Abstract
Perinatal stress disrupts normal development of the hypothalamo-pituitary-adrenal (HPA) axis. Adult male (but not female) rats previously subjected to a stress such as neonatal maternal separation (NMS) are characterized by chronic elevation of plasma corticosterone (Cort) levels and an abnormally elevated hypoxic ventilatory response through mechanisms that remain unknown. The present study tested the hypothesis that a chronic increase of plasma Cort levels alone augments the ventilatory response to hypoxia in adult rats. Three groups of Sprague–Dawley male and female rats were used (control, placebo and Cort implants). Rats subjected to chronic Cort elevation received a subcutaneous Cort implant (300 mg) 14 days prior to ventilatory measurements, whereas sham-operated rats received placebo implants. Controls received no treatment. Plasma Cort levels and body weight profiles were measured to assess protocol efficiency. Whole body plethysmography was used to measure ventilatory activity and metabolic indices during normoxia and following a 20 min period of moderate hypoxia (12% O2). Male rats implanted with Cort showed a ventilatory response to hypoxia higher than placebo-treated rats; this effect was mainly due to a larger tidal volume response. In females, Cort treatment increased the breathing frequency response but the effect on minute ventilation was not significant. Taken together, these data show that chronic elevation of Cort alone increases the ventilatory response to hypoxia, but in a sex-specific manner. These data raise important questions regarding the mechanisms underlying the sexual dimorphism of this effect and the potential link between HPA axis dysfunction and respiratory disorders related to abnormal ventilatory chemoreflex.
Early life exposition to a stressor has several consequences for the development of the central nervous system (CNS) that persist throughout life. The impact of exposing offspring to different forms of stress has been addressed using various models such as neonatal maternal separation (NMS) (Wigger & Neumann, 1999; Lehmann & Feldon, 2000) and neonatal isolation/deprivation (NI; Lai et al. 2006). Published findings have shown that mother–pup interactions play an important role in early life programming of the hypothalamo-pituitary-adrenal (HPA) axis (Genest et al. 2004; Lai et al. 2006; Slotten et al. 2006). Neonatal maternal separation disrupts development of the circuits regulating HPA axis activity and ultimately results in a persistent increase in blood plasma Cort levels (Francis et al. 1999; Francis & Meaney, 1999; Liu et al. 2000), a condition that predisposes to several anomalies ranging from anxiety and hypertension (Hofer, 1994; Boccia & Pedersen, 2001; Anand et al. 2003) to immunosuppression (Brown-Borg et al. 1993; Wrona & Trojniar, 2005).
We have shown that NMS has functional consequences that extend beyond behaviour since it alters normal development of the respiratory control system (Genest et al. 2004, 2007a, b; Kinkead et al. 2005a, b, c). The neuroendocrine and respiratory phenotypes that develop following NMS include an increase in plasma adreno-corticotropic hormone (ACTH) and Cort levels as well as an increase in the hypoxic ventilatory response (Genest et al. 2004). These traits are sex specific as they are apparent in males only; however, the mechanisms underlying this manifestation of respiratory plasticity remain unknown (Genest et al. 2004). In light of these data, we tested the hypothesis that chronic Cort elevation is sufficient to augment the ventilatory response to hypoxia in the adult rat. To do so, we used a pharmacological approach to chronically increase plasma Cort levels in male and female rats by implanting slow release Cort pellets to raise hormonal level within a physiologically relevant range. We then used whole body plethysmography to compare metabolic indices and ventilatory activity of awake, unrestrained rats subjected to Cort treatment to data obtained in control and placebo-treated animals under normoxic and hypoxic conditions.
Results show that in adult male rats, chronic elevation of plasma Cort alone augments the hypoxic ventilatory response. In females, however, the Cort treatment augments the frequency component of the hypoxic response, but the net changes in minute ventilation are not statistically significant. These discrepancies further illustrate the differences in male and female physiology with respect to neuroendocrine modulation of ventilatory control. These results have been reported previously in abstract form (Fournier et al. 2006).
Methods
Experimental animals
Experiments were performed on 52 adult Sprague–Dawley rats aged between 12 and 16 weeks: 25 males weighing between 326 g and 474 g, and 27 females weighing between 244 g and 332 g on the day of surgery. All animals were purchased from Charles River Canada (St-Constant, Quebec, Canada). Animals were supplied with food and water ad libitum and maintained in standard animal care conditions (21°C, 12 : 12 h dark–light cycle: lights on at 06.00 h and off at 18.00 h). Laval University Animal Care Committee approved the experimental procedures and the protocols were in accordance with the guidelines detailed by the Canadian Council on Animal Care.
Anaesthesia and surgical procedures
Telemetry
All 52 animals received a surgical intervention for the implantation of a fixed telemetric probe transponder (E-mitter, Mini Mitter, Bend, OR, USA) to measure core body temperatures (Tb) during ventilatory measurements. Rats were anaesthetized with isoflurane (2–2.5% in air). The probe was inserted in the peritoneum and sutured behind the internal wall of the abdominal cavity (Montandon et al. 2006; Genest et al. 2007a). Following this procedure, rats subjected to the placebo (8 males, 7 females) or the Cort treatment (10 males, 13 females) received subcutaneous implants (see below). Control rats received surgery solely for the implantation of the telemetric probe (7 males, 7 females).
Pellet implantation
In addition to telemetric probe implantation, each Cort-treated rat received three 100 mg pellets of Cort (Innovative Research of America, Sarasota, FL, USA). According to the manufacturer's information, Cort release occurs at a constant rate over 21 days. The dosage of the pellet used in this experiment was based on the protocol established by Orchinik et al. (2001) and preliminary data. The intervention for implanting pellets consisted of incising the upper back of the animal at the level of the scapula and gently introducing the pellets subcutaneously. Placebo-treated animals received three placebo pellets obtained from the same supplier.
For all groups of rats, postsurgical care consisted of subcutaneous injections of a non-steroid anti-inflammatory (ketoprofen: 2 mg kg−1), an antibiotic (baytril: 5 mg kg−1) and 5 ml of lactated Ringer solution given immediately after surgery, 24 h, and 48 h post-operation.
Body weight profiles
Individual body weights (Wb) were recorded every 2 days from the day of surgery until the day of blood withdrawal, which occurred on average 4 ± 1 days following hypoxic exposure (day 14).
