Abstract
Histamine is a major mast cell mediator of immunoneural signalling in the gut and mast cells play a role in the pathophysiology of functional and inflammatory bowel diseases. Histamine receptors are therefore promising drug targets to treat gut disorders. We aimed to study the so far unknown effect of histamine on neural activity in the human enteric nervous system (ENS) and to identify the pharmacology of histamine response. We used fast imaging techniques in combination with the potentiometric dye di-8-ANEPPS to monitor directly membrane potential changes and thereby neuronal excitability in the human submucous plexus from surgical specimens of 110 patients (2137 neurones, 273 ganglia). Local microejection of histamine resulted in action potential discharge in 37% of neurones. This excitatory effect was mimicked by the H1 agonist HTMT-dimaleat, H2 agonist dimaprit, H3 agonist (R)-(−)-α-methylhistamine and H4 agonist 4-methylhistamine. The excitatory actions of the agonists were specifically and selectively blocked by the H1, H2, H3 or H4 receptor antagonists pyrilamine, ranitidine, clobenpropit or J1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine (JNJ 7777120), respectively. Clobenproprit reduced the excitatory response to histamine. Unlike in the guinea-pig ENS (R)-(−)-α-methylhistamine had no presynaptic actions in human submucous plexus. Application of agonists revealed receptor clustering which was as follows: 29% H1/H3, 27% H2, 20% H1/H2/H3, 10% H3, 7% H1/H2 and 7% H2/H3. Histamine excites human enteric neurones and this effect involves all four histamine receptors; most striking was the identification of an excitatory H3 mediated component and the discovery of H4 mediated neuronal excitation. These data may form the basis of identification of new targets to treat inflammatory and functional gut disorders.
The behaviour of the normal and diseased bowel is determined by integrative functions of the enteric nervous system (ENS). The activity level of the ENS is modulated by other integrative systems, among which the most prominent is the enteric immune system (Wood, 2004; Lomax et al. 2005). The close apposition between inflammatory/immune cells and enteric nerves forms the anatomical basis of neuroimmune interactions in the gut (Stead et al. 1989). Intestinal mast cells are key players in immunoneural communication and they play an important role in the regulation of gastrointestinal functions. Changes in mast cell density and the stimulation-dependent mediator release profile of human intestinal mast cells strongly indicate the involvement of mast cells in disorders associated with allergic reaction, bacterial or parasitic infections, inflammatory bowel diseases, and irritable bowel syndrome (IBS) (Raithel et al. 1995; Maurer et al. 2003; He, 2004; Bischoff & Crowe, 2005; Barbara et al. 2006). Recently, we described an excitatory effect of a mast cell mediator cocktail on human submucous neurones, demonstrating the functional role for a mast cell ENS axis in human intestine (Schemann et al. 2005). Upon stimulation, mast cells may release a number of mediators; among the most prominent is histamine. There are four G-protein-coupled histamine receptor subtypes, H1–H4 (Haas & Panula, 2003; MacGlashan, 2003; Xie & He, 2005; Celanire et al. 2005; de Esch et al. 2005). The current concepts on neuroimmune interaction in the gut are based on the role of histamine as a neuromodulator in the guinea-pig ENS, where histamine has two main actions (Wood, 2004). First, histamine evokes activation of enteric nerve cells mainly involving H2 receptors (Nemeth et al. 1984; Frieling et al. 1993). Second, histamine acts at presynaptic H3 receptors to suppress release of acetylcholine and somatostatin from enteric nerves as well as release of noradrenalin from sympathetic terminals (Tamura et al. 1988; Liu et al. 2000).
Such data cannot be transferred to the human system, in particular considering species-specific neurochemical, neurophysiological and neuropharmacological properties of human enteric neurones (Schneider et al. 2001; Schemann et al. 2002; Schemann & Neunlist, 2004). Recent data support the relevance of mast cells for symptom generation and pathogenesis of IBS. Mast cells in colonic mucosal biopsy specimens from IBS patients are more densely packed, release more histamine and are closer apposed to nerves than in normal subjects (Barbara et al. 2004). The close apposition and the increased release of mast cell mediators correlate with the symptom score in IBS patients (Barbara et al. 2004). In the human intestine, histamine influences a variety of gut functions including fluid and electrolyte transport (Crowe et al. 1990; Stack et al. 1995; Keely et al. 1995).
Although drugs that modulate actions of histamine appear promising novel targets to treat symptoms associated with functional and inflammatory bowel diseases, successful development of such drugs requires knowledge of histamine effects in the human ENS and the receptors involved (Wood, 2006). Therefore, it was the aim of this study to characterize the so far unknown effects of histamine on human enteric neurones and to identify its pharmacology by employing specific H1, H2, H3 or H4 receptor agonists and antagonists.
Methods
Tissue samples
Human tissue samples of small and large bowel were obtained from 110 patients undergoing surgery at the Departments of Surgery at the Medical Clinic Freising and the Medical Clinic of the Technische Universität München. Samples were taken from macroscopically unaffected areas as determined by visual inspection of the pathologists (S.S and C.v.W). Diagnoses that led to the surgery were as follows: carcinoma of small or large bowel (79 patients), colon polyps (4 patients), diverticulitis (18 patients), stenosis (3 patients), Morbus Crohn (2 patients), peritonitis (1 patient), ulcerative colitis (1 patient), dehiscence (1 patient), recurrent bleeding (1 patient). All procedures were approved by the ethics committee of the Technische Universität München (project approval 744/02).
