | −0.8 | 0.54 | 37.3 | 117.5 | 73.4 |
| ±0.074 | ±1.17 | ±0.6 | ±0.014 | ±0.2 | ±0.05 | ±0.9 | ±1.8 | ±2.1 | |
| Min1 | 1.190* | 14.97 | 16.0* | 0.736* | −63.2* | 0.41 | 33.8* | 122.9 | 74.4 |
| ±0.069 | ±0.03 | ±0.9 | ±0.005 | ±6.5 | ±0.02 | ±0.6 | ±5.1 | ±2.9 | |
| Min 2 | 1.163* | 15.01 | 15.7* | 0.739* | −62.0* | 0.39 | 38.1 | 136.0* | 85.4* |
| ±0.062 | ±0.01 | ±0.8 | ±0.005 | ±4.5 | ±0.02 | ±0.6 | ±4.2 | ±2.6 | |
| Min 3 | 1.129* | 15.24 | 15.4* | 0.736* | −60.3* | 0.39 | 38.4 | 134.7* | 84.6* |
| ±0.059 | ±0.16 | 0.9 | ±0.008 | ±5.4 | ±0.02 | ±1.0 | ±4.9 | ±3.0 | |
| End | 1.157* | 14.98 | 15.6* | 0.738* | −68.6* | 0.39 | 38.0 | 141.7* | 88.4* |
| ±0.076 | ±0.02 | ±1.0 | ±0.008 | ±6.2 | ±0.02 | ±0.8 | ±4.6 | ±2.1 | |
| Rec 1 | 1.164* | 16.67 | 16.0* | 0.366 | −0.8 | 0.93* | 37.3 | 129.8* | 80.5 |
| ±0.122 | ±1.55 | ±1.3 | ±0.023 | ±0.1 | ±0.12 | ±1.9 | ±3.2 | ±2.7 | |
| Post | |||||||||
| Eupnoea | 0.994 | 10.67 | 9.1 | 0.328 | −0.6 | 0.58 | 35.0 | 117.7 | 75.0 |
| ±0.134 | ±0.57 | ±1.2 | ±0.022 | ±0.1 | ±0.09 | ±1.8 | ±4.2 | ±2.9 | |
| Min 1 | 1.157 | 14.92* | 15.6* | 0.726* | −68.3* | 0.40 | 32.7 | 120.7 | 74.3 |
| ±0.081 | ±0.03 | ±1.1 | ±0.008 | ±6.8 | ±0.03 | ±1.6 | ±5.0 | ±2.5 | |
| Min 2 | 1.115 | 15.03* | 15.1* | 0.737* | −66.8* | 0.38 | 35.0 | 132.2* | 84.5* |
| ±0.078 | ±0.01 | ±1.1 | ±0.007 | ±6.0 | ±0.03 | ±1.8 | ±4.8 | ±2.7 | |
| Min 3 | 1.093 | 15.00* | 14.8* | 0.721* | −65.2* | 0.38 | 36.6 | 132.7* | 85.5* |
| ±0.071 | ±0.01 | ±1.0 | ±0.012 | ±5.6 | ±0.02 | ±1.6 | ±4.7 | ±2.9 | |
| End | 1.080 | 15.05* | 14.7* | 0.696* | −71.6* | 0.39 | 36.2 | 135.4* | 86.1* |
| ±0.064 | ±0.02 | ±0.9 | ±0.012 | ±6.9 | ±0.02 | ±1.7 | ±4.9 | ±2.4 | |
| Rec 1 | 1.097 | 16.76* | 16.0* | 0.364 | −0.8 | 0.93* | 34.7 | 125.7 | 80.6 |
| ±0.086 | ±1.39 | ±1.6 | ±0.022 | ±0.1 | ±0.13 | ±1.8 | ±3.2 | ±3.2 | |
Values are means ±s.e.m. (n = 8).
Significantly different from eupnoea (P < 0.05).
Definition of terms: VT, tidal volume; fb, breathing frequency; 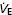 , minute ventilation; TI/TTOT duty cycle [inspiratory time (TI)/total time (TTOT)]; Peak Pm, peak mouth pressure; VT/TI, mean inspiratory flow rate;
, minute ventilation; TI/TTOT duty cycle [inspiratory time (TI)/total time (TTOT)]; Peak Pm, peak mouth pressure; VT/TI, mean inspiratory flow rate; 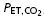 , end-tidal carbon dioxide; SBP, systolic blood pressure; DBP, diastolic blood pressure; Min1–3, minutes 1–3 of resistive breathing; Rec1, first minute of recovery.
