Abstract
We investigated the responses of morphologically identified myenteric neurons of the guinea-pig ileum to inflammation that was induced by the intraluminal injection of trinitrobenzene sulphonate, 6 or 7 days previously. Electrophysiological properties were examined with intracellular microelectrodes using in vitro preparations from the inflamed or control ileum. The neurons were injected with marker dyes during recording and later they were recovered for morphological examination. A proportion of neurons with Dogiel type I morphology, 45% (32/71), from the inflamed ileum had a changed phenotype. These neurons exhibited an action potential with a tetrodotoxin-resistant component, and a prolonged after-hyperpolarizing potential followed the action potential. Of the other 39 Dogiel type I neurons, no changes were observed in 36 and 3 had increased excitability. The afterhyperpolarizing potential (AHP) in Dogiel type I neurons was blocked by the intermediate conductance, Ca2+-dependent K+ channel blocker TRAM-34. Neurons which showed these phenotypic changes had anally directed axonal projections. Neither a tetrodotoxin-resistant action potential nor an AHP was seen in Dogiel type I neurons from control preparations. Dogiel type II neurons retained their distinguishing AH phenotype, including an inflection on the falling phase of the action potential, an AHP and, in over 90% of neurons, an absence of fast excitatory transmission. However, they became hyperexcitable and exhibited anodal break action potentials, which, unlike control Dogiel type II neurons, were not all blocked by the h current (Ih) antagonist Cs+. It is concluded that inflammation selectively affects different classes of myenteric neurons and causes specific changes in their electrophysiological properties.
Persistent pain, hypersensitivity and functional disorders of visceral organs during and following inflammation have been attributed to changes in the afferent neurons innervating the site of inflammation. The inflammation-induced increases in the excitability of neurons involve changes in activity, expression and distribution of ion channels. For example, the tetrodotoxin (TTX)-resistant voltage-gated Na+ current is enhanced in the afferent neurons innervating stomach (Bielefeldt et al. 2002), ileum (Stewart et al. 2003) and colon (Beyak et al. 2004) after the induction of inflammation in these organs. Changes in the expression of Na+ channels in dorsal root ganglion (DRG) neurons innervating the inflamed hindpaws (Black et al. 2004; Coggeshall et al. 2004) and their contribution to hyperalgesia (Khasar et al. 1998) have also been demonstrated. Inflammation was associated with decreases in A-type K+ currents in afferent neurons after inflammation in the bladder (Yoshimura & De Groat, 1999), stomach (Dang et al. 2004) and joints (Takeda et al. 2006), as well as a decreased delayed rectifier type K+ current in primary afferents after inflammation in the ileum (Stewart et al. 2003) and decreases in both types of voltage-gated K+ currents in masseter muscle afferents (Harriott et al. 2006). Roles of voltage-activated Ca2+ channels in inflammation-induced hyperexcitability of joint afferents and the contribution of these channels to post-inflammatory hyperalgesia have been reported (Neugebauer et al. 1996; Sluka, 1998; Saegusa et al. 2001).
Changes in electrophysiological properties of neurons intrinsic to the gastrointestinal tract, most notably hyperexcitability, following inflammation occur in the guinea-pig colon (Frieling et al. 1994; Linden et al. 2003; Lomax et al. 2005, 2006) and jejunum (Palmer et al. 1998). The hyperexcitability of enteric neurons might contribute to disorders of motility, secretion and hypersensitivity during and following gastrointestinal inflammation (Sharkey & Kroese, 2001; De Giorgio et al. 2004; Sharkey, 2006). Although changes in many types of membrane current in dorsal root and trigeminal ganglia primary afferent neurons have been studied during and following inflammation of the visceral organs, changes in only two currents, the current that underlies the late afterhyperpolarizing potential (AHP), and the hyperpolarization-activated cation current, Ih, have been implicated in the hyperexcitability of enteric neurons in the inflamed colon (Linden et al. 2003; Lomax et al. 2005). The late AHP following an action potential in the non-inflamed intestine is a distinctive feature of Dogiel type II neurons. However, whether other neuron types or other currents are affected is not known.
To investigate these questions, we have examined changes in electrophysiological properties in morphologically identified neurons of the guinea-pig ileum in the present work. In this region, neuronal types have been documented more thoroughly than in other parts of the intestine (Brookes, 2001; Furness, 2006). In fact, all types of neurons in the myenteric ganglia of the guinea-pig small intestine have been identified by morphology, electrophysiological properties, projections to targets and physiological function. The present study reveals that in the inflamed ileum changes in properties occur in both Dogiel type II neurons and other neuron types, and that the changes are sufficient that electrophysiological characteristics can no longer be used to identify functional classes of neurons.
Methods
All experiments were performed on guinea-pigs (150–275 g) of either sex from the inbred Hartley strain colony of the Department of Anatomy and Cell Biology at the University of Melbourne. All procedures were conducted according to the Code of Practice of the National Health and Medical Research Council of Australia and were approved by the University of Melbourne Animal Experimentation Ethics Committee. All animals were maintained in a controlled environment at 21°C on a 12: 12 h light–dark cycle with free access to food and water. At the time of taking tissue, animals were stunned by a blow to the head and killed by cutting the carotid arteries and severing the spinal cord.
Induction of inflammation
Guinea-pigs were anaesthetized with a mixture of xylazine (20 mg kg−1) and ketamine hydrochloride (100 mg kg−1; Troy Laboratories, Australia), given intramuscularly. TNBS (2,4,6-trinitrobenzenesulphonic acid; Wako Industries, Nagoya, Japan) was given in an amount of 30 mg kg−1 in 1 ml of 30% ethanol. The abdomen was opened by a 1.5 cm incision in the mid-line and the distal part of the ileum was exteriorized. TNBS was injected into the lumen of the ileum, approximately 8 cm proximal to the ileocaecal junction, through a 30 gauge needle over the course of 1 min. The intestine was temporally occluded just distal to the injection site during the TNBS injection and for a further minute following injection. This restricted the region of inflammation to the intestine proximal to the injection. A fine silk ligature (5.0) was tied loosely around a nearby blood vessel to mark the injection site for later location. The intestine was then returned to the abdominal cavity, the abdominal wall and peritoneum were closed with sutures, and the skin was closed with stainless steel staples. The guinea-pigs were housed individually and monitored during recovery from anaesthesia (2–3 h) and then housed together, with free access to food and water. The guinea-pigs showed no signs of stress, exhibited apparently normal exploratory behaviour, and ate soon after they awoke from anaesthesia. Animals were taken at 6 and 7 days after TNBS injection. Segments of inflamed ileum were removed for electrophysiological studies and for histological assessment of the level of inflammation. Animals were weighed prior to administration of TNBS and daily following surgery.
Control data are from guinea-pigs of the same colony that were in the same age range, but were not subjected to surgery. In addition, changes were investigated in tissue from nine sham-operated animals. These animals underwent identical surgery, including the injection of 30% ethanol into the lumen of the distal ileum. Tissue was taken 6 and 7 days later.
Assessment of inflammation
Gross morphological damage was assessed by visual inspection of the segments at the time of tissue removal and this was later correlated with histological assessment. To provide a histological grading, three parameters were graded, 0–3, flattening of the mucosa (0 = normal, 3 = severe flattening), presence of haemorrhagic sites (0 = none, 3 = numerous sites) and circular muscle change (thickening and disorganization of muscle bundles, 0 = normal, 3 = substantial thickening and disorganization). Samples adjacent to those used for electrophysiology were taken into phosphate buffered saline (PBS, 0.9% NaCl in 0.01 m sodium phosphate buffer) containing 1 μm nicardipine, pinned on balsa board with the mucosa facing upwards, and fixed in a formaldehyde (2%) and picric acid (0.2%) mixture in 0.1 m sodium phosphate buffer, pH 7.0 overnight at +4°C. The next day the tissue was washed with 3 × 10 min dimethylsulphoxide (DMSO: Merck, Australia), followed by 3 × 10 min PBS. Once all fixative was removed, the sample was stored in PBS–sucrose (30%)–azide 0.1%; Sigma overnight, then 50: 50 OCT compound Tissue-Tek: PBS–sucrose–azide, again overnight, prior to cryosectioning. Transverse sections (12 μm) were cut and placed onto 1% gelatin-coated glass slides. The sections were allowed to dry at room temperature for at least 1 h then processed for standard haematoxylin and eosin staining. Following staining they were dehydrated through graded ethanol solutions, transferred into histolene and mounted using permanent mounting medium.