Ventilatory and metabolic measurements
Ventilatory measurements were performed using a whole body, flow-through plethysmography system (model PLY3223, Buxco Electronics, Sharon, CT, USA) according to a protocol previously described (Kinkead et al. 2001; Genest et al. 2004, 2007a, b; Montandon et al. 2006). Briefly, the rat was placed in the plethysmographic chamber and the resting respiratory and metabolic measurements were started once the animal was calm and the breathing activity was stable. The acclimatization period typically lasted between 30 and 60 min. The breathing frequency (f), tidal volume (VT), minute ventilation 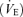 and inspiratory flow (TI) were all recorded using data acquisition software (IOX, EMKA Technologies, Falls Church, VA, USA). After 10 min of normoxic measurements, N2 was added to the inflowing gas mixture to reach 12% O2. The composition of the hypoxic mixture delivered to the chamber was monitored continuously with an O2 analyser and rats were exposed to this stimulus for 20 min. All measurements were made between 09.00 h and 13.00 h to minimize fluctuations associated with circadian rhythms.
and inspiratory flow (TI) were all recorded using data acquisition software (IOX, EMKA Technologies, Falls Church, VA, USA). After 10 min of normoxic measurements, N2 was added to the inflowing gas mixture to reach 12% O2. The composition of the hypoxic mixture delivered to the chamber was monitored continuously with an O2 analyser and rats were exposed to this stimulus for 20 min. All measurements were made between 09.00 h and 13.00 h to minimize fluctuations associated with circadian rhythms.
During ventilatory measurements, the barometric pressure, body temperature, chamber temperature and humidity were recorded for subsequent calculation of VT expressed in ml (BTPS) per 100 g of body weight (Wb) according to the equations provided by Drorbough & Fenn (1955). Given the between group differences in body weights (Wb), the calculations of the minute ventilation 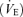 , the consumption of oxygen
, the consumption of oxygen 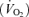 , and the ratio
, and the ratio 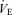 /
/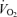 were also corrected using allometric factors according to the equations described by Mortola et al. (1994). The flow rate and composition of the gas mixtures flowing in and out of the chamber was analysed with an oxygen analyser (model S-3A, Ametek, Pittsburgh, PA, USA) and a carbon dioxide analyser (model CD-3A, Ametek, Pittsburgh, PA, USA) for subsequent calculation of oxygen consumption
were also corrected using allometric factors according to the equations described by Mortola et al. (1994). The flow rate and composition of the gas mixtures flowing in and out of the chamber was analysed with an oxygen analyser (model S-3A, Ametek, Pittsburgh, PA, USA) and a carbon dioxide analyser (model CD-3A, Ametek, Pittsburgh, PA, USA) for subsequent calculation of oxygen consumption 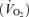 and carbon dioxide
and carbon dioxide 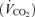 production in an open system (Mortola & Dotta, 1992). These measurements were taken under baseline conditions and at the end of the hypoxic period.
production in an open system (Mortola & Dotta, 1992). These measurements were taken under baseline conditions and at the end of the hypoxic period.
Plasma corticosterone measurements
Following respiratory measurements, rats were returned to the animal care facility where they recovered on average 4 ± 1 days before killing was performed using deep anaesthetisia with ketamine–xylazine 5% (0.2 mg (100 g)−1). As soon as the animal lost consciousness and no longer responded to toe pinch, a blood sample was collected through intracardiac puncture. Blood samples were collected between 09.00 h and 13.00 h and transferred into a K2-EDTA vacutainer tube (Becton Dickinson). Blood plasma was separated by centrifugation at 3500 r.p.m. and 4°C for 15 min before being stored at −80°C until assayed.
Corticosterone levels were determined using the Correlate(tm)-EIA ELISA Kits (Assay Design Inc., Ann Arbor, MI, USA), and a microplate spectrophotometer (u-Quant, Bio-Tek Instruments Inc., Winooski, VT, USA). According to the manufacturer, the sensitivity of the kit is 27 pg ml−1. By default, the enzyme immunoassay procedure quantifies free plasma Cort only. Knowing that female rats possess a greater amount of Cort binding globulin (CBG) in their blood plasma when compared to male rats (Breuner & Orchinik, 2002; Toran-Allerand et al. 2005), the analysis was repeated in the presence of a steroid displacement reagent to measure total plasma Cort. The relative proportion of effective (percentage free) Cort was assessed by calculating the ratio of free to total Cort for both male and female rats.
Data analysis
Body weights
Body weights were expressed as a percentage change from pre-surgery values.
Ventilatory activity
Normoxic measurements of ventilatory variables (Vt, 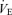 , f and TI) were obtained by averaging the last 10 min of stable recording using the Datanalyst software (EMKA Technologies). For all ventilatory measurements, steady state hypoxic values were obtained by averaging data from the 14th to the 20th minute of hypoxic exposure. For selected variables, the hypoxic ventilatory response was expressed as a percentage change from baseline.
, f and TI) were obtained by averaging the last 10 min of stable recording using the Datanalyst software (EMKA Technologies). For all ventilatory measurements, steady state hypoxic values were obtained by averaging data from the 14th to the 20th minute of hypoxic exposure. For selected variables, the hypoxic ventilatory response was expressed as a percentage change from baseline.
Corticosterone
Corticosterone concentrations were calculated from the parameters of the standard curve linearized by a log–log transformation.
Statistical analysis
Body weight profiles, plasma Cort levels, and normalized ventilatory data were compared using a two-way ANOVA (sex and treatment). Absolute (non-normalized) respiratory data were analysed using a three-way ANOVA; sex, hypoxia, and treatment (Statview 5.0, SAS Institute, Cary, NC, USA). A repeated measures design was used when appropriate. ANOVA was followed by Fisher's post hoc test when P ≤ 0.05, unless specified otherwise, and results of post hoc tests appear in figures only. ANOVA results are reported in the text and the factors of interest are specified. Data are expressed as means ± standard error of the mean (s.e.m.) and differences considered statistically significant at P ≤ 0.05, unless otherwise stated.
Results
Body weight profiles
Prior to surgery, the body weight of females was lower than males regardless of the group condition (P < 0.0001; data not shown). Overall, the weight change seen over the course of the experiments (expressed as a percentage change from presurgery weight) was greater in males than in females (sex effect: P < 0.0001). The weight increase seen in nontreated rats (placebo + controls) was greater in males than females (sex × treatment, P = 0.004; Fig. 1). Note, however, that the relative weight loss associated with chronic Cort treatment was similar for males and females (−7% for both; Fig. 1).
