Abstract
Interstitial cells of Cajal-like cells (ICC-LCs) in the urethra may act as electrical pacemakers of spontaneous contractions. However, their properties in situ and their interaction with neighbouring urethral smooth muscle cells (USMCs) remain to be elucidated. To further explore the physiological role of ICC-LCs, spontaneous changes in [Ca2+]i (Ca2+ transients) were visualized in fluo-4 loaded preparations of rabbit urethral smooth muscle. ICC-LCs were sparsely distributed, rather than forming an extensive network. Ca2+ transients in ICC-LCs had a lower frequency and a longer half-width than those of USMCs. ICC-LCs often exhibited Ca2+ transients synchronously with each other, but did not often show a close temporal relationship with Ca2+ transients in USMCs. Nicardipine (1 μm) suppressed Ca2+ transients in USMCs but not in ICC-LCs. Ca2+ transients in ICC-LCs were abolished by cyclopiazonic acid (10 μm), ryanodine (50 μm) and caffeine (10 mm) or by removing extracellular Ca2+, and inhibited by 2-aminoethoxydiphenyl borate (50 μm) and 3-morpholino-sydnonimine (SIN-1; 10 μm), but facilitated by increasing extracellular Ca2+ or phenylephrine (1–10 μm). These results indicated that Ca2+ transients in urethral ICC-LCs in situ rely on both Ca2+ release from intracellular Ca2+ stores and Ca2+ influx through non-L-type Ca2+ channel pathways. ICC-LCs may not act as a coordinated pacemaker electrical network as do ICC in the gastrointestinal (GI) tract. Rather they may randomly increase excitability of USMCs to maintain the tone of urethral smooth muscles.
Smooth muscles in the urethra generate spontaneous contractions, which are tonically augmented by neurally released noradrenaline (NAd) through the activation of α1-adrenoceptors, to maintain a sustained tone (Andersson & Wein, 2004). Underlying these contractions is spontaneous electrical activity, termed spontaneous transient depolarizations (STDs) and slow waves (Hashitani et al. 1996; Hashitani & Edwards, 1999). STDs are initiated by mean of the spontaneous release of Ca2+ from intracellular stores, which activates Ca2+-activated chloride channels. Summed STDs result in larger depolarizations which activate L-type Ca2+ channels to compose the plateau phase of slow waves and contract smooth muscles (Hashitani et al. 1996; Hashitani & Edwards, 1999).
Although this spontaneous activity was originally assumed to be generated within USMCs themselves, extensive research using isolated cells taken from the urethra has now revealed that this ‘myogenic’ activity may originate from ICC-LCs. Isolated ICC-LCs but not USMCs exhibit spontaneous Ca2+ oscillations and spontaneous transient inward currents (STICs), which depend on Ca2+ release from intracellular Ca2+ stores and the subsequent activation of Ca2+-activated chloride channels, respectively (Sergeant et al. 2000, 2001; Johnston et al. 2005). Therefore, ICC-LCs may be responsible for the initiation and propagation of electrical activity recorded from intact tissue preparations of the urethra, and act as electrical pacemaker cells as do ICC located in the myenteric region (ICC-MY) of the GI tract (Dickens et al. 1999; Sanders et al. 2006).
The frequencies of STDs and of STICs recorded in isolated urethral ICC-LCs are increased by bath-applied NAd (Sergeant et al. 2000, 2002). Moreover, spontaneous Ca2+ transients recorded from isolated ICC-LCs are diminished by either nitric oxide (NO) or cyclic GMP (Sergeant et al. 2006b). This is consistent with the effects of NAd and sodium nitroprusside, an NO donor, on the frequency of slow waves recorded in the intact circular smooth muscles of the rabbit urethra (Hashitani et al. 1996), suggesting that ICC-LCs may also play an important role in the neurally mediated regulation of spontaneous excitation as do intramuscular ICC (ICC-im) in the GI tract (Ward et al. 2000; Suzuki et al. 2003). This hypothesis was further supported by a recent report showing frequent points of contact between Kit-positive ICC-LCs and nerves, particularly nitrergic nerves (Lyons et al. 2007).
Since the primary step of spontaneous activity in the urethra is Ca2+ release from intracellular stores in ICC-LCs, blockade of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) with cyclopiazonic acid (CPA) would be expected to suppress urethral smooth muscle contractions. However, CPA, which has been shown to abolish STICs in isolated ICC-LCs (Sergeant et al. 2001), increased the amplitude and duration of spontaneous contractions in a majority of preparations of rabbit urethra (Hashitani et al. 2006). Similar heterogeneity was observed for the effects of CPA on slow waves or spontaneous Ca2+ transients in the rabbit urethra. Thus, it is important to know if CPA effectively prevents spontaneous activity in urethral ICC-LCs in situ, and thus if ICC-LCs may be able to generate pacemaking activity via Ca2+ store-independent mechanisms.
The mechanical characteristics of the urethral smooth muscles, which display sustained tone (Bridgewater et al. 1993), are clearly different from those of GI smooth muscles, which generate phasic contractions for peristalsis. Therefore, even though ICC-LCs in the urethra may act as primary pacemaker cells, as do ICC in the GI tract, either the initiation or propagation of spontaneous activity in the urethra may not be similar to that in the GI tract where highly coordinated oscillators, i.e. ICC-MY and ICC-IM, drive the bulk of the smooth muscles within the wall.
The aim of the present study was to visualize spontaneous Ca2+ transients in ICC-LCs of the rabbit urethra in situ to compare their properties with those of USMCs in situ and also with previously reported characteristics of isolated ICC-LCs. We also investigated the mechanisms underlying the initiation and propagation of the spontaneous Ca2+ transients in the urethra, focusing particularly on the interactions between ICC-LCs and USMCs.
Methods
Tissue preparation
Male rabbits, weighing 2–3 kg, were killed by exsanguinations under pentobarbitone anaesthesia. This procedure has been approved by the animal experimentation ethics committee of the Physiological Society of Japan. The urethra and bladder were removed, and the urethra was dissected free of the bladder approximately 3 cm distal of the bilateral ureter entry. The dorsal wall of the urethra was then opened longitudinally and the mucosa and periurethral connective tissues were dissected away. The outer striated muscle and longitudinal smooth muscle were then carefully removed leaving the circular muscle layers intact. Since the division into circular and longitudinal smooth muscle layers is not as clear as in the GI tract wall, circular muscle sections lying close to the submucosal border were used for experiments.