Tissue preparation for electrophysiology
Segments of inflamed ileum, 2–3 cm long, were taken from the region of inflammation in TNBS-treated animals and from control (untreated or sham-operated) animals, 8–10 cm proximal to the ileocaecal junction. The oral end was marked with a fine pin passed through the tissue. The segments were placed in physiological saline (composition (mm): NaCl 118, KCl 4.8, NaHCO3 25, NaH2PO4 1.0, MgSO4 1.2, glucose 11.1, CaCl2 2.5; equilibrated with 95% O2–5% CO2) and initially kept at room temperature. The solution contained 3 μm nicardipine and 1 μm hyoscine (both from Sigma-Aldrich, Sydney, Australia) to inhibit muscle movement. The mucosa, submucosa and circular smooth muscle were carefully removed to expose the myenteric plexus. The preparation was pinned to the Sylgard elastomer base of a recording dish (volume 1 ml) which was placed on the stage of an inverted microscope and continuously superfused (4 ml min−1) with physiological saline that had been pre-heated to yield a bath temperature of 34–35°C. The tissue was equilibrated with perfusate for 1–2 h before recording commenced.
Electrophysiological recordings
Neurons were impaled with conventional borosilicate glass microelectrodes filled with 1% biocytin (Sigma-Aldrich) in 1 m KCl. Electrode resistances were 100–170 MΩ. Recordings were made using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA, USA). Signals were digitized at 1–10 kHz, using a Digidata 1322A interface (Axon Instruments) and stored using PC-based data acquisition software (Axoscope 8.2, Axon Instruments). Measurements of electrophysiological properties were made after allowing the impalements to stabilize for at least 15 min without applying intracellular holding current. At this time the ability of the cell to fire an action potential was assessed. Action potentials were evoked both by extracellular stimulation to induce antidromically activated action potentials and by injection of brief intracellular current pulses. For analysis of the late AHPs following action potentials, only intracellular current pulses were used, in order to differentiate AHPs from slow inhibitory postsynaptic potentials. Only cells that were able to fire an action potential, had resting membrane potentials (RMPs) more negative than −40 mV, and were adequately filled with dye to reveal their morphology were included in the electrophysiological analysis. The properties of the action potentials and late AHPs in all groups were recorded at resting membrane potential, except that when TRAM-34 was applied the membrane potential was held at −60 mV. Compounds were applied by addition to the superfusion solution: tetrodotoxin (TTX) from Alomone Laboratories, Israel and TRAM-34 synthesized in-house (Nguyen et al. 2007). Evoked responses were measured prior to drug application and after at least 20 min in the presence of drug in the extracellular solution.
Small intracellular hyperpolarizing current pulses (duration 500 ms, intensity 0.03 nA, yielding voltage shifts of 5–10 mV) were used to determine input resistance (Rin) and cell capacitance (Cin). Excitability was assessed by injecting 0.5–2 s depolarizing current pulses, at an intensity of 0.02–0.3 nA at 20 s intervals, through the recording electrode while a holding current was used to maintain the resting membrane potential at −60 mV.
Electrical stimuli were applied to internodal strands using a fine tungsten stimulating electrode (10–50 μm tip diameter), insulated except at the tip. Stimuli were delivered via an ISO-Flex stimulator controlled by a Master-8 programmable pulse generator (both from AMPI, Jerusalem, Israel). Fast excitatory postsynaptic potentials (EPSPs) were evoked by extracellular pulses of 0.1 ms duration and 0.3–0.5 mA intensity at 10 s intervals while the membrane potential was held at −90 mV. Membrane potential, Rin, and numbers of action potentials in response to depolarizing pulses were determined using in-house analysis routines written in Igor Pro 4.0 analysis software (WaveMetrics, USA). For analysis of cell excitation we also observed the presence or absence of anodal break excitation prior to and after the presynaptic nerve stimulation.
Neuron identification
Biocytin was passed from the recording electrodes into the neurons during impalement. Once a neuron in a ganglion had been injected with biocytin, a diagram of the positions of the ganglion and of the impaled neuron was prepared so that the neuron could be later identified under the microscope. If further recordings were taken, the electrode was moved to a fresh ganglion to avoid ambiguity of cell identity. At the end of each experiment, the tissue was fixed overnight in 2% formaldehyde plus 0.2% picric acid in 0.1 m sodium phosphate buffer (pH 7.0), cleared in 3 × 10 min each of DMSO, and washed in PBS (3 × 10 min). Fixed tissue was stored at 4°C in PBS containing sodium azide (0.1%).
Preparations were incubated overnight at 4°C. The tissues were then washed (3 × 10 min) in PBS prior to incubation with streptavidin coupled to Texas red (Amersham Biosciences, USA), 1: 400, for 2 h at room temperature. Preparations were then washed (3 × 10 min) in PBS and mounted on glass slides using buffered glycerol (pH 8.4).
To analyse the morphologies and projections of the impaled neurons, preparations in which impaled nerve cells had been identified were removed from the slides and washed in PBS, prior to conversion of the streptavidin, bound to the biocytin, to a permanent deposit (Clerc et al. 1998). This was achieved using goat anti-streptavidin antiserum coupled to biotin (Vector Laboratories, Burlingame, CA, USA), diluted at 1: 50 and incubated overnight at 4°C. The biotin was in turn localized using an avidin–biotin–horseradish peroxidase kit (Vectastain, Vector Laboratories). The horseradish peroxidase was reacted with diaminobenzidine and hydrogen peroxide to yield a permanent deposit. Cell shapes, positions and projections were evaluated on an Olympus BH microscope under positive-low phase contrast optics, and drawn with the aid of a camera lucida drawing tube at ×400 or ×1000 magnification.
Statistics
Electrophysiological data are presented as mean ± s.e.m. Statistical differences were determined by one-way ANOVA with Tukey–Kramer post hoc test for multiple group comparisons. Differences were considered statistically significant at P < 0.05.
Results
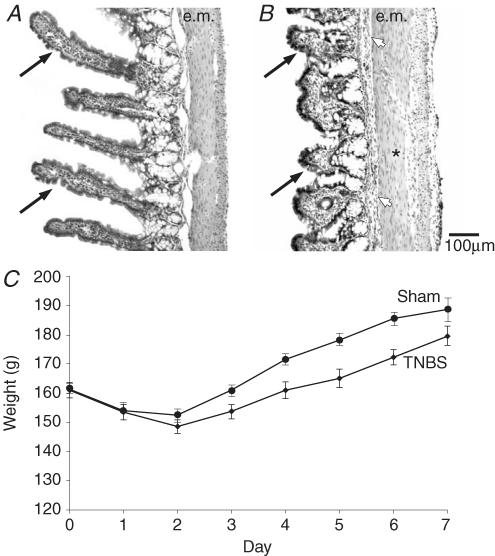
The administration of TNBS caused inflammation in 5–8 cm of the ileum that was characterized by small regions of vascular dilatation, including haemorrhagic foci, ulceration and flattening of the mucosa and muscle thickening (Fig. 1A and B), as has been previously reported (Martinolle et al. 1997). After the administration of TNBS, guinea-pigs lost weight (Fig. 1C). The average weight loss on day 2 after injection of TNBS was 8–10% of the weight prior to surgery. After this initial weight loss, the animals regained weight at a slower rate than sham-operated controls (Fig. 1C). On days 2 and 3 after the TNBS, the animals often had diarrhoea, sometimes with visual evidence of blood in the faeces. This was usually resolved on day 4, and by days 6 and 7 the guinea-pigs had normal appearance and formed faecal pellets, similar to untreated animals. The animals took food and water on all days following the surgery.
Figure 1. Changes indicative of inflammation: reduced villus height and body weight loss.
A and B, haematoxylin and eosin staining of the ileum from control (A), and from a guinea-pig 6 days after the intraluminal injection of 2,4,6-trinitrobenzenesulphonic acid (TNBS, B). Comparison of the images shows the substantial decrease in the heights of villi after TNBS (arrows). There is also a slight thickening of the external muscle (e.m.) and enlarged lymphatic vessels (*). The submucosal layer is more prominent (arrowhead). C, time course of body weight loss and recovery after injection of TNBS into the guinea-pig ileum (♦, mean ±s.e.m., n = 155). Changes in body weight in sham controls reveal a smaller weight loss and quicker post-operative weight gain (•, n = 9).
Electrophysiology experiments utilized myenteric plexus-longitudinal muscle preparations from 155 guinea-pigs with TNBS-induced ileitis and from 48 control (untreated) animals. In addition, neurons from 9 sham-operated controls were examined. All neurons were characterized electrophysiologically, labelled by intracellular injection of biocytin during recording, and were later analysed morphologically. Only neurons that had both electrophysiological characteristics and morphological identification were used for analysis. In total, 167 neurons from inflamed tissues and 48 neurons from control (untreated) guinea-pigs were included in the analysis.
Morphologically, neurons were classified into one of two categories: multiaxonal Dogiel type II neurons (n = 93 in the inflamed group and n = 26 in the control group) that had large round or oval cell bodies, and uniaxonal neurons (n = 74 in the inflamed group and n = 22 in the control group). Uniaxonal neurons from inflamed tissues were classified as Dogiel type I neurons (n = 71) that had a single axon and irregular lamellar dendrites and as filamentous interneurons (n = 3) that had a single axon and long filamentous dendrites (Dogiel, 1899; Brehmer et al. 1999; Furness, 2006). Of the 71 Dogiel type I neurons, the axons of 7 neurons could be traced into residual circular muscle, identifying these neurons as circular muscle motor neurons (CMMNs). All uniaxonal neurons were further identified as ascending or descending neurons according to the direction of projection of their axons.