Tissue preparation and neuroimaging technique
The multisite optical recording technique (MSORT) is a fast imaging technique that allows us to record neural activity, in particular action potential discharge, in the human ENS and has been previously described in detail (Neunlist et al. 1999; Schemann et al. 2005; Michel et al. 2005). After removal from the patient, the tissue was placed in cold oxygenated sterile Krebs solution containing (mm): 117 NaCl, 4.7 KCl, 1.2 MgCl2.6H2O, 1.2 NaH2PO4, 25 NaHCO3, 2.5 CaCl2 2H2O and 11 glucose (all chemicals from Sigma, Steinheim, Germany). The tissue was dissected to obtain preparations of the inner submucous plexus (5 × 10 mm final size) and then placed in a recording chamber and continuously perfused with Carbogen (5% CO2–95% O2, equilibrated at pH 7.4)-gassed 37°C Krebs solution. The tissue chamber was mounted onto an Olympus IX 50 microscope (Olympus, Hamburg, Germany) equipped with a 150 W xenon arc lamp (Osram, Munich, Germany). Individual ganglia were stained with the fluorescent voltage sensitive dye Di-8-ANEPPS (1-(3-sulphonatopropyl)-4-[β-[2-(di-n-octylamino)-6-naphthyl]vinyl]pyridinium betaine, Molecular Probes Mobitec, Göttingen, Germany) by local pressure application through a microejection pipette loaded with 20 μm Di-8-ANEPPS. Controlled illumination of the preparation for 1.3–5 s was achieved by a software operated shutter (Uniblitz D122, Vincent Associates, New York, NY, USA). Longer exposures were not used because they often caused dye bleaching and thus compromise repeated measurements in the same ganglion. Dye staining of the nerve cells did not change their electrophysiological properties (Neunlist et al. 1999). Recordings from Di-8-ANEPPS stained neurones were made with a 40× oil immersion objective (UAPO/340 Olympus, Hamburg, Germany) by using a filter cube equipped with a 545 ± 15 nm excitation interference filter, a 565 nm dichroic mirror and a 580 nm barrier filter (Olympus, Hamburg, Germany). Signals were acquired with a frequency of 1.6 kHz and processed by an array of 464 photodiodes (RedShirt Imaging, Decatour, GA, USA). The MSORT setup allows measurement of relative changes in fluorescence (ΔF/F), which is linearly related to changes in the membrane potential (Neunlist et al. 1999). It is important to emphasize that the photodiode system used for this study is an AC-coupled system with a time constant of 500 ms. This allowed the recordings of action potential and fast synaptic potentials with the compromise that slowly developing, small amplitude changes in membrane potential are not detected. Therefore, all agonist-evoked excitation is reflected by the increase of action potential discharge, but the underlying slow depolarization of the membrane potential is not seen in any of the traces. Electrical stimulation of interganglionic fibre tracts with a 25 μm Teflon-coated platinum electrode connected to a stimulator with a constant current isolation unit was used to evoke fast excitatory postsynaptic potentials (fast EPSPs).
Drug application
As the availability of human tissue is limited we needed to use local drug application techniques to be able to study more than one ganglion per preparation and more than one agonist per ganglion. Tissue exposure to the drugs by addition of the agonists to the superfusing Krebs solution was therefore not feasible. Histamine receptor agonists were applied to single ganglia by pressure ejection from micropipettes (20 psi, up to 500 ms duration, ejection speed 55 ± 27 nl s−1, approximately 200 μm from the ganglion). Pipettes were filled with histamine (100 μm, Sigma), the H1 agonist HTMT-dimaleat (10 μm), the H2 agonist dimaprit (50 μm), the H3 agonist (R)-(−)-α-methylhistamine (1 μm) (all from Tocris Cookson, Bristol, UK), or the H4 agonist 4-methylhistamine (50 μm, provided by GlaxoSmithKline, Harlow, UK).
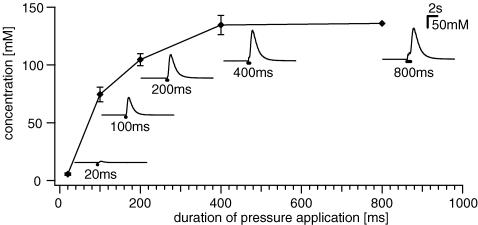
We performed control experiments in order to calculate the dilution factor and to provide a good estimate of the concentration of the microejected drug at the ganglionic level (Fig. 1). For this purpose we used a self-made concentration element consisting of two separate compartments each filled with 10 mm KCl and connected via a KCl-filled agar bridge. The potential difference between the two chambers was measured via silver–silver chloride electrodes. The recording tip of one of the electrodes was similar in shape and size to a human submucous ganglion (130 × 220 μm). We then positioned a microejection pipette about 200 μm away from the electrode tip and applied 1 m KCl with pressure microejection using the same settings as for our tissue studies. The changes in potential were plotted against the duration of the pressure pulse. The Nernst equation was then used to convert the potential differences into molar KCl concentrations (Fig. 1). Depending on the duration of the pressure pulse we estimated that any substance applied via pressure ejection pulses will be diluted by 1: 200 (20 ms pulse duration) to 1: 8 (400 ms pulse duration) once it reaches the ganglion (Fig. 1).
Figure 1. Drug dilution with pressure pulse microejection measured with a concentration element.
The duration of pressure ejection pulses of 1 m KCl (X-axis) is plotted against the calculated concentration of KCl (Y-axis) at the tip of the electrode. Insets illustrate representative traces of changes in KCl concentration for different pulse durations. Each data point represents 2–10 experiments. See Methods for further explanation.
Histamine receptor antagonists were added to the Krebs solution superfusing the preparations. We used the H1 antagonist pyrilamin (1 μm), the H2 antagonist ranitidine (10 μm), the H3 antagonist clobenpropit (10 μm) (all Sigma, Schnelldorf, Germany), and the H4 antagonist 1-[(5-chloro-1H-indol-2-yl)carbonyl]-4-methylpiperazine (JNJ 7777120; 1–50 μm; provided by Johnson & Johnson Pharmaceutical Research & Development, L.L.C., San Diego, CA, USA); they were perfused for 45 min before re-application of the agonists. The concentrations of the agonists and antagonists were based on similar experiments in the guinea-pig ENS, reported agonist and antagonist specificity and affinity to human histamine receptors and own preliminary studies that were aimed to determine agonist concentrations that produced consistent and reproducible responses (Nemeth et al. 1984; Tamura et al. 1988; Frieling et al. 1993; Liu et al. 2000; Lim et al. 2005). Additional substances used were hexamethonium (Sigma) and ω-conotoxin GVIA (conotoxin, Alomone Laboratories, Jerusalem, Israel). All substances were dissolved in Krebs solution.
