Abstract
Here the hypothesis that skeletal muscle Ca2+–calmodulin-dependent kinase II (CaMKII) expression and signalling would be modified by endurance training was tested. Eight healthy, young men completed 3 weeks of one-legged endurance exercise training with muscle samples taken from both legs before training and 15 h after the last exercise bout. Along with an ∼40% increase in mitochondrial F1-ATP synthase expression, there was an ∼1-fold increase in maximal CaMKII activity and CaMKII kinase isoform expression after training in the active leg only. Autonomous CaMKII activity and CaMKII autophosphorylation were increased to a similar extent. However, there was no change in α-CaMKII anchoring protein expression with training. Nor was there any change in expression or Thr17 phosphorylation of the CaMKII substrate phospholamban with training. However, another CaMKII substrate, serum response factor (SRF), had an ∼60% higher phosphorylation at Ser103 after training, with no change in SRF expression. There were positive correlations between the increases in CaMKII expression and SRF phosphorylation as well as F1ATPase expression with training. After training, there was an increase in cyclic-AMP response element binding protein phosphorylation at Ser133, but not expression, in muscle of both legs. Taken together, skeletal muscle CaMKII kinase isoform expression and SRF phosphorylation is higher with endurance-type exercise training, adaptations that are restricted to active muscle. This may contribute to greater Ca2+ mediated regulation during exercise and the altered muscle phenotype with training.
Skeletal muscles adapt to the functional demands placed on them (Holloszy & Booth, 1976; Baar et al. 2006), but the precise mechanisms of remodelling are unknown. It is believed that these adaptations occur through repeated stimuli by which there are coordinated changes in the specific protein degradation and synthesis which contribute to the overall change in the expression of a particular protein (Booth et al. 1982). In particular, there is emerging evidence that acute exercise increases the transcriptional activity of certain genes (Keller et al. 2001; Pilegaard et al. 2000, 2003) through activation of transcription factors (Irrcher & Hood, 2004; McGee et al. 2006; Wright et al. 2007) via various signalling pathways (Hawley et al. 2006), which has been conceptually termed excitation–transcription coupling (Chin, 2004). The understanding of the signalling mechanisms by which skeletal muscle becomes more fatigue resistant and has greater oxidative capacity with endurance exercise training is important as this may aid in the development of strategies to retard the development of insulin resistance (Hawley & Houmard, 2004; Kiens, 2006) as well as muscle dysfunctions (Chakkalakal et al. 2006).
Many signalling pathways may play a role in exercise induced skeletal muscle adaptation (for review see Hawley et al. 2006). Of note, AMPK expression and activity are higher after training (Frøsig et al. 2004), probably providing increased signalling to mitochondrial biogenesis because mice deficient in the α2-AMPK subunit have 20–25% lower mitochondrial enzyme expression in muscle (Jørgensen et al. 2007). On the other hand, in these mice the exercise-training induced increase in mitochondrial biogenesis is not impaired (Jørgensen et al. 2005, 2007), suggesting that other contraction induced signalling molecules are important for this adaptive process to exercise training. One such candidate is Ca2+ signalling (Chin, 2004). In particular, studies of mice where skeletal muscle proteins involved in Ca2+ homeostasis were manipulated show that changes in Ca2+ handling can alter skeletal muscle phenotype (e.g. oxidative capacity and contractile properties) regardless of motor nerve innervation (Chin et al. 2003; Song et al. 2004; Racay et al. 2006). It has been shown that Ca2+ signalling may increase gene expression of myosin-IIa (Allen & Leinwand, 2002), troponin-I slow isoform (Juretić et al. 2007), GLUT4 (Ojuka et al. 2002), PGC1α (Ojuka et al. 2003) and mitochondrial genes (Ojuka et al. 2003; Freyssenet et al. 2004), but probably not hexokinase-II (Halseth et al. 2000). Furthermore, it is known that increases in intracellular Ca2+ can affect protein turnover in skeletal muscle (Lewis et al. 1982) and during exercise various Ca2+ signalling molecules are activated including conventional protein kinase C (Richter et al. 1987), Ca2+–calmodulin-dependent kinases (CaMK; Rose et al. 2003, 2005, 2006; Liu et al. 2005) and protein phosphatase 2B (PP2B/calcineurin; Liu et al. 2001; Tothova et al. 2006). While PP2B is probably involved in motor nerve determined fibre type profile (Olson & Williams, 2000; Schiaffino & Serrano, 2002), there is accumulating evidence that it is not involved in many of the phenotypic modifications of skeletal muscle with repeated exercise (Parsons et al. 2004; Garcia-Roves et al. 2006; Huang et al. 2006).
Of the multifunctional CaMKs, CaMKI and CaMKIV have been implicated as putative signalling molecules in the adaptive process to exercise (McKinsey et al. 2000a, b; Wu et al. 2002; Smith et al. 2007), but since neither of these are expressed in skeletal muscle (Rose & Hargreaves, 2003; Chin, 2004; Akimoto et al. 2005; Rose et al. 2006), this is probably not the case. On the other hand, CaMKII isoforms are expressed in skeletal muscle (Bayer et al. 1998; Damiani et al. 2000; Rose et al. 2006) and studies of non-human mammals show that CaMKII activity and expression are higher with training (Antipenko et al. 1999; Flück et al. 2000b). These latter observations suggest that endurance training in man might also lead to increased CaMKII expression/activity in skeletal muscle. In addition, CaMKII is enriched in skeletal muscle nuclei (Flück et al. 2000a) and can phosphorylate proteins involved in the transcriptional machinery such as serum response factor (Flück et al. 2000a), myogenin (Tang et al. 2004), histone deacytalase 4 (Liu et al. 2005) and possibly cAMP response element binding protein (Hook & Means, 2001). Thus, CaMKII is an attractive candidate for activity dependent skeletal muscle adaptation as also hypothesized by others (Chin, 2004, 2005; Hood et al. 2006).
