Abstract
Recent studies suggest that altered neural regulation of the gastrointestinal microvasculature contributes to the pathogenesis of inflammatory bowel disease. Therefore, we employed video microscopy techniques to monitor nerve-evoked vasoconstrictor responses in mouse colonic submucosal arterioles in vitro and examined the effect of 2,4,6-trinitrobenzene sulphonic acid (TNBS) colitis. Nerve stimulation (2–20 Hz) caused frequency-dependent vasoconstrictor responses that were abolished by tetrodotoxin (300 nm) and guanethidine (10 μm). The P2 receptor antagonist suramin (100 μm) or the α1-adrenoceptor antagonist prazosin (100 nm) reduced the vasoconstriction and the combination of suramin and prazosin completely abolished responses. Nerve-evoked constrictions of submucosal arterioles from mice with TNBS colitis were inhibited by prazosin but not suramin. Superfusion of ATP (10 μm) resulted in large vasoconstrictions in control mice but had no effect in mice with colitis whereas constrictions to phenylephrine (3 μm) were unaffected. P2X1 receptor immunohistochemistry did not suggest any alteration in receptor expression following colitis. However, Western blotting revealed that submucosal P2X1 receptor expression was increased during colitis. In contrast to ATP, αβ-methylene-ATP (1 μm), which is resistant to catabolism by nucleotidases, constricted control and TNBS arterioles. This indicates that reduced purinergic transmission to submucosal arterioles may be due to increased degradation of ATP during colitis. These data comprise the first description of the neural regulation of mouse submucosal arterioles and identify a defect in sympathetic regulation of the GI vasculature during colitis due to reduced purinergic neurotransmission.
Maintenance of the intestinal mucosal barrier limits activation of the enteric immune system and is thought to be an important regulator of intestinal inflammation (Fiocchi, 1998). Mucosal microvascular dysfunction, which alters mucosal barrier function, may be a contributing factor to the chronic relapsing and remitting course of inflammatory bowel diseases (IBD) (Thornton & Solomon, 2002; Hatoum & Binion, 2005). In contrast to acute inflammation where blood flow to affected sites is transiently increased, blood flow to chronically inflamed regions of gut in IBD patients is reduced (Hatoum & Binion, 2005).
The microvasculature of the gastrointestinal tract is subject to a complex hierarchy of neural control mechanisms. In the guinea-pig gastrointestinal tract, sympathetic postganglionic neurons provide vasoconstrictor input to submucosal arterioles while enteric submucosal vasomotor neurons and extrinsic primary afferent neurons innervate the vessels and release vasodilator substances (Vanner & Surprenant, 1996). Submucosal arterioles determine mucosal blood flow as they are the primary resistance vessels of the splanchnic vasculature (Lundgren, 1984; Granger et al. 1989). Therefore, a disruption in the balance between vasoconstrictor and vasodilator influences may lead to altered blood flow to the GI mucosa.
While there is evidence of anatomical remodelling of the gut microvasculature (Anthony et al. 1997; Anthony, 1999) and angiogenesis (Danese et al. 2006) in IBD, there are also emerging data that point to a role for aberrant neural regulation of GI arterioles in these conditions (Hatoum et al. 2003a). Hatoum et al. (2003a) demonstrated that submucosal arterioles from IBD patients did not dilate in response to acetylcholine due to changes in endothelial release of vasodilators, suggesting that enteric cholinergic vasodilator innervation of these arterioles is altered in IBD. Moreover, a recent in vivo study of mouse colonic submucosal arterioles demonstrated that bradykinin-induced vasodilations were reduced during dextran sodium sulphate (DSS) colitis due to an endothelial defect (Mori et al. 2005). In addition, several recent studies have shown that inflammation markedly alters the innervation of the GI tract (Collins, 1996; Mawe et al. 2004; Beyak & Vanner, 2005; Lomax et al. 2005a). These studies indicate that inflammation affects multiple neural regulatory pathways in the GI tract. Thus, the aim of the present study was to test the hypothesis that GI inflammation alters the neural regulation of blood flow in the GI tract, with a particular emphasis on the vasoconstrictor innervation by sympathetic neurons.
Although great strides have been made in defining the neural circuitry that regulates blood flow in the gastrointestinal tract (Vanner & Surprenant, 1996; Vanner & MacNaughton, 2004), the majority of these experiments have been performed in a single species, the guinea-pig. Given the increased availability of immunologically characterized models of IBD in mice compared to other species, our goal was to determine the extent and mechanism of altered neural control of the GI vasculature in the 2,4,6-trinitrobenzene sulphonic acid (TNBS) model of colitis in mice (Strober et al. 2002). Therefore, we first characterized the normal neural regulation of mouse colon submucosal arterioles and then compared these characteristics to data obtained in tissue from mice with TNBS colitis.
Methods
Male CD-1 mice weighing 25–35 g were obtained from Charles River Laboratories (Montreal, PQ, Canada). Experimental protocols were approved by the Queen's University Animal Care Committee and conformed to the Guidelines of the Canadian Council of Animal Care. Mice were anaesthetized by isoflurane inhalation and killed by cervical transection and exsanguination. Distal colon was removed and placed in Krebs solution (mm: 126 NaCl, 2.5 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 5 KCl, 25 NaHCO3, and 11 glucose) that was gassed with 95% O2–5% CO2. The colon was opened along the mesenteric border, pinned flat in a Sylgard (Dow-Corning)-lined Petri dish that contained Krebs solution. Submucosal preparations were obtained following removal of the mucosa and muscularis externae, with care taken not to touch or break submucosal arterioles. The preparations were subsequently pinned in small organ baths, mounted on the stage of an inverted microscope and continuously superfused with Krebs solution warmed to yield a bath temperature of 35–36°C.