All Dogiel type II neurons and about half of the Dogiel type I neurons from the inflamed intestine were hyperexcitable, whereas no hyperexcitable neurons were encountered in preparations from control (untreated) or sham-operated guinea-pigs.
Populations of neurons with late AHPs
For neurons from inflamed intestine, late AHPs following soma action potentials were observed in 32/71 Dogiel type I and 1/3 filamentous neurons (Fig. 2) and 93/93 neurons that had Dogiel type II morphology (Fig. 7). Uniaxonal neurons with late AHPs comprised 44.6% of all uniaxonal neurons from the inflamed ileum and 45% of Dogiel type I neurons (Fig. 2B). In control small intestine, some filamentous neurons (about 5% of all neurons), but not Dogiel type I neurons, have AH electrophysiology (Song et al. 1997; Clerc et al. 1998). Thirty-one Dogiel type I neurons with late AHPs had descending axons and one had a small cell body and projected locally to a remnant of circular muscle. The processes of two descending Dogiel type I neurons with late AHPs were traced to the circular muscle. Of neurons from the control ileum, 0/22 uniaxonal neurons and 26/26 Dogiel type II neurons had late AHPs.
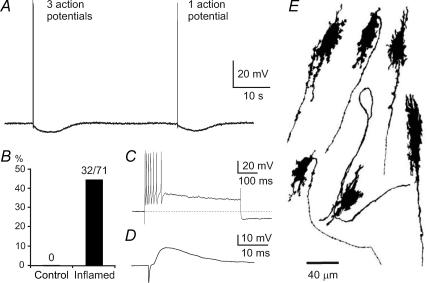
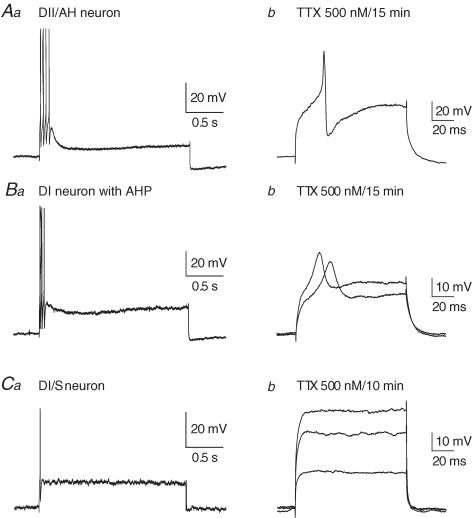
Figure 2. The occurrence of afterhyperpolarizing potentials (AHPs) in myenteric neurons with Dogiel type I (DI) morphology from the inflamed ileum.
A, examples of late AHPs in a DI neuron. A train of three action potentials as well as a single action potential, elicited by brief intracellular current pulses, are followed by late AHPs lasting about 8 s. B, late AHPs did not occur in these neurons from control ileum (0/22), whereas about 45% (32/71) of DI neurons from the inflamed ileum exhibited late AHPs. C, a depolarizing current pulse of 100 pA and 500 ms induced 7 action potentials. Early AHPs, lasting less than 10 ms, are seen after each action potential. Following the action potential burst, the development of the late AHP is apparent as a sag in the depolarization and a hyperpolarization of about 15 mV after the end of current injection. D, a fast excitatory postsynaptic potential elicited by stimulation of presynaptic inputs to a DI neuron with a late AHP. Fast EPSPs were prominent in all DI neurons with late AHPs. E, examples of the shapes of DI neurons that exhibited late AHPs. Each of these neurons is from the inflamed intestine and was filled with dye during recording. The typical short lamellar dendrites and single axon, sometimes with axonal spines, of DI neurons are seen.
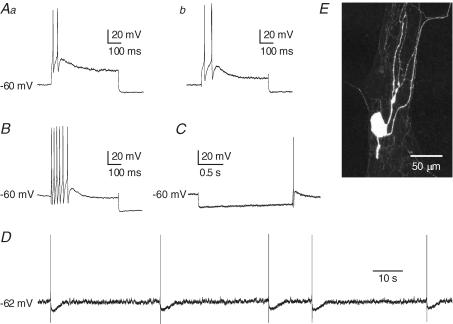
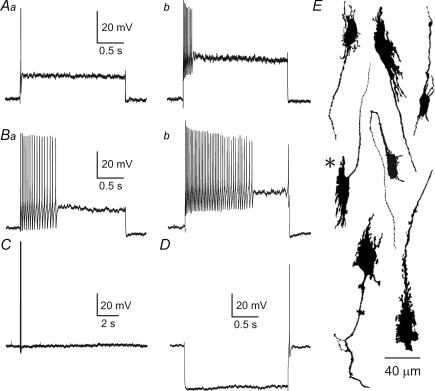
Figure 7. Action potential generation in Dogiel type II (DII) neurons from the inflamed ileum.
Aa and b, action potential firing in response to depolarizing current pulse (200 pA, 500 ms) of DII neurons from control (Aa) and sham-operated (Ab) ileum. B, action potentials in response to intracellular depolarizing current (200 pA, 500 ms) in a DII neuron from the inflamed ileum. C, anodal break action potential that was observed in a DII neuron from the inflamed intestine. Anodal break action potentials were much more common after inflammation. D, spontaneous action potentials arising at a resting membrane potential of −62 mV in a DII neuron from the inflamed intestine. Spontaneous action potentials were never seen in Dogiel type II neurons from control intestine, under the conditions used in this study, even when the resting membrane potential was more depolarized than −62 mV. E, fluorescence image of the neuron from which the record in B was taken. This is a typical DII neuron with a smooth-surfaced oval cell body and several long, axon-like processes.
Dogiel type I neurons with late AHPs in the inflamed ileum
These neurons had prominent late AHPs after a single action potential induced by an intracellular current pulse, with amplitudes similar to AHP amplitudes in Dogiel type II neurons from the inflamed or control ileum (Fig. 3G). However, the durations at half-amplitude of late AHPs in these Dogiel type I neurons were significantly briefer than in Dogiel type II neurons from both inflamed and control ileum (P < 0.05 for both, Fig. 3H).
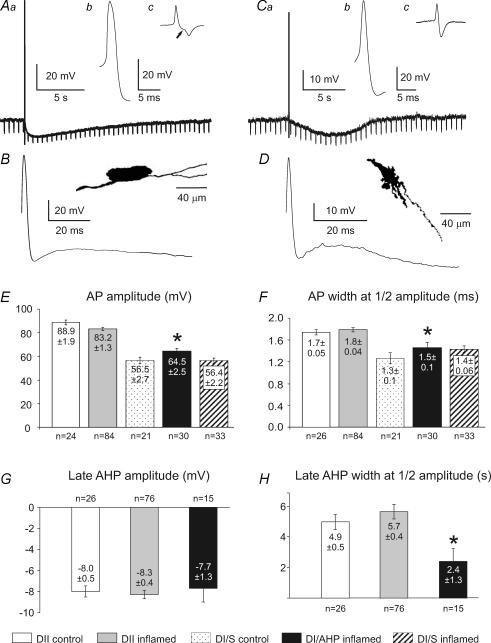
Figure 3. Properties of afterhyperpolarizing potentials in myenteric Dogiel type II (DII) and Dogiel type I (DI) neurons from the inflamed ileum.
Aa, b and c, late AHP and action potential in a DII neuron. The AHP following a single action potential was associated with significant reduction of the input resistance, as indicated by the decrease in the voltage changes caused by hyperpolarizing current injection (Aa). The action potential had a hump on the falling phase (Ab), demonstrated in the first time derivative (dV/dt) of the action potential (Ac), which clearly shows the biphasic falling phase (arrow). B, the action potential and after-potentials of the neuron in A at greater sweep speed. It can be seen that the membrane potential depolarizes after the early AHP, before the beginning of the late AHP is apparent. The inset shows the shape of the neuron from which the records in A were taken. This is a typical DII neuron, with an oval cell body and multiple long processes (camera lucida drawing after dye filling). Ca, b and c, the action potential and afterhyperpolarization in a DI neuron. The late AHP following a single action potential was also associated with a reduction in input resistance (Ca). In both cells the action potential was recorded with the membrane potential held at −60 mV. The action potential did not have a hump on its falling phase (Cb), which is emphasized by the lack of inflection in the first derivative of the trace (Cc). D, the action potential and after potentials, showing the delay before the onset of the late AHP. The inset shows the neuron from which the records in C and D were taken. This is a typical DI neuron, with lamellar dendrites and a single axon. E and F, action potential (AP) amplitudes and widths at half-amplitude of the APs of DII neurons were not significantly affected by inflammation. G, amplitudes of late AHPs of DII neurons from control and inflamed ileum and DI neurons from the inflamed ileum. There are no significant differences. H, widths at half-amplitude of late AHPs of DII neurons from control and inflamed ileum and DI neurons from the inflamed ileum. The late AHP of the DI neurons was briefer than that of DII neurons (P < 0.05). Means ± s.e.m. and numbers of neurons analysed for each parameter are given in the histograms. *Significantly different from Dogiel type II neurons from the inflamed ileum (P < 0.05).