Figure 1.
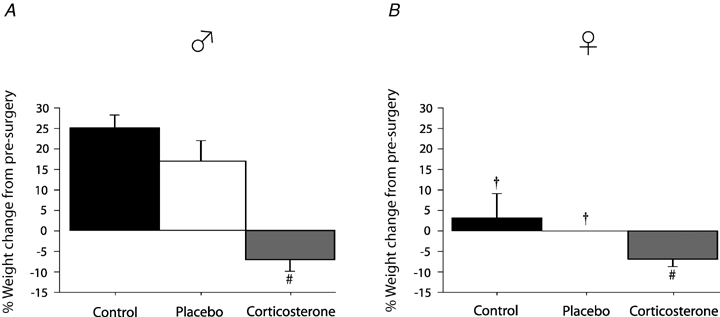
Comparison of body weight before (day 0) and 14 days after surgical implantation of corticosterone pellets (300 mg), placebo implants, or control treatment (telemetry probe for temperature measurement only) Data were obtained in male (A) and female rats (B) and are expressed as a percentage change from pre-surgery weight. #Value different from control condition (P < 0.05). †Value different from corresponding male value (P < 0.05).
Plasma corticosterone
Plasma samples were collected on average 4 ± 1 days after hypoxic exposure. Corticosterone treatment increase plasma levels of free Cort (treatment effect: P = 0.01). This effect tended to be sex specific (sex × treatment: P = 0.07) since a significant free Cort increase was observed only in males (Fig. 2A). Total Cort levels (bound + free) were higher than free plasma Cort levels but were not affected by treatment or sex (P > 0.1 for both; Fig. 2B). Expressing bio-available (free) Cort levels as a ratio of the total plasma levels showed that chronic Cort treatment increased the percentage of free Cort levels but the increase was greater in males than females (sex × treatment: P = 0.03; Fig. 2C).
Figure 2.
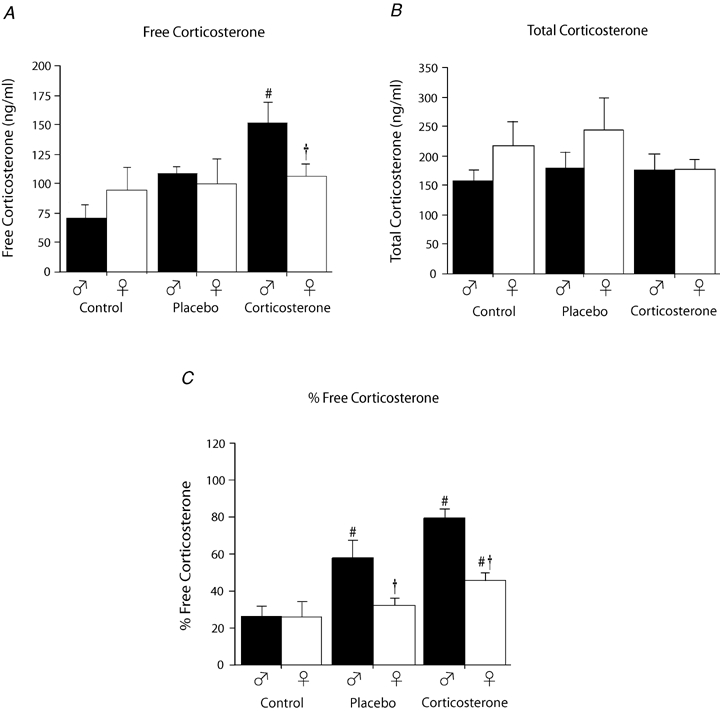
Comparison of ‘resting’ free plasmatic corticosterone (A), total corticosterone (B) levels and the percentage of free corticosterone (C) between control, placebo and corticosterone treated rats Data were obtained in male (black) and female rats (white) 4 ± 1 days after ventilatory measurements were completed (i.e. 18 ± 1 day post surgery). A and B, data are expressed in ng ml−1; C, data are expressed as ratio (percentage). #Value different from the control condition (P < 0.05). †Value different from corresponding male value (P < 0.05).
Breathing frequency (f)
Throughout the experiment, breathing frequency of females was lower than males (P < 0.0001; Fig. 3). Hypoxia increased the breathing frequency of all rats (P < 0.0001; Fig. 3) and the response observed in Cort treated animals was the greatest (hypoxia × treatment: P = 0.02). Despite suggestive trends, this effect was not sex specific (hypoxia × sex × treatment: P = 0.2; Fig. 2). The lower breathing frequency observed in females contributes (at least in part) to this result because expressing these data as the percentage change from baseline suggest that the breathing frequency response of females tends to be greater than males (sex effect: P = 0.1). Moreover, the only group which showed a significant difference from controls (treatment effect: P = 0.07) was Cort treated females (Fig. 6). Analysis of the frequency response on a minute by minute basis during the early stage of hypoxia (first 10 min) revealed no effect of sex or treatment on the time course of the response (P > 0.3 for both males and females; data not shown).
Figure 3.
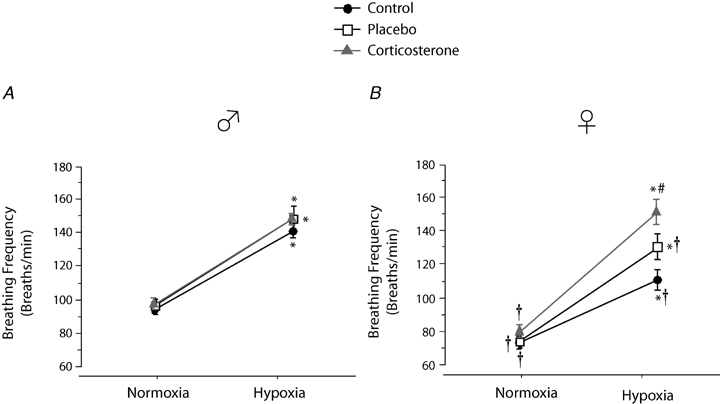
Comparison of the breathing frequency measurements obtained in control, placebo, and corticosterone treated male (A) and female rats (B) Data were obtained during normoxia (baseline) and following 20 min exposure to moderate hypoxia (12% O2). Data are expressed in breaths min−1. *Value different from normoxia. #Value different from the control condition (P < 0.05). †Value different from corresponding male value (P < 0.05).
