Abstract
Phosphodiesterase type 5 (PDE5) acts specifically on cyclic guanosine monophosphate (cGMP) and terminates cGMP-mediated signalling. PDE5 has a well established role in vascular smooth muscle, where specific inhibitors of PDE5 such as sildenafil correct erectile dysfunction by augmenting cGMP-mediated vascular relaxation. However, the role of PDE5 outside of the vasculature has received little attention. The present study tested PDE5 inhibitors on the cGMP-mediated modulation of K+ channels in the neurohypophysis (posterior pituitary). Photolysis of caged-cGMP enhanced current through Ca2+-activated K+ channels, and this enhancement recovered in about 2 min. Sildenafil essentially eliminated this recovery, suggesting that the reversal of K+ current enhancement depends on cGMP breakdown. Activation of nitric oxide synthase during trains of activity in pituitary nerve terminals enhances excitability. When trains of stimulation were applied at regular intervals, sildenafil enhanced the excitability of neurohypophysial nerve terminals and increased the action potential firing probability. T-1032, a compound with high specificity for PDE5 over PDE6, had a similar action. Voltage imaging in intact neurohypophysis with a voltage sensitive absorbance dye showed that T-1032 reduced the failure of propagating action potentials during trains of activity. This indicates that PDE5 activity limits action potential propagation in neurohypophysial axons. Immunoassay of oxytocin, a neuropeptide hormone secreted by the posterior pituitary, demonstrated that sildenafil increased electrically evoked release. Thus, PDE5 plays an important role in the regulation of neurohypophysial function, and blockade of this enzyme can enhance the use-dependent facilitation of neurohypophysial secretion.
Signalling by nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) plays an important role in the relaxation of vascular smooth muscle (Ignarro, 2002). cGMP is degraded by phosphodiesterases, and the cGMP specific enzyme phosphodiesterase type 5 (PDE5) catabolizes cGMP in the vasculature. Specific inhibitors of PDE5 such as sildenafil, vardenafil and tadalafil have dramatically improved the treatment of erectile dysfunction by amplifying NO/cGMP signalling and promoting vascular relaxation (Corbin et al. 2002; Rotella, 2002; Carson & Lue, 2005). Virtually all reported actions of PDE5 inhibitors have been on vascular smooth muscle, and potential PDE5 targets outside of the vasculature have received little attention.
NO/cGMP signalling has been shown to play an important role in the modulation of ion channels and synaptic transmission (White, 1999; Ahern et al. 2002). Both NO and cGMP modulate ion channels in the peptidergic nerve terminals of the neurohypophysis (posterior pituitary) (Ahern et al. 1999; Ahern et al. 2000; Klyachko et al. 2001), a gland with especially high levels of the enzyme that initiates NO/cGMP signalling, neuronal NO synthase (Bredt et al. 1990). These nerve terminals release two neuropeptide hormones, vasopressin, which regulates cardiovascular function and blood volume, and oxytocin, which functions primarily in reproduction. When Ca2+ enters pituitary nerve terminals during intense electrical activity, NO synthase is activated. NO then activates soluble guanylate cyclase and the resulting production of cGMP and activation of cGMP-dependent protein kinase increases the activity of large conductance Ca2+-activated K+ channels (BK channels). Action potentials are then altered in a manner that reduces failures during repetitive activity, so that a train of action potentials produces a greater influx of Ca2+ (Klyachko et al. 2001). To learn more about the termination of this use-dependent facilitation, we investigated the effect of PDE5 specific inhibitors on posterior pituitary nerve terminals. A primary motivation for these experiments was an awareness that oxytocin has a number of important functions in reproduction, including major roles in sexual arousal and orgasm (Pedersen et al. 1992; Meston & Frohlich, 2000). These roles of oxytocin add to the interest of a potential involvement of PDE5 in neurohypophysial function, and in the actions of PDE5 blockers on the release of neurohypophysial hormones.
Methods
The posterior pituitary was isolated from male Sprague–Dawley rats aged 2–3 months after rendering animals unconscious by placing in a chamber with elevating levels of CO2. All procedures followed NIH guidelines for animal care and were approved by the University of Wisconsin Research Animal Resources Center. For patch clamp recordings, slices 70 μm thick were cut with a vibratome and used immediately (Klyachko et al. 2001). Slices were prepared in physiological saline consisting of (mm): 125 NaCl, 4 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 10 glucose, bubbled with 95% O2–5% CO2 (pH 7.3 when bubbled with this gas mixture). Except where noted, this solution was used to bathe tissue during experiments. Measured osmolarities for all solutions used in this study were 280–290 mosmol l−1. Patch clamp recordings were made using patch pipettes filled with (mm): 130 KCl, 10 NaCl, 10 Hepes, 4 Mg-ATP, 0.3 GTP, 2 cAMP, and 5 EGTA, with pH adjusted to 7.3 with KOH. Patch pipettes filled with this solution had resistances ranging from 4 to 6 MΩ. In current clamp experiments the patch pipette solution was modified by reducing EGTA to 0.2 mm and omitting cAMP. The reduction in EGTA allowed intracellular Ca2+ to rise and activate NO synthase. Patch clamp recordings were made with an EPC-7 patch clamp amplifier interfaced to an Apple Macintosh computer. Recordings were performed in physiological saline at room temperature (22–25°C). cGMP was applied by photolysis of caged cGMP (guanosine 3′,5′-cyclic monophosphate P-1-(2-nitrophenyl)ethyl ester; Calbiochem) added to the patch pipette solution (1 mm). Photolysis was achieved by illumination through the microscope objective with a Rapp flash lamp.