To study presynaptic effects of the H3 receptors it was important to perform comparative studies in guinea-pig enteric neurones; methods and techniques were identical to the ones used for human submucous plexus and described in detail elsewhere (Schemann et al. 2005). To obtain myenteric plexus longitudinal muscle preparations, segments of guinea-pig ileum (male Dunkin–Hartley; 300–500 g; Charles River, Kisslegg, Germany) were quickly removed after killing the animals by cervical dislocation and exsanguination. The procedures used are in accordance with the German ethical guidelines for animal experiments.
Data analysis and statistics
Di-8-ANEPPS incorporates into the outer membrane revealing the outline of individual cell bodies. The overlay of signals and ganglion image allowed us to analyse the response of individual cells. The total number of neurones for each ganglion was determined by visual inspection of images from the Di-8-ANEPPS stained ganglion taken with a high resolution video camera (Cohu 4910, Cohu Inc., San Diego, CA, USA) (Neunlist et al. 1999; Schemann et al. 2005; Michel et al. 2005). For analysis the optical signals (traces of all photodiodes) were superimposed onto the image of the ganglion thus allowing us to calculate for each ganglion the percentage of nerve cells responding to a compound (Michel et al. 2005). To test correlation between percentage of histamine or agonists responding cells with age, sex or gut region, the Pearson product moment correlation was used. A P-value < 0.05 was considered statistically significant. Differences in action potential discharge before and after drug application were tested with Student's paired t test (SigmaStat 3.1, Systat Software Inc., Erkrath, Germany).
Results
Recordings were performed in 2137 neurones from 273 ganglia of 110 subjects (63 male, 47 female). The mean age was 65 years (range 25–88 years) for male and 68 years (range 30–83 years) for female subjects. Recordings were made from 1 jejunum, 13 ileum, 93 colon and 3 rectum specimens. No correlation existed between the number of histamine or agonists sensitive cells and age, sex or gut region.
To improve readability, numbers of neurones, ganglia and subjects are given in sequence without further specification, e.g. a result based on experiments in 30 neurones from 6 ganglia from 10 patients is presented as (30/6/10).
Histamine excited human submucous neurones: involvement of H1, H2 and H3 receptors
Pressure application of histamine evoked action potential discharge in 37.4% of human submucous neurones (800/109/52) (Fig. 2). The spike rate increased with the duration of the pressure application (Fig. 3). Histamine never decreased spontaneous spike discharge (180/55/31) (Fig. 2).
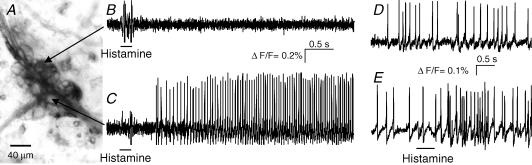
Figure 2. Histamine evoked action potential discharge in a subset of human submucous neurones.
A, fluorescence image of a Di-8-ANEPPS labelled submucous ganglion. Individual neurones of the ganglion can be distinguished by the strong staining of the outer membrane. B and C, the change in fluorescence intensity is shown on the right for two of these neurones to pressure application of 200 ms histamine (horizontal bars). The neurone shown in the upper trace did not respond to histamine while the neurone in the bottom trace responded after a short delay with a discharge of action potentials that lasted throughout the recording period of 4 s. D, the deflections in the traces during application of histamine are pressure ejection artefacts. E, one spontaneously active neurone from a different ganglion; 500 ms histamine application to the same neuron evoked increased spike discharge without changing the discharge pattern of ongoing activity. Note that underlying slow depolarizations of membrane potential are not detected by the AC-coupled photodiode system.
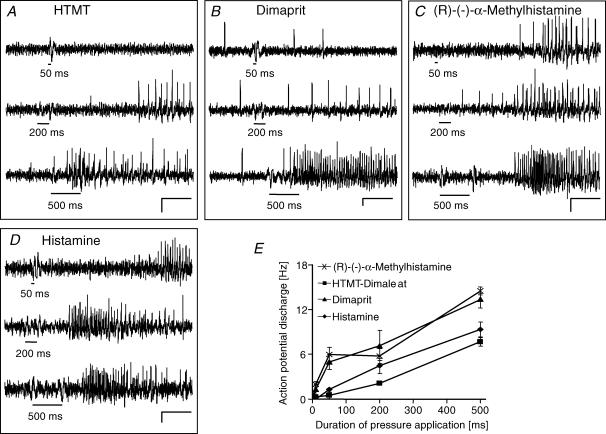
Figure 3. Histamine, the H1, H2 and H3 agonists evoked spike discharge which increased with duration of pressure application.
A–D, the H1 agonist HTMT-dimaleat, the H2 agonist dimaprit, the H3 agonist (R)-(−)-α-methylhistamine and histamine evoked spike discharge which increased with pressure application of 50 ms, 200 ms and 500 ms (black bars mark duration of drug application). Scale bars indicate changes in fluorescence intensity (ΔF/F = 0.2%) and time (0.5 s). The deflections in the traces during application of the substances are pressure ejection artefacts. E, illustration of the results for histamine and the receptor agonists after pressure application of 10 ms, 50 ms, 200 ms and 500 ms (each data point from n = 11–90 neurons). Note that underlying slow depolarizations of membrane potential are not detected by the AC-coupled photodiode system.
