Abstract
Glucocorticoids act primarily in a feed-forward fashion on brain to activate CNS pathways that implement wanting appropriate to physiological needs. Thus, depending on the available conditions, elevated glucocorticoids may augment the behavioural want to run, fight or feed. Although glucocorticoids stimulate intake of chow, fat and sucrose, insulin appears to sculpt calorie-associated desires toward foods high in fat, acting through hepatic branch afferents of the vagus nerve. Both conditions of reduced food allowance and chronic stress excite glucocorticoid-augmented central neural networks that may lead toward ultimate abdominal obesity.
Adrenal glucocorticoids (GC) are well known to mobilize substrate from peripheral energy depots such as muscle and fat for use in hepatic gluconeogenesis, insuring a plentiful supply of glucose for use under conditions of challenge when flight or fight may be necessary. However, GC also have marked and complementary effects on the brain, that serve to augment behaviours, autonomic and neuroendocrine outflows, and learning and memory that are particularly associated with body energy balance and maintenance of life during challenging periods. During the past decade our lab has been exploring the roles of energy stores, GC and insulin on feeding behaviours and central stress responses (Pecoraro et al. 2006).
Below we review studies that show when adrenalectomized (ADX) rats are provided with high-density sucrose solutions, the rats normalize caloric stores and central corticotropin-releasing factor (CRF) expression to those levels that are observed in sham-operated animals. Drinking the pleasurable, high density calories restores neuroendocrine, autonomic outflows and energy stores to normal in rats without GC. This finding suggested a new working model for feedback in the hypothalamo-pituitary adrenal (HPA) axis that we have since tested. Ingestion of sweet (sucrose and saccharin) and fat (lard) substances, and searching for rewarding food, and memory for it, is proportional to the circulating GC environment, again invoking the powerful effects of GC on shaping behaviours associated with feeding. GC also stimulate insulin secretion, and we have found that it is the interaction between GC and insulin that modulates the choice of fat (lard) intake. The action of insulin is on the liver, probably through insulin receptors, and mediated through hepatic branch vagal afferents to the brain to provoke lard intake. In the absence of insulin, lard eating does not persist beyond one day in diabetic rats. Thus, through a variety of different mechanisms, the GC insure caloric intake particularly intake of high-density, pleasurable calories. In the presence of chronic stressors, this type of feeding may become habitual. If increased intake of comfort foods does become a habit, abdominal obesity may result, leading to many of the current ills of our society (Dallman et al. 2007).
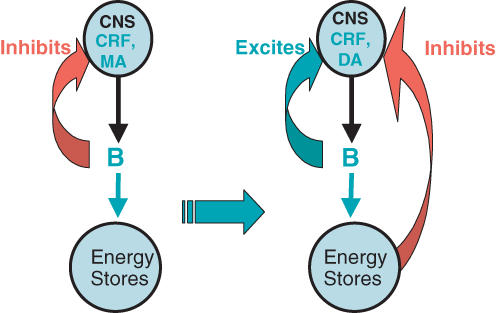
Adrenalectomy and sucrose: a new model of feedback regulation in the HPA axis (Fig. 1)
Figure 1. New working model for regulation of activity in the HPA axis.
The schema on the left shows the standard GC-mediated feedback of HPA function on the brain as well as the fact that the GC act on peripheral energy stores. The schema on the right shows our current working model. Note that the effect of GC on brain is now excitatory, and that there is a signal from energy stores that now is inhibitory on the brain and HPA axis (GC, glucocorticoids; CNS, central nervous system; CRF, corticotropin-releasing factor; MA, monoamines; DA, dopamine).
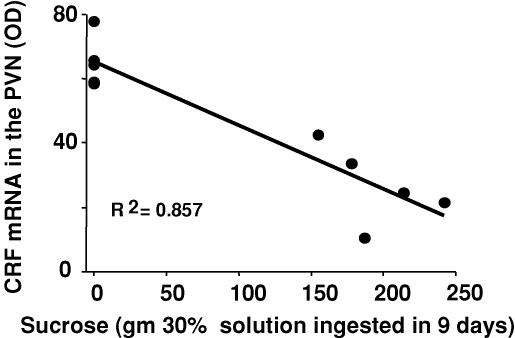
When ADX rats are given saline to drink (thus preventing sodium depletion due to loss of aldosterone) and chow to eat ad libitum, there is a constellation of metabolic consequences that occurs in the absence of GC. Male rats eat slightly less than normal, increase body weight at slower rates, have decreased fat mass and increased general sympathetic tone; all of these effects are corrected by supplying corticosterone (Akana et al. 1985; Dallman et al. 2003a). However, to our great surprise, we also found that allowing ADX rats to drink a solution of 32% sucrose, as well as chow and saline ad libitum in the absence of corticosterone, prevented the metabolic deficiencies from occurring (Bell et al. 2000; Laugero et al. 2001). Providing sucrose as well as saline to drink restored thermogenesis (as measured by uncoupling protein-1) to normal, and thus appeared to reduce sympathetic outflow induced by ADX (Bell et al. 2000). Moreover, voluntary sucrose drinking also prevented the well-known changes in central CRF that normally occur both in the hypothalamus and amygdala after ADX (Fig. 2).
Figure 2. Sucrose ingestion is inversely related to CRF mRNA in the paraventricular nuclei (PVN).
In bilaterally adrenalectomized rats, the total sucrose intake during the 9 days it was available is tightly related to the expression of hypothalamic CRF. CRF mRNA was restored to values similar to those in sham-operated rats (5 points on the right) reducing the normally elevated CRF seen in adrenalectomized rats (4 points on the left) to values similar to rats with intact adrenals (data from Laugero et al. 2001).
When trying to understand how sucrose drinking had such marked effects not only peripherally, but also on brain CRF, we found that there was a quite strong correlation (r = −0.64) with mesenteric fat mass (Dallman et al. 2003b), and we suggested the new model of feedback regulation of HPA axis function shown in Fig. 1. It appears that the brain is informed of fat storage, particularly in the mesenteric fat, and that this information reduces activity in central CRF systems. The limbic CRF system appears to be recruited by chronic stressors, and represents a critical component of the chronic stress response system (Dallman et al. 2006).
We have tested this hypothesis by providing sucrose to adrenalectomized rats replaced with corticosterone and subjected to cold. Under conditions of low, clamped corticosterone concentrations, sucrose, and increased fat mass, were important in diminishing the central responses and increasing thermogenic responses to cold (Bell et al. 2002). Although we hypothesize that there is neural feedback from fat depots to the central nervous system, this has not been identified, yet. Nonetheless, it seems clear in intact animals, as well, that increasing energy stores in the form of fat depot weight, reduces central neural responses to either acute (la Fleur et al. 2005a), or chronic (Pecoraro et al. 2004) stressors.
Some actions of glucocorticoids on brain and feeding behaviour
GC infused directly into the brain ventricular system of ADX rats excite, rather than inhibit both hypothalamic CRF and pituitary adrenocorticotropin hormone (ACTH) secretion, and they also appear to negate the effect of drinking sucrose on metabolism in these rats (Laugero et al. 2002). Thus, in brain, the GC appear to act on the HPA axis in a feed-forward, rather than in the canonical feedback fashion that is usually envisioned (see Fig. 1).
In keeping with central stimulation of CRF, the GC also stimulate behaviours associated with nutrient gain (Dallman et al. 2005). The GC appear to stimulate ongoing behaviour that is dependent on the context, and simply seem to intensify the drive to perform the behaviour; this may well be a consequence of the effects of GC on dopamine secretion in the shell of the nucleus accumbens, the so-called pleasure centre (Barrot et al. 2000). Fighting (Haller et al. 2000; Mikics et al. 2004), risk assessment (Mikics et al. 2005) search (Pecoraro et al. 2005) and running behaviours (Leshner, 1971) are all augmented by the adrenal steroids, as is feeding behaviour.
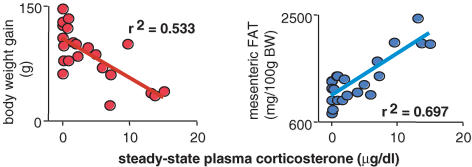
Glucocorticoids increase the voluntary intake of palatable foods in a dose-related manner (Bell et al. 2000; Bhatnagar et al. 2000; la Fleur et al. 2004); however, they do not stimulate chow intake in the presence of normal insulin concentrations (Strack et al. 1995; la Fleur et al. 2004). When ADX rats are provided with corticosterone replacement that results in steady-state concentrations in plasma, insulin concentrations increase pari passu with corticosterone (Akana et al. 1985; Strack et al. 1995; Bell et al. 2000), Together, these hormones act to increase fat storage, particularly mesenteric fat, although with high GC there is still marked peripheral catabolism and a decreased rate of body weight gain. (Fig. 3).
Figure 3. Corticosterone has opposite effects on body weight gain and mesenteric fat weight.
In adrenalectomized, corticosterone-treated rats allowed sucrose to drink ad libitum, body weight decreases (left panel) as mesenteric fat weight increases (right panel) showing a central shift of calorie storage (data from Bell et al. 2000).
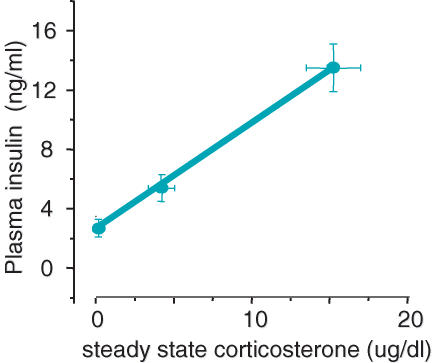
Examining the streptozotocin-diabetic rat, la Fleur showed clearly that ADX rats ate increasing amounts of chow with increasing corticosterone, but that they would not eat lard, unless they were also infused with insulin that provided low circulating concentrations (la Fleur et al. 2004). Of course, increasing GC stimulates increasing concentrations of insulin in the circulation (Fig. 4), and it may be this action of the GC that indirectly results in increased intake of pleasurable foods. The GC may provide the wanting for calories, but the increased insulin may determine what food is wanted in conditions of choice. In her studies, la Fleur also showed that specific hepatic vagal afferents to hypothalamus and amygdala were involved in insulin-induced lard intake in diabetic rats (la Fleur et al. 2003, 2005b).
Figure 4. As corticosterone increases, circulating insulin increases.
In adrenalectomized, corticosterone-treated rats allowed lard to eat ad libitum, plasma insulin increased with steady-state corticosterone concentrations. This also occurs in similarly treated rats allowed only chow, but the extent of the effect is greater when the rats are also given the choice of lard to eat (data from la Fleur et al. 2004).
Actions of insulin and the hepatic branch of the vagus nerve on lard intake
We continued to study where insulin acts to stimulate lard intake in streptozotocin-diabetic rats given a subcutaneous pellet of corticosterone that produced steady-state plasma concentrations that were slightly below the circadian maximum. Such corticosterone replacement blocks endogenous ACTH and adrenal secretion in the absence of chronic stressors (Akana et al. 1992) and stimulates high caloric intake of either chow or lard, depending on the circulating insulin concentrations (la Fleur et al. 2004).
In the first study, we compared the effects of insulin infusions into the jugular versus the superior mesenteric veins (Warne et al. 2006). With 5 days of ad libitum lard availability, both groups of insulin-infused rats ate roughly the same amount of lard as non-diabetic controls, although the vehicle-infused rats ate very little lard. Moreover, all of the diabetic rats were equally hyperphagic, although the insulin infusion resulted in decreased chow intake, compensating for the increased calories ingested as lard. Although the insulin infusions decreased circulating glucose somewhat, because the dose was low (3 U day−1), all diabetic rats were markedly hyperglycaemic. However, the jugular insulin infusion restored subcutaneous fat depot weight to normal and increased body weight markedly whereas the mesenteric insulin infusion restored mesenteric fat depot weight without increasing body weight (Warne et al. 2006). Both sites of insulin infusion reinstated lard ingestion and total intake was regulated, but the infusion site clearly determined the metabolic outcome.
Next, we compared c-Fos immunoreactivity in brains of rats that were made diabetic, given corticosterone and infused into the jugular or superior mesenteric vein with either saline or insulin. In order to obtain an acute lard-feeding stimulus, we remove lard but not chow for 8 h on the fifth day and restored it for an hour in the dark activity period before collecting brains. The results showed two patterns of c-Fos staining: one associated with circulating insulin concentrations which were the same in the insulin-infused groups, and the other associated with a large amount of lard ingestion that was only observed in the rats infused with insulin into the mesenteric vein. The lard-associated c-Fos patterns suggested that the known opiatergic ‘pleasure’ network from the nucleus of the tractus solitarius throughout the brain, including the ventral tegmental area, n. accumbens and other limbic memory sites were affected by eating appreciable amounts of lard (Warne et al. 2007b). These results suggested strongly that a vagal afferent signal stimulated by insulin accompanies lard eating.
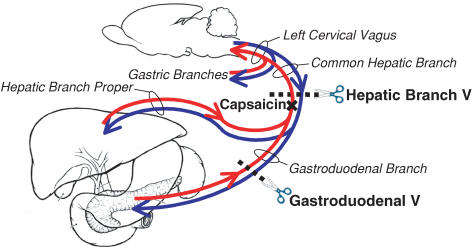
In subsequent studies that are still ongoing, we have attempted to differentiate among hepatic branch vagal afferent and efferent neural actions that might mediate the effects of insulin. Figure 5 shows a schematic design of our manipulations. Cutting the hepatic branch of the vagus (Warne et al. 2007a) restored lard intake to normal in diabetic rats without insulin, suggesting strongly that normally, in the absence of insulin, the hepatic branch of the vagus inhibits the ingestion of lard. Infusion of insulin, however, over-rides the inhibitory signal. The results of treating the hepatic branch vagus with capsaicin to denervate hepatic afferent input to brain are detailed in this special issue of The Journal of Physiology (Warne et al.).
Figure 5. Outline of paradigm to determine how the hepatic branch vagus modulates lard intake.
In published and ongoing experiments we are testing the role of the hepatic branch of the vagus (which includes afferents and efferents to both the liver and gastroduodenum), the gastroduodenal branch of the hepatic vagus, and the role of afferent nerves from both sites. The anatomy is indicated for the part of the vagus nerve of interest. Scissors and dotted lines denote severing the nerve; the x indicates the site of painting the nerve with capsaicin in an attempt to destroy afferent fibres without impairing the efferents (experiments of Warne et al. 2006).
We are continuing these studies with the anticipation that we will be able to evolve our understanding of the role(s) of the hepatic branch of the vagus nerve, both the afferent and efferent components, as well as the hepatic actions of insulin, on the drive of rats to voluntarily ingest lard. There were marked alterations in brain c-Fos patterns of expression, and we anticipate altered expression of hypothalamic and limbic brain neuromodulators as a consequence of eating lard and the various vagal manoeuvers, as was found under slightly different conditions after hepatic branch vagotomy (la Fleur et al. 2005b).
Conclusions
GC and insulin are intimately entwined in regulating both the amount and choice of food intake and caloric disposition (Dallman et al. 1993). Both act in the periphery and the central nervous system, generally in opposing directions. GC act centrally to motivate caloric intake and the learning, memories and behaviours associated with this, although they may induce neuronal apoptosis (Sapolsky, 1992). By contrast, insulin acts at the hypothalamus (Woods & Porte, 1983) as well as on the reward system (Figlewicz et al. 2004, 2006) to limit caloric intake. Insulin, like GC, promotes learning and memory but is anti-apoptotic (van der Heide et al. 2006). Peripherally, GC reduce calories stored in fat and protein and increase insulin, whereas insulin increases calories stored in both sites (Leibel et al. 1989) and also seems to act systemically through over-riding a normal inhibitory hepatic signal to promote the ingestion of lard calories (Warne et al. 2007a). Together these hormones synergize to greatly expand mesenteric fat depots, putting stored calories in a site that is highly labile and where fat can be readily mobilized by the sympathetic nervous system for immediate use by the liver.
These actions of GC and insulin are obviously highly evolutionarily useful, allowing organisms to respond to and have the stored energy available to escape threat. However, today's wealthy societies may have outstripped this usefulness for the evolved actions of these hormones. Life in the 21st century is fast-paced, food is abundant and readily available; furthermore, there is a perception that persistent stressors are increasing and may lead to stress-associated diseases (Rozanski et al. 1999). Tellingly, a recent report shows prospective evidence that job strain and poor social support is significantly associated with increased body mass index and abdominal obesity (Brunner et al. 2007). It may be that the evolutionarily key interactions between the stress hormones and insulin are now our undoing, given the current environment.
Acknowledgments
We are very grateful for the help generously given by Drs Susan F Akana, Abigail Ginsberg and Ms Hart Horneman in realizing the results of many of the experiments discussed. We also are pleased to acknowledge that the work was supported by grants from the National Institutes of Health (DA16994, DK28172).
References
- Akana SF, Cascio CS, Shinsako J, Dallman MF. Corticosterone: narrow range required for normal body and thymus weight and ACTH. Am J Physiol Regul Integr Comp Physiol. 1985;249:R527–R532. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- Akana S, Scribner K, Bradbury M, Strack A, Walker C-D, Dallman M. Feedback sensitivity of the rat hypothalamo-pituitary-adrenal axis and its capacity to adjust to exogenous corticosterone. Endocrinology. 1992;131:585–594. doi: 10.1210/endo.131.2.1322275. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF, Dallman MF. Sucrose intake and corticosterone interact with cold to modulate behavior, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. J Neuroendocrinol. 2002;14:330–342. doi: 10.1046/j.1365-2826.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF. Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol. 2000;12:461–470. doi: 10.1046/j.1365-2826.2000.00488.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF. Corticosterone facilitates saccharin intake in adrenalectomized rats. Does corticosterone increase stimulus salience? J Neuroendocrinol. 2000;12:453–460. doi: 10.1046/j.1365-2826.2000.00487.x. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II study. Am J Epidemiol. 2007;165:828–837. doi: 10.1093/aje/kwk058. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Laugero KD, Gomez F, Manalo S, Bell ME, Bhatnagar S. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003a;79:3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Pecoraro NC, Warne JP, la Fleur SE, Foster MT. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr Alzheimer Res. 2007;4:199–204. doi: 10.2174/156720507780362236. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of comfort food. Proc Soc Nat Acad Sci U S A. 2003b;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE, Warne JP, Ginsberg AB, Laugero KC, Houshyar H, Strack AM, Bhatnagar S, Bell ME. Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Frontiers Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AD, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Phys Behav. 2006;89:611–618. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Haller J, Halasz J, Mikics E, Kruk MR, Makara GB. Ultradian corticosterone rhythm and the propensity to behave aggressively in male rats. J Neuroendocrinol. 2000;12:937–940. doi: 10.1046/j.1365-2826.2000.00568.x. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Akana SF, Manalo S, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–2185. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005a;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Ji H, Manalo S, Friedman MI, Dallman MF. The hepatic vagus mediates fat-induced inhibition of diabetic hyperphagia. Diabetes. 2003;52:2321–2330. doi: 10.2337/diabetes.52.9.2321. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Manalo S, Roy M, Houshyar H, Dallman MF. Hepatic vagotomy alters limbic and hypothalamic neuropeptide responses to insulin-dependent diabetes and voluntary lard ingestion. Eur J Neurosci. 2005b;21:2733–2742. doi: 10.1111/j.1460-9568.2005.04125.x. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expresion of corticotropin-releasing factor mRNA and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001a;142:2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Gomez F, Manalo S, Dallman MF. Corticosterone infused intracerebroventricularly inhibits energy storage and stimulates the hypothalamo-pituitary axis in adrenalectomized rats drinking sucrose. Endocrinology. 2002;143:4552–4562. doi: 10.1210/en.2002-220613. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Edens NK, Fried SK. Physiologic basis for control of body fat distribution in humans. Annu Rev Nutrition. 1989;9:417–443. doi: 10.1146/annurev.nu.09.070189.002221. [DOI] [PubMed] [Google Scholar]
- Leshner AI. The adrenals and the regulatory nature of running wheel activity. Physiol Behav. 1971;6:551–558. doi: 10.1016/0031-9384(71)90204-6. [DOI] [PubMed] [Google Scholar]
- Mikics E, Barsy B, Barsvari B, Haller J. Behavioral specificity of non-genomic glucocorticoid effects in rats: effects of risk assessment in the elevated plus-maze and the open-field. Horm Behav. 2005;48:152–162. doi: 10.1016/j.yhbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Mikics E, Kruk MR, Haller J. Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology. 2004;29:618–635. doi: 10.1016/S0306-4530(03)00090-8. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Dallman MF, Warne JP, Ginsberg AB, Laugero KD, la Fleur SE, Houshyar H, Gomez F, Akana SF, Bhargava A. From Malthus to motive: how the HPA axis engineers the phenotype, yoking needs to wants. Prog Neurobiol. 2006;79:247–340. doi: 10.1016/j.pneurobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pecoraro NC, Roy M, Dallman MF. Glucocorticoids dose-dependently remodel energy stores and amplify incentive reality effects. Psychoneuroendocrinology. 2005;30:815–825. doi: 10.1016/j.psyneuen.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, the Aging Brain, and the Mechanisms of Neuron Death. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Strack AM, Sebastian RJ, Schwartz MW, Dallman MF. Glucocorticoids and insulin: reciprocal signals for energy balance. Am J Physiol Regul Integr Comp Physiol. 1995;268:R142–R149. doi: 10.1152/ajpregu.1995.268.1.R142. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the CNS: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Warne JP, Foster MT, Horneman HF, Pecoraro NC, Ginsberg AB, Akana SF, Dallman MF. Afferent signaling through the common hepatic branch of the vagus inhibits lard intake and modifies plama metabolic levels. J Physiol. 2007;583:455–467. doi: 10.1113/jphysiol.2007.135996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JP, Foster MT, Horneman HF, Pecoraro NC, Ginsberg AB, Akana SF, Dallman MF. Hepatic branch vagotomy, like insulin replacement, promotes voluntary lard intake in streptozotocin-diabetic rats. Endocrinology. 2007a;14:3288–3298. doi: 10.1210/en.2007-0003. [DOI] [PubMed] [Google Scholar]
- Warne JP, Horneman H, Ginsberg AB, Pecoraro NC, Foster MT, Akana SF, Dallman MF. Mapping brain c-Fos immunoreactivity after insulin-induced voluntary lard intake: insulin- and lard-associated patterns. J Neuroendocrinol. 2007b doi: 10.1111/j.1365-2826.2007.01593.x. in press. [DOI] [PubMed] [Google Scholar]
- Warne JP, Horneman H, Wick EC, Bhargava A, Pecoraro NC, Ginsberg AB, Akana SF, Dallman MF. Comparison of superior mesenteric versus jugular venous infusions of insulin in streptozotocin-diabetic rats on the choice of caloric intake, body weight and fat stores. Endocrinology. 2006;147:5443–5451. doi: 10.1210/en.2006-0702. [DOI] [PubMed] [Google Scholar]
- Woods SC, Porte D., Jr The role of insulin as a satiety factor in the central nervous system. Adv Metab Disord. 1983;10:457–468. doi: 10.1016/b978-0-12-027310-2.50024-4. [DOI] [PubMed] [Google Scholar]