For release measurements, the whole posterior pituitary (neurointermediate lobe) was placed in a small chamber (volume ∼0.5 ml) and perfused with physiological saline supplemented with 1 mm l-arginine. Stimulus was applied to the axon bundle through a constant-current stimulus isolator with a glass micropipette (diameter 100–200 μm). Prior to stimulation, the solution was changed from the physiological saline given above to a solution with the same composition but with (mm): 20 Hepes, 0 NaHCO3, 0 NaH2PO4, with pH adjusted to 7.3 with NaOH. The perfusion was then stopped for about 15 min, and basal release was measured 5 min after this solution change. Electrical stimulation was initiated immediately thereafter, and consisted of five trains lasting 10 s applied at 1 min intervals. Each train was composed of 150–250 μA, 0.2 ms pulses at 25 Hz. Five minutes after the end of stimulation the bath was gently mixed with a micropipette and three separate 10 or 20 μl samples were collected from the vicinity of the tissue. Perfusion of the oxygenated physiological saline was restored immediately after sample collection and drug was added to the bathing solution for 30–60 min. The perfusion was then stopped and the solution was replaced with Hepes buffered saline in a repeat of the above protocol for the second round of measurements.
Samples were assayed for oxytocin with the Correlate-EIA enzyme immunoassay kit (Assay Designs, Ann Arbor, MI, USA). Absorbance at 405 nm was measured with an automated microplate reader. Concentration was determined with reference to a standard curve obtained for each sample plate.
Action potential propagation was monitored by imaging membrane potential in the intact posterior pituitary. The entire posterior pituitary was stained immediately after removal from an animal by incubating with 0.05 mg ml−1 RH482 (a voltage-sensitive absorbance dye purchased from Hayashibara, Okayama, Japan) in physiological saline for 45 min at room temperature. The posterior pituitary was then placed, intermediate lobe down, in an imaging chamber and superfused with physiological saline at room temperature. Electrical stimulation at a low frequency was initiated as soon as the placement was complete, and continued for 10 min to establish a baseline. In about half of these experiments the saline was supplemented with 1 mm l-arginine, but this supplement had no effect so we combined these data. Voltage imaging was carried out with instrumentation modelled after Wu & Cohen (1993) and described in detail by Chang & Jackson (2006). A 100 W halogen bulb provided illumination. Incident light was filtered with a 700 nm filter with a 25 nm bandpass. Transmitted light was collected with a 20× Olympus objective (N.A. = 0.95), imaged with a 464 element hexagonal photodiode fibre-optic camera, amplified to 5 mV pA−1 of photocurrent, and read into a PC with a program written in this laboratory (Chang, 2006). Signals were low-pass filtered at 5 kHz. For stimulation with trains a 100 ms high-pass filter was applied and full frames were sampled at 0.3 ms intervals. For single shock responses there was no high-pass filter and full frames were sampled at 0.2 ms intervals. A physiological saline-filled glass micropipette with a ∼50 μm tip provided monopolar stimulation from a stimulus isolator triggered by the computer used for data acquisition. Stimulation consisted of 150–200 μA, 0.2 ms pulses applied to the base of the posterior pituitary near the site of entry of the neural stalk. Optical signals corresponding to electrically evoked responses to low-frequency shocks were stable for over an hour.
Sildenafil was isolated from commercial Viagra. The water soluble fraction showed a single component by thin layer chromatography and HPLC. Mass spectrometry and NMR confirmed this to be sildenafil. One sample was purified with Sephadex chromatography (Francis et al. 2003), and a few patch clamp experiments performed with this material gave the same results. Vardenafil was isolated from commercial Levitra. T-1032 was purchased from Sigma-Aldrich and dissolved as a stock solution of 0.2 mm in DMSO. This stock was diluted > 2000-fold for use in experiments to reduce DMSO to 0.05% or less.
Results
Enhancement of whole-terminal K+ current
Whole-terminal patch clamp recordings revealed current through voltage-activated K+ channels of nerve terminals in slices of the rat posterior pituitary (Fig. 1). Previous experiments using whole-terminal recording, single-channel recording, and pharmacological manipulations established the initial transient component as current predominantly through A-type K+ channels. A large conductance Ca2+-activated K+ channel (BK channel) opens almost as rapidly as the A-current and makes a modest contribution to the peak current, but because the BK channel inactivates slowly and incompletely, it accounts for nearly all of the sustained current seen at the end of a 200 ms pulse (Bielefeldt et al. 1992). More recent work showed that the sustained component of current can be selectively enhanced by cGMP through the activation of cGMP-dependent protein kinase. Single-channel recording in excised patches demonstrated that this action of cGMP results from the modulation of BK channels (Klyachko et al. 2001).