Action potential (AP) amplitudes and durations were not significantly different between Dogiel type I neurons with late AHPs and Dogiel type I neurons that did not exhibit late AHPs from either inflamed or control ileum (Fig. 3E and F). However, the action potential amplitudes of Dogiel type I neurons with late AHPs were significantly lower compared with Dogiel type II neurons from both inflamed and control ileum (P < 0.001 for both, Fig. 3E). There was a significant difference between AP durations of Dogiel type I neurons with late AHPs and Dogiel type II neurons from both the inflamed (P < 0.01) and control ileum (P < 0.05, Fig. 3F).
An action potential component that was not blocked by TTX (0.5–1 μm) was prominent in Dogiel type I neurons with late AHPs (Fig. 4Ba and b). This is well established for AH/Dogiel type II neurons from control intestine (North, 1973; Hirst et al. 1974; Wood, 1987) and was confirmed in Dogiel type II neurons from the inflamed intestine (Fig. 4Aa and b). The amplitude of the TTX-resistant component of the action potential in Dogiel type I neurons with late AHPs was smaller than in Dogiel type II neurons.
Figure 4. Sensitivities to tetrodotoxin (TTX) of action potentials of Dogiel type II (DII) and Dogiel type I (DI) neurons from the inflamed ileum.
Aa and b, action potentials in response to intracellular depolarizing current (200 pA, 2 s) in a DII neuron from the inflamed ileum (A) and in the same neuron 15 min after exposure to TTX (500 nm). Depolarization (300 pA, 100 ms) still elicited an active event, although its amplitude was reduced (Ab). Ba and b, for a DI neuron from the inflamed ileum, in which late AHPs were observed, the action potential induced by a 200 pA, 2 s depolarizing pulse in control solution (Ba) was reduced but not blocked by TTX (Bb, 200 and 250 pA, 100 ms pulses). Ca and b, the action potential in DI neurons without late AHPs from the inflamed ileum in response to intracellular depolarizing current (200 pA, 2 s, Ca) was completely blocked by TTX (Cb, 150, 250 and 300 pA, 100 ms pulses). In all cases the membrane potential prior to depolarization was held at −60 mV.
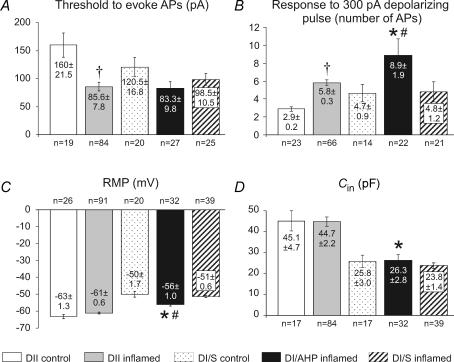
Dogiel type I neurons with late AHPs, like Dogiel type II neurons from the inflamed small intestine, were hyperexcitable (Figs 2C and 7B). The threshold for depolarizing current pulses to evoke an action potential in Dogiel type I neurons with late AHPs was significantly lower than for Dogiel type II neurons from the control ileum (P < 0.01), but not different to Dogiel type II neurons from the inflamed ileum or Dogiel type I neurons without late AHPs from either inflamed or control ileum (P > 0.05, Fig. 5A). The number of action potentials fired in response to the maximum (300 pA) depolarizing current pulse in Dogiel type I neurons with late AHPs was significantly higher compared with all other groups from both inflamed and control ileum (P < 0.05 for all, Fig. 5B). The proportion of Dogiel type I neurons with late AHPs that exhibited anodal break action potentials (47%, 9/19 tested) was higher than that of Dogiel type I neurons without late AHPs from the inflamed ileum (24%, 8/33 tested).
Figure 5. Comparisons of electrophysiological properties of Dogiel type II (DII) and Dogiel type I (DI) neurons from the control and inflamed ileum.
Five groups are compared: DII neurons from control and inflamed ileum, DI neurons from control, DI neurons that exhibited late AHPs from the inflamed ileum and DI neurons that did not exhibit late AHPs from the inflamed ileum. A, thresholds for action potential generation in DII neurons from the inflamed ileum were significantly lower than from control (P < 0.001). Differences between DI neurons were not significant. B, excitability, measured as the numbers of action potentials elicited by 500 ms depolarizing pulses, was significantly greater for DI/AHP neurons from the inflamed intestine compared with DI neurons from control or DI/S neurons from the inflamed ileum (P < 0.05). Excitability was also increased for DII neurons from the inflamed ileum compared with DII neurons from the control ileum (P < 0.05). C, the resting membrane potential (RMP) was not changed in DII neurons, although DI/AHP neurons from the inflamed ileum were more hyperpolarized than DI/S inflamed or control DI neurons (P < 0.05), but less hyperpolarized than DII neurons from the inflamed ileum (P < 0.001). D, there were no significant differences in neuron capacitance. DI/AHP neurons from the inflamed intestine were significantly smaller than DII neurons. Values of mean ± s.e.m. and numbers of neurons analysed for each parameter are incorporated into the histograms. *Significantly different from Dogiel type II neurons from the inflamed ileum (P < 0.05). #Significantly different from Dogiel type I/S neurons from the inflamed ileum (P < 0.05). †Significantly different from Dogiel type II neurons from the control ileum (P < 0.05).
Dogiel type I neurons with late AHPs were more hyperpolarized than Dogiel type I neurons without late AHPs from both inflamed (P < 0.01) and control (P < 0.05, Fig. 5C) ileum, but were less hyperpolarized than Dogiel type II neurons from both inflamed and control ileum (P < 0.001 for both, Fig. 5C).
The input resistance (Rin) in Dogiel type I neurons with late AHPs (185 ± 16 MΩ, n = 32) was not significantly different from other groups of neurons (P > 0.05, Tukey–Kramer multiple comparisons test). Rin values were 172 ± 14 MΩ for Dogiel type I/S neurons from the inflamed ileum (n = 39) and 176 ± 2 MΩ for Dogiel type I neurons from the control ileum (n = 14).
The cell capacitance (Cin) of Dogiel type I neurons with late AHPs was similar to those of Dogiel type I neurons without late AHPs from both inflamed and control ileum (Fig. 5D). Cin of Dogiel type I neurons with late AHPs was significantly different from Dogiel type II neurons from both the inflamed (P < 0.001) and control ileum (P < 0.01).
Currents and channels involved in generating the late AHP in Dogiel type I neurons from the inflamed ileum
The late AHPs in Dogiel type II neurons are caused by opening of Ca2+ channels and activation of intermediate conductance Ca2+-activated potassium (IKCa) channels (Mao et al. 2006; Nguyen et al. 2007). The Ca2+ enters primarily through N-type voltage-gated Ca2+ channels in myenteric Dogiel type II neurons (Rugiero et al. 2002). The inflection/hump on the repolarizing phase of the action potential, due to activation of these voltage-sensitive Ca2+ channels, is generally used for identification of AH/Dogiel type II neurons (Schutte et al. 1995). Analysis of the first time derivatives (dV/dt) of the action potentials revealed an inflection on the action potential repolarizing phase in 10 out of 32 Dogiel type I neurons with late AHPs, but in most (22/32) Dogiel type I neurons with a late AHP, the hump was not observed (Fig. 3C).
Changes in input resistance during the late AHPs in Dogiel type I neurons were similar to those in Dogiel type II neurons (Fig. 3A and C). In both neuron types, Rin was reduced during the hyperpolarization of the late AHP, indicating that K+ conductance was increased. Input resistances, measured as averages of six values of Rin during hyperpolarizing current pulses before and at the peak of AHPs, decreased by 28% in Dogiel type I neurons with late AHPs, from 180 ± 10 to 130 ± 3 MΩ and by 53% in Dogiel type II neurons from 202 ± 15 to 96 ± 3 MΩ. In both Dogiel type II neurons and in Dogiel type I neurons with late AHPs, a depolarization occurred after the fast AHP and before the late AHP (Fig. 3B and D). Thus, in each neuron type there was a delay between the repolarization of the action potential and the beginning of the AHP, as has been described for Dogiel type II neurons, in which an after-depolarizing potential contributes to the delay (Wood & Mayer, 1978; Vogalis et al. 2002b).
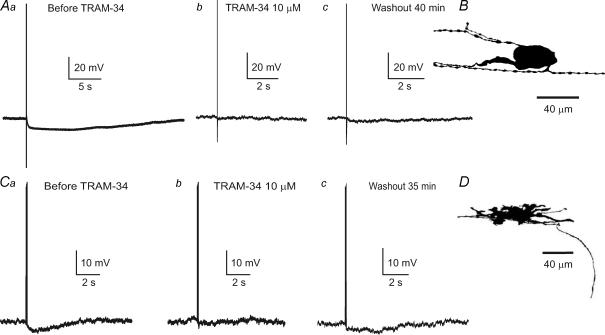
We used the selective IKCa channel blocker, TRAM-34, to determine whether these channels underlie the late AHPs in Dogiel type I neurons from the inflamed ileum, as they do in Dogiel type II neurons. TRAM-34 (10 μm) blocked the late AHPs in both Dogiel type I and Dogiel type II neurons from the inflamed ileum by 98 ± 2% (Fig. 6). For both types of neurons, at least 30 min application of TRAM-34 was required to block the late AHPs. The slow block of IKCa channels was possibly due to the action of TRAM-34 being at the cytoplasmic face of the channel (Wulff et al. 2000). There was a slow and incomplete recovery of the AHPs after washout of TRAM-34 in both neuron types (Fig. 6).
