Abstract
It is established that the gut peptide galanin reduces neuronal excitability via galanin receptor subtypes GALR1 and GALR3 and increases excitability via subtype GALR2. We have previously shown that galanin potently reduces mechanosensitivity in the majority of gastro-oesophageal vagal afferents, and potentiates sensitivity in a minority. These actions may have implications for therapeutic inhibition of gut afferent signalling. Here we investigated which galanin receptors are likely to mediate these effects. We performed quantitative RT-PCR on RNA from vagal (nodose) sensory ganglia, which indicated that all three GALR subtypes were expressed at similar levels. The responses of mouse gastro-oesophageal vagal afferents to graded mechanical stimuli were investigated before and during application of galanin receptor ligands to their peripheral endings. Two types of vagal afferents were tested: tension receptors, which respond to circumferential tension, and mucosal receptors which respond only to mucosal stroking. Galanin induced potent inhibition of mechanosensitivity in both types of afferents. This effect was totally lost in mice with targeted deletion of Galr1. The GALR1/2 agonist AR-M961 caused inhibition of mechanosensitivity in Galr1+/+ mice, but this was reversed to potentiation in Galr1−/− mice, indicating a minor role for GALR2 in potentiation of vagal afferents. We observed no functional evidence of GALR3 involvement, despite its expression in nodose ganglia. The current study highlights the complex actions of galanin at different receptor subtypes exhibiting parallels with the function of galanin in other systems.
Modulation of vagal afferent signalling from the upper gastrointestinal tract has important implications in treatment of gastrointestinal dysfunction. Particularly relevant disorders include functional dyspepsia and gastro-oesophageal reflux disease. So far we have demonstrated potent inhibition of vagal afferent mechanosensitivity by metabotropic receptors to the amino acids γ-amino butyric acid (GABA) and glutamate (Page & Blackshaw, 1999; Page et al. 2005c). Moreover, these effects may be accompanied by inhibition of transient lower oesophageal sphincter relaxation and gastro-oesophageal reflux (Blackshaw et al. 1999; Lehmann et al. 1999; Lidums et al. 2000), with therapeutic benefit (Blackshaw, 2001; Zhang et al. 2002). In addition to afferent modulation by amino acids, peptidergic modulation of vagal afferent fibres by opioids has also been demonstrated (Ozaki et al. 2000).
We have recently identified a novel role for galanin in modulation of extrinsic afferents from the upper gastrointestinal tract of mice and ferrets (Page et al. 2005b). Galanin is a 29–30 amino acid brain–gut neuropeptide, with distribution and functions throughout the gastrointestinal tract as well as central nervous system. It has previously been shown to regulate a variety of functions including neurotransmitter release, nociception, spinal reflexes and feeding behaviour (Kask et al. 1997; Crawley, 1999; Liu & Hokfelt, 2002; Wiesenfeld-Hallin et al. 2005).
Galanin may have excitatory or inhibitory effects on motor function or neuronal excitability depending on which of the three distinct receptor subtypes (GALR1, 2 and 3) it binds (Branchek et al. 1998, 2000). As such galanin may have contrasting effects: pro- and anti-nociceptive effects in the spinal cord (Wynick et al. 2001); contraction or relaxation of gastrointestinal smooth muscle directly or via neuronal actions (Botella et al. 1995; Ren et al. 2001); and effects on signalling in central gastric vagal pathways with both inhibition and excitation demonstrated (Yuan et al. 2002; Tan et al. 2004). These divergent effects of galanin were reflected in our in vitro gastro-oesphageal vagal afferent preparation (Page et al. 2005b) and we speculated that they may be determined by variable expression of different galanin receptor subtypes on different afferent endings; however, this remained to be examined. The predominant effect of galanin we observed was inhibition of vagal afferent mechanosenstivity (Page et al. 2005b). GALR1 is negatively coupled to adenylate cyclase via inhibitory Gi G-protein and can influence neuronal Ca2+ and inwardly rectifying K+ currents. GALR2 is coupled via Go and Gq/11 G-proteins leading to activation of cellular inositol trisphosphate production and protein kinase C activity (Branchek et al. 2000). The physiological role of GALR3 remains relatively unexplored. The receptor utilizes a signalling pathway linked through G-proteins to inhibition in a similar way to GALR1 (Branchek et al. 2000). The diverse actions of galanin in the gut are likely to be the result of activation of multiple receptors with differing signal transduction pathways. The inhibitory effects we observed would thus be mediated via either GALR1 or GALR3 and the potentiating effects via GALR2. The relatively higher frequency at which we encountered inhibitory effects compared to the excitatory effects suggests that agonists of GALR1 or GALR3 may have more therapeutic potential for reducing mechanosensory function than antagonists of GALR2.
Targeting specific receptors in order to reduce vagal afferent signalling is important in order to minimize side effects of therapeutic intervention and to gain a better understanding of mechanisms of modulation of vagal afferent endings. To date, the specific role of each galanin receptor in gastro-oesophageal vagal afferent mechanosensation is yet to be determined. In order to better understand the contribution of individual receptor subtypes involved in modulation of mechanosensitivity, we used specific galanin receptor ligands, Galr1−/− mice and electrophysiological recordings of mechanical responsiveness in single vagal afferent fibres.
Methods
All studies were performed in accordance with the guidelines of the Animal Ethics Committees of the Royal Adelaide Hospital and the Institute for Medical and Veterinary Science, Adelaide, and also the Animal Ethics Committee of the University of Adelaide.
Generation of galanin receptor 1 (Galr1)-null mutant mice
The methods used to generate Galr1-null mutants have been described in detail previously (Jacoby et al. 2002; Blakeman et al. 2003). Briefly, W9.5 embryonic stem cells were transfected with a Galr1 gene targeting construct and correctly targeted clones were isolated and injected into C57BL/6 blastocysts. The resultant chimaeric mice were mated with C57BL/6 mice to produce heterozygous (Galr1+/−) mice that carried the Galr1-null allele in their germ line. These heterozygotes were backcrossed to C57BL/6 mice to generate Galr1+/−for mating to produce Galr1−/− and Galr1+/+ littermates for analysis. Galr1−/− mice on a C57BL/6 background exhibit normal growth rates and lifespan and display no overt phenotype.