Figure 6.
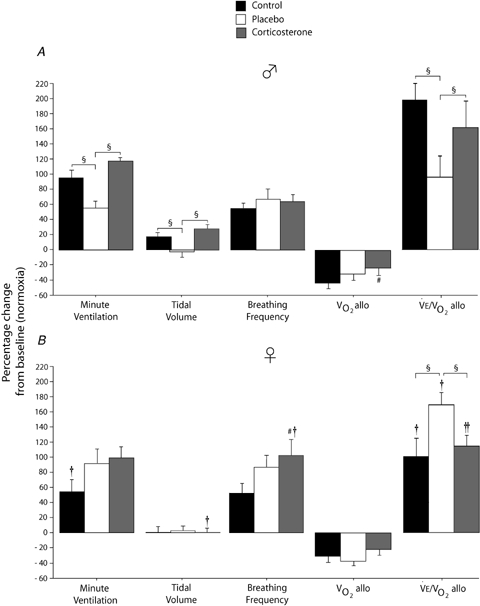
Effects of experimental treatments (control, black bars), placebo implants (white bars), corticosterone implants (grey bar) on the ventilatory response to moderate hypoxia (12% O2; 20 min) Results for selected ventilatory variables expressed as a percentage change from normoxic values. Data were obtained in males (A) and females (B) and are expressed as means ± 1 s.e.m.§Values different at P < 0.05. #Value different from the control condition (P < 0.05). † and †† indicate values different from corresponding male value at P < 0.05 and P < 0.1, respectively.
Tidal volume (VT)
In males, chronic treatment increased baseline Vt both in placebo and Cort treated rats. This effect did not occur in females (sex × treatment: P = 0.04; Fig. 4). Overall, hypoxia increased Vt (hypoxia effect: P = 0.04); however, the hypoxic response was augmented by Cort treatment in males only (hypoxia × sex × treatment: P = 0.01; Fig. 4). Expressing these data as a percentage change from baseline confirmed these observations (sex × treatment: P = 0.02) and showed that unlike any other groups, Vt of placebo treated males decreased during hypoxia (Fig. 6).
Figure 4.
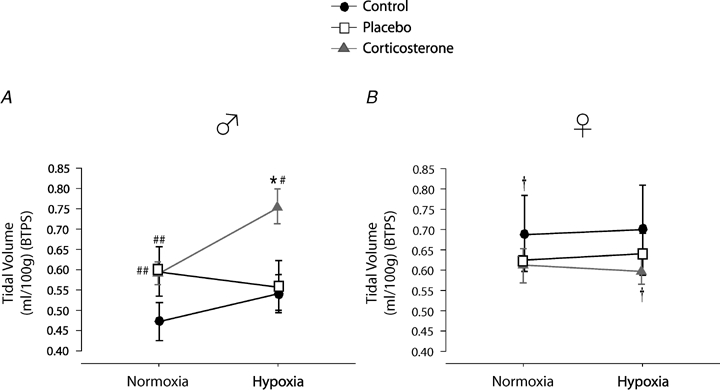
Comparison of the tidal volume measurements obtained in control, placebo, and corticosterone treated male (A) and female rats (B) Data were obtained during normoxia (baseline) and following 20 min exposure to moderate hypoxia (12% O2). Data are expressed in ml 100 g−1 under BTPS conditions. *Value different from normoxia (P < 0.05). # and ## indicate values different from the corresponding control value at P < 0.05 and P < 0.1, respectively.
Minute ventilation 
Minute ventilation was affected by sex and treatment (P = 0.05 for both; Fig. 5). This was noticeable under baseline conditions where Cort treated females breathed less than Cort treated males (Fig. 5). Hypoxia increased minute ventilation of all animals (P < 0.0001) and subjecting rats to the chronic Cort treatment resulted in a sex-specific (males only) increase in the hypoxic ventilatory response (hypoxia × sex × treatment: P = 0.05; Fig. 5). Expressing the hypoxic response as a percentage change from baseline confirmed these observations (sex × treatment: P = 0.03; Fig. 6). Note that in males, the largest difference was between placebo and Cort treated rats (Fig. 6). Correcting 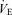 for allometric factors yielded similar results (hypoxia × sex × treatment: P = 0.03; Table 1).
for allometric factors yielded similar results (hypoxia × sex × treatment: P = 0.03; Table 1).
Figure 5.
Comparisons of the minute ventilation measurements obtained in male (A) and female rats (B) under normoxia and following 20 min exposure to moderate hypoxia (12% O2) Data are expressed in ml min−1 (100 g)−1 under BTPS conditions. *Value significantly different from normoxia (P < 0.05). #Value different from the control condition (P < 0.05). †Value different from corresponding male value (P < 0.05).
Table 1.