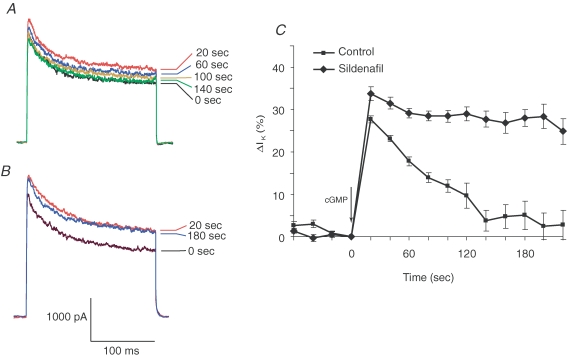
Figure 1. The effect of sildenafil on cGMP-induced enhancement of K+ current in pituitary nerve terminals.
A and B, K+ current was activated by steps from −110 mV to 50 mV. Photolysis of caged cGMP (immediately after the 0 s trace) enhanced BK channel activity. In a control experiment (A), the cGMP-activated current declined back to its original level, as indicated by the sequence of current traces recorded at regular intervals. With sildenafil (1 μm) included in the patch pipette solution (B) cGMP increased the K+ current, and the ensuing decay was almost completely blocked. A trace recorded 180 s after uncaging shows essentially no recovery. C, the time course of the cGMP effect on BK channel current (the final current at the end of a voltage step) shows the fast decay in control experiments and the almost complete block of this decay by sildenafil (means ± standard error of the mean; n= 6 for controls and for sildenafil). Current was normalized to the precGMP value at t= 0 and expressed as the percentage increase.
When whole-terminal recordings were made using a patch pipette solution that had been supplemented with caged-cGMP, a photolytic pulse of light from a flash lamp released cGMP and enhanced voltage-activated K+ current. The sustained component (at the end of the voltage pulse) due to BK channels showed the greatest increase (Fig. 1A). Test pulses applied at 20 s intervals showed that this enhancement peaked within 20 s and decayed back to baseline in about 2 min (Fig. 1A). When recordings were made with 1 μm sildenafil included in the patch pipette solution, the cGMP-induced increase in BK channel activity became essentially irreversible (Fig. 1B). The time course of current change shows this behaviour for averaged control and sildenafil experiments (Fig. 1C). Previous experiments with the non-selective phosphodiesterase inhibitor isobutylmethylxanthene indicated that a phosphodiesterase regulates the reversal of this cGMP-mediated enhancement of BK channel activity (Klyachko et al. 2001). The results in Fig. 1 suggest that this enzyme is PDE5, although phosphodiesterase type 6 (PDE6) is also sensitive to sildenafil (Carson & Lue, 2005) and experiments presented below will address this issue.
Enhancement of action potentials
Activation of the NO/cGMP signalling cascade enhances excitability in pituitary nerve terminals, increasing the probability that action potentials will fire when driven at high frequencies. This enhancement of action potentials allows stimulus trains to produce higher elevations in cytosolic Ca2+ (Klyachko et al. 2001). BK channel modulation by cGMP contributes to this enhancement of excitability by amplifying the action potential after-hyperpolarization and accelerating the recovery of voltage-gated Na+ channels from inactivation. This effect of cGMP on excitability can be initiated by the NO/cGMP signalling cascade when the intraterminal Ca2+ concentration rises during trains of activity. Since the posterior pituitary is driven in vivo by trains of impulses in characteristic ranges of frequency (Poulain & Wakerley, 1982; Nordmann & Stuenkel, 1986), we studied the responses of nerve terminals to trains of impulses under current clamp. To improve the detection of an enhancement in excitability, we adjusted the current pulse amplitude so that ∼15% of the pulses evoked action potentials (Fig. 2, top traces). The initial action potential firing probability was thus low so that increases in excitability were revealed as an increase in action potential firing. In control experiments, the initial low probability of action potential firing failed to rise as stimulus trains were applied at regular 1 min intervals (Fig. 2A, lower traces). The absence of enhancement presumably reflects the efficient degradation of cGMP during the intervals between the trains. In recordings with sildenafil the low initial action potential firing rate rose dramatically with successive trains (Fig. 2B). This increase was blocked by the NO synthase inhibitor 7-nitroindazole (7-NI) (Fig. 2C), indicating that it depends on the NO/cGMP signalling cascade. The averaged results for these experiments are shown in Fig. 2D. These experiments demonstrated that phosphodiesterase can limit the activity-dependent enhancement of excitability in posterior pituitary nerve terminals.
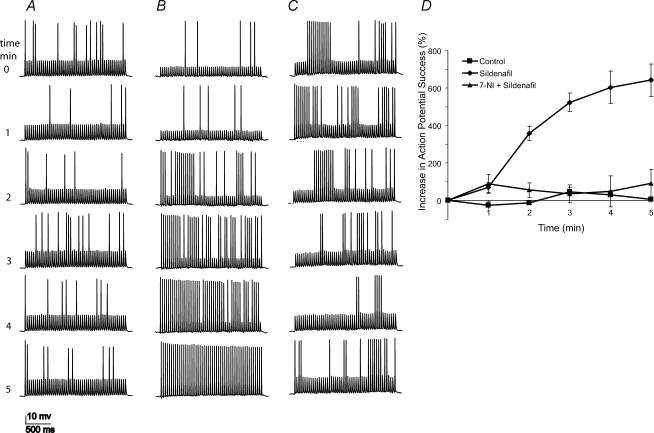
Figure 2. Sildenafil increased the action potential success rate.