Immunohistochemistry
To identify cells expressing Kit immunoreactivity, preparations which contained several muscle bundles were incubated for 1 h in nominally Ca2+ free physiological salt solution (PSS) containing rat monoclonal antibodies raised against the Kit protein (ACK-2, diluted 1: 100, Cymbus Biotechnology Ltd, Hampshire, UK). The tissue was washed and then incubated for another 1 h in anti-rat IgG antibody labelled with a fluorescent marker (IgG-Alexa Fluor 488, diluted 1: 250, Molecular Probes, OR, USA). After washing with PSS, preparations were observed using an inverted fluorescence microscope (IX70, Olympus) equipped with an electron multiplier CCD camera (C9100, Hamamatsu Photonics). Kit-positive ICC-LCs were also viewed under Nomarski optics. On some occasions, preparations which had been incubated for ACK-2 with IgG-Alexa Fluor 488, were subsequently loaded with fura-2 AM (10 μm; Molecular Probes) as previously described (Hashitani et al. 2006). Preparations, loaded with fura-2, were illuminated with ultraviolet light (wavelengths 340 and 380 nm) and the emission fluorescence was measured through a barrier filter (peak transmission 510 nm), using a micro photoluminescence measurement system (Aquacosmos, Hamamatsu Photonics).
Intracellular calcium measurements
To visualize changes in the concentration of intracellular calcium ([Ca2+]i; Ca2+ transients) recorded from USMCs and ICC-LCs, different loading conditions, i.e. ‘normal’ and ‘light’ loadings, respectively, were applied. For visualizing Ca2+ transients in circular USMCs, preparations were pinned out on a Sylgard plate (silicone elastomer, Dow Corning Corp., Midland, MI, USA) which had a window of some 1.5 mm × 3 mm in the centre. To minimize tissue distortion due to smooth muscle contractions, preparations were stretched radially using 15–20 L-shaped tungsten wires (20 μm in diameter). After 30 min incubation with warmed (36°C) PSS, spontaneous muscle contractions were visually detected, and preparations were then incubated in low-Ca2+ PSS ([Ca2+]o= 0.1 mm) containing 3–5 μm fluo-4 AM (FluoroPure™ grade special packaging, Molecular Probes) and cremophor EL (0.01%, Sigma) for 45–60 min at room temperature (‘normal’ loading). Following incubation, the preparations were superfused with dye-free, warmed (36°C) PSS at a constant flow rate (about 2 ml min−1) for 30 min
To visualize Ca2+ signals in ICC-LCs of the rabbit urethra in situ, preparations were incubated in low Ca2+ physiological salt solution (PSS) ([Ca2+]o= 0.1 mm) containing fluo-4 AM (0.1–1 μm) and cremophor EL (0.01%) for 15–30 min at 36°C (‘light’ loading). Although USMCs' Ca2+ signals were hardly detected in this loading condition, increasing [Ca2+]o from 0.1 mm to 0.5 mm enhanced USMCs' Ca2+ signals to a measurable level, and thus allowed the investigation of temporal relationships of Ca2+ signals between ICC-LCs and USMCs. Following incubation, the preparations were superfused with dye-free, warmed (36°C) PSS at a constant flow (about 2 ml min−1) for 30 min.
The recording chamber was mounted on the stage of an inverted fluorescence microscope (IX70, Olympus) equipped with an electron multiplier CCD camera (C9100, Hamamatsu Photonics) and a high speed scanning polychromatic light source (C7773, Hamamatsu Photonics). Preparations were viewed under either a water-immersion objective (UPlanApo 60, Olympus) or an air objective (UPlanApo 20, Olympus) and illuminated at 495 nm. For the ×60 objective, the Sylgard plate was turned over and then placed at the bottom of the recording chamber so that the preparation now faced the glass bottom of the chamber. The fluorescence emission in a variable sized rectangular window was measured through a barrier filter above 515 nm, and images were obtained every 35–200 ms (frame interval) with an exposure time of 17.4–58.7 ms using a micro-photoluminescence measurement system (Aquacosmos, Hamamatsu Photonics). Relative changes in [Ca2+]i were expressed as the ratio (F/F0) of the fluorescence generated by an event (F) against baseline (F0).
Isometric tension recordings
To detect changes in muscle tension and [Ca2+]i in USMCs simultaneously, one end of the preparations was pinned out on a Sylgard plate, and the other end was tied by a nylon thread which connected to a force transducer. Isometric tension changes were digitized using a Digidata 1200 interface and stored on a personal computer for later analysis.
Solutions and drugs
The ionic composition of PSS was as follows (mm): NaCl, 119; KCl, 5.0; CaCl2, 2.5; MgCl2, 2.0; NaHCO3, 25.0; NaH2PO4, 1.0; and glucose, 11.0. The solution was bubbled with 95% O2 and 5% CO2 to maintain pH in the recording bath at approximately 7.4. High Ca2+ solution or nominally Ca2+ free solution was prepared by either increasing or omitting CaCl2 from the composition of PSS, respectively.
Drugs used were 3-morpholino-sydnonimine (SIN-1) hydrochloride, 2-aminoethoxydiphenyl borate (2-APB), caffeine, cyclopiazonic acid (CPA), nicardipine, phenylephrine hydrochloride and ryanodine (all from Sigma, St Louis, MO, USA). These drugs were dissolved in distilled water except CPA, nicardipine, 2-APB and ryanodine, which were dissolved in dimethyl sulphoxide (DMSO). Caffeine was directly dissolved in PSS to obtain its final concentration. The final concentration of these solvents in physiological saline did not exceed 1: 1000.
Calculations and statistics
Measured values are expressed as means ± standard deviation. Statistical significance was tested using Student's t test, and probabilities of less than 5% were considered significant. The synchronicity of Ca2+ signals between ICC-LC and either ICC-LC or USMC were analysed using the cross-correlation function of Clampfit 10 software (Axon Instruments–Molecular Devices, Union City, CA, USA).
Results
Identification of ICC-LCs in situ in the rabbit urethra
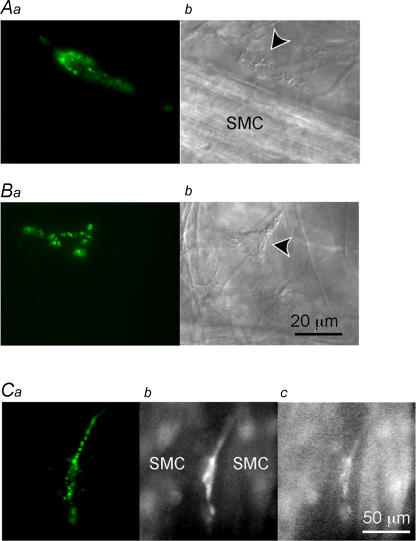
Consistent with recent reports (McHale et al. 2006; Lyons et al. 2007), Kit-positive cells which we have designated as ICC-LCs, were sparsely distributed in the rabbit urethral preparations, being situated predominately within the connective tissue between the smooth muscle bundles. ICC-LCs were also scattered amongst the smooth muscle cells within muscle bundles. ICC-LCs had either spindle-shaped cell bodies, some 60–100 μm in length and less than 10 μm in width (Fig. 1Aa), or stellate-shaped cell bodies with a few processes (Fig. 1Ba). The general morphology of ICC-LCs which had been identified by their Kit immunoreactivity was also visualized using Nomarski optics (Fig. 1Ab and Bb).
