Abstract
This study examined cerebrovascular reactivity and ventilation during step changes in CO2 in humans. We hypothesized that: (1) end-tidal PCO2 (PET,CO2) would overestimate arterial PCO2 (Pa,CO2) during step variations in PET,CO2 and thus underestimate cerebrovascular CO2 reactivity; and (2) since PCO2 from the internal jugular vein (Pjv,CO2) better represents brain tissue PCO2, cerebrovascular CO2 reactivity would be higher when expressed against Pjv,CO2 than with Pa,CO2, and would be related to the degree of ventilatory change during hypercapnia. Incremental hypercapnia was achieved through 4 min administrations of 4% and 8% CO2. Incremental hypocapnia involved two 4 min steps of hyperventilation to change PET,CO2, in an equal and opposite direction, to that incurred during hypercapnia. Arterial and internal jugular venous blood was sampled simultaneously at baseline and during each CO2 step. Cerebrovascular reactivity to CO2 was expressed as the percentage change in blood flow velocity in the middle cerebral artery (MCAv) per mmHg change in Pa,CO2 and Pjv,CO2. During hypercapnia, but not hypocapnia, PET,CO2 overestimated Pa,CO2 by +2.4 ± 3.4 mmHg and underestimated MCAv-CO2 reactivity (P < 0.05). The hypercapnic and hypocapnic MCAv-CO2 reactivity was higher (∼97% and ∼24%, respectively) when expressed with Pjv,CO2 than Pa,CO2 (P < 0.05). The hypercapnic MCAv–Pjv,CO2 reactivity was inversely related to the increase in ventilatory change (R2= 0.43; P < 0.05), indicating that a reduced reactivity results in less central CO2 washout and greater ventilatory stimulus. Differences in the PET,CO2, Pa,CO2 and Pjv,CO2–MCAv relationships have implications for the true representation and physiological interpretation of cerebrovascular CO2 reactivity.
Measurement of cerebrovascular reactivity to CO2 has been widely applied in clinical practice to evaluate cerebral vascular function – e.g. in patients with carotid artery stenosis (Widder et al. 1994), hypertension (Serrador et al. 2005), stroke (Wijnhoud et al. 2006) and heart failure (Xie et al. 2005), and a related impairment has been linked to cerebral ischaemic events (Cosentino & Volpe, 2005; Wijnhoud et al. 2006). The acute manipulation of Pa,CO2 through hyperventilation has been used as an intervention to rapidly reduce intracranial pressure or to adjust cerebral blood flow (CBF) to metabolic needs. Furthermore, changes in cerebrovascular CO2 reactivity affect stability of the ventilatory responsiveness to CO2 via alterations in the degree of washout in central chemoreceptor hydrogen [H+]; these changes have been documented in a range of physiological (Cummings et al. 2007) and pathophysiological (Xie et al. 2005; Ainslie et al. 2007) conditions.
In most instances CBF reactivity is expressed as the percentage change in CBF per mmHg change in Pa,CO2, or end-tidal CO2(PET,CO2) obviating the more invasive Pa,CO2 measurement. The tight correlation between the percentage of change in middle cerebral artery blood flow velocity (MCAv) measured by transcranial Doppler ultrasonography during PET,CO2 variations (Markwalder et al. 1984; Ide et al. 2003) has encouraged the use of transcranial Doppler (TCD) ultrasonography to measure CO2 cerebrovascular reactivity. However, several considerations are important when using Pa,CO2 (or PET,CO2) to investigate cerebral blood flow reactivity. First, PET,CO2 has been shown to underestimate Pa,CO2 at rest (Robbins et al. 1990) and to overestimate Pa,CO2 during exercise (Jones et al. 1979; Robbins et al. 1990). Similarly, a positive PET,CO2–Pa,CO2 gradient is seen in animals exposed to increased CO2 (Jennings & Chen, 1975; Oliven et al. 1985; Tojima et al. 1988). Such alterations in the Pa,CO2–PET,CO2 relationship may have implications for the true representation and physiological interpretation of cerebrovascular reactivity to CO2. In humans, it is not known how the Pa,CO2–PET,CO2 relationship is altered throughout the hypercapnic and hypocapnic range. To address these inaccuracies, Jones et al. (1979) developed a regression equation using PET,CO2 and tidal volume to provide an estimate of Pa,CO2 (ePa,CO2) during exercise to compensate for the overestimation of Pa,CO2 by PET,CO2. Thus, we reasoned that since hypercapnia evokes an increase in ventilation (and tidal volume), this empirical equation could also be used to estimate Pa,CO2 during step changes in PET,CO2. Therefore, cerebrovascular CO2 reactivity during hypercapnia and hypocapnia could be expressed in three different ways (Pa,CO2, ePa,CO2 and PET,CO2) and compared accordingly.
The second consideration when investigating CBF reactivity is whether cerebrovascular reactivity to CO2 might be even better expressed as a percentage of brain tissue PCO2. This idea evolves from a study by Shapiro and colleagues who showed that changes in CBF corelated more closely with jugular venous CO2 tension (Pjv,CO2) than Pa,CO2 (r= 0.83 versus r= 0.72, respectively), suggesting that Pa,CO2 is not the effective stimulus for cerebral vasodilatation (Shapiro et al. 1966). In contrast, Severinghaus & Lassen (1967) provided data to indicate that brain tissue PCO2 (based on Pjv,CO2) was not the ultimate determinant of CBF during a single step of hypocapnia, and that Pa,CO2– or arterial wall PCO2– may have an important role. Whether there is a differential control of CBF via brain tissue PCO2 or Pa,CO2 during hypercapnia and hypocapnia remains to be established. With respect to ventilation, however, it is clear that the central contribution to the ventilatory response to CO2 is determined not by Pa,CO2 but by changes in brain tissue PCO2 and [H+] (Ahmad & Loeschcke, 1982; Smith et al. 2006). Thus, the stimulus at the central chemoreceptor level might also be better represented by the PCO2 of the venous cerebral outflow (Fencl, 1986; Xie et al. 2006).