Arteriolar diameter measurements
Vasoconstrictions were monitored by continuously measuring the outside diameter of individual submucosal arterioles using a computer-assisted videomicroscopy system (Diamtrak; Flinders University of South Australia), as previously described (Neild, 1989). Briefly, a Eurysys Piccolo (Bock Optronics, Missisauga, Ontario, Canada) frame-grabber board in a PC was used to digitize television images of the arteriole. Cursors were placed on either side of the arteriole and were able to follow the outside of the arteriole as it constricted or dilated. The distance between the cursors was a measure of the outside diameter of arterioles and the resolution of the system was < 1 μm. The arterioles studied in control and TNBS mice were second order arterioles, i.e. after the first branch within the submucosa. Data were saved to the hard drive of a PC for analysis using Diamtrak.
In our preliminary studies in mouse colon, prostaglandin F2α (PGF2α; 400 nm) increased the likelihood of seeing constrictions in response to lower frequency stimuli. The amplitude of constrictions to 20 Hz trains of electrical stimuli was not altered in the presence of PGF2α (15.8 ± 1.7 versus 16.38 ± 1.6 μm, n = 13 arterioles each, P = 0.8), so measurements of constrictions following nerve stimulation were made in the presence of PGF2α. Perivascular nerve terminals were extracellularly stimulated (with a Grass SD10 stimulator) using 6 s trains at frequencies between 2 and 20 Hz applied via a bipolar silver chloride stimulation electrode placed either side of the arteriole of interest. Maximal constriction amplitude (in μm) was measured for each neurogenic constriction.
Immunohistochemistry
Segments of distal colon were removed and placed in a Sylgard-lined Petri dish containing Krebs solution. The colon was opened along the line of mesenteric attachment and dissected as above to reveal the submucosa. The tissue was then fixed at 4°C for 60 min in 3.7% buffered formalin (for tyrosine hydroxylase immunohistochemistry) or overnight in 4% paraformaldehyde (for P2X1 receptor immunohistochemistry). Following 3 × 10 min washes in phosphate buffered saline (PBS), tissues were incubated for an hour in normal sheep serum in 1% Triton X-100. Tissues were subsequently washed 3 times in PBS before incubation overnight in the primary antiserum (rabbit anti-P2X1 receptor antiserum; 1: 500 code no. 5224 from Chemicon, Temecula, CA, USA; or rabbit anti-tyrosine hydroxylase antiserum; 1: 500, code no. 152 from Chemicon; Lourenssen et al. 2005). Primary antisera were then removed and the preparations were washed for 3 × 10 min in PBS, followed by a 2 h incubation in goat anti-rabbit alexa fluor 555 (1: 500; Invitrogen, Carlsbad, CA, USA). Following a final 3 × 10 min wash in PBS, preparations were mounted on slides in buffered glycerol and coverslipped. The slides were then analysed with an epifluorescence microscope (Olympus BX51). Images were acquired using a Coolsnap CCD camera (Photometrics Inc., Tucson, AZ, USA) and Image Pro software (Media Cybernetics, Silverspring, MD, USA).
Western blotting
Dissections of colonic submucosa were performed as above. Tissue was then transferred to150 μl ice-cold lysis buffer and sonicated for 15 s. Following 30 min of solubilization the solution was centrifuged at 13,400g. for 1 min. An aliquot containing 10 μg of total protein was removed and separated on a 10% polyacyrlamide gel. The separated proteins were then electroblotted onto ployvinylidene difluoride (PVDF) membrane and immersed in 5% non-fat milk in PBS containing 0.05% Tween (PBST) for 1 h. The blot was washed with PBST and incubated overnight at 4°C in polyclonal rabbit anit-P2X1 antibody (Chemicon) at a 1: 1000 dilution in 5% non-fat milk. After subsequent washes in PBST, the blot was incubated in donkey HRP-labelled secondary rabbit antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 1 h, washed in PBST and finally developed with a chemiluminescent substrate (Pierce, Rockford, IL, USA). The blot was then stripped at 60°C for 1 h in 02 m glycine pH 2.5 containing 0.05% Tween 20, washed briefly and then incubated for 1 h in a 1: 20 000 dilution of mouse anti-β-actin antibody (Sigma Aldrich, St Louis, MO, USA). After extensive washes (3 × 15 min) in PBST, the secondary antibody (Peroxidase-conjugated AffiniPure Donkey anti-mouse; Jackson ImmunoResearch) was added at 1: 10 000 dilution for 1 h. The blot was developed as previously described, scanned and imported into ImagePro software in order to measure the integral optical density (IOD) of individual bands. The ratio of the IOD of P2X1 bands to β-actin was calculated for each protein sample.
TNBS model of colitis
Animals were anaesthetized by intraperitoneal injection of a combination of midazolam (1.25 mg ml−1) and hypnorm (0.315 mg ml−1 fentanyl and 10 mg ml−1 fluanisone; 0.1 ml of mixture per 20 g body wt). A midline laparotomy was performed, and the descending colon was carefully exposed. Under a dissecting microscope, a microlitre syringe (Hamilton, Reno, NV, USA) equipped with a 32-gauge needle was used to inject 100 μl TNBS (0.6 mg ml−1 in 40% ethanol) into the colonic lumen. The abdomen was irrigated with warm saline and sutured closed. After surgery, animals were allowed to recover on a warming blanket and were given free access to food and water. Following recovery from anaesthesia, animals were monitored for signs of pain, altered feeding habits, or weight loss. Animals that displayed behaviour consistent with ongoing pain or failure to thrive were killed. Five to eight days later, the mice were killed by isoflurane overdose followed by exsanguination.
Assessment of inflammation
The severity of inflammation was assigned a macroscopic damage score according to four categories: the presence of mucosal erosion; hyperaemia; petecchial haemoerrhage and adhesions (Moore et al. 2002). Colons were assigned a score from 0 to 4 for each of these categories (maximum score would be 16). Animals that had a damage score of at least 4 were considered inflamed.