Determination of galanin receptor transcript expression in nodose ganglia using quantitative RT-PCR
Five male or female mice (20–30g) were killed via CO2 inhalation and the nodose ganglia were removed bilaterally as previously described (Page et al. 2004, 2005a). RNA quantification was determined by measuring the absorbance at 260 nm (A260) using a spectrophotometer (Biorad, New South Wales, Australia). RNA quality was estimated by the A260 and A280 ratio. Quantitative reverse transcription polymerase chain reactions (RT-PCR) reactions were performed as described in detail previously (Hughes et al. 2007). Briefly, quantitative RT-PCR reactions were performed using a Chromo4 (MJ Research, Biorad) real-time instrument attached to a PTC-200 Peltier thermal cycler (MJ Research) and analysed using Opticon Monitor software (MJ Research). Quantitative RT-PCR reactions were performed using a Qiagen QuantiTect SYBR Green RT-PCR one-step kit (Qiagen, Australia) according to the manufacturer's specifications, with specific Quantitect Primer Assays (Qiagen) optimized and validated for the detection of the known sequence of mouse galanin receptors 1, 2 and 3 and β-actin transcripts contained in the National Center for Biotechnology Information (NCBI) reference sequence database (http://www.ncbi.nlm.nih.gov/RefSeq). All product lengths were restricted below 150 bp to maximize efficiency of the SYBR Green reaction. These primer assays were used under the following conditions: reverse transcription: 50°C for 30 min; initial PCR activation: 95°C for 15 min; PCR cycles 94°C for 15 s, 55°C for 30 s and 72°C for 30 s repeated for 44 cycles. A melting curve program verified the specificity and identity of the RT-PCR products and no primer dimers were observed. Confirmation of the amplified products were resolved by 3% agarose gel electrophoresis and visualized via ethidium bromide staining. Each assay was run in at least triplicate in separate experiments. Control PCRs were performed by substituting RNA template with distilled RNAse-free water. All assays were validated for linearity of amplification efficiency and quantitative standard curves obtained using serial dilutions of RNA. Calculations for relative expression of mRNA transcripts were performed using the comparative cycle threshold (CT) method, comparing to the internal reference gene β-actin using the calculation ΔCT (CT of target transcript – CT of β-actin). To determine relative expression of these transcripts in whole nodose ganglia, the ΔΔCT was calculated using the formula: ΔΔCT = ΔCT [β-actin] −ΔCT [galanin receptor] and the relative fold differences calculated using the formula 2−2ΔΔCT. Quantitative data are expressed as mean ±s.d., and significant differences in transcript expression determined by a Mann–Whitney U test at a significance level of P < 0.05.
In vitro mouse gastro-oesophageal afferent preparation
Galr1+/+, Galr1−/− and C57BL/6 mice fed ad libitum (20–30 g) were killed via CO2 inhalation and the thorax was opened by a midline incision. No overt phenotypic differences between genotypes were noted at this stage. The stomach and oesophagus with attached vagal nerves were removed and placed in a modified Krebs solution containing (mm): NaCl 118.1, KCl 4.7, NaHCO3 25.1, NaH2PO4 1.3, MgSO4.7H2O 1.2, CaCl2 1.5, citric acid 1.0 and glucose 11.1, bubbled with 95% O2–5% CO2. The temperature was maintained at 4°C during dissection to prevent metabolic degradation. After further dissection, the preparation was opened out longitudinally along the oesophagus and greater curve of the stomach. The preparation was then placed mucosa side up in the organ bath. This preparation has been described in detail previously (Page et al. 2002, 2005b). Nifedipine (1 μm) was also added to the Krebs solution to prevent smooth muscle contraction. In a previous preliminary study we have shown that nifedipine has no effect on the mechanical sensitivity of gastro-oesophageal vagal afferents (Page et al. 2006).
Characterization of gastro-oesophageal vagal afferent properties
Two types of mechanosensitive afferents were studied, those responding to mucosal stroking but not to circular tension (mucosal receptors) and those responding to mucosal stroking and circular tension (tension receptors) as reported previously (Page et al. 2002).
Location of receptive fields of all types of afferent fibre was determined by mechanical stimulation throughout the preparation with a brush. Accurate quantification of mechanical responses was performed differently according to the primary adequate stimulus for the type of fibre. Mechanical thresholds of both types of fibre were determined using calibrated von Frey hairs. The most reproducible, stimulus-dependent responses of these afferents to mucosal stroking were evoked when the probe was moved at a rate of 5 mm s−1 across the receptive field rather than being kept static. Because the receptive fields are small (< 1 mm2), a single test at each intensity is prone to missing the centre of the receptive field on some occasions. Therefore, we minimized experimenter error by measuring the mean response to the middle eight of 10 standard strokes given at 1 s intervals. Because the von Frey hair was bent throughout the stroking stimulus, the receptive field was subjected to an even force as the hair passed over it. This protocol was found to give highly reproducible data and was therefore used to assess effects of galanin on vagal afferents. Tension–response curves were also obtained for all afferent fibres, which were used in combination with von Frey thresholds to determine whether the receptive fields of fibres were located in the mucosa or the muscle layer. Tension stimuli were applied via fine suture silk attached to an unpinned point adjacent to the mechanoreceptive fields. The thread was attached to a cantilever via a pulley close to the preparation. Reference standard weights were then placed on the opposite end of the cantilever. Each weight was applied as a step and maintained for 1 min, and the response was measured as the mean discharge evoked over this period. Because all responses to tension were similarly slowly adapting, this method of assessment was considered representative of physiological responsiveness. The tension–response curves were produced by applying weights to the cantilever system in the range of 1–5 g. A recovery period of at least 1 min was allowed between each tension stimulus.