, end-tidal carbon dioxide; SBP, systolic blood pressure; DBP, diastolic blood pressure; Min1–3, minutes 1–3 of resistive breathing; Rec1, first minute of recovery.
Table 2.
Group mean data for the Exp group during resistive breathing
| VT (l) | fb (breaths min−1) |
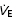 (l min−1) (l min−1) |
TI/TTOT | Peak Pm (cmH2O) | VT/TI (l s−1) |
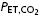 (mmHg) (mmHg) |
SBP (mmHg) | DBP (mmHg) | |
|---|---|---|---|---|---|---|---|---|---|
| Pre | |||||||||
| Eupnoea | 0.781 | 12.93 | 8.0 | 0.304 | −0.8 | 0.54 | 39.6 | 113.6 | 69.9 |
| ±0.104 | ±1.65 | ±0.5 | ±0.018 | ±0.1 | ±0.05 | ±1.1 | ±1.7 | ±1.5 | |
| Min 1 | 1.095* | 14.99 | 14.9* | 0.723* | −56.8* | 0.38* | 35.1* | 110.7 | 66.6 |
| ±0.058 | ±0.03 | ±0.8 | ±0.013 | ±6.8 | ±0.02 | ±1.0 | ±3.8 | ±2.9 | |
| Min 2 | 1.068* | 14.99 | 14.5* | 0.722* | −55.6* | 0.37* | 36.6 | 121.6 | 74.4 |
| ±0.057 | ±0.03 | ±0.8 | ±0.016 | ±5.9 | ±0.02 | ±1.5 | ±2.5 | ±2.5 | |
| Min 3 | 1.055 | 15.14 | 14.4* | 0.713* | −59.4* | 0.37* | 38.5 | 126.9* | 77.1* |
| ±0.063 | ±0.12 | ±0.9 | ±0.017 | ±6.2 | ±0.02 | ±0.9 | ±4.5 | ±4.5 | |
| End | 1.007 | 14.79 | 13.6* | 0.722* | −51.0* | 0.35* | 37.7 | 133.0* | 82.3* |
| ±0.066 | ±0.13 | 1.0 | ±0.015 | ±5.7 | ±0.02 | ±0.8 | ±3.2 | ±2.8 | |
| Rec 1 | 0.942 | 17.35* | 13.7* | 0.348 | −0.9 | 0.80* | 38.3 | 124.5* | 75.9 |
| ±0.130 | ±1.62 | ±1.1 | ±0.010 | ±0.1 | ±0.07 | ±2.2 | ±2.5 | ±1.8 | |
| Post | |||||||||
| Eupnoea | 0.940 | 10.98 | 8.5 | 0.304 | −0.5 | 0.56 | 36.8 | 112.8 | 70.3 |
| ±0.117 | ±1.04 | ±0.9 | ±0.014 | ±0.1 | ±0.05 | ±1.6 | ±2.1 | ±1.2 | |
| Min 1 | 1.079 | 14.87 | 14.4* | 0.689* | −61.8* | 0.39* | 31.6* | 108.9 | 63.7* |
| ±0.023 | ±0.12 | ±0.3 | ±0.018 | ±5.2 | ±0.01 | ±1.4 | ±4.0 | ±2.8 | |
| Min 2 | 1.080 | 15.02 | 14.6* | 0.709* | −64.6* | 0.38* | 34.9 | 117.5 | 70.6 |
| ±0.033 | ±0.02 | ±0.4 | ±0.014 | ±6.3 | ±0.01 | ±1.6 | ±3.0 | ±1.9 | |
| Min 3 | 1.015 | 15.00 | 13.7* | 0.690* | −63.4* | 0.37* | 36.9 | 121.3* | 73.6 |
| ±0.038 | ±0.03 | ±0.5 | ±0.019 | ±7.2 | ±0.01 | ±1.3 | ±3.0 | ±2.2 | |
| End | 0.985 | 15.01 | 13.3* | 0.693* | −56.4* | 0.36* | 36.9 | 119.7* | 73.2 |
| ±0.049 | ±0.04 | ±0.6 | ±0.014 | ±6.3 | ±0.02 | ±1.8 | ±3.1 | ±2.1 | |
| Rec 1 | 1.080 | 16.00* | 13.2* | 0.343 | −0.8 | 0.68 | 34.2 | 117.7 | 72.7 |
| ±0.178 | ±2.24 | ±1.5 | ±0.016 | ±0.1 | ±0.07 | ±1.7 | ±2.1 | ±1.2 | |
Values are means ±s.e.m. (n = 8).
