Abstract
Sensitization of purinergic P2X receptors is one of the mechanisms responsible for exaggerated pain responses to inflammatory injuries. Prostaglandin E2 (PGE2), produced by inflamed tissues, is known to contribute to abnormal pain states. In a previous study, we showed that PGE2 increases fast inactivating ATP currents that are mediated by homomeric P2X3 receptors in dorsal root ganglion (DRG) neurons isolated from normal rats. Protein kinase A (PKA) is the signalling pathway used by PGE2. Little is known about the action of PGE2 on ATP currents after inflammation, although the information is crucial for understanding the mechanisms underlying inflammation-induced sensitization of P2X receptors. We therefore studied the effects of PGE2 on P2X3 receptor-mediated ATP currents in DRG neurons dissociated from complete Freund's adjuvant (CFA)-induced inflamed rats. We found that PGE2 produces a large increase in ATP currents. PKCɛ, in addition to PKA, becomes involved in the modulatory action of PGE2. Thus, PGE2 signalling switches from a solely PKA-dependent pathway under normal conditions to both PKA- and PKC-dependent pathways after inflammation. Studying the mechanisms underlying the switch, we demonstrated that cAMP-responsive guanine nucleotide exchange factor 1 (Epac1) is up-regulated after inflammation. The Epac agonist CPT-OMe mimics the potentiating effect of PGE2 and occludes the PKC-mediated PGE2 action on ATP currents. These results suggest that Epac plays a critical role in P2X3 sensitization by activation of de novo PKC-dependent signalling of PGE2 after inflammation and would be a useful therapeutic target for pain therapies.
ATP has been established as an important transmitter signalling sensory information in dorsal root ganglion (DRG) neurons (Burnstock, 2000; North, 2002). It activates purinergic homomeric P2X3 and heteromeric P2X2/3 receptors in small and medium diameter DRG neurons and transmits sensory information from the periphery to the spinal cord (Bardoni et al. 1997; Burgard et al. 1999; Gu, 2003; Engelman & MacDermott, 2004). ATP release is dramatically increased after cell damage and stress (Ferguson et al. 1997; Cook & McCleskey, 2002). Rats with inflammation and nerve injury often respond to ATP or its analogue, α,β-methylene ATP (α,β-meATP), with exaggerated nocifensive behaviours (e.g. rapid paw withdrawal, increasing tail flicks, chewing and paw flinching) (Hamilton et al. 1999; Tsuda et al. 2000; Chen et al. 2005) and elevated cell firing in sensory neurons (Hamilton et al. 2001). We found that P2X3 receptor-mediated ATP currents are enhanced in DRG neurons isolated from inflamed or nerve injured rats (Xu & Huang, 2002; Chen et al. 2005). Studying the mechanisms underlying the enhanced ATP responses, we demonstrated that total P2X3 receptor expression increases after inflammation (Xu & Huang, 2002) and P2X3 receptor trafficking to the cell membrane is enhanced after nerve injury (Chen et al. 2005). We further showed that repeated high-frequency electrical stimulation (as occurs under injurious conditions) increases membrane expression of P2X3 receptors, resulting in a prolonged increase in ATP currents (Xu & Huang, 2004). The increase is mediated by calcium–calmodulin protein kinase II (CaMKII). Thus, protein kinases are likely to play important roles in the P2X3 sensitization.
PGE2 is a well-known lipid mediator that is synthesized and released from damaged or inflamed tissue surrounding terminals of nociceptive sensory neurons. It increases the excitability and capsaicin-induced currents in DRG neurons (Lopshire & Nicol, 1998; Hu et al. 2002) and potentiates the release of calcitonin gene-related protein (CGRP) and substance P from neurons (Southall & Vasko, 2001; Kopp et al. 2002). Behavioural studies show that after carrageenan-induced inflammation, PGE2-induced hyperalgesia lasts long after acute nociceptive responses subside and the hyperalgesia can be antagonized by PKCɛ inhibitors (Khasar et al. 1999; Aley et al. 2000; Parada et al. 2003). This change in signalling suggests that modulation of receptor activity by an inflammatory mediator, such as PGE2, is a dynamic process. Although PGE2 has been shown to augment the effect of α,β-meATP on nociceptive behaviour in rats (Hamilton et al. 1999), the mechanism of PGE2 action has not yet been explored. We have shown, in a separate study, that PGE2 increases P2X3 receptor-mediated ATP currents in normal DRG neurons and PKA mediates the PGE2 action (Wang et al. 2007). Since P2X3 receptors play such important roles in the sensitization of sensory neurons after injury (Xu & Huang, 2002; North, 2004; Chen et al. 2005), it is important to determine whether PGE2 also increases P2X3 receptor-mediated currents in sensory neurons after inflammation. We further asked if the signalling pathway of PGE2 changes. If it does, what is the mechanism underlying the change? We show here that the enhancing effect of PGE2 on ATP currents is greatly exaggerated after inflammatory injury. The increase is the result of activation of PKCɛ in addition to PKA by PGE2. Epac, a guanine nucleotide exchange factor for small GTPases of the Rap family (Bos, 2003), targeted by cAMP to exchange GDP to GTP, is critically important in activating PKCɛ-dependent signalling of PGE2 after injury.
Methods
Animals
All animal procedures were in accordance with the guidelines of the National Institutes of Health and with those of the International Association for the Study of Pain and were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. Sprague–Dawley rats 25–35 days old were used in patch recordings and Western blotting. Older rats (8–10 weeks old) were used in behavioural experiments. A total of 82 rats were used. To induce inflammation, animals were lightly anaesthetized with isoflurane (5% for 2–5 min). Complete Freund's adjuvant (CFA) (Mycobacterium butyricum (10 mg ml−1) (Difco, Detroit, MI, USA), was mixed in a peanut oil–saline (1: 1) emulsion and the mixture (100 μl) was injected into the plantar surface of the left hindpaw (Xu & Huang, 2002). The injection produced localized inflammation characterized by redness, oedema and hyperalgesia in the hindpaw. Treated animals that could not resume normal activity or showed signs of polyarthritic development were killed. To label DRG neurons that innervated the inflamed hindpaw, retrograde tracer Texas red dextran (1% dissolved in saline) was injected into the hindpaw (5 μl per injection point) 3 days prior to the CFA treatment. At end of the study the animals were killed by a rising concentraion of carbon dioxide.