Effect of galanin on the mechanosensitivity of vagal afferents
After mechanical sensitivity of the gastro-oesophageal vagal afferent had been established, the effect of galanin on mechanical sensitivity was determined. Galanin (1 nm) was added to the superfusing solution and allowed to equilibrate for 20 min after which time the tension–response and stroke–response curves were re-determined. This equilibration period was observed so as to ensure penetration of the drug into all layers of the tissue. This procedure was repeated for galanin at increasingly higher doses (3–10 nm). The concentration of galanin chosen was based on previous experiments on C57BL/6 mouse and ferret gastro-oesophageal vagal afferents in which galanin had a dose-dependent effect between 1 and 10 nm (Page et al. 2005b). Time-controlled experiments were performed in which there was no significant change in the mechanical responses over a comparable duration. Concentration–response curves were obtained in tissue from matched Galr1+/+ and Galr1−/− mice for tension receptors. Because the effects of galanin on tension receptors were similar in the Galr1+/+ littermate mice and unmatched C57/BL6 mice, the latter were used as controls for studies of mucosal afferents. This was necessary because of the limited availability of Galr1+/+ littermates.
Effect of galanin and SNAP 37889 on the mechanosensitivity of gastro-oesophageal vagal afferents
After mechanical sensitivity of the gastro-oesophageal vagal afferent had been established in C57BL/6 mice, the effect of galanin on mechanical sensitivity was determined. The maximal concentrations of galanin (1 nm for tension receptors, 10 nm for mucosal receptors) were added to the superfusing solution and allowed to equilibrate for 20 min after which time the tension–response and stroke–response curves were re-determined. The GALR3-selective antagonist SNAP 37889 (10 nm for tension receptors and 100 nm for mucosal receptors) was then added to the superfusing solution along with the galanin and allowed to equilibrate for 20 min. Mechanical response relationships were then re-determined. These concentrations were calculated based on the relative efficacy of galanin in our preparation and the published potency of SNAP 37889 (Swanson et al. 2005). SNAP 37889 is at least 200 times more selective for GALR3 than GALR1 and 2 (Swanson et al. 2005).Time-controlled experiments were performed in which there was no significant change in the mechanical responses over a comparable duration.
Effect of AR-M961 on the mechanosensitivity of gastro-oesophageal vagal afferents
After mechanical sensitivity of tension receptor gastro-oesophageal vagal afferents had been established in C57BL/6 mice, the effect of galanin on mechanical sensitivity was determined. Galanin (100 nm) was added to the superfusing solution and allowed to equilibrate for 20 min after which time the tension–response curve was re-determined. Galanin was then removed from the superfusate and no further tests performed for 20 min to ensure complete washout of galanin from the tissue. The tension–response curve was then re-determined. The GALR2 agonist AR-M961 (10 nm) was then added to the Krebs solution and the preparation was left for a further 20 min equilibration period. The tension–response curve was then again re-determined. This procedure was repeated for a higher dose of AR-M961 (100 nm). AR-M961 has been shown to have potent agonistic activity at both GALR1 and GALR2, with an IC50 of 0.403 nm and 1.74 nm, respectively (Liu et al. 2001). Time-controlled experiments were performed in which there was no significant change in the mechanical responses over a comparable duration.
In a separate series of experiments using Galr1−/− mice, the effect of AR-M961 alone was determined. After mechanical sensitivity of tension receptors had been established, AR-M961 (100 nm) was added to the superfusing solution and the preparation was allowed to equilibrate for 20 min. The tension–response curve was then re-determined.
Data recording and analysis
Afferent impulses were amplified with a biological amplifier (DAM 50; World Precision Instruments, Sarasota, FL, USA), filtered (band-pass filter-932; CWE Inc., Ardmore, PA, USA) and monitored using an oscilloscope (DL 1200 A; Yokogawa, Tokyo). Single units were discriminated on the basis of action potential shape, duration and amplitude using Spike 2 software (Cambridge Electronic Design, Cambridge, UK). All data were recorded and analysed off-line using a personal computer (IBM Thinkpad). Peristimulus time histograms and discharge traces were displayed using Spike 2 software. Data are expressed as means ±s.e.m., with number (n) of individual afferents given in all instances. Differences between stimulus–response curves were evaluated using two-way ANOVA. Differences were considered significant if P < 0.05.
Drugs
Stock solutions of all drugs were kept frozen and diluted to their final concentration in Krebs solution on the day of the experiment. SNAP 37889 was a kind donation from AstraZeneca (Mölndal, Sweden). AR-M961 was a kind donation from Ralf Schmidt at AstraZeneca (Montreal, Canada). Galanin was obtained from Auspep Pty Ltd (Victoria, Australia).
Results
Expression of galanin receptors in mouse nodose ganglion
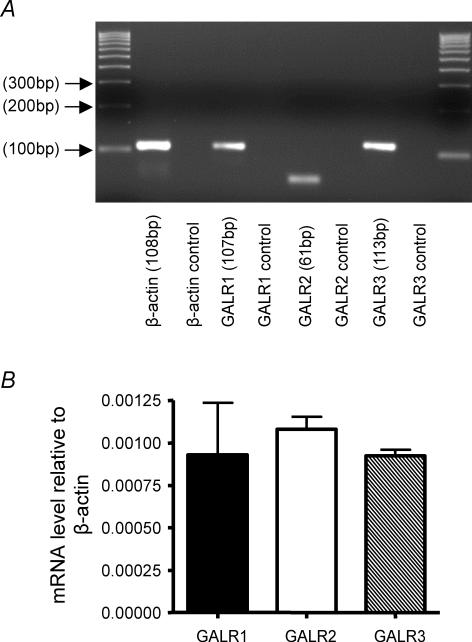
Using RT-PCR and gel electrophoresis we were able to confirm the presence and size of the amplified products generated by the Quantitect Primer Assays specific for galanin receptors GALR1, GALR2 and GALR3 and β-actin (Fig. 1A), showing intense single bands corresponding to the predicted sizes of the galanin receptors and β-actin transcripts (Fig. 1A). Quantitative RT-PCR analysis compared relative expression of galanin receptors in whole nodose ganglia. Using β-actin as a reference CT value, quantitative RT-PCR analysis of whole nodose ganglia revealed that there was no significant difference in the relative transcript expression between galanin receptor subtypes (Fig. 1B). We also measured quantitative expression of GALR2 and GALR3 in the nodose ganglia of Galr1−/− mice, which showed no difference in their levels relative to β-actin (data not shown) compared with wild-types; these data indicate no adaptive regulation of other galanin receptors in Galr1−/− mice.
Figure 1. Transcript expression and relative expression of galanin receptors in whole mouse nodose ganglia.
