Abstract
Sympatho-excitatory manoeuvres are used to study vascular responsiveness in humans, but it is unclear if circulating adrenaline attenuates peripheral vasoconstriction during these manoeuvres. We hypothesized that vasoconstrictor responses to three manoeuvres (neck pressure, unilateral thigh-cuff release and isometric handgrip) would be greater after the administration of the β-adrenergic blocker propranolol. Seven men and six women underwent these manoeuvres while beat-by-beat arterial pressure (finger photoplethysmography), femoral mean blood velocity (Doppler ultrasound) and femoral artery diameter (edge-detection software) were measured. Femoral vascular conductance was calculated as flow/pressure. Propranolol had no effect on baseline femoral vascular conductance (P > 0.05). As a result of neck pressure, femoral vascular conductance was reduced 23.9 ± 3.5% before vs. 33.2 ± 3.2% after infusion of propranolol (P = 0.033). After thigh-cuff release, femoral vascular conductance declined 50.2 ± 5.8% before vs. 57.4 ± 9.6% after propranolol infusion (P = 0.496). During handgrip, femoral vascular conductance was reduced 47.2 ± 9.6% before vs. 55.2 ± 9.2% after propranolol administration (P = 0.447). After handgrip, women had a greater rise in conductance than men (women: 153 ± 16.2%; men: 36.4 ± 10.6%; P < 0.001), which was blunted by 54.8% by propranolol (P < 0.001 vs. control), but unaffected by propranolol in men (P = 0.355 vs. control). The finding that β-adrenergic receptor-mediated vasodilatation minimally affects vascular responses to these sympatho-excitatory manoeuvres reinforces their utility in the investigation of sympathetic vascular regulation in humans. Interestingly, post-handgrip hyperaemia is greater in women than men and is, in part, β-adrenergic receptor mediated.
Sympatho-excitatory manoeuvres have historically been utilized to investigate the role of the sympathetic nervous system in cardiovascular regulation. While manoeuvres such as externally applied neck pressure and isometric handgrip exercise were initially implemented to study resultant changes in heart rate, venous occlusion plethysmography has been used to study vascular changes in response to sympatho-excitatory manoeuvres such as isometric handgrip exercise (Seals, 1989; Taylor et al. 1991), cold pressor test (Jacob et al. 2000), lower body negative pressure (Rowell & Seals, 1990) and mental stress (Halliwill et al. 1997; Lindqvist et al. 1997), as well as in response to pharmacological treatments (Pawelczyk & Levine, 2002). Furthermore, the increased availability of ultrasonography has given investigators another tool to examine the sympathetic nervous system's control of the vasculature (Shoemaker et al. 2000; Keller et al. 2003).
A potential confound in this line of research is the release of catecholamines, most specifically adrenaline from the adrenal medulla, in response to these sympatho-excitatory manoeuvres (Taylor et al. 1991; Jones et al. 1996). In humans, intravenous adrenaline infusions sufficient to increase plasma adrenaline concentrations within normal physiological ranges have evoked reductions in both systemic and calf vascular resistance (Stratton et al. 1985; Freyschuss et al. 1986). These reductions in vascular resistance are thought to be due to β2-adrenergic receptor-mediated vasodilatation of vascular smooth muscle (Johnsson, 1975; Hjemdahl & Linde, 1983; Dawes et al. 1997; Lindqvist et al. 1997). If adrenaline release during sympatho-excitatory manoeuvres is robust enough to cause vasodilatation of vascular smooth muscle, this vasodilatation could potentially attenuate part of the sympathetically mediated vasoconstriction being examined. In addition, there may be sex-based differences in responses to sympatho-excitation, as a consequence of either reduced sympathetic neural outflow (Ettinger et al. 1996) or greater β2-adrenergic receptor sensitivity in women (Kneale et al. 2000), which may be partially dependent upon the menstrual cycle phase (Ettinger et al. 1998).
Previously, systemic (MacDonald et al. 1966) and regional (Eklund & Kaijser, 1976) β-adrenergic blockade has been found to increase systemic and contralateral forearm vascular resistance in response to sustained handgrip exercise. More recently, Reed et al. (2000) demonstrated that the increased forearm vascular conductance evoked by ischaemic handgrip exercise after stellate ganglion blockade was abolished after the intra-arterial administration of propranolol, which suggested β-adrenergic receptor-mediated vasodilatation during sympatho-excitation in humans. However, previous investigations have not examined leg vascular responses to these manoeuvres and the role of circulating adrenaline in vascular responses to other sympatho-excitatory manoeuvres (e.g. neck pressure and unilateral thigh-cuff release) is not well defined.
The focus of our investigation therefore was to assess the influence of β-adrenergic receptor-mediated vasodilatation on the vascular responses to three commonly used sympatho-excitatory manoeuvres: neck pressure, unilateral thigh-cuff release and isometric handgrip exercise. Our goal was to determine whether β-adrenergic receptor-mediated vasodilatation attenuated leg vasoconstrictor responses to these manoeuvres by measuring responses before and after β-adrenergic blockade. We tested the hypothesis that the reductions in femoral vascular conductance mediated by brief (5 s) bouts of neck pressure would be unaffected (due to the short timeframe of the response), whereas reductions in femoral vascular conductance mediated by unilateral thigh-cuff release and isometric handgrip, which induce more prolonged vasoconstriction, would be greater after the administration of the β-adrenergic antagonist propranolol.
Methods
All study protocols were approved by the Institutional Review Board of the University of Oregon and conducted in accordance with the Declaration of Helsinki. Each subject gave his or her written consent prior to participation in the study.
Subjects
Thirteen subjects (7 males, 6 females, age 18–33 years) volunteered to participate in the study. Subjects were healthy, normotensive, non-smokers, ranging in fitness from sedentary to moderately aerobically trained, and taking no medications with the exception of oral contraceptives.
Subjects were screened in order to exclude individuals from participation in the study who had a carotid sinus above or below the region encased by the neck collar used for the neck pressure manoeuvre. Each subject had his or her carotid arteries imaged via a Doppler ultrasound machine equipped with a 10.0 MHz linear vascular probe (GE Vingmed System 5, Horton, Norway).
Study visits
Subjects were studied on two different occasions, separated by 2–14 days. The purpose of the first visit was to familiarize subjects to the sympatho-excitatory manoeuvres performed in the study and test day-to-day reproducibility of the responses to these manoeuvres by comparing data collected during the first visit to the control data collected during visit two. During visit one, subjects underwent four trials of neck pressure, one trial of unilateral thigh-cuff release and one trial of isometric handgrip exercise. During visit two, subjects underwent the same series of sympatho-excitatory manoeuvres before and after the intravenous administration of the β-adrenergic antagonist propranolol. The manoeuvres were not randomized because the half-life of propranolol (3–5 h) is substantially longer than the post-infusion duration of the study. The order of the manoeuvres was selected to progress from least likely (neck pressure) to most likely (handgrip) to release adrenaline, in order to minimize this potential confound on subsequent responses to sympatho-excitation. For both visits, subjects reported to the laboratory at least 2 h post-prandial, having refrained from alcohol consumption or exercise for 24 h and consumption of caffeine for 12 h. Female subjects had a negative pregnancy test on both visits. In addition, female subjects were studied during the early follicular phase of their menstrual cycle or during the placebo phase of the oral contraceptive cycle for the second visit to minimize the effects of reproductive hormones on autonomic cardiovascular regulation (Minson et al. 2000). Ambient temperature was maintained between 21 and 23°C during all experiments.
Measurements
For each visit, subjects were instrumented while supine. Heart rate and blood pressure were measured throughout all experimental procedures. Heart rate was measured by a five-lead electrocardiogram (Q710, Quinton Instruments, Bothell, WA, USA). Beat-to-beat arterial pressure was measured by finger photoplethysmography (Finometer, TNO, Amsterdam, The Netherlands) with the right hand supported at heart level and verified with an automated sphygmomanometer (Dinamap Pro100 vital systems monitor, Critikon, Tampa, FL, USA).