Figure 1. Identification of ICC-LCs in the rabbit urethra Panels.
a show fluorescent images of ICC-LCs in the rabbit urethra stained using ACK2 antibody against Kit labelled with Alexa 488. Panels b show micrographs of preparations viewed with Nomarski optics. A, ICC-LC (arrow head) which had a spindle-shaped cell body is shown lying in parallel with a muscle bundle (SMC). B, another ICC-LC having a stellate-shaped cell body is shown located in the connective tissue between the muscle bundles. C, in a different preparation, which had been loaded with fura-2, ICC-LCs identified by immunoreactivity against Kit (a) had higher F340 fluorescence than neighbouring smooth muscle cells (SMC, b), whilst having similar F380 fluorescence (c).
In preparations which had been loaded with Kit antibody and fura-2, ICC-LCs identified by their immunoreactivity for Kit (Fig. 1Ca) generally had a higher F340 fluorescence than that of USMCs (Fig. 1Cb), whilst having similar F380 fluorescence to that of USMCs (Fig. 1Cc). ICC-LCs had higher basal fluorescence in either fura-2 or fluo-4 loaded preparations, which were not stained with Kit antibody suggesting that the Kit antibody little affected ICC-LCs viability.
For the following functional studies, ICC-LCs were identified by their high basal fluorescence, general morphology, location and slower Ca2+ signals (see details below). Therefore, we were not able to tell whether or not all ICC-LCs were Kit-positive, and thus could not exclude the possibility that we have investigated heterogeneous populations of cells.
Spontaneous Ca2+ transients recorded from USMCs of the rabbit urethra
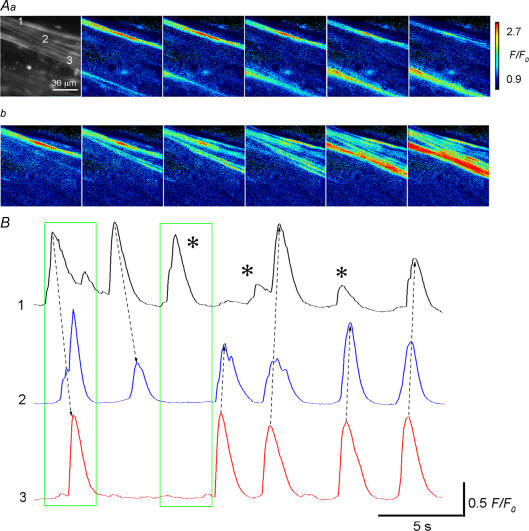
Under ‘normal’ fluo-4 loading conditions, USMCs generated spontaneous Ca2+ transients at a frequency of 10.8 ± 4.3 min−1 (n = 24). USMC Ca2+ transients had an amplitude of 0.36 ± 0.12 F/F0 and a half-amplitude duration (half-width) of 0.69 ± 0.23 s (n = 24). These values of the frequency and half-width were similar to those of fura-2 loaded urethra preparations (Hashitani et al. 2006). USMC Ca2+ transients occurred either as non-propagated Ca2+ transients (Fig. 2Aa) or intercellular Ca2+ waves within a muscle bundle (Fig. 2Ab). Unlike intercellular Ca2+ waves in detrusor smooth muscle bundles of the guinea-pig bladder (Hashitani et al. 2001), the Ca2+ waves originated from a single site often failed to spread across muscle bundles (Fig. 2B).
Figure 2. Spontaneous Ca2+ transients recorded from USMCs in the rabbit urethra.
Aa, a series of frames at intervals of 0.1 s demonstrating two non-propagating Ca2+ transients generated by USMCs within a muscle bundle. b, another series of frames at intervals of 0.1 s demonstrate an intercellular Ca2+ wave within the same smooth muscle bundle. B, Ca2+ transients initiated in USMC (1) sometimes spread across a muscle bundle to trigger Ca2+ transients in USMC (3) and vice versa. On other occasions the Ca2+ wave stopped at USMC (2) or did not propagate at all (asterisks). Numbers for traces in B correspond to those in Aa. The first and second frames correspond to images in Ab and Aa, respectively. Dotted arrows indicate the direction of Ca2+ wave propagation.
To investigate the correlation between spontaneous USMC Ca2+ transients and muscle contractions, changes in muscle tension were simultaneously recorded with [Ca2+]i. Unloaded urethral preparations generated spontaneous contractions 14.3 ± 3.2 min−1 (n = 6). After ‘normal’ fluo-4 loading, the preparations exhibited spontaneous contractions 13.7 ± 2.8 min−1, and these values were not significantly different from control values (P > 0.4, n = 6), indicating that ‘normal’ fluo-4 loading did not disrupt USMC activity. Although the frequency of spontaneous contractions were similar to those of USMC Ca2+ transients, there was no correlation between muscle contractions and Ca2+ transients in any particular muscle bundle within the preparations, presumably arising from a low synchronicity between bundles.
After ‘normal’ loading conditions ICC-LCs were readily identified by their high basal fluorescence intensity (see Fig. 1C) and seen either to be separately distributed or to form linear connections with a few neighbouring ICC-LCs. Under these conditions ICC-LCs seldom displayed spontaneous Ca2+ transients.
Spontaneous Ca2+ transients recorded from ICC-LCs of the rabbit urethra
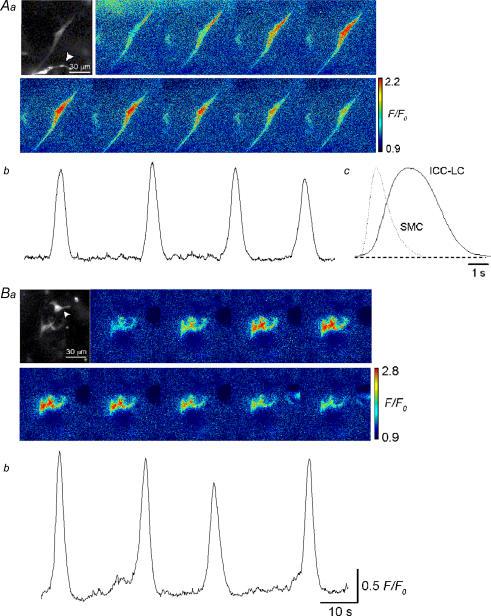
To visualize Ca2+ transients in ICC-LCs more consistently, the ‘light’ loading of the fluo-4 protocol was used. Both spindle- (Fig. 3Aa) and stellate-shaped (Fig. 3Ba) ICC-LCs generated spontaneous Ca2+ transients. Spontaneous Ca2+ transients recorded from spindle-shaped ICC-LCs occurred at a rate of 0.7–9 min−1 (3.1 ± 1.6 min−1, n = 39) and an amplitude of 0.75 ± 0.31 F/F0 (n = 39, Fig. 3 Ab). Their half-width ranged between 1.9 and 12.6 s (mean 3.9 ± 1.8 s, n = 39, Fig. 3Ca), significantly longer than the half-widths of Ca2+ transients in USMCs (0.69 ± 0.23 s, n = 24, P < 0.05, Fig. 3Cb), which were measured under ‘normal’ loading condition. Stellate-shaped ICC-LCs generated spontaneous Ca2+ transients at a rate of 0.96–7.5 min−1 (3.2 ± 1.8 min−1, n = 24), which had an amplitude of 0.79 ± 0.35 F/F0 (n = 24, Fig. 3Bb) and half-width ranging between 1.9 and 6.1 s (mean 3.7 ± 1.3 s, n = 24). These values were not significantly different from those of Ca2+ transients in spindle-shaped ICC-LCs (P > 0.2).