A, RT-PCR products separated on a 3% agarose gel to confirm the presence of GALR1, GALR2 and GALR3 transcripts. The sizes of the amplified products were confirmed showing intense single bands corresponding to the predicted sizes of GALR1, GALR2, GALR3 and β-actin transcripts. In addition, this validated the products obtained during quantitative analysis. B, quantitative RT-PCR revealed there to be no significant difference in transcript expression between galanin receptor subtypes (P > 0.05, Mann–Whitney U test). Experiments were performed at least in triplicate. GALR1, 2 and 3 receptor expression are calculated relative to β-actin mRNA levels.
Effect of galanin on the mechanosensitivity of gastro-oesophageal vagal afferents
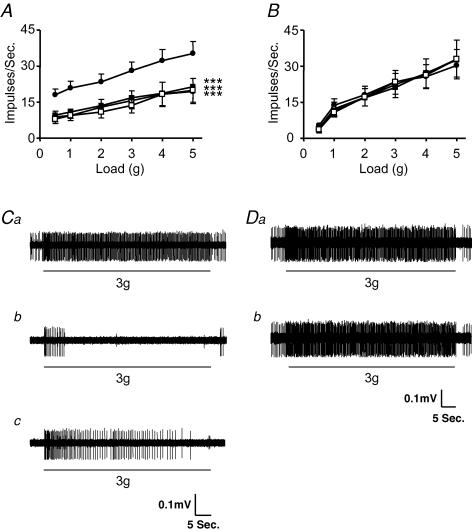
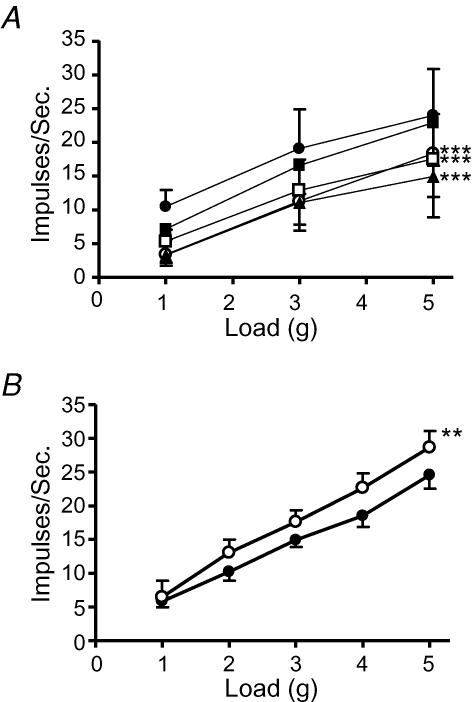
The effect of galanin on the mechanosensitivity of mouse tension-sensitive gastro-oesophageal vagal afferents is illustrated in Fig. 2. Galanin (1–10 nm) potently and significantly reduced the response of tension receptors from Galr1+/+ mice (n = 7; Fig. 2A and C). This inhibitory effect was reversed upon washout of galanin from the superfusate (Fig. 2Cc). These data confirm our previous observations (Page et al. 2005b). However, galanin (1–10 nm) had no effect on the mechanosensitivity of tension receptors from Galr1−/− mice (n = 13; Fig. 2B and D).
Figure 2. The effect of galanin on mouse gastro-oesophageal vagal tension receptors.
Stimulus–response functions of tension receptors to circumferential tension from Galr1+/+ (A, n = 7) and Galr1−/− (B, n = 13) mice. The responses are before (•) and after exposure to galanin (1 (○), 3 (▪) and 10 nm (□)). ***Significant difference from control using a two-way ANOVA (P < 0.001). C, original recording of a tension receptor response in Galr1+/+ animal to circular tension with a 3 g weight before (Ca), during (Cb) and after washout (Cc) of galanin (10 nm). D, original recording in Galr1−/− animal of a tension receptor response to circular tension with a 3 g weight before (Da) and during (Db) exposure to galanin (10 nm).
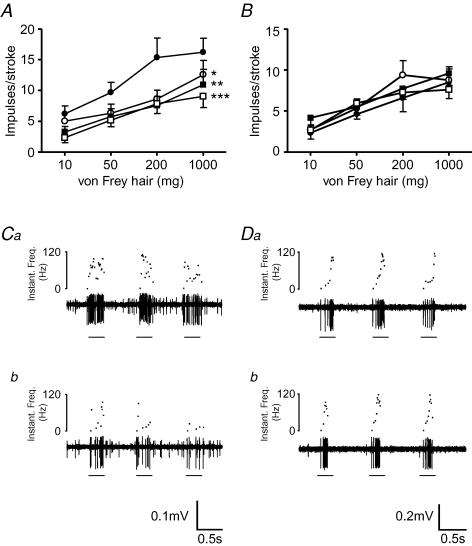
The effect of galanin on the mechanosensitivity of mucosal receptors is illustrated in Fig. 3. Galanin (1–10 nm) significantly reduced their response in C57BL/6 mice (n = 7; Fig. 3A and C), but had no effect on the mechanosensitivity of mucosal receptors from Galr1−/− mice (n = 8; Fig. 3B and D).
Figure 3. The effect of galanin on mouse gastro-oesophageal vagal mucosal receptor.
Stimulus–response functions from wild-type (A, n = 7) and Galr1−/− (B, n = 8) mice of mucosal receptors to mucosal stroking with calibrated von Frey hairs (10–1000 mg). The responses are before (•) and after exposure to galanin (1 (○), 3 (▪) and 10 nm (□)). Asterisks indicate significant difference from control using a two-way ANOVA (*P < 0.05, **P < 0.01 and ***P < 0.001). C, original recording of a mucosal receptor response from wild-type mouse to mucosal stroking with a 200 mg von Frey hair before (Ca) and during (Cb) exposure to galanin (10 nm). D, original recording from Galr1−/− mouse of a mucosal receptor response to mucosal stroking with a 200 mg von Frey hair before (Da) and during (Db) exposure to galanin (10 nm).
Mechanosensitivity of mucosal and tension receptors in the absence of galanin showed no significant differences (P > 0.05, two-way ANOVA) in control stimulus–response curves between Galr1−/−and wild-type mice (data from Figs 2A and B, and 3A and B).
