Abstract
In this study we investigated whether long-term consumption of a moderate/high fat (MHF), high-energy diet can affect the gene expression of the Y1 receptor (Y1R) for neuropeptide Y (NPY) in the dorsomedial (DMH), ventromedial (VMH), arcuate (ARC) and paraventricular (PVN) hypothalamic nuclei of male and female Y1R/LacZ transgenic mice, carrying the murine Y1R promoter linked to the LacZ gene. MHF diet-fed male mice showed an increased consumption of metabolizable energy that was associated with a significant increase in body weight as compared with chow-fed controls. In parallel, consumption of a MHF diet for 8 weeks significantly decreased Y1R/LacZ transgene expression in the DMH and VMH of male mice whereas no changes were found in the ARC and PVN. Leptin treatment reduced body weight of both MHF diet- and chow-fed male mice but failed to prevent the decrease in Y1R/LacZ transgene expression apparent in the DMH and VMH of male mice after 8 weeks of MHF diet intake. Conversely, no significant changes of metabolizable energy intake, body weight or hypothalamic β-galactosidase expression were found in MHF diet-fed female Y1R/LacZ transgenic mice. A gender-related difference of Y1R/LacZ transgenic mice was also observed in response to leptin treatment that failed to decrease body weight of both MHF diet- and chow-fed female mice. Results herein demonstrate that Y1R/LacZ FVB mice show a sexual dimorphism both on energy intake and on nucleus-specific regulation of the NPY Y1R system in the hypothalamus. Overall, these results provide new insights into the mechanism by which diet composition affects the hypothalamic circuit that controls energy homeostasis.
The hypothalamus plays a critical role in integrating neural and hormonal signals that influence whole body energy balance, which, when altered, could result in obesity (Schwartz et al. 2000). Among those factors that play a major role in regulating body weight, adiposity and energy metabolism are leptin and neuropeptide Y (NPY) (Sahu, 2003; Kalra & Kalra, 2004). Leptin, a hormone produced by adipocytes, acts as a feedback signal to the hypothalamus to reduce food intake and stimulate thermogenesis (Campfield et al. 1995; Halaas et al. 1995). An increase in body fat increases leptin levels that, in turn, reduces food intake, while a decrease in body fat leads to decreased levels of the circulating hormone and to a stimulation of food intake (Elmquist et al. 1998). Mutations that result in leptin deficiency, or in leptin resistance, are associated with massive obesity (Elmquist et al. 1998).
NPY is found in high concentration in the hypothalamus of both rodents and humans and it exerts opposite effects of those of leptin (Chronwall et al. 1985; Hokfelt et al. 1998). NPY has a potent ability to stimulate feeding, reduce energy expenditure and induce obesity and it opposes the anorectic effect of leptin (Stanley et al. 1986; Kalra et al. 1991). Hypothalamic NPY-containing neurons express leptin receptors and leptin was shown to negatively regulate arcuate (ARC) NPY gene expression (Stephens et al. 1995). Increased hypothalamic NPY activity has been suggested in some forms of genetic obesity linked to defective leptin signalling, including the ob/ob, db/db and fa/fa Zucker rats (Wilding et al. 1993; Schwartz et al. 1996; Kim et al. 2000) and genetic depletion of NPY attenuates obesity in ob/ob mice (Erickson et al. 1996).
NPY Y1, Y2 and Y5 receptors are expressed in the hypothalamic sites involved in the regulation of ingestive behaviour and energy balance (Parker & Herzog, 1999). Studies in genetically modified mice and pharmacological studies revealed that the Y1 receptor (Y1R) plays a crucial role in the control of energy homeostasis (Herzog, 2003; Pedrazzini, 2004; Eva et al. 2006). The potent anorectic effect of selective Y1R antagonists suggests that Y1R is physiologically involved in appetite regulation (Kanatani et al. 1996; Wieland et al. 1998). mutant mice lacking the Y1R feed and grow normally but exhibit a significantly reduced feeding response to NPY or to the NPY receptor agonist PYY3-36 (Kanatani et al. 2000).
During negative energy balance, for example during food restriction, exercise, thyrotoxicosis, lactation or anorexia, there is an increase in the synthesis of NPY in the ARC and of the peptide concentration in NPYergic terminals (Dryden et al. 1994; Ishii et al. 2003; Bi et al. 2005; Chance et al. 2007) that are associated with the down-regulation of Y1R in the PVN (for review see Eva et al. 2006), suggesting that the level of NPY signalling is strongly influenced by the nutritional status. A number of investigators have examined the effect of diet-induced obesity on the hypothalamic NPY system, but no single conclusion can be made from these studies. In diet-induced obesity models, when there is positive energy balance, ARC NPY mRNA and protein content increase (Levin & Dunn-Meynell, 1997; Huang et al. 2003), decrease (Wilding et al. 1992; Bergen et al. 1999; Hansen et al. 2004) or remain unchanged (Wilding et al. 1992), and alteration of hypothalamic NPY gene expression was shown to be dependent on the genetic background of the different strains of rodents and on the type of treatment. Similarly, conflicting results have been reported on the expression levels of the Y1R subtype which was found to be either decreased (Beck et al. 2001), increased (Schaffhauser et al. 2002) or unchanged (Widdowson, 1997; Huang et al. 2003) in the hypothalamus of genetically obese and obesity prone rodents. Moreover, it is unclear whether diet composition may affect the expression of hypothalamic Y1R receptors (Schaffhauser et al. 2002; Huang et al. 2003). These discrepancies possibly reflect the limitation of analyses done on whole hypothalamus and further studies are needed to show region-specific changes of Y1R expression in different hypothalamic nuclei.