Given the outlined literature, it would appear that potential difference in cerebrovascular reactivity when expressed against Pa,CO2 or Pjv,CO2, and the potential relationship to CO2 ventilatory drive, has not been fully described in humans. Therefore the aims of this study were (1) to compare the accuracy of PET,CO2 and ePa,CO2 for predicting Pa,CO2 during step hyper- and hypocapnia changes, and (2) to compare CBF and ventilatory sensitivities to Pa,CO2, Pjv,CO2, ePa,CO2 and PET,CO2. Based on the aforementioned studies, we tested two original hypotheses: first, that PET,CO2 but not ePa,CO2 would overestimate Pa,CO2 during step changes in PCO2, and therefore result in apparent lower cerebrovascular CO2 reactivity; and second that because the changes in cerebral vascular tone occurring with changes in CO2 tension serve to limit changes in brain tissue PCO2 and thus Pjv,CO2, both PET,CO2 and Pa,CO2 reactivities would be lower when compared with Pjv,CO2. We also reasoned that, if MCAv–Pjv,CO2 reactivity determines brain PCO2, in those individuals with a low MCAv–Pjv,CO2 reactivity there would theoretically be less CO2 washout at the level of the central chemoreceptors and therefore a greater ventilatory stimulus.
Methods
Subjects
Twelve healthy individuals (aged 27 ± 4 years (mean ±s.d.), body mass index 24 ± 4 kg m−2, 10 male, 2 female) volunteered for this study, which was approved by the Lower South Regional Ethics committee and conformed to the standards set by the Declaration of Helsinki. All participants received verbal and written explanations of the experimental procedures, including risks involved in the study and written informed consent was obtained. Participants were non-smokers and not on any medication and none had a known history of cardiovascular, neurological or respiratory disease.
Experimental design
Following full familiarization of each subject to the experimental protocol (excluding cannulation), participants arrived at the laboratory (> 1 week) having abstained from exercise and alcohol for 24 h, and having not consumed items containing caffeine, or a heavy meal for 4 h.
Cannulation of internal jugular vein and radial artery
Following placement of three-lead ECG and peripheral O2 saturation monitor, participants were positioned in the Trendelenberg position for placement of the internal jugular vein catheter under local anaesthesia (1% lidocaine). A 16-gauge, 5 inch catheter (Arrow International) was advanced to the right jugular bulb using the Seldinger technique and the position was confirmed by ultrasound. Another catheter (20 gauge BD Insyte) was placed, under local anaesthesia into the radial artery. Both catheters were regularly infused with normal saline (0.9% NaCl) to maintain patency. After cannulation participants rested quietly in the supine position, breathing room air, for at least 30 min to allow the setup of monitoring equipment (see below). During this time the participants also acclimatized to the breathing apparatus, after which baseline measurements were obtained. Participants then underwent the tests of cerebrovascular reactivity to CO2 described below.
Experimental protocol
CO2 vasoreactivity
Incremental hypercapnia was induced by switching the inspired gas from room air to 4% CO2 (in 21% O2 with the balance N2; Hypercapnia I) for 4 min, then 8% CO2 (in 21% O2 with the balance N2; Hypercapnia 2) for 4 min. The PET,CO2 was recorded during the final 30 s of each hypercapnic exposure. Following the incremental hypercapnia, subjects breathed room air to ensure that parameters returned to baseline values. Participants were then instructed to increase their rate and depth of breathing to generate two levels (4 min each level) of decremental hypocapnia to match, in an equal and opposite direction, the rise in PET,CO2 incurred during the incremental hypercapnia steps. Training for the voluntary hyperventilation was performed during the familiarization session, during which verbal feedback was provided to assist subjects to reach and maintain the target levels of hyperventilation. In the current study, the hypercapnia steps were conducted first to allow the individual changes in PET,CO2 to be recorded in order to determine the required PET,CO2 changes needed for the two hypocapnia steps. This order was important since previous reports indicate that prior hypocapnia (but not prior hypercapnia) may cause persistent cerebral vasoconstriction, thus influencing the normal MCAv-CO2 response to hypercapnia (Ide et al. 2003). Although it has been reported that prior hypercapnia does not influence the CBF response to hypocapnia (Ide et al. 2003), full recovery was permitted between the hyper- and hypocapnia tests to rule out any prior influence of the hypercapnia. Cerebrovascular reactivity to CO2 was expressed as the percentage (%) change in MCAv per mmHg change in PET,CO2, Pa,CO2, an empirical equation involving PET,CO2 and tidal volume (ePa,CO2) or the Pjv,CO2. As used in other studies, MCAv was expressed at the percentage change from the baseline to allow between-study comparisons and to reduce interindividual variability that is unrelated to the experimental manipulation (Ide et al. 2003; Xie et al. 2005; Ainslie et al. 2007; Cummings et al. 2007). The ePa,CO2 was calculated by using the regression equation developed by Jones et al. (1979):
where tidal volume (VT) is in litres.
Monitoring equipment
Blood flow velocity in the right middle cerebral artery was measured using a 2 MHz pulsed Doppler ultrasound system (DWL Doppler, Sterling, VA, USA) using search techniques described elsewhere (Aaslid et al. 1982). Beat-to-beat arterial blood pressure and heart rate were monitored using finger photoplethysmography (Finometer, TPD Biomedical Instrumentation, the Netherlands) and ECG, respectively. End-tidal CO2 was sampled from a leak-free mask and measured by a gas analyser (model CD-3A CO2 analyser, AEI Technologies, Pittsburgh, PA, USA). All data were acquired continuously at 200 Hz using an analog-to-digital converter (Powerlab/16SP ML795; ADInstruments, Colorado Springs, CO, USA) interfaced with a computer and were subsequently analysed using commercially available software (Chart version 5.02, ADInstruments).
Analytical measurements
Blood samples were drawn simultaneously from the arterial and jugular catheters. Timed collections were drawn twice (separated by 10 min) during baseline air breathing and during the final 30 s of each of the hypercapnic and hypocapnic challenges. Before procuring samples 1–2 ml of arterial and venous blood was aspirated from the catheter's dead space and disregarded. Arterial and venous blood was then drawn slowly over a 20 s period through the 20 G cannulas to ensure adequate flow and to avoid haemolysis. Immediately after acquisition, the arterial and venous blood samples were transferred to capillary tubes for measurement of arterial and venous blood gases (NPT7 Series, Radiometer, Copenhagen). Commercial standards were used to calibrate the blood gas analyser before starting the tests. The reproducibility of the blood gas measurements (and PET,CO2) at rest was assessed from the coefficient of variation of the difference between the two baseline collections divided by 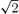 (Atkinson & Nevill, 1998). The within-test coefficient of variation for PET,CO2, Pa,CO2 and Pjv,CO2 was < 0.5%.