Effect of a GALR3 antagonist on the inhibitory effect of galanin
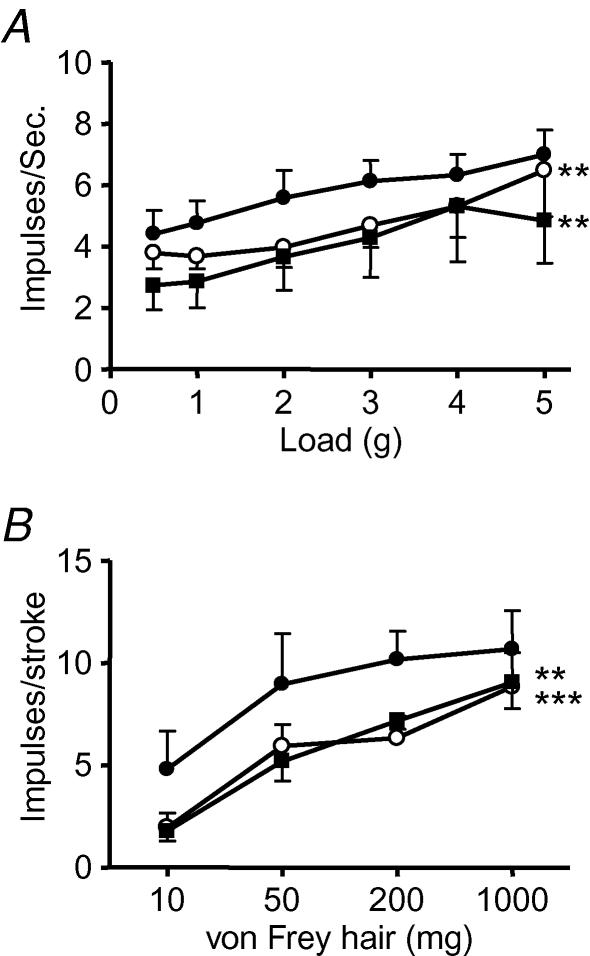
The effect of galanin and the GALR3-selective antagonist SNAP 37889 on wild-type mouse gastro-oesophageal vagal afferents is illustrated in Fig. 4. Galanin (1 nm) alone significantly reduced the response of tension receptors to circular tension (n = 5; Fig. 4A). When the GALR3 antagonist SNAP 37889 (10 nm) was added to the superfusate along with galanin (1 nm), there was no significant change in the response to circular tension compared with galanin (1 nm) alone (Fig. 4A). Galanin (10 nm) also significantly reduced the responses of mucosal receptors (Fig. 4B), which were unchanged by SNAP 37889 (100 nm; Fig. 4B).
Figure 4. The effect of galanin and SNAP 37889 on the mechanosensitivity of gastro-oesophageal vagal afferents.
Stimulus–response functions of tension (A, n = 5) and mucosal (B, n = 5) receptors to circumferential tension and mucosal stroking. The responses are before (•), after exposure to galanin (○: A, 1 nm; B, 10 nm) and after exposure to galanin and SNAP 37889 (▪: A, 10 nm; B, 100 nm). Asterisks indicate significant difference from control using a two-way ANOVA (**P < 0.01 and ***P < 0.001).
Effect of a GALR1/2 agonist on the mechanosensitivity of gastro-oesophageal vagal afferents
The effect of AR-M961 on the mechanosensitivity of tension-sensitive gastro-oesophageal vagal afferents is illustrated in Fig. 5. In preparations obtained from wild-type mice, galanin (100 nm) significantly reduced the response of tension receptors to circular tension (n = 5; Fig. 5A). After washout of galanin, the GALR1/2 agonist AR-M961 (10 and 100 nm) mimicked the inhibitory effect of galanin (100 nm), significantly reducing the response of tension receptors to circular tension (Fig. 5A). By contrast, in preparations obtained from Galr1−/− mice the GALR1/2 agonist AR-M961 (100 nm; n = 6) significantly increased the response of tension receptors to circular tension (Fig. 5B).
Figure 5. The effect of galanin and AR-M961 on the mechanosensitivity of gastro-oesophageal vagal tension receptors.
Stimulus–response functions of tension receptors to circumferential tension from wild-type (A; n = 5) and GalR1−/− (B; n = 6) mice. A, the responses of wild-type tension receptors before (•), after exposure to galanin (○; 100 nm), after washout of galanin (▪), after exposure to AR-M961 (□; 10 nm) and finally after exposure to AR-M961 (▴; 100 nm). B, the responses of Galr1−/− tension receptors before (•) and after exposure to AR-M961 (○; 100 nm). Asterisks indicate significant difference from control using a two-way ANOVA (**P < 0.01 and ***P < 0.001).
Discussion
The current data demonstrate potent modulation of responses to mechanical stimuli by exogenous galanin in vagal tension and mucosal receptors of the stomach and oesophagus in mice. As previously demonstrated, both facilitatory and inhibitory effects of exogenous galanin were observed, but inhibitory effects were predominant (Page et al. 2005b). The inhibitory effects of galanin were lost in Galr1−/− mice, showing that they occur via activation of the inhibitory GALR1 receptor. We previously hypothesized that GALR3, also an inhibitory receptor, may mediate at least part of galanin-induced inhibition. However, the lack of any residual effect of galanin in the Galr1-knockout mouse indicated that GALR3 did not contribute to its effects. This notion was further supported by the finding that the selective GALR3 antagonist SNAP 37889 (Swanson et al. 2005) failed to reverse the inhibitory effects of galanin. This study also revealed an inhibitory effect of the GALR1 and GALR2 agonist, AR-M961 (Liu et al. 2001), on wild-type mice. However when applied exogenously in Galr1 −/− mice this drug had a potentiating effect, implicating a functional role for the GALR2 receptor in modulation of vagal afferent sensitivity. We detected all three galanin receptor transcripts in whole nodose ganglia representing cell bodies which transport receptor protein to the periphery. Using quantitative RT-PCR, we also demonstrated similar relative expression of all three galanin receptors in the whole nodose ganglion, suggesting that although GALR3 has no functional role in the fibres we studied, it is expressed in vagal afferents.