Selective H1, H2 or H3 receptor agonists mimicked the excitatory histamine response (Fig. 3). Microejection of the H1 agonist HTMT-dimaleat (341/43/25), the H2 agonist dimaprit (579/75/41) or the H3 agonist R-(−)-α-methylhistamine (619/70/38) evoked action potential discharge which increased in frequency with the duration of the pressure pulse (Fig. 3). Co-application of the selective agonists and histamine revealed that 63%, 60% and 82% of histamine responsive cells were activated by the H1 agonist (34/4/4), the H2 agonist (87/15/12), and the H3 agonist (48/8/5), respectively. This action profile indicated that neither one of the agonists could evoke a response in all histamine responsive neurones, suggesting a certain receptor clustering. This was addressed by coapplication of all three histamine receptor agonists (94/10/8). Out of the 94 neurones, 41 responded at least to one of the agonists. The highest proportion of cells responded to the H1 and the H3 but not to the H2 agonist, and are hence termed H1/H3. All combinations except a response to the H1 agonist only were observed (H1/H3 = 29%, H2 = 27%, H1/H2/H3 = 20%; H3 = 10%, H1/H2 = 7%, H2/H3 = 7%) (Fig. 4).
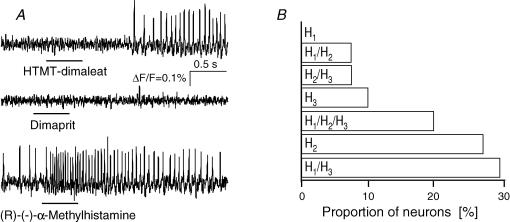
Figure 4. Responses to histamine receptor agonists suggested distinct receptor clustering.
A, the same submucous neurone responded to the H1 agonist HTMT-dimaleat and the H3 agonist (R)-(−)-α-methylhistamine, but not to the H2 agonist dimaprit (each 500 ms pressure application). This type of response corresponded to the code H1/H3. B, results of such experiments from 94 neurons revealed that most neurons responded to the H1 and H3 agonist but not to the H2 agonist. All possible codes occurred, except one, which is a response to the H1 agonist only. Note that underlying slow depolarizations of membrane potential are not detected by the AC-coupled photodiode system.
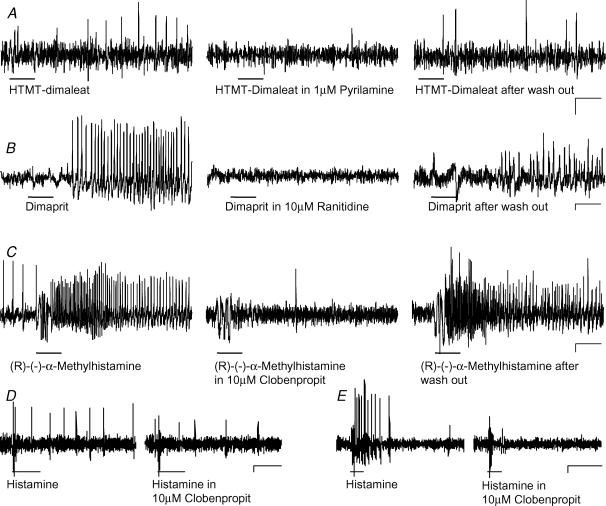
Selective H1, H2 or H3 receptor antagonists were used to demonstrate agonist specificity in 15 preparations derived from 11 subjects (Fig. 5). Perfusion of the H1 antagonist pyrilamin (1 μm) almost abolished the H1 agonist evoked spike discharge (5.4 ± 2.2 Hz versus 0.5 ± 0.5 Hz; 13/5/5). The selective H2 antagonist ranitidine (10 μm) totally abolished the H2 agonist evoked spike discharge (14.1 ± 5.8 Hz versus 0 Hz; 22/5/5). Likewise, the H3 antagonist clobenpropit (10 μm) nearly blocked the H3 agonist evoked spike discharge (11.5 ± 8.5 Hz versus 0.1 ± 0.2 Hz; 25/8/7) (Fig. 5).
Figure 5. Responses to histamine and histamine receptor agonists were blocked by selective receptor antagonists.
A, effect of the H1 agonist HTMT-dimaleat was blocked by the H1 antagonist pyrilamine and recovered after wash out of the antagonist. B, effect of the H2 agonist dimaprit was blocked by the H2 antagonist ranitidine and recovered after wash out of the antagonist. C, effect of the H3 agonist (R)-(−)-α-methylhistamine was blocked by the H3 antagonist clobenpropit and recovered after wash out of the antagonist. Histamine and histamine receptor agonists were applied by pressure application (black bars) before, during and after perfusion of the antagonists. D, histamine evoked spike discharge is decreased in the presence of clobenproprit. E, in this neurone clobenprobrit blocked the response to histamine. The deflections in the traces during application of the agonists are pressure ejection artefacts. The effects of the agonists were restored after wash out periods of 30 min to 1 h. Traces in A, B and C are from different neurones. Scale bars represent time (0.5 s) and changes in fluorescence intensity (ΔF/F = 0.1%). Note that underlying slow depolarizations of membrane potential are not detected by the AC-coupled photodiode system.