(Atkinson & Nevill, 1998). The within-test coefficient of variation for PET,CO2, Pa,CO2 and Pjv,CO2 was < 0.5%.
Statistical analysis
All data were analysed using the SPSS statistics package (SPSS Inc., Chicago, IL, USA). End-tidal CO2 and ePa,CO2 were averaged over the 20 s period during the simultaneous arterial and venous blood draws. Agreement between the indirect estimates of Pa,CO2 (PET,CO2, ePa,CO2) with actual Pa,CO2 during the step changes in CO2 was assessed in a number of ways. (1) Using the final data points for each incremental change in CO2, a one-way ANOVA was used to assess the statistical differences to compare absolute change in Pa,CO2 with the indirect estimates (Table 1). The Bonferroni–Dunn test was used for post hoc analysis when a significant effect was found. (2) The bias (mean absolute difference between Pa,CO2) (Bland & Altman, 1986) and relative bias (bias/Pa,CO2 value × 100%) of the end-tidal and ePa,CO2 values at baseline and during the last 30 s of each step change in CO2. Additionally, the 95% limits of agreement were calculated as ±1.96 ×s.d. of the bias (Bland & Altman, 1986). The slope of MCAv with Pa,CO2, PET,CO2 and ePa,CO2 was determined by the least squares linear regression analysis for each subject in the hyper- and hypocapnic range. Independent analysis confirmed that, in the current study, the relationship between MCAv–PCO2 change (end-tidal, arterial or jugular vein) was better described as a linear response (R2 > 0.89), rather than exponential (R2 < 0.80) or sigmoidal (R2 < 0.85). Stepwise linear regression equations were constructed with Pa,CO2 or Pjv,CO2 as the dependant variables to determine how well these correlated with PET,CO2. When each PET,CO2 value was regressed against their corresponding Pa,CO2 or Pjv,CO2 tensions, correlation coefficients of R2= 0.92 and R2= 0.79, respectively, were found (P < 0.05). It was revealed that when multiple regression analysis was performed, addition of any other physiological variable (e.g. frequency, ventilation, tidal volume) as a second independent variable did not improve the correlation. Thus, based upon the PET,CO2 responses, a multiple regression equation was constructed to predict Pa,CO2 and Pjv,CO2. Previous data (n= 14; Ainslie et al. unpublished data) from our laboratory in which Pa,CO2 and PET,CO2 were sampled (using the same experimental set-up in the supine posture) over a range of Pa,CO2 values (23–63 mmHg; 69 sample points), was used to validate the regression equation. All group data are expressed as a means ± standard deviation (s.d.). Significance was established at an alpha level of P < 0.05.
Table 1.
Haemodynamic and respiratory changes during step changes in PCO2
| Baseline | + 4% | + 8% | Hypo 1 | Hypo 2 | |
|---|---|---|---|---|---|
| MCAv (cm s−1) | 61 ± 15 | 71 ± 16 | 107 ± 23*† | 52 ± 15* | 40 ± 12*† |
| Pa,CO2 (mmHg) | 40 ± 4 | 44 ± 4 | 54 ± 4*† | 34 ± 3.1* | 23.0 ± 4.3*† |
| PET,CO2 (mmHg) | 42 ± 3 | 47 ± 3 | 60 ± 3.0*† | 36 + 4* | 23 + 4.0*† |
| ePa,CO2 (mmHg) | 42 ± 3 | 46 ± 3* | 55 ± 3*† | 36 ± 4* | 24 ± 4*† |
| Pjv,CO2 (mmHg) | 50 ± 6 | 52 ± 4 | 58 ± 5*† | 44 ± 4* | 41 ± 7*† |
| TV (l) | 0.6 ± 0.3 | 0.9 ± 0.4* | 2 ± 0.3*† | 0.9 ± 0.5* | 1.2 ± 0.5* |
| Ventilation (l min−1) | 7 ± 2 | 12 ± 4 | 33 ± 13*† | 9 ± 3 | 24 ± 9*† |
| MAP (mmHg) | 88 ± 9 | 89 ± 9 | 98 ± 11 | 86 ± 9 | 80 ± 15 |
| HR (b min−1) | 59 ± 10 | 62 ± 9 | 74 ± 8 | 59 ± 7 | 69 ± 8 |
Values are mean ±s.d.PET,CO2, end-tidal CO2; ePa,CO2, regression equation using both end-tidal PCO2 and tidal volume to estimat ePa,CO2; Pa,CO2, arterial PCO2.
Different from baseline (P < 0.05);
different from + 4 and hypo 1 (P < 0.05).
Results
Changes during hyper- and hypocapnia
Incremental hypercapnia caused a progressive increase in Pa,CO2 and MCAv whereas decremental hypocapnia caused a progressive decline in Pa,CO2 and MCAv (Table 1). The Pjv,CO2 was higher than Pa,CO2, PET,CO2 and ePa,CO2 at baseline and during the step changes in PCO2 (Table 1). There were no significant changes in either mean blood pressure or heart rate during incremental hypercapnia or hypocapnia (Table 1).