Electrophysiology
ATP currents were recorded from small or medium (mean size: 25 pF, ∼28 μm in diameter) acutely dissociated neurons isolated from L4–5 DRGs in normal rats or rats 3–5 days after CFA injection. Rats were anaesthetized intraperitoneally (i.p.) with sodium pentobarbital (50 mg kg−1). DRGs were then excised from the animals and put in an ice-cold, oxygenated dissecting solution, containing (mm): 135 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgCl2, 10 glucose and 10 Hepes, pH 7.2 (osmolarity, 305 mosmol l−1). After removal of the connective tissue, the ganglia were put in a dissecting solution containing collagenase IV (1.0–1.5 mg ml−1; Boehringer Mannheim, Indianapolis, IN, USA) and trypsin (1.0 mg ml−1; Sigma, St Louis, MO, USA) and incubated for 1 h at 34.5°C. At the end of treatment, DRGs were taken out of the enzyme solution, washed and put into another dissecting solution containing DNAase (0.5 mg ml−1; Sigma). Cells were then dissociated by trituration with fire-polished glass pipettes and placed on acid-cleaned glass coverslips. The experiments were conducted 2 h after plating. During experiments, cells were superfused (0.5 ml min−1) at room temperature with an external solution (130 mm NaCl, 5 mm KCl, 2 mm KH2PO4, 2.5 mm CaCl2, 1 mm MgCl2, 10 mm Hepes, 10 mm glucose, pH 7.3; osmolarity, 295–300 mosmol l−1). ATP (Sigma) was applied through an electronic valve for 2 s. The rate of solution exchange was determined by a change in the junction potential as the external NaCl concentration switched from 130 to 65 mm. The time constant for our solution change was 0.21 ms. This exchange rate was fast and would not limit peak ATP responses. Current recordings were made under voltage-clamp conditions using the whole-cell patch recording technique. In most experiments, 10 μm ATP was used in normal neurons and 5 μm ATP was used in inflamed neurons so that ATP currents of normal and inflamed neurons were of similar amplitudes. An identical ATP concentration (5 μm) was used in the experiments where direct comparisons between normal and CFA neurons were made. Membrane potential was held at −60 mV. Unless indicated, patch-clamp electrodes had a resistance of 3–5 MΩ when filled with the pipette solution containing (mm): 145 potassium gluconate, 10 NaCl, 10 Hepes, 10 glucose, 5 BAPTA and 1 CaCl2, pH 7.25 adjusted with KOH; osmolarity, 290 mosmol l−1). The currents were filtered at 2–5 kHz and sampled at 100 μs per point.
Behavioural experiments
Nocifensive, i.e. flinching, responses to a α,β-meATP application were conducted using a described method (Chen et al. 2005). Briefly, rats were placed under individual plexiglass domes and acclimatized for 1 h. α,β-meATP (50 nmol in 50 μl phosphate-buffered saline (PBS)) was injected intradermally at the plantar surface of the left hindpaw using a 30-gauge needle. Following the injection, the rat was immediately put back under the dome. Flinching behaviour was assessed by determining paw withdrawal duration (PW duration), i.e. the accumulative duration that the hindpaw was lifted in the air in a 1 min time bin. PW duration, instead of the more common paw flinching frequency, was used to assess paw flinching responses to α,β-meATP because paw lift duration varied with pain states or concentration of agonists. Under more severe nociceptive conditions, e.g. inflammation, in response to α,β-meATP application, rats not only lifted the injected paw more frequently, but also left the paw in the air for a longer period. The frequency of flinching, i.e. number of paw lifts per minute, would not be an accurate measure of nociception. Therefore, accumulative PW duration, e.g. 5 (flinches) × 0.5 s + 2 × 2 s + 1 × 4 s = 10.5 s in 1 min, which is a better measure of nociception, was used. To study the effect of PGE2, α,β-meATP and PGE2 were applied to the paw simultaneously. All behavioural studies were performed under blind conditions. To study the protein kinase-dependent effects of PGE2, the PKA antagonist H89 was applied 20 min prior to PGE2 and ATP application and the PKC antagonist bisindoylmalemide (Bis) was administered 5 min before PGE2 and ATP application. Different exposure times for H89 and Bis were used to accommodate the difference in the onset of drug effects.
Western blotting
L4–L5 DRGs of normal rats or ipsilateral to the injected paw of CFA rats were dissected out and homogenized in RIPA lysis buffer (SC24948, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The cell lysates were then centrifuged at 15, 000 g for 12 min at 4°C and total proteins were obtained. The concentrations of protein samples in homogenates were determined using a BCA reagent (Pierce, Rockford, IL, USA). Fifty micrograms of total proteins were loaded onto each well of a 7% Tris-HCl SDS-PAGE gel. After electrophoresis, the proteins were electrotransferred onto a polyvinylidene difluoride membrane (Bio-Rad). The membrane was then incubated in a blocking buffer (Tris-Buffered-Saline Triton X-100 with 5% w/v fat-free dry milk) overnight at 4°C. Samples were probed with primary rabbit anti-Epac1 antibody (1 : 500; provided by Dr Xiaodong Cheng, UTMB) and secondary goat anti-rabbit IgG–HRP antibody (1 : 2000; Santa Cruz Biotechnology). The immunoreactive proteins were detected by enhanced chemiluminescence (ECL kit; Amersham Biosciences, Arlington Heights, IL, USA). The bands recognized by the primary antibody were visualized by exposing the blotting membrane onto an X-ray film. Actin was probed with mouse anti-actin (1 : 1000; Chemicon, Temecula, CA, USA) and anti-mouse HRP-conjugated secondary antibody (1 : 400; Chemicon) to normalize the variation in sample loading.
Drugs
Bisindolylmaleimide I (Bis), H89 dihydrochloride (H89), protein kinase A inhibitor 6–22 amide (PKA-I), PKCɛ translocation inhibitor peptide (PKCɛ-I) and 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (CPT) were purchased from Calbiochem (La Jolla, CA, USA). ATP, α,β-meATP, A-316491 and brefeldin A (BFA) were from Sigma; PGE2, sulprostone, butaprost, SC-1220 were from Cayman Chemical (Ann Arbor, MI, USA); Texas red dextran was from Molecular Probes (Carlsbad, CA, USA). Except for PKA-I, PKCɛ-I, CPT, ATP, α,β-meATP and Texas red dextran, all other compounds were prepared in DMSO as stocks at concentrations at least 1000 times more concentrated than the final concentrations used in experiments. Stock solutions were stored at −20°C and diluted immediately before use. The period of application for a drug was determined according to the time required for the drug effect to reach a steady state.
Data analysis
All data are expressed as mean ±s.e.m. Differences between two means were analysed with Student's paired or unpaired t test. Comparisons between multiple means were done with one-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc test. A P < 0.05 was considered significant.