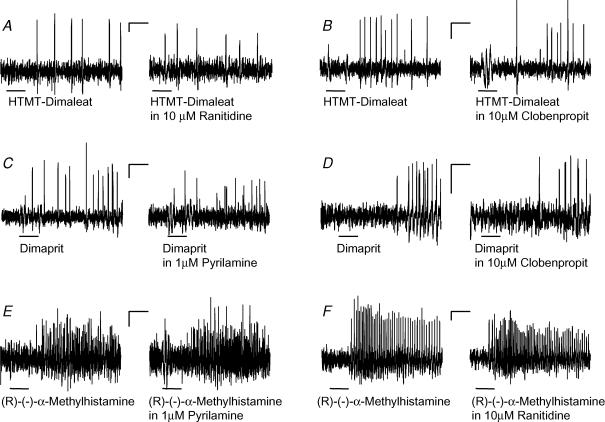
Specificity of agonists and antagonists was further verified by studying whether agonist responses showed cross-sensitivity to other histamine receptor antagonists (75/17/10). Results from these studies revealed that the H1 agonist response was not changed in the presence of H2 (2.87 ± 0.8 Hz versus 2.88 ± 1.1 Hz) or H3 (9.9 ± 9.4 Hz versus 9.0 ± 10.8 Hz) antagonists. The H2 agonist induced response was not affected by the H1 (3.9 ± 2.1 Hz versus 4.3 ± 1.7 Hz) or H3 (3.1 ± 0.9 Hz versus 3.0 ± 0.1 Hz) antagonists, and the H3 agonist evoked response was not affected by the H1 (8.8 ± 2.6 Hz versus 10.6 ± 3.6 Hz) or H2 (5.9 ± 2.4 Hz versus 6.6 ± 3.4 Hz) antagonists (Fig. 6).
Figure 6. The excitatory effects of the histamine agonists on human submucous neurones were specific with no indication of cross-reactivity.
A–B, the responses to pressure application of the H1 agonist HTMT-dimaleat (black bars) remained in the presence of the H2 antagonist ranitidine (A) or the H3 antagonist clobenpropit (B). C–D, likewise the responses to pressure application of the H2 agonist dimaprit (black bars) remained in the presence of the H1 antagonist pyrilamine (C) or the H3 antagonist clobenpropit (D). E–F, the responses to pressure application of the H3 agonist (R)-(−)-α-methylhistamine (black bars) remained in the presence of the H1 antagonist pyrilamine (E) or the H2 antagonist ranitidine (F). The deflections in the traces during application of the agonists are pressure ejection artefacts. Traces in A–E were from different neurones. Scale bars represent time (0.5 s) and changes in fluorescence intensity (ΔF/F = 0.1%). Note that underlying slow depolarizations of membrane potential are not detected by the AC-coupled photodiode system.
H3 and H4 receptor mediated responses
Most noteworthy was the excitatory response to (R)-(−)-α-methylhistamine. Because of the close homology between H3 and H4 receptors we performed experiments to specifically rule out cross-reactivity.
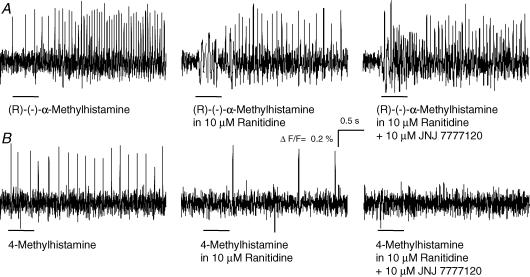
The specificity of the H3 agonist evoked excitation was supported by the finding that the response to (R)-(−)-α-methylhistamine was not changed during perfusion of the H4 receptor specific antagonist JNJ 7777120 at concentrations of 1, 10, or 50 μm (129/7/7) (Fig. 7).
Figure 7. H3 and H4 receptor mediated excitation in the human submucous plexus was receptor specific.
A, pressure application of the H3 agonist (R)-(−)-α-methylhistamine evoked action potential discharge (left panel) which was not changed during perfusion of the H2 antagonist ranitidine (centre panel) or the H4 antagonist 10 μm JNJ 7777120 (right panel). B, pressure application of the H4 agonist 4-methylhistamine evoked action potential discharge (left panel). The response was diminished but not abolished during perfusion with the H2 antagonist ranitidine (centre panel). Additional perfusion of the H4 antagonist JNJ 7777120 totally blocked the response to 4-methylhistamine (right panel). All traces are from the same neurone. The deflections in the traces during application of the agonists are pressure ejection artefacts. Note that underlying slow depolarizations of membrane potential are not detected by the AC-coupled photodiode system.
Recently, 4-methylhistamine, formerly used in some studies as an H2 agonist, has been identified as the first potent and selective H4 agonist (Lim et al. 2005). Without ranitidine in the bath pressure application of 10 μm or 50 μm 4-methylhistamine evoked action potential discharge in 26% (26/2/2), or 38% (14/2/2) of the neurones, respectively (Fig. 7). In the presence of the H2 antagonist ranitidine the proportion of neurones that responded to 10 μm or 50 μm 4-methy-lhistamine decreased to 3.8% (79/7/4) or 8% (88/8/4), respectively. The remaining 4-methylhistamine response was abolished by perfusion of the H4 antagonist JNJ 7777120 (10 or 50 μm, 64/6/6) (Fig. 7). The mean frequency of action potential discharge after pressure application of 4-methylhistamine in the presence of ranitidine was 1.2 ± 0.3 Hz.
Pressure application of the H4 agonist 4-methylhistamine (50 μm) and the H3 agonist (R)-(−)-α-methylhistamine (1 μm) onto the same neurones (189/15/9) in the presence of ranitidine provided additional evidence for the existence of both receptor subtypes (Fig. 7). 4-Methylhistamine induced an effect in 21, whereas (R)-(−)-α-methylhistamine induced an effect in 81 of these neurones. Importantly, all cells which responded to the H4 agonist were also activated by the H3 agonist but not vice versa. Thus, the H4 mediated excitation occurred in about 11% of human submucous neurones (183/12/10) and we calculated that about 27% of histamine sensitive neurones responded to 4-methylhistamine.
The results of the above experiments allow three main conclusions. First, the excitatory response to (R)-(−)-α-methylhistamine was indeed mediated by H3 receptors. Second, H4 receptor activation evoked excitatory response in human submucous plexus neurones. Third, a small subset of H3 sensitive neurones was also activated by the H4 agonist.
We performed experiments to show involvement of H3 receptors in the histamine response. Responses to pressure application of histamine were compared before and during perfusion of clobenpropit (45/7/5). Histamine evoked action potential discharge was significantly reduced in all neurones in the presence of clobenproprit (2.2 ± 1.6 Hz versus 0.4 ± 0.5 Hz) (Fig. 5). A total block of the histamine response was seen in 60% of the neurones.