Figure 3. Spontaneous Ca2+ transients recorded from ICC-LCs in situ in the rabbit urethra.
Aa, a series of frames at interval 1 s demonstrating Ca2+ transients recorded from a spindle-shaped ICC-LC. b, the ICC-LC generated four spontaneous Ca2+ transients over 90 s. c, averaged Ca2+ transients recorded from ICC-LCs had much longer durations than those of the overlaid averaged USMC Ca2+ transients. Ba, another series of frames at intervals of 1 s demonstrate Ca2+ transients recorded from a pair of stellate-shaped ICC-LCs. b, in the same preparation, ICC-LCs generated four spontaneous Ca2+ transients over 90 s. Note that prominently bright ICC-LCs (arrowheads in Aa and Ba) did not exhibit any Ca2+ signals.
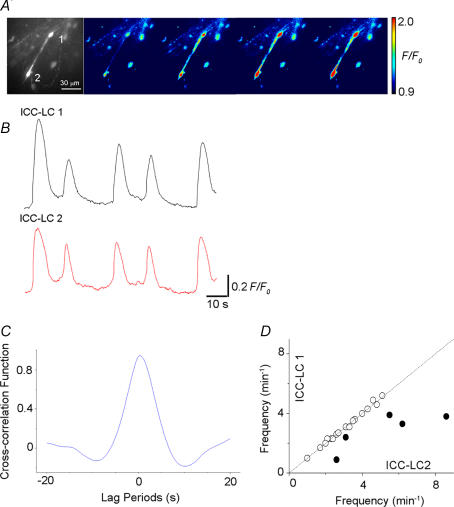
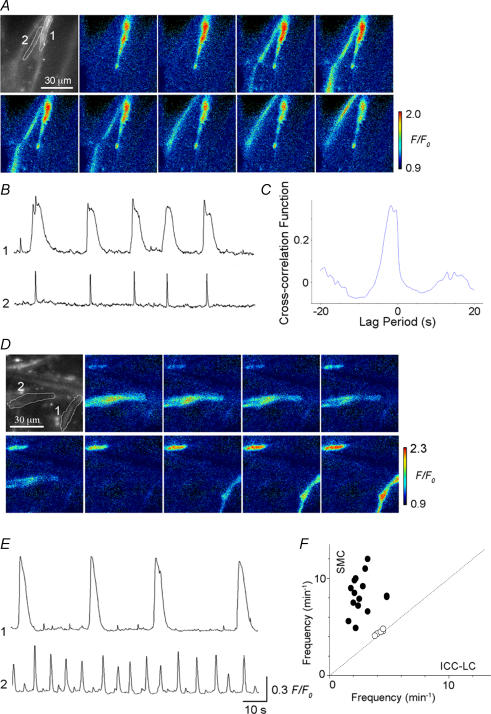
In 22 preparations where more than two ICC-LCs generated Ca2+ transients within a field of view (135 μm × 135 μm, ×60 objective), the temporal relationship between ICC-LCs was examined. In 17 out of 22 preparations, pairs of ICC-LCs exhibited synchronous Ca2+ transients (Fig. 4A, B and D). Figure 4C illustrates the cross-correlogram for one of these pairs of ICC-LCs generating synchronous Ca2+ transients, clearly demonstrating a peak near lag period zero and therefore a close temporal correlation (Fig. 4C). In the remaining five preparations, no temporal relationship was observed between pairs of ICC-LCs (Fig. 4D).
Figure 4. Analysis of the temporal relationship of Ca2+ transients between a pair of ICC-LCs.
A, a series of frames at intervals of 1 s demonstrating a pair of ICC-LCs (1 and 2) generating spontaneous Ca2+ transients. B, in the same preparation synchronous Ca2+ transients were generated by a pair of ICC-LCs. C, a cross-correlogram for this pair of ICC-LCs had a peak near lag period zero. D, a comparison of mean frequency between pairs of ICC-LCs showed a close temporal correlation between ICC-LCs for 17 of the 22 pairs of ICC-LCs investigated (○) but not for the remaining five pairs of ICC-LCs (•).
Interaction between ICC-LCs and USMCs in the rabbit urethra
In 21 preparations, spontaneous Ca2+ transients in ICC-LCs were observed simultaneously with those of USMCs within a field of view (×60 objective). Under ‘light’ loading conditions, USMCs generated spontaneous Ca2+ transients at a frequency of 8.7 ± 3.5 min−1, and had an amplitude of 0.28 ± 0.15 F/F0 and a half-width of 0.62 ± 0.12 s (n = 28). In five preparations, ICC-LCs and USMCs generated synchronous Ca2+ transients (Fig. 5A and B). A cross-correlogram for ICC-LCs and USMCs showed a peak near lag period zero, suggesting a close temporal correlation between the two cell types (Fig. 5C). The peak correlation values were consistently smaller than those of the correlograms for pairs of ICC-LCs as the configuration of USMC Ca2+ transients was fairly different from those of ICC-LCs. In the remaining 16 preparations, USMCs generated Ca2+ transients independently from those of ICC-LCs (Fig. 5D and E). It should be noted that the frequencies of USMC Ca2+ transients was never lower than those of ICC-LCs, and that synchronicity between USMCs and ICC-LCs was invariably observed at the lowest frequency of USMC Ca2+ transients (Fig. 5F).
Figure 5. Analysis of the temporal relationship of Ca2+ transients between ICC-LCs and USMCs.
Aa, a series of frames at intervals of 0.2 s demonstrating USMC Ca2+ transients originating from an ICC-LC. B, in the same preparation, synchronous Ca2+ transients were generated by an ICC-LC (1) and USMC (2). C, a cross-correlogram for ICC-LCs and USMCs showed a peak near lag period zero. D, in a different preparation, a series of frames at intervals of 0.2 s demonstrating USMC Ca2+ transient and subsequent Ca2+ transient in a pair of ICC-LCs. E, Ca2+ transients in ICC-LCs (1) occurred independently from those of USMCs (2). Scale bars also apply to traces in B. Numbers for traces in B and E correspond to those in A and D. F, a comparison of mean frequency between ICC-LCs and USMCs demonstrates a close temporal correlation between the two types of cells in five preparations but not in the remaining 16 preparations.