Agreement of PET,CO2 and ePa,CO2 with Pa,CO2 during changes in PCO2
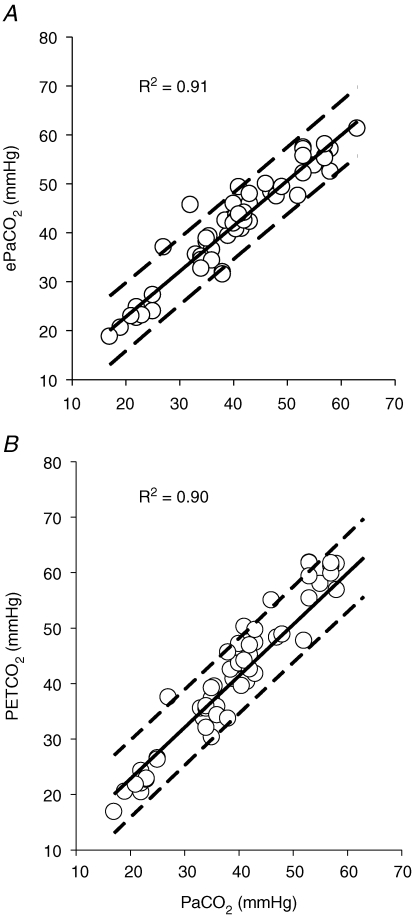
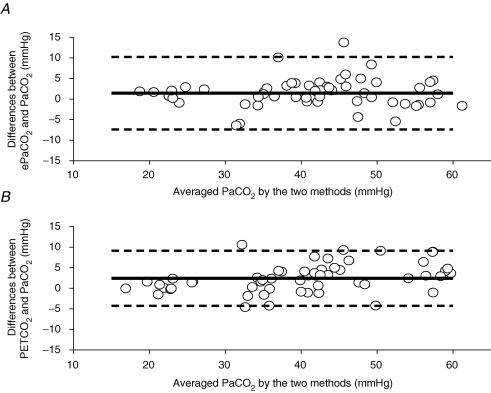
Pooled linear regressions for PET,CO2 and ePa,CO2 with Pa,CO2 during hyper- and hypocapnia using the individual values obtained for each step change in PCO2 are shown in Fig. 1. Bland and Altman analyses were carried out to compare the estimates (PET,CO2 and ePa,CO2) of Pa,CO2 the data throughout the entire range of changes in PCO2 for all the subjects. The Bland and Altman plot is presented for each indirect method in Fig. 2. The PET,CO2–Pa,CO2 difference was greater than the ePa,CO2–Pa,CO2 difference (2.4 ± 3.4 mmHg versus 1.4 ± 4.5 mmHg; P < 0.0.5), indicating an overestimation of Pa,CO2 by PET,CO2. This overestimation occurred at baseline and during hypercapnia, but not during hypocapnia (Table 2). At baseline, the limits of agreement (± 1.96 s.d. of the difference between estimated and actual Pa,CO2) were not different between ePa,CO2 (2.01 ± 3.72 mmHg) and PET,CO2 (1.95 ± 4.1 mmHg). Similarly, at baseline the relative bias for ePa,CO2 and PET,CO2 was 4.9 ± 2.1% and 5.0 ± 3.27%, respectively. However, during incremental hypercapnia, in contrast to hypocapnia, there was a progressive increase in both absolute and relative bias of PET,CO2 which was not seen with ePa,CO2 (Table 2). Stepwise multiple regression yielded the following equations to better predict Pa,CO2 or Pjv,CO2 by PET,CO2:
| (1) |
| (2) |
Figure 1. Pooled linear regressions forePa,CO2(A) and end-tidal CO2 (B) againstPa,CO2.
Data points were obtained at baseline and during the last 30 s of each step change in PCO2. Dotted lines represent 95% confidence intervals.
Figure 2. Bland and Altman plot of differences between estimates (ePa,CO2, A; end-tidal CO2, B) and measuredPa,CO2 for the whole group of subjects.
Dotted lines represent 95% confidence intervals and continuous line represents the mean bias. Individual points in the hypocapnic range are clustered near zero illustrating that there is little difference between ePa,CO2 (A) or PET,CO2 (B) and Pa,CO2 in this range. However, in the hypercapnic range the spread of values increases, indicating the values of the estimates vary further from measured Pa,CO2 values. This is clearly seen in the increase in bias in the hypercapnic steps in Table 1. These plots as well as the values in Table 1 also show that in the hypocapnic range PET,CO2 seems to better agree with measured Pa,CO2 values (lower bias), whereas in the hypercapnic range ePa,CO2 agrees better with measured Pa,CO2 values than PET,CO2.
Table 2.
Comparison of absolute and relative bias of estimate of PCO2 at baseline and during step changes in PCO2
| Pa,CO2 | PET,CO2 | ePa,CO2 | |
| Baseline | |||
| Mean ±s.d. (mmHg) | 40 ± 4 | 42 ± 3* | 42 ± 3* |
| B ± lA (mmHg) | — | 2 ± 4 | 2 ± 4 |
| RB ±s.d. (%) | — | 5 ± 2 | 5 ± 3 |
| 4% CO2 | |||
| Mean ±s.d. (mmHg) | 43 ± 4 | 47 ± 3* | 46 ± 3 |
| B ± lA (mmHg) | — | 4 ± 7 | 2 ± 6 |
| RB ±s.d. (%) | — | 8 ± 4 | 5 ± 3 |
| 8% CO2 | |||
| Mean ±s.d. (mmHg) | 55 ± 4 | 60 ± 3*† | 56 ± 3 |
| B ± lA (mmHg) | — | 5 ± 6 | 0.2 ± 6 |
| RB ±s.d. (%) | — | 9 ± 3 | 0.4 ± 3 |
| Hypo 1 | |||
| Mean ±s.d. (mmHg) | 35 ± 3 | 36 ± 4 | 34 ± 3 |
| B ± lA (mmHg) | — | 2 ± 9 | 2 ± 12 |
| RB ±s.d. (%) | — | 5 ± 5 | 5 ± 6 |
| Hypo 2 | |||
| Mean ±s.d. (mmHg) | 23 ± 4 | 23 ± 4 | 24 ± 4 |
| B ± lA (mmHg) | — | 0.3 ± 3 | 0.9 ± 3 |
| RB ±s.d. (%) | — | 1 ± 1 | 4 ± 1 |
Values are mean ±s.d.PET,CO2, end-tidal CO2; ePa,CO2, regression equation using both end-tidal PCO2 and tidal volume to estimat ePa,CO2; Pa,CO2, arterial PCO2; s.d., standard deviation; RB, relative bias; B, bias; LA, 95% limits of agreement (±1.96 ×s.d. of the bias).
Different from Pa,CO2 (P < 0.05);
different from ePa,CO2 (P < 0.05).
Addition of another physiological variable (e.g. frequency, ventilation, tidal volume) as a second independent variable did not increase the precision of either regression equation. The developed regression equation was used to predict Pa,CO2 from PET,CO2 from previously collected data in our laboratory using the same experimental set-up in the supine posture. Predicted values for Pa,CO2 were close to the measured values (R2= 0.97; P < 0.05): the comparison of the predicted and measured values for Pa,CO2 showed a s.d. of 0.87 Torr.