Drugs
Drugs were applied to submucosal preparations by addition to the superfusate. Tissues were incubated in each drug for at least 3 min before nerve stimulation or vasoconstrictor application. The following drugs were purchased from Sigma-Aldrich, St Louis, MO, USA: Prazosin; 2-aminoethoxydiphenyl borate (2-APB); tetrodotoxin; guanethidine; ATP (magnesium salt); αβ-methylene-ATP. Pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS) and suramin were purchased from Tocris (Ellisville, MO, USA) and PGF2α (U46619) was obtained from Caymen Chemical (Ann Arbor, MI, USA).
Data analysis
Population data are expressed as mean ±s.e.m. Student's t test for paired or unpaired data was used to discern statistically significant differences between groups of data. When more than two groups were compared, a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test was used. A P-value of < 0.05 was taken to indicate significance. Data analysis and graphing were performed using Graphpad Prism (GraphPad Software Inc., San Diego, CA, US) and SigmaPlot (Systat Software Inc., San Jose, CA, USA).
Results
Vasoconstrictor regulation in normal mice
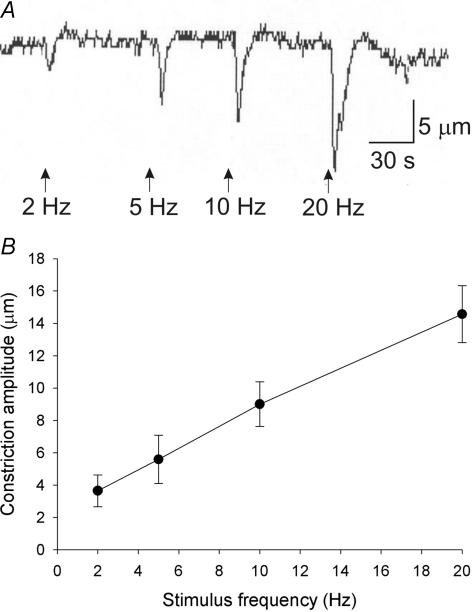
Electrical field stimulation (pulse duration 0.5 ms; 20–30 V stimulus intensity) in the presence of prostaglandin F2α resulted in vasoconstrictions (Fig. 1A) that were highly reproducible and did not run down during the course of a typical experiment (2 h). Stimulus trains were applied for 6 s, and the amplitude of resulting vasoconstrictions increased with increasing stimulus frequency (Fig. 1B). In a minority of arterioles, constrictions were followed by after-dilatations; these dilatations were not studied further in the present investigation. In many arterioles it was difficult to elicit constrictions to lower frequency trains; thus, pharmacological analysis was performed only on constrictions to 5 and 20 Hz stimuli.
Figure 1. Frequency dependence of mouse colon submucosal arteriole vasoconstriction to nerve stimulation.
A, raw data traces of arteriolar diameter responses to nerve stimulation at various frequencies. Nerve stimulation decreased arteriolar diameter (downward deflections). The amplitude of evoked constrictions increased with increasing stimulus frequencies. B, mean ±s.e.m. frequency–response data for nine arterioles from control mouse colon submucosal arterioles.
Stimulus-evoked vasoconstrictions were completely abolished by TTX (300 nm; control: 18.6 ± 2.7 μm versus TTX: 0 ± 0 μm; n = 4; P < 0.01, paired t test), demonstrating that the stimulus parameters used did not have any direct effect on the arterioles. In submucosal arterioles from guinea-pig ileum, nerve-mediated constrictions were due to release of neurotransmitter from the perivascular terminals of sympathetic postganglionic neurons (Evans & Surprenant, 1992). To determine whether a similar situation is at play in mouse colon, we examined the effect of the sympathetic neurotoxin guanethidine (10 μm) on nerve-mediated vasoconstrictions. Guanethidine abolished all vascular responses to nerve stimulation (control 21.9 ± 0.7 μm versus guanethidine: 0.6 ± 0.7 μm; n = 8; P < 0.001, paired t test). Taken together, these data indicate that EFS causes vasoconstriction due to action potential-dependent release of neurotransmitter from sympathetic nerve terminals (Hirst et al. 1996).
We next wished to identify the neurotransmitters and receptors involved in vasoconstrictor responses to nerve stimulation in mouse colon submucosal arterioles. In a previous study of guinea-pig ileum it was found that ATP or a related purine is the only sympathetic neurotransmitter that constricts submucosal arterioles in response to nerve stimulation (Evans & Surprenant, 1992). This conclusion was based on the observations that adrenoceptor antagonists had no effect on evoked constrictions whereas the P2 receptor antagonist suramin abolished nerve-evoked constrictions. In the present study, suramin (100 μm) decreased the amplitude of evoked constrictions in some but not all arterioles studied (reductions in amplitude of more than 15% were observed in 25 of 38 arterioles studied at 20 Hz). In no cases did suramin abolish the constriction to nerve stimulation. Suramin did, however, abolish vasoconstrictions caused by superfusion of ATP (10 μm; data not shown). Prazosin (100 nm), an α1-adrenoceptor antagonist, consistently reduced the amplitude of evoked constrictions (n = 8 arterioles). When expressed as percentage inhibition, suramin and prazosin on their own blocked constrictions to 20 Hz stimuli by 26 and 40%, respectively (Fig. 2). However, the combination of suramin plus prazosin abolished responses to nerve stimulation (n = 16 arterioles), which is more than would be would be predicted on the basis of their individual inhibitory effects. One explanation for this finding is that sufficient noradrenaline or ATP is released in response to nerve stimulation to compensate for the component (purinergic or adrenergic) inhibited by either antagonist. Alternatively, postjunctional synergy between P2X receptors and α1-adrenoceptors, as described in rat mesenteric arteries (Ralevic & Burnstock, 1990), may account for these data.
Figure 2. Pharmacology of sympathetic vasoconstrictions.
A and B, raw data illustrating the inhibitory effect of the P2 receptor antagonist suramin and the α1-adrenoceptor antagonist prazosin on vasoconstrictions to 20 Hz electrical stimuli. C, summary data showing that suramin (n = 38) and prazosin (n = 8) individually reduced evoked vasoconstrictions and that the combination of suramin plus prazosin (n = 16) abolished neurally mediated vasoconstrictions. *P < 0.001, one-way ANOVA plus Tukey's multiple comparisons test.