Role of L-type Ca2+ channels in generating Ca2+ transients in ICC-LCs and USMCs
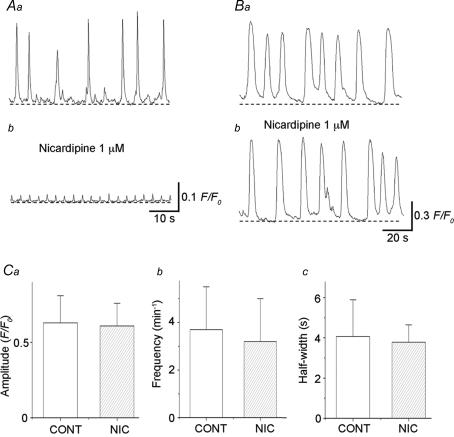
Nicardipine (1 μm) abolished (n = 4) or greatly reduced the amplitude of Ca2+ transients in USMCs (from 0.46 ± 0.25 F/F0 to 0.066 ± 0.014 F/F0, n = 6, Fig. 6Aa and b). In preparations which had been treated with nicardipine (1 μm), nicardipine-resistant Ca2+ transients occurred at a frequency of 12.3 ± 3.9 min−1 (10.8 ± 4.0 min−1 in control, P > 0.05) and had a half-width 0.51 ± 0.12 s (0.69 ± 0.22 s in control, P > 0.05).
Figure 6. Effects of nicardipine on spontaneous Ca2+ transients recorded from USMCs and ICC-LCs in the urethra.
Aa, spontaneous Ca2+ transients recorded form USMCs of the rabbit urethra were strongly suppressed by nicardipine (1 μm) (Ab). Ba, in another preparation, ICC-LC Ca2+ transients generated spontaneous Ca2+ transients which were not inhibited by nicardipine (1 μm) (Bb). C, summary of the effects of nicardipine on ICC-LC Ca2+ transients. Nicardipine (1 μm) did not significantly change amplitude (a), frequency (b) or half-width (c) of ICC-LC Ca2+ transients.
As summarized in Fig. 6C, nicardipine (1 μm) did not significantly alter the amplitude of Ca2+ transients of ICC-LCs (0.63 ± 0.18 F/F0 in control, 0.61 ± 0.15 F/F0 in nicardipine, n = 9, P > 0.05, Fig. 6Ba and b), nor reduce their frequency (3.7 ± 1.8 min−1 in control, 3.2 ± 1.2 min−1 in nicardipine, n = 9, P > 0.05) or half-width (4.1 ± 1.8 s in control, 3.8 ± 0.86 s in nicardipine, n = 9, P > 0.05).
Since the blockade of L-type Ca2+ channels did not suppress Ca2+ transients in ICC-LCs, subsequent experiments on ICC-LC Ca2+ transients were carried out in the presence of nicardipine (1 μm) unless otherwise stated to further diminish muscle contractions.
Role of intracellular Ca2+ stores in generating Ca2+ transients of ICC-LCs and USMCs
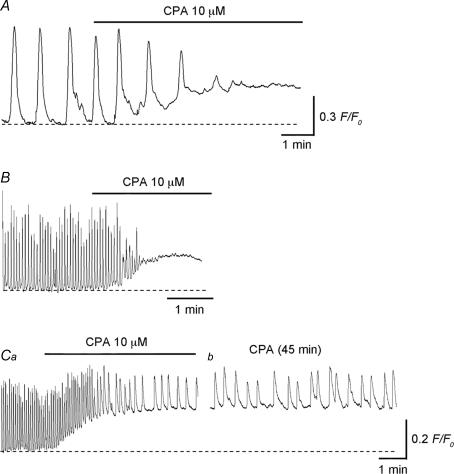
Irrespective of the presence (n = 6) or absence (n = 4) of nicardipine (1 μm), ICC-LC Ca2+ transients were abolished by CPA (10 μm) within 5 min, indicating that ICC-LC Ca2+ transients rely on Ca2+ release from intracellular stores (Fig. 7A). CPA also increased the basal Ca2+ level by 0.28 ± 0.12 F/F0 (n = 10).
Figure 7. Role of intracellular Ca2+ stores in generating spontaneous Ca2+ transients in ICC-LCs and USMCs of the urethra.
CPA (10 μm) abolished spontaneous Ca2+ transients recorded from ICC-LC (A) and USMC (B). A and B were recorded from different preparations. Ca, in other preparations, CPA reduced the frequency of spontaneous Ca2+ transients recorded from USMCs. b, in the same preparations which had been treated with CPA for 45 min, spontaneous Ca2+ transients occurred but with a markedly reduced frequency and amplitude.
CPA (10 μm) abolished USMC Ca2+ transients within 5 min in seven preparations (Fig. 7B). In the remaining five preparations, CPA reduced the frequency of USMC Ca2+ transients but did not prevent their generation (Fig. 7Ca and b). In preparations which had been treated with CPA (10 μm) for 45 min, USMC Ca2+ transients were generated at a frequency of 2.3 ± 0.46 min−1 (9.7 ± 2.6 min−1 in control, P < 0.05, n = 5), and had an amplitude of 0.29 ± 0.089 F/F0 (0.52 ± 0.11 F/F0 in control, P < 0.05) and a half-width of 1.8 ± 0.52 s (0.63 ± 0.13 s in control, P < 0.05). CPA also increased basal Ca2+ levels by 0.25 ± 0.11 F/F0 (n = 12).
Role of extracellular Ca2+ in generating Ca2+ transients of ICC-LCs
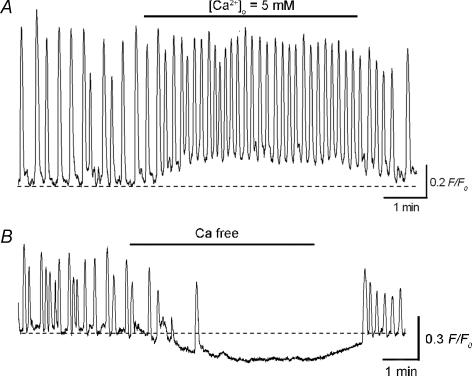
Although ICC-LC Ca2+ transients were insensitive to nicardipine, increasing [Ca2+]o from 2.5 mm to 5 mm accelerated their generation (Fig. 8A). In high Ca2+ solution, ICC-LC Ca2+ transients were generated at a frequency of 9.1 ± 2.1 min−1 (3.5 ± 1.3 min−1 in control, P < 0.05, n = 5), and had an amplitude of 0.53 ± 0.1 F/F0 (0.76 ± 0.17 F/F0 in control, P < 0.05) and a half-width of 2.9 ± 0.35 s (3.6 ± 0.79 s in control, P < 0.05). High Ca2+ solution also increased basal Ca2+ levels by 0.19 ± 0.09 F/F0 (n = 5).