Effect of chronic elevation of corticosterone on selected ventilatory and metabolic variables measured in male and female rats
| Females | Males | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia (12%) | Normoxia | Hypoxia (12%) | |||||||||
| CTRL n = 7 | SHAM n = 7 | Cort n = 13 | CTRL n = 7 | SHAM n = 7 | Cort n = 13 | CTRL n = 7 | SHAM n = 8 | Cort n = 10 | CTRL n = 7 | SHAM n = 8 | Cort n = 10 | |
| Tb (°C) | 37.5 ± 0.2 | 38.0 ± 0.3 | 38.1 ± 0.2 | 37.3 ± 0.2 | 37.6 ± 0.3 | 37.6 ± 0.2 | 38.2 ± 0.4 | 37.9 ± 0.3 | 37.5 ± 0.2 | 37.7 ± 0.4 | 37.5 ± 0.3 | 37.2 ± 0.2 |
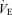 (ml min−1 (100 g)−1) (ml min−1 (100 g)−1)
|
49.5 ± 9.9 | 44.0 ± 3.0 | 43.9 ± 2.7 | 67.2 ± 9.7* | 78.5 ± 7.0* | 86.8 ± 8.9* | 43.8 ± 3.5 | 51.1 ± 6.7 | 56.0 ± 2.8† | 85.4 ± 7.5* | 82.3 ± 10.8* | 122.8 ± 8.8†* |
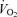 (ml STPD (100 g)−1) (ml STPD (100 g)−1) |
2.5 ± 0.1 | 2.6 ± 0.3 | 2.3 ± 0.2 | 2.0 ± 0.2* | 1.9 ± 0.2* | 2.1 ± 0.2 | 2.6 ± 0.2 | 2.2 ± 0.1 | 2.6 ± 0.1 | 1.8 ± 0.1* | 1.8 ± 0.1* | 2.3 ± 0.2# |
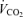 (ml STPD (100 g)−1) (ml STPD (100 g)−1) |
2.2 ± 0.2 | 2.3 ± 0.2 | 2.1 ± 0.2 | 1.7 ± 0.1** | 1.9 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.4 ± 0.1 | 1.4 ± 0.1* | 1.8 ± 0.1** | 1.8 ± 0.1* |
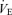 / /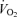
|
19.4 ± 3.1 | 17.5 ± 1.3 | 19.9 ± 1.5 | 77.0 ± 14.4*†† | 85.7 ± 8.1*† | 78.6 ± 6.7*† | 15.4 ± 2.8 | 28.0 ± 5.7 | 22.3 ± 1.8 | 49.6 ± 4.2* | 48.7 ± 6.7* | 55.9 ± 6.0* |
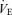 / /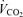
|
20.9 ± 3.5 | 19.8 ± 1.6 | 21.7 ± 1.7 | 65.5 ± 11.2* | 80.4 ± 7.0*† | 88.7 ± 8.8*†† | 22.8 ± 3.3 | 26.3 ± 5.5 | 24.2 ± 2.5 | 60.3 ± 7.9* | 48.6 ± 8.7* | 67.9 ± 6.1* |
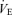 (allometric) (allometric) |
85.8 ± 17.9 | 73.9 ± 4.2† | 81.7 ± 6.1† | 111.0 ± 22.0 | 122.4 ± 12.0* | 134.4 ± 12.4†* | 85.7 ± 6.5 | 114.4 ± 14.9# | 110.0 ± 5.1## | 138.1 ± 11.4* | 139.5 ± 14.6 | 197.7 ± 12.8*# |
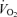 (allometric) (allometric) |
4.1 ± 0.2†† | 4.2 ± 0.4 | 3.9 ± 0.3† | 2.8 ± 0.2* | 2.6 ± 0.3* | 3.0 ± 0.2* | 4.9 ± 0.3# | 4.1 ± 0.2 | 4.8 ± 0.2 | 2.6 ± 0.2* | 2.8 ± 0.2* | 3.5 ± 0.3*# |
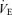 / /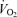 (allometric) (allometric) |
20.4 ± 3.3 | 18.4 ± 1.4†† | 21.0 ± 1.6 | 42.8 ± 10.5 | 49.5 ± 5.2* | 44.0 ± 4.2*†† | 18.3 ± 2.4 | 29.9 ± 6.1 | 23.8 ± 2.0 | 53.5 ± 4.6* | 52.7 ± 7.3* | 60.4 ± 6.5* |
Data are expressed as means ±s.e.m.
Value different from normoxia (P < 0.05).
Value different from males (P < 0.05).
Value different from controls (P < 0.05).
Value different from controls at P < 0.1. Allometric correction factors: 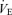 baseline: W0.47;
baseline: W0.47; 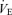 hypoxia: W0.62;
hypoxia: W0.62; 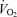 baseline: W0.52;
baseline: W0.52; 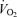 hypoxia: W0.68, where W refers to the animal's weight. From Mortola et al. (1994).
hypoxia: W0.68, where W refers to the animal's weight. From Mortola et al. (1994).
Selected metabolic indices
During normoxia, neither Cort treatment nor sex alters body temperature (P > 0.7 for both, Table 1). Hypoxia lowered body temperature (hypoxia effect: P < 0.0001); however, this decrease could not be detected in post hoc tests. This response tended to be greater in Cort-treated females (hypoxia × sex × treatment: P = 0.07; Table 1).
Analysing 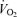 revealed no differences related to sex (P = 0.93) or treatment (P = 0.24).
revealed no differences related to sex (P = 0.93) or treatment (P = 0.24). 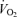 decreased during hypoxia (P < 0.0001) and this response tended to be less in Cort treated rats (hypoxia × treatment: P = 0.06). This effect was similar in males and females (hypoxia × sex × treatment: P = 0.23). Allometric correction of
decreased during hypoxia (P < 0.0001) and this response tended to be less in Cort treated rats (hypoxia × treatment: P = 0.06). This effect was similar in males and females (hypoxia × sex × treatment: P = 0.23). Allometric correction of 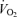 data produced similar results (hypoxia effect: P < 0.001; hypoxia × treatment: P = 0.1; hypoxia × sex × treatment: P = 0.2) except that (1)
data produced similar results (hypoxia effect: P < 0.001; hypoxia × treatment: P = 0.1; hypoxia × sex × treatment: P = 0.2) except that (1) 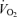 measurements obtained in males were greater than females (sex effect: P = 0.05), especially noticeable under baseline conditions, and (2) the
measurements obtained in males were greater than females (sex effect: P = 0.05), especially noticeable under baseline conditions, and (2) the 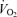 decrease of Cort treated rats was statistically significant (Table 1). These results were confirmed by data normalization which showed that, during hypoxia, the decrease in allometric
decrease of Cort treated rats was statistically significant (Table 1). These results were confirmed by data normalization which showed that, during hypoxia, the decrease in allometric 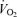 was less in Cort treated rats (treatment effect: P = 0.05; Fig. 6).
was less in Cort treated rats (treatment effect: P = 0.05; Fig. 6).
As expected, hypoxia increased the 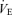 /
/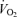 ratio in all rats (P < 0.0001; Table 1). Although the response was greater in females than in males (hypoxia × sex: P < 0.0001), the
ratio in all rats (P < 0.0001; Table 1). Although the response was greater in females than in males (hypoxia × sex: P < 0.0001), the 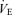 /
/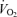 increase was not affected by treatment (hypoxia × sex × treatment: P = 0.19). However, these results are biased by body weight because correction of the
increase was not affected by treatment (hypoxia × sex × treatment: P = 0.19). However, these results are biased by body weight because correction of the 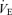 /
/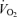 ratio according to allometric equations showed that, overall, allometric
ratio according to allometric equations showed that, overall, allometric 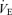 /
/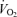 is greater in males than females (sex effect: P = 0.05) and that this response tended to be greater in Cort treated males (hypoxia × sex × treatment: P = 0.07; Table 1). Expressing these results as a percentage change from baseline confirmed this analysis and revealed a strong, sex-specific effect of sham treatment which is the opposite of Cort treatment (sex × treatment: P = 0.001; Fig. 6).
is greater in males than females (sex effect: P = 0.05) and that this response tended to be greater in Cort treated males (hypoxia × sex × treatment: P = 0.07; Table 1). Expressing these results as a percentage change from baseline confirmed this analysis and revealed a strong, sex-specific effect of sham treatment which is the opposite of Cort treatment (sex × treatment: P = 0.001; Fig. 6).