Here we examine CaMKII activity and expression of CaMKII isoforms in skeletal muscle of humans before and after short-term endurance exercise training. Potential downstream targets such as serum response factor and cAMP response element binding protein were also examined as well as particular genes which these might activate such as the β-subunit of the mitochondrial F1-ATP synthase.
Methods
Subjects and intervention
The specific details of subjects and the training regime have been reported (Frøsig et al. 2004, 2007). Eight healthy, young men (age: 25 ± 1 years, BMI: 24.6 ± 0.5 kg m−2) completed 3 weeks of one-legged knee-extension endurance exercise training with muscle biopsy samples taken from the vastus lateralis of both the active and passive leg before training and 15 h after the last exercise bout. Subjects consumed a standardized diet (i.e. ∼56% carbohydrate, 29% fat, 15% protein; 13.5 ± 0.2 MJ day−1) for two days prior to sampling. The exercise training consisted of dynamic knee extension exercise (70–85% peak work load; PWL) for 1–2 h per session, four to six sessions per week. Within each exercise session there was also a small bout (i.e. 5–7 min) of exercise at 100% of PWL in order to recruit all motor units of the vastus lateralis (Gollnick et al. 1974) and thereby train all muscle fibres. Subjects gave informed consent prior to participation and the study was carried out in accordance with the Declaration of Helsinki.
Tissue preparation
All materials were from Sigma-Aldrich (USA) unless stated otherwise. For skeletal muscle sample protein extraction, samples (15–20 mg) were freeze-dried and then homogenized while in an ice slurry (i.e. 0°C) in a buffer (15 μl per mg tissue, original weight) containing 50 mm Tris (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 2 mm sodium orthovanadate, 1 mm PMSF, 1 mm dithiothreitol, 1 mm benzamidine, 0.5% (v/v) protease inhibitor cocktail, and 1% (v/v) Nonidet P-40, using a polytron homogenizer (PT 1200, Kinematica) until no visible particles remained. The homogenates were mixed thoroughly by end-over-end rotation at 4°C for 30 min, and then spun at 6000 g for 10 min at 4°C. The clarified supernatant was taken and stored at −80°C until required. A small aliquot of each lysate was taken prior to storage for total protein concentration analysis.
Analytic techniques
Protein concentration of tissue extracts was determined in triplicate by the bicinchoninic acid (BCA) method using bovine serum albumin standards (Pierce Biotechnology Inc., Rockford, IL, USA) and BCA assay reagents (Pierce Biotechnology). A maximal coefficient of variance of 5% was accepted between replicates. Samples were immunoblotted for protein expression and phosphorylation according to Rose et al. (2005). The primary antibodies used were anti-F1-ATPase-β (Santa Cruz Biotechnlogy Inc., Santa Cruz, CA, USA; sc-16689), anti-CaMKII (BD Biosciences–Pharmingen, USA; 612624), anti-phospho-Thr287-CaMKII (Cell Signalling Technology, Inc., MA, USA; 3361), anti-phospholamban (Cyclacel, UK; 010-14), anti-phosphoThr17-phospholamban (Cyclacel, UK; 010-13), anti-serum response factor (Santa Cruz Biotechnology; sc-335), anti-phospho-Ser103-serum response factor (Cell Signalling Technology; 4261), anti-cAMP response element binding protein (Cell Signalling Technology; 9192) and anti-phospho-Ser133-cAMP response element binding protein (Cell Signalling Technology; 9191). Secondary antibodies were from DakoCytomation (Glostrup, Denmark). Band intensity was quantified by Kodak imaging software (Kodak 1D 3.5, USA). Preliminary experiments demonstrated that the amounts of protein loaded were within the dynamic range for the conditions used and the results obtained (data not shown), and that the phosphospecific antibodies were indeed phosphospecific (data not shown; Rose et al. 2006). To measure kinase activity muscle extracts were analysed according to Rose & Hargreaves (2003). In brief, CaMKII activity of muscle extracts was measured in the presence (maximal activity) or absence (autonomous activity) of Ca2+-calmodulin with autocamtide-2 (Upstate Biotech., USA) as the peptide substrate. This assay has been shown to be specific for CaMKII as using a specific inhibitor for CaMKII reduces the majority of phosphotransfer activity by lysate proteins in the presence or absence of calmodulin (Rose & Hargreaves, 2003).
Calculations and statistics
Statistical analyses were performed using SigmaStat v. 3.1 with two-way ANOVA for repeated measures used with Student–Neuman–Kuels post hoc testing. Correlations and regression analyses were performed using SPSS 14.0. Differences were considered to be significant when P was less than 0.05.
Results
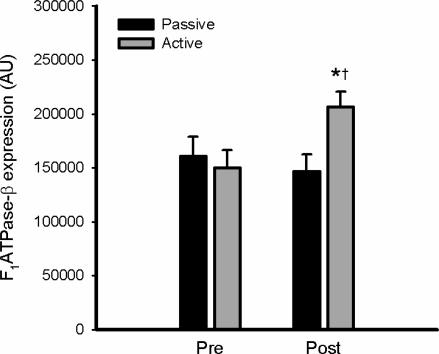
The subject characteristics and general adaptations to training have been reported (Frøsig et al. 2004). In particular, similar to other studies (Dela et al. 1992; Kiens et al. 1993; Kristiansen et al. 2000), this training protocol resulted in significant increases in functional aerobic work capacity (∼16%) and skeletal muscle mitochondrial enzymes (CS: ∼37%; βHAD: ∼35%) as well as increases in hexokinase-II (∼300%) and glucose transporter-4 (∼50%), changes which were restricted to the active leg (Frøsig et al. 2004; Frøsig et al. 2007). In addition, there was also a 42 ± 8% increase in the expression of the β-subunit of the mitochondrial F1-ATP synthase (F1ATPase-β) in skeletal muscle of the active leg after training (Figs 1 and 2).