Figure 8. Role of extracellular Ca2+ in generating spontaneous Ca2+ transients in ICC-LCs of the urethra.
A, in a urethral smooth muscle preparation, ICC-LC generated spontaneous Ca2+ transients. Increasing [Ca2+]o from 2.5 mm to 5 mm accelerated ICC-LC Ca2+ transients and increased basal Ca2+ concentration. B, in a different preparation, ICC-LC generated spontaneous Ca2+ transients. Switching from normal PSS to nominally Ca2+ free solution rapidly abolished ICC-LC Ca2+ transients.
In contrast, switching from normal PSS to nominally Ca2+ free solution immediately prevented their generation (n = 4, Fig. 8B) and also reduced basal Ca2+ levels by 0.22 ± 0.09 F/F0 (n = 4). These results indicated that the generation of ICC-LC Ca2+ transients requires a supply of Ca2+ from extracellular space through a pathway other than L-type Ca2+ channels.
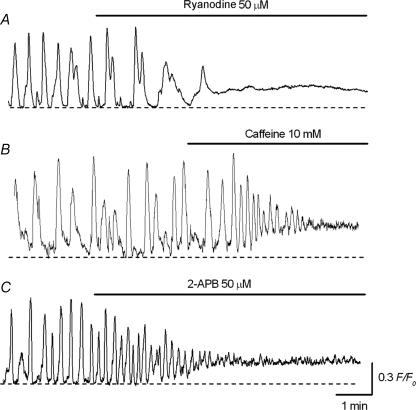
Effects of ryanodine, caffeine and 2-APB in generating Ca2+ transients of ICC-LCs
Since Ca2+ release from intracellular stores is involved in the generation of ICC-LC Ca2+ transients, the contributions due to ryanodine and InsP3 receptors were investigated. Ryanodine (50 μm) first reduced the amplitude of spontaneous Ca2+ transients recorded in ICC-LCs, and subsequently prevented their generation within 5 min (Fig. 9A) in association with an increase in basal Ca2+ levels by 0.15 ± 0.07 F/F0 (n = 5). In contrast, caffeine (10 mm) initially increased the frequency of spontaneous Ca2+ transients in ICC-LCs and reduced their amplitude. Subsequently it abolished the generation of ICC-LC Ca2+ transients within 5 min (n = 4, Fig. 9B) and this was accompanied by an increase in basal Ca2+ levels by 0.19 ± 0.08 F/F0 (n = 4).
Figure 9. Effects of ryanodine, caffeine and 2-APB on spontaneous Ca2+ transients in urethral ICC-LCs.
A, ryanodine (50 μm) prevented the generation of spontaneous Ca2+ transients recorded in ICC-LCs. B, in another preparation, caffeine (10 mm) initially increased the frequency, and then abolished the discharge of spontaneous Ca2+ transients recorded in ICC-LC. C, in a different preparation, 2-APB (50 μm) greatly suppressed ICC-LC spontaneous Ca2+ transients.
In 5 of 9 preparations, 2-APB (50 μm) reduced the amplitude of spontaneous Ca2+ transients in ICC-LCs, and then almost completely suppressed their generation within 10 min (Fig. 9C). In the remaining four preparations, 2-APB reduced the amplitude of ICC-LC Ca2+ transients. In four preparations which had been treated with 2-APB (50 μm) for 20 min, ICC-LC Ca2+ transients occurred at a frequency of 3.6 ± 2.8 min−1 (2.8 ± 2.1 min−1 in control, P > 0.05, n = 4), and had an amplitude of 0.21 ± 0.081 F/F0 (0.55 ± 0.17 F/F0 in control, P < 0.05) and half-width of 2.4 ± 0.3 s (3.0 ± 0.3 s in control, P < 0.05). 2-APB also increased the basal Ca2+ level by 0.11 ± 0.05 F/F0 (n = 9).
Role of nitrergic and α-adrenergic simulation in modulating Ca2+ transients of ICC-LCs
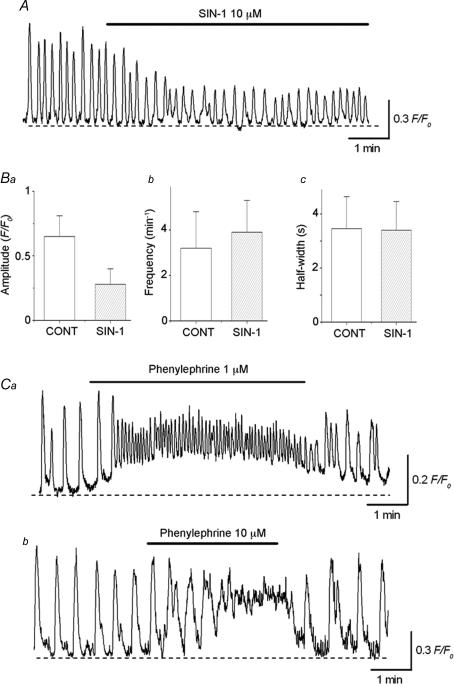
To investigate whether ICC-LCs in situ may be capable of responding to nitrergic and α-adrenergic stimulation during neuromuscular transmission in the urethra, the effects of SIN-1, which decays to release NO (Feelisch et al. 1989), and phenylephrine on ICC-LC Ca2+ transients were also examined.
SIN-1 (10 μm) reduced the amplitude of ICC-LC Ca2+ transients (n = 6, Fig. 10A) or abolished their generation (n = 2). In six preparations which had been treated with SIN-1 for 15 min, ICC-LC Ca2+ transients occurred at a frequency of 3.9 ± 1.4 min−1, and had a half-width of 3.4 ± 1.1 s and amplitude of 0.28 ± 0.12 F/F0. These results were summarized in Fig. 10B, and demonstrate that SIN-1 inhibits ICC-LC Ca2+ transients by reducing their amplitude.
Figure 10. Role of nitrergic and α-adrenergic stimulation in the modulation of spontaneous Ca2+ transients recorded from the urethral ICC-LCs.
SIN-1 (10 μm;A) reduced the amplitude (Ba) of spontaneous Ca2+ transients recorded from ICC-LC, but did not significantly alter either their frequency (Bb) or half-width (Bc). Ca, in another preparation, bath-applied phenylephrine (1 μm) increased the frequency of spontaneous Ca2+ transients recorded from ICC-LC and raised basal Ca2+ levels. b, a higher concentration of phenylephrine (10 μm) further accelerated ICC-LC Ca2+ transients which summed to create a sustained rise in the basal Ca2+ concentration.