Femoral artery mean blood velocities were measured using an ultrasound probe (10 MHz linear-array vascular probe, GE Vingmed System 5). The entire width of the artery was insonated with an angle of 60 deg. Five subjects (2 men, 3 women) had their common femoral arteries isonated distal to the inquinal ligament, approximately 2–3 cm proximal to the bifurcation. Due to difficulties obtaining an image of the common femoral arteries, the superficial femoral arteries of the eight remaining subjects (5 men, 3 women) were isonated approximately 2–3 cm distal to the bifurcation. The Doppler ultrasound machine was interfaced with a computer equipped with a custom edge-detection software system (Woodman et al. 2001) in order to record femoral artery images for subsequent analysis. During measurements, an inflatable cuff was placed on the right ankle and inflated to supra-systolic pressure (240 mmHg) to control for the effect of random opening and closing of the numerous arteriovenous anastomoses found in the foot cutaneous vasculature.
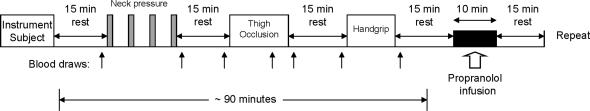
In addition, on the second visit a venous catheter was inserted into the antecubital region of the dominant arm to obtain blood samples and infuse propranolol. Figure 1 presents an overview of the visit two protocol.
Figure 1.
Protocol schematic.
Protocol
Maximal voluntary handgrip force
After instrumentation, the subject's maximal voluntary handgrip force (Recording hand dynamometer, Stoelting Co., Wood Dale, IL, USA) was determined using a minimum of three trials made with his or her non-dominant arm. Each trial was separated by 1 min of rest and subjects were encouraged to give their best effort while avoiding gasping breaths, Valsalva manoeuvres or contraction of other muscle groups.
Neck pressure
Subjects were fitted with a neck collar for application of externally applied positive neck pressure. The neck collar encased the anterior two-thirds of the neck and was fitted to form a seal between the mandible and the clavicles and sternum. The neck collar was connected to a programmable pressure controller (PPC-1000, Engineering Development Laboratory, Inc., Newport News, VA, USA) set to apply 50 mmHg of pressure to the carotid sinus regions, thereby unloading the carotid baroreceptors and eliciting a baroreflex-mediated rise in muscle sympathetic nerve activity and a reduction in cardiac parasympathetic nerve activity (Rea & Eckberg, 1987; Fritsch et al. 1991; Eckberg & Fritsch, 1993). Five-second bouts of neck pressure were R-wave activated via a custom-made controller interfaced to the neck pressure system.
Between neck pressure manoeuvres, subjects maintained constant respiration rates (12 breaths min−1) by breathing to a metronome. To minimize respiratory-influenced modulations in heart rate and blood pressure, neck pressure stimuli were administered during a voluntary end-expiration apnoea. The voluntary apnoea was sustained for 3 s prior to, 5 s during, and 5 s after application of neck pressure (total 13 s of apnoea). Subjects underwent four neck pressure manoeuvres separated by 1 min rest periods.
Unilateral thigh-cuff release
A thigh cuff, 19 cm in width (Hokanson, Bellevue, WA, USA) was inflated to supra-systolic pressure (220 mmHg) for a period of 10 min, then was abruptly deflated, thereby unloading the cardiopulmonary and arterial baroreceptors and eliciting a baroreflex-mediated rise in muscle sympathetic nerve activity and reduction in cardiac parasympathetic nerve activity (Fadel et al. 2001). To minimize respiratory-influenced modulations in heart rate and blood pressure, subjects maintained a constant breathing frequency and cuff deflation was coordinated with normal end-expiration.
Isometric handgrip exercise
Isometric handgrip exercise at 35% of maximal voluntary contraction force was performed with the non-dominant arm to the point of fatigue, thereby eliciting a rise in muscle sympathetic nerve activity (Mark et al. 1985; Seals, 1989). Subjects maintained a constant breathing frequency with a metronome. Moreover, they were coached to avoid gasping breaths, Valsalva manoeuvres or recruitment of accessory muscle groups, while being encouraged to maintain the target force as long as possible. The point of fatigue was identified by an inability to maintain handgrip force within 10% of the goal force for more than 2 s despite verbal encouragement, as well as the attainment of a maximal perceived exertion rating (Borg, 1970).
Propranolol administration
Propranolol hydrochloride (Inderal) (0.15 mg kg−1, maximum 10 mg) was infused via the antecubital intravenous catheter at a rate of 1 mg min−1, followed by infusion of 10 ml of normal saline. This dose administered intravenously has been shown to block β-receptors in the peripheral circulation, thereby increasing systemic vascular resistance in response to isometric handgrip exercise (MacDonald et al. 1966) and abolishing the reduction in forearm vascular resistance evoked by infusion of adrenaline (Johnsson, 1975). Likewise, this dose effectively prevents the rise in heart rate in response to subsequent administration of isoproterenol (isoprenaline) (Epstein et al. 1965; Holmberg et al. 1965). In addition, Hansson and colleagues (Hansson et al. 1974) showed that graded intravenous infusions of propranolol did not elicit further significant reductions in heart rate or increases in total peripheral resistance when dosages were increased from 0.10 mg kg−1 to 0.22 mg kg−1. Subjects were monitored during the subsequent 15 min supine rest period, prior to undergoing a second series of sympatho-excitatory manoeuvres.
Plasma catecholamines
To measure plasma catecholamine concentrations, blood samples were obtained from the antecubital intravenous catheter 1 min before and 1 min after each sympatho-excitatory manoeuvre. In addition, blood samples were taken at minute seven of the unilateral thigh occlusion. Samples were collected in pre-chilled heparinized tubes, centrifuged and stored at −80°C until analysed by high-performance liquid chromatography with electrochemical detection.
Data analysis
The quadrature output of the Doppler ultrasound band of blood velocities was demodulated so a continuous analog signal of mean blood velocity could be obtained. This signal, along with the electrocardiogram and arterial pressure tracing, were digitized and stored on a computer and analysed off-line using signal processing software (Windaq, Dataq Instruments, Akron, OH, USA). Beat-by-beat mean blood velocity in the femoral artery was determined by averaging the mean velocity curve for each cardiac cycle via R-wave gated analysis. Femoral artery diameter was determined on a beat-to-beat basis via custom edge-detection wall tracking software (Woodman et al. 2001) and linearly interpolated with femoral mean blood velocity to calculate mean femoral artery blood flow. Femoral artery blood flow was divided by mean arterial pressure to calculate femoral vascular conductance. Neck pressure data for each subject were averaged across four trials under both the control and blockade conditions. Unilateral thigh-cuff release and isometric handgrip exercise data were averaged over 10 s time periods.
Statistical analysis
Results were analysed using a mixed model analysis of variance with SAS (Version 9.1, SAS Institute, Inc., Cary, NC, USA) Differences were considered significant when P < 0.05. All values are reported as means ± s.e.m. With the exception of isometric handgrip, men and women had similar responses to the sympatho-excitatory manoeuvres so all but the isometric handgrip data were combined.