Results
The effect of PGE2 on ATP currents is enhanced under inflammatory conditions
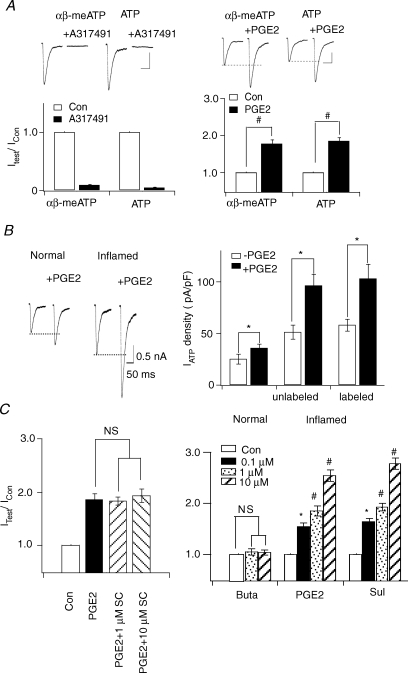
To study the effect of PGE2 on ATP currents in DRG neurons of inflamed rats (referred to from hereon as inflamed neurons), CFA was injected into the rat left paw to induce inflammation. DRG neurons were then isolated from the injected rats 3–5 days later when the inflammatory state had reached a plateau level (Guo & Huang, 2001). We first confirmed that fast-inactivating ATP currents are mediated by P2X3 receptors in inflamed neurons. The P2X1 and P2X3 receptor agonist, α,β-meATP (North, 2002) and ATP activated similar current responses in the same inflamed neuron (Fig. 1A). Both current responses could be blocked by the specific P2X3 receptor antagonist A317491 (0.5 μm) (Jarvis et al. 2002) (Fig. 1A). On studying the effect of PGE2 on ATP currents, we found that PGE2 exerted similar enhancing effects on fast-inactivating α,β-meATP- and ATP-evoked currents. Like those observed in normal neurons (Wang et al. 2007), PGE2 had no effect on slow-inactivating ATP currents (data not shown).
Figure 1. PGE2 effects on fast-inactivating ATP currents are enhanced after inflammation.
A, the effect of PGE2 on ATP currents is mediated by homomeric P2X3 receptors in inflamed rats. ATP (5 μm) and α,β-meATP (5 μm)-evoked fast-inactivating currents were blocked by the P2X3 antoginst A317491. PGE2 potentiated α,β-meATP and ATP-evoked currents similarly. B, actions of PGE2 on ATP (5 μm)-induced currents in both normal and inflamed cells. Left, examples of ATP currents in normal or inflamed neurons before and after PGE2. Right, prior to PGE2 treatment, the mean peak current density of fast ATP currents in inflamed neurons was 2.04-fold larger than that in normal neurons. PGE2 produced a much larger increase in ATP currents in inflamed neurons and in Texas red dextran-labelled inflamed neurons. C, EP3 receptors mediated the PGE2 actions on ATP currents in inflamed neurons. The EP2 agonist, butaporst (Buta), had no effects on ATP currents. The EP3/EP1 agonist, sulprostone (Sul), mimicked the effects of PGE2 and the EP1 antagonist, SC-19220 (SC), could not block PGE2-elicited potentiation.
We then determined the extent the potentiation of ATP currents by PGE2 in inflamed neurons. In a previous study, we found that ATP currents in DRG neurons were greatly enhanced after inflammation (Xu & Huang, 2002). Prior to PGE2 treatment, the peak current density of fast ATP responses evoked in DRG neurons isolated from inflamed rats was 2.04-fold larger than those measured in normal cells (at 5 μm ATP, normal: 25 ± 5 pA pF−1, n= 12; inflamed: 51 ± 7 pA pF−1, n= 50) (Fig. 1B). PGE2 enhanced the fast-inactivating ATP currents in inflamed neurons. The magnitude of the increase was much larger than the enhancement obtained in normal cells (IATP (with PGE2)/IATP: 1.31 ± 0.07, n= 12 for normal neurons; 1.84 ± 0.08, n= 50 for inflamed neurons) (Fig. 1A). Thus, PGE2 exerts a larger effect on ATP currents after inflammation. We further confirmed the results by recording neurons that were known to innervate the inflamed paw. The retrograde tracer, Texas red dextran (1%), was injected into the hindpaw, which was subsequently treated with CFA. As in unlabelled inflamed neurons, PGE2 produced a large potentiation of fast-inactivating ATP currents in labelled inflamed neurons (labelled neurons: without PGE2: 56 ± 6 pA pF−1; IATP (with PGE2)/IATP= 1.78 ± 0.87, n= 11) (Fig. 1B).
The prostanoid receptor (EP receptor) type mediating the PGE2 action was determined in inflamed neurons. The EP1 antagonist SC-19220, at 1 and 10 μm, could not block the enhancing effect of PGE2; the EP2 agonist butaporst (1 and 10 μm) did not have any effect on ATP currents. Only the EP3 agonist sulprostone dose-dependently increased ATP currents, mimicking the action of PGE2. Even though high concentrations of sulprostone can activate EP1 receptors (Boie et al. 1997; Narumiya et al. 1999), the observed effect of sulprostone is probably not due to activation of EP1 receptors because of the inability of SC-19220 to block the PGE2 effect. We suggest that EP3 receptors mediate the enhancing effect of PGE2 in inflamed neurons (Fig. 1C), as observed in normal neurons (Wang et al. 2007). The participation of EP4 receptors was not studied because suitable agonists and antagonists were not available.