Figure 6. Inhibition of late AHPs by a blocker of intermediate conductance Ca2+-activated potassium (IKCa) channels, TRAM-34.
Aa, b and c, late AHP in a Dogiel type II neuron from the inflamed intestine before the (Aa) and after application of TRAM-34 (10 μm, Ab). The amplitude of late AHP did not recover even after 40 min of washout with Krebs solution (Ac). B, the shape of the neuron from which the records in A were taken. Ca, b and c, late AHP in a Dogiel type I neuron was also blocked by TRAM-34 (10 μm). D, the shape of the Dogiel type I neuron with a late AHP from which the records in C were taken. The membrane potential in both neurons was held at −60 mV.
Dogiel type II neurons
These neurons from the inflamed ileum were hyperexcitable compared with controls. They exhibited a lower threshold of depolarizing current pulse necessary to evoke an action potential compared with Dogiel type II neurons from the control ileum (P < 0.001, Fig. 5A) and they yielded a greater number of action potentials in response to the maximum (300 pA) depolarizing current pulse (P < 0.05, Figs 5B and 7B).
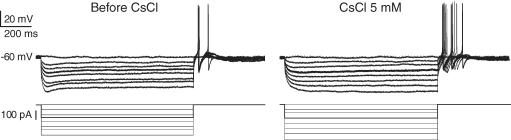
A further indication of the hyperexcitability of Dogiel type II neurons from the inflamed ileum was the high proportion of neurons exhibiting anodal break action potentials: 39% (29/75, Fig. 7C) compared with 23% (6/26) of Dogiel type II neurons from the control ileum, and spontaneous action potentials that occurred at the resting membrane potential in some (5/93) Dogiel type II neurons from the inflamed ileum (Fig. 7D) compared with 0/26 Dogiel type II neurons from the control ileum. Anodal break action potentials were completely blocked by Cs+ (5 mm) in 3/8 tested Dogiel type II neurons from the inflamed ileum, but in the other 5 Dogiel type II neurons from the inflamed ileum Cs+ had no effect on anodal break action potentials (Fig. 8). In all neurons from the inflamed ileum, the sag in the voltage response to hyperpolarizing current, which is due to the hyperpolarization-activated cation current (Ih), was blocked by Cs+.
Figure 8. Persistence of anodal break action potentials in DII neurons from the inflamed ileum after additin of Cs+.
In control solution there was an obvious sag in the voltage response to imposed hyperpolarization, which is due to the activation of a hyperpolarization activated cation current (Ih). Ih persists after the end of hyperpolarizing steps, and this tail current has been implicated as a cause of the anodal break. However, when Ih was blocked by Cs+ (5 mm), anodal break action potentials were still observed in some DII neurons from the inflamed intestine.
The cell input resistance in Dogiel type II neurons from the inflamed ileum (191 ± 9.4 MΩ, n = 86) was higher than in Dogiel type II neurons from the control ileum (144.3 ± 10.5 MΩ, n = 26, P < 0.05).
Action potential amplitude and duration, resting membrane potential and cell capacitance in Dogiel type II neurons from the inflamed ileum were not significantly different from those in Dogiel type II neurons from the control ileum (for all P > 0.05, Figs 3E and F, and 5C and D). The late AHP amplitude and its duration in Dogiel type II neurons from the inflamed and control ileum were also similar (for both P > 0.05, Fig. 3G and H).
Fast synaptic transmission in neurons with late AHPs from the inflamed ileum
The properties of fast EPSPs in all neurons were measured as averages of 20 fast EPSPs that were recorded with a holding current that set the cell membrane potential at −90 mV. Amongst Dogiel type II neurons from the inflamed ileum, only 1/52 tested received a fast synaptic input. The average amplitude of fast EPSPs recorded from this neuron was 8.6 ± 1.1 mV and duration at half-amplitude was 8.1 ± 0.3 ms. All Dogiel type I neurons with late AHPs had prominent fast synaptic inputs. The parameters of these fast EPSPs were measured in 18 of these neurons. The average amplitude was 14.9 ± 1.1 mV and the duration at half-amplitude was 13.7 ± 3.4 ms. The amplitudes and the durations of fast EPSPs in Dogiel type I neurons with late AHPs were not significantly different from these parameters in either Dogiel type I neurons without late AHPs from the inflamed intestine or from control intestine (P > 0.05 for both). Values of fast EPSP amplitude and duration in Dogiel type I neurons without late AHPs from the inflamed intestine were 20.2 ± 2.3 mV and 16.3 ± 1.1 ms (n = 23), respectively; in neurons from control intestine, the fast EPSP amplitude was 18.8 ± 1.4 mV and the width at half-amplitude was 19.8 ± 1.7 ms (n = 13).
Properties of uniaxonal neurons without late AHPs from the inflamed ileum
Forty-one neurons with S type electrophysiological properties (Hirst et al. 1974) have been recorded from the inflamed ileum and subsequently identified morphologically. They were 39 Dogiel type I cells (26 with descending, 9 with ascending axons, 2 local circular muscle motor neurons, 1 local tertiary plexus neuron and 1 secretomotor neuron) and 2 filamentous neurons (1 with a descending and 1 with an ascending axon). Four of the descending Dogiel type I neurons had axons that projected to and ramified in the circular muscle and therefore these neurons were almost certainly descending CMMNs. The secretomotor neuron was identified by its morphology (Furness, 1985). Electrophysiological properties of these neurons were compared with the properties of 22 Dogiel type I/S neurons from the control intestine.
The majority of Dogiel type I neurons with S electrophysiological properties from the inflamed ileum were not hyperexcitable (25/28 tested) in response to depolarizing current pulses (Fig. 9Aa and b). The threshold for depolarization to evoke an action potential was not significantly different to that of Dogiel type I/S neurons from the control intestine (Fig. 5A). The number of action potentials in response to a 300 pA depolarizing pulse in Dogiel type I/S neurons from the inflamed ileum was similar to that in Dogiel type I/S neurons from the control ileum.
Figure 9. Excitability and morphology of Dogiel type I neurons without AHPs from the inflamed ileum.
Aa and b, excitability of a DI neuron with typical S electrophysiological properties (no AHP) from the inflamed ileum. Such neurons were 25/28 DI/S neurons that were tested. The threshold for depolarization to evoke an action potential was about 100 pA (Aa). The number of action potentials in response to a 300 pA depolarizing pulse was up to six (Ab). Ba and b, records from a hyperexcitable DI/S neuron from the inflamed ileum. These were 3/28 of the DI/S neurons. B, the hyperexcitable neurons responded with multiple action potentials to a 100 pA depolarizing current pulse. Bb, response to 300 pA depolarizing current pulses revealed their hyperexcitability. C, the three hyperexcitable neurons did not have a late AHP, but had prominent anodal break action potentials (D). Shapes of DI/S neurons without AHPs from the inflamed intestine are shown at the right (E). The records in Ba and b were from the neuron marked with an asterisk. Holding potential was −50 mV in A, Aa, B, Ba and D.
The other 3/28 tested Dogiel type I/S neurons from the inflamed ileum were hyperexcitable (Fig. 9Ba and b). The hyperexcitable neurons had low thresholds for action potential initiation in response to depolarization (55 ± 10 pA, n = 3) and fired in average 34 ± 11 action potentials in response to a 300 pA depolarizing current pulse (n = 3). This subgroup consisted of one ascending and two descending Dogiel type I neurons. All three neurons were localized in the middle of their ganglion. The resting membrane potentials in these three neurons (−50 ± 1.4 mV) were similar to those of other Dogiel type I/S neurons from the inflamed ileum (−51 ± 1.5 mV). Anodal break action potentials that were rarely seen in Dogiel type I/S cells in these series of experiments (5/28 cells), were prominent in 2 out of 3 hyperexcitable Dogiel type I/S neurons (Fig. 9E). The average cell capacitance for these neurons was 35.5 ± 4.2 pF (n = 3) and the average input resistance was 184 ± 33 MΩ (n = 3); these parameters were not statistically different from those in other Dogiel type I/S neurons from the inflamed ileum. In all other respects, these three neurons were also not different from the majority of Dogiel type I/S cells from the inflamed ileum, therefore all parameters were included in data for the combined Dogiel type I/S group. All electrophysiological properties of Dogiel type I/S neurons from the inflamed ileum were not significantly different from those in Dogiel type I/S neurons from the control intestine (Figs 3 and 5).