Significantly different from eupnoea (P < 0.05).
Definition of terms: VT, tidal volume; fb, breathing frequency; 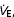 , minute ventilation; TI/TTOT, duty cycle [inspiratory time (TI)/total time (TTOT)]; Peak Pm, peak mouth pressure; VT/TI, mean inspiratory flow rate;
, minute ventilation; TI/TTOT, duty cycle [inspiratory time (TI)/total time (TTOT)]; Peak Pm, peak mouth pressure; VT/TI, mean inspiratory flow rate; 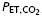 , end-tidal carbon dioxide; SBP, systolic blood pressure; DBP, diastolic blood pressure; Min1–3, minutes 1–3 of resistive breathing; Rec1, first minute of recovery.
, end-tidal carbon dioxide; SBP, systolic blood pressure; DBP, diastolic blood pressure; Min1–3, minutes 1–3 of resistive breathing; Rec1, first minute of recovery.
Respiratory muscle strength
Baseline MIP strength was similar between the Exp and Sham groups prior to training (P > 0.05) (Exp: −125.0 ± 10.0 cmH2O; Sham: −141.2 ± 11.2 cmH2O); however, MEP values were significantly greater (P < 0.01) in the Sham group (Sham: 158.9 ± 7.4 cmH2O; Exp: 127.2 ± 6.2 cmH2O). The MIP and MEP training responses for both groups are displayed in Fig. 1. A significant increase in MIP following training was observed in the Exp group but not the Sham group. All subjects in the Exp group displayed some rise in MIP after 5 weeks of training, with a group mean improvement of 17%. Although there was a trend for improvements in MIP in the Sham group (+6%), this finding was neither consistent across all subjects, nor significant (P > 0.05). No significant changes in MEP were observed in either group following training.
Figure 1.
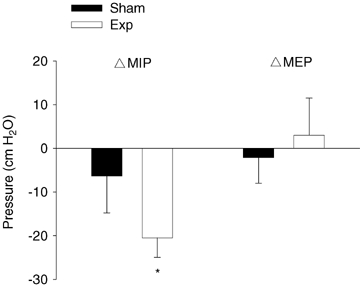
Changes in maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) following 5 weeks of inspiratory muscle training in the Sham (n = 8) and Exp (n = 8) groups Values are means ±s.e.m.*Post-training value significantly different from pre-training value (P < 0.05).
Respiratory measures during resistive breathing
The mean RBT was 535 ± 52 s in duration (range: 266–996 s). Over the course of the RBT, subjects in both groups had difficulty achieving the target 60% MIP level with mean peak Pm values of 47.3 ± 1.5% and 43.9 ± 3.3% of MIP in the Sham and Exp groups, respectively. The RBT duration and Pm values were held constant for pre- and post-IMT trials. These pressure values combined with the imposed breathing pattern corresponded to a substantial amount of force with mean fb×∫Pm values over 200 times eupnoeic levels for both groups (Fig. 3). All subjects were able to maintain the desired breathing pattern during the RBTs (fb= 15 breaths min−1; TI/TTOT= 0.70) (Tables 1 and 2). As expected, the prescribed breathing protocol did result in some degree of hyperventilation for all subjects during all RBTs, with fb and 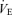 values significantly above rest. This hyperventilation resulted in significant drops in
values significantly above rest. This hyperventilation resulted in significant drops in 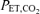 during the first 30–60 s of the RBTs in some subjects but was adequately compensated for with the administration of supplemental CO2 during subsequent minutes.
during the first 30–60 s of the RBTs in some subjects but was adequately compensated for with the administration of supplemental CO2 during subsequent minutes.
Figure 3.