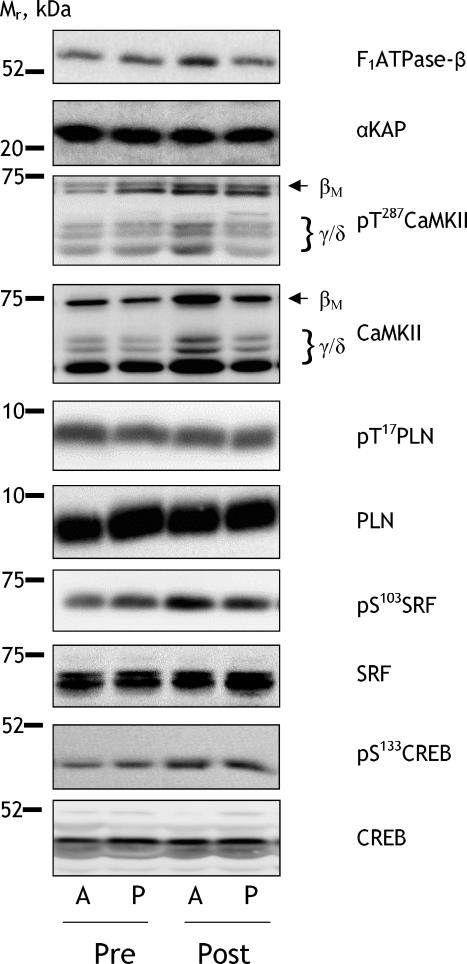
Figure 1. Representative immunoblots.
Skeletal muscle samples from the vastus lateralis muscle of the passive (P) and active (A) leg before (Pre) and after (Post) 3 week of exercise training were extracted and lysates were immunoblotted for total and phosphorylated proteins as described in Methods. Isoforms of CaMKII as well as the relative electrophoretic mobility (Mr) of proteins are indicated. F1ATPase-β: β-subunit of F1-ATP synthase; αKAP: αCaMKII kinase anchoring protein; CaMKII: Ca2+–calmodulin-dependent protein kinase II; PLN: phospholamban; SRF: serum response factor; CREB: cAMP response element binding protein.
Figure 2. Endurance exercise training increases skeletal muscle F1ATPase-β expression.
Skeletal muscle samples from the vastus lateralis muscle of the passive and active leg before (Pre) and after (Post) 3 weeks of exercise training were extracted and lysates were immunoblotted for β-subunit of F1-ATP synthase expression. Data are means ±s.e.m., n = 8; *different from Pre, P < 0.01; †different from Passive, P < 0.01.
Effect of training on CaMKII activity and expression
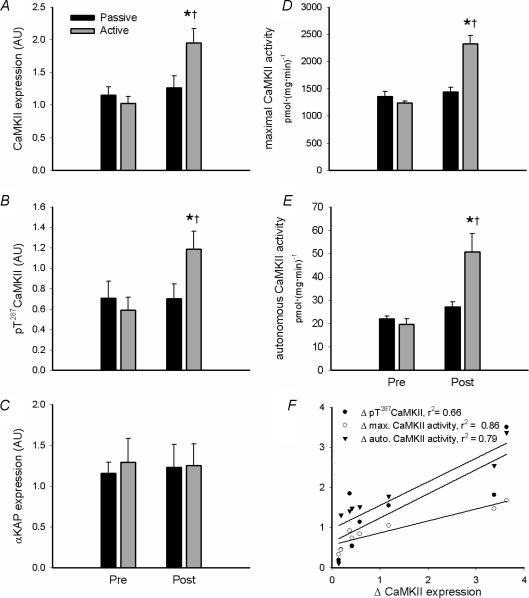
Representative immunoblots are shown in Fig. 1. In response to 3 weeks of training, there was an ∼1-fold increase in the expression of CaMKII kinase isoforms (i.e. βM, γ and δ) but not the α isoform (αKAP; Fig. 3A and C). Similar to CaMKII kinase isoform expression, there was an ∼90% increase in maximal CaMKII activity (Fig. 3D). Furthermore, there was an increase in autonomous (i.e. Ca2+–CaM independent) CaMKII activity and CaMKII phosphorylation at Thr287 (Fig. 3B and E). There were strong positive correlations between the increases in total CaMKII kinase isoform expression and maximal (r2 = 0.86, P < 0.01) and autonomous (r2 = 0.79, P < 0.05) CaMKII activity as well as pT287-CaMKII (r2 = 0.66, P < 0.05) with training (Fig. 3F), indicating that these changes were attributable to the increase in CaMKII expression. Importantly, the changes that were observed were restricted to skeletal muscle of the active leg.
Figure 3. Endurance exercise training increases skeletal muscle CaMKII kinase isoform expression and activity.
Skeletal muscle samples from the vastus lateralis muscle of the passive and active leg before (Pre) and after (Post) 3 weeks of exercise training were extracted and lysates were immunoblotted for Ca2+–calmodulin-dependent protein kinase II expression (CaMKII; A and C) and phospho-Thr287CaMKII (B). Skeletal muscle extracts were assayed in vitro for Ca2+–calmodulin-dependent protein kinase II (CaMKII) activity in the presence (i.e. maximal activity; D) or absence (i.e. autonomous activity; middle panel; E) of Ca2+ and calmodulin. Shown in F are correlations between the changes in maximal CaMKII activity and CaMKII phosphorylation and activities of skeletal muscle of the active leg. Data are means ±s.e.m., n = 8; *different from Pre, P < 0.05; †different from Passive, P < 0.05.