Vasomotor control in TNBS colitis
TNBS instillation caused severe transmural inflammation in the distal colon of mice. Colonic damage scores of normal mice were 0 whereas the mean damage score of TNBS colons was 7.5 ± 0.47 (n = 17 mice). Preparations of submucosa were obtained from the slightly less inflamed margins of inflamed regions and examined using the same techniques as for control preparations. Resting outside diameters of submucosal arterioles were significantly higher in TNBS colons compared to controls (control: 47.1 ± 1.0 μm, n = 35 arterioles; TNBS: 60.6 ± 2.0 μm, n = 24 arterioles; P < 0.01, unpaired t test), despite the fact that arterioles from the same level of the vascular tree were always selected.
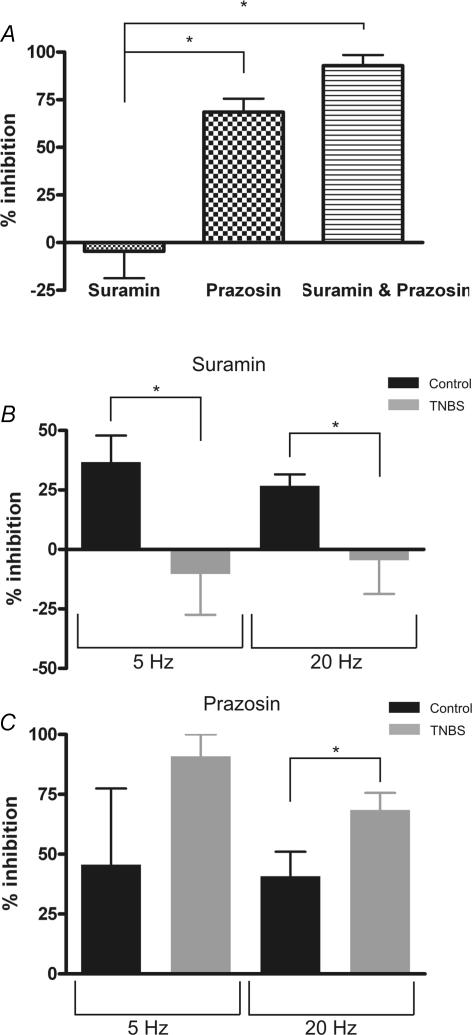
Nerve stimulation at 20 Hz in TNBS preparations evoked constrictions of similar amplitude to normal mice (control: 15.8 ± 1.8 μm versus TNBS: 15.4 ± 2.2 μm; n = 17 and 12 arterioles, respectively). However, in contrast to control mice, suramin had little or no inhibitory effect on nerve evoked constrictions (Fig. 3A, 3 of 10 constrictions were reduced by more than 15% by suramin). Prazosin, on the other hand, significantly reduced the amplitude of evoked constrictions in 5 of 5 arterioles (Fig. 3A) and the combination of suramin plus prazosin abolished all constrictions to nerve stimulation in TNBS arterioles (n = 9). We directly compared the percentage inhibition by suramin (100 μm) of sympathetic vasoconstrictions evoked by 5 and 20 Hz between control and TNBS colitis submucosal preparations (Fig. 3B). Suramin was significantly more effective at reducing evoked constrictions in control versus TNBS colitis arterioles at both stimulus frequencies. In contrast, prazosin (100 nm), inhibited a significantly larger percentage of constrictions to 20 Hz stimuli in TNBS colitis compared to controls (Fig. 3C). These data indicate that inflammation altered purinergic signalling between sympathetic neurons and submucosal arterioles. The alteration in purinergic neurotransmission during inflammation could result from effects on purinergic transmitter release from sympathetic nerve terminals and subsequent catabolism by ectonucleotidases, or could be due to altered arteriolar responsiveness to extracellular purines.
Figure 3. Pharmacology of sympathetic vasoconstrictions in arterioles from TNBS colitis mice.
A, mean ±s.e.m. data which demonstrate that suramin (n = 10) had no inhibitory effect on vasoconstrictions evoked by 20 Hz stimuli in these preparations whereas prazosin (n = 5) inhibited a large component of evoked constrictions. The combination of suramin plus prazosin (n = 9) abolished the constriction response to nerve stimulation but there is no statistical difference between the effect of prazosin alone and prazosin plus suramin. B, comparison of the percentage inhibition by suramin (100 μm) of sympathetic vasoconstrictor responses at 5 and 20 Hz in control and TNBS arterioles. Suramin had significantly less effect on constrictions in TNBS colon compared to controls. C, prazosin had significantly more effect at 20 Hz on constrictions in TNBS colon versus controls. This indicates that the adrenergic component of sympathetic vasoconstrictions compensated for the colitis-induced loss of purinergic signalling. *P < 0.05, one way ANOVA with Tukey's multiple comparisons test for A and unpaired t test with Welch's correction for B and C (comparisons of control versus TNBS).
Mechanism of purinergic defect
Immunohistochemistry for tyrosine hydroxylase (TH) was used to examine whether the sympathetic innervation of submucosal arterioles was anatomically remodelled in TNBS colitis, thus accounting for alterations in sympathetic vascular control. TH immunoreactivity was confined to axons and varicosities that formed a perivascular network around arterioles and within submucosal ganglia (Li et al. 1998) (Fig. 4). We counted the number of TH-immunoreactive varicose nerve fibres across the width of submucosal arterioles (diameters 40–60 μm) from the colons of three controls and three animals with TNBS colitis. Control colonic submucosal arterioles were surrounded by 5.3 ± 0.5 immunoreactive axons (n = 7 arterioles) compared to 4.6 ± 0.4 axons in TNBS colons (n = 7; P = 0.29, unpaired t test; Fig. 4C). Although vascular innervation by TH-immunoreactive axons was anatomically unaffected by TNBS colitis, the density of TH-immunoreactive axons in the submucosal plexus appeared reduced (data not shown).