Comparison of cerebrovascular and ventilatory CO2 reactivity determined with end-tidal, arterial PCO2 or internal jugular vein PCO2
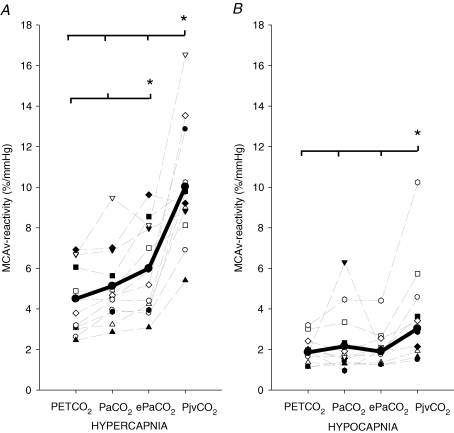
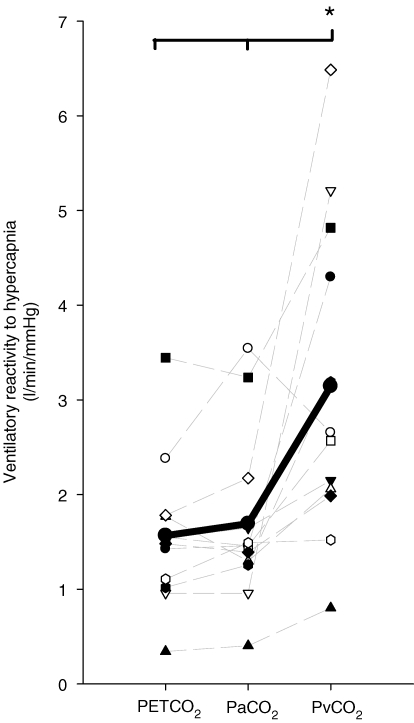
The MCAv-CO2 reactivity in the hyper- and hypocapnic range as determined with Pa,CO2, PET,CO2, ePa,CO2 or Pjv,CO2 is displayed in Fig. 3. In both the hyper- and hypocapnia range, MCAv–Pjv,CO2 reactivity was higher than with all other methods. Moreover, this reactivity was ∼97% greater in the hypercapnia range when compared with a ∼24% increase in the hypocapnia range. During hypercapnia, in contrast to hypocapnia, the slope of the MCAv–PET,CO2 relationship was smaller than the slopes of both the MCAv–ePa,CO2 and MCAv–Pa,CO2 relationships (P < 0.05; Fig. 3). The slope of the ventilatory response to hypercapnia was also steeper when presented against Pjv,CO2 than PET,CO2 or Pa,CO2 (3.0 ± 1.8 l min−1 mmHg−1, 1.6 ± 0.8 l min−1 mmHg−1 and 1.7 ± 0.9 l min−1 mmHg−1, respectively; P < 0.05; Fig. 4). In addition to the elevation in the slope of MCAv–Pjv,CO2, as expected there was a significant rightward shift in the x-axis intercept of the ventilation–Pjv,CO2 plot when compared with the ventilation–Pa,CO2 and ventilation–PET,CO2 plot (46.3 ± 6.8 mmHg, 34.6 ± 4.9 mmHg and 37.0 ± 4.8 mmHg, respectively; P < 0.05). During hypercapnia, the MCAv–Pjv,CO2 reactivity was related to the degree of ventilatory change (R2= 0.43; P < 0.05). No relationships were evident between MCAv–Pa,CO2 reactivity and the degree of ventilatory change.
Figure 3. Calculation of cerebrovascular CO2 reactivity usingPET,CO2, Pa,CO2, ePa,CO2andPjv,CO2during hypercapnia (A) and hypocapnia (B).
Each faint line represents an individual reactivity. Bold lines represent group average. Note: Pjv,CO2–MCAv reactivity was higher during both hypercapnia (∼97%, A) and hypocapnia (∼24%, B) when compared with Pa,CO2–MCAv reactivity. There seemed to be three visual outliers in both A and B; however, upon closer examination, these data points are within 2 s.d. of the sample mean and therefore not classed as statistical outliers. Further, these data points do not reflect any non-compliance or technical error, and all other data (i.e. heart rate, MCAv, BP, end-tidal CO2) were normal during each test.
Figure 4. Calculation of ventilatory CO2 reactivity usingPET,CO2, Pa,CO2, ePa,CO2andPjv,CO2during hypercapnia.
Each faint line represents an individual reactivity. Bold line represents group average.
Discussion
The novel integration of ventilation, CBF, and end-tidal, arterial and jugular vein PCO2 during step steady-state changes in CO2 has produced three new findings: (1) there is a systemic overestimation of Pa,CO2 by PET,CO2 during hypercapnia but not hypocapnia; (2) cerebrovascular and ventilatory CO2 reactivity to hypercapnia are underestimated by PET,CO2 when compared with Pa,CO2, whereas both PET,CO2 and Pa,CO2 reactivities are lower when compared with Pjv,CO2; and (3) during hypercapnia, the slope of the MCAv–Pjv,CO2 relationship was inversely related to the degree of ventilatory change, possibly because of the reduced CO2 washout occurring with decreasing MCAv reactivity, leading ultimately a greater ventilatory stimulus at the central chemoreceptors. Collectively, these findings suggest that knowledge of the changes in cerebral tissue or venous PCO2 is critical for the correct interpretation of data related to the sensitivities of both cerebral blood flow and ventilation to CO2, as well as their corelationship.
Arterial versus end-tidal CO2 monitoring
Our data indicate that caution is required when assuming that PET,CO2 accurately reflects Pa,CO2 during hypercapnia. To circumvent the problem that PET,CO2 significantly overestimates Pa,CO2 during exercise, a regression equation using both PET,CO2 and tidal volume to estimate Pa,CO2 was developed (Jones et al. 1979). It should be noted that the PET,CO2–Pa,CO2 gradient is relatively fixed in one body position with constancy of respiratory rate, minute volume, and circulatory stability. A change in any of these variables affects the PET,CO2–Pa,CO2 gradient; this has been quantified for changes in pulmonary dead-space (Bjurstedt et al. 1962) and with ventilation–perfusion ratio changes induced by standing (Immink et al. 2006), whereas the relationship between dynamic changes in pulmonary and circulatory variables and PET,CO2 is nonlinear and complex (Gisolf et al. 2004). Based on our data set, limited to the supine posture with controlled changes in CO2, we developed a new regression equation to provide a better estimate of Pa,CO2 during supine resting conditions (Pa,CO2= 2.367 + 0.884 PET,CO2). In contrast to exercise (Jones et al. 1979), tidal volume was not required as a second independent variable to increase the precision of either regression equation. A possible reason for this difference is that the magnitude of the tidal volume change evoked by hypercapnia in the supine posture is lower than that during upright exercise and insufficient to affect the PET,CO2–Pa,CO2 difference.