Sources of endogenous galanin
Galanin has previously been demonstrated in enteric nerves of several species (Ekblad et al. 1985; Melander et al. 1985; Furness et al. 1987) including humans (Singaram et al. 1991, 1994), as well as in vagal afferents in various species (Calingasan & Ritter, 1992; Page et al. 2005b) with these galanin-containing neurones being shown to project to the stomach. Circulating galanin levels found in previous studies are equal to or greater than doses used in this investigation (Harling et al. 1991) implicating endogenous galanin in the vicinity of afferent terminals as a strong candidate for modulation of vagal afferent neurotransmission. Endogenous galanin may thus reach endings either by release locally from vagal afferent endings, or systemically following release by neural or endocrine cells intrinsic to the gut. We found that baseline mechanosensitivity of mucosal and tension receptors were unchanged in mice lacking GALR1, which may argue against a role for endogenous galanin in ongoing modulation of mechanosensitivity. However, our previous data indicate an increased mechanosensitivity in some ferret mechanoreceptors after application of the non-selective galanin receptor antagonist galantide (Page et al. 2005b). Therefore a number of factors may determine the access of endogenous galanin to the endings, including an intact vascular supply, dimensions of the tissue, and species.
Galanin receptors on vagal afferents
The effects of galanin are mediated by three distinct receptors that activate multiple second messenger pathways to modulate cell activity. GALR1 and GALR3 are coupled positively to potassium channels and may reduce levels of cyclic AMP, giving rise to hyperpolarization. GALR2 is coupled positively to phospholipase C resulting in cellular excitation (Branchek et al. 2000). There are abundant reports of expression of GALR1 and GALR2 in sensory neurones (Xu et al. 1996; Sten Shi et al. 1997; O'Donnell et al. 1999; Waters & Krause, 2000), but to date GALR3 has received little attention, and its existence in sensory neurones is controversial (Waters & Krause, 2000; Mennicken et al. 2002). Ours are the first data demonstrating the expression of galanin receptors in the nodose ganglion, although a previous report of galanin binding sites in the human nodose ganglion has been published (Sweerts et al. 2000).
GALR1
Based on anatomical and functional evidence, galanin has been suggested to play a predominantly inhibitory role in intrinsic reflexes of the gut (Pham et al. 2002; Liu et al. 2003), and on nociceptive processing at the spinal level where galanin has been shown to exert an analgesic effect (Liu & Hokfelt, 2002). Studies of the effects of exogenous galanin on sensory endings also showed a mainly inhibitory effect (Heppelmann et al. 2000; Flatters et al. 2003; Page et al. 2005b). The present study reflected these findings demonstrating that the effects of galanin on gastro-oesophageal vagal afferent mechanosensitivity are predominantly inhibitory. Based on the widespread distribution of GALR1 mRNA in central and peripheral nervous systems, it is proposed that this receptor mediates many of the inhibitory actions of galanin in feeding (Crawley, 1999), nociception (Liu & Hokfelt, 2002; Blakeman et al. 2003), gut motility and secretion (Liu et al. 2003; Sternini et al. 2004). In the present study we had the opportunity to test directly the hypothesis that GALR1 predominates by examining afferent function in the Galr1−/− mouse. Previously studies in this mouse have focused on the role of GALR1 in the brain and its inhibitory role in epilepsy, anxiety and memory (Jacoby et al. 2002; Fetissov et al. 2003; Holmes et al. 2003; Wrenn et al. 2004; McColl et al. 2006). Galr1−/− mice also show thermal hyperalgesia and increased neuropathic pain presumably due to reduced inhibition by galanin within the spinal cord (Blakeman et al. 2003). Our data show that galanin also serves an inhibitory role in the peripheral nervous system. As described in other studies of Galr1-null mutant mice raised on the same background, no other overt phenotypic differences could be observed, suggesting that the changes in mechanical responsiveness and effects of galanin were directly attributable to the loss of the receptor.
Role of GALR2
GALR2 is likely to mediate the excitatory effects we observed, as it is positively coupled (Branchek et al. 2000). Our findings with the GALR1 and GALR2 agonist AR-M961 initially reinforced our findings with galanin, showing predominantly inhibition mediated through GALR1, and therefore that GALR1 is functionally more active than GALR2 in modulation of vagal afferent mechanosensitivity. In the Galr1 null mutant, however, AR-M961 resulted in an increase in mechanosensitivity, therefore revealing a role for GALR2, albeit a minor one. This observation was supported by observations we made of two afferents from wild-type mice in which galanin augmented mechanosensitivity, where this effect was mimicked by the selective GALR2 agonist AR-M1896 (Liu et al. 2001) (data not shown).
Role of GALR3
A functional role for GALR3 in addition to the GALR1 in modulation of extrinsic afferents was ruled out by the finding that the selective GALR3 antagonist SNAP 37889 (Swanson et al. 2005) failed to reverse any of the inhibitory effects of galanin on both tension and mucosal afferents. Also the inhibitory effect of galanin was totally absent in the Galr1−/− mice. There is an apparent conflict with the finding that GALR3 was found in equal abundance to GALR1 and GALR2 in the nodose ganglion, where the cell bodies of the afferents are located. However, our recent estimates of gastric-projecting nodose neurones indicate that they represent only around 10–15% of the total number of neurons (Page et al. 2005b). Therefore GALR3 expression may be restricted to cell bodies of vagal afferents innervating other structures. Immunohistochemical detection of GALR3 has so far proven equivocal, so this hypothesis remains untested and will form part of further investigation in this area. Alternatively, a role for GALR3 other than in ligand-mediated actions remains a possibility. Measurements of GALR3 in gut tissue by similar methods, on the other hand, showed considerably lower expression compared with GALR1 and GALR2 (Anselmi et al. 2005a, b), and a function for GALR3 has yet to be ascribed within the gut.