Figure 4. Immunohistochemical analysis of sympathetic neuroanatomy in normal (A) and TNBS colitis (B) colons.
Submucosal preparations were stained with antiserum that detects tyrosine hydroxylase (TH), a vital enzyme for catecholamine synthesis. Immunoreactivity was present in a perivascular plexus along submucosal arterioles and was also detected in varicosities that surround neurons in the submucosal plexus. Although there was a decrease in the amount of staining in the submucosal plexus, the innervation of submucosal arterioles by TH-immunoreactive fibres was not reduced during colitis (C).
Postjunctional responsiveness to sympathetic vasoconstrictors was assessed by measuring the amplitude of constrictions to superfusion of ATP (10 μm; n = 17), a P2 receptor agonist and phenylephrine (3 μm; n = 9), a selective α1-adrenoceptor agonist. Both agonists caused robust arteriolar constrictions in normal mice. The response to ATP desensitized markedly during application (Fig. 5A), consistent with P2X1 receptor activation underlying the majority of submucosal arteriolar responses to purines (Vulchanova et al. 1996; but also see Gitterman & Evans 2000). The constriction to ATP was abolished in TNBS preparations (n = 7, Fig. 5B), indicating that the altered pharmacology of sympathetic vasomotor control in the gut of TNBS animals was due to an abrogated response to purinergic vasoconstrictors. In contrast, TNBS colitis had no effect on adrenergic vasoconstrictions (n = 12; Fig. 5C).
Figure 5. Responsiveness of submucosal arterioles to sympathetic vasoconstrictors in normal and TNBS-inflamed colons.
A, superfusion of ATP caused large vasoconstrictions that rapidly desensitize in arterioles from control colons. TNBS colitis abolished the responsiveness of arterioles to ATP. B, summary data on constriction amplitude to ATP (control: n = 17; TNBS: n = 7) and phenylephrine (control: n = 9; TNBS: n = 12), an α-adrenoceptor agonist in control and TNBS colons. Colitis abolished constrictions to ATP whereas constrictions to phenylephrine were unaffected. *P < 0.01.
In an effort to define the mechanism underlying defective purinergic vasomotor control in this model of colitis, we tested the following possible hypotheses: colitis alters downstream signalling pathways involved in purinergic but not adrenergic neurotransmission; colitis reduces purinergic receptor expression on submucosal arterioles; colitis leads to irreversible desensitization of purinergic receptors; and colitis leads to increased degradation of extracellular ATP.
P2X1 receptors are ligand-gated ion channels that allow calcium influx which can directly cause vasoconstriction of submucosal arterioles and small mesenteric arteries (Gitterman & Evans, 2001; Lamont et al. 2006). α-Adrenergic receptors are seven-transmembrane-domain G protein-coupled receptors that cause vasoconstriction by activating IP3 receptors on the sarcoplasmic reticulum leading to Ca2+ release from intracellular stores (Li et al. 2003). Consistent with this, superfusion of preparations with zero-Ca2+ Krebs solution (Ca2+ replaced with Mg2+) abolished the response to ATP (n = 3) but not phenylephrine (n = 3; Fig. 6A). However, blockade of L-type calcium channels with 1 μm nifedipine did not reduce constrictions to ATP (control: 11.7 ± 0.9 μm versus nifedipine: 11.0 ± 1.2 μm; P = 0.28, paired t test, n = 6). Although zero-Ca2+ Krebs solution did not abolish the response to phenylephrine, it did significantly reduce the amplitude of phenylephrine constrictions. This is consistent with zero extracellular Ca2+ depleting sarcoplasmic reticulum Ca2+ stores, which would blunt the IP3 receptor-mediated response to phenylephrine. In addition, 2-aminoethoxy-diphenylborate (2-APB; 100 μm; n = 10), which interferes with IP3 receptor signalling, blocked constrictions to phenylephrine but did not alter constrictions to ATP (n = 5; Fig. 6B). These data indicate that purinoceptor and adrenoceptor activation have divergent signalling pathways leading to vasoconstriction, one of which is sensitive (purinergic) to inflammation while the other (adrenergic) is resistant. These separate pathways may indicate that the defect in purinergic signalling is due to inflammation reducing the influx of extracellular Ca2+ into vascular myocytes following purinergic transmitter release, rather than downstream events such as myofilament activation following increased intracellular Ca2+.
Figure 6. Signalling pathways downstream of receptor activation differ between purinergic and adrenergic constrictions.
A, purinergic constrictions rely on Ca2+ influx and were abolished by superfusion with zero-Ca2+ Krebs solution (n = 3 each for ATP and phenylephrine). B, purinergic constrictions were unaltered by 2-APB (n = 5), an IP3 receptor antagonist, whereas constrictions to phenylephrine (n = 10) were abolished following blockade of IP3 receptor-mediated release of sarcoplasmic reticulum stores of Ca2+. *P < 0.05.
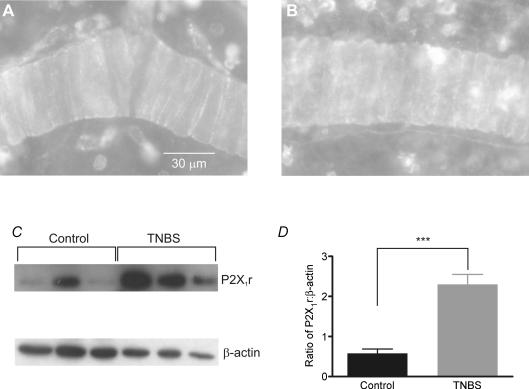
We used immunohistochemical analysis of P2X1 receptor expression to examine whether colitis altered receptor localization. P2X1 receptor immunoreactivity was restricted to arterioles and an unidentified cell type that is likely to be an immunocyte (Fig. 7A; representative of preparations from 4 mice). Preincubation of antiserum with neutralizing peptide abolished all staining, as did application of a mismatched secondary antibody with the P2X1 antiserum (data not shown). Preparations from animals with TNBS colitis did not exhibit any marked alteration in P2X1 receptor immunoreactivity on arterioles although more non-vascular cells were immunoreactive for P2X1 receptor (Fig. 7B; representative of preparations from 3 TNBS mice). Western blot analysis revealed a significant increase in P2X1 receptor protein in the colonic submucosa of TNBS colitis animals (Fig. 7C and D; n = 6 animals for each condition).