Results
Baseline haemodynamics
Baseline haemodynamics, under both the control condition and after administration of propranolol, are shown in Table 1. The only baseline haemodynamic variable on which propranolol had an effect was heart rate. After administration of propranolol, baseline heart rate prior to each manoeuver was reduced compared with control (neck pressure: control 57.1 ± 2.0 beats min−1vs. blockade 50.2 ± 1.6 beats min−1, P < 0.001; unilateral thigh-cuff release: control 57.1 ± 2.1 beats min−1vs. blockade 51.0 ± 1.3 beats min−1, P < 0.001; isometric handgrip: control 58.8 ± 1.9 beats min−1vs. blockade 53.5 ± 1.3 beats min−1,P < 0.001) and this effect was comparable across manoeuvres (P = 0.334). In addition, the average time between propranolol infusion and study completion was 83 min with a maximum of 87 min. In contrast, the half-life of propranolol is 3–5 h. Taken together, this suggests that blockade was maintained throughout the second part of the study. Supine resting mean blood velocity, femoral artery diameter, mean arterial pressure and femoral vascular conductance were not different between control and blockade conditions (all P > 0.05).
Table 1.
Baseline haemodynamics
| Control | Propranolol | |
|---|---|---|
| Heart rate (beats min−1) | 58.8 ± 1.9 | 53.5 ± 1.7* |
| Mean blood velocity (cm s−1) | 4.1 ± 0.7 | 3.9 ± 0.5 |
| Diameter (mm) | 6.6 ± 0.3 | 6.7 ± 0.3 |
| Flow (ml min−1) | 87.3 ± 15.7 | 85.2 ± 13.3 |
| Mean arterial pressure (mmHg) | 78.8 ± 2.0 | 80.7 ± 3.5 |
| Femoral vascular conductance (ml min−1 mmHg−1) | 1.1 ± 0.2 | 1.1 ± 0.2 |
P < 0.05 vs. control. Values are means ± s.e.m.
Neck pressure
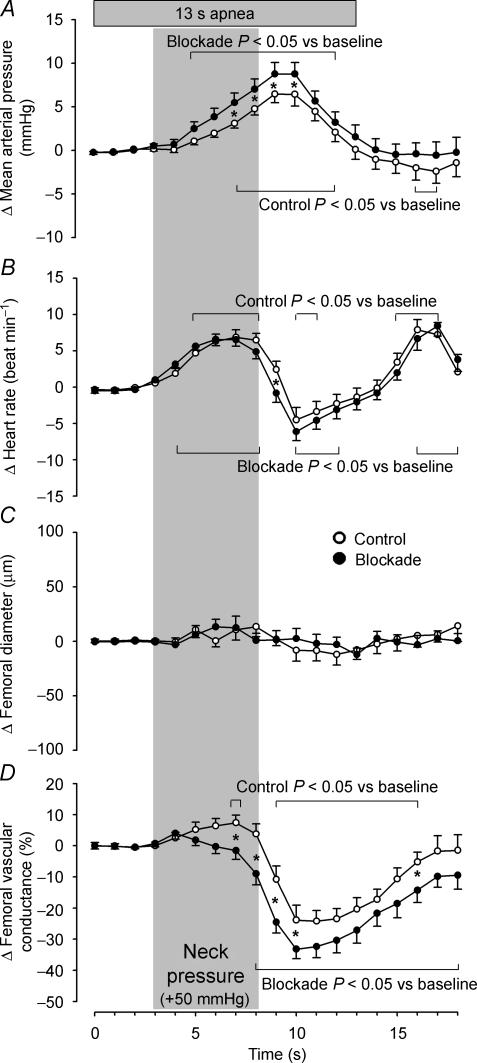
The haemodynamic responses to external neck pressure, averaged over four trials per subject, are expressed as changes from baseline in Fig. 2. Neck pressure induced a rise in mean arterial pressure under both the control and blockade conditions, which peaked 6 s after onset of neck pressure (control Δ 6.44 ± 0.99 mmHg; blockade Δ 8.73 ± 1.09 mmHg; both P < 0.001 vs. baseline) and was augmented by propranolol (P = 0.047 vs. control). Heart rate increased in a similar manner under control and blockade conditions, both peaking 4 s after the onset of neck pressure (control Δ 6.87 ± 1.31 beats min−1; blockade Δ 6.53 ± 1.08 beats min−1; both P < 0.001 vs. baseline). There were no differences in femoral artery diameter in response to neck pressure during either the control or the blockade trials, as diameter did not change from baseline under either condition (all P > 0.129). However, Fig. 2D shows a greater decline in femoral vascular conductance (both P < 0.001 vs. baseline), expressed as per cent change from baseline, in the propranolol (Δ −33.2 ± 3.2%) vs. the control (Δ −23.9 ± 3.5%) trials (P = 0.033), both reaching a nadir 7 s after the onset of neck pressure. During visit one, neck pressure trials induced a 21.6 ± 4.6% reduction in femoral vascular conductance (P = 0.789 vs. visit two control).
Figure 2. Averaged changes in mean arterial pressure (A), heart rate (B), femoral artery diameter (C) and femoral vascular conductance (D) elicited by neck pressure under control (○) and propranolol (•) conditions.
Data are averaged over four trials under each condition and expressed as changes from baseline. Vertical shaded bar indicates 5 s period of neck pressure. Brackets above and below graphs indicate a difference from baseline under each condition. *P < 0.05 vs. control. Values are means ± s.e.m.
Unilateral thigh-cuff release
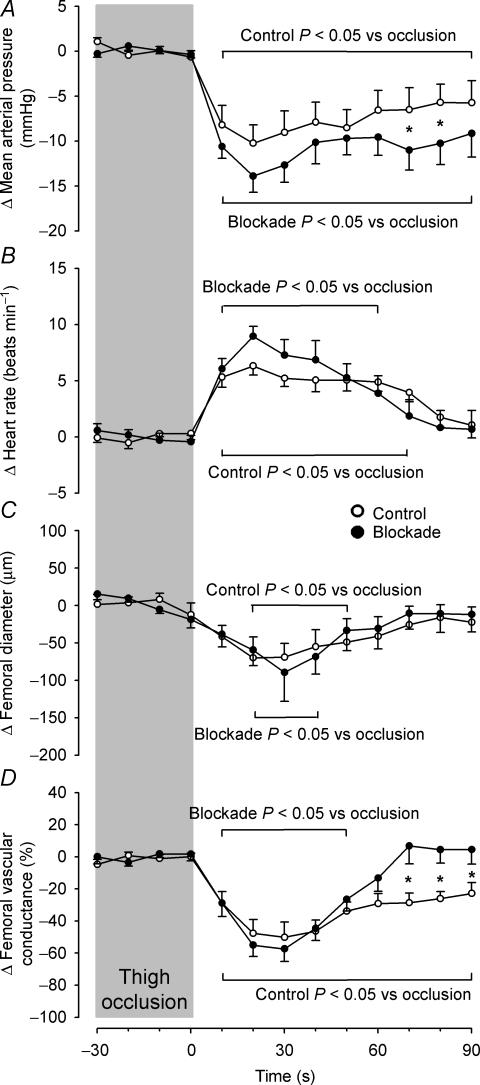
The hemodynamic responses to unilateral thigh-cuff release, expressed as changes from baseline immediately preceding cuff release, are displayed in Fig. 3. Thigh-cuff release evoked a profound drop in mean arterial pressure under both conditions, reaching a nadir at 20 s post-cuff release (control Δ −10.3 ± 1.9 mmHg; blockade Δ −13.9 ± 1.9 mmHg; both P < 0.001 vs. baseline). This response did not differ between conditions until 70 s post-cuff release (control Δ−6.5 ± 1.9 mmHg; blockade Δ−11.0 ± 1.9 mmHg; P = 0.045), at which time mean arterial pressure had nearly returned to pre-occlusion baseline under both conditions. This decrease in blood pressure evoked an abrupt rise in heart rate under both conditions (control Δ 6.3 ± 1.0 beats min−1; blockade Δ 8.9 ± 1.2 beats min−1; both P < 0.001 vs. baseline), which peaked 20 s post-cuff release and was not different in the control vs. the blockade trials (P = 0.078). Femoral artery diameter decreased post-cuff release under both conditions, reaching a nadir 20 s post-cuff release during control trials (Δ−70.0 μm) and 30 s post-cuff release during blockade trials (Δ−89.3 μm; both P < 0.003 vs. baseline), although there were no differences in this response between conditions (P = 0.521). Thigh-cuff release elicited similar, marked reductions in femoral vascular conductance, expressed as per cent change from baseline, under both the control and blockade conditions, reaching a nadir at 30 s post-cuff release (control Δ−50.2 ± 5.8%; blockade Δ−57.4 ± 9.6%; both P < 0.001 vs. baseline). However, these responses diverged at 70 s post-cuff release, as femoral vascular conductance was slower to approach pre-occlusion baseline in the control trials (control Δ−28.6 ± 5.8%; blockade Δ 6.7 ± 9.6%; P = 0.002). During visit one, unilateral thigh-cuff release resulted in a 45.3 ± 10.7% reduction in femoral vascular conductance 30 s post-cuff release (P = 0.747 vs. visit two control).