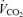 was not affected by sex (P = 0.37) or treatment (P = 0.21). Hypoxia decreased
was not affected by sex (P = 0.37) or treatment (P = 0.21). Hypoxia decreased 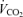 (P < 0.0001) and this response was not affected by sex or treatment either (hypoxia × sex × treatment: P = 0.2; Table 1). Consequently, hypoxia significantly increased the
(P < 0.0001) and this response was not affected by sex or treatment either (hypoxia × sex × treatment: P = 0.2; Table 1). Consequently, hypoxia significantly increased the 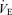 /
/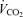 ratio (P < 0.0001). This response was greater in females (hypoxia × sex: P = 0.0003) and was augmented by treatment (hypoxia × treatment: P = 0.05). This effect was not sex specific (hypoxia × sex × treatment: P = 0.13; Table 1).
ratio (P < 0.0001). This response was greater in females (hypoxia × sex: P = 0.0003) and was augmented by treatment (hypoxia × treatment: P = 0.05). This effect was not sex specific (hypoxia × sex × treatment: P = 0.13; Table 1).
Discussion
This study shows that chronic elevation of plasma corticosterone levels (Cort) leads to persistent, sex-specific (males only) enhancement of the hypoxic ventilatory response in adult rats. In addition to its effects on respiratory control, this study also revealed important sexual dimorphism in the way that Cort is managed by the animal because even though Cort treatment elicited similar weight loss in both sexes, the increase in plasma Cort levels measured in females was marginal in comparison with males. These results are important because in humans, abnormally elevated chemoreflexes are likely to contribute to sleep apnoea and hypertension (Smith & Pacchia, 2007) and predispose to respiratory instability during sleep (Younes et al. 2001; Kara et al. 2003; Younes, 2004). Consequently, neuroendocrine disorders leading to chronic Cort elevation, regardless of their origin, could contribute to the emergence of disease related to respiratory control dysfunction.
Critique of methods
In male rats, our protocol efficiently raised free plasma Cort to levels similar to those reported in adult rats previously subjected to conditions such as chronic stress, neonatal maternal separation, or perinatal maternal food restriction which interfere with normal hypothalamo-pituitary-adrenal (HPA) axis function (Dumont et al. 2000; Leonhardt et al. 2002; Genest et al. 2004). Our inability to elevate Cort levels significantly in females was not anticipated given that, relative to body weight, the dose administered to females was higher than males. It is possible that, contrary to the information supplied by the manufacturer, Cort delivery rate was not constant throughout the protocol and that Cort levels peaked during the early days that followed implantation such that Cort release from the pellets was complete well before the end of the protocol. However, it is difficult to conceive why this would have occurred in females but not in males. The use of a higher dose was attempted in preliminary experiments (400 mg), but the overall condition of these females in the days that followed Cort implantation clearly showed that this dose exceeded the rats' ability to manage such high Cort levels. Females are known to have higher plasma levels of Cort binding globulin (CBG) which help reduce (and thus regulate) plasma levels of free Cort (Breuner & Orchinik, 2002). However, total Cort levels achieved were not higher in Cort treated rats of either sex. While this observation is consistent with the higher Cort excretion rate associated with higher plasma Cort levels (Ohmori et al. 1997), it would imply that Cort excretion rate is further enhanced in females than in males. Owing to the fact that Cort is a liposoluble molecule, adipose tissue (which is proportionally higher in females) may help remove free Cort from the circulation in females. However, these possibilities remain to be addressed experimentally.
In conclusion, our inability to show a significant increase in plasma Cort levels (free or total) in females raise our awareness to the numerous factors that one must consider during experimental hormone manipulation, especially in females. These caveats are the basis of the debate concerning the indicators and factors that determine bioavailability (and thus effectiveness) of the Cort molecule (Mendel, 1989; Petersen et al. 2006). While resolving this issue is well beyond the scope of the present study, our physiological measurements (e.g. body weight) clearly show that our protocol was efficient to chronically elevate plasma Cort levels in both sexes and indicate that the relative (percentage) of free Cort levels is a valuable indicator of Cort bioavailability, especially in females in which a weight loss was observed even though free Cort levels were not increased significant.
Effects of placebo versus corticosterone treatment on the hypoxic ventilatory response
We initially anticipated that placebo treatment would have little effect on our measurements such that these data could be pooled with those of controls as it is commonly done to increase statistical power. However, data analysis showed that this procedure was not appropriate because in some instances, results obtained in placebo treated rats (especially males) were misleading as they were different from controls and the opposite of those obtained in Cort treated rats (see Fig. 6). The factors contributing to this placebo effect on ventilatory measurements are unclear but since the experimental and handling procedures performed on Cort and placebo treated rats were exactly the same, this suggests that the matrix used to contain Cort in the pellets exerts undesirable (and unknown) side-effects which are distinct from those caused by Cort alone. Since this important and unexpected effect was common to both placebo and Cort treated rats, we believe that placebo treated animals are the best reference group to assess the effects of chronic Cort treatment on the hypoxic ventilatory response. Figure 6 clearly shows that using control rats as a sole reference group would be misleading because in males, comparing Cort treated rats to controls only would underestimate the effects of Cort treatment on ventilatory control, whereas in females, the effects of Cort would be exaggerated. These results emphasize the importance of using adequate control and sham groups in experimental design.