In conclusion, we have revealed a novel role for galanin in modulation of extrinsic afferent function in the upper gastrointestinal tract whereby divergent effects of galanin are mediated via GALR1 and GALR2 subtypes. In particular, functional evidence suggests that the inhibitory effects are mediated through GALR1 and the excitatory effects through GALR2, whereas we observed no functional evidence of GALR3 involvement. The current study once again highlights the complex actions of galanin at different receptor subtypes exhibiting parallels with the function of galanin in somatic sensory innervation, spinal innervation of the gastrointestinal tract and fits alongside roles already demonstrated for galanin in intrinsic control of gut secretion and motility. Should the effect of galanin in mice translate to humans as previously demonstrated with other neuromodulators of vagal afferent sensitivity, GALR1 agonists could be of therapeutic use in the treatment of diseases of altered extrinsic afferent signalling from the gastrointestinal tract.
Acknowledgments
Dr John Bekkers from Australian National University is acknowledged for maintenance of the Galr1−/− colony. This study was supported by the National Health and Medical Research Council of Australia and the University of Adelaide.
References
- Anselmi L, Lakhter A, Hirano AA, Tonini M, Sternini C. Expression of galanin receptor messenger RNAs in different regions of the rat gastrointestinal tract. Peptides. 2005a;26:815–819. doi: 10.1016/j.peptides.2004.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi L, Stella SL, Jr, Lakhter A, Hirano A, Tonini M, Sternini C. Galanin receptors in the rat gastrointestinal tract. Neuropeptides. 2005b;39:349–352. doi: 10.1016/j.npep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA. Receptors and transmission in the brain-gut axis: potential for novel therapies. IV. GABAB receptors in the brain-gastroesophageal axis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G311–G315. doi: 10.1152/ajpgi.2001.281.2.G311. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Staunton E, Lehmann A, Dent J. Inhibition of transient LES relaxations and reflux in ferrets by GABA receptor agonists. Am J Physiol Gastrointest Liver Physiol. 1999;277:G867–G874. doi: 10.1152/ajpgi.1999.277.4.G867. [DOI] [PubMed] [Google Scholar]
- Blakeman KH, Hao JX, Xu XJ, Jacoby AS, Shine J, Crawley JN, Iismaa T, Wiesenfeld-Hallin Z. Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience. 2003;117:221–227. doi: 10.1016/s0306-4522(02)00779-0. [DOI] [PubMed] [Google Scholar]
- Botella A, Delvaux M, Fioramonti J, Frexinos J, Bueno L. Galanin contracts and relaxes guinea pig and canine intestinal smooth muscle cells through distinct receptors. Gastroenterology. 1995;108:3–11. doi: 10.1016/0016-5085(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Branchek T, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Branchek T, Smith KE, Walker MW. Molecular biology and pharmacology of galanin receptors. Ann N Y Acad Sci. 1998;863:94–107. doi: 10.1111/j.1749-6632.1998.tb10687.x. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Ritter S. Presence of galanin in rat vagal sensory neurons: evidence from immunohistochemistry and in situ hybridization. J Auton Nerv Syst. 1992;40:229–238. doi: 10.1016/0165-1838(92)90205-u. [DOI] [PubMed] [Google Scholar]
- Crawley JN. The role of galanin in feeding behavior. Neuropeptides. 1999;33:369–375. doi: 10.1054/npep.1999.0049. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Rokaeus A, Hakanson R, Sundler F. Galanin nerve fibers in the rat gut: distribution, origin and projections. Neuroscience. 1985;16:355–363. doi: 10.1016/0306-4522(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Jacoby AS, Brumovsky PR, Shine J, Iismaa TP, Hokfelt T. Altered hippocampal expression of neuropeptides in seizure-prone GALR1 knockout mice. Epilepsia. 2003;44:1022–1033. doi: 10.1046/j.1528-1157.2003.51402.x. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Fox AJ, Dickenson AH. In vivo and in vitro effects of peripheral galanin on nociceptive transmission in naive and neuropathic states. Neuroscience. 2003;116:1005–1012. doi: 10.1016/s0306-4522(02)00947-8. [DOI] [PubMed] [Google Scholar]
- Furness JB, Costa M, Rokaeus A, McDonald TJ, Brooks B. Galanin-immunoreactive neurons in the guinea-pig small intestine: their projections and relationships to other enteric neurons. Cell Tissue Res. 1987;250:607–615. doi: 10.1007/BF00218954. [DOI] [PubMed] [Google Scholar]
- Harling H, Messell T, Poulsen SS, Rasmussen TN, Holst JJ. Galanin and vasoactive intestinal polypeptide: coexistence and corelease from the vascularly perfused pig ileum during distension and chemical stimulation of the mucosa. Digestion. 1991;50:61–71. doi: 10.1159/000200741. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Just S, Pawlak M. Galanin influences the mechanosensitivity of sensory endings in the rat knee joint. Eur J Neurosci. 2000;12:1567–1572. doi: 10.1046/j.1460-9568.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN. Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology. 2003;28:1031–1044. doi: 10.1038/sj.npp.1300164. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol. 2007;500:863–875. doi: 10.1002/cne.21204. [DOI] [PubMed] [Google Scholar]
- Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107:195–200. doi: 10.1016/s0169-328x(02)00451-5. [DOI] [PubMed] [Google Scholar]
- Kask K, Berthold M, Bartfai T. Galanin receptors: involvement in feeding, pain, depression and Alzheimer's disease. Life Sci. 1997;60:1523–1533. doi: 10.1016/s0024-3205(96)00624-8. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Antonsson M, Bremner-Danielsen M, Flardh M, Hansson-Branden L, Karrberg L. Activation of the GABAB receptor inhibits transient lower esophageal sphincter relaxations in dogs. Gastroenterology. 1999;117:1147–1154. doi: 10.1016/s0016-5085(99)70400-2. [DOI] [PubMed] [Google Scholar]
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. Control of transient lower esophageal sphincter relaxations and reflux by the GABAB agonist baclofen in normal subjects. Gastroenterology. 2000;118:7–13. doi: 10.1016/s0016-5085(00)70408-2. [DOI] [PubMed] [Google Scholar]