A–C, stimulation with trains of pulses (train duration 2 s, frequency 25 Hz; individual pulses were 3–4 ms and 120–200 pA) at 1 min intervals shows that activity can enhance action potentials (times in minutes indicated by numbers to the left). A, in a control experiment, the initial action potential success rate remained roughly constant with successive trains. B, with sildenafil (1 μm), the action potential success rate improved dramatically. C, blockade of NO synthase with 7-NI (200 μm) prevented the enhancement of action potentials by sildenafil. D, plot of action potential success rate versus time. The action potential success rate was computed for the entire 2 s train, with the initial value subtracted and used to normalize changes. Average values were plotted with standard errors of the mean (controls, n= 6; sildenafil, n= 6; sildenafil + 7-NI, n= 7.)
Selective blockade of PDE5
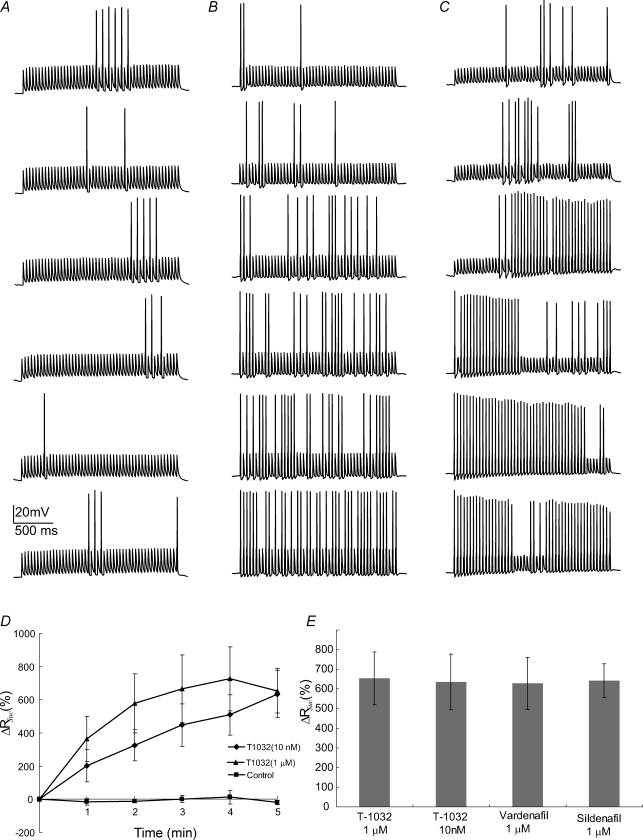
In order to evaluate the specific involvement of PDE5 we tested two additional inhibitors, T-1032 and vardenafil. Sildenafil shows a > 100-fold specificity for PDE5 over other forms of PDE, except for PDE6, for which the specificity is only 7-fold (Carson & Lue, 2005). Vardenafil has a > 460-fold preference for other isoforms but has only a 3-fold preference over PDE6. T-1032 is a particularly useful compound to evaluate a possible involvement of PDE6 because it has a 28-fold preference for PDE5 over PDE6 (Ukita et al. 2001). We found that, like sildenafil, both vardenafil (data not shown) and T-1032 (Fig. 3) promoted use-dependent enhancement of action potentials. Action potentials evoked by trains of pulses were used to compare recordings in which 1 μm T-1032 was added to the patch pipette solution (Fig. 3B). In control nerve terminals we used the same amount of DMSO but no drug (Fig. 3A). The enhancement of action potentials by T-1032 was very clear and this result is further illustrated in a plot of success rate versus train number (Fig. 3D). When the concentration of T-1032 was reduced to 10 nm, the amount of enhancement remaimed roughly the same, although the effect developed more slowly. The slower time course most likely reflects the time required for diffusion of T-1032 from the patch pipette into the nerve terminal. The increase in action potential success rate after six trains was indistinguishable for both concentrations of T-1032, as well as for sildenafil and vardenafil (Fig. 3E). Note that 10 nm T-1032 is still well above the IC50 of 1 nm for PDE5 inhibition, but well below the IC50 of 28 nm for PDE6 inhibition (Ukita et al. 2001). At 10 nm, the block of PDE6 by T-1032 should be only 26%. The equal efficacy of these two concentrations of T-1032 thus provides strong evidence that these inhibitors are acting on PDE5 rather than PDE6. These results thus establish PDE5 as an important signalling protein in terminating the activity of the NO/cGMP cascade in the posterior pituitary.
Figure 3. T-1032 altered action potential success rate.
A–C, T-1032 enhancement of action potential success rate appeared with successive trains (pulse protocol as in Fig. 2). Without T-1032 (A, control with 0.05% DMSO), the action potential success rate remained unchanged. With T-1032 (10 nm in B, and 1 μm in C), the action potential success rate increased. D, plot of action potential success rate versus time. Values were calculated in the same way as with sildenafil (Fig. 2). E, various drug additions all produced similar enhancement of action potentials for the 6th train compared to the first.