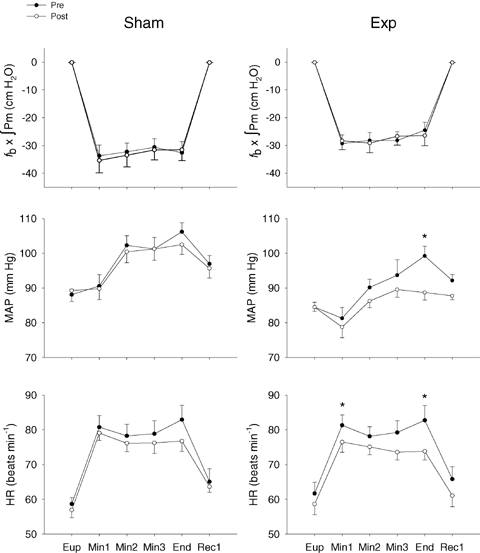
Group mean heart rate, mean arterial pressure (MAP) and inspiratory muscle force generation (fb×∫Pm) during resistive breathing before (•) and after (○) inspiratory muscle training in the Sham (n = 8) and Exp (n = 8) groups Eup, eupnoea; Min1–3, minutes 1–3 of resistive breathing; End, final minute of resistive breathing; Rec1, first minute of recovery. Values are means ±s.e.m.*Significantly different from post-training value (P < 0.05).
Cardiovascular measures during resistive breathing
Prior to training, the resistive breathing resulted in significant and sustained HR (Sham: +41%; Exp: +35%) and MAP (Sham: +21%; Exp: +17%) increases in both training groups. The rise in HR occurred within the first minute of resistive breathing and tended to return to near baseline levels within a minute of recovery. The rises in MAP were more gradual, typically occurring within 2 or 3 min of resistive breathing, and failing to normalize within the first minute of recovery. Fig. 2A and B show a trace of one representative Exp subject during the RBTpre and RBTpost and demonstrate an attenuated blood pressure response. The mean HR and MAP responses for both the Sham and Exp groups are displayed in Fig. 3. Following training in the Sham group, HR and MAP were again elevated in response to the RBT (HR: +36%; MAP: +15%). There were no differences in HR or MAP between the pre- and post-RBT tests of the Sham group at any time point (P > 0.05). In contrast, the Exp group exhibited significantly blunted HR and MAP responses during the RBTpost (HR: +27%; MAP: +4%).
Figure 2.
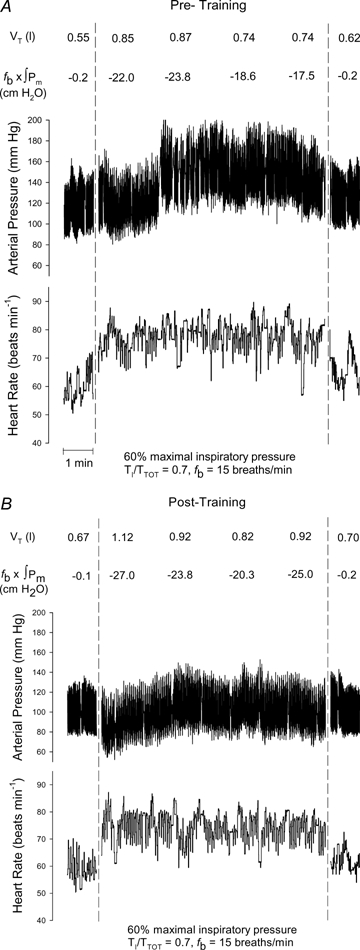
Raw data from an individual Exp subject during resistive breathing Resistive breathing at 60% of MIP, prolonged duty cycle of 0.70 and a breathing frequency of 15 breaths min−1 at baseline (A) and following five weeks of inspiratory muscle training (B). VT, tidal volume; fb×∫Pm, inspiratory muscle force generation.
Discussion
Main findings
The purpose of this study was to investigate the effects of IMT on the cardiovascular responses to resistive inspiratory muscle work. To this end, subjects were required to perform a resistive breathing task before and after a 5 week period of inspiratory muscle training. During the RBTpre, subjects in both groups exhibited a respiratory muscle metaboreflex as evidenced by a time-dependent rise in HR and MAP. Following training in the experimental group, the HR response to the same absolute workload was blunted and the MAP response was nearly abolished; however, in the Sham group there were no significant differences in the rise of HR or MAP between baseline and post-training tests. We interpret this to indicate a training-induced attenuation of the activity of chemosensitive afferent fibres in response to resistive inspiratory muscle work.