Chronic elevation of plasma corticosterone levels augments the hypoxic ventilatory response: comparison with neonatal maternal separation and potential mechanisms
The free Cort levels measured in our Cort treated rats (mean value: 163.9 ng ml−1; data range: 99.8–313.2 ng ml−1) are comparable to those measured in adult male rats previously subjected to NMS (Genest et al. 2004; mean value: 139.9 ng ml−1; data range: 39.3–352.2 ng ml−1). Clearly, the means by which chronic elevation of Cort levels were achieved in this study is very distinct, yet the consequences on the hypoxic ventilatory response of male rats are strikingly similar as both approaches augment the hypoxic ventilatory response by ∼25%, owing mainly to an increase in the tidal volume response (Genest et al. 2004; Genest et al. 2007a). This comparison must be done cautiously since, as we discussed previously, placebo treatment attenuates the hypoxic ventilatory response of male rats. However, comparing placebo and Cort treated rats show that the magnitude of the hypoxic ventilatory response correlates with percentage free Cort levels in males (r = 0.43; P = 0.06) but not females (r =−0.068; P = 0.8; data not shown). Despite the limitations inherent to correlative evidence, these data suggest that Cort levels affect the hypoxic ventilatory response in a sex-specific fashion.
Data obtained thus far indicate that NMS has extensive effects on the respiratory control system of male rats because it affects both the peripheral (chemosensory) and the central components of the hypoxic chemoreflex (Genest et al. 2004, 2007a; Kinkead et al. 2005a, b, c). Although the present study allowed us to assess the functional consequences of our Cort treatment in a physiologically relevant context, the approach used did not address the mechanisms underlying this manifestation of respiratory plasticity. Unlike NMS rats, however, chronic Cort treatment had no effect on the immediate breathing frequency response to hypoxia. This suggests that chronic Cort treatment mainly interferes with central components of the respiratory control system as it does not appear to affect carotid body function.
The lower metabolic response to hypoxia observed in Cort treated males suggests that this hormone affects performance of the preoptic area of the hypothalamus, the main neural structure regulating the reduction in body temperature during hypoxia (Bicego et al. 2007). Preliminary data from our laboratory suggest that this effect occurs in NMS males also (R. Gulemetova, unpublished observation). However, the magnitude of the Cort effect on this response is not sufficient to exclude the likelihood that chronic Cort treatment also affects CNS structures regulating the hypoxic ventilatory response. The current literature provides little information on the impact of glucorticoids on the ventilatory control system. In that regard, Joseph et al. (1998) have shown that in young adult males (250–320 g), chronic dexamethasone administration (1 mg kg−1 daily for 10 days) augments tyrosine hydroxylase (TH) activity in the carotid body and reduces it in brainstem catecholaminergic areas (NTS, A5, locus coeruleus, and parabrachial nucleus), but reduces the hypoxic ventilatory response (Joseph et al. 1998). The factors underlying the differences between these results and those reported here are unclear but indicate that the nature of the steroid used (dexamethasone versus Cort) and/or the administration procedure (daily injection versus chronic implants) are important experimental parameters that can affect the functional (respiratory) outcome differently. The effects of glucocorticoids on CNS structures involved in the regulation of the hypoxic ventilatory response have not been addressed directly, but there is evidence indicating that Cort regulates dopaminergic neurotransmission (Czyrak et al. 2003) and that chronic stress increases noradrenaline levels in brainstem and pontine regions relevant to respiratory control (e.g. NTS, A1/C1, and locus coeruleus; Lachuer et al. 1994). Our data showing that NMS increases TH mRNA expression in carotid bodies of male (but not female) rats are consistent with these results and indicate that these structures warrant further investigation (Kinkead et al. 2005c).
The functional consequences of perinatal stress exposure on CNS development and function are strikingly different between males and females. Unlike males, NMS does not affect HPA axis function in females because ‘resting’ plasma ACTH and corticosterone levels measured in NMS females are not different from controls (Genest et al. 2004). Furthermore, the hypoxic ventilatory response of NMS females is lower than controls (Genest et al. 2004). The neuroendocrine mechanisms ‘protecting’ HPA axis development in the face of neonatal stress are still unknown, but ovarian hormones are prime candidates for such action and they act on the regulation of Cort release. In the present study, females were not entirely immune to the stress associated with the placebo procedure which, unlike males, tended to contribute to (rather than mask) the effects of chronic Cort elevation on the hypoxic ventilatory response. However, this ‘protection’ was relatively effective because even though Cort treatment had a marginal effect on the hypoxic ventilatory response, female rats lost weight to the same extent as males over the course of the treatment.
Perspectives
Factors such as chronic or perinatal stress can affect HPA axis function in ways that will ultimately lead to chronic Cort elevation, a condition commonly associated with behavioural and psychiatric disorders both in humans and in animal models (Graham et al. 1999; Gunnar, 2003; Nicolson, 2004). However, since enhancement of the hypoxic ventilatory response predicts adverse prognosis in patients with chronic heart failure (Ponikowski et al. 2001) and predisposes to respiratory instability and recurrent apnoeas during sleep (Younes et al. 2001; Smith et al. 2003), the present study suggests that chronic Cort elevation may exacerbate and/or contribute to such cardio-respiratory disorders. While further studies are necessary to determine the mechanisms by which Cort affects the respiratory control system, these results provide valuable clues to understand the pathophysiology of respiratory disease and develop new intervention strategies.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR). This work was also supported by the Canada Research Chair in Respiratory Neurobiology and by the Fondation de la Recherche sur les Maladies Infantiles. We would like to express our most sincere appreciation for the support and technical assistance of Sophie-Emanuelle Genest, Sylvie Viger, Gaspard Montandon and Evelyne Vachon.