Figure 3. Averaged changes in mean arterial pressure (A), heart rate (B), femoral artery diameter (C) and femoral vascular conductance (D) elicited by unilateral thigh-cuff release under control (○) and propranolol (•) conditions.
Data are expressed as changes from baseline immediately preceding cuff release. Vertical shaded bar indicates the last 30 s of thigh occlusion. Brackets above and below graphs indicate a difference from baseline under each condition. *P < 0.05 vs. control.
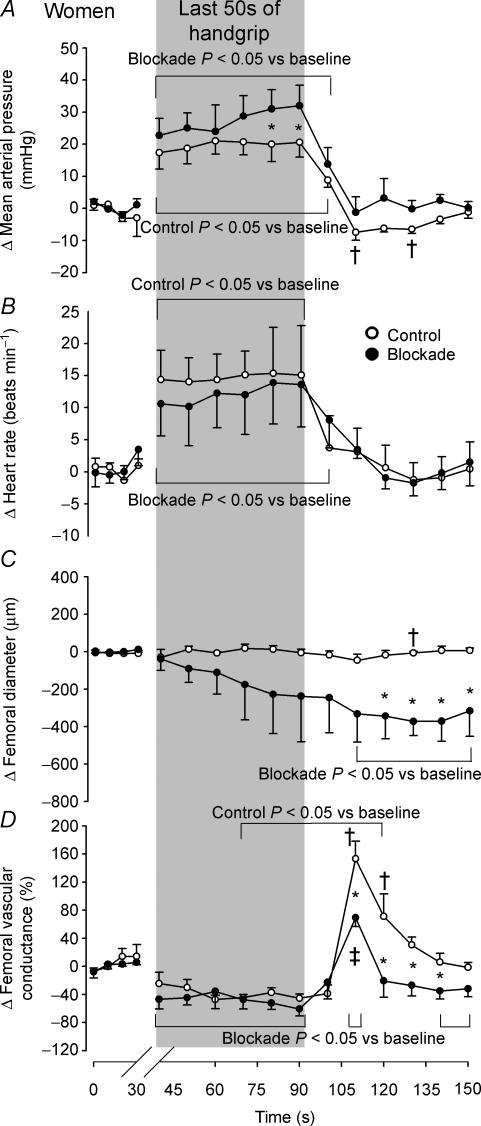
Isometric handgrip exercise
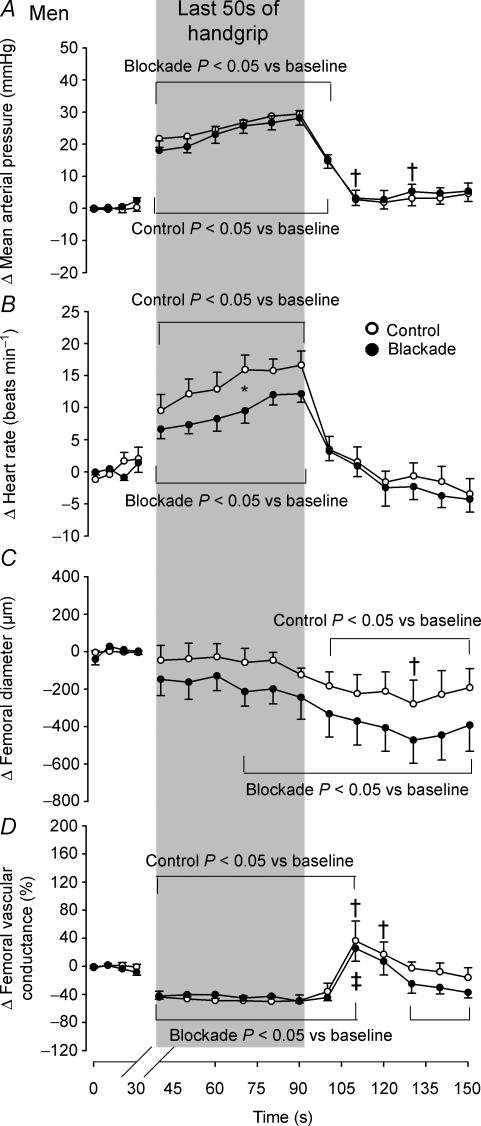
Men and women responded differently to isometric handgrip exercise to fatigue, thus the hemodynamic changes evoked by this manoeuvre in men are displayed in Fig. 4 and those changes in women are displayed in Fig. 5. In men, the average duration of isometric handgrip exercise was 184.1 ± 13.3 s in the control trials and 162.6 ± 11.2 s in the blockade trials (P = 0.029). In women, the average duration of isometric handgrip exercise was 185 ± 12.7 s in the control trials (P = 0.968 vs. men) and 160.1 ± 16.1 s in the blockade trials (P = 0.110 vs. control; P = 0.897 vs. men). In men, the handgrip-induced rise in mean arterial blood pressure that peaked just prior to cessation of handgrip (control Δ 29.5 ± 2.6 mmHg; blockade Δ 28.2 ± 2.6 mmHg; both P < 0.001 vs. baseline; Fig. 4) was the same under both control and blockade conditions (P = 0.701), whereas as women approached fatigue, they exhibited a greater rise in mean arterial pressure in the blockade condition (control Δ 20.6 ± 3.9 mmHg; blockade Δ 31.9 ± 3.9 mmHg; P = 0.033; both P < 0.001 vs. baseline; Fig. 5). In men, the rise in heart rate induced by handgrip (both P < 0.001 vs. baseline) peaked just prior to cessation of handgrip and was attenuated by propranolol (control Δ 16.6 ± 2.4 beats min−1; blockade Δ 12.2 ± 1.9 beats min−1; P = 0.021), while the women exhibited a similar increase in heart rate (both P < 0.001 vs. baseline), which peaked 10 s before handgrip release, in both the control and blockade trials (control Δ 15.3 ± 3.6 beats min−1; blockade Δ 13.9 ± 3.1 beats min−1; P = 0.719).
Figure 4. Averaged changes in men in mean arterial pressure (A), heart rate (B), femoral artery diameter (C) and femoral vascular conductance (D) elicited by isometric handgrip exercise to fatigue under control (○) and propranolol (•) conditions.
Data are expressed as changes from baseline and the vertical shaded bar indicates the last 50 s of handgrip. Brackets above and below graphs indicate a difference from baseline under each condition. *P < 0.05 vs. control. †Control P < 0.05 vs. women. ‡Blockade P < 0.05 vs. women.
Figure 5. Averaged changes in women in mean arterial pressure (A), heart rate (B), femoral artery diameter (C) and femoral vascular conductance (D) elicited by isometric handgrip exercise to fatigue under control (○) and propranolol (•) conditions.