Our observation that PET,CO2 overestimates Pa,CO2 during steady state increases in CO2, is consistent with several human studies using CO2 rebreathing (Denison et al. 1969a,b; Laszlo et al. 1971). Although this PET,CO2–Pa,CO2 overestimation was present at comparable time points to the current study, Laszlo et al. (1971) considered this to be a transient phenomenon since the PET,CO2 and Pa,CO2 were similar after 10 min. Also, in animals PET,CO2 overestimates Pa,CO2 during rebreathing (Guyatt et al. 1973) and steady-state methods (Jennings & Chen, 1975; Tojima et al. 1988) of CO2 administration. Whilst it appears that a definitive mechanism for the PET,CO2–Pa,CO2 gradient has not been established, Gurtner & Traystman (1979) proposed the ‘charged membrane hypothesis’, which ultimately results in the production of CO2 in the region of the negatively charged alveolar capillary wall and in turn creates a positive gradient for CO2 between the alveolar gas and pulmonary arterial blood.
One noteworthy finding of the present study was that PET,CO2 was in good agreement with Pa,CO2 during hypocapnia. The physiological basis for this effect is unclear. Based on previous literature (Domino et al. 1993; Dorrington & Talbot, 2004), hypocapnia might be expected to increase the PET,CO2–Pa,CO2 gradient by increasing the venous admixture, an effect owing to hypocapnia-induced bronchoconstriction (Oliven et al. 1985) and pulmonary vasodilatation (Dorrington & Talbot, 2004). From our current data, we cannot provide further mechanistic insight to explain this disparity; however, because Pa,CO2 was highly reproducible at baseline, and selective changes occurred between hypercapnia (increase in PET,CO2–Pa,CO2 gradient) and hypocapnia (decrease in PET,CO2–Pa,CO2 gradient), it seems unlikely that this finding is due to technical artifact.
Arterial or internal jugular vein PCO2 as the independant variable for cerebrovascular reactivity testing?
Our data are consistent with earlier human-based reports which have indicated that CO2 inhalation causes an increase in internal jugular venous PCO2 to a lesser degree than the corresponding increase in systemic arterial blood (Kety & Schmidt, 1948; Fencl, 1986). As mentioned, Shapiro et al. (1966) showed a higher correlation of jugular venous CO2 tension, when compared with arterial PCO2, with the associated changes in CBF during elevations in CO2, indicating that brain tissue CO2 tension, rather than Pa,CO2, provides a better correlate of the physiological stimulus. Conversely, during hypocapnia experimental data indicate that Pa,CO2 (or arterial wall PCO2) may have a more important role than Pjv,CO2 (Severinghaus & Lassen, 1967). In the current study, cerebrovascular CO2 reactivity was higher in both the hyper- and hypocapnic range when presented against Pjv,CO2 when compared with Pa,CO2. It is noteworthy that this increase in Pjv,CO2–CO2 reactivity was highest in the hypercapnic range (∼97%) compared with the hypocapnic range (∼24%). It is tempting to speculate that these hyper-/hypocapnic reactivity differences may reflect a differential control of CBF via brain tissue PCO2 or Pa,CO2 during hypercapnia (Shapiro et al. 1966) and hypocapnia (Severinghaus & Lassen, 1967); however, it should be acknowledged that, whilst Pjv,CO2 is likely to be a closer index of brain tissue PCO2 than Pa,CO2 (Fencl, 1986; Xie et al. 2006), clarifying the mechanisms involved is difficult knowing only arterial–venous PCO2 gradients and not information related to the CO2 flux across the brain.
Consistent with previous reports (Ide et al. 2003; Xie et al. 2005, 2006; Cummings et al. 2007), MCAv-CO2 reactivity was greater in the hypercapnic range than the hypocapnic range (Fig. 4). One logical possibility is that the greater CBF response with hypercapnia limits the change in brain tissue PCO2 and thus Pjv,CO2. In the hypocapnic range, a reduced CBF reactivity suggests a greater change in Pjv,CO2 with changes in Pa,CO2 and thus a lesser slope in the Pjv,CO2–CBF response (i.e. the denominator (ΔPjv,CO2) for ΔMCAv/ΔPjv,CO2 gets higher). The mechanisms underlying this greater reactivity to hypercapnia compared with hypocapnia may be related to a greater influence of vasodilator mediators on intracranial vascular tone compared with vasoconstrictive mediators (Toda & Okamura, 1998).
Integration of ventilation and cerebral blood flow
Changes in cerebrovascular CO2 reactivity affect stability of the ventilatory responsiveness to CO2 via alterations in the degree of washout in central chemoreceptor hydrogen [H+] (Xie et al. 2006; Ainslie et al. 2007; Cummings et al. 2007). In humans, to avoid the need for invasive procedures, these ventilatory and cerebrovascular responses to CO2 are typically estimated using a change Pa,CO2 as an index. One important consideration is that the medullary central chemoreceptors are not stimulated directly by Pa,CO2; rather, they are simulated by [H+] via alterations in brain tissue CO2 tension. This stimulus at the central chemoreceptor level is therefore more likely to be better represented by the PCO2 of the venous cerebral outflow or by a mean of arterial and cerebral venous PCO2 (Fencl, 1986; Xie et al. 2006). In further support of this, during hypercapnia, MCAv–Pjv,CO2 reactivity (but not MCAv–Pa,CO2 reactivity) was inversely related to the increase in ventilation (R2= 0.43; P < 0.05). Whilst not establishing cause-and-effect, we interpret these findings to indicate that, in those individuals with a reduced MCAv–Pjv,CO2, there is less CO2 washout at the level of the central chemoreceptors and therefore a greater ventilatory stimulus. If the changes in MCAv reflect the changes in the posterior circulation supplying the medulla (see Methodological considerations), then it seems reasonable to assume that differences in CBF reactivity and related CO2 washout may cause changes in central chemoreceptor stimulation. These findings support the conclusion that cerebrovascular responsiveness to CO2 is an important determinant of eupnoeic ventilation and of hypercapnic ventilatory responsiveness in humans, primarily via its effects at the level of the central chemoreceptors (Xie et al. 2006).