The potentiating actions of PGE2 on ATP currents are mediated by both PKA and PKC after inflammation
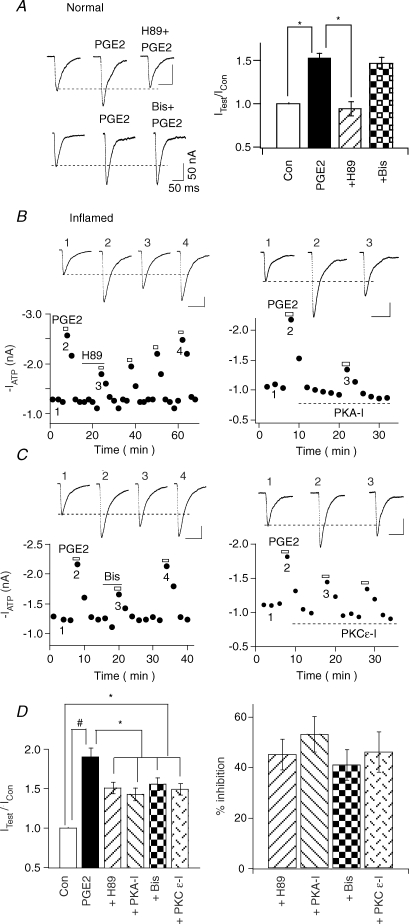
We next compared the role of protein kinases in the actions of PGE2 in normal and inflamed neurons. As observed previously (Wang et al. 2007), the membrane-permeant PKA inhibitor H89 (1 μm) blocked PGE2 enhancement of ATP currents (n= 7) in normal DRG neurons while the membrane-permeant PKC inhibitor Bis (1 μm) had no effect on the PGE2 action (Fig. 2A). These results suggest that PKA, but not PKC, is involved in the modulatory action of PGE2. We then studied the signalling of PGE2 in inflamed rats. Although H89 could inhibit the enhancing effect of PGE2 on ATP currents, the block was incomplete (Fig. 2B, left). The inhibition by H89 was 45 ± 6% (n= 10) (Fig. 2D, left). The more specific PKA antagonist, PKA-I, also partially blocked PGE2-induced enhancement (% of inhibition: 53 ± 7%, n= 5) (Fig. 2B right and D). The effects of the PKC antagonist, Bis, on the action of PGE2 were then studied. Unlike those observed in normal neurons, Bis became effective in inhibiting the potentiation of PGE2 (% of inhibition: 41 ± 6%, n= 10) (Fig. 2C, left, and D). To determine the PKC subtype that was responsible for the PKC action, the effect of a specific PKCɛ inhibitor peptide, PKCɛ-I, was also examined. PKCɛ-I blocked the effect of PGE2 by a similar amount as Bis (% inhibition by PKCɛ-I: 46 ± 8%, n= 4) (Fig. 2C, right, and D), suggesting that PKCɛ is a major PKC isoform responsible for the action of PKC. Thus, a switch from PKC-independent to PKC-dependent PGE2 action occurs after inflammation.
Figure 2. Switching of PGE2 signalling after inflammation.
A, in normal rats, PGE2-induced potentiation of ATP currents was blocked by the PKA inhibitor H89, but was not affected by the PKC inhibitor Bis. Left, examples of the effects of H89 and Bis. Right, IATP (with PGE)/IATP: PGE2 = 1.52 ± 0.06 (n= 10), PGE2 + H89 = 0.94 ± 0.07 (n= 4), PGE2 + Bis = 1.42 ± 0.08 (n= 4) (*P < 0.05). B, PGE2-induced potentiation of ATP currents in inflamed rats was blocked by PKA inhibitors. Left, in inflamed neurons, PGE2-induced increase of ATP currents could only be partially blocked by H89. The blocking effect of H89 was slowly reversed after an extensive washout of H89. Right, PKA-I perfused into cells through the patch pipette also blocked PGE2 effect partially. The blocking effect of intracellular PKA-I was not reversible. C, the enhancement in inflamed rats was also sensitive to PKC antagonists. Left, PGE2 potentiating effect of ATP could be partially blocked by Bis. Bis blocking effect was reversible. Right, intracellularly perfused PKCɛ-I irreversibly and partially inhibited the PGE2 modulatory action. D, PKA- and PKC-dependent actions of PGE2 in inflamed rats. Left, the pool data indicate that both the PKA antagonists H89 or PKA-I, and the PKC antagonists Bis or PKCɛ blocked the potentiating effects of PGE2 partially. Right, the percentages of inhibition by the PKA and PKC antagonist were calculated using the relation {[IATP (with PGE2) −IATP]−[IATP (with PGE + antagonist) −IATP]}/[IATP (with PGE) −IATP]× 100.
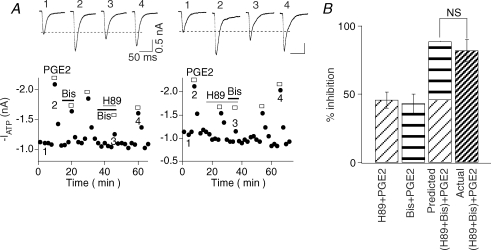
We then determined if PKA and PKC pathways act independently. The pathways were sequentially blocked by applying Bis first and then H89 (Fig. 3A, left). In this set of experiments, Bis alone again decreased PGE2 potentiation partially (% of inhibition: 43 ± 7%, n= 6). H89, in the presence of Bis, reduced the PGE2 potentiation by an additional 41 ± 6% (n= 6). We next switched the order of inhibitor applications, i.e. H89 first and then Bis (Fig. 3A, right). H89 alone blocked PGE2 action by 44 ± 6% (n= 5). Bis, in the presence of H89, inhibited the PGE2 effects by an additional 40 ± 7% (n= 5). The total block exerted by the two inhibitors applied simultaneously was 82 ± 8% (n= 11), which was not significantly different from the predicted sum of the individual effects of H89 plus the effect of Bis (i.e. 44 + 43 = 87%) (Fig. 3B). These results suggest that PKA and PKC pathways act independently and no crosstalk exists between them.
Figure 3. No crosstalk exists between PKA- and PKC-dependent actions of PGE2.
A, left, we first applied Bis and then Bis + H89 together. Right, in another experiment, H89 was applied alone first and then applied with Bis. B, pooled data indicate that H89 alone blocked PGE2 enhancement by 44 ± 6% (n= 5), Bis alone blocked PGE2 enhancement by 43 ± 7% (n= 6). H89 and Bis applied together blocked PGE2 enhancement by 82 ± 8% (n= 11). The amount of block is not significantly different from the predicted sum of the effects of H89 and Bis (44 + 43 = 87%).
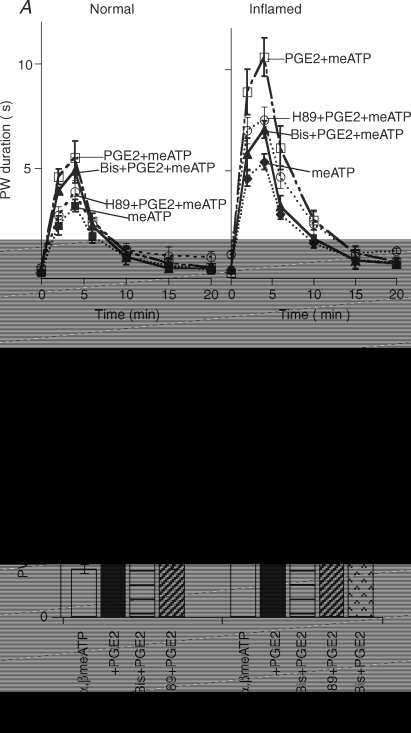
We also determined whether the inflammation-induced switch in PGE2 signalling can be observed behaviourally (Fig. 4). The P2X receptor agonist, α,β-meATP, has been shown to elicit paw withdrawal repeatedly, i.e. paw flinching, a sign of hyperalgesia, in normal rats (Hamilton et al. 1999; Chen et al. 2005). Paw withdrawal duration (PW duration), i.e. the accumulative duration that the hindpaw was lifted in the air in a 1 min time bin, was the parameter used to assess paw flinching responses to α,β-meATP (see Methods for detail). PGE2 (5 nmol per 50 μl) increased α,β-meATP-induced PW duration in both normal and inflamed rats. In normal rats, the potentiating effect of PGE2 was completely blocked by H89 (0.5 nmol per 50 μl), and was not affected by the PKC antagonist Bis (0.5 nmol per 50 μl). In CFA-treated rats, aside from H89, Bis also exerted inhibitory effects on the α,β-meATP-induced flinching responses. Application of H89 and Bis together completely blocked the potentiating effect of PGE2. These observations show that inflammation indeed triggers PKC-dependent actions of PGE2. The large potentiation of ATP responses by PGE2 after inflammatory injury is likely to contribute to the much-exaggerated hyperaglesia.