Therefore, the primary aim of this study was to examine the effect of long-term exposure to a high-energy, moderate/high fat (MHF) diet on the Y1R gene expression in four hypothalamic nuclei (ARC, PVN, DMH and VMH) that are involved in the regulation of energy balance (Schwartz et al. 2000). To this purpose we used, as a model, transgenic mice (Y1R/LacZ) carrying the murine Y1R promoter linked to the LacZ gene, that were created on a FVB/n background (Oberto et al. 1998). We previously demonstrated that changes in energy balance during pregnancy, fasting, leptin treatment or glucose administration can modulate Y1R/LacZ transgene expression in a tissue-specific manner, suggesting that changes in transgene expression may reflect changes of Y1R steady state and therefore alteration of NPY Y1R signal transduction (Zammaretti et al. 2001; Oberto et al. 2003; Eva et al. 2006). In addition, since gender differences in leptin responsiveness have been reported for FVB mice (Harris et al. 2002), both female and male mice were included in the study.
Methods
Experimental animals
Y1R/LacZ mice from transgenic line 62 of our breeding colony (Oberto et al. 1998) were employed in this study. Y1R/LacZ transgenic mice were created and maintained on a FVB inbred background and were genetically identical. Mice were housed conventionally with a 12 h light–dark cycle and with constant temperature (21 ± 2°C). Animal care and handling throughout the experimental procedure were in strict accordance with the European Community Council Directive, 24th November 1986 (86/609/EEC) and the protocol was approved by the Animal Investigation Committee of the Ministero dell'Università e della Ricerca Scientifica e Tecnologica.
Treatments
Three-week-old male (initial body weight 11–14 g) or female (initial body weight 7–12 g) Y1R/LacZ transgenic mice were studied. The animals were randomly assigned to four groups: two groups (of chow-fed males, chow-fed females) had free access to standard mouse chow (Morini, S. Polo D'Enza, Re, Italy), which contains 2.63 kCal g−1 with 60% of the metabolizable energy content as carbohydrates, 7% as fibre, 26% as protein and 8% as fat. The remaining two groups of animals (MHF diet-fed males and MHF diet-fed females) were fed with a highly palatable MHF diet (slightly modified diet reported by Lauterio et al. 1994; Harlan Italy, S. Pietro al Natisone, Udine, Italy). This diet contains 3.56 kCal g−1 with 45% of the metabolizable energy content as carbohydrates, 7% as fibre, 18% as protein and 31% as fat. This dietary model was chosen for two main reasons. First, it is relatively low in fat compared with other models and thus is more in line with the average diet in developed countries consisting of 30–45% of energy from fat. Second, given that the MHF diet enables identification of susceptible and obesity-resistant populations (Lauterio et al. 1994; Lauterio et al. 1999), this dietary model can be useful to study gender-dependent differences in the hypothalamic circuit that controls feeding behaviour and obesity.
After an 8 week period, each group of mice (chow-fed males, chow-fed females, MHF diet-fed males and MHF diet-fed females) was further divided into two subgroups and treated for 3 days with a single daily intraperitoneal injection of 1 μg g−1 murine recombinant leptin (LEP; Sigma-Aldrich, Milano, Italy) or saline. All mice were killed by cervical dislocation immediately after the treatment at the end of the day 3. Brains were quickly removed, placed in 10% embedding medium (Bio-optica, Milano, Italy) in PBS, frozen on crushed dry ice, and stored at −80°C until assayed.
Body weight and food consumption determination
Body weight was measured at 16.00 h on the 7th day of each week throughout experiments. After a 8 week period, the groups of chow-fed males, chow-fed females, MHF diet-fed males and MHF diet-fed females treated with leptin were further weighed daily at 16.00 h immediately after the hormone administration.
Food intake was monitored in three independent experiments. All animals from each group of mice (chow-fed males, chow-fed females, MHF diet-fed males and MHF diet-fed females) were housed in standard cages (each containing 5–6 animals of the same group). Mean food consumption (mean grams per mouse) was determined at 1 week intervals throughout experiments by measuring on the 7th day of each week, at 16.00 h, the amount of food consumed per cage and dividing it by the number of mice housed in the cage. The amount of food consumed per cage during these time intervals was estimated by subtracting the residual food recovered from each cage from the total amount presented. After the measurement the mice were given fresh quantities of their diets. Caloric intake was obtained by multiplying the mean food intake per mouse by diet metabolizable energy and results were expressed as mean kilocalories per mouse.
β-Galactosidase staining
Y1R/LacZ expression was determined by β-galactosidase staining of mice brain coronal sections, as previously described (Zammaretti et al. 2001; Oberto et al. 2003). Briefly, frozen brains were cut on a cryostat at −20°C and 25 μm sections were collected on clean slides starting from a level corresponding to the end of the anterior commissure. Sections were dehydrated with acetone–chloroform (1: 1), air dried and shortly fixed in 2.5% glutaraldehyde in PBS (each step for 5 min on ice), and incubated overnight at 37°C in a solution containing 1 mg ml−1 of 5-broma-4-chloro-3-indolyl-beta-D-galacto pyrnoside (X-gal; Inalco, Milano, Italy), 5 mm potassium ferricyanide, 5 mm potassium ferrocyanide, 2 mm MgCl2 and 0.01% Triton X-100 in PBS. After washing in water, sections were counterstained with nuclear fast red, dried and coverslipped with DPX mounting medium (Fluka, Buchs, Switzerland).