Fast EPSPs were recorded in all Dogiel type I/S neurons from the inflamed ileum (n = 39). As was mentioned above, the amplitudes and durations of fast EPSPs were not significantly different from Dogiel type I neurons of inflamed and control ileum.
Discussion
The present results show that 45% (32/71) of Dogiel type I neurons from inflamed ileum had an electrophysiological phenotype that was distinctly different from that normally exhibited by these neurons. The phenotypically changed neurons exhibited prominent late AHPs and in many respects their electrophysiological phenotype resembled that of Dogiel type II neurons. In addition, neurons with Dogiel type II morphology became more excitable following inflammation, but retained the major characteristics that define their electrophysiological phenotype: a large amplitude action potential with a hump on the falling phase and a prominent, long-lasting, late AHP. In both control and inflamed ileum very few Dogiel type II neurons exhibited fast excitatory synaptic potentials.
Phenotypically changed Dogiel type I neurons
The prolonged AHPs in transformed Dogiel type I neurons were associated with a conductance increase and were blocked by TRAM-34 (10 μm), which is highly selective for intermediate conductance, Ca2+-activated potassium (IKCa) channels over other classes of K+ channel (Wulff et al. 2000). In enteric Dogiel type II neurons, IKCa channels are activated following entry of Ca2+ during the action potential, augmented by Ca2+-activated Ca2+ release from intracellular stores (Hillsley et al. 2000; Vogalis et al. 2002a). Dogiel type I neurons from control intestine, in earlier work identified by their S electrophysiological phenotype, do not appear to have a Ca2+ contribution to the action potential, which is completely blocked by tetrodotoxin (North, 1973; Hirst et al. 1974; Wood, 1987). We found that Dogiel type I neurons with late AHPs had a TTX-resistant component of their action potential. Thus, the transformation of Dogiel type I neurons could be due to the activation of pre-existing IKCa channels following the emergence of a Ca2+ component of the action potential. Whether there is de novo or augmented expression of IKCa channels in these Dogiel type I neurons remains to be determined.
In normal intestine, Dogiel type II neurons have broader action potentials compared with those of Dogiel type I neurons and a prominent inflection (hump) on their repolarizing phase due to the Ca2+ component. Previously, the inflection on the repolarizing phase of an action potential and the presence of a TTX-resistant component were considered to be reliable features for identification of AH/Dogiel type II neurons (Hirst et al. 1985; Schutte et al. 1995; Furness et al. 1998). Our results show that this cannot be applied to the classification of neurons from the inflamed intestine, as 30% of the phenotypically changed Dogiel type I neurons had action potentials with inflections. The absence of inflection in some of the neurons may be due to differences in the kinetics of Ca2+ currents activated during the action potential, or to other differences, such as in the properties of delayed rectifier and/or large conductance (BK) potassium channels that are involved in repolarization. The amplitudes and durations of action potentials in Dogiel type I neurons with late AHPs were similar to those in unchanged Dogiel type I cells.
Without their morphological identification by dye filling at the time of recording, the transformed Dogiel type I neurons could easily be misidentified as AH cells, which are normally neurons with Dogiel type II morphology. However, one distinguishing electrophysiological characteristic did remain: these Dogiel type I neurons with late AHPs, but very rarely Dogiel type II cells from the control or inflamed ileum, had fast synaptic inputs. The amplitudes and durations of fast EPSPs in Dogiel type I neurons with late AHPs were not significantly different compared with amplitudes and durations of fast EPSPs in Dogiel type I/S neurons from the inflamed or control ileum. This is an interesting observation, because inflammatory mediators, applied acutely to myenteric ganglia, reduce the amplitudes of fast EPSPs (Xia et al. 1999; Gao et al. 2002). Our observations suggest that this reduction, if it occurs in vivo, does not persist. Our observations are consistent with those of Krauter et al. (2007), who found that fast EPSPs had greater amplitude than control 6 days after TNBS was injected into the lumen of the colon.
Late AHPs have been previously reported in a small subpopulation of uniaxonal neurons from non-inflamed small intestine or colon. Late AHPs in these neurons were less prominent than in Dogiel type II neurons, and the AHP was sometimes only observed after a volley of action potentials (Song et al. 1997; Clerc et al. 1998; Lomax et al. 1999; Tamura et al. 2001; Nurgali et al. 2003). The late AHPs in transformed Dogiel type I neurons were significantly briefer in duration compared with Dogiel type II neurons from both the inflamed and control ileum, but they were of similar amplitude (about 8 mV) and occurred after a single action potential. A late AHP following a burst of action potentials has been reported in highly excitable Dogiel type I neurons which are located at the corners of the ganglia close to internodal strands in the myenteric plexus of the guinea-pig ileum (Smith et al. 1999). The AHP in these cells was reduced by apamin, which blocks SK but not IKCa channels, and does not affect the AHP in other enteric neurons (Tack & Wood, 1992; Vogalis et al. 2002a). The uniaxonal neurons in which we found AHPs after inflammation were not these neurons: they differed in size, shape and projections (see below).
The majority of Dogiel type I neurons with changed phenotype (31/32) had descending axons. The other neuron was deduced to be a local circular muscle motor neuron, from its shape and the projection of its axon to the muscle. In the non-inflamed small intestine, three classes of Dogiel type I neurons with descending axons have been identified in the myenteric plexus: inhibitory circular muscle motor neuron that are immunoreactive for nitric oxide synthase (NOS) and comprise 16% of all neurons in the myenteric plexus; NOS-immunoreactive descending interneurons (5%); and 5-HT-immunoreactive descending interneurons (2%, Furness, 2006). Except in rare cases, we were not able to distinguish between the circular muscle motor neurons and interneurons, due to the removal of the circular muscle layer during preparation for electrophysiology. Three neurons (1 local, 2 descending) were traced to the circular muscle, and none to the longitudinal muscle. The cell capacitance of Dogiel type I neurons with late AHPs was not different from the cell capacitance in unchanged Dogiel type I neurons, indicating that these cells have the same size as other Dogiel type I neurons, on average.
Dogiel type I neurons with late AHPs from the inflamed ileum had low thresholds for depolarizing current pulses to evoke action potentials, they fired more action potentials to depolarization, but only 1/32 exhibited spontaneous action potentials. The lower threshold for generation of action potentials might be contributed by a reduced threshold for activation of voltage-activated Na+ channels in these neurons after inflammation. In addition, a higher incidence of anodal break action potentials was observed in Dogiel type I neurons with AHPs (47%), compared with Dogiel type I/S neurons from the inflamed ileum (24%).
Changes in Dogiel type II neurons
Dogiel type II neurons from the inflamed ileum were hyperexcitable, but their electrophysiological phenotype was very similar to that of Dogiel type II neurons from control ileum. In both cases the neurons had large amplitude action potentials with an inflection on the falling phase and a component of the action potential was resistant to tetrodotoxin. The action potentials were followed by prolonged AHPs. After inflammation, the neurons had higher input resistances, reduced thresholds for depolarizing current pulses to evoke action potentials, fired more action potentials to depolarization and exhibited spontaneous action potentials. This conversion from a low excitability or unexcitable state to a hyperexcitable state is typical of AH/Dogiel type II neurons that are exposed to inflammatory mediators, for example, histamine (Tamura & Wood, 1992), interleukins (Xia et al. 1999) or leukotrienes (Liu et al. 2003). Enhancement of neuronal excitability and phenotypic changes in myenteric neurons suggest a plasticity of enteric neurons that is initiated through the actions of various inflammatory mediators released during TNBS-induced ileitis. Further mechanisms may be involved in the maintenance of the changes.
Spontaneous action potentials are not observed in Dogiel type II neurons from control ileum under the conditions used in this study. Despite the fact that spontaneous action potentials occurred in the neurons from inflamed ileum, they were not depolarized relative to Dogiel type II neurons of control ileum. This, and the lower threshold for generation of action potentials by intracellular current injection, suggests that there was a reduced threshold for activation of voltage-sensitive Na+ currents in Dogiel type II neurons after inflammation.
Action potentials that follow hyperpolarizing voltage steps in Dogiel type II neurons (anodal break action potentials) have been attributed to the Cs+-sensitive hyperpolarization-activated cation current, Ih (Galligan et al. 1990; Rugiero et al. 2002; Xiao et al. 2004). The incidence of anodal break action potentials in Dogiel type II neurons was 39% in the inflamed ileum, compared with 23% in the control ileum. It has been reported that Ih is increased during TNBS-induced inflammation in AH myenteric neurons in the distal colon (Linden et al. 2003). However, the present study indicated that the increase in the prominence of anodal break action potentials was not simply due to a change in Ih. Although Ih was blocked by CsCl in the Dogiel type II neurons, we continued to observe anodal break action potentials in some neurons. The resistance of these events to CsCl suggests that activation of other current(s) was involved in the generation of anodal break action potentials in AH neurons from the inflamed intestine. The other current that can cause anodal break firing is the low-voltage-activated T-type Ca2+ current (Jahnsen & Llinas, 1984; Huguenard, 1996). T currents have not been previously reported in enteric neurons and we are currently investigating the properties of this current and its channels in myenteric neurons.