Lack of evidence for H3 receptor mediated presynaptic effects in human submucous neurones
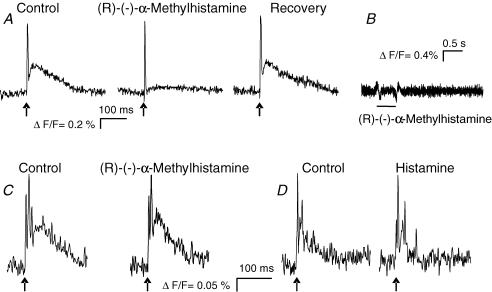
Electrophysiological experiments on guinea-pig myenteric neurones showed that histamine had a presynaptic inhibitory action to reduce fast EPSP amplitude via activation of presynaptic H3 receptors (Nemeth et al. 1984; Tamura et al. 1988). We used the same preparation and successfully reproduced these data in order to demonstrate that the optical recording technique allowed us to detect inhibition of fast EPSPs. We could readily demonstrate a decrease in fast EPSP amplitude after spritz application of 100 μm histamine (79/10/8) or 1 μm (R)-(−)-α-methylhistamine (28/4/4) (Fig. 8). However, spritz application of either substance had no effect on fast EPSPs in the human submucous plexus (36/8/4) (Fig. 8). The fast EPSP amplitudes expressed as relative changes in fluorescence remained constant after histamine (0.64 ± 0.40%ΔF/F versus 0.67 ± 0.40%ΔF/F, P = 0399) or (R)-(−)-α-methylhistamine application (0.422 ± 0.221%ΔF/F versus 0.424 ± 0.210%ΔF/F, P = 0311). Even 15 min perfusion of 1 μm (R)-(−)-α-methylhistamine did not change fast EPSP amplitudes (0.53 ± 0.19%ΔF/F versus 0.54 ± 0.15%ΔF/F, P = 0.81) in human submucous neurones (6/2/2). As expected from previous studies (Tamura et al. 1988) (R)-(−)-α-methylhistamine had no excitatory effect in guinea-pig myenteric neurones (Fig. 8), a finding that further supported a human specific H3 mediated neuronal excitation.
Figure 8. The H3 receptor agonist (R)-(−)-α-methylhistamine inhibited fast EPSPs in guinea-pig myenteric neurones but had no effect on fast EPSPs in human submucous neurons.
A, electrical stimulation of an interganglionic fibre tract (arrows) evoked a compound action potential (steep upstroke) originating from nerve fibre passing through the ganglion (see Schemann et al. 2005) followed by a subthreshold fast EPSP in a guinea-pig myenteric neurone (left trace). Shortly after pressure application of (R)-(−)-α-methylhistamine, the fast EPSP was almost abolished (centre panel) and recovered several minutes after the application (right panel). B, pressure application of (R)-(−)-α-methylhistamine did not evoke any postsynaptic response in a guinea-pig myenteric neurone. C, electrical stimulation of an interganglionic fibre tract (arrows) in the human submucous plexus evoked fast EPSP (left panel). The fast EPSP remained unchanged after pressure application of (R)-(−)-α-methylhistamine (right panel). D, electrical stimulation of an interganglionic fibre tract (arrow) evoked fast EPSP triggering several action potentials in a different neurone of the human submucous plexus (left panel) which was unchanged after spritz application of histamine (right panel).
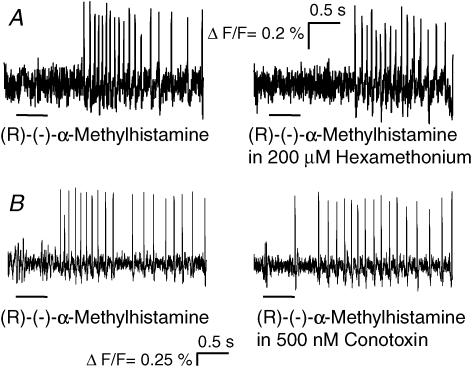
The H3 agonist-evoked excitation in the human submucous plexus is mediated postsynaptically rather than by presynaptic facilitation because its effect is not changed in the presence of the nicotinic receptor blocker hexamethonium (200 μm) (10/3/1) or the calcium channel blocker ω-conotoxin (500 nm) (24/5/3) or in calcium depleted Krebs solution (13/3/2) (Fig. 9).
Figure 9. The H3 agonist (R)-(−)-α-methylhistamine directly excited human submucous neurones.
A–B, the response to pressure application of (R)-(−)-α-methylhistamine remained after nicotinic blockade with hexamethonium (A) or blockade of synaptic transmission by conotoxin (B). The deflections in the traces during application of the agonists are pressure ejection artefacts. Note that underlying slow depolarizations of membrane potential are not detected by the AC-coupled photodiode system.
Discussion
The present study revealed that the mast cell mediator histamine has potent excitatory effects in the human ENS. Mast cells are located in close proximity to nerves and the proximity of activated mast cells to nerves correlates with the frequency and severity of abdominal pain, suggesting a functional mast cell–nerve interface relevant for immunoneural signalling (Barbara et al. 2004). It is therefore conceivable that mast cell mediators, among them histamine, reach enteric nerves by volume transmission. This is supported by the finding that increased activity of enteric nerves in sensitized guinea-pigs is reduced by antagonists of histamine receptors suggesting that histamine released from mast cells is able to reach enteric neurons (Frieling et al. 1994).