Quantification of transgene expression as determined by β-galactosidase histochemistry
The expression of the transgene appears as blue dots. Quantification of the Y1R/LacZ transgene expression was made by computer-assisted morphometrical analysis as previously described (Zammaretti et al. 2001; Oberto et al. 2003). Sections were counterstained with neutral fast red and hypothalamic nuclei were identified on the basis of the mouse brain atlas of Franklin and Paxinos (Franklin & Paxinos, 1997). For each mouse, two standardized sections of comparable levels of the PVN (around bregma −0.70 to −0.82 mm), rostral ARC (around bregma −1.34), rostral DMH (around bregma −1.46), rostral VMH (around bregma −1.22) were chosen. Selected sections were placed on a Leica Diaplan microscope, observed by means of a ×10 objective, and the corresponding image was transferred, via a black and white CCD camera (PCO, VC44, Keilheim, Germany) to a digitizing board (Scion LG-3, Scion Co., Frederick, MD, USA) placed in a PowerPC 8200 Macintosh computer. Acquisition and analysis of the images were performed using the software NIH-Image (version 1.62, a freeware by W. Rasband, NIH, Bethesda, MA, USA). Sections were observed and digitized first by using a built-in green filter to better identify the nuclei extension. A line, drawn following the boundaries of the selected nuclei, defined the area of interest (AOI, Figs 3 and 4). The same section was then digitized using a built-in red filter obtaining a strong enhancement of the histochemical signal, but losing the definition of the nuclear boundaries. The AOI selected on the first image was finally superimposed on the second image to delimit the region in which dots should be counted. Using a manual thresholding method, dots were selected and the corresponding image was binarized. For each animal and nucleus the cumulative number of dots and the cumulative areas of the analysed sections were considered to obtain the density expression of the transgene expressed as dots per μm2.
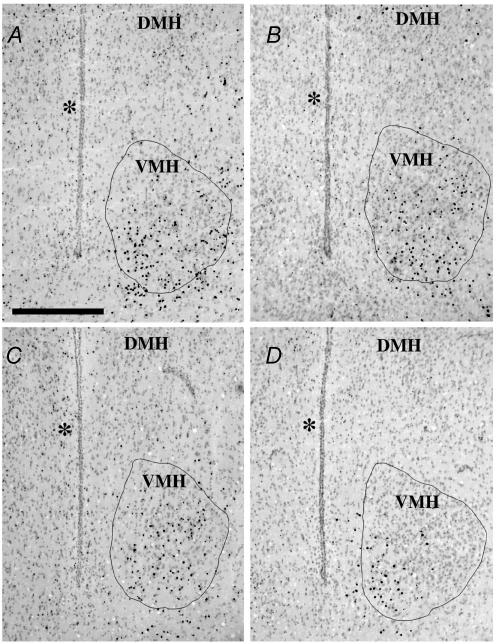
Figure 3. Coronal sections illustrating different expression of the Y1R/LacZ transgene in the rostral level of the ventromedial (VMH) nucleus of male mice from different experimental groups.
A, standard chow-fed male mouse; B, standard chow-fed male mouse treated for 3 days with 1 μg g−1 of murine recombinant leptin; C, MHF diet-fed male mouse; D, MHF diet-fed male mouse treated for 3 days with 1 μg g−1 of murine recombinant leptin. Pictures were digitized using a red filter to enhance the histochemical staining. The closed lines in each picture represent the area of interest (AOI) for counting the number of positive dots. The AOI was drawn following the boundaries of the ventromedial nucleus (VMH) on the green filter-digitized image. DMH, dorsomedial nucleus. * indicates third ventricle; scale bar, 400 μm.
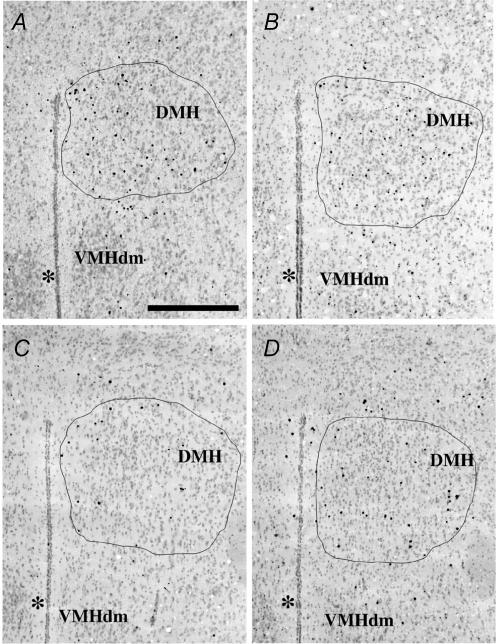
Figure 4. Coronal sections illustrating different expression of the Y1R/LacZ transgene in the rostral level of the dorsomedial (DMH) nucleus of male mice from different experimental groups.
A, standard chow-fed male mouse; B, standard chow-fed male mouse treated for 3 days with 1 μg g−1 of murine recombinant leptin; C, MHF diet-fed male mouse; D, MHF diet-fed male mouse treated for 3 days with 1 μg g−1 of murine recombinant leptin. Pictures were digitized as described in Fig. 3. The AOI was drawn following the boundaries of the dorsomedial nucleus (DMH) on the green filter-digitized image. VMHdm, ventromedial hypothalamic nucleus, pars dorsomedialis. * indicates third ventricle; scale bar, 400 μm.
Data analysis
A repeated measures analysis of variance (ANOVA) was used to compare mean body weight over time and the appropriate contrasts were analysed by unpaired t test. Food consumption, energy intake and transgene expression were examined using one-way ANOVA and Newman–Keuls test for multiple comparisons. All data are expressed as means ±s.e.m. and the level of statistical significance was set at P < 0.05 for all comparisons.