The Ih current has been implicated in limiting the amplitude and duration of the AHP (Galligan et al. 1990), and its enhancement has been suggested to decrease the magnitude of the late AHP in myenteric AH neurons of the distal colon following inflammation (Linden et al. 2003). However, in the present study there were no differences in the amplitudes and durations of the late AHPs in Dogiel type II neurons from the control and inflamed ileum. Consistent with our results, Lomax et al. (2005) did not detect any difference in the effects of 2 mm CsCl on the AHP of submucosal AH neurons from the inflamed and control distal colon. Moreover, these authors showed, by quantitative analysis of the amplitude of the Ih current (measured as the amplitude of the ‘sag’ that occurred during 500 ms hyperpolarization current injection), that there is no difference in the Ih amplitude between inflamed and control AH neurons. Nevertheless, a reduction of AHP magnitude was observed in submucosal AH neurons, which was associated with a reduced duration of action potential, and presumably a smaller influx of Ca2+ (Lomax et al. 2005).
Dogiel type I neurons without phenotypic change
The electrophysiological phenotype of the majority of Dogiel type I neurons from the inflamed guinea-pig ileum was not different from the Dogiel type I neurons of the control ileum; that is, these neurons had the S type phenotype, and accordingly we refer to these as Dogiel type I/S neurons. Although the majority of Dogiel type I/S neurons from the inflamed ileum (36/39) were not detectably different from Dogiel type I/S neurons from the control ileum, three Dogiel type I/S neurons from inflamed intestine were hyperexcitable. They had low thresholds for depolarizing current pulses to evoke action potentials (53 ± 10 pA) and fired on average 34 ± 11 action potentials in response to 300 pA depolarizing current pulses.
Hyperexcitable S neurons have been previously reported in the non-inflamed guinea-pig ileum by Smith et al. (1999). The authors described tonic S neurons characterized by low resting membrane potentials, high input resistance (about 500 MΩ), due to a small size of their cell bodies, low threshold for action potential generation, firing of action potentials throughout a depolarizing pulse and spontaneous fast EPSPs. Morphologically they were identified as motor neurons and ascending interneurons located at the corners of large ganglia close to internodal strands. In our experiments the hyperexcitable Dogiel type I/S neurons had cell capacitance of 33.5 ± 3.5 pF and input resistance of 196.3 ± 27 MΩ; these parameters were not statistically different from those in other Dogiel type I/S neurons from the inflamed ileum. In all other respects these three neurons were not different from the majority of Dogiel type I/S neurons. Morphologically they were identified as one ascending Dogiel type I and two descending Dogiel type I neurons. A hyperexcitable filamentous neuron was also encountered. These neurons were localized in the middles of their ganglia.
In a study in non-inflamed intestine with intact mucosa, Kunze et al. (1997) reported that some S neurons that received ongoing input from Dogiel type II neurons were hyperexcitable. We encountered spontaneously active Dogiel type II neurons in our experiments in inflamed ileum which could feasibly have provided slow excitatory inputs to the Dogiel type I/S neurons and contributed to their hyperexcitability.
Other phenotypic changes
In the present work, we have investigated phenotypic changes in the electrophysiological properties of enteric neurons. It is probable that other changes occur, for example in neurochemistry, including neuropeptide and receptor expression. However, there is no evidence of substantial morphological change. All the filled neurons could be classified into the morphological types that have been documented in the normal intestine, and it seems unlikely that the neurons switch their shapes and projections. Future studies will be needed to carefully examine which other changes occur in the neurons.
Comparison with previous studies
The only previous electrophysiological study of post-inflammatory changes in myenteric neurons of the small intestine was following infection with Trichinella spiralis (Palmer et al. 1998). In that study, an enhanced excitability of neurons with the AH electrophysiological phenotype was reported, but whether the AH-type neurons after inflammation included both Dogiel type II and Dogiel type I neurons was not determined, because cells were not identified by morphology. Palmer et al. (1998) found that 53% of AH neurons from Trichinella spiralis-infected guinea-pigs had fast EPSPs. In our study, all 32 Dogiel type I neurons with AHPs had fast EPSPs, but only 1 of 93 Dogiel type II neurons from the inflamed ileum had a fast EPSP. This suggests that the AH cell population identified by Palmer et al. included both Dogiel type II and Dogiel type I neurons. In other regions also, the most prominent change observed in electrophysiological properties was the enhanced excitability of AH neurons. This has been reported in both myenteric (Linden et al. 2003) and submucosal neurons (Lomax et al. 2005, 2006; O'Hara et al. 2007) from the guinea-pig distal colon. The majority of myenteric neurons (61%) were identified morphologically in the study of Linden et al. (2003), although no illustrations were included. They reported that changed excitability occurred in AH/Dogiel type II neurons.
In each place, different mechanisms have been uncovered. As is discussed above, we have found that increased excitability of Dogiel type II neurons was associated with a lowered voltage threshold for action potential generation and possibly the de novo appearance of a T-like current, whereas in myenteric neurons of the distal colon an enhanced h current was implicated (Linden et al. 2003) and in submucosal neurons there was a reduced Ca2+ influx during the action potential that had the consequence of reducing the AHP (Lomax et al. 2005, 2006).
Further studies are required to define what impact the phenotypic changes in neurons found in this study have on changes in motility or secretion that occur in the gastrointestinal tract after inflammation (Kellow, 2002; De Giorgio et al. 2004; Lomax et al. 2004; Wood, 2004). Modulation of the AHP by compounds affecting IKCa channels have profound effects on the motility of the non-inflamed intestine (Ferens et al. 2007), so it is feasible that the de novo appearance of the AHP in descending Dogiel type I interneurons or inhibitory motor neurons will affect motility. Whether the changes in currents and channels are due to the direct effects of inflammatory mediators or are secondary responses in the cascade of events following inflammation remains open, and a difficult problem to resolve.
Conclusion
The present study demonstrates that inflammation has different effects on the electrophysiological properties of different types of neurons in the small intestine. We revealed that phenotypic changes in a subset of Dogiel type I neurons that occur after inflammation involve the unmasking of IKCa-like channels that are involved in the generation of the late AHP. As normally occurs in Dogiel type II neurons, activation of TTX-resistant voltage-activated inward currents and IKCa-like channels occurred in the Dogiel type I neurons with late AHPs. The changes were sufficient that electrophysiological characteristics could no longer be used to identify functional classes of neurons. Therefore morphological identification is important to define the functional classes of neurons affected by inflammation.
Acknowledgments
This work was supported by a project grant, number 400020, from the National Health and Medical Research Council (NHMRC) of Australia. K.N. is supported by a NHMRC Peter Doherty (Biomedical) Fellowship, number 400472. We thank Professor J. C. Bornstein for the use of laboratory facilities. Louise Pontell is thanked for technical assistance.