- Liu HX, Brumovsky P, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hokfelt T. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci U S A. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Hokfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci. 2002;23:468–474. doi: 10.1016/s0165-6147(02)02074-6. [DOI] [PubMed] [Google Scholar]
- Liu S, Hu HZ, Gao C, Gao N, Xia Y, Wood JD. Actions of galanin on neurotransmission in the submucous plexus of guinea pig small intestine. Eur J Pharmacol. 2003;471:49–58. doi: 10.1016/s0014-2999(03)01798-9. [DOI] [PubMed] [Google Scholar]
- McColl CD, Jacoby AS, Shine J, Iismaa TP, Bekkers JM. Galanin receptor-1 knockout mice exhibit spontaneous epilepsy, abnormal EEGs and altered inhibition in the hippocampus. Neuropharmacology. 2006;50:209–218. doi: 10.1016/j.neuropharm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A, Fahrenkrug J, Tatemoto K, Mutt V. Distribution of galanin-like immunoreactivity in the gastro-intestinal tract of several mammalian species. Cell Tissue Res. 1985;239:253–270. doi: 10.1007/BF00218003. [DOI] [PubMed] [Google Scholar]
- Mennicken F, Hoffert C, Pelletier M, Ahmad S, O'Donnell D. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J Chem Neuroanat. 2002;24:257–268. doi: 10.1016/s0891-0618(02)00068-6. [DOI] [PubMed] [Google Scholar]
- O'Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- Ozaki N, Sengupta JN, Gebhart GF. Differential effects of μ-, δ-, and κ-opioid receptor agonists on mechanosensitive gastric vagal afferent fibers in the rat. J Neurophysiol. 2000;83:2209–2216. doi: 10.1152/jn.2000.83.4.2209. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. GABAB receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci. 1999;19:8597–8602. doi: 10.1523/JNEUROSCI.19-19-08597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Martinez-Salgado C, Wemmie JA, Brennan TJ, Symonds E, Omari T, Lewin GR, Welsh MJ, Blackshaw LA. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology. 2004;127:1739–1747. doi: 10.1053/j.gastro.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005a;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Page AJ, O'Donnell TA, Blackshaw LA. Inhibition of mechanosensitivity in visceral primary afferents by GABAB receptors involves calcium and potassium channels. Neuroscience. 2006;137:627–636. doi: 10.1016/j.neuroscience.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Page AJ, Slattery JA, O'Donnell TA, Cooper NJ, Young RL, Blackshaw LA. Modulation of gastro-oesophageal vagal afferents by galanin in mouse and ferret. J Physiol. 2005b;563:809–819. doi: 10.1113/jphysiol.2004.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Young RL, Martin CM, Umaerus M, O'Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005c;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Pham T, Guerrini S, Wong H, Reeve J, Jr, Sternini C. Distribution of galanin receptor 1 immunoreactivity in the rat stomach and small intestine. J Comp Neurol. 2002;450:292–302. doi: 10.1002/cne.10311. [DOI] [PubMed] [Google Scholar]
- Ren J, Hu HZ, Starodub AM, Wood JD. Galanin suppresses calcium conductance and activates inwardly rectifying potassium channels in myenteric neurones from guinea-pig small intestine. Neurogastroenterol Motil. 2001;13:247–254. doi: 10.1046/j.1365-2982.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- Singaram C, Sengupta A, Sugarbaker DJ, Goyal RK. Peptidergic innervation of the human esophageal smooth muscle. Gastroenterology. 1991;101:1256–1263. doi: 10.1016/0016-5085(91)90075-v. [DOI] [PubMed] [Google Scholar]
- Singaram C, Sengupta A, Sweet MA, Sugarbaker DJ, Goyal RK. Nitrinergic and peptidergic innervation of the human oesophagus. Gut. 1994;35:1690–1696. doi: 10.1136/gut.35.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sten Shi TJ, Zhang X, Holmberg K, Xu ZQ, Hokfelt T. Expression and regulation of galanin-R2 receptors in rat primary sensory neurons: effect of axotomy and inflammation. Neurosci Lett. 1997;237:57–60. doi: 10.1016/s0304-3940(97)00805-7. [DOI] [PubMed] [Google Scholar]
- Sternini C, Anselmi L, Guerrini S, Cervio E, Pham T, Balestra B, Vicini R, Baiardi P, D'Agostino G, L & Tonini M. Role of galanin receptor 1 in peristaltic activity in the guinea pig ileum. Neuroscience. 2004;125:103–112. doi: 10.1016/j.neuroscience.2003.12.043. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, Hokfelt T, et al. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci U S A. 2005;102:17489–17494. doi: 10.1073/pnas.0508970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweerts BW, Jarrott B, Lawrence AJ. [125I]-galanin binding sites in the human nodose ganglion. Life Sci. 2000;67:2685–2690. doi: 10.1016/s0024-3205(00)00859-6. [DOI] [PubMed] [Google Scholar]
- Tan Z, Fogel R, Jiang C, Zhang X. Galanin inhibits gut-related vagal neurons in rats. J Neurophysiol. 2004;91:2330–2343. doi: 10.1152/jn.00869.2003. [DOI] [PubMed] [Google Scholar]
- Waters SM, Krause JE. Distribution of galanin-1-2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience. 2000;95:265–271. doi: 10.1016/s0306-4522(99)00407-8. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Crawley JN, Hokfelt T. Galanin and spinal nociceptive mechanisms: recent results from transgenic and knock-out models. Neuropeptides. 2005;39:207–210. doi: 10.1016/j.npep.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, et al. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur J Neurosci. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- Wynick D, Thompson SW, McMahon SB. The role of galanin as a multi-functional neuropeptide in the nervous system. Curr Opin Pharmacol. 2001;1:73–77. doi: 10.1016/s1471-4892(01)00006-6. [DOI] [PubMed] [Google Scholar]
- Xu ZQ, Shi TJ, Landry M, Hokfelt T. Evidence for galanin receptors in primary sensory neurones and effect of axotomy and inflammation. Neuroreport. 1996;8:237–242. doi: 10.1097/00001756-199612200-00048. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Dey L, Xie JT, Aung HH. Gastric effects of galanin and its interaction with leptin on brainstem neuronal activity. J Pharmacol Exp Ther. 2002;301:488–493. doi: 10.1124/jpet.301.2.488. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lehmann A, Rigda R, Dent J, Holloway RH. Control of transient lower oesophageal sphincter relaxations and reflux by the GABAB agonist baclofen in patients with gastro-oesophageal reflux disease. Gut. 2002;50:19–24. doi: 10.1136/gut.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]