Figure 7. Immunohistochemical and immunoblot detection of P2X1 receptor in submucosal arterioles.
A, in control colon submucosa, receptor immunoreactivity was localized to smooth muscle cells within arterioles. B, TNBS colitis did not affect P2X1 receptor immunoreactivity in arterioles but increased immunoreactivity in non-vascular cell types, probably reflecting influx of immune cells during colitis. C, Western blotting of submucosal protein with a P2X1 receptor antiserum detected a band at the predicted molecular weight of ∼59 kDa; β-actin antiserum revealed a band at ∼40 kDa. D, analysis of the ratio of densitometry measurements of P2X1 receptor and β-actin bands in submucosal preparations from 6 control and 6 TNBS colons. TNBS colitis significantly increased P2X1 receptor expression. ***P < 0.001, unpaired t test with Welch's correction.
We attempted to evoke prolonged desensitization of purinergic receptors by incubating dissected preparations of colonic submucosa from control mice for 3 h in ATP (10 μm) prior to washout of ATP and reapplication of ATP while monitoring vasoconstriction. ATP was reapplied at hourly intervals during the 3 h incubation to circumvent the effect of ecto-ATPases on receptor activation (Westfall et al. 2002). We found no difference in ATP responsiveness between preparations that had been preincubated for 3 h in ATP (12.9 ± 2.4 μm; n = 4) or normal Krebs solution (14.7 ± 3.4 μm; n = 9; P = 0.64, unpaired t test with Welch's correction). This indicates that prolonged desensitization of purinergic receptors does not lead to irreversible receptor desensitization and thus is not likely to account for altered responsiveness to ATP of arterioles from TNBS colitis mice.
Our finding that P2X1 receptor expression was increased by colitis, coupled with the lack of evidence of receptor desensitization following prolonged exposure to purinergic agonist led us to hypothesize that colitis alters purinergic neurotransmission by enhancing the degradation of extracellular purines. We tested this hypothesis by examining the responses of submucosal arterioles from normal and colitis mice to the degradation-resistant P2X1 and P2X3 receptor agonist αβ-methylene-ATP. In contrast to the lack of responsiveness of arterioles to ATP in colitis (Fig. 8A) the same arterioles constricted readily to β-methylene-ATP (Fig. 8B and C). In agreement with Western blot analysis that suggested increased expression of submucosal P2X1 receptor in colitis, constrictions to αβ-methylene-ATP were significantly larger in arterioles from inflamed colons compared to controls (Fig. 8C).
Figure 8. The purinergic agonistαβ-methylene-ATP, but not ATP, constricts arterioles in TNBS colon.
Responses to ATP and αβ-methylene-ATP were compared in arterioles from 12 control mice and 6 mice with TNBS colitis. ATP and αβ-methylene ATP cause equal constrictions in control mice (P = 0.98; paired t test), but in TNBS arterioles there is a marked difference in the responsiveness of arterioles (###P < 0.0001, paired t test). A and C, vasoconstrictions of arterioles to ATP is abolished in TNBS colitis (***P < 0.0001, unpaired t test with Welch's correction) whereas arteriolar responsiveness to αβ-methylene-ATP is increased in colitis (B and C; ***P < 0.0001, unpaired t test with Welch's correction).
Discussion
The main findings of the present study are that noradrenaline and a purine are released as cotransmitters from perivascular sympathetic nerve terminals in the submucosa of mouse distal colon. During TNBS colitis, the purinergic component of this cotransmission is markedly reduced.
Synaptic transmission to submucosal arterioles
Noradrenaline and ATP or a related purine are commonly released as cotransmitters from sympathetic varicosities (Westfall et al. 2002; Burnstock, 2004). They bind their respective receptors and in the majority of vascular beds cause vasoconstriction (Huidobro-Toro & Donoso, 2004). In submucosal arterioles of guinea-pig ileum, ATP is the sole sympathetic neurotransmitter that binds postjunctional receptors to cause vasoconstriction (Evans & Surprenant, 1992). Neuropeptide Y (NPY) and noradrenaline are also released from perivascular sympathetic varicosities in this preparation but act as neuromodulators rather than directly causing vasoconstriction (Evans & Surprenant, 1992; Lewis et al. 1999). In the present study, we observed direct postjunctional roles for ATP and noradrenaline in sympathetic vasoconstriction of submucosal arterioles. Adrenergic and purinergic receptor antagonists both reduced sympathetic vasoconstrictions and the combination of both antagonists abolished the vasoconstriction. This is in contrast to the finding in guinea-pig submucosal arterioles that suramin abolished sympathetic vasoconstrictions whereas prazosin had no effect (Evans & Surprenant, 1992). Whether this finding reflects species-dependent or regional differences in the pharmacology of sympathetic vasoconstriction (guinea-pig ileum versus mouse distal colon) is a matter for future investigation.
Colitis alters pharmacology
Injection of TNBS into the colons of mice results in a predominantly Th1-mediated transmural inflammation of the colon, associated with infiltration of colonic tissue by macrophages and T-lymphocytes, that is thought to resemble Crohn's disease (Strober et al. 2002). The P2 receptor antagonist suramin did not have any effect on sympathetic vasoconstrictions in TNBS colitis, whereas in control mice suramin reduced the amplitude of vasoconstrictions (Fig. 3B). In contrast, colitis did not alter the inhibitory effect of prazosin on sympathetic vasoconstrictions. This suggests that the alteration in sympathetic vasoregulation during colitis is restricted to purinergic signalling pathways.