Data are expressed as changes from baseline and the vertical shaded bar indicates the last 50 s of handgrip. Brackets above and below graphs indicate a difference from baseline under each condition. *P < 0.05 vs. control. †Control P < 0.05 vs. men. ‡Blockade P < 0.05 vs. men.
In response to handgrip exercise, men showed a reduction in femoral artery diameter that reached a nadir 40s after cessation of handgrip and was not different between the control and blockade conditions (control Δ−278.4 ± 85.5 μm; blockade Δ−472.7 ± 91.3 μm; P = 0.101 vs. control; both P < 0.003 vs. baseline; Fig. 4). In contrast, no change in femoral artery diameter was found in women during the control trials (P = 0.044 vs. men at 40 s post-handgrip), but a decline in femoral artery diameter, that reached a nadir 40 s post-handgrip, was seen in response to isometric handgrip with blockade (Δ−372.5 ± 139.4 μm; P = 0.022 vs. control; Fig. 5). Isometric handgrip-induced similar reductions in femoral vascular conductance, expressed as per cent changes from baseline, were found in men and women under both the control and blockade conditions (men: control Δ−48.9 ± 10.6%; blockade Δ−49.7 ± 10.1%; P = 0.944, women: control Δ−45.3 ± 16.2%; blockade Δ−59.7 ± 15.4%; P = 0.389; all P < 0.007 vs. baseline). During recovery from handgrip, however, while men had a moderate increase in femoral vascular conductance during both the control and blockade trials (both P < 0.014 vs. baseline), women experienced a 153 ± 16.2% rise in femoral vascular conductance 20 s post-handgrip under the control condition (P < 0.001 vs. baseline; P < 0.001 vs. men), which was blunted by 54.8% with propranolol (P < 0.001 vs. control; P < 0.020 vs. men). Visit one isometric handgrip-mediated reductions in femoral vascular conductance were similar to those of the visit two control trials in both men (Δ−40.1 ± 12.4%; P = 0.519 vs. control) and women (Δ−37.2 ± 12.7%; P = 0.585 vs. control).
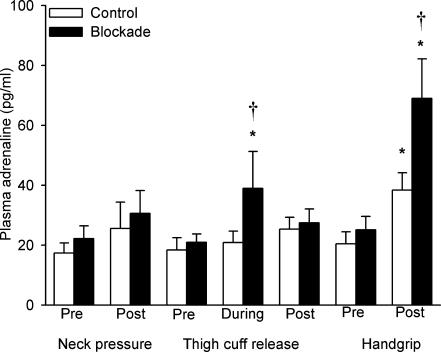
Circulating adrenaline
Figure 6 shows the plasma adrenaline concentrations, under both the control and blockade conditions, before and 1 min after each sympatho-excitatory manoeuvre, as well as at minute seven of unilateral thigh occlusion. Propranolol did not alter baseline plasma adrenaline levels, or plasma adrenaline concentrations in response to neck pressure (both P > 0.286 vs. control). Moreover, post-neck pressure adrenaline levels did not differ significantly from baseline concentrations in either the control or blockade conditions (both P > 0.087). Likewise, plasma adrenaline levels prior to unilateral thigh occlusion were not affected by propranolol (P = 0.689 vs. control), nor did the concentrations differ significantly from baseline levels, or between conditions, after thigh-cuff release (all P > 0.104). However, under the blockade condition, plasma adrenaline concentrations during thigh occlusion (minute 7 of 10) increased from baseline (39 ± 12.4 vs. 21 ± 2.7 pg ml−1; P = 0.017). Furthermore, these adrenaline levels during thigh occlusion were greater during the blockade vs. the control trials (39 ± 12.4 vs. 20.9 ± 3.8 pg ml−1; P = 0.003).
Figure 6. Averaged plasma adrenaline concentrations measured before and after neck pressure, unilateral thigh-cuff release and isometric handgrip to fatigue, as well as at the seventh minute of thigh occlusion.
Open bars denote control trials and filled bars denote propranolol trials. *P < 0.05 vs. pre-manoeuver. †P < 0.05 vs. control.
Propranolol did not affect plasma adrenaline concentrations prior to isometric handgrip exercise (P = 0.506 vs. control). However, adrenaline levels rose from 20.4 ± 4.1 to 38.4 ± 5.8 pg ml−1 in the control condition and from 25.1 ± 4.5 to 69 ± 13.2 pg ml−1 in the blockade condition (both P < 0.001). Moreover, these post-handgrip adrenaline levels were substantially greater in the blockade trials when compared with the control trials (P < 0.001). In men, plasma adrenaline concentrations in response to isometric handgrip rose from 23.3 ± 4.1 to 42.6 ± 4.1 pg ml−1 in the control condition and from 29.4 ± 7.9 to 74.6 ± 7.9 pg ml−1 in the blockade condition. Plasma adrenaline concentrations in women rose from 9.8 ± 7.5 to 23.3 ± 7.5 pg ml−1 in the control condition and from 9.1 ± 14.4 to 48.6 ± 14.4 pg ml−1 in the blockade condition. Unfortunately, due to loss of catheter patency in some subjects, we have insufficient subjects to statistically compare the adrenaline response to handgrip between men and women.
Circulating noradrenaline
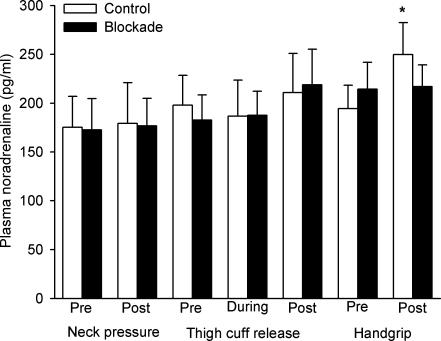
Figure 7 shows plasma noradrenaline concentrations under both experimental conditions, before and 1 min after each sympatho-excitatory manoeuvre, as well as at the seventh minute of unilateral thigh occlusion. Plasma noradrenaline levels prior to neck pressure, unilateral thigh occlusion and isometric handgrip were not altered by propranolol (all P > 0.467 vs. control).
Figure 7. Averaged plasma noradrenaline concentrations measured before and after neck pressure, unilateral thigh-cuff release and isometric handgrip to fatigue, as well as at the seventh minute of thigh occlusion.
Open bars denote control trials and filled bars denote propranolol trials. *P < 0.05 vs. pre-manoeuvre.
Plasma noradrenaline concentrations did not change in response to neck pressure (P > 0.829; both conditions) or unilateral thigh-cuff release (P > 0.059; both conditions), nor did they differ between the experimental conditions (both P > 0.751). Furthermore, there were no changes from baseline in plasma noradrenaline levels during thigh occlusion and no differences were found between the control and blockade trials at this time point (all P > 0.065).
Under the control condition, plasma noradrenaline concentrations increased from 194.3 ± 24.1 to 249.7 ± 32.9 pg ml−1 in response to isometric handgrip (P = 0.039). In contrast, there was no difference between plasma noradrenaline levels before and after isometric handgrip exercise under the blockade condition (P = 0.891).
Discussion
The objective of the current study was to determine whether β-adrenergic receptor-mediated vasodilatation attenuates leg vasoconstrictor responses to three commonly used sympatho-excitatory manoeuvres. In contrast to our hypothesis, the β-adrenergic antagonist propranolol mildly enhanced reductions in femoral vascular conductance in response to neck pressure, but did not alter reductions in femoral vascular conductance in response to unilateral thigh-cuff release or isometric handgrip exercise. Propranolol markedly attenuated the unexpected and profound post-handgrip hyperaemia found in women, whereas the post-handgrip hyperaemic response was considerably smaller in men and unaffected by propranolol.