References
- Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–G855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Ozaki N, Gebhart GF. Mild gastritis alters voltage-sensitive sodium currents in gastric sensory neurons in rats. Gastroenterology. 2002;122:752–761. doi: 10.1053/gast.2002.31901. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108:237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Brehmer A, Schrodl F, Neuhuber W. Morphological classifications of enteric neurons – 100 years after Dogiel. Anat Embryol. 1999;200:125–135. doi: 10.1007/s004290050267. [DOI] [PubMed] [Google Scholar]
- Brookes SJH. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Clerc N, Furness JB, Bornstein JC, Kunze WAA. Correlation of electrophysiological and morphological characteristics of myenteric neurons of the duodenum in the guinea-pig. Neuroscience. 1998;82:899–914. doi: 10.1016/s0306-4522(97)00318-7. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Tate S, Carlton SM. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett. 2004;355:45–48. doi: 10.1016/j.neulet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol. 2004;286:G573–G579. doi: 10.1152/ajpgi.00258.2003. [DOI] [PubMed] [Google Scholar]
- De Giorgio R, Guerrini S, Barbara G, Stanghellini V, De Ponti F, Corinaldesi R, Moses PL, Sharkey KA, Mawe GM. Inflammatory neuropathies of the enteric nervous system. Gastroenterology. 2004;126:1872–1883. doi: 10.1053/j.gastro.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Dogiel AS. Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere. Arch Anat Physiol Leipzig Anat Abt Jg. 1899;1899:130–158. [Google Scholar]
- Ferens D, Baell D, Lessene G, Smith JE, Furness JB. Effects of modulators of Ca2+-activated, intermediate-conductance potassium channels on motility of the rat small intestine, in vivo. Neurogastroenterol Motil. 2007;19:383–389. doi: 10.1111/j.1365-2982.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with trichinella spiralis. Gastroenterology. 1994;107:1602–1609. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Oxford: Blackwell Publishing; 2006. p. 274. [Google Scholar]
- Furness JB, Costa M, Gibbins IL, Llewellyn-Smith IJ, Oliver JR. Neurochemically similar myenteric and submucous neurons directly traced to the mucosa of the small intestine. Cell Tissue Res. 1985;241:155–163. doi: 10.1007/BF00214637. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Tatsumi H, Shen KZ, Surprenant A, North RA. Cation current activated by hyperpolarization (IH) in guinea-pig enteric neurons. Am J Physiol Gastrointest Liver Physiol. 1990;259:G966–G972. doi: 10.1152/ajpgi.1990.259.6.G966. [DOI] [PubMed] [Google Scholar]
- Gao C, Liu S, Hu H-Z, Gao N, Kim GY, Xia Y, Wood JD. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- Harriott AM, Dessem D, Gold MS. Inflammation increases the excitability of masseter muscle afferents. Neuroscience. 2006;141:433–442. doi: 10.1016/j.neuroscience.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Kenyon JL, Smith TK. Ryanodine-sensitive stores regulate the excitability of AH neurons in the myenteric plexus of guinea-pig ileum. J Neurophysiol. 2000;84:2777–2785. doi: 10.1152/jn.2000.84.6.2777. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Johnson SM, van Helden DF. The calcium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985;361:297–314. doi: 10.1113/jphysiol.1985.sp015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellow JE. Advances in the management of irritable bowel syndrome. J Gastroenterol Hepatol. 2002;17:503–507. doi: 10.1046/j.1440-1746.2002.02759.x. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Krauter EM, Linden DR, Sharkey KA, Mawe GM. Synaptic plasticity in myenteric neurons of the guinea pig distal colon: Presynaptic mechanisms of inflammation-induced synaptic facilitation. J Physiol. 2007;581:787–800. doi: 10.1113/jphysiol.2007.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WAA, Bertrand PP, Furness JB, Bornstein JC. Influence of the mucosa on the excitability of myenteric neurons. Neuroscience. 1997;76:619–634. doi: 10.1016/s0306-4522(96)00408-3. [DOI] [PubMed] [Google Scholar]
- Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003;547:589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hu HZ, Gao C, Gao N, Wang G, Wang X, Gao X, Xia Y, Wood JD. Actions of cysteinyl leukotrienes in the enteric nervous system of guinea-pig stomach and small intestine. Eur J Pharmacol. 2003;459:27–39. doi: 10.1016/s0014-2999(02)02820-0. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2004;17:4–15. doi: 10.1111/j.1365-2982.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 2005;564:863–875. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, O'Hara JR, Hyland NP, Mawe GM, Sharkey KA. Persistent alterations to enteric neural signalling in guinea pig colon following resolution of colitis. Am J Physiol Gastrointest Liver Physiol. 2006;292:482–491. doi: 10.1152/ajpgi.00355.2006. [DOI] [PubMed] [Google Scholar]
- Lomax AEG, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv Syst. 1999;76:45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Mao Y, Wang B, Kunze W. Characterization of myenteric sensory neurons in the mouse small intestine. J Neurophysiol. 2006;96:998–1010. doi: 10.1152/jn.00204.2006. [DOI] [PubMed] [Google Scholar]
- Martinolle JP, Garcia-Villar R, Fioramonti J, Bueno L. Altered contractility of circular and longitudinal muscle in TNBS-inflamed guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1258–G1267. doi: 10.1152/ajpgi.1997.272.5.G1258. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Vanegas H, Nebe J, Rumenapp P, Schaible HG. Effects of N- and L-type calcium channel antagonists on the responses of nociceptive spinal cord neurons to mechanical stimulation of the normal and the inflamed knee joint. J Neurophysiol. 1996;76:3740–3749. doi: 10.1152/jn.1996.76.6.3740. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Matsuyama H, Baell J, Hunne B, Fowler CJ, Smith JE, Nurgali K, Furness JB. Effects of compounds that influence KCNN4 channels on after-hyperpolarizing potentials, and determination of IK channel sequence, in guinea pig enteric neurons. J Neurophysiol. 2007;97:2024–2031. doi: 10.1152/jn.00935.2006. [DOI] [PubMed] [Google Scholar]
- North RA. The calcium-dependent slow after-hyperpolarization in myenteric plexus neurone with tetrodotoxin-resistant action potentials. Br J Pharmacol. 1973;49:709–711. doi: 10.1111/j.1476-5381.1973.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurgali K, Furness JB, Stebbing MJ. Correlation of electrophysiology, shape and synaptic properties of myenteric AH neurons of the guinea-pig distal colon. Autonomic Neurosci. 2003;103:50–64. doi: 10.1016/s1566-0702(02)00212-6. [DOI] [PubMed] [Google Scholar]
- O'Hara JR, Lomax AE, Mawe GM, Sharkey KA. Ileitis alters neuronal and enteroendocrine signalling in guinea-pig distal colon. Gut. 2007;56:186–194. doi: 10.1136/gut.2006.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Wong Riley M, Sharkey KA. Functional alterations in jejunal myenteric neurons during inflammation in nematode-infected guinea pigs. Am J Physiol Gastrointest Liver Physiol. 1998;275:G922–G935. doi: 10.1152/ajpgi.1998.275.5.G922. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Gola M, Kunze WAA, Reynaud J-C, Furness JB, Clerc N. Analysis of whole cell currents by patch clamp of guinea-pig myenteric neurones in intact ganglia. J Physiol. 2002;538:447–463. doi: 10.1113/jphysiol.2001.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa H, Kurihara T, Zong S, Kazuno A, Matsuda Y, Nonaka T, Han W, Toriyama H, Tanabe T. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 2001;20:2349–2356. doi: 10.1093/emboj/20.10.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte IWM, Kroese ABA, Akkermans LMA. Somal size and location within the ganglia for electrophysiologically identified myenteric neurons of the guinea pig ileum. J Comp Neurol. 1995;355:563–572. doi: 10.1002/cne.903550406. [DOI] [PubMed] [Google Scholar]
- Sharkey KA. Visceral sensation and colitis: inflammation and hypersensitivity do not always go hand in hand. Neurogastroenterol Motil. 2006;18:87–90. doi: 10.1111/j.1365-2982.2005.00743.x. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Kroese ABA. Consequences of intestinal inflammation of the enteric nervous system: Neuronal activation induced by inflammatory mediators. Anat Rec. 2001;262:79–90. doi: 10.1002/1097-0185(20010101)262:1<79::AID-AR1013>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sluka KA. Blockade of N- and P/Q-type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J Pharmacol Exp Ther. 1998;287:232–237. [PubMed] [Google Scholar]
- Smith TK, Burke EP, Shuttleworth CWR. Topographical and electrophysiological characteristics of highly excitable S neurones in the myenteric plexus of the guinea-pig ileum. J Physiol. 1999;517:817–830. doi: 10.1111/j.1469-7793.1999.0817s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZM, Brookes SJH, Ramsay GA, Costa M. Characterization of myenteric interneurons with somatostatin immunoreactivity in the guinea-pig small intestine. Neuroscience. 1997;80:907–923. doi: 10.1016/s0306-4522(96)00605-7. [DOI] [PubMed] [Google Scholar]
- Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol. 2003;552:797–807. doi: 10.1113/jphysiol.2003.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack JF, Wood JD. Electrical behaviour of myenteric neurones in the gastric antrum of the guinea-pig. J Physiol. 1992;447:49–66. doi: 10.1113/jphysiol.1992.sp018990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Yoshida S, Matsumoto S. Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience. 2006;138:621–630. doi: 10.1016/j.neuroscience.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Tamura K, Ito H, Wade PR. Morphology, electrophysiology, and calbindin immunoreactivity of myenteric neurons in the guinea pig distal colon. J Comp Neurol. 2001;437:423–437. doi: 10.1002/cne.1293. [DOI] [PubMed] [Google Scholar]
- Tamura K, Wood JD. Effects of prolonged exposure to histamine on guinea pig intestinal neurons. Dig Dis Sci. 1992;37:1084–1088. doi: 10.1007/BF01300291. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR, Furness JB. TEA- and apamin-resistant KCa channels in guinea-pig myenteric neurons: slow AHP channels. J Physiol. 2002a;538:421–433. doi: 10.1113/jphysiol.2001.012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR, Lohman R-J, Furness JB. Action potential afterdepolarization mediated by a Ca2+-activated cation conductance in myenteric AH-neurons. Neuroscience. 2002b;115:375–393. doi: 10.1016/s0306-4522(02)00410-4. [DOI] [PubMed] [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 67–100. [Google Scholar]
- Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Wood JD, Mayer CJ. Intracellular study of electrical activity of Auerbach's plexus in guinea–pig small intestine. Pflugers Archive European Journal of Physiology. 1978;374:265–275. doi: 10.1007/BF00585604. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hänsel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Nat Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH, Wood JD. IL-1b and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J Clin Invest. 1999;103:1309–1316. doi: 10.1172/JCI5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Nguyen TV, Ngui K, Strijbos PJLM, Selmer IS, Neylon CB, Furness JB. Molecular and functional analysis of hyperpolarisation-activated nucleotide gated (HCN) channels in the enteric nervous system. Neuroscience. 2004;129:603–614. doi: 10.1016/j.neuroscience.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, De Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]