We found that all four histamine receptors were involved in the excitatory response, which markedly differs from findings reported in animal models. In guinea-pig enteric neurones the excitatory histamine response was mainly mediated by H2 receptors (Nemeth et al. 1984; Frieling et al. 1993). Besides the strong involvement of H1 receptors and the discovery of an H4 mediated component of the neuronal excitatory histamine response, one of the most striking results was the H3 receptor mediated excitatory effect in human submucous plexus. Several lines of evidence indicated that the (R)-(−)-α-methylhistamine evoked excitation was indeed an H3 receptor specific effect. First, H1, H2 or H4 antagonists did not affect the (R)-(−)-α-methylhistamine response. Second, experiments applying H1, H2 and H3 agonists to the same neurones revealed different receptor patterns making cross-reactivity highly unlikely. Third, interaction with the closely related H4 receptor can be ruled out because the H4 specific antagonist JNJ 7777120 did not change response to the H3 agonist and application of H3 and H4 agonists to the same neurones showed that more cells responded to the H3 than to the H4 agonist. So far, H3 mediated actions on nerve activity in the brain or guinea-pig ENS have been described as inhibitory, often involving presynaptic localization of the H3 receptors (Nemeth et al. 1984; Frieling et al. 1993; Xie & He, 2005). Our results demonstrated that neither histamine nor the H3 agonist decreased the amplitude of fast EPSPs in the human submucous plexus, yet we could readily demonstrate H3 mediated suppression of fast EPSPs in the guinea-pig ENS.
To the best of our knowledge, there has been no report on excitatory actions mediated by H3 receptors and the human ENS may be unique or one of the first examples for such excitatory actions. Diverse H3 receptor mediated effects may become more common with the identification of numerous isoforms of the human H3 receptor (Hancock et al. 2003; Bakker, 2004) and the species related pharmacological heterogeneity of H3 receptors (Ireland-Denny et al. 2001).
A recent study suggested expression of H1, H2 and H4, but not H3, receptors in human intestine (Sander et al. 2006). The failure of H3 expression in this study is in contrast to the prominent H3 receptor mediated neuronal excitation in our study, but may be explained by a relatively low expression of H3 receptors or expression of H3 isoforms that may not have been recognized by the probes used in the study of Sander et al. (2006). It is important to mention, that H3 expression did occur in two of their patients, one control and one patient with irritable bowel syndrome (Sander et al. 2006).
Pharmacologically, we distinguished subpopulations of human submucous neurones based on the response pattern to various histamine receptor agonists. We had to use relatively long recording periods to identify the pharmacology of the responses and this compromised subsequent immunohistochemical evaluation of the neurochemical coding of particular subpopulations. Since we studied histamine effects on submucous neurones, the obvious target tissues include epithelial cells, vascular smooth muscle or immune cells. Our study suggests that it is important to consider all four histamine receptors when studying the functional relevance of neurally mediated histamine responses in the human gut. While some data on human intestine supported a role of nerves in mediating mast cell mediator evoked ion secretion (Crowe & Perdue, 1993) others suggested no involvement of enteric nerves based on the TTX insensitivity of the histamine responses (Stack et al. 1995). These findings were based on acute histamine effects. In future studies it may be worthwhile to specifically investigate neurally mediated responses after long-term histamine applications that closely reflect chronically high histamine levels in the diseased gut. Such experiments in guinea-pig colon revealed histamine induced cyclical increases in ion secretion that involved excitation of enteric neurones (Cooke et al. 1995).
In view of the blockbuster status of H1 and H2 antagonists, similar expectations for drugs targeting H3 or H4 receptors have been recently expressed (Leurs et al. 2005). Thus H4 receptor antagonists may represent a novel pharmacological approach to treat inflammatory diseases including colitis (Thurmond et al. 2004; Leurs et al. 2005; Varga et al. 2005). Plasticity in histamine receptor expression in patients with food allergy and IBS, in particular an increase in H1 and H2 receptor mRNA levels, emphasizes the potential clinical benefit of drugs that act on histaminergic pathways (Sander et al. 2006). Our findings on histamine mediated neural actions and the human-specific pharmacology may be of help to develop such compounds.
Acknowledgments
This study was supported by DFG Sche 267/5-1, 5-2, 5-3.
References
- Bakker RA. Histamine H3-receptor isoforms. Inflamm Res. 2004;53:509–516. doi: 10.1007/s00011-004-1286-9. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, de Giorgio R, Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil. 2006;18:6–17. doi: 10.1111/j.1365-2982.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089–1113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Celanire S, Wijtmans M, Talaga P, Leurs R, de Esch IJ. Keynote review: Histamine H3 receptor antagonists reach out for the clinic. Drug Discov Today. 2005;10:1613–1627. doi: 10.1016/S1359-6446(05)03625-1. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Wang YZ, Reddix R, Javed N. Cholinergic and VIP-ergic pathways mediate histamine H2 receptor-induced cyclical secretion in the guinea pig colon. Am J Physiol Gastrointest Liver Physiol. 1995;268:G465–G470. doi: 10.1152/ajpgi.1995.268.3.G465. [DOI] [PubMed] [Google Scholar]
- Crowe SE, Perdue MH. Anti-immunoglobulin E-stimulated ion transport in human large and small intestine. Gastroenterology. 1993;105:764–772. doi: 10.1016/0016-5085(93)90894-i. [DOI] [PubMed] [Google Scholar]
- Crowe SE, Sestini P, Perdue MH. Allergic reactions of rat jejunal mucosa. Ion transport responses to luminal antigen and inflammatory mediators. Gastroenterology. 1990;99:74–82. doi: 10.1016/0016-5085(90)91232-u. [DOI] [PubMed] [Google Scholar]