Vasoconstrictor responses to neck pressure
Neck pressure has been used to probe the carotid baroreflex-leg vasoconstrictor response in a number of settings including rest and exercise (Keller et al. 2003, 2004a, b; Ichinose & Nishiyasu, 2005). Both the time-course and the relative reductions in femoral vascular conductance in the current study are similar to these prior studies and consistent with the notion that a brief 5 s stimulus to the carotid baroreceptors produces a robust vasoconstrictor response in the skeletal muscle vascular beds of the leg that becomes most evident ∼7 s after the onset of neck pressure. Under resting control conditions, the reduction in vascular conductance is in the order of 20–25%, as illustrated in Fig. 2. Contrary to our expectations, this reduction in femoral vascular conductance was mildly augmented by propranolol. This greater reduction in femoral vascular conductance with propranolol was accomplished primarily by the elimination of the brief initial increase in conductance seen in response to neck pressure in the control condition. There are at least four possible explanations for this observation.
First, one possible explanation is that a rise in circulating adrenaline in response to neck pressure during control trials led to a β-adrenergic receptor-mediated vasodilatation which attenuated leg vasoconstrictor responses, and that this was subsequently blocked by propranolol administration. However, since the effect of propranolol on the neck pressure response was first apparent within 5 s of the onset of neck pressure, it seems unlikely that this effect could be mediated by a change in circulating levels of adrenaline.
Second, it is possible that propranolol altered the cardiac output response to neck pressure, and that this indirectly led to changes in femoral vascular conductance. However, the overall blood pressure response to neck pressure is mediated primarily by changes in vascular tone, and, to a lesser degree heart rate (Ogoh et al. 2002), with no notable changes in stroke volume (Ogoh et al. 2003). In the current study, the pressure response to neck pressure was not reduced by propranolol; thus, it seems improbable that propranolol mediated its effects on the vascular response via an effect on the heart.
Third, it is possible that propranolol, working via central nervous system β-adrenergic receptors, modified responsiveness of the carotid baroreflex such that there was a greater rise in sympathetic nerve activity to the leg. However, as the heart rate response was largely unaffected by administration of propranolol, it seems unlikely that blockade of central nervous system β-adrenergic receptors had an effect on overall baroreflex function.
Lastly, it is possible that neurally released noradrenaline in response to neck pressure activated β-adrenergic receptors in the leg, and that this was blocked by propranolol administration. While the affinity of noradrenaline for β-adrenergic receptors is modest in comparison to its affinity for β-adrenergic receptors, outflow of noradrenaline from sympathetic nerves could activate a small portion of β-adrenergic receptors.
Vasoconstrictor responses to unilateral thigh-cuff release
To our knowledge, this is the first study to use unilateral thigh-cuff release as a stimulus to probe baroreflex-leg vasoconstrictor responses. This methodology has been used mostly to assess baroreflex-heart rate responses. As illustrated in Fig. 3, thigh-cuff release evoked a marked drop in mean arterial pressure of 10–15 mmHg that reached a nadir after ∼20 s, similar to what has been reported by others (Fadel et al. 2001, 2003). This fall in pressure produced a robust vasoconstrictor response in the skeletal muscle vascular beds of the leg that was most evident ∼20–30 s after the release of the cuff. Under resting control conditions, the reduction in vascular conductance was in the order of 50–60%. This reduction in femoral vascular conductance was not affected by propranolol, but the time-course of recovery from the nadir was more rapid after propranolol administration. Thus, unlike neck pressure, it does not appear that there is β-adrenergic receptor-mediated vasodilatation during the baroreflex-mediated vasoconstrictor response to thigh-cuff release.
On the other hand, we recognize that the neck pressure response is largely an ‘open-loop’ baroreflex response. In other words, because of the brief stimulus employed, the time-course of this response is short enough that alterations in the vascular response do not have time to feedback and change the net response. In contrast to this, the response to thigh-cuff release is largely closed-loop and, thus, an alteration in the vascular response might be offset by further changes in sympathetic neural outflow, creating the appearance of no net change in the response. In other words, we think it is plausible that the same β-adrenergic mechanism that we believe blunts the vasoconstrictor response to neck pressure could also blunt the vasoconstrictor response to thigh-cuff release, but that this effect is normally masked by greater elevations in nerve activity. However, it is less clear how or why this might result in the more sustained vasoconstriction seen in the absence of β-adrenergic blockade. In the end, it is likely that direct recordings of muscle sympathetic nerve activity may be needed to further explore this possibility.
Vasoconstrictor response to isometric handgrip exercise
Isometric handgrip exercise to fatigue leads to vasoconstriction in the contralateral forearm and both calves, as measured by venous occlusion plethysmography (Seals, 1989; Saito et al. 1990; Taylor et al. 1991), and across the whole-leg, as measured by femoral artery Doppler ultrasound (Shoemaker et al. 2000). Similar to these classic studies, we found progressive increases in arterial pressure that corresponded with a vasoconstrictor response in the vascular beds of the leg. Under resting control conditions, the reduction in vascular conductance was in the order of 50–60%, as illustrated in Figs 4 and 5.
Previous studies have found that both the systemic (MacDonald et al. 1966) and the contralateral forearm (Eklund & Kaijser, 1976) vasoconstrictor responses to isometric handgrip exercise were augmented by β-adrenergic blockade. In addition, intra-arterial administration of propranolol severely blunted the contralateral forearm vasodilator response seen during ischaemic handgrip exercise when sympathetically mediated vasoconstriction has been circumvented by stellate ganglion blockade (Reed et al. 2000). These observations led us to hypothesize that propranolol would augment the reduction in femoral vascular conductance observed in response to isometric handgrip to fatigue. In contrast to our hypothesis, however, propranolol did not alter the femoral vasoconstrictor response to this sympatho-excitatory manoeuvre, despite elevations in circulating adrenaline.
It is unclear how best to reconcile these observations. One possible explanation is that administration of propranolol in our study led to a diminished sympathetic vasoconstrictor response during activation of the muscle metaboreflex (Mark et al. 1985; Sinoway et al. 1989). In other words, when competition from β-adrenergic receptor-mediated vasodilatation was absent, sympathetic neural outflow did not increase as much. Along these lines, plasma noradrenaline concentrations correlate strongly with muscle sympathetic nerve activity during a variety of stressors (Victor et al. 1987; Wallin et al. 1987; Eckberg et al. 1988). The finding that handgrip exercise produced a smaller rise in noradrenaline after propranolol administration is consistent with the notion of a smaller rise in sympathetic outflow during blockade.
An alternative explanation is that there may be heterogeneity of limb vascular responsiveness to circulating adrenaline or the balance between vasoconstrictor and vasodilator responses. Along these lines, lower body negative pressure (Jacobsen et al. 1992), cold pressor stimulation (Jacob et al. 2000) and isometric handgrip exercise (Rusch et al. 1981) evoke greater vasoconstriction in the calf than the forearm. In addition, intra-arterial infusions of phenylephrine result in greater reductions in calf vs. forearm vascular conductance, which suggests enhanced α-adrenergic receptor-mediated vasoconstriction in the legs compared with the arms (Pawelczyk & Levine, 2002). Moreover, blunted vascular responses have been found in the legs vs. the arms in response to intra-arterial infusions of both endothelium-dependent and endothelium-independent vasodilatator agents (Jacob et al. 2000; Newcomer et al. 2004). Thus, differences in how the limbs respond to neurally mediated vasoconstriction and hormonally mediated vasodilatation may explain why prior work with propranolol has been able to document β-adrenergically mediated vasodilatation in the contralateral forearm in response to handgrip exercise (Eklund & Kaijser, 1976; Reed et al. 2000) whereas the current study has not shown this in the leg.