Effect of contraction on putative CaMKII substrates
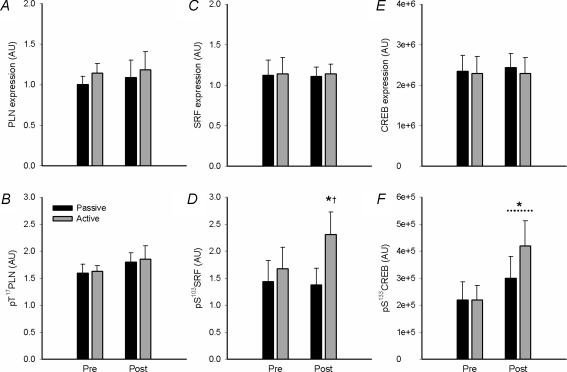
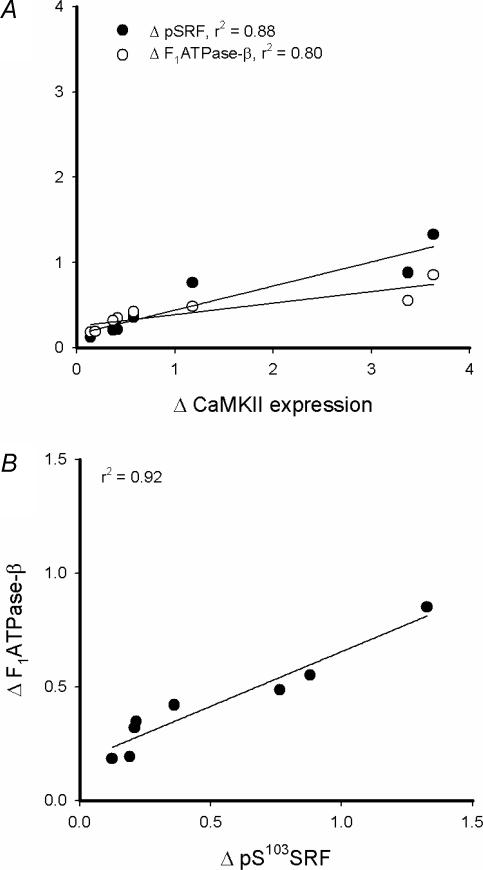
Representative immunoblots are shown in Fig. 1. There were no effects of training on phospholamban expression or phosphorylation at Thr17 (Fig. 4A and B). Serum response factor (SRF) phosphorylation at Ser103 was higher in skeletal muscle of the active leg with training, without changes in SRF expression (Fig. 4C and D). cAMP response element binding protein (CREB) phosphorylation at Ser133 was higher in skeletal muscle of both legs after training, with no changes in CREB expression (Fig. 4E and F). There was a positive correlation between the increases in total CaMKII kinase isoform expression and SRF phosphorylation (r2 = 0.88; P < 0.01) and F1ATPase-β expression (r2 = 0.80; P < 0.05; Fig. 5A), but not CREB phosphorylation (r2 = 0.01; P > 0.5; data not shown). There was a strong positive correlation between the increases in skeletal muscle SRF phosphorylation and F1ATPase-β expression with training (r2 = 0.92, P < 0.01; Fig. 5B). There was no relationship between changes in CREB phosphorylation and F1ATPase-β expression with training (r2 = 0.03; P > 0.5; data not shown).
Figure 4. Effects of endurance-type exercise on phosphorylation and expression of putative skeletal muscle CaMKII substrates.
Skeletal muscle samples from the vastus lateralis muscle of the passive and active leg before (Pre) and after (Post) 3 week of exercise training were extracted and lysates were immunoblotted for phospholamban (PLN) expression (A) and Thr17 phosphorylation (B); serum response factor (SRF) expression (C) and Ser103 phosphorylation (D); and cAMP-response element binding protein (CREB) expression (E) and phospho-Ser133CREB (F). Data are mean ±s.e.m., n = 8; * different from Pre, P < 0.05; † different from Passive, P < 0.05.
Figure 5. Changes in CaMKII kinase isoform expression positively correlate with changes in SRF phosphorylation and F1ATPase-β expression with endurance exercise training.
Shown are correlations between the changes in Ca2+–calmodulin-dependent kinase II (CaMKII) expression and serum response factor (SRF) Ser103 phosphorylation and mitochondrial F1-ATP synthase β-subunit expression of skeletal muscle of the active leg with endurance exercise training (A). Shown in B is a correlation between changes in SRF Ser103 phosphorylation and F1ATPase-β expression.
Discussion
The main finding of this study is that skeletal CaMKII expression and maximal activity (Fig. 3) are increased by short-term endurance training in humans. The increase in maximal CaMKII activity was probably due to higher CaMKII expression as these variables covaried closely (Fig. 3F). Furthermore, autonomous (i.e. Ca2+–CaM independent) CaMKII activity and phosphorylation at Thr287 were increased to a similar extent (Fig. 3). The increases in CaMKII autonomous activity and phosphorylation were also closely related to changes in CaMKII expression indicating that these changes were caused primarily by higher CaMKII expression with training (Fig. 3F). Importantly, it has been previously reported that AMP activated protein kinase (AMPK) expression and activity are also increased in trained muscle at rest (Frøsig et al. 2004). Thus, together with the present data, it is apparent that effects of physical training may not only be evoked during the training periods themselves during which AMPK and CaMKII are activated (Rose et al. 2006; Wojtaszewski et al. 2000), but enhanced AMPK and CaMKII expression/activity in the rest periods between training bouts may also affect muscle gene expression.