Figure 4. PGE2 effects on paw flinches depend on PKC in inflamed rats.
A, time course of α,β-meATP action. α,β-meATP (50 nmol in 50 μl) injection resulted in repeated flinchings, quantified as paw withdrawal durations (PW duration). PGE2 (5 nmol in 50 μl), co-injected with α,β-meATP, increased the flinch responses. In normal rats, the increase was insensitive to Bis (0.5 nmol in 50 μl). In inflamed rats, PGE2 elicited a larger increase in flinch responses. The increase, unlike in normal rats, could be inhibited by Bis. B, averaged data obtained from 3 to 6 rats in each group. In normal rat group, H89 (0.5 nmol in 50 μl) blocked the PGE2 enhancement and Bis had no significant effect on PW duration. In inflamed rat group, PGE2 caused a much larger enhancement. H89 inhibited the PW duration and Bis also blocked the flinching responses.
Epac is critical for PKC-dependent signalling of PGE2 after inflammation
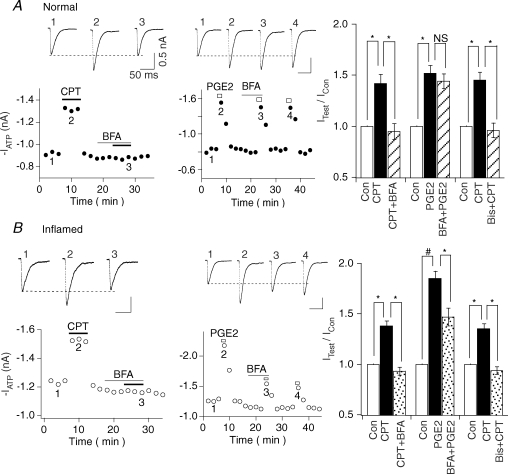
PGE2 actions on ATP currents were found to switch from PKA-dependent in normal conditions to both PKA- and PKC-dependent after inflammatory injury. To determine the mechanism of the switch, we studied the role of Epac in the action of PGE2 (Fig. 5). We first determined if Epac participates in the modulatory action of PGE2. The specific Epac agonist, CPT (10 nm−1μm), which activates both Epac1 and Epac2 (Bos, 2003), was bath-applied and was found to potentiate ATP responses both in normal and inflamed cells. Since specific antagonists for Epac have not yet become available, BFA was used as an Epac antagonist (Zhong & Zucker, 2005; Huang & Hsu, 2006). BFA is known to block the exchange factor (GEF) for ADP-ribosylation factor (Arf) (Arf-GEF), a small G protein important for cellular trafficking (Shin & Nakayama, 2004). These Arf-GEFs of the BFA-inhibited guanine nucleotide-exchange protein 1/2 (BIG1/2) and GEFs families are structurally related to Epac (Cherfils & Melancon, 2005; Zhong & Zucker, 2005), although the mechanism of action underlying the action of BFA on Epac is not entirely clear. We first determined if BFA inhibits the effect of Epac on ATP currents. At 100 nm, a concentration 30–1000 times lower than that used to inhibit Arf (Zeeh et al. 2006), BFA had a minimal effect on ATP currents (Fig. 5). However, BFA could completely block CPT-induced enhancement of ATP currents in both normal and inflamed neurons (Fig. 5A and B, left panels), suggesting that BFA indeed blocks the action of Epac. In addition, Bis was used to confirm that the CPT action on ATP currents is PKC-mediated. We found that Bis (1 μm) could completely block the CPT enhancement of ATP currents both in normal and inflamed neurons (normal: CPT/Con = 1.45 + 0.08, (Bis + CPT)/Con = 0.96 + 0.07, n= 4; inflamed: CPT/Con = 1.37 + 0.05, (Bis + CPT)/Con = 0.94 + 0.04, n= 4). We then examined the effect of BFA on the modulatory action of PGE2. In the normal state, BFA did not affect the enhancing effect of PGE2 (IATP (with PGE2)/IATP= 1.52 ± 0.08, IATP (with PGE2 + BFA)/IATP= 1.44 ± 0.08, n= 5) (Fig. 5A, middle and right). In contrast, in the inflammatory state, BFA partially blocked the action of PGE2 on ATP currents (IATP (with PGE2)/IATP= 1.85 ± 0.07, IATP (with PGE2 + BFA)/IATP= 1.47 ± 0.09, n= 5) (Fig. 5B, middle and right). These results suggest that although P2X3 receptors are capable of responding to activation of Epac both in the normal and inflammatory states, PGE2 can activate the Epac pathway only after inflammatory injury.
Figure 5. PGE2 activates the Epac pathway only in inflamed neurons.
A, PGE2 action on ATP currents does not involve Epac in normal cells. Left, the Epac agonist CPT (10 nm), enhanced ATP (10 μm) responses in normal cells (IATP (with CPT)/IATP= 1.42 ± 0.09, n= 5). Pretreatment with BFA (100 nm) completely blocked the effect of CPT. Middle, BFA had no effect on PGE2-enhancing action in normal cells. Right, bar graph indicates combined data from 5 cells in each group. We also showed that Bis completely blocked CPT-induced enhancement of ATP currents. B, PGE2 effects are partially mediated by Epac in inflamed cells. Left, CPT (10 nm) enhanced ATP (5 μm) responses in inflamed cells (IATP (with CPT)/IATP= 1.38 ± 0.07, n= 5). BFA (100 nm) completely blocked the effect of CPT. Middle, BFA partially blocked the PGE2 enhancement of ATP currents in inflamed cells. Right, bar graph shows that BFA partially reduced PGE2 enhancement of ATP responses after inflammation. Furthermore, the effect of CPT is PKC mediated because its enhancement of ATP currents was completely inhibited by Bis.