Vasodilator response to termination of isometric handgrip exercise
Interestingly, the post-handgrip hyperaemic responses were vastly different between men and women in the current study. The greater hyperaemia in women was attributable to a greater β-adrenergic receptor-mediated vasodilatation as it was blunted by more than 50% after propranolol administration. This would implicate a greater adrenaline release in women in response to final stages of isometric exercise; however, we have inadequate sampling of plasma adrenaline to address this possibility. Notably, muscle sympathetic nerve activity falls silent following the release of isometric handgrip (Mark et al. 1985). As such, passive vasodilatation may explain much of the post-handgrip hyperaemia that was not blocked by propranolol in both men and women (Ettinger et al. 1996, 1998; Jones et al. 1996). However, it is likely that the differences seen between sexes may be attributed to either greater circulating levels of adrenaline, greater β2-adrenergic receptor density, or greater receptor sensitivity in women. These factors may be partially dependent upon the menstrual cycle phase (Mills et al. 1996; Kneale et al. 2000).
Methodological considerations
During each sympatho-excitatory manoeuvre, femoral artery diameter was determined on a beat-to-beat basis, rather than assuming a constancy of diameter during interventions. These data may provide some insight into responsiveness of the large conduit arteries of the legs. Consistent with previous reports, neck pressure did not change femoral artery diameter (Keller et al. 2003, 2004b; Ichinose & Nishiyasu, 2005). In contrast, both unilateral thigh-cuff release and isometric exercise resulted in reductions in femoral artery diameter under one or more of the conditions we studied. Declines in femoral artery diameter after thigh-cuff release tracked with the decline in arterial pressure, consistent with the compliance of this artery. In contrast, femoral artery diameter began to decline in the latter stages of the handgrip exercise while arterial pressure was increasing. This is suggestive of α-adrenergic-mediated vasoconstriction of the conduit vessel, although diminished vascular sheer stress (Koller & Kaley, 1991; Saltin et al. 1998; Clifford & Hellsten, 2004) as a result of vasoconstriction of the distal resistance vessels or propagated vasoconstriction (Green et al. 1996; Segal & Jacobs, 2001) may have contributed to this reduction in femoral artery diameter. Recently Wray et al. (2005) reported a greater change in vessel diameter for a given change in shear stress in the brachial and deep femoral arteries, compared with the common femoral artery. Those results suggest heterogeneity of vascular responses, not only between limbs, but of different regions of the vasculature in the same limb. In the current investigation, we measured responses in either the superficial or the common femoral artery. However, we did not note any differences between how these vessels responded to the specific manoeuvres we employed. Regardless of the underlying mechanisms for changes in diameter, this highlights the importance of tracking changes in vessel diameter during sympatho-excitatory manoeuvers when the overall goal is to determine blood flow and/or vascular responses.
Conclusions
Sympatho-excitatory manoeuvres, such as isometric handgrip exercise, neck pressure and unilateral thigh-cuff release have traditionally been used to study the sympathetic nervous system and how it regulates the cardiovascular system under a variety of circumstances (Seals, 1989; Halliwill et al. 1996; Fadel et al. 2003; Keller et al. 2004a). Such manoeuvres have allowed investigators to study how stress responses differ with age (Taylor et al. 1991), sex (Ettinger et al. 1996), race (Ray & Monahan, 2002), fitness level (Fadel et al. 2001) and disease (Eckberg et al. 1986; Floras et al. 1989; Sopher et al. 1990). A thorough understanding of these manoeuvres, including their potential limitations, is therefore crucial. The results of the current study suggest that β-adrenergic receptor-mediated vasodilatation minimally affects leg vascular responses to these commonly used sympatho-excitatory manoeuvres. Interestingly, we have observed a profound skeletal muscle hyperaemia during recovery from isometric handgrip exercise that is greater in women than men and this hyperaemia is blunted by propranolol. This suggests that some aspects of β-adrenergic receptor-mediated vasodilatation may vary across populations and stressors. However, the primary findings from this study add further validity to the use of these manoeuvres in the study of sympathetic vascular regulation in humans.
Acknowledgments
The authors would like to express their gratitude to all of the subjects who participated in this series of studies. We also wish to thank Julie B. Manson, Martin C. Anderson and Brenna M. Lynn for technical assistance during these studies. These studies were conducted by Thomas K. Pellinger in partial fulfilment for the degree of Doctor of Philosophy in the Department of Human Physiology at the University of Oregon. This research was supported by a grant from the National Heart, Lung and Blood Institute HL-65305 and the American Heart Association 555623Z.
References
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by β-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Fritsch JM. How should human baroreflexes be tested? News Physiol Sci. 1993;8:7–12. doi: 10.1152/physiologyonline.1993.8.1.7. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Harkins SW, Fritsch JM, Musgrave GE, Gardner DF. Baroreflex control of plasma norepinephrine and heart period in healthy subjects and diabetic patients. J Clin Invest. 1986;78:366–374. doi: 10.1172/JCI112586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Rea RF, Andersson OK, Hedner T, Pernow J, Lundberg JM, Wallin BG. Baroreflex modulation of sympathetic activity and sympathetic neurotransmitters in humans. Acta Physiol Scand. 1988;133:221–231. doi: 10.1111/j.1748-1716.1988.tb08401.x. [DOI] [PubMed] [Google Scholar]
- Eklund B, Kaijser L. Effect of regional α- and β-adrenergic blockade on blood flow in the resting forearm during contralateral isometric handgrip. J Physiol. 1976;262:39–50. doi: 10.1113/jphysiol.1976.sp011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S, Robinson BF, Kahler RL, Braunwald E. Effects of β-adrenergic blockade on the cardiac response to maximal and submaximal exercise in man. J Clin Invest. 1965;44:1745–1753. doi: 10.1172/JCI105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol. 1996;80:245–251. doi: 10.1152/jappl.1996.80.1.245. [DOI] [PubMed] [Google Scholar]
- Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol. 1998;85:2075–2081. doi: 10.1152/jappl.1998.85.6.2075. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Stromstad M, Hansen J, Sander M, Horn K, Ogoh S, Smith ML, Secher NH, Raven PB. Arterial baroreflex control of sympathetic nerve activity during acute hypotension: effect of fitness. Am J Physiol Heart Circ Physiol. 2001;280:H2524–H2532. doi: 10.1152/ajpheart.2001.280.6.H2524. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Stromstad M, Wray DW, Smith SA, Raven PB, Secher NH. New insights into differential baroreflex control of heart rate in humans. Am J Physiol Heart Circ Physiol. 2003;284:H735–H743. doi: 10.1152/ajpheart.00246.2002. [DOI] [PubMed] [Google Scholar]
- Floras JS, Sinkey CA, Aylward PE, Seals DR, Thoren PN, Mark AL. Postexercise hypotension and sympathoinhibition in borderline hypertensive men. Hypertension. 1989;14:28–35. doi: 10.1161/01.hyp.14.1.28. [DOI] [PubMed] [Google Scholar]
- Freyschuss U, Hjemdahl P, Juhlin-Dannfelt A, Linde B. Cardiovascular and metabolic responses to low dose adrenaline infusion: an invasive study in humans. Clin Sci (Lond) 1986;70:199–206. doi: 10.1042/cs0700199. [DOI] [PubMed] [Google Scholar]