Enhancement of action potential propagation
In order to evaluate the effect of PDE5 on the propagation of action potentials during trains of activity, we imaged electrically evoked voltage changes in isolated posterior pituitary (Obaid & Salzberg, 1996). The voltage-sensitive absorbance dye, RH482, was used to provide an optical signal that enabled us to monitor the propagation of action potentials over a ∼700 μm region. Electrical stimulation elicited action potentials, which were recorded as absorbance changes through most of the imaged area (Fig. 4A). The magnitudes of the optical signals were typically ∼0.1% of the resting light intensity, which is typical for RH482 and other dyes of this class in intact neural tissue. Optical signals at a succession of four locations separated by 66 μm showed that the spikes propagate away from the site of stimulation (Fig. 4B). The colours of the traces in Fig. 4B correspond to the colours used to highlight selected traces in Fig. 4A. In Fig. 4B the propagation velocity was estimated as 0.12 m s−1 from the slope of a plot of time of peak versus distance. Note that the stimulation polarity (cathodal) produced downward signals in the vicinity of the stimulus electrode (Fig. 4A). The positive-going signals are thus action potentials, and they were reversibly blocked by 300 nm tetrodotoxin (data not shown).
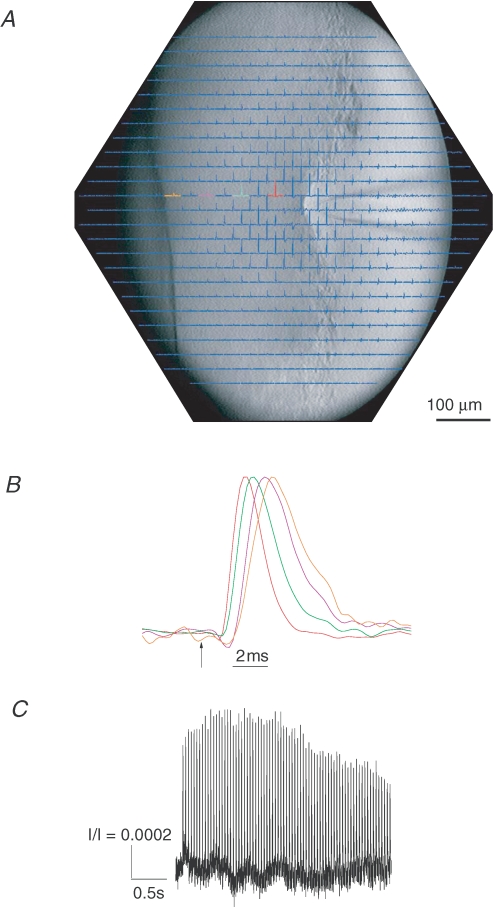
Figure 4. Propagation of action potentials through the posterior pituitary.
An array of photodiodes collected light over a 700 μm region of posterior pituitary. A, all 464 channels are displayed with the position of each signal corresponding to the site in a CCD image superimposed. Each trace is 100 ms in duration, with the stimulus applied 50 ms after the start. Each trace is an average of 4 trials at 3 s intervals. The distance between fields of adjacent diodes is 33 μm. The stimulating electrode is visible on the right and a nylon fibre that holds the preparation in place is visible on the left. Note that because of the increasing thickness of the tissue from right to left, the image is only in focus in one vertical stretch, and the left side appears darker. B, four traces from a series of locations were normalized and expanded in time to show the propagation of action potentials away from the site of stimulation. Colours of traces correspond to those in A. C, an optical response to a stimulus consisting of a single 3 s, 25 Hz train of pulses. This trace was a single trial from the location of the magenta trace in Fig. 4A, which is ∼200 μm from the stimulating electrode.
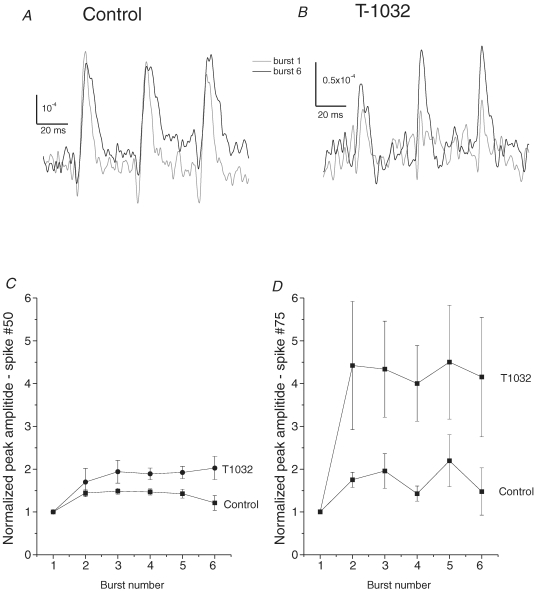
Figure 4C shows the optical response to one 3 s, 25 Hz train of pulses. As the train continued, a decline in the amplitude of the optical signals suggested that with sustained stimulation action potentials failed in a significant fraction of axons. We applied six such trains at 1 min intervals to assess the facilitation of propagation. Focusing on the final three spikes of such trains and comparing the first and sixth train, we saw weak enhancement of spikes in control experiments (Fig. 5A), and significant enhancement in experiments with 1 μm T-1032 (Fig. 5B). This enhancement was evaluated for 10 control experiments and 11 experiments in 1 μm T-1032. Either control physiological saline or 1 μm T-1032 was present from the start of the recording. In each experiment a baseline was established with responses to well-separated shocks at 1 min intervals (as in Fig. 4A and B), and after 10 min, train stimulation was initiated. The peak amplitudes of the 50th and 75th (final) spikes were then determined and averaged to generate a plot of amplitude versus burst number. The 50th spike showed a ∼40% enhancement in control experiments and ∼90% enhancement in experiments with 1 μm T-1032 (Fig. 5C). The 75th spike showed slightly greater enhancement in control experiments, but in T-1032 the 75th spike was enhanced slightly more than 4-fold (Fig. 5D). These results indicate that blockade of phosphodiesterase enhances action potential propagation in neurohypophysial axons during repeated trains of stimulation. Neither T-1032 nor sildenafil had any effect on responses to well separated shocks applied at 1 or 2 min intervals (data not shown). Thus, the enhancement of propagation by drugs was only seen with stimulation at physiologically relevant frequencies.