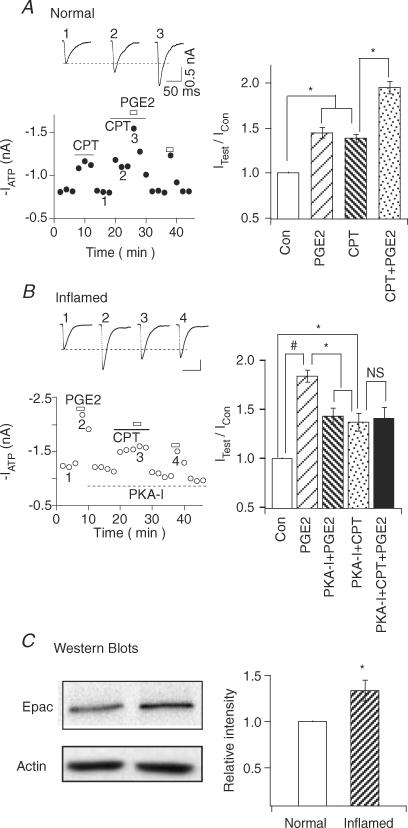
We further substantiated the involvement of Epac by determining if CPT can occlude the effect of PGE2 (Fig. 6). In normal cells, only PKA was involved in the action of PGE2. CPT, which activates the Epac-mediated PKC pathway, should not affect the effect of PGE2. This was indeed the case. CPT, by itself, increased ATP currents (IATP (with CPT)/IATP= 1.39 ± 0.05, n= 4) (Fig. 6A). When CPT and PGE2 were applied together, ATP currents were further enhanced (IATP (with CPT + PGE2)/IATP= 1.95 ± 0.09, n= 4). The potentiations of IATP by PGE2 with CPT (i.e. IATP (with CPT + PGE2)/IATP (with CPT) = 1.40 ± 0.06, n= 4) and without CPT (i.e. IATP (with PGE2)/IATP= 1.45 ± 0.06, n= 4) were similar. Thus, CPT could not occlude the potentiating effect of PGE2 in normal neurons. For inflamed cells, both PKA and PKC are involved in the action of PGE2 (Fig. 2). To determine if the PGE2-induced PKC signalling is Epac-mediated, we perfused PKA-I intracellularly to block PGE2-induced PKA activation (IATP (with PGE2)/IATP= 1.84 ± 0.06; IATP (with PGE2 + PKA-I)/IATP= 1.43 ± 0.08, n= 5) (Fig. 6B). CPT was then applied to the cell to activate the Epac-dependent pathway. CPT alone increased ATP responses (IATP (with CPT + PKA-I)/IATP= 1.37 ± 0.09, n= 5). PGE2, applied in the presence of CPT, could not potentiate ATP currents any further (IATP (with PGE2 + CPT + PKA-I)/IATP= 1.41 ± 0.11, n= 5). Only after washout of CPT, could PGE2 again potentiate ATP responses. Thus, CPT indeed occluded the effect of PGE2 in inflamed neurons. These results provide additional evidence that PGE2 modulation of ATP currents involves the Epac-dependent pathway after inflammation.
Figure 6. CPT occludes the PGE2 action and Epac1 is up-regulated in inflamed cells.
A, CPT does not occlude the effect of PGE2 in normal neurons. Left, application of CPT (10 nm) to normal neurons potentiated ATP (10 μm) currents. PGE2 produced similar potentiation of ATP responses with and without CPT. Right, combined data showed that CPT was not able to occlude the PGE2 action on ATP currents in normal neurons. B, CPT occludes the effect of PGE2 in the presence of PKA-I in inflamed neurons. Left, in an inflamed cell, PGE2 produced a large enhancement of ATP (5 μm) currents. The cell was then intracellularly perfused with 200 nm PKA-I to block the PKA pathway. Application of CPT enhanced ATP currents. However, PGE2 could no longer potentiate the currents in the presence of CPT. After washout of CPT, PGE2 again potentiated ATP currents, but to a smaller extent due to the block of PKA pathway by PKA-I. Right, combined data showing that CPT occludes the PGE2 enhancement of ATP currents in inflamed neurons. C, Epac1 expression is enhanced in the inflammatory state. Left, Western blots of Epac1 in L4–5 ganglia obtained from normal rats (left) or from rats 5 days after inflamed treatment (right). Actin control of each sample was given. Right, mean intensity of Epac1 expression. The relative intensity of Epac1 was increased by 34%± 11% (n= 5) after CFA treatment
The mechanisms triggering the activation of the Epac pathway by PGE2 following inflammation are not known. One possibility is a change in Epac expression. To explore this possibility, the expression of Epac1 in normal and inflammatory states was examined using Western analyses (Fig. 6C). The level of Epac1 in DRGs of CFA-treated rats was 34% higher than that of normal rats (inflamed/normal DRGs = 1.34 ± 0.11, n= 5). These results suggest that up-regulation of Epac1 could contribute, in part, to the appearance of the PKC-dependent pathway of PGE2 after inflammation.
Discussion
We showed that PGE2 reversibly potentiates homomeric P2X3 receptor-mediated fast-inactivating ATP currents in both normal and inflamed DRG neurons (Fig. 1). Under normal conditions, the potentiating effect of PGE2 is dependent solely on PKA (Fig. 2). After inflammation, the PGE2 effect on ATP currents is greatly enhanced (Fig. 1). The increase results from the appearance of a PKC-dependent, in addition to the PKA-dependent, action of PGE2 (Figs 2, 3 and 7). PKCɛ is the isoform involved (Fig. 2). Since the PKA and PKC effects are additive (Fig. 3), there is no crosstalk between the PKA- and PKC-mediated actions of PGE2. In addition, we found that PGE2 enhancement of α,β-meATP-induced nocifensive behavioural responses are inhibited by the PKC antagonist, Bis, only in inflamed rats (Fig. 4). Thus, the appearance of PKC-dependent signalling of PGE2 is also responsible for the exaggerated nocifensive behaviours in the inflammatory state.
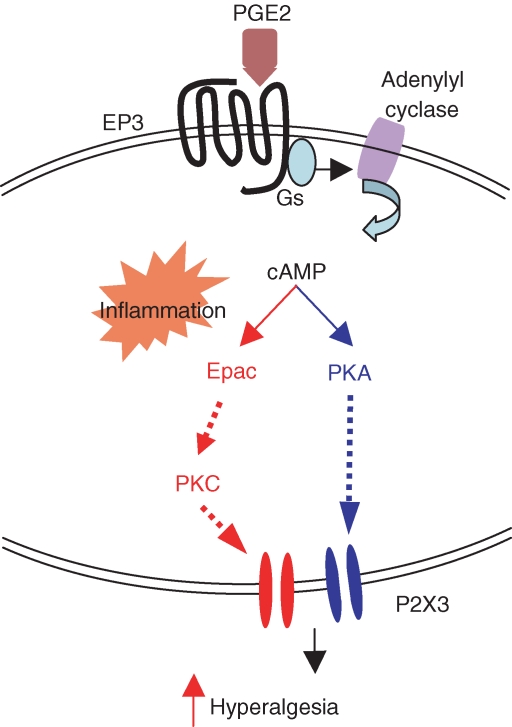
Figure 7. Proposed signalling pathways for PGE2 enhancement of P2X3 receptor-mediated responses in normal and inflamed rats.