In contrast, bath applied phenylephrine (1 μm) increased the frequency of ICC-LC Ca2+ transients (from 3.5 ± 0.9 min−1 to 13.2 ± 3.4 min−1, P < 0.05, n = 4) and caused a rise in the Ca2+ level (0.21 ± 0.08 F/F0, n = 4, Fig. 10Ca). Phenylephrine (1 μm) also reduced the amplitude (from 0.63 ± 0.12 F/F0 to 0.27 ± 0.09 F/F0, P < 0.05, n = 4) and half-width (from 3.4 ± 0.7 s to 1.4 ± 0.3 s, P < 0.05, n = 4) of ICC-LC Ca2+ transients.
A higher concentration of phenylephrine (10 μm) increased the frequency of ICC-LC Ca2+ transients, which resulting in a sustained rise in the Ca2+ level (0.31 ± 0.12 F/F0, n = 4, Fig. 10Cb), indicating that ICC-LCs are capable of responding to α-adrenergic stimulation by increasing their frequency of Ca2+ transient discharge.
Discussion
Spontaneous Ca2+ transients in ICC-LCs recorded in the rabbit urethra in situ were insensitive to nicardipine, an L-type Ca2+ channels blocker, which strongly suppressed Ca2+ transients in USMCs. Instead these Ca2+ transients were dependent on the Ca2+ release from intracellular Ca2+ stores. At the concentration used in the present study (50 μm), ryanodine could produce a closed state of ryanodine receptor Ca2+ channels to inhibit Ca2+ release from intracellular stores (Sutko et al. 1997). Indeed, it reduced the amplitude of ICC-LC Ca2+ transients before any appreciable rise in basal Ca2+ level. In contrast, caffeine increased the frequency of ICC-LC Ca2+ transients, suggesting that it may stimulate Ca2+ release though the opening of ryanodine receptors. Therefore, ryanodine and caffeine may affect ryanodine receptors in opposite ways, but both eventually prevent the generation of ICC-LCs. However, ryanodine could also increase Ca2+ permeability of intracellular stores to decrease the Ca2+ store content (Stuko et al. 1997). This may account for the continued increase in basal Ca2+ levels presumably due to the capacitative Ca2+ entry. 2-APB, which has been widely used as a blocker for InsP3-induced Ca2+ release, also suppressed ICC-LC Ca2+ transients. These results are in good agreement with studies using isolated ICC-LCs, which revealed that InsP3 receptors are required to coordinate localized Ca2+ transients resulting from ryanodine receptor activation (Sergeant et al. 2006a). However, 2-APB caused an increase in basal Ca2+ which could not be explained by its inhibitory action on InsP3-induced Ca2+ release. Therefore, we cannot exclude the possibility that 2-APB-induced inhibition of ICC-LC Ca2+ transients might be attributed to an action on either SERCA (Bilmen et al. 2002) or capacitative Ca2+ entry (Prakriya & Lewis, 2001).
Although Ca2+ transients in ICC-LCs were not diminished by nicardipine, removal of extracellular Ca2+ caused an immediate cessation of Ca2+ transients, suggesting that Ca2+ influx from the extracellular space may be tightly coupled with Ca2+-induced Ca2+ release via ryanodine receptors to initiate Ca2+ transients in ICC-LCs. This is again consistent with the results obtained from freshly isolated ICC-LCs in the rabbit urethra, in which spontaneous Ca2+ oscillations depend on [Ca2+]o but were not inhibited by nifedipine (Johnson et al. 2005). Recently it has been demonstrated that Ca2+ entry during the reverse mode activation of the sodium–calcium exchanger (NCX) may be responsible for this Ca2+ influx (Bradley et al. 2006). One might expect that increased [Ca2+]i would suppress Ca2+ influx through NCX, and thus inhibit ICC-LC Ca2+ transients. However, neither a low concentration of phenylephrine nor increasing extracellular Ca2+, which caused substantial increases in [Ca2+]i, prevented the generation of ICC-LC Ca2+ transients, suggesting that the inhibition of ICC-LC Ca2+ transients by blockers of intracellular Ca2+ handling was not due to an increase in [Ca2+]i.
In the present study, the blockade of SERCA with CPA abolished spontaneous Ca2+ transients in ICC-LCs, confirming that their generation depends on Ca2+ release from intracellular stores. CPA also either abolished USMC Ca2+ transients or markedly reduced their frequency, suggesting that ICC-LCs may be responsible for their generation. Since the resting membrane potential of USMCs (between −40 mV and −50 mV) was close to the threshold of L-type Ca2+ channel activation (Hashitani et al. 1996), it is likely that the excitability of USMCs in CPA-treated preparations was increased by either membrane depolarizations or raised basal Ca2+ level. However, we could not exclude the possibility that a small population of USMCs may be capable of generating spontaneous activity by Ca2+ store-independent mechanisms as do detrusor smooth muscle cells (Hashitani et al. 2004) after disruption of the ‘primary’ ICC-LC pacemaker. Alternatively, heterogeneous subpopulations of ICC-LCs with different sensitivities to CPA might exist.
Since ICC-LCs are capable of responding to both nitrergic and α-adrenergic stimulation, ICC-LCs may be targeted by autonomic nerves which play an essential role in generating both contraction and relaxation of the urethral smooth muscle wall (Andersson & Wein, 2004). Indeed spinous-formed cells expressing cGMP immunoreactivity form a network surrounding smooth muscle bundles particularly in the longitudinal smooth muscle layer (Waldeck et al. 1998). However, CPA-treated urethral smooth muscles are also able to respond to both nitrergic and α-adrenergic stimulation, suggesting that USMCs may also be directly involved in neuromuscular transmission (Hashitani et al. 2006). Unlike bladder in which cyclic GMP (cGMP) was increased in ICC-LCs but not in detrusor smooth muscle cells, addition of a NO donor caused uniform increases in cGMP in USMCs (Smet et al. 1996), indicating that they are capable of responding to NO. This paper also demonstrated that nitric oxide synthase-immunoreactive nerve terminals provide a dense innervation to USMCs. This perhaps suggests that neuromuscular transmission in the urethra may not be exclusively targeting ICC-LCs.
One of the major aims of this study was to investigate the temporal correlation between ICC-LCs and USMCs in generating spontaneous activity in the urethra. In the guinea-pig gastric antrum (Hennig et al. 2004) and mouse ileum (Park et al. 2006), spontaneous Ca2+ waves initiated from ICC-MY spread through the ICC-MY network and activated muscle layers. Simultaneous recordings of muscle tension, Ca2+ and membrane potential of the gastric antrum demonstrate that all signals occur at the same frequency and duration, indicating that pacemaking electrical activity generated by ICC-MY directly triggers smooth muscle contraction (Dickens et al. 2000). ICC-LCs in the urethra often exhibited synchronous Ca2+ transients, suggesting that ICC-LCs within a small cluster may be electrically well-coupled. However, ICC-LCs did not form an extensive network (see Lyons et al. 2007), nor did their Ca2+ transients consistently exhibit a temporal correlation with neighbouring USMCs' Ca2+ transients.