Inspiratory muscle strength changes with training
We detected an average MIP improvement of approximately 21 cmH2O in the Exp group. This 17% increase in MIP is within the range of mean training improvements (8–41%) observed by others employing a similar 50% MIP training protocol (Sonetti et al. 2001; Guenette et al. 2006; McConnell & Lomax, 2006). The Sham group results are similar to those seen in other studies (Sonetti et al. 2001; Volianitis et al. 2001; Downey et al. 2006). The fact that there were no significant changes in MEP in either group further suggests that the MIP improvements observed in the Exp subjects were a result of the IMT training.
Sympathetic attenuation with inspiratory muscle training
During the RBTpre it is believed that there were both central and peripheral factors working to increase HR and MAP above resting values. The increased central command associated with the increased inspiratory efforts probably resulted in significant vagal withdrawal (Hollander & Bouman, 1975). Additionally, the greater contractile force associated with the RBT presumably increased the mechanical deformation of the diaphragm and increased the activity of the mechanically sensitive (type III) afferent fibres within this muscle (Duron, 1981; Jammes & Speck, 1995). Typically, the changes to HR and MAP induced by mechanoreceptors appear earlier and are of a lesser magnitude than those of the more delayed-acting metaboreceptors. The initial HR and MAP responses in our study are similar to those of others (St Croix et al. 2000; Sheel et al. 2001) who have manipulated mechanoreceptor or central command input by employing extremely high resistive (95% MIP) breathing or hyperpnoea tasks in the absence of significant metaboreceptor activity. We therefore believe the HR and MAP increases observed in the early minutes of the RBT to be mechanoreceptor and centrally mediated.
The prolonged duty cycle and high inspiratory resistance of this task has been shown to bring about fatigue by restricting blood flow to the diaphragm and reducing its mechanical efficiency (Bellemare & Grassino, 1982a, b; Bellemare et al. 1983). Over time, an accumulation of lactic acid and other metabolic by-products is believed to result in the sympathetically mediated metaboreflex (Dempsey et al. 2002). The delayed and gradual time-course of the rise in MAP observed in the Exp subjects prior to training and in the Sham subjects, both before and after IMT, is consistent with this reflex as observed by others (St Croix et al. 2000; Sheel et al. 2001; McConnell & Lomax, 2006). In contrast, HR failed to respond in a delayed fashion and observed a more abrupt increase. As HR is more vulnerable to central influences (Victor et al. 1989), it is possible this mechanism played a greater role in the HR time-course and, as a result, masked the role played by the metaboreflex.
We exposed subjects to nearly identical Pm, fb, VT and TI/TTOT values during the pre- and post-RBTs. As a result, the absolute amount of inspiratory work and the degree of mechanical deformation experienced by the diaphragm during the RBTpost was no less than that during the RBTpre. Despite these control measures, we observed a blunted HR and MAP response during the RBTpost of the Exp group in comparison to pre-training values and values in the Sham group. There are numerous possibilities as to why this may have occurred. Given the MIP improvements with IMT, it may be suggested that subjects in the Exp group were working at a lower relative intensity during the RBTpost and that this contributed to our findings. However, this is unlikely to be the case as the MIP improvements were offset by the slightly greater Pm generated during the RBTpost (Exp: 44.3 ± 10.0% MIP; Sham: 50.2 ± 9.0% MIP). A second possibility is that there may have been less mechanoreceptor activity during the RBTpost due to a reduced responsiveness to a given mechanical stimulus. This may have occurred due to conditioning of the type III receptors to the repeated mechanical deformations experienced during the IMT. Such an adaptation is hypothesized to have occurred in training studies of other skeletal muscles (Sinoway et al. 1996; Fisher & White, 1999). Sinoway et al. (1996) have suggested that this effect may be related to the muscle acidosis associated with training, as it has been shown in the anaesthetized feline model that the mechanically sensitive type III afferents have a reduced discharge frequency following repeated lactic acid exposure (Sinoway et al. 1993). However, mechanoreceptor attenuation if present, should have been apparent from the onset of the RBTpost and would not solely explain the growing disparity between the MAP values of the RBTpre and RBTpost over time.