- Fritsch JM, Smith ML, Simmons DT, Eckberg DL. Differential baroreflex modulation of human vagal and sympathetic activity. Am J Physiol Regul Integr Comp Physiol. 1991;260:R635–R641. doi: 10.1152/ajpregu.1991.260.3.R635. [DOI] [PubMed] [Google Scholar]
- Green DJ, O'Driscoll G, Blanksby BA, Taylor RR. Control of skeletal muscle blood flow during dynamic exercise: contribution of endothelium-derived nitric oxide. Sports Med. 1996;21:119–146. doi: 10.2165/00007256-199621020-00004. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol. 1997;504:211–220. doi: 10.1111/j.1469-7793.1997.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol. 1996;495:279–288. doi: 10.1113/jphysiol.1996.sp021592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L, Zweifler AJ, Julius S, Hunyor SN. Hemodynamic effects of acute and prolonged β-adrenergic blockade in essential hypertension. Acta Med Scand. 1974;196:27–34. doi: 10.1111/j.0954-6820.1974.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P, Linde B. Influence of circulating NE and Epi on adipose tissue vascular resistance and lipolysis in humans. Am J Physiol Heart Circ Physiol. 1983;245:H447–H452. doi: 10.1152/ajpheart.1983.245.3.H447. [DOI] [PubMed] [Google Scholar]
- Holmberg G, Levi L, Mathe A, Rosen A, Scott HM. Comparison of the plasma catecholamine levels and the influence of propranolol on peripheral hemodynamic manifestations of emotional stress in labile hypertensive and normal subjects. Circulation. 1965;32(Suppl. 2):115–116. [Google Scholar]
- Ichinose M, Nishiyasu T. Muscle metaboreflex modulates the arterial baroreflex dynamic effects on peripheral vascular conductance in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1532–H1538. doi: 10.1152/ajpheart.00673.2004. [DOI] [PubMed] [Google Scholar]
- Jacob G, Costa F, Shannon J, Robertson D, Biaggioni I. Dissociation between neural and vascular responses to sympathetic stimulation: contribution of local adrenergic receptor function. Hypertension. 2000;35:76–81. doi: 10.1161/01.hyp.35.1.76. [DOI] [PubMed] [Google Scholar]
- Jacobsen TN, Nielsen HV, Kassis E, Amtorp O. Subcutaneous and skeletal muscle vascular responses in human limbs to lower body negative pressure. Acta Physiol Scand. 1992;144:247–252. doi: 10.1111/j.1748-1716.1992.tb09293.x. [DOI] [PubMed] [Google Scholar]
- Johnsson G. Influence of metoprolol and propranolol on hemodynamic effects induced by adrenaline and physical work. Acta Pharmacol Toxicol (Copenh) 1975;36:59–68. doi: 10.1111/j.1600-0773.1975.tb03322.x. [DOI] [PubMed] [Google Scholar]
- Jones PP, Spraul M, Matt KS, Seals DR, Skinner JS, Ravussin E. Gender does not influence sympathetic neural reactivity to stress in healthy humans. Am J Physiol Heart Circ Physiol. 1996;270:H350–H357. doi: 10.1152/ajpheart.1996.270.1.H350. [DOI] [PubMed] [Google Scholar]
- Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol. 2004a;561:283–293. doi: 10.1113/jphysiol.2004.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Ogoh S, Greene S, Olivencia-Yurvati A, Raven PB. Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol. 2004b;561:273–282. doi: 10.1113/jphysiol.2004.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Wasmund WL, Wray DW, Ogoh S, Fadel PJ, Smith ML, Raven PB. Carotid baroreflex control of leg vascular conductance at rest and during exercise. J Appl Physiol. 2003;94:542–548. doi: 10.1152/japplphysiol.00817.2002. [DOI] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol. 2000;36:1233–1238. doi: 10.1016/s0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 1991;260:H862–H868. doi: 10.1152/ajpheart.1991.260.3.H862. [DOI] [PubMed] [Google Scholar]
- Lindqvist M, Melcher A, Hjemdahl P. Attenuation of forearm vasodilator responses to mental stress by regional β-blockade, but not by atropine. Acta Physiol Scand. 1997;161:135–140. doi: 10.1046/j.1365-201X.1997.00192.x. [DOI] [PubMed] [Google Scholar]
- MacDonald HR, Sapru RP, Taylor SH, Donald KW. Effect of intravenous propranolol on the systemic circulatory response to sustained handgrip. Am J Cardiol. 1966;18:333–344. doi: 10.1016/0002-9149(66)90051-8. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Ziegler MG, Nelesen RA, Kennedy BP. The effects of the menstrual cycle, race, and gender on adrenergic receptors and agonists. Clin Pharmacol Ther. 1996;60:99–104. doi: 10.1016/S0009-9236(96)90172-1. [DOI] [PubMed] [Google Scholar]
- Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol. 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Monteiro F, Wasmund WL, Raven PB. Haemodynamic changes during neck pressure and suction in seated and supine positions. J Physiol. 2002;540:707–716. doi: 10.1113/jphysiol.2001.013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol. 2003;550:317–324. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk JA, Levine BD. Heterogeneous responses of human limbs to infused adrenergic agonists: a gravitational effect? J Appl Physiol. 2002;92:2105–2113. doi: 10.1152/japplphysiol.00979.2001. [DOI] [PubMed] [Google Scholar]
- Ray CA, Monahan KD. Sympathetic vascular transduction is augmented in young normotensive blacks. J Appl Physiol. 2002;92:651–656. doi: 10.1152/japplphysiol.00788.2001. [DOI] [PubMed] [Google Scholar]
- Rea RF, Eckberg DL. Carotid baroreceptor-muscle sympathetic relation in humans. Am J Physiol Regul Integr Comp Physiol. 1987;253:R929–R934. doi: 10.1152/ajpregu.1987.253.6.R929. [DOI] [PubMed] [Google Scholar]
- Reed AS, Tschakovsky ME, Minson CT, Halliwill JR, Torp KD, Nauss LA, Joyner MJ. Skeletal muscle vasodilatation during sympathoexcitation is not neurally mediated in humans. J Physiol. 2000;525:253–262. doi: 10.1111/j.1469-7793.2000.t01-1-00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB, Seals DR. Sympathetic activity during graded central hypovolemia in hypoxemic humans. Am J Physiol Heart Circ Physiol. 1990;259:H1197–H1206. doi: 10.1152/ajpheart.1990.259.4.H1197. [DOI] [PubMed] [Google Scholar]
- Rusch NJ, Shepherd JT, Webb RC, Vanhoutte PM. Different behavior of the resistance vessels of the human calf and forearm during contralateral isometric exercise, mental stress, and abnormal respiratory movements. Circ Res. 1981;48:I118–I130. [PubMed] [Google Scholar]
- Saito M, Mano T, Iwase S. Changes in muscle sympathetic nerve activity and calf blood flow during static handgrip exercise. Eur J Appl Physiol Occup Physiol. 1990;60:277–281. doi: 10.1007/BF00379396. [DOI] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Herr MD, Sinoway LI. Dissociation of muscle sympathetic nerve activity and leg vascular resistance in humans. Am J Physiol Heart Circ Physiol. 2000;279:H1215–H1219. doi: 10.1152/ajpheart.2000.279.3.H1215. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- Sopher SM, Smith ML, Eckberg DL, Fritsch JM, Dibner-Dunlap ME. Autonomic pathophysiology in heart failure: carotid baroreceptor-cardiac reflexes. Am J Physiol Heart Circ Physiol. 1990;259:H689–H696. doi: 10.1152/ajpheart.1990.259.3.H689. [DOI] [PubMed] [Google Scholar]
- Stratton JR, Pfeifer MA, Ritchie JL, Halter JB. Hemodynamic effects of epinephrine: concentration-effect study in humans. J Appl Physiol. 1985;58:1199–1206. doi: 10.1152/jappl.1985.58.4.1199. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation during sustained isometric exercise in young and older men. Am J Physiol Regul Integr Comp Physiol. 1991;261:R1061–R1069. doi: 10.1152/ajpregu.1991.261.5.R1061. [DOI] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Morlin C, Hjemdahl P. Muscle sympathetic activity and venous plasma noradrenaline concentrations during static exercise in normotensive and hypertensive subjects. Acta Physiol Scand. 1987;129:489–497. doi: 10.1111/j.1748-1716.1987.tb08088.x. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol. 2001;91:929–937. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol. 2005;99:81–86. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]