- de Esch IJ, Thurmond RL, Jongejan A, Leurs R. The histamine H4 receptor as a new therapeutic target for inflammation. Trends Pharmacol Sci. 2005;26:462–469. doi: 10.1016/j.tips.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Frieling T, Cooke HJ, Wood JD. Histamine receptors on submucous neurons in guinea pig colon. Am J Physiol Gastrointest Liver Physiol. 1993;264:G74–G80. doi: 10.1152/ajpgi.1993.264.1.G74. [DOI] [PubMed] [Google Scholar]
- Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after sensitization to milk antigen. Am J Physiol Gastrointest Liver Physiol. 1994;267:G1087–G1093. doi: 10.1152/ajpgi.1994.267.6.G1087. [DOI] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Hancock AA, Esbenshade TA, Krueger KM, Yao BB. Genetic and pharmacological aspects of histamine H3 receptor heterogeneity. Life Sci. 2003;73:3043–3072. doi: 10.1016/j.lfs.2003.06.003. [DOI] [PubMed] [Google Scholar]
- He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309–318. doi: 10.3748/wjg.v10.i3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland-Denny L, Parihar AS, Miller TR, Kang CH, Krueger KM, Esbenshade TA, Hancock AA. Species-related pharmacological heterogeneity of histamine H3 receptors. Eur J Pharmacol. 2001;433:141–150. doi: 10.1016/s0014-2999(01)01518-7. [DOI] [PubMed] [Google Scholar]
- Keely SJ, Stack WA, O'Donoghue DP, Baird AW. Regulation of ion transport by histamine in human colon. Eur J Pharmacol. 1995;279:203–209. doi: 10.1016/0014-2999(95)00156-f. [DOI] [PubMed] [Google Scholar]
- Leurs R, Bakker RA, Timmerman H, de Esch IJ. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov. 2005;4:107–120. doi: 10.1038/nrd1631. [DOI] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. J Pharmacol Exp Ther. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- Liu S, Xia Y, Hu H, Ren J, Gao C, Wood JD. Histamine H3 receptor-mediated suppression of inhibitory synaptic transmission in the submucous plexus of guinea-pig small intestine. Eur J Pharmacol. 2000;397:49–54. doi: 10.1016/s0014-2999(00)00228-4. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Fernandez E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005;17:4–15. doi: 10.1111/j.1365-2982.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- MacGlashan D., Jr Histamine: a mediator of inflammation. J Allergy Clin Immunol. 2003;112:S53–S59. doi: 10.1016/s0091-6749(03)01877-3. [DOI] [PubMed] [Google Scholar]
- Maurer M, Theoharides T, Granstein RD, Bischoff SC, Bienenstock J, Henz B, Kovanen P, Piliponsky AM, Kambe N, Vliagoftis H, Levi-Schaffer F, Metz M, Miyachi Y, Befus D, Forsythe P, Kitamura Y, Galli S. What is the physiological role of mast cells? Exp Dermatol. 2003;12:886–910. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- Michel K, Zeller F, Langer R, Nekarda H, Kruger D, Dover TJ, Brady CA, Barnes NM, Schemann M. Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology. 2005;128:1317–1326. doi: 10.1053/j.gastro.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Nemeth PR, Ort CA, Wood JD. Intracellular study of effects of histamine on electrical behaviour of myenteric neurones in guinea-pig small intestine. J Physiol. 1984;355:411–425. doi: 10.1113/jphysiol.1984.sp015427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Peters S, Schemann M. Multisite optical recording of excitability in the enteric nervous system. Neurogastroenterol Motil. 1999;11:393–402. doi: 10.1046/j.1365-2982.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Raithel M, Matek M, Baenkler HW, Jorde W, Hahn EG. Mucosal histamine content and histamine secretion in Crohn's disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol. 1995;108:127–133. doi: 10.1159/000237129. [DOI] [PubMed] [Google Scholar]
- Sander LE, Lorentz A, Sellge G, Coeffier M, Neipp M, Veres T, Frieling T, Meier PN, Manns MP, Bischoff SC. Selective expression of histamine receptors H1R, H2R and H4R, but not H3R, in the human intestinal tract. Gut. 2006;55:498–504. doi: 10.1136/gut.2004.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Michel K, Ceregrzyn M, Zeller F, Seidl S, Bischoff SC. Human mast cell mediator cocktail excites neurons in human and guinea-pig enteric nervous system. Neurogastroenterol Motil. 2005;17:281–289. doi: 10.1111/j.1365-2982.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- Schemann M, Michel K, Peters S, Bischoff SC, Neunlist M. Cutting-edge technology. III. Imaging and the gastrointestinal tract: mapping the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2002;282:G919–G925. doi: 10.1152/ajpgi.00043.2002. [DOI] [PubMed] [Google Scholar]
- Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16:55–59. doi: 10.1111/j.1743-3150.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, Schemann M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn's disease. Neurogastroenterol Motil. 2001;13:255–264. doi: 10.1046/j.1365-2982.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- Stack WA, Keely SJ, O'Donoghue DP, Baird AW. Immune regulation of human colonic electrolyte transport in vitro. Gut. 1995;36:395–400. doi: 10.1136/gut.36.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- Tamura K, Palmer JM, Wood JD. Presynaptic inhibition produced by histamine at nicotinic synapses in enteric ganglia. Neuroscience. 1988;25:171–179. doi: 10.1016/0306-4522(88)90016-4. [DOI] [PubMed] [Google Scholar]
- Thurmond RL, Desai PJ, Dunford PJ, Fung-Leung WP, Hofstra CL, Jiang W, Nguyen S, Riley JP, Sun S, Williams KN, Edwards JP, Karlsson L. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J Pharmacol Exp Ther. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- Varga C, Horvath K, Berko A, Thurmond RL, Dunford PJ, Whittle BJ. Inhibitory effects of histamine H4 receptor antagonists on experimental colitis in the rat. Eur J Pharmacol. 2005;522:130–138. doi: 10.1016/j.ejphar.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Wood JD. Histamine, mast cells, and the enteric nervous system in the irritable bowel syndrome, enteritis, and food allergies. Gut. 2006;55:445–447. doi: 10.1136/gut.2005.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, He S. Roles of histamine and its receptors in allergic and inflammatory bowel diseases. World J Gastroenterol. 2005;11:2851–2857. doi: 10.3748/wjg.v11.i19.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]