PGE2 binds EP3 receptors, which activates Gs and adenylyl cyclase, thus increasing the level of cAMP. In normal sensory neurons, cAMP activates PKA alone resulting in an increase in P2X3 receptor-mediated currents and nocifensive responses (route on the right). After inflammation, in addition to the PKA-mediated pathway, cAMP also activates Epac which leads to the activation of the PKC signalling pathway (route on the left). Activation of the PKC-dependent pathway further increases the potentiation of P2X3 receptor-mediated responses by PGE2, giving rise to exaggerated hyperalgesic behavioural responses.
Our results suggest that EP3 is the receptor subtype mediating the PGE2-induced increase in ATP currents in inflamed neurons (Fig. 1), similar to that observed in normal DRG neurons (Wang et al. 2007). Thus, the appearance of PKC signalling does not seem to result from a change in the prostanoid receptor subtype that mediates the PGE2 action. Four EP receptor subtypes, i.e. EP1, 2, 3 and 4, have been found in DRG neurons (Oida et al. 1995; Southall & Vasko, 2001). Among the EP3A–D splice variants, EP3A and EP3B mRNA could not be detected in DRGs and EP3D expression has not been documented (Southall & Vasko, 2001). Only EP3C and EP4 receptors were found to mediate the PGE2-elicited cAMP production and facilitate peptide release (Southall & Vasko, 2001). Our results are consistent with the idea that PGE2 activates Gs-coupled EP3C receptors to potentiate ATP currents in normal and inflamed DRG neurons. The involvement of EP4 receptors in PGE2 enhancement of ATP currents has yet to be determined. Since the level of PGE2 binding and the functional effects of receptor subtype activation can change during chronic PGE2 exposure or inflammation (Southall et al. 2002; Bar et al. 2004), it would be of interest to determine the expression and functions of splice variants of EP receptors in inflamed DRG neurons in the future.
Activation of certain splice variants of EP3, e.g. EP3D receptors, has been shown to directly activate PKC (Asboth et al. 1996; Zacharowski et al. 1999). Both PKA and PKC were found to be involved in PGE2-induced modulation of tetrodotoxin-resistant Na+ channel activity in sensory neurons (Gold et al. 1998). Since PKC activity is increased in inflamed cells (Sluka et al. 1997; Wu et al. 2005), the appearance of PKC signalling of PGE2 could result from inflammation-induced, but Epac-independent, changes in PKC activity. This seems unlikely because we found that PKA-independent action of PGE2 can be blocked both by the PKC inhibitors PKCɛ-I or Bis, and by the Epac inhibitor BFA (inhibition by PKCɛ-I = 46 ± 8%, by Bis = 41 ± 6%, by BFA = 38 ± 8%) (Figs 2 and 5).
We found that Epac plays a central role in producing the PKC-mediated effects of PGE2 (Figs 5 and 6). Pretreatment with low concentration of the Epac activator, CPT, occludes the effects of PGE2 on ATP currents in inflamed neurons, but does not do so in normal neurons (Fig. 6). BFA blocks CPT-induced potentiation of ATP currents in both normal and inflamed neurons, but can inhibit the PGE2 effect on ATP currents only in inflamed neurons. These results, taken together, strongly suggest that Epac is essential for the appearance of PKC-dependent action of PGE2 on ATP currents in the inflammatoty state. Since the expression of Epac1 increases significantly after inflammation (Fig. 6), we propose that Epac1 up-regulation could be one of the reasons underlying the Epac-mediated action of PGE2 after inflammation. We have yet to determine if inflammation changes the expression of another major Epac, Epac2, in DRGs. In addition, our conclusion does not exclude the possibility that other factors, such as up-regulation of cAMP after inflammation, contribute to the activation of Epac.
The CFA-induced change in PGE2 signalling observed in our study appears to be closely related to the primed state induced by carrageenan (Aley et al. 2000; Parada et al. 2005). Aley et al. (2000) have found that a single injection of carrageenan into the rat paw induces acute inflammation that lasts for ∼72 h. After the acute hyperalgesia subsides, application of PGE2, 5-hydroxytryptamine (5HT) or adenosine produces similar hyperalgesia but lasts up to 3 weeks. These prolonged hyperalgesia, termed hyperalgesic priming, can be blocked by PKCɛ and cAMP inhibitors, suggesting a recruitment of the cAMP/PKCɛ pathway (Parada et al. 2003, 2005). Our CFA-induced hyperalgesia usually lasts for ∼2 weeks (Guo & Huang, 2001), a period similar to the primed state (Aley et al. 2000; Parada et al. 2003). The PKC-dependent PGE2 modulation of ATP currents we observed also occurs only after inflammation (Figs 2 and 3). It remains to be determined whether Epac is directly involved in the cAMP/PKCɛ pathway to give rise to the carrageenan-primed hyperalgesia (Parada et al. 2003, 2005).
In normal rats, the Epac agonist, CPT, at 10 μm, has been shown to promote PKCɛ translocation to the membrane of DRG neurons (Hucho et al. 2005). At an even higher concentration (5 nm per 2.5 μl = 1 mm), CPT can mimic the effect of adrenaline (epinephrine)-induced mechanical hyperalgesia (Hucho et al. 2005). The receptors targeted by the PKCɛ translocation have yet to be determined. The concentrations of CPT used in the study of Hucho et al. (2005) were 10- to 1000-fold higher than the concentration used by us to mimic PGE2 effects on P2X3 receptors (Figs 5 and 6). We found that CPT, at 10 nm−1 μm, potentiated fast ATP currents (Fig 5) but blocked the currents at high concentration (≥ 10 μm) (data not shown). We further demonstrated that CPT occludes PGE2-induced potentiation of ATP currents only in inflamed neurons, even though CPT potentiates ATP currents in both normal and inflamed neurons (Fig. 6). Although one cannot completely eliminate the possibility that a change in the Epac concentration in normal and inflamed neurons may contribute to the observed differences in the CPT effects, CPT-induced hyperalgesia in normal rats examined by Hucho et al. (2005) is unlikely to be related to the pathway switching after CFA-induced inflammation observed here. This suggestion is consistent with the study of Dina et al. (2003), who showed that the β2-adrenergic receptor agonist adrenaline, induces acute hyperalgesia that is sensitive to a PKCɛ inhibitor. This hyperagesia is different from primed PGE2-induced hyperalgesia (Aley et al. 2000) in several important ways. Adrenaline-induced hyperalgesia can neither be potentiated by carrageenan priming (Parada et al. 2005) nor requires priming to recruit cytoskeleton and PKCɛ signalling (Dina et al. 2003). Because of the large concentration difference in the CPT requirement for PGE2 potentiation of ATP currents (this study) and for PKCɛ translocation (Hucho et al. 2005), and because of the important differences in the characteristics of adrenaline-induced (Dina et al. 2003)) and primed PGE2-induced hyperalgesia (Parada et al. 2005), further studies are needed to determine the relationship among different targets and phenomena.