Figure 5. Enhancement of AP propagation through the posterior pituitary by T-1032.
The posterior pituitary was stimulated with 6 trains (3 s, 25 Hz), as in Fig. 4C, at 1 min intervals. A, the optical recordings of the final three spikes of a train are displayed for the 1st and 6th train. In this control experiment there was little enhancement of responses. B, optical recordings from an experiment in 1 μm T-1032 show that the final 3 spikes of the 6th burst of a train are greater than the final 3 spikes of the first. C and D, the 50th spikes in trains from multiple experiments were averaged to assess the effect of 1 μm T-1032 on action potential propagation. In each experiment a spatial map was prepared of the difference in spike amplitude of the 50th (C) or 75th (D) spike for the 1st versus the 5th or 6th train. Three diodes from this map showing the largest enhancement were then selected and the enhancements at these locations were averaged to produce a number for the enhancement in one experiment. The enhancements for 10 control experiments and 11 T-1032 experiments were then averaged and plotted versus burst number. The error bars are the standard errors of the mean, with n taken as the number of pituitaries for that condition.
Enhancement of evoked oxytocin release
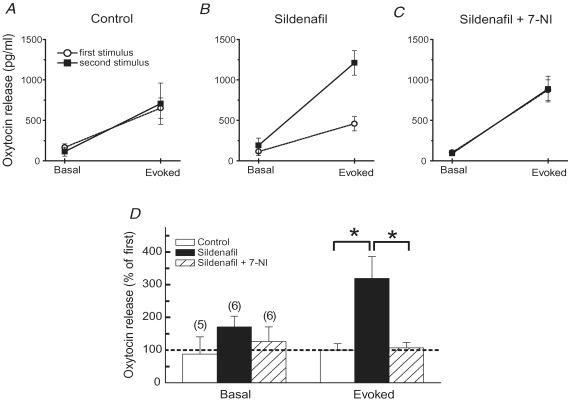
To determine the impact of these drug-induced changes in channel function and excitability on hormone secretion, we measured oxytocin release from the isolated posterior pituitary using an ELISA-based assay. Basal oxytocin release was measured first, followed by the release evoked by electrical stimulation with 25 Hz trains. The stimulus trains employed here generally increased the release of oxytocin about 5-fold over basal release. Thirty to sixty minutes after the first stimulation, basal release had returned to a level similar to that measured prior to stimulation. A subsequent stimulus train again produced the same ∼5-fold enhancement (Fig. 6A). This protocol of two successive rounds of basal and evoked release was then used to test sildenafil, applying the drug after the first round. (A higher concentration of sildenafil, 15 μm, was used in these experiments compared to the patch clamp experiments because here the drug had to diffuse into tissue whereas in the patch clamp experiments the drug was applied directly to the nerve terminal interior.) Sildenafil increased basal release slightly, and this change was not significant. This weak effect on basal release is consistent with the failure of cGMP to induce exocytosis in pituitary nerve terminals (Klyachko et al. 2001). However, sildenafil increased the stimulus-evoked release 3-fold over that seen in the first round (Fig. 6B). 7-NI blocked this action of sildenafil (Fig. 6C), indicating that the enhancement of release depended on the NO/cGMP cascade. These results are summarized in Fig. 6D, and demonstrate a clear effect of sildenafil on electrically evoked oxytocin release.
Figure 6. Sildenafil enhancement of oxytocin release from isolated posterior pituitary.
Basal and evoked release were measured in two rounds as described in Methods. A, in the control experiment basal and evoked release did not change between the two rounds. Electrical stimulation of the axon bundle (5 trains of 10 s and 25 Hz) increased release ∼5-fold over the basal level. B, adding sildenafil (15 μm) to the bathing solution after the first round increased the evoked release in the second round 3-fold. C, adding sildenafil with 7-NI (200 μm) left the release in the 2nd round identical to that in the 1st (as in the control). D, bar graph illustrating the effects of sildenafil and sildenafil + 7-NI on basal and evoked release. For control, n= 5; for sildenafil, n= 6, for sildenafil + 7-NI, n= 6. *Indicates P < 0.05 by the t test.