Results
Effects of the MHF diet on Y1R/LacZ mice body weight
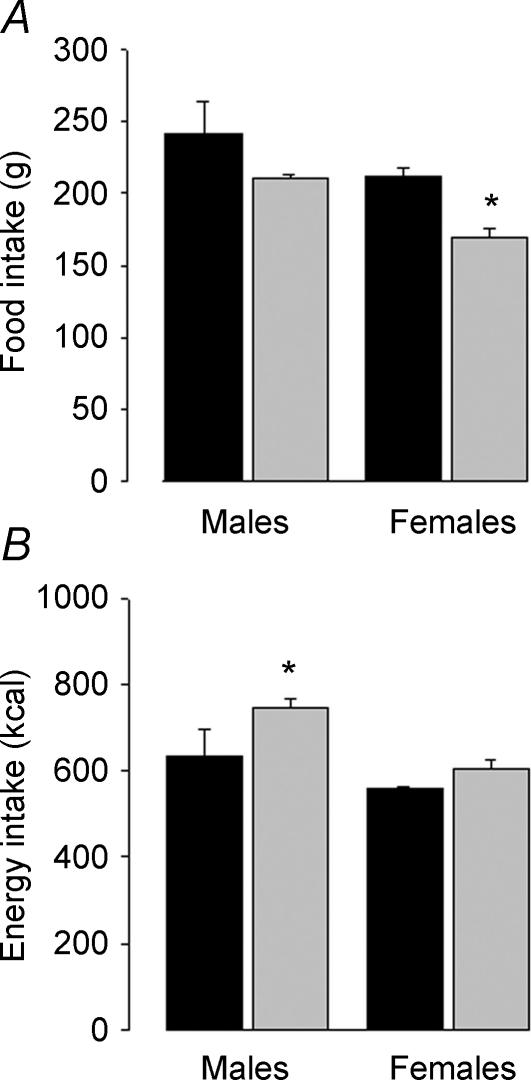
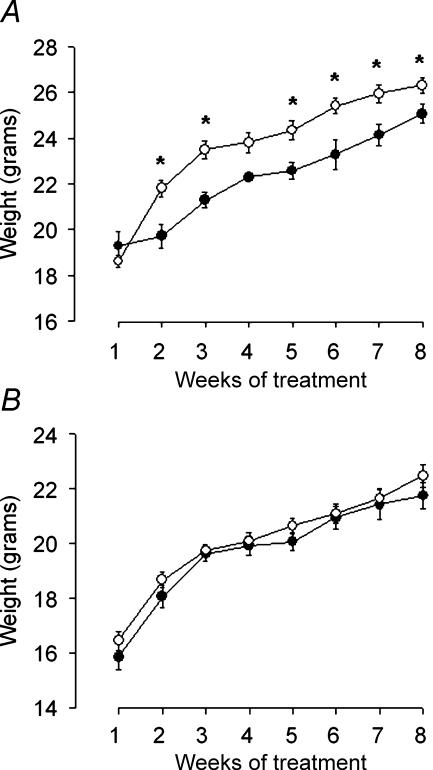
A different response of male and female mice to ingestion of MHF diet for 8 weeks was observed. In males, MHF diet-fed mice consumed the same amount of food, as compared with chow-fed mice, during the 8 weeks of treatment (Fig. 1A), resulting in a significant increase in their cumulative energy intake (Fig. 1B). Analysis of variance for repeated measurements detected a significant effect of the diet on body weight of MHF diet-fed mice as compared with chow-fed ones (Fig. 2A). However, despite their tendency to gain weight more rapidly than controls, Y1R/LacZ mice only poorly responded to the MHF diet, being 6–8% heavier of chow-fed controls at the end of the experiment.
Figure 1. Cumulative food intake (A) and caloric intake (B) of Y1R/LacZ transgenic male or female mice fed either standard chow (black bars) or MHF diet (grey bars) for 8 weeks.
Data are the sum of mean grams per mouse (A) and mean kilocalories per mouse (B) that were determined on the 7th day of each week and are means ±s.e.m. from three experiments (N = 5–6). One-way ANOVA; A, F3,8 = 6.062, P = 0.019. *P < 0.05 versus chow-fed females and MHF diet-fed males, by Newman–Keuls test; B, F3,8 = 6.043 P = 0.019. *P < 0.05 versus chow-fed males and MHF diet-fed females, by Newman–Keuls test.
Figure 2. Weight changes of Y1R/LacZ transgenic male (A) or female (B) mice fed a MHF diet (○) compared with controls fed standard chow (•).
Body weight was determined on the 7th day of each week and data are means ±s.e.m. of values from 7 (chow-fed males), 20 (MHF diet-fed males), 14 (chow-fed females) or 28 (MHF diet-fed females) mice. One-way ANOVA for repeated measurements: A, F1,25 = 6.725 P = 0.0157. *P < 0.05 versus chow-fed mice, by unpaired t test; B, F1,40 = 0.854, P = 0.361.
Conversely, in females, MHF diet-fed mice had a significantly lower food intake than chow-fed mice (Fig. 1A), and no significant effects of diet were evident when the cumulative intake of metabolizable energy was calculated (Fig. 1B). Moreover the ingestion of the MHF diet did not significantly increase body weight of females during the 8 weeks of treatment (Fig. 2B).
The subcutaneous injection of 1 μg g−1 of leptin for 3 days decreased the body weight of both chow-fed (P = 0.009) and MHF diet-fed (P = 0.053) male mice. Conversely, the same treatment failed to affect the body weight of chow- or MHF diet-fed female mice (Table 1). The treatment with saline for 3 or 7 days failed to affect body weight of chow-fed male and female mice (data not shown; Zammaretti et al. 2001).
Table 1.
Effect of the treatment with 1 μg g−1 of murine recombinant leptin for 3 days on the body weight of Y1R/LacZ transgenic male or female mice fed with standard chow or with a MHF diet for 8 weeks
| Males weight (g) | Females weight (g) | |||
|---|---|---|---|---|
| Days of treatment | Chow-fed mice | MHF-fed mice | Chow-fed mice | MHF-fed mice |
| d0 | 22.69 ± 0.35 | 26.77 ± 0.44 | 21.74 ± 0.62 | 23.18 ± 0.91 |
| d3 | 21.82 ± 0.45* | 25.09 ± 0.74a | 21.22 ± 0.56 | 22.68 ± 0.96 |
Data are the mean ±s.e.m. from 6 (chow-fed males), 8 (chow-fed females), 9 (MHF fed males) and 12 (MHF fed females) determinations. One-way ANOVA for repeated measurements: males, overall effect: F1,13 = 30.06, P = 0.001; effect of leptin treatment, F1,1 = 7.42, P = 0.017.