Methodological considerations
Although transcranial Doppler ultrasound measures flow velocity, rather than blood flow, in the MCA, the majority of research suggests that MCAv is a reliable index of regional CBF due to little or no change in the diameter of the MCA (Giller et al. 1993; Valdueza et al. 1997; Serrador et al. 2000). Our reported values for cerebrovascular CO2 reactivity are consistent with those reported previously in healthy humans using MRI (van der Zande et al. 2005; Wise et al. 2007). An important consideration is whether steady-state MCAv was reached during the last minute of each of the 4 min hypo- hypercapnia steps. The MCAv changes and reactivity reported in this study are comparable with those of previous studies using 90 s increments in end-tidal CO2 (Ide et al. 2003) or using 5 min incremental steps of inspired CO2 (Xie et al. 2006), suggesting that the 240 s steps of hypercapnia is sufficiently long for the MCAv responses to unfold. With transcranial Doppler, it is possible to assess only the major changes in regional CBF-CO2 reactivity; however, it is known that the cerebrovascular responses to CO2 are qualitatively similar throughout the brain, although more pronounced in grey versus white matter and in cerebellar and brainstem regions versus cortical areas (Ramsay et al. 1993; van der Zande et al. 2005). It seems reasonable to suggest that changes in regional MCAv reactivity should reflect blood flow in other region of the brain, including the posterior circulation supplying the medulla, where the change of CO2 and pH is directly sensed by the central chemoreceptors and thereby affects the respiratory neurons (Kawai et al. 1996). Previous data from our laboratory indicate that during step changes in PCO2, there is a close relationship (R2= 0.82; Ainslie et al. unpublished data) between the changes in MCAv (regional) and global CBF as determined using the Fick equation (CBF = cerebral metabolic oxygen consumption/arterio-venous O2 content difference), assuming that cerebral metabolic oxygen consumption was unchanged during changes in Pa,CO2. This technique and related assumptions has been reported in detail previously (Wasserman & Patterson, 1961; Shapiro et al. 1966; Severinghaus & Lassen, 1967). Therefore, in the present study, it seems reasonable to assume that changes in MCAv adequately reflect global changes in CBF.
In summary, there is a systematic overestimation of Pa,CO2 by PET,CO2 during step changes in PCO2; this overestimation results in a lower MCAv-CO2 reactivity. Both PET,CO2 and Pa,CO2 reactivities, however, are lower when compared with MCAv–Pjv,CO2 reactivity. During hypercapnia, a lower MCAv–Pjv,CO2 reactivity may result in less CO2 washout and greater ventilatory stimulus and the level of the central chemoreceptors. Differences in these relationships have implications for the true representation and interpretation of cerebrovascular CO2 reactivity. These results may serve as the point of reference for ‘normal’ responses for comparison against pathological disorders.
Acknowledgments
The authors wish to thank Dr Kevin Cummings for critical feedback and review of the manuscript. Financial support from the Department of Physiology, University of Otago.
References
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Ahmad HR, Loeschcke HH. Transient and steady state responses of pulmonary ventilation to the medullary extracellular pH after approximately rectangular changes in alveolar PCO2. Pflugers Arch. 1982;395:285–292. doi: 10.1007/BF00580791. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Burgess K, Subedi P, Burgess KR. Alterations in cerebral dynamics at high altitude following partial acclimatization in humans: wakefulness and sleep. J Appl Physiol. 2007;102:658–664. doi: 10.1152/japplphysiol.00911.2006. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- Bjurstedt H, Hesser CM, Liljestrand G, Matell G. Effects of posture on alveolar-arterial CO2 and O2 differences and on alveolar dead space in man. Acta Physiol Scand. 1962;54:65–82. doi: 10.1111/j.1748-1716.1962.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Cosentino F, Volpe M. Hypertension, stroke, and endothelium. Curr Hypertens Rep. 2005;7:68–71. doi: 10.1007/s11906-005-0057-5. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Swart M, Ainslie PN. Morning attenuation in cerebrovascular CO2 reactivity in humans is associated with a lowered cerebral oxygenation and an augmented ventilatory response to CO2. J Appl Physiol. 2007;102:1891–1898. doi: 10.1152/japplphysiol.01437.2006. [DOI] [PubMed] [Google Scholar]
- Denison D, Edwards R, Jones G, Pope H. Comparison of rebreathing estimates with direct measurements of mixed venous PCO2 and PO2 in man. J Physiol. 1969a;202:75–76. [PubMed] [Google Scholar]
- Denison D, Edwards R, Jones G, Pope H. Direct and rebreathing estimates of the O2 and CO2 pressures in mixed venous blood. Respir Physiol. 1969b;7:326–334. doi: 10.1016/0034-5687(69)90016-4. [DOI] [PubMed] [Google Scholar]
- Domino K, Swenson E, Polissar N, Lu Y, Eisenstein B, Hlastala M. Effect of inspired CO2 on ventilation and perfusion heterogeneity in hyperventilated dogs. J Appl Physiol. 1993;75:1306–1314. doi: 10.1152/jappl.1993.75.3.1306. [DOI] [PubMed] [Google Scholar]
- Dorrington K, Talbot N. Human pulmonary vascular responses to hypoxia and hypercapnia. Pflugers Arch. 2004;449:1–15. doi: 10.1007/s00424-004-1296-z. [DOI] [PubMed] [Google Scholar]
- Fencl V. Acid–Base Blance in Cerebral Fluids. Baltimore, MD, USA: Weaverly Press; 1986. [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. [PubMed] [Google Scholar]