There are several potential mechanisms that could underlie the reduction in purinergic participation in sympathetic vasoconstriction during colitis. It could be due to a selective reduction in release of the purinergic transmitter in colitis or a reduction in arteriolar responsiveness to the transmitter. Although we were unable to directly measure extracellular purine levels following nerve stimulation, we observed a complete absence of responsiveness of arterioles from TNBS colons to application of ATP, whereas arterioles from control mice always constricted to ATP. This indicates that the loss of purinergic involvement in sympathetic vasomotor control in TNBS colitis is due to a reduction in postjunctional responsiveness to ATP or enhanced extracellular degradation of ATP.
In order to test the hypothesis that colitis altered postjunctional responsiveness to ATP, we utilized immunohistochemistry and Western blotting to examine whether major differences in P2X1 receptor expression occurred in the colitis blood vessels. While the immunohistochemistry findings do not exclude the possibility that subtle changes in receptor distribution have occurred, Western blot data indicate that P2X1 receptor expression is markedly up-regulated in colitis. This finding was unexpected given our data on the lack of vasoconstrictor effect of ATP during colitis. However, P2X1 receptor expression in submucosal preparations was not limited to arteriolar smooth muscle cells. Immunocytes also stained with the antiserum, and the number of P2X1 receptor-immunoreactive immunocytes increased during TNBS colitis. While we did not identify which particular immune cells express the P2X1 receptor, previous studies have found evidence of P2X1 receptor mRNA in neutrophils, lymphocytes and macrophages (reviewed in Bours et al. 2006); each of these cells types is known to be increased in TNBS colitis (Wirtz et al. 2007). Therefore, the influx of inflammatory cells during colitis may account for some of the observed increase in P2X1 receptor protein expression, although it seems likely that vascular myocyte expression of the receptor is also increased during colitis. This conclusion is supported by the observation that the amplitude of constrictions to a degradation-resistant ATP analogue, αβ-methylene-ATP, is increased in colitis (Fig. 8). It is possible that the increased responsiveness/expression of vascular P2X1 receptors is a compensatory response to the reduction in purinergic neurotransmission during colitis.
Extracellular concentrations of ATP are normally maintained in the submicromolar range by a variety of ectonucleotidases and nucelosidases, which exist in soluble form or are expressed on cell membranes (Westfall et al. 2002; Gendron et al. 2002). Inflammation may lead to a large increase in release of purines due to cellular damage (Bours et al. 2006). Whether the increased degradation of ATP in TNBS colitis is in response to increased extracellular concentrations of purines during inflammation or whether this phenomenon is solely due to an increasing number of immune cells which express ecto-ATPases (Bours et al. 2006) during colitis is unclear. However, a previous study in guinea-pig colitis found evidence of increased purinergic signalling between enteric neurons during colitis (Lomax et al. 2005b), which suggests that the up-regulation of ecto-ATPase activity by inflammation may be restricted to vascular tissue.
Physiological significance
The present findings and a previous report of increased purinergic contribution to fast excitatory postsynaptic potentials in guinea-pig colitis indicate that purinergic neurotransmission is particularly labile and prone to alterations during GI inflammation. Extracellular purines are signalling molecules that are released under a variety of conditions and bind receptors on many cell types, including neurons, endothelial cells, smooth muscle cells and immune cells (Bours et al. 2006; Burnstock, 2007). Due to the requirement to tightly regulate extracellular purine concentrations during inflammation, it appears that ectonucleotidase activity is increased during colitis. Although there are reports of increased vascular ectonculeotidase activity during hypoxia, the present study is the first evidence that implies that colitis up-regulates these enzymes. In the vasculature, increased ectonucleotidase activity, in particular CD73, which converts AMP to adenosine, is a protective adaptation following hypoxia as it increases extracellular adenosine concentrations (Thompson et al. 2004; Zernecke et al. 2006). If other nucleotidases, such as CD39, which hydolyses ATP, are also up-regulated, this protective response to inflammation may have the side-effect of decreasing the purinergic regulation of blood flow.
The findings of this study demonstrate that sympathetic vasoconstrictor regulation of submucosal arterioles is abnormal in the inflamed colon. Previous in vivo and in vitro studies in tissues from normal and inflamed mouse and human colonic submucosa identified a defect in vasodilator function following inflammation (Hatoum et al. 2003b; Mori et al. 2005). Taken together with the present findings, it appears that inflammation affects multiple neural pathways that regulate GI blood flow. There is strong evidence that defective mucosal barrier function contributes to the pathogenesis of IBD. Precise regulation of blood flow to the mucosa, which depends of maintenance of appropriate perfusion pressure and compensatory constrictor and dilator responses within the microcirculation (Lundgren, 1984), is critical to mucosal barrier function. Therefore, altered vascular regulation during colitis, as described in the present study, may perpetuate gastrointestinal inflammation. Future studies will utilize intravital microscopy to characterize the impact of altered purinergic transmission on mucosal blood flow in the mouse colon.
Acknowledgments
This study was supported by a Faculty Transition Award to A.L. from the Canadian Association of Gastroenterology, Crohn's and Colitis Foundation of Canada (CCFC) and Canadian Institutes of Health Research and by operating grants to A.L. and S.V. from the CCFC. We are grateful to Iva Kosatka for expert technical assistance with the TNBS model of colitis, to Dr Ian Spreadbury for helpful discussions and to Dr Michael Blennerhasset for providing the antityrosine hydroxylase antiserum.