The increase in kinase isoform expression and activity of CaMKII was perhaps expected as other studies have shown that regular running in rats (Flück et al. 2000b) and chronic low frequency motor nerve stimulation of rabbits (Antipenko et al. 1999) resulted in increases in CaMKII activity of fast-twitch muscles. Other work from the present laboratory has shown that CaMKIIβM expression was higher in gastrocnemius muscle of mice after 4 weeks of voluntary wheel running (J. T. Treebak, A. J. Rose, M. Hargreaves, J. F. P. Wojtaszewski & E. A. Richter, unpublished observations). On the other hand, studies show that denervation of rat soleus muscle resulted in higher CaMKIIγ expression (Chin, 2004) and skeletal muscle CaMKIIδ expression is higher during muscle regeneration (Abraham & Shaw, 2006). These increases may be a compensatory adaptation to counter muscle atrophy (Chin, 2004). In any case, isoform expression appears to be sensitive to differing levels of muscle activity.
In contrast to the expression of kinase isoforms of CaMKII, there was no difference in α-CaMKII kinase anchoring protein (αKAP) with training (Fig. 3C). αKAP is a truncated non-kinase splice variant of the CaMKIIα isoform expressed in neuronal cells which is expressed at relatively high levels in skeletal muscle (Bayer et al. 1998). αKAP is proposed to anchor CaMKII holoenzymes to surface membranes of intracellular organelles such as nuclei and sarcoplasmic reticulum (SR; Bayer et al. 1998; Nori et al. 2003) and it has been hypothesized that this results in directing CaMKII to its specific substrates in skeletal muscle (Bayer & Schulman, 2001). This suggests that while there may be an increase in the expression of kinase isoforms of CaMKII in the present study, this increase may not be localized to intracellular organelles such as nuclei and SR.
Importantly, the changes that were observed were restricted to the active leg, indicating that the mechanisms behind these increases were attributable to factors arising within the active muscle. Conceptually, the increase in CaMKII expression could be due to either increased synthesis or lower degradation. Using microarray analyses, Mahoney et al. (2005) were not able to detect changes in CaMKII isoform mRNA levels 3 and 48 h after exhaustive endurance exercise. However, mice that over-express the calpain inhibitor calpastatin have 3-fold higher CaMKII expression in gastrocnemius muscle (Otani et al. 2007) indicating that CaMKII expression is at least partially regulated by proteolysis. Clearly, further studies are warranted to examine the mechanisms behind the higher CaMKII kinase isoform expression with exercise training.
Perhaps a more important matter to arise from this study is the functional consequences of higher CaMKII expression and activity in skeletal muscle with endurance exercise training. In particular, the higher CaMKII expression may result in greater sensitivity of Ca2+–CaM signalling through CaMKII substrates and functional effects during exercise as well as at rest. When CaMKII substrates were examined, there was no effect on phospholamban (PLN) expression or phosphorylation at Thr17 (Fig. 4A and B), which is a known CaMKII substrate and is phosphorylated during contractions (Rose et al. 2006). This suggests that the higher CaMKII expression did not result in higher CaMKII signalling towards PLN in vivo. However, it may be that with the higher CaMKII expression there is greater regulation of PLN phosphorylation during exercise, and given that PLN phosphorylation enhances SERCA activity (Simmerman & Jones, 1998) this may result in better Ca2+ homeostasis in trained muscle, as has been observed in rats (Inashima et al. 2003). However, it should be noted that there are no studies that have examined the effects of training on skeletal muscle Ca2+ regulation with exercise in humans or Ca2+ kinetics in isolated muscle fibres. Clearly this is an important area for further work given that endurance exercise training results in better muscle performance (Holloszy & Booth, 1976) which may be related to improved Ca2+ homeostasis in skeletal muscle during exercise (Allen & Westerblad, 2002).
Another important function of CaMKII in skeletal muscle is likely to be regulation of gene expression. Indeed, CaMKII is enriched in skeletal muscle nuclei (Flück et al. 2000a; Nori et al. 2002), and nuclear CaMKII is activated with contractions (Liu et al. 2005). The transcription factor serum response factor (SRF) is a CaMKII substrate (Flück et al. 2000a) and the phosphorylation of SRF, but not expression, was higher in trained muscle (Fig. 4C and D), indicative of higher basal CaMKII signalling to this substrate with training. However, it should be noted that SRF may be phosphorylated by other kinases potentially activated by muscle activity (Heidenreich et al. 1999; Lange et al. 2005). Importantly, while the regulation of SRF activity is complex (Pipes et al. 2006), SRF phosphorylation increases its DNA binding activity (Rivera et al. 1993) and thus may result in higher expression of genes that it activates such as the mitochondrial F1-ATP synthase-β (Nelson et al. 1995) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; Esterbauer et al. 1999), the latter of which may be responsible for coordinated changes in oxidative capacity with endurance training (Baar, 2004). Indeed, similar to SRF phosphorylation, mitochondrial enzymes were up-regulated in the active leg after training (Frøsig et al. 2004), including the β subunit of the F1-ATP synthase (Fig. 2), an expected result since chronic low frequency electrical stimulation of rabbit fast-twitch muscle increases F1ATPase-β mRNA expression (Williams et al. 1987). Indeed, the magnitude of increase in SRF phosphorylation and F1ATPase-β expression after training covaried between subjects (Fig. 5-B). Given that long-term Ca2+-ionophore treatment of muscle cells to raise intracellular Ca2+ can also increase F1ATPase-β expression (Freyssenet et al. 2004), and that the F1ATPase-β gene promoter contains serum response elements (Nelson et al. 1995), this suggests that chronic muscle activity may act via a CaMKII–SRF pathway to increase expression of some nuclear encoded mitochondrial proteins.