P < 0.01 versus chow-fed mice at d0
P = 0.053 versus MHF-fed mice at d0; females, overall effect: F1,18 = 1.384, P = 0.25.
Effects of the MHF diet on Y1R/LacZ transgene expression in the VMH and DMH
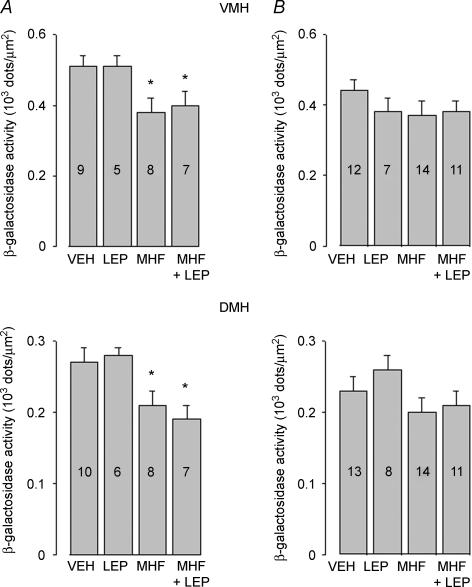
Y1R/LacZ transgene expression was determined by histochemical β-galactosidase staining of brain coronal sections using the chromogenic substrate X-gal. Ingestion of MHF diet for 8 weeks decreased β-galactosidase staining in VMH (Fig. 3A and C) and DMH (Fig. 4A and C) of male mice as compared with chow-fed mice. Leptin treatment (1 μg g−1 for 3 days) failed to abrogate this effect (Figs 3D and 4D). Quantitative analysis (summarized in Fig. 5) demonstrated that 8 week ingestion of the MHF diet decreased β-galactosidase expression in VMH and DMH of male mice by 25% and 22%, respectively, as compared with chow-fed mice. Conversely, no significant changes of β-galactosidase staining were observed in VMH and DMH of MHF diet-fed female mice (Fig. 5B). Leptin treatment failed to modify Y1R/LacZ expression in the VMH and DMH of chow-fed or MHF diet-fed male and female mice (Fig. 5).
Figure 5. Effect of exposure to a MHF diet for 8 weeks on β-galactosidase in the ventromedial (VMH) (upper panel) and dorsomedial (DMH) (lower panel) of 11-week-old male (A) and female (B) Y1R/LacZ transgenic mice.
Mice were fed with standard chow and treated for 3 days with saline (VEH); fed with standard chow and treated for 3 days with 1 μg g−1 of murine recombinant leptin (LEP); fed with a MHF diet for 8 weeks, then treated for 3 days with saline (MHF); fed with a MHF diet for 8 weeks, then treated for 3 days with 1 μg g−1 of murine recombinant leptin (MHF + LEP). Data are expressed as density of blue dots and are means ±s.e.m.; numbers within each column indicate the number of animals used for the determination. VMH, one-way ANOVA: A, F1,3 = 3.6764, P = 0.0254. *P < 0.05 versus VEH by Newman–Keuls test; B, F1,3 = 1.1757, P = 0.331. DMH, one-way ANOVA: A, F1,3 = 6.1503, P = 0.025. *P < 0.05 versus VEH and LEP by Newman–Keuls test; B, F1,3 = 1.5731, P = 0.21.
Lack of effects of ingestion of the MHF diet on Y1R/LacZ transgene expression in PVN and ARC
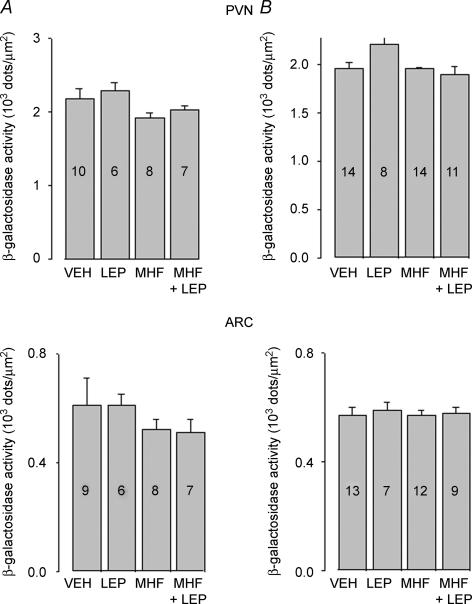
Quantitative analysis of Y1R/LacZ transgene expression in PVN and ARC demonstrated that exposure of Y1R/LacZ male mice to the MHF diet for 8 weeks did not induce significant changes on β-galactosidase expression (Fig. 6A). Leptin treatment also failed to affect β-galactosidase expression in the PVN and ARC of chow-fed and MHF diet-fed male mice (Fig. 6A). Similarly, neither the exposure to MHF diet nor the leptin treatment affected transgene expression in the PVN and ARC of female Y1R/LacZ mice (Fig. 6B).
Figure 6. Effect of exposure to a MHF diet for 8 weeks on β-galactosidase in the paraventricular (PVN) (upper panel) and arcuate (ARC) (lower panel) of male (A) or female (B) Y1R/LacZ transgenic mice.
Mice were treated as in Fig. 5. Data are expressed as density of blue dots and are means ±s.e.m.; numbers within each column indicate the number of animals used for the determination. PVN, one-way ANOVA: A, F1,3 = 2.37, P = 0.0927; B, F1,3 = 2.207, P = 0.101. ARC, one-way ANOVA: A, F1,3 = 0.675, P = 0.575; B, F1,3 = 0.9114, P = 0.1768.
Discussion
In the present study we examined the effect of a moderate to high fat, high-energy diet on the Y1R gene expression in the hypothalamus of Y1R/LacZ transgenic mice, which were created and maintained on a FVB/n inbred mouse strain. Although the susceptibility of the FVB mouse strain to obesity is not well studied, it was previously reported that FVB mice fed with a high fat diet become obese and show an increase in circulating leptin that is correlated with body weight and adiposity, indicative of leptin resistance (Frederich et al. 1995; Martin et al. 2006).
Data presented here show that, in Y1R/LacZ transgenic male mice, consumption of a MHF diet for 8 weeks promotes a significant increase of body weight that is associated with a decrease of Y1R gene expression in the DMH and VMH. Conversely, Y1R gene expression in the ARC and PVN was not altered in this obese model, despite the well documented role of ARC NPY neurons in feeding regulation. In addition, the decreased expression of Y1R/LacZ transgene was only observed in MHF diet-fed male mice but not in MHF diet-fed females, which failed to gain weight over the course of the experiment, suggesting that the susceptibility to develop obesity of male mice correlates with changes in the Y1R gene expression in the VMH and DMH.
NPY neurons of ARC, DMH and VMH belong to complex pathways involved in the control of feeding behaviour and they can be differentially regulated in response to hyperphagia and excessive body weight gain (Beck, 2006). In animal models of obesity with a deficiency in leptin signalling, ARC NPY system is up-regulated (for review see Beck, 2006; Eva et al. 2006). Conversely, in other rodent models of genetic obesity, for instance those where the hypothalamic melanocortin signalling is reduced, NPY expression was found to be decreased in ARC and increased in DMH and VMH (Kesterson et al. 1997; Guan & Van der Ploeg, 1998; Bi et al. 2001). Additionally, diet-induced obese mice showed a profound increase in the NPY mRNA expression both in the DMH and the VMH, and a decrease in the ARC (Guan et al. 1998a). It has been postulated that the activation of NPY signalling in the DMH and VMH can be a compensatory mechanism to counteract ARC NPY neuron deficiency, in order to maintain adequate food intake (Beck, 2006).
Indeed, increased neuronal activity in DMH was found to contribute to the development of obesity caused by ingestion of a high fat diet (Bellinger & Bernardis, 2002). Mice susceptible to diet-induced obesity have increased c-fos expression in DMH when fed a high fat diet (Xin et al. 2000), whereas lesions of rat DMH attenuate high fat diet-induced weight gain (Bellinger & Bernardis, 2002).
In our dietary obesity model, MHF-fed Y1R/LacZ transgenic male mice consume the same amount of food as compared with chow-fed mice in spite of the higher energy content of MHF diet. Thus, we speculate that MHF diet consumption may activate the anabolic NPYergic neurons both in the VMH and DMH, leading to the down-regulation of the Y1R.
On the other hand, the fact that we did not observe significant changes of the Y1R/LacZ transgene expression in the PVN is not surprising, given that in several rodent models of genetic and dietary obesity the expression of the NPY Y5R subtype was consistently shown to be altered in the PVN (Widdowson, 1997; Xin & Huang, 1998; Beck et al. 2001; Schaffhauser et al. 2002). The decrease of Y1R/LacZ expression in DMH and VMH may therefore only partially reflect alterations of NPY receptors in the hypothalamus of MHF-fed male mice. Measurement of NPY release in VMH, DMH and PVN could provide information on the causal relationship between changes in NPY Y1R transmission and weight gain in MHF-fed male mice.
Furthermore, since the decrease of Y1R gene expression was only observed in MHF diet-fed Y1R/LacZ transgenic mice, that develop obesity over the course of the treatment, the possibility that the down-regulation of Y1R is secondary to the weight gain cannot be excluded. Recent studies have shown that the expression of hypothalamic Y1R can be regulated in a nucleus-specific manner, independently of the activity of the NPY system (Chance et al. 2007). Thus, the changes in DMH and VMH Y1R gene transcriptional activity could be an integral part of NPY dysfunction in our dietary obesity model.
Further studies will be needed to identify the role of leptin in the susceptibility of male mice when eating a MHF diet. Previous studies have shown that obesity-prone mice exposed to a high fat diet develop leptin resistance progressively, and that leptin insensitivity becomes apparent after 8 weeks of treatment (Lin et al. 2000; Martin et al. 2006). Accordingly, we showed that leptin treatment decreases body weight of both chow-fed mice and mice fed with a MHF diet for 8 weeks, although, in the latter case, it failed to reach a fully significant effect. Conversely, we were unable to relate the leptin-induced decrease in body weight with Y1R gene expression since leptin treatment failed to prevent the decrease in Y1R/LacZ transgene expression in VMH and DMH of MHF diet-fed male mice. This finding could reflect a level of leptin resistance in MHF diet-fed male mice that, in turn, could compromise leptin-dependent regulation of VMH and DMH NPY Y1R system.
However, given that in MHF-fed mice, leptin levels presumably increase to reflect the energy status of the animals, we cannot exclude the possibility that higher doses of leptin could counteract the reduction of Y1R/LacZ transgene expression observed in DMH and VMH of male mice.
The second major finding of this study is that, by using a dietary model that enables identification of susceptible and obesity-resistant populations (Lauterio et al. 1994; Lauterio et al. 1999), we could demonstrate that the FVB mouse strain shows a sexual dimorphism in the response to high fat diet and leptin sensitivity. Y1R/LacZ transgenic female mice consumed a significantly lower amount of food than chow-fed female mice and MHF diet-fed male mice, and did not show body weight gain or changes of Y1R/LacZ transgene expression in the hypothalamus. We also demonstrated a gender-related difference in the FVB mouse strain in response to leptin treatment that failed to decrease body weight of both MHF diet- and chow-fed female mice. This observation is in line with previous studies showing that peripheral infusion of leptin produces a greater weight loss in male FVB mice than in females, and it suggests that leptin responsiveness of FVB mice is sex related (Harris et al. 2002).
The molecular mechanisms responsible for the sex difference in the effect of both the diet manipulation and leptin administration remain to be clarified. However, we could speculate that this sexual dimorphism may depend on differences in sex hormones and/or in oestrogen receptor isoform distribution. Indeed, steroid environment may affect Y1R-mediated signalling (Musso et al. 2000; Xu et al. 2000; Hill et al. 2004) as well as the expression of leptin receptors (Bennett et al. 1998) in the hypothalamus. Moreover, the expression of NPY and Y1R can be differentially regulated depending on the region-specific ratio between oestrogen receptor α and oestrogen receptor β (Musso et al. 2000; Titolo et al. 2006) and this mechanism could mediate, at least in part, the effects of oestrogen on energy balance (Acosta-Martinez et al. 2007).
On the other hand, given that gender differences in responsiveness to leptin treatment were reversed for FVB mice compared with those observed in C57BL/6J mice (Sarmiento et al. 1997; Harris et al. 2003), the smaller response to high energy diet and leptin treatment in female FVB mice might represent a peculiarity specific to the FVB/N mouse strain.
In conclusion, the novel finding of this study is that Y1R/LacZ FVB mice show a sexual dimorphism in response to a MHF, high-energy diet that affects feeding behaviour and energy intake as well as the expression of hypothalamic Y1R receptor gene in a nucleus-specific manner.
Although the physiological basis of this regulatory mechanism remains to be determined, our dietary obesity model appears to be useful to better understand the complex mechanisms involved in the gender-dependent regulation of specific gene expression patterns of distinct nuclei in the hypothalamus. The findings presented herein may therefore be of significance for understanding the overall effects of NPY Y1R transmission in the hypothalamic nuclei involved in feeding behaviour and energy homeostasis.
Acknowledgments
This work has been supported by Telethon, project no. D.82 to C.E. F.Z. was supported by a Telethon fellowship (project no. D.82 to C.E.).
References
- Acosta-Martinez M, Horton T, Levine JE. Estrogen receptors in neuropeptide Y neurons: at the crossroads of feeding and reproduction. Trends Endocrinol Metab. 2007;18:48–50. doi: 10.1016/j.tem.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Beck B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1159–1185. doi: 10.1098/rstb.2006.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B, Richy S, Dimitrov T, Stricker-Krongrad A. Opposite regulation of hypothalamic orexin and neuropeptide Y receptors and peptide expressions in obese Zucker rats. Biochem Biophys Res Commun. 2001;286:518–523. doi: 10.1006/bbrc.2001.5420. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bennett PA, Lindell K, Karlsson C, Robinson IC, Carlsson LM, Carlsson B. Differential expression and regulation of leptin receptor isoforms in the rat brain: effects of fasting and oestrogen. Neuroendocrinology. 1998;67:29–36. doi: 10.1159/000054295. [DOI] [PubMed] [Google Scholar]
- Bergen HT, Mizuno T, Taylor J, Mobbs CV. Resistance to diet-induced obesity is associated with increased proopiomelanocortin mRNA and decreased neuropeptide Y mRNA in the hypothalamus. Brain Res. 1999;851:198–203. doi: 10.1016/s0006-8993(99)02186-1. [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R254–R260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Chance WT, Xiao C, Dayal R, Sheriff S. Alteration of NPY and Y1 receptor in dorsomedial and ventromedial areas of hypothalamus in anorectic tumor-bearing rats. Peptides. 2007;28:295–301. doi: 10.1016/j.peptides.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O'Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- Dryden S, Frankish H, Wang Q, Williams G. Neuropeptide Y and energy balance: one way ahead for the treatment of obesity? Eur J Clin Invest. 1994;24:293–308. doi: 10.1111/j.1365-2362.1994.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front Neuroendocrinol. 2006;27:308–339. doi: 10.1016/j.yfrne.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998a;9:3415–3419. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain Res Mol Brain Res. 1998;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Jovanovska V, Morris MJ. Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem. 2004;88:909–916. doi: 10.1046/j.1471-4159.2003.02217.x. [DOI] [PubMed] [Google Scholar]
- Harris RB, Mitchell TD, Hebert S. Leptin-induced changes in body composition in high fat-fed mice. Exp Biol Med (Maywood) 2003;228:24–32. doi: 10.1177/153537020322800103. [DOI] [PubMed] [Google Scholar]
- Harris RB, Mitchell TD, Mynatt RL. Leptin responsiveness in mice that ectopically express agouti protein. Physiol Behav. 2002;75:159–167. doi: 10.1016/s0031-9384(01)00653-9. [DOI] [PubMed] [Google Scholar]
- Herzog H. Neuropeptide Y and energy homeostasis: insights from Y receptor knockout models. Eur J Pharmacol. 2003;480:21–29. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- Hill JW, Urban JH, Xu M, Levine JE. Estrogen induces neuropeptide Y (NPY) Y1 receptor gene expression and responsiveness to NPY in gonadotrope-enriched pituitary cell cultures. Endocrinology. 2004;145:2283–2290. doi: 10.1210/en.2003-1368. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, Landry M, Bao L, Schalling M, Koistinaho J, DeArmond SJ, Prusiner S, Gong J, Walsh JH. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Huang XF, Han M, Storlien LH. The level of NPY receptor mRNA expression in diet-induced obese and resistant mice. Brain Res Mol Brain Res. 2003;115:21–28. doi: 10.1016/s0169-328x(03)00174-8. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Hypothalamic neuropeptide Y/Y1 receptor pathway activated by a reduction in circulating leptin, but not by an increase in circulating ghrelin, contributes to hyperphagia associated with triiodothyronine-induced thyrotoxicosis. Neuroendocrinology. 2003;78:321–330. doi: 10.1159/000074885. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci U S A. 1991;88:10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. NPY and cohorts in regulating appetite, obesity and metabolic syndrome: beneficial effects of gene therapy. Neuropeptides. 2004;38:201–211. doi: 10.1016/j.npep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Ishihara A, Asahi S, Tanaka T, Ozaki S, Ihara M. Potent neuropeptide Y Y1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 1996;137:3177–3182. doi: 10.1210/endo.137.8.8754736. [DOI] [PubMed] [Google Scholar]
- Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, Fukami T, Morin N, MacNeil DJ, Van der Ploeg LH, Saga Y, Nishimura S, Ihara M. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141:1011–1016. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol Endocrinol. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- Kim EM, O'Hare E, Grace MK, Welch CC, Billington CJ, Levine AS. ARC POMC mRNA and PVN a-MSH are lower in obese relative to lean zucker rats. Brain Res. 2000;862:11–16. doi: 10.1016/s0006-8993(00)02060-6. [DOI] [PubMed] [Google Scholar]
- Lauterio TJ, Bond JP, Ulman EA. Development and characterization of a purified diet to identify obesity-susceptible and resistant rat populations. J Nutr. 1994;124:2172–2178. doi: 10.1093/jn/124.11.2172. [DOI] [PubMed] [Google Scholar]
- Lauterio TJ, Davies MJ, DeAngelo M, Peyser M, Lee J. Neuropeptide Y expression and endogenous leptin concentrations in a dietary model of obesity. Obes Res. 1999;7:498–505. doi: 10.1002/j.1550-8528.1999.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Levin BE. Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R382–R387. doi: 10.1152/ajpregu.1999.276.2.R382. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA. Dysregulation of arcuate nucleus preproneuropeptide Y mRNA in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1365–R1370. doi: 10.1152/ajpregu.1997.272.5.R1365. [DOI] [PubMed] [Google Scholar]
- Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- Musso R, Maggi A, Eva C. 17-beta-estradiol stimulates mouse neuropeptide Y-Y-1 receptor gene transcription by binding to estrogen receptor alpha in neuroblastoma cells. Neuroendocrinology. 2000;72:360–367. doi: 10.1159/000054605. [DOI] [PubMed] [Google Scholar]
- Oberto A, Mele P, Zammaretti F, Panzica G, Eva C. Evidence of altered neuropeptide Y content and neuropeptide Y1 receptor gene expression in the hypothalamus of pregnant transgenic mice. Endocrinology. 2003;144:4826–4830. doi: 10.1210/en.2003-0197. [DOI] [PubMed] [Google Scholar]
- Oberto A, Tolosano E, Brusa R, Altruda F, Panzica G, Eva C. The murine Y1 receptor 5′ upstream sequence directs cell-specific and developmentally regulated LacZ expression in transgenic mice CNS. Eur J Neurosci. 1998;10:3257–3268. doi: 10.1046/j.1460-9568.1998.00336.x. [DOI] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11:1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T. Importance of NPY Y1 receptor-mediated pathways: assessment using NPY Y1 receptor knockouts. Neuropeptides. 2004;38:267–275. doi: 10.1016/j.npep.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Sarmiento U, Benson B, Kaufman S, Ross L, Qi M, Scully S, DiPalma C. Morphologic and molecular changes induced by recombinant human leptin in the white and brown adipose tissues of C57BL/6 mice. Laboratory Invest. 1997;77:243–256. [PubMed] [Google Scholar]
- Schaffhauser AO, Madiehe AM, Braymer HD, Bray GA, York DA. Effects of a high-fat diet and strain on hypothalamic gene expression in rats. Obes Res. 2002;10:1188–1196. doi: 10.1038/oby.2002.161. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) a to ERb in clonal hypothalamic neurons. Mol Endocrinol. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- Widdowson PS. Regionally-selective down-regulation of NPY receptor subtypes in the obese Zucker rat. Relationship to the Y5 ‘feeding’ receptor. Brain Res. 1997;758:17–25. doi: 10.1016/s0006-8993(97)00160-1. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding JP, Gilbey SG, Bailey CJ, Batt RA, Williams G, Ghatei MA, Bloom SR. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology. 1993;132:1939–1944. doi: 10.1210/endo.132.5.7682936. [DOI] [PubMed] [Google Scholar]
- Wilding JP, Gilbey SG, Mannan M, Aslam N, Ghatei MA, Bloom SR. Increased neuropeptide Y content in individual hypothalamic nuclei, but not neuropeptide Y mRNA, in diet-induced obesity in rats. J Endocrinol. 1992;132:299–304. doi: 10.1677/joe.0.1320299. [DOI] [PubMed] [Google Scholar]
- Xin X, Storlien LH, Huang XF. Hypothalamic c-fos-like immunoreactivity in high-fat diet-induced obese and resistant mice. Brain Res Bull. 2000;52:235–242. doi: 10.1016/s0361-9230(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Xin XG, Huang XF. Down-regulated NPY receptor subtype-5 mRNA expression in genetically obese mouse brain. Neuroreport. 1998;9:737–741. doi: 10.1097/00001756-199803090-00032. [DOI] [PubMed] [Google Scholar]
- Xu M, Urban JH, Hill JW, Levine JE. Regulation of hypothalamic neuropeptide Y Y1 receptor gene expression during the estrous cycle: role of progesterone receptors. Endocrinology. 2000;141:3319–3327. doi: 10.1210/endo.141.9.7642. [DOI] [PubMed] [Google Scholar]
- Zammaretti F, Panzica G, Eva C. Fasting, leptin treatment, and glucose administration differentially regulate Y1 receptor gene expression in the hypothalamus of transgenic mice. Endocrinology. 2001;142:3774–3782. doi: 10.1210/endo.142.9.8404. [DOI] [PubMed] [Google Scholar]