Discussion
This study has shown that PDE5 inhibitors act on the peptidergic nerve terminals of the posterior pituitary to promote excitability and enhance the electrically evoked release of the neuropeptide oxytocin. Although vasopressin was not tested in the present study, the high success rate of patch clamp recordings in the demonstration of enhanced excitability at the single-nerve-terminal level would suggest that PDE5 inhibitors can produce a similar enhancement of vasopressin release. The modulation of BK channels by cGMP has a unique voltage dependence that enhances the after-hyperpolarization of action potentials (Klyachko et al. 2001). By blocking the recovery of BK channel activity after enhancement by NO and cGMP, these drugs increase the magnitude of the after-hyperpolarization, and promote the recovery of Na+ channels between spikes. Action potentials are particularly susceptible to propagation failure at the large axonal varicosities of the posterior pituitary (Jackson & Zhang, 1995; Obaid & Salzberg, 1996; Muschol et al. 2003). We have found that action potential failure during 25 Hz stimulus trains is reduced by PDE5 inhibitors both in single nerve terminals (Figs 2 and 3) and in axons > 200 μm away from the site of electrical stimulation (Figs 4 and 5). The enhancement of release by PDE5 inhibitors would thus appear to result from more effective action potential invasion of the terminal arbor of the posterior pituitary. In this way sildenafil and similar drugs can enable action potentials to propagate through large varicosities, reach a greater number of nerve terminals, and trigger more release.
The molecular components of the NO/cGMP signalling cascade are widespread in nerve terminals (Bredt et al. 1990; Ahern et al. 2002; Jaffrey et al. 2002). Thus, it is likely that the signalling molecules at work in the posterior pituitary govern release dynamics in other parts of the nervous system. Some of the basic forms of plasticity seen in the posterior pituitary have been identified in other peptidergic systems (Agoston et al. 1988; Peng & Horn, 1991), where it is possible that the NO/cGMP cascade could make a similar contribution to use-dependent facilitation. To the extent that these mechanisms operate in the nervous system, sildenafil and other PDE5 inhibitors may serve as useful pharmacological tools in testing hypotheses concerning the role of use-dependent facilitation of presynaptic function.
The selective facilitation of evoked release over basal release (Fig. 6) parallels the medicinal action of sildenafil and other PDE5 inhibitors in their selectivity for responses to sexual stimulation. Sildenafil and related drugs act on the corpus cavernosa to correct erectile dysfunction (Corbin et al. 2002; Rotella, 2002). However, oxytocin is released into the circulation during orgasm (Pedersen et al. 1992; Meston & Frohlich, 2000), and the results presented here suggest that this release could be augmented by a PDE5 inhibitor. Oxytocin also serves as a signal in central neuronal pathways associated with the erectile response (Argiolas & Melis, 2005). If PDE5 inhibitors influence central oxytocin nerve terminals in the same way as peripheral nerve terminals of the posterior pituitary, then the activity of these central oxytocin pathways would be enhanced. There is now convincing evidence that PDE5 inhibitors enhance sexual function in women (Berman et al. 2003; Caruso et al. 2003; Caruso et al. 2006), thus raising the question of whether these drugs act exclusively on vascular function. The increasing recreational use of PDE5 inhibitors (Smith & Romanelli, 2005) may also be relevant to the hypothesis of additional loci of action. Thus, it is hoped that the present findings will motivate studies in humans to evaluate roles for changes in oxytocin release in connection with PDE5 blockers. Given the importance of oxytocin in a wide range of reproductive functions (Pedersen et al. 1992), it should be of interest to use PDE5 inhibitors in experiments to test the role of this enzyme, especially in cases where use-dependent facilitation has been hypothesized.
Acknowledgments
This work was supported by NIH grant NS30016.