References
- Anthony A. Vascular anatomy in gastrointestinal inflammation. J Clin Pathol. 1999;52:381–384. doi: 10.1136/jcp.52.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony A, Dhillon AP, Pounder RE, Wakefield AJ. Ulceration of the ileum in Crohn's disease: correlation with vascular anatomy. J Clin Pathol. 1997;50:1013–1017. doi: 10.1136/jcp.50.12.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyak MJ, Vanner S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol Motil. 2005;17:175–186. doi: 10.1111/j.1365-2982.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996;111:1683–1699. doi: 10.1016/s0016-5085(96)70034-3. [DOI] [PubMed] [Google Scholar]
- Danese S, de la Sans MMC, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A, Fiocchi C. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Surprenant A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br J Pharmacol. 1992;106:242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Gendron FP, Benrezzak O, Krugh BW, Kong Q, Weisman GA, Beaudoin AR. Purine signaling and potential new therapeutic approach: possible outcomes of NTPDase inhibition. Curr Drug Targets. 2002;3:229–245. doi: 10.2174/1389450023347713. [DOI] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br J Pharmacol. 2000;131:1561–1568. doi: 10.1038/sj.bjp.0703760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries: dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol. 2001;132:1201–1208. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DN, Kvietis PR, Korthuis RJ, Premen AJ. Microcirculation of the intestinal mucosa. In: Wood JD, editor. Handbook of Physiology, section 6, The Gastrointestinal System, vol. I, Motility and Circulation. Bethesda, MD, USA: American Physiological Society; 1989. pp. 1405–1474. [Google Scholar]
- Hatoum OA, Binion DG. The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflamm Bowel Dis. 2005;11:304–313. doi: 10.1097/01.mib.0000160772.78951.61. [DOI] [PubMed] [Google Scholar]
- Hatoum OA, Binion DG, Otterson MF, Gutterman DD. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology. 2003a;125:58–69. doi: 10.1016/s0016-5085(03)00699-1. [DOI] [PubMed] [Google Scholar]
- Hatoum OA, Miura H, Binion DG. The vascular contribution in the pathogenesis of inflammatory bowel disease. Am J Physiol Heart Circ Physiol. 2003b;285:H1791–H1796. doi: 10.1152/ajpheart.00552.2003. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Choate JK, Cousins HM, Edwards FR, Klemm MF. Transmission by post-ganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience. 1996;73:7–23. doi: 10.1016/0306-4522(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro JP, Donoso MV. Sympathetic co-transmission: the coordinated action of ATP and noradrenaline and their modulation by neuropeptide Y in human vascular neuroeffector junctions. Eur J Pharmacol. 2004;500:27–35. doi: 10.1016/j.ejphar.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lamont C, Vial C, Evans RJ, Wier WG. P2X1 receptors mediate sympathetic post-junctional Ca2+ transients (jCaTs) in mesenteric small arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3106–H3113. doi: 10.1152/ajpheart.00466.2006. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Evans RJ, Neild TO. Inhibition of vasoconstriction and Ca2+ currents mediated by neuropeptide Y Y2 receptors. J Smooth Muscle Res. 1999;35:147–156. doi: 10.1540/jsmr.35.147. [DOI] [PubMed] [Google Scholar]
- Li PL, Lee HC, Nelson MT, Meininger GA, Van BC. Novel Ca2+ signalling mechanisms in vascular myocytes: symposium overview. Acta Physiol Scand. 2003;179:339–352. doi: 10.1046/j.0001-6772.2003.01216.x. [DOI] [PubMed] [Google Scholar]
- Li ZS, Fox-Threlkeld JE, Furness JB. Innervation of intestinal arteries by axons with immunoreactivity for the vesicular acetylcholine transporter (VAChT) J Anat. 1998;192:107–117. doi: 10.1046/j.1469-7580.1998.19210107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, Fernandez E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005a;17:4–15. doi: 10.1111/j.1365-2982.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 2005b;564:863–875. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenssen S, Wells RW, Blennerhassett MG. Differential responses of intrinsic and extrinsic innervation of smooth muscle cells in rat colitis. Exp Neurol. 2005;195:497–507. doi: 10.1016/j.expneurol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Lundgren O. Microcirculation of the gastrointestinal tract, and pancreas. In: Renkin EM, Michel CC, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol IV, Microcirculation. Bethesda, MD, USA: American Physiological Society; 1984. pp. 799–863. [Google Scholar]
- Mawe GM, Collins SM, Shea-Donohue T. Changes in enteric neural circuitry and smooth muscle in the inflamed and infected gut. Neurogastroenterol Motil. 2004;16(Suppl. 1):133–136. doi: 10.1111/j.1743-3150.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1045–G1051. doi: 10.1152/ajpgi.00406.2001. [DOI] [PubMed] [Google Scholar]
- Mori M, Stokes KY, Vowinkel T, Watanabe N, Elrod JW, Harris NR, Lefer DJ, Hibi T, Granger DN. Colonic blood flow responses in experimental colitis: time course and underlying mechanisms. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1024–G1029. doi: 10.1152/ajpgi.00247.2005. [DOI] [PubMed] [Google Scholar]
- Neild TO. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26:48–52. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Postjunctional synergism of noradrenaline and adenosine 5′-triphosphate in the mesenteric arterial bed of the rat. Eur J Pharmacol. 1990;175:291–299. doi: 10.1016/0014-2999(90)90567-p. [DOI] [PubMed] [Google Scholar]
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton M, Solomon MJ. Crohn's disease: in defense of a microvascular aetiology. Int J Colorectal Dis. 2002;17:287–297. doi: 10.1007/s00384-002-0408-5. [DOI] [PubMed] [Google Scholar]
- Vanner S, Macnaughton WK. Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil. 2004;16(Suppl. 1):39–43. doi: 10.1111/j.1743-3150.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol Gastrointest Liver Physiol. 1996;271:G223–G230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther. 2002;303:439–444. doi: 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bidzhekov K, Ozuyaman B, Fraemohs L, Liehn EA, Luscher-Firzlaff JM, Luscher B, Schrader J, Weber C. CD73/ecto-5′-nucleotidase protects against vascular inflammation and neointima formation. Circulation. 2006;113:2120–2127. doi: 10.1161/CIRCULATIONAHA.105.595249. [DOI] [PubMed] [Google Scholar]