The intervening steps between Epac and PKC have yet to be determined. The small G proteins downstream of Epac that are involved in PGE2 action are not known. Likely candidates are Rap1 and Rap2B. Epac1 is known to activate Rap1 to regulate extracellular signal-regulated kinase (ERK) signalling (Kawasaki et al. 1998; Enserink et al. 2002). In β-adrenergic receptor expressed HEK cells or neuroblasoma N1E-115 cells, Rap2B, which is activated by Epac1, is found to regulate the activation PLCɛ and is critically important in mediating adrenaline (in HEK cells)- or PGE1 (in N1E115)-induced increase in intracellular Ca2+ and inositol phosphate formation (Schmidt et al. 2001). Phosphatidylinositol (PI)-PLC and PLD/PKCɛ pathways appear to be involved in the adrenaline-induced hyperalgesia in normal rats (Hucho et al. 2005).
We showed here that the P2X3 receptor is the downstream target of PGE2-induced activation of PKA and PKC (Fig. 7). There are several serine and threonine residues in the intracellular N- and C-termini of the P2X molecule that are potential sites for phosphorylation. It is unclear if PGE2-induced protein kinases directly phosphorylate P2X3 receptors. The PKC activators, including substance P, bradykinin and phorbol ester (PMA), are found to potentiate P2X1, P2X2/3 and P2X3 receptor-mediated currents in the oocyte or HEK293 expression system (Boue-Grabot et al. 2000; Paukert et al. 2001; Vial et al. 2004b). The conserved PKC phosphorylation site in the N-terminal domain of P2X1 and P2X2 receptors, i.e. threonine 18 (T18), is crucial for receptor functions. T18, which is constitutively phosphorylated, is responsible for the slow inactivation of P2X2 receptor currents (Boue-Grabot et al. 2000; Vial et al. 2004b). For P2X3 receptors, the corresponding P2X3-T12A mutant yields no measurable ATP currents (Paukert et al. 2001). A plausible explanation is that without phosphorylation at the T12 site, the mutant P2X3 receptor inactivates at such a fast rate that no significant current would flow through the channel. The PKC sites in the C-terminus of P2X receptors are thought to stabilize the kinetic properties of the receptor-mediated currents (Boue-Grabot et al. 2000). Recent reports further suggest that PKC may regulate P2X3 receptor activity by phosphorylating serine and threonine residues located in the extracellular sites (ectodomain) of P2X3 receptors (Wirkner et al. 2005) or by indirectly interacting with the P2X3 receptor through an accessory protein (Brown & Yule, 2007). Less data are available on the PKA phosphorylation site on P2X receptors. One study showed that phosphorylation of Ser431 in the C-terminus of P2X2 receptors reduces the amplitude of expressed P2X2 receptors (Chow & Wang, 1998). Another study showed that cAMP increases P2X4 currents by activating PKA in HEK293 cells (Brown et al. 2004). Since PGE2 does not affect the kinetics of P2X3-mediated ATP currents, PKA and PKC, induced by PGE2, are more likely to phosphorylate regulatory proteins associated with P2X3 receptors than to phosphorylate P2X3 receptors directly (Vial et al. 2004a).
We have shown previously that the expression of P2X3 and P2X2/3 receptors and the trafficking of P2X3 receptors to the cell membrane are increased after inflammation and nerve injuries, giving rise to large ATP currents and exaggerated α,β-meATP-induced hyperalgesic responses (Xu & Huang, 2002; Chen et al. 2005). Here, we observed that P2X3 receptor-mediated responses are dynamically modulated by PGE2. The modulation switches from a solely PKA-dependent pathway in the normal state to both PKA- and PKC-dependent pathways in the inflammatory state (Fig. 7). This change could partly be attributed to the up-regulation of Epac1 (Fig. 6), which leads to a stronger modulation of P2X3 receptors by PGE2 and further enhances fast-inactivating ATP currents. Since Epac plays such an important role in triggering exaggerated ATP and hyperalgesic responses, antagonizing Epac up-regulation in sensory neurons could be a new strategy for treatment of patients with chronic pain.
Acknowledgments
The authors thank Drs William D. Willis and Richard E Coggeshall for comments on the manuscript and Dr Xiaodong Cheng for providing the Epac1 antibody. The work is supported by National Institutes of Health grants (NS30045, DA13668, DE17813).
References
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asboth G, Phaneuf S, Europe-Finner GN, Toth M, Bernal AL. Prostaglandin E2 activates phospholipase C and elevates intracellular calcium in cultured myometrial cells: involvement of EP1 and EP3 receptor subtypes. Endocrinology. 1996;137:2572–2579. doi: 10.1210/endo.137.6.8641211. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Natura G, Telleria-Diaz A, Teschner P, Vogel R, Vasquez E, Schaible HG, Ebersberger A. Changes in the effect of spinal prostaglandin E2 during inflammation: prostaglandin E (EP1–EP4) receptors in spinal nociceptive processing of input from the normal or inflamed knee joint. J Neurosci. 2004;24:642–651. doi: 10.1523/JNEUROSCI.0882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boie Y, Stocco R, Sawyer N, Slipetz DM, Ungrin MD, Neuschafer-Rube F, Puschel GP, Metters KM, Abramovitz M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot E, Archambault V, Seguela P. A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X2 ATP-gated channels. J Biol Chem. 2000;275:10190–10195. doi: 10.1074/jbc.275.14.10190. [DOI] [PubMed] [Google Scholar]
- Brown DA, Bruce JI, Straub SV, Yule DI. cAMP potentiates ATP-evoked calcium signaling in human parotid acinar cells. J Biol Chem. 2004;279:39485–39494. doi: 10.1074/jbc.M406201200. [DOI] [PubMed] [Google Scholar]
- Brown DA, Yule DI. Protein kinase C regulation of P2X3 receptors is unlikely to involve direct receptor phosphorylation. Biochim Biophys Acta. 2007;1773:166–175. doi: 10.1016/j.