References
- Anand D, Stevenson CJ, West CR, Pharoah PO. Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child. 2003;88:135–138. doi: 10.1136/adc.88.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicego KC, Barros RCH, Branco LGS. Physiology of temperature regulation: Comparative aspects. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:616–639. doi: 10.1016/j.cbpa.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Klemcke HG, Blecha F. Lymphocyte proliferative responses in neonatal pigs with high or low plasma cortisol concentration after stress induced by restraint. Am J Vet Res. 1993;54:2015–2020. [PubMed] [Google Scholar]
- Czyrak A, Mackowiak M, Chocyk A, Fijal K, Wedzony K. Role of glucocorticoids in the regulation of dopaminergic neurotransmission. Pol J Pharmacol. 2003;55:667–674. [PubMed] [Google Scholar]
- Drorbough JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–86. [PubMed] [Google Scholar]
- Dumont EC, Kinkead R, Trottier J, Gosselin I, Drolet G. Effect of chronic psychogenic stress exposure on enkephalin neuronal activity and expression in the rat hypothalamic paraventricular nucleus. J Neurochem. 2000;75:2200–2211. doi: 10.1046/j.1471-4159.2000.0752200.x. [DOI] [PubMed] [Google Scholar]
- Fournier S, Allard M, Gulemetova R, Kinkead R. Chronic elevation of corticosterone increases the ventilatory response to hypoxia in adult rats. FASEB J. 2006;20:A374. [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor–norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Genest SE, Balon N, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and enhancement of the hypoxic ventilatory response: the role of GABAergic neurotransmission within the paraventricular nucleus of the hypothalamus. J Physiol. 2007a;583:299–314. doi: 10.1113/jphysiol.2007.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rat. J Physiol. 2004;554:543–557. doi: 10.1113/jphysiol.2003.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation induces sex-specific augmentation of the hypercapnic ventilatory response in awake rat. J Appl Physiol. 2007b;102:1416–1421. doi: 10.1152/japplphysiol.00454.2006. [DOI] [PubMed] [Google Scholar]
- Graham YP, Heim C, Goodman SH, Miller AH, Nemeroff CB. The effects of neonatal stress on brain development: implications for psychopathology. Dev Psychopathol. 1999;11:545–565. doi: 10.1017/s0954579499002205. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Integrating neuroscience and psychological approaches in the study of early experiences. Ann N Y Acad Sci. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr. 1994;397(suppl):9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Joseph V, Dalmaz Y, Cottet-Emard JM, Pequignot JM. Dexamethasone's influence on tyrosine hydroxylase activity in the chemoreflex pathway and on the hypoxic ventilatory response. Pflugers Arch. 1998;435:834–839. doi: 10.1007/s004240050591. [DOI] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K, Somers VK. Chemoreflexes – physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Dupenloup L, Valois N, Gulemetova R. Stress-induced attenuation of the hypercapnic ventilatory response in awake rats. J Appl Physiol. 2001;90:1729–1735. doi: 10.1152/jappl.2001.90.5.1729. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Genest SE, Gulemetova R, Lajeunesse Y, Laforest S, Drolet G, Bairam A. Neonatal maternal separation and early life programming of the hypoxic ventilatory response in rats. Respir Physiol Neurobiol. 2005a;149:313–324. doi: 10.1016/j.resp.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Gulemetova R, Bairam A. Neonatal maternal separation enhances phrenic responses to hypoxia and carotid sinus nerve stimulation in the adult anesthetised rat. J Appl Physiol. 2005b;99:189–196. doi: 10.1152/japplphysiol.00070.2005. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Joseph V, Lajeunesse Y, Bairam A. Neonatal maternal separation enhances dopamine D2 receptor and tyrosine hydroxylase mRNA expression levels in carotid body of rats. Can J Physiol Pharmacol. 2005c;83:76–84. doi: 10.1139/y04-106. [DOI] [PubMed] [Google Scholar]
- Lachuer J, Delton I, Buda M, Tappaz M. The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo-pituitary-adrenal axis. Brain Res. 1994;638:196–202. doi: 10.1016/0006-8993(94)90650-5. [DOI] [PubMed] [Google Scholar]
- Lai MC, Holmes GL, Lee KH, Yang SN, Wang CA, Wu CL, Tiao MM, Hsieh CS, Lee CH, Huang LT. Effect of neonatal isolation on outcome following neonatal seizures in rats – the role of corticosterone. Epilepsy Res. 2006;68:123–136. doi: 10.1016/j.eplepsyres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal seperation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Lesage J, Dufourny L, Dickes-Coopman A, Montel V, Dupouy JP. Perinatal maternal food restriction induces alterations in hypothalamo-pituitary-adrenal axis activity and in plasma corticosterone-binding globulin capacity of weaning rat pups. Neuroendocrinology. 2002;75:45–54. doi: 10.1159/000048220. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- Montandon G, Bairam A, Kinkead R. Long-term consequences of neonatal caffeine on ventilation, occurrence of apneas, and hypercapnic chemoreflex in male and female rats. Pediatr Res. 2006;59:519–524. doi: 10.1203/01.pdr.0000203105.63246.8a. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Dotta A. Effects of hypoxia and ambient temperature on gaseous metabolism of newborn rats. Am J Physiol Regul Integr Comp Physiol. 1992;263:R267–R272. doi: 10.1152/ajpregu.1992.263.2.R267. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Matsuoka T, Saiki C, Naso L. Metabolism and ventilation in hypoxic rats: effect of body mass. Respir Physiol. 1994;97:225–234. doi: 10.1016/0034-5687(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Ohmori S, Kurokouchi K, Kanda K, Kawano S, Ito T, Izumi R, Yasukawa K, Inazu M, Murata Y, Seo H. Effect of bisphosphonate administration on the excretion of stress hormones in tail-suspended rats. Environ Med. 1997;41:9–12. [PubMed] [Google Scholar]
- Orchinik M, Carroll SS, Li YH, McEwen BS, Weiland NG. Heterogeneity of hippocampal GABAA receptors: regulation by corticosterone. J Neurosci. 2001;21:330–339. doi: 10.1523/JNEUROSCI.21-01-00330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen HH, Andreassen TK, Breiderhoff T, Brasen JH, Schulz H, Gross V, Grone HJ, Nykjaer A, Willnow TE. Hyporesponsiveness to glucocorticoids in mice genetically deficient for the corticosteroid binding globulin. Mol Cell Biol. 2006;26:7236–7245. doi: 10.1128/MCB.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJS. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KCF. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: Gender-dependent effects. Brain Res. 2006;1097:123–132. doi: 10.1016/j.brainres.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Smith CA, Nakayama H, Dempsey JA. The essential role of carotid body chemoreceptors in sleep apnea. Can J Physiol Pharmacol. 2003;81:774–779. doi: 10.1139/y03-056. [DOI] [PubMed] [Google Scholar]
- Smith ML, Pacchia CF. Sleep apnoea and hypertension: role of chemoreflexes in humans. Exp Physiol. 2007;92:45–50. doi: 10.1113/expphysiol.2006.033753. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17α-Estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Wrona D, Trojniar W. Suppression of natural killer cell cytotoxicity following chronic electrical stimulation of the ventromedial hypothalamic nucleus in rats. J Neuroimmunol. 2005;163:40–52. doi: 10.1016/j.jneuroim.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]