The main role for SRF in skeletal muscle is probably growth and maturation of myofibers (Li et al. 2005), and thus perhaps hypertrophy, and skeletal muscle SRF expression has been shown to be up-regulated by load-induced hypertrophy (Flück et al. 2000b). Hence, further studies are warranted to investigate the effect of resistance type exercise on SRF and CaMKII and their roles therein. These studies are important, as CaMKII expression/activity have been shown to be altered in dystrophic skeletal muscle (Damiani et al. 1996; Abraham & Shaw, 2006) and Ca2+–CaM and CaMKII signalling has been proposed to be a potential pathway by which degenerative muscular diseases can be retarded (Abraham & Shaw, 2006; Chakkalakal et al. 2006).
Another substrate of CaMKs (Hook & Means, 2001) which has been hypothesized to be involved in mitochondrial biogenesis with exercise (Akimoto et al. 2005) downstream of Ca2+ signalling (Carrasco & Hidalgo, 2006), namely cAMP response element binding protein (CREB), was also examined. There were no changes in the expression of CREB with training, but there was an increase CREB Ser133 phosphorylation in muscle of the active and inactive leg after training (Fig. 4E and F). CREB phosphorylation at Ser133 increases DNA binding activity and activates the transcription of several genes (Carrusco & Hidalgo, 2006). There are few other studies on skeletal muscle CREB with exercise; however, Widegren et al. (1998) showed that CREB phosphorylation was higher in inactive muscle only after exercise and concluded that this may be due to stimulation via humoral factors. Indeed, CREB phosphorylation/activity can be induced by hormones (Viguerie et al. 2006; Zheng et al. 2004) and metabolites (Hashimoto et al. 2007) that increase with acute exercise and thus the general increase in CREB phosphorylation after training may be mediated by humoral stimuli. In any case, in contrast to other adaptations (Frøsig et al. 2004 and Results) CREB phosphorylation was not restricted to active muscle and did not correlate with changes in CaMKII or F1ATPase-β expression (see Results), which suggests that CREB may not be important for the adaptive process to repeated muscle contraction, at least downstream of CaMKII and AMPK.
In summary, the expression of kinase isoforms CaMKII and SRF phosphorylation in skeletal muscle are higher at rest after endurance-type exercise training. Thus, the effects of physical training may not only be evoked during acute exercise, but the enhanced CaMKII activity in the rest periods between training bouts may also affect skeletal muscle gene expression. Thus, along with other signalling pathways such as MAPK, AMPK and calcineurin and their downstream transcription factors (Hawley et al. 2006; Hood et al. 2006), the increase in CaMKII expression and signalling may contribute to the altered skeletal muscle phenotype with training.
Acknowledgments
Betina Bolmgren is acknowledged for technical assistance. Financial support is acknowledged from the Copenhagen Muscle Research Centre, the Danish Medical and Natural Science Research Councils, the Danish Diabetes Association, the European Union (an Integrated Project, contract number LSHM-CT-2004-005272), as well as the Novo-Nordisk Research and Lundbeck Foundations. A.J.R. was supported by a postdoctoral fellowship from the Carlsberg Foundation and from the European Union. B.K. acknowledges the financial support of the Danish Ministry of Food, Agriculture and Fisheries, as well as the Danish Ministry of Family and Consumer Affairs. J.F.P.W. was supported by a Hallas Møller fellowship from the Novo Nordisk Foundation.
References
- Abraham ST, Shaw C. Increased expression of δCaMKII isoforms in skeletal muscle regeneration: Implications in dystrophic muscle disease. J Cell Biochem. 2006;97:621–632. doi: 10.1002/jcb.20669. [DOI] [PubMed] [Google Scholar]
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- Allen DL, Leinwand LA. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulate preferential activation of the IIa myosin heavy chain promoter. J Biol Chem. 2002;277:45323–45330. doi: 10.1074/jbc.M208302200. [DOI] [PubMed] [Google Scholar]
- Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2002;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antipenko A, Frias JA, Parra J, Cadefau JA, Cusso R. Effect of chronic electrostimulation of rabbit skeletal muscle on calmodulin level and protein kinase activity. Int J Biochem Cell Biol. 1999;31:303–310. doi: 10.1016/s1357-2725(98)00112-5. [DOI] [PubMed] [Google Scholar]
- Baar K. Involvement of PPARγ co-activator-1, nuclear respiratory factors 1 and 2, and PPARα in the adaptive response to endurance exercise. Proc Nutr Soc. 2004;63:269–273. doi: 10.1079/PNS2004334. [DOI] [PubMed] [Google Scholar]
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- Bayer KU, Harbers K, Schulman H. αKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle. EMBO J. 1998;17:5598–5605. doi: 10.1093/emboj/17.19.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, Schulman H. Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem Biophys Res Commun. 2001;289:917–923. doi: 10.1006/bbrc.2001.6063. [DOI] [PubMed] [Google Scholar]
- Booth FW, Nicholson WF, Watson PA. Influence of muscle use on protein synthesis and degradation. Exerc Sport Sci Rev. 1982;10:27–48. [PubMed] [Google Scholar]