- Gisolf J, Wilders R, Immink R, Van Lieshout J, Karemaker J. Tidal volume, cardiac output and functional residual capacity determine end-tidal CO2 transient during standing up in humans. J Physiol. 2004;554:579–590. doi: 10.1113/jphysiol.2003.056895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner G, Traystman R. Gas-to-blood PCO2 differences during severe hypercapnia. J Appl Physiol. 1979;47:67–71. doi: 10.1152/jappl.1979.47.1.67. [DOI] [PubMed] [Google Scholar]
- Guyatt A, Yu C, Lutherer B, Otis A. Studies on alveolar-mixed venous CO2 and O2 gradients in the rebreathing dog lung. Respir Physiol. 1973;17:178–194. doi: 10.1016/0034-5687(73)90060-1. [DOI] [PubMed] [Google Scholar]
- Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 2003;95:129–137. doi: 10.1152/japplphysiol.01186.2002. [DOI] [PubMed] [Google Scholar]
- Immink RV, Secher NH, Roos CM, Pott F, van Madsen PL, Lieshout JJ. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol. 2006;96:609–614. doi: 10.1007/s00421-006-0136-6. [DOI] [PubMed] [Google Scholar]
- Jennings D, Chen C. Negative arterial-mixed expired PCO2 gradient during acute and chronic hypercapnia. J Appl Physiol. 1975;38:382–388. doi: 10.1152/jappl.1975.38.3.382. [DOI] [PubMed] [Google Scholar]
- Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial PCO2 in exercise. J Appl Physiol. 1979;47:954–960. doi: 10.1152/jappl.1979.47.5.954. [DOI] [PubMed] [Google Scholar]
- Kawai A, Ballantyne D, Muckenhoff K, Scheid P. Chemosensitive medullary neurones in the brainstem–spinal cord preparation of the neonatal rat. J Physiol. 1996;492:277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo G, Clark T, Pope H, Campbell E. Differences between alveolar and arterial PCO2 during rebreathing experiments in resting human subjects. Respir Physiol. 1971;12:36–52. doi: 10.1016/0034-5687(71)90099-5. [DOI] [PubMed] [Google Scholar]
- Markwalder TM, Grolimund P, Seiler RW, Roth F, Aaslid R. Dependency of blood flow velocity in the middle cerebral artery on end-tidal carbon dioxide partial pressure – a transcranial ultrasound Doppler study. J Cereb Blood Flow Metab. 1984;4:368–372. doi: 10.1038/jcbfm.1984.54. [DOI] [PubMed] [Google Scholar]
- Oliven A, Cherniack N, Deal E, Kelsen S. The effects of acute bronchoconstriction on respiratory activity in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;131:236–241. doi: 10.1164/arrd.1985.131.2.236. [DOI] [PubMed] [Google Scholar]
- Ramsay SC, Murphy K, Shea SA, Friston KJ, Lammertsma AA, Clark JC, Adams L, Guz A, Frackowiak RS. Changes in global cerebral blood flow in humans: effect on regional cerebral blood flow during a neural activation task. J Physiol. 1993;471:521–534. doi: 10.1113/jphysiol.1993.sp019913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PA, Conway J, Cunningham DA, Khamnei S, Paterson DJ. A comparison of indirect methods for continuous estimation of arterial PCO2 in men. J Appl Physiol. 1990;68:1727–1731. doi: 10.1152/jappl.1990.68.4.1727. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol. 2005;98:151–159. doi: 10.1152/japplphysiol.00471.2004. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW, Lassen N. Step hypocapnia to separate arterial from tissue PCO2 in the regulation of cerebral blood flow. Circ Res. 1967;20:272–278. doi: 10.1161/01.res.20.2.272. [DOI] [PubMed] [Google Scholar]
- Shapiro W, Wasserman AJ, Patterson JJ. Mechanism and pattern of human cerebrovascular regulation after rapid changes in blood CO2 tension. J Clin Invest. 1966;45:913–922. doi: 10.1172/JCI105406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Rodman J, Chenuel B, Henderson K, Dempsey JA. Response time and sensitivity of the ventilatory response to CO2 in unanesthetized intact dogs: central vs. peripheral chemoreceptors. J Appl Physiol. 2006;100:13–19. doi: 10.1152/japplphysiol.00926.2005. [DOI] [PubMed] [Google Scholar]
- Toda N, Okamura T. Cerebral vasodilators. Jpn J Pharmacol. 1998;76:349–367. doi: 10.1254/jjp.76.349. [DOI] [PubMed] [Google Scholar]
- Tojima H, Kuriyama T, Fukuda Y. Arterial to end-tidal PCO2 difference varies with different ventilatory conditions during steady state hypercapnia in the rat. Jpn J Physiol. 1988;38:445–457. doi: 10.2170/jjphysiol.38.445. [DOI] [PubMed] [Google Scholar]
- Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhaupl KM. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. AJNR Am J Neuroradiol. 1997;18:1929–1934. [PMC free article] [PubMed] [Google Scholar]
- van der Zande FH, Hofman PA, Backes WH. Mapping hypercapnia-induced cerebrovascular reactivity using BOLD MRI. Neuroradiology. 2005;47:114–120. doi: 10.1007/s00234-004-1274-3. [DOI] [PubMed] [Google Scholar]
- Wasserman AJ, Patterson JL., Jr The cerebral vascular response to reduction in arterial carbon dioxide tension. J Clin Invest. 1961;40:1297–1303. doi: 10.1172/JCI104359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widder B, Kleiser B, Krapf H. Course of cerebrovascular reactivity in patients with carotid artery occlusions. Stroke. 1994;25:1963–1967. doi: 10.1161/01.str.25.10.1963. [DOI] [PubMed] [Google Scholar]
- Wijnhoud AD, Koudstaal PJ, Dippel DW. Relationships of transcranial blood flow Doppler parameters with major vascular risk factors: TCD study in patients with a recent TIA or nondisabling ischemic stroke. J Clin Ultrasound. 2006;34:70–76. doi: 10.1002/jcu.20193. [DOI] [PubMed] [Google Scholar]
- Wise RG, Pattinson KT, Bulte DP, Chiarelli PA, Mayhew SD, Balanos GM, O'Connor DF, Pragnell TR, Robbins PA, Tracey I, Jezzard P. Dynamic forcing of end-tidal carbon dioxide and oxygen applied to functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2007;27:1521–1532. doi: 10.1038/sj.jcbfm.9600465. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, Russell D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med. 2005;172:371–378. doi: 10.1164/rccm.200406-807OC. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Morgan BJ, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol. 2006;577:319–329. doi: 10.1113/jphysiol.2006.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]