The frequency of USMC Ca2+ transients was never lower than that of ICC-LCs; synchronicity between USMCs and ICC-LCs also consistently occurred at the lowest frequency of USMC Ca2+ transients. If multiple ICC-LCs including those located out of the field of view or beyond the plane of focus were connected to a smooth muscle bundle within a well coupled electrical syncytium, excitation arising from USMCs or ICC-LCs should be transmitted in both directions equally well so that the frequency of Ca2+ transients in ICC-LCs and USMCs should not be very different. However, USMCs often generated non-propagating Ca2+ transients, suggesting that cell-to-cell coupling between USMCs may be relatively weak and that USMCs can generate Ca2+ transients themselves without input from ICC-LCs. Furthermore, we were not able to demonstrate any correlation between muscle contractions and USMC Ca2+ transients, although they occurred at a similar frequency. It seems most likely that individual ICC-LCs are driving USMC bundles independently of other ICC-LCs. In addition, ICC-LCs might have a longer refractory period than USMCs, which may account for their slower time course. We envisage that randomly occurring Ca2+ transients in urethral ICC-LCs increase USMC excitability within individual muscle bundles and that the tensions in these bundles sum to produce a sustained contraction of the urethral wall to maintain urinary continence (Bridgewater et al. 1993).
ICC-LCs have now been identified throughout the urinary tract, although their physiological functions are still to be elucidated (Hashitani 2006). Interestingly, spontaneous Ca2+ transients recorded from ICC-LCs in both suburotherial layer (Wu et al. 2004) and detrusor smooth muscle layers (Hashitani et al. 2004) of the bladder have low frequencies (about 0.5–2.5 min−1) and long durations (some 15 s) as do ICC-LCs in the urethra. However, in the bladder spontaneous Ca2+ transients recorded from detrusor ICC-LCs occur independently of those in the smooth muscle cells arising from the spontaneous generation of action potentials (Hashitani et al. 2004). Moreover, single ICC-LCs of the mouse renal pelvis, where atypical smooth muscle cells may initiate spontaneous action potential discharge (Lang et al. 2006), generate large, long (10 s) inward currents at a low frequency (1–3 min−1). ICC-LCs in the urethra may consist of distinct subpopulations, where some ICC-LCs with a relatively fast time course may act as electrical pacemakers, whilst others with a slower time course may play another supportive role. In mouse ileum, a subpopulation of ICC-MY display spontaneous Ca2+ transients which fire at a low frequency and do not appear to drive smooth muscle Ca2+ transients (Yamazawa & Iino, 2002).
In conclusion, properties of ICC-LCs in situ in the rabbit urethra are very similar to those of isolated ICC-LCs, suggesting that they may act as a primary pacemaker in generating spontaneous contractions. However, signal transmission from ICC-LCs to USMCs may be much less extensive than that between ICC and smooth muscle cells in the GI tract, and thus electrical pacemaking signals generated by ICC-LCs may be ‘less securely’ transmitted to smooth muscles.
Acknowledgments
The authors wish to thank Dr R. J. Lang for his critical reading of the manuscript and Dr F. R. Edwards for his helpful suggestions on cross-correlation analysis. This project was supported by research grants from the Japan Society for the Promotion of Science (No.17390443) to H.H.
References
- Andersson K-E, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- Bilmen JG, Wootton LL, Godfrey RE, Smart OS, Michelangeli F. Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenyl borate (2-APB). 2-APB reduces both Ca2+ binding and phosphoryl transfer from ATP, by interfering with the pathway leading to the Ca2+-binding sites. Eur J Biochem. 2002;269:3678–3687. doi: 10.1046/j.1432-1033.2002.03060.x. [DOI] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, Johnston L, Large RJ, Matsuda T, Baba A, McHale NG, Thornbury KD, Sergeant GP. Contribution of reverse Na+–Ca2+ exchange to spontaneous activity in interstitial cells of Cajal in the rabbit urethra. J Physiol. 2006;574:651–661. doi: 10.1113/jphysiol.2006.110932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater M, Macneill HF, Brading AF. Regulation of urethral tone in pig urethral smooth muscle. J Urol. 1993;150:223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomica T. Identification of rhythmically active cells in guinea–pig stomach. J Physiol. 1999;514:513–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GDS. Vagal inhibition in the antral region of guinae pig stomach. Am J Physiol Gastrointest Liver Physiol. 2000;279:G388–G399. doi: 10.1152/ajpgi.2000.279.2.G388. [DOI] [PubMed] [Google Scholar]
- Feelisch M, Ostrowski J, Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14(Suppl. 11):S13–S22. [PubMed] [Google Scholar]
- Hashitani H. Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol. 2006;576:707–714. doi: 10.1113/jphysiol.2006.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Kohri K, Suzuki H. Heterogeneous CPA sensitivity of spontaneous excitation in smooth muscle of the rabbit urethra. Br J Pharmacol. 2006;148:340–349. doi: 10.1038/sj.bjp.0706729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Hirst GD, Park KJ, Smith CB, Sanders KM, Ward SM, Smith TK. Propagation of pacemaker activity in the guinea-pig antrum. J Physiol. 2004;556:585–599. doi: 10.1113/jphysiol.2003.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Tonta MA, Zoltkowski BZ, Meeker WF, Wendt I, Parkington HC. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. 2006;576:695–705. doi: 10.1113/jphysiol.2006.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons AD, Gardiner TA, McCloskey KD. Kit-positive interstitial cells in the rabbit urethra: structural relationships with nerves and smooth muscle. BJU Int. 2007;99:687–694. doi: 10.1111/j.1464-410X.2006.06617.x. [DOI] [PubMed] [Google Scholar]
- McHale NG, Hollywood MA, Sergeant GP, Shafei M, Thornbury KT, Ward SM. Organization and function of ICC in the urinary tract. J Physiol. 2006;576:689–694. doi: 10.1113/jphysiol.2006.116657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, Sanders KM. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290:C1411–C1427. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006a;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol. 2001;280:C1349–C1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Ca2+ signalling in urethral interstitial cells of Cajal. J Physiol. 2006a;576:715–720. doi: 10.1113/jphysiol.2006.115956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Johnston L, McHale NG, Thornbury KD, Hollywood MA. Activation of the cGMP/PKG pathway inhibits electrical activity in rabbit urethral interstitial cells of Cajal by reducing the spatial spread of Ca2+ waves. J Physiol. 2006b;574:167–181. doi: 10.1113/jphysiol.2006.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol. 2002;283:C885–C894. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- Smet PJ, Jonavicius J, Marshall VR, de Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GDS. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck K, Ny L, Persson K, Andersson K-E. Mediators and mechanisms of relaxation in rabbit urethral smooth muscle. Br J Pharmacol. 1998;123:617–624. doi: 10.1038/sj.bjp.0701645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol. 2004;559:231–243. doi: 10.1113/jphysiol.2004.067934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]