Although mechanoreceptor adaptations may have contributed to the blunted MAP and HR we do not feel that they entirely explain the attenuated responses observed during the latter stages of the RBTpost. Alternatively, we propose that following IMT, the aerobic capacity of the respiratory muscles may have been improved thereby reducing the imbalance between diaphragm blood flow and metabolic demand during the RBTpost. The resulting reduction in metabolite accumulation would attenuate the activity of not only the type IV chemosensitive afferents but also the mechanically sensitive type III afferents, which evidence suggests are influenced by local metabolite concentrations as well (Sinoway et al. 1993). Changes to the enzyme profile and fibre type composition of the diaphragm have been documented following treadmill training in rats (Vrabas et al. 1999) and in clinical populations that experience chronically high inspiratory muscle workloads (Tikunov et al. 1997; Ribera et al. 2003). We believe that such aerobic adaptations of the diaphragm were also present following IMT in our Exp group. Additionally, during IMT the diaphragm probably experienced repeated metabolite exposures that may have led to a decreased responsiveness of the chemoreceptors to a given metabolic stimulus. Such a training effect has previously been proposed by Sinoway et al. (1992) who showed that the forearms of trained individuals exhibited a lesser sympathetic response to a given pH change when compared with those of untrained individuals. Similar adaptations at the level of the metaboreceptors in this study would explain the blunted HR and MAP responses during the final minutes of the RBTpost.
Debate exists as to whether or not the respiratory metaboreflex serves to improve the perfusion of the ischaemic diaphragm. Although the increased sympathetic stimulus associated with this reflex has been shown to restrict limb blood flow, the sympathetic outflow is likely to also target the vasculature of the respiratory muscles. However, based on studies of isolated arteriole preparations, it has been suggested that in comparison with the arterioles of the leg, those of the diaphragm exhibit less of a vasoconstrictive response to sympathetic input (Aaker & Laughlin, 2002). This implies that during activation of the metaboreflex there was a relative increase in blood flow to the diaphragm in comparison with the locomotor muscles.
Relevance for exercise performance
Is respiratory muscle work an important determinant of the ability to perform dynamic exercise? Two lines of evidence suggest that activation of the respiratory muscle metaboreflex contributes to increased sympathetic tone and redistribution of blood flow during dynamic exercise. Firstly, increasing or decreasing the work of breathing during heavy cycle exercise has effects on leg blood flow (Harms et al. 1997). It appears there is a threshold or critical point for activation of this reflex that does not occur during submaximal exercise (Wetter et al. 1999) or non-fatiguing inspiratory efforts (Sheel et al. 2002). In the present study, we believe that IMT may have raised the so-called ‘ischaemic threshold’ required to elicit significant increases in MAP. Such a threshold change has previously been demonstrated (Mostoufi-Moab et al. 1998) to occur at the forearm following a period of handgrip training. Secondly, directly altering the work of breathing during maximal exercise has been shown to have effects on locomotor muscle fatigue (Romer et al. 2006) and exercise performance (Harms et al. 2000) in endurance athletes. Indeed, respiratory muscle unloading and loading results in a 14% improvement and 15% decline, respectively, in the time to fatigue during maximal exercise (Harms et al. 2000). This has also been demonstrated in exercising heart failure patients (Mancini et al. 1997; O'Donnell et al. 1999). Most recently, McConnell & Lomax (2006) have shown that inspiratory muscle work performed immediately prior to plantar exercise will expedite plantar flexor fatigue and that IMT can raise the threshold of inspiratory muscle work necessary to elicit this effect. Collectively, our findings coupled with those of others point to important reflexes originating in the respiratory muscles which have consequences for exercise performance.
Methodological considerations
There are a number of limitations to this study that are worthy of mention. Firstly, we employed a volitional pressure generation test (MIP and MEP) rather than an electrically evoked test for the assessment of inspiratory and expiratory muscle strength. Although the improvement in MIP in the Exp group strongly suggests an increase in global inspiratory muscle strength, it is unclear what physiological adaptations were behind such increases. Increased central drive, increased motor unit recruitment, increased inspiratory muscle contractility, and inspiratory muscle hypertrophy are all possible explanations for the MIP increase observed after IMT.
Inspiratory muscle fatigue was not assessed in this study. Therefore, we cannot be certain as to whether fatigue did or did not occur. However, based upon the current understanding of the conditions necessary to induce inspiratory muscle fatigue (Bellemare & Grassino, 1982a, b; Bellemare et al. 1983), and based upon the findings of others who have assessed inspiratory muscle fatigue following the same RBT protocol (Sheel et al. 2001) as that employed in this study, we would speculate that the peak Pm values, TI/TTOT values and duration of the RBTs were sufficient to induce inspiratory muscle fatigue in our subjects prior to training.
Summary
In summary, our findings demonstrate that 5 weeks of resistive IMT is capable of increasing inspiratory muscle strength and attenuating the time-dependent rise in HR and MAP that occurs with resistive inspiratory work in healthy males. We attribute the attenuated cardiovascular response to a reduced activity of chemically sensitive afferent fibres innervating the inspiratory muscles. Such an effect may have been caused by an increased oxidative capacity of the inspiratory muscles, resulting in a reduced metabolite accumulation with resistive work. Alternatively, the observed attenuation may be due to a desensitization or decline in responsiveness of the type III and IV afferents to chemical stimulants, resulting from a conditioned response to repeated exposure to the accumulated metabolites associated with IMT. Our findings of reduced cardiovascular responsiveness to high levels of inspiratory work suggest that IMT may have the potential to improve whole-body exercise performance.
Acknowledgments
We thank our subjects for their enthusiastic participation. This study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Foundation for Innovation. J.D.W. and J.A.G. were supported by graduate scholarships from NSERC and the Michael Smith Foundation for Health Research (MSFHR). A.W.S. was supported by a Scholar Award from the MSFHR and a New Investigator award from the Canadian Institutes of Health Research.
References
- Aaker A, Laughlin MH. Diaphragm arterioles are less responsive to α1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol. 2002;92:1808–1816. doi: 10.1152/japplphysiol.01152.2001. [DOI] [PubMed] [Google Scholar]
- ATS. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Grassino A. Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol. 1982a;53:1190–1195. doi: 10.1152/jappl.1982.53.5.1190. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Grassino A. Evaluation of human diaphragm fatigue. J Appl Physiol. 1982b;53:1196–1206. doi: 10.1152/jappl.1982.53.5.1196. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Wight D, Lavigne CM, Grassino A. Effect of tension and timing of contraction on the blood flow of the diaphragm. J Appl Physiol. 1983;54:1597–1606. doi: 10.1152/jappl.1983.54.6.1597. [DOI] [PubMed] [Google Scholar]
- Boutellier U, Buchel R, Kundert A, Spengler C. The respiratory system as an exercise limiting factor in normal trained subjects. Eur J Appl Physiol Occup Physiol. 1992;65:347–353. doi: 10.1007/BF00868139. [DOI] [PubMed] [Google Scholar]
- Boutellier U, Piwko P. The respiratory system as an exercise limiting factor in normal sedentary subjects. Eur J Appl Physiol Occup Physiol. 1992;64:145–152. doi: 10.1007/BF00717952. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- Downey AE, Chenoweth LM, Townsend DK, Ranum JD, Ferguson CS, Harms CA. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir Physiol Neurobiol. 2006;156:137–146. doi: 10.1016/j.resp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Duron B. Intercostal and diaphragmatic muscle afferents. In: Hornbein TF, editor. Regulation of Breathing, Part I. New York: Marcel Decker; 1981. pp. 473–540. [Google Scholar]
- Fairbarn MS, Coutts KC, Pardy RL, McKenzie DC. Improved respiratory muscle endurance of highly trained cyclists and the effects on maximal exercise performance. Int J Sports Med. 1991;12:66–70. doi: 10.1055/s-2007-1024658. [DOI] [PubMed] [Google Scholar]
- Fisher WJ, White MJ. Training-induced adaptations in the central command and peripheral reflex components of the pressor response to isometric exercise of the human triceps surae. J Physiol. 1999;520:621–628. doi: 10.1111/j.1469-7793.1999.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenette JA, Martens AM, Lee AL, Tyler GD, Richards JC, Foster GE, Warburton DE, Sheel AW. Variable effects of respiratory muscle training on cycle exercise performance in men and women. Appl Physiol Nutr Metab. 2006;31:159–166. doi: 10.1139/h05-016. [DOI] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res. 2000;856:240–244. doi: 10.1016/s0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975;38:272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Speck DF. Respiratory control by respiratory and diaphragmatic muscle afferents. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Marcel Dekker; 1995. pp. 543–582. [Google Scholar]
- Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- McConnell AK, Lomax M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J Physiol. 2006;577:445–457. doi: 10.1113/jphysiol.2006.117614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini D, Donchez L, Levine S. Acute unloading of the work of breathing extends exercise duration in patients with heart failure. J Am Coll Cardiol. 1997;29:590–596. doi: 10.1016/s0735-1097(96)00556-6. [DOI] [PubMed] [Google Scholar]
- Mostoufi-Moab S, Widmaier EJ, Cornett JA, Gray K, Sinoway LI. Forearm training reduces the exercise pressor reflex during ischemic rhythmic handgrip. J Appl Physiol. 1998;84:277–283. doi: 10.1152/jappl.1998.84.1.277. [DOI] [PubMed] [Google Scholar]
- O'Donnell DE, D'Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med. 1999;160:1804–1811. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- Ribera F, N'Guessan B, Zoll J, Fortin D, Serrurier B, Mettauer B, Bigard X, Ventura-Clapier R, Lampert E. Mitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:873–879. doi: 10.1164/rccm.200206-519OC. [DOI] [PubMed] [Google Scholar]
- Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol. 2003;95:1159–1169. doi: 10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Pegelow DF, Dempsey JA. Threshold effects of respiratory muscle work on limb vascular resistance. Am J Physiol Heart Circ Physiol. 2002;282:H1732–H1738. doi: 10.1152/ajpheart.00798.2001. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Shenberger J, Leaman G, Zelis R, Gray K, Baily R, Leuenberger U. Forearm training attenuates sympathetic responses to prolonged rhythmic forearm exercise. J Appl Physiol. 1996;81:1778–1784. doi: 10.1152/jappl.1996.81.4.1778. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Rea RF, Mosher TJ, Smith MB, Mark AL. Hydrogen ion concentration is not the sole determinant of muscle metaboreceptor responses in humans. J Clin Invest. 1992;89:1875–1884. doi: 10.1172/JCI115792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonetti DA, Wetter TJ, Pegelow DF, Dempsey JA. Effects of respiratory muscle training versus placebo on endurance exercise performance. Respir Physiol. 2001;127:185–199. doi: 10.1016/s0034-5687(01)00250-x. [DOI] [PubMed] [Google Scholar]
- Spengler CM, Roos M, Laube SM, Boutellier U. Decreased exercise blood lactate concentrations after respiratory endurance training in humans. Eur J Appl Physiol Occup Physiol. 1999;79:299–305. doi: 10.1007/s004210050511. [DOI] [PubMed] [Google Scholar]
- Stuessi C, Spengler CM, Knopfli-Lenzin C, Markov G, Boutellier U. Respiratory muscle endurance training in humans increases cycling endurance without affecting blood gas concentrations. Eur J Appl Physiol. 2001;84:582–586. doi: 10.1007/s004210100408. [DOI] [PubMed] [Google Scholar]
- Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation. 1997;95:910–916. doi: 10.1161/01.cir.95.4.910. [DOI] [PubMed] [Google Scholar]
- Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- Volianitis S, McConnell AK, Koutedakis Y, McNaughton L, Backx K, Jones DA. Inspiratory muscle training improves rowing performance. Med Sci Sports Exerc. 2001;33:803–809. doi: 10.1097/00005768-200105000-00020. [DOI] [PubMed] [Google Scholar]
- Vrabas IS, Dodd SL, Powers SK, Hughes M, Coombes J, Fletcher L, Demirel H, Reid MB. Endurance training reduces the rate of diaphragm fatigue in vitro. Med Sci Sports Exerc. 1999;31:1605–1612. doi: 10.1097/00005768-199911000-00017. [DOI] [PubMed] [Google Scholar]
-
Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on
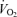 and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
and leg blood flow during submaximal exercise. J Appl Physiol. 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]