- Carrasco MA, Hidalgo C. Calcium microdomains and gene expression in neurons and skeletal muscle cells. Cell Calcium. 2006;40:575–583. doi: 10.1016/j.ceca.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Michel SA, Chin ER, Michel RN, Jasmin BJ. Targeted inhibition of Ca2+/calmodulin signaling exacerbates the dystrophic phenotype in mdx mouse muscle. Hum Mol Genet. 2006;15:1423–1435. doi: 10.1093/hmg/ddl065. [DOI] [PubMed] [Google Scholar]
- Chin ER. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc Nutr Soc. 2004;63:279–286. doi: 10.1079/PNS2004335. [DOI] [PubMed] [Google Scholar]
- Chin ER. Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol. 2005;99:414–423. doi: 10.1152/japplphysiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- Chin ER, Grange RW, Viau F, Simard AR, Humphries C, Shelton J, Bassel-Duby R, Williams RS, Michel RN. Alterations in slow-twitch muscle phenotype in transgenic mice overexpressing the Ca2+ buffering protein parvalbumin. J Physiol. 2003;547:649–663. doi: 10.1113/jphysiol.2002.024760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Angelini C, Pelosi M, Sacchetto R, Bortoloso E, Margreth A. Skeletal muscle sarcoplasmic reticulum phenotype in myotonic dystrophy. Neuromuscul Disord. 1996;6:33–47. doi: 10.1016/0960-8966(95)00016-x. [DOI] [PubMed] [Google Scholar]
- Damiani E, Sacchetto R, Margreth A. Variation of phospholamban in slow-twitch muscle sarcoplasmic reticulum between mammalian species and a link to the substrate specificity of endogenous Ca2+-calmodulin-dependent protein kinase. Biochim Biophys Acta. 2000;1464:231–241. doi: 10.1016/s0005-2736(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Dela F, Mikines KJ, von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol Endocrinol Metab. 1992;263:E1134–E1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Oberkofler H, Krempler F, Patsch W. Human peroxisome proliferator activated receptor γ coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics. 1999;62:98–102. doi: 10.1006/geno.1999.5977. [DOI] [PubMed] [Google Scholar]
- Flück M, Booth FW, Waxham MN. Skeletal muscle CaMKII enriches in nuclei and phosphorylates myogenic factor SRF at multiple sites. Biochem Biophys Res Commun. 2000a;270:488–494. doi: 10.1006/bbrc.2000.2457. [DOI] [PubMed] [Google Scholar]
- Flück M, Waxham MN, Hamilton MT, Booth FW. Skeletal muscle Ca2+-independent kinase activity increases during either hypertrophy or running. J Appl Physiol. 2000b;88:352–358. doi: 10.1152/jappl.2000.88.1.352. [DOI] [PubMed] [Google Scholar]
- Freyssenet D, Irrcher I, Connor MK, Di Carlo M, Hood DA. Calcium-regulated changes in mitochondrial phenotype in skeletal muscle cells. Am J Physiol Cell Physiol. 2004;286:C1053–C1061. doi: 10.1152/ajpcell.00418.2003. [DOI] [PubMed] [Google Scholar]
- Frøsig C, Jørgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Frøsig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JFP. Effects of endurance exercise training on insulin signalling in human skeletal muscle – Interactions at the level of PI3-K, Akt and AS160. Diabetes. 2007. in press. [DOI] [PubMed]
- Garcia-Roves PM, Huss J, Holloszy JO. Role of calcineurin in exercise-induced mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2006;290:E1172–E1179. doi: 10.1152/ajpendo.00633.2005. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halseth AE, O'Doherty RM, Printz RL, Bracy DP, Granner DK, Wasserman DH. Role of Ca2+ fluctuations in L6 myotubes in the regulation of the hexokinase II gene. J Appl Physiol. 2000;88:669–673. doi: 10.1152/jappl.2000.88.2.669. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007 doi: 10.1096/fj.07-8174com. in press. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Zierath JR. Signalling mechanisms in skeletal muscle: role in substrate selection and muscle adaptation. Essays Biochem. 2006;42:1–12. doi: 10.1042/bse0420001. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Houmard JA. Preventing insulin resistance through exercise: a cellular approach. Med Sci Sports Exerc. 2004;36:1187–1190. doi: 10.1249/01.mss.0000132382.95142.71. [DOI] [PubMed] [Google Scholar]
- Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill MA, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, Nordheim A. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol. 2006;209:2265–2275. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- Hook SS, Means AR. Ca2+/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- Huang YC, Dennis RG, Baar K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am J Physiol Cell Physiol. 2006;291:C11–C17. doi: 10.1152/ajpcell.00366.2005. [DOI] [PubMed] [Google Scholar]
- Inashima S, Matsunaga S, Yasuda T, Wada M. Different time course of changes in sarcoplasmic reticulum and myosin isoforms in rat soleus muscle at early stage of hyperthyroidism. Acta Physiol Scand. 2003;180:79–87. doi: 10.1046/j.0001-6772.2003.01220.x. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Hood DA. Regulation of Egr-1, SRF, and Sp1 mRNA expression in contracting skeletal muscle cells. J Appl Physiol. 2004;97:2207–2213. doi: 10.1152/japplphysiol.00388.2004. [DOI] [PubMed] [Google Scholar]
- Jørgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of α2-AMPK in basal, training- and AICAR-induced GLUT4, hexokinase II and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Jørgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Juretić N, Urzúa U, Munroe DJ, Jaimovich E, Riveros N. Differential gene expression in skeletal muscle cells after membrane depolarization. J Cell Physiol. 2007;210:819–830. doi: 10.1002/jcp.20902. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen S, Gade J, Wojtaszewski JF, Kiens B, Richter EA. Glucose uptake is increased in trained vs. untrained muscle during heavy exercise. J Appl Physiol. 2000;89:1151–1158. doi: 10.1152/jappl.2000.89.3.1151. [DOI] [PubMed] [Google Scholar]
- Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- Lewis SE, Anderson P, Goldspink DF. The effects of calcium on protein turnover in skeletal muscles of the rat. Biochem J. 1982;204:257–264. doi: 10.1042/bj2040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, Nordheim A, Olson EN. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci U S A. 2005;102:1082–1087. doi: 10.1073/pnas.0409103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19:1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- McGee SL, Sparling D, Olson AL, Hargreaves M. Exercise increases MEF2- and GEF DNA-binding activity in human skeletal muscle. FASEB J. 2006;20:348–349. doi: 10.1096/fj.05-4671fje. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000a;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000b;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Luciakova K, Li R, Betina S. The role of thyroid hormone and promoter diversity in the regulation of nuclear encoded mitochondrial proteins. Biochim Biophys Acta. 1995;1271:85–91. doi: 10.1016/0925-4439(95)00014-u. [DOI] [PubMed] [Google Scholar]
- Nori A, Lin PJ, Cassetti A, Villa A, Bayer KU, Volpe P. Targeting of α-kinase-anchoring protein (αKAP) to sarcoplasmic reticulum and nuclei of skeletal muscle. Biochem J. 2003;370:873–880. doi: 10.1042/BJ20021624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J. 2003;17:675–681. doi: 10.1096/fj.02-0951com. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ Am J Physiol Endocrinol Metab. 2002;282:E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- Otani K, Polonsky KS, Holloszy JO, Han DH. Inhibition of calpain results in impaired contraction-stimulated GLUT4 translocation in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;291:E544–E548. doi: 10.1152/ajpendo.00510.2005. [DOI] [PubMed] [Google Scholar]
- Parsons SA, Millay DP, Wilkins BJ, Bueno OF, Tsika GL, Neilson JR, Liberatore CM, Yutzey KE, Crabtree GR, Tsika RW, Molkentin JD. Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J Biol Chem. 2004;279:26192–26200. doi: 10.1074/jbc.M313800200. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- Racay P, Gregory P, Schwaller B. Parvalbumin deficiency in fast-twitch muscles leads to increased ‘slow-twitch type’ mitochondria, but does not affect the expression of fiber specific proteins. FEBS J. 2006;273:96–108. doi: 10.1111/j.1742-4658.2005.05046.x. [DOI] [PubMed] [Google Scholar]
- Richter EA, Cleland PJ, Rattigan S, Clark MG. Contraction-associated translocation of protein kinase C in rat skeletal muscle. FEBS Lett. 1987;217:232–236. doi: 10.1016/0014-5793(87)80669-5. [DOI] [PubMed] [Google Scholar]
- Rivera VM, Miranti CK, Misra RP, Ginty DD, Chen RH, Blenis J, Greenberg ME. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol Cell Biol. 1993;13:6260–6273. doi: 10.1128/mcb.13.10.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303–309. doi: 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Kiens B, Richter EA. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J Physiol. 2006;574:889–903. doi: 10.1113/jphysiol.2006.111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002;23:569–575. doi: 10.1016/s0165-6147(02)02111-9. [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- Smith JA, Collins M, Grobler LA, Magee CJ, Ojuka EO. Exercise and CaMK activation both increase the binding of MEF2A to the Glut4 promoter in skeletal muscle in vivo. Am J Physiol Endocrinol Metab. 2007;292:E413–420. doi: 10.1152/ajpendo.00142.2006. [DOI] [PubMed] [Google Scholar]
- Song Q, Young KB, Chu G, Gulick J, Gerst M, Grupp IL, Robbins J, Kranias EG. Overexpression of phospholamban in slow-twitch skeletal muscle is associated with depressed contractile function and muscle remodeling. FASEB J. 2004;18:974–976. doi: 10.1096/fj.03-1058fje. [DOI] [PubMed] [Google Scholar]
- Tang H, Macpherson P, Argetsinger LS, Cieslak D, Suhr ST, Carter-Su C, Goldman D. CaM kinase II-dependent phosphorylation of myogenin contributes to activity-dependent suppression of nAChR gene expression in developing rat myotubes. Cell Signal. 2004;16:551–563. doi: 10.1016/j.cellsig.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Tothova J, Blaauw B, Pallfacchina G, Rudolf R, Argentini C, Reggani C, Schiaffino S. NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. J Cell Sci. 2006;119:1604–1611. doi: 10.1242/jcs.02875. [DOI] [PubMed] [Google Scholar]
- Viguerie N, Clement K, Barbe P, Courtine M, Benis A, Larrouy D, Hanczar B, Pelloux V, Poitou C, Khalfallah Y, Barsh GS, Thalamas C, Zucker JD, Langin D. In vivo epinephrine-mediated regulation of gene expression in human skeletal muscle. J Clin Endocrinol Metab. 2006;89:2000–2014. doi: 10.1210/jc.2003-031733. [DOI] [PubMed] [Google Scholar]
- Widegren U, Jiang XJ, Krook A, Chibalin AV, Björnholm M, Tally M, Roth RA, Henriksson J, Wallberg-Henriksson H, Zierath JR. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB J. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- Williams RS, Garcia-Moll M, Mellor J, Salmons S, Harlan W. Adaptation of skeletal muscle to increased contractile activity. Expression nuclear genes encoding mitochondrial proteins. J Biol Chem. 1987;262:2764–2767. [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Wang ZM, Delbono O. Ca2+ calmodulin kinase and calcineurin mediate IGF-1-induced skeletal muscle dihydropyridine receptor α1S transcription. J Membr Biol. 2004;197:101–112. doi: 10.1007/s00232-003-0645-8. [DOI] [PubMed] [Google Scholar]