Author's present address
V. Klyachko: Smilow Neuroscience Program, NYU School of Medicine, NY 10016, USA.
References
- Agoston DV, Conlon JM, Whittaker VP. Selective depletion of the acetylcholine and vasoactive intestinal peptide of the guinea-pig myenteric plexus by differential mobilization of distinct transmitter pools. Exp Brain Res. 1988;72:535–542. doi: 10.1007/BF00250599. [DOI] [PubMed] [Google Scholar]
- Ahern GP, Hsu S-F, Jackson MB. Direct actions of nitric oxide on rat neurohypophysial K+ channels. J Physiol. 1999;520:165–176. doi: 10.1111/j.1469-7793.1999.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern GP, Hsu S-F, Klyachko VA, Jackson MB. Induction of persistent sodium currents by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. doi: 10.1074/jbc.M003090200. [DOI] [PubMed] [Google Scholar]
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. Central control of penile erection: Role of the paraventricular nucleus of the hypothalamus. Prog Neurobiol. 2005;76:1–21. doi: 10.1016/j.pneurobio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Berman JR, Berman LA, Toler SM, Gill J, Haughie S. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol. 2003;170:2333–2338. doi: 10.1097/01.ju.0000090966.74607.34. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Rotter JL, Jackson MB. Three potassium channels in rat posterior pituitary nerve endings. J Physiol. 1992;458:41–67. doi: 10.1113/jphysiol.1992.sp019405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005;96:257–280. doi: 10.1111/j.1464-410X.2005.05614.x. [DOI] [PubMed] [Google Scholar]
- Caruso S, Intelisano G, Farina M, Di Mari L, Agnello C. The function of sildenafil on female sexual pathways: a double-blind, crossover, placebo-controlled pilot study. European J Obstetrics Gynecol Reprod Biol. 2003;110:201–206. doi: 10.1016/s0301-2115(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Caruso S, Rugolo S, Agnello C, Intelisano G, Di Mari L, Cianci A. Sildenafil improves sexual functioning in premenopausal women with type 1 diabetes who are affected by sexual arousal disorder: a double-blind, crossover, placebo-controlled pilot study. Fertil Steril. 2006;85:1496–1501. doi: 10.1016/j.fertnstert.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Chang PY. Biophysics. Madison: University of Wisconsin; 2006. Heterogeneous spatial patterns of long-term potentiation in hippocampal slices. PhD. thesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PY, Jackson MB. Heterogeneous spatial patterns of long-term potentiation in rat hippocampal slices. J Physiol. 2006;576:427–443. doi: 10.1113/jphysiol.2006.112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JD, Francis SH, Webb DJ. Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction. Urology. 2002;60(Suppl. 2B):4–11. doi: 10.1016/s0090-4295(02)01686-2. [DOI] [PubMed] [Google Scholar]
- Francis SH, Raja Sekhar K, Rouse AB, Grimes KA, Corbin JD. Single step isolation of sildenafil from commercially available Viagra tablets. Int J Impotence Res. 2003;15:369–372. doi: 10.1038/sj.ijir.3901040. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- Jackson MB, Zhang SJ. Action potential propagation and propagation block by GABA in rat posterior pituitary nerve terminals. J Physiol. 1995;483:597–611. doi: 10.1113/jphysiol.1995.sp020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey SR, Benfenati F, Snowman AM, Czernik AJ, Snyder SH. Neuronal nitric-oxide synthase localization mediated by a terniary complex with synapsin and CAPON. Proc Natl Acad Sci U S A. 2002;99:3199–3204. doi: 10.1073/pnas.261705799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko VA, Ahern GP, Jackson MB. cGMP-mediated facilitation in nerve terminals by enhancement of the spike after-hyperpolarization. Neuron. 2001;31:1015–1025. doi: 10.1016/s0896-6273(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57:1012–1030. doi: 10.1001/archpsyc.57.11.1012. [DOI] [PubMed] [Google Scholar]
- Muschol M, Kosterin P, Ichikawa M, Salzberg BM. Activity-dependent depression of excitability and calcium transients in the neurohypophysis suggests a model of ‘stuttering conduction’. J Neurosci. 2003;23:11352–11362. doi: 10.1523/JNEUROSCI.23-36-11352.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann JJ, Stuenkel EL. Electrical properties of axons and neurohypophysial nerve terminals and their relationship to secretion in the rat. J Physiol. 1986;380:521–539. doi: 10.1113/jphysiol.1986.sp016300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid AL, Salzberg BM. Micromolar 4-aminopyridine enhances invasion of a vertebrate neurosecretory terminal arborization: optical recording of action potential propagation using an ultrafast photodiode-mosfet camera and a photodiode array. J Gen Physiol. 1996;107:353–368. doi: 10.1085/jgp.107.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Jirikowski G, Insel TR, editors. Oxytocin in Maternal, Sexual, and Social Behaviors. Annals of the New York Academy of Sciences. Vol. 652. 1992. [PubMed] [Google Scholar]
- Peng YY, Horn JP. Continuous repetitive stimuli are more effective than bursts for evoking LHRH release in bullfrog sympathetic ganglia. J Neurosci. 1991;11:85–95. doi: 10.1523/JNEUROSCI.11-01-00085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurons secreting oxytocin and vasopressin. Neurosci. 1982;7:773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Rotella DP. Phosphodiesterase 5 inhibitors: current status and potential applications. Nature Rev Drug Discov. 2002;1:674–682. doi: 10.1038/nrd893. [DOI] [PubMed] [Google Scholar]
- Smith KM, Romanelli F. Recreational use and misuse of phosphodiesterase 5 inhibitors. J Am Pharm Assoc. 2005;45:63–72. [PubMed] [Google Scholar]
- Ukita T, Nakamura Y, Kubo A, Yamamoto Y, Moritani Y, Saruta K, Higashijima T, Kotera J, Takagi M, Kikkawa K, Omori K. Novel, potent, and selective phosphodiesterase 5 inhibitors: synthesis and biological activities of a series of 4-aryl-1-isoquinolinone derivatives. J Med Chem. 2001;44:2204–2218. doi: 10.1021/jm000558h. [DOI] [PubMed] [Google Scholar]
- White RE. Cyclic GMP and ion channel regulation. Adv Second Messenger Phosphoprotein Res. 1999;33:251–277. doi: 10.1016/s1040-7952(99)80013-5. [DOI] [PubMed] [Google Scholar]
- Wu JY, Cohen LB. Fast multisite optical measurements of membrane potential. In: Mason WT, editor. Fluorescent and Luminescent Probes for Biological Activity. London: Academic Press; 1993. pp. 389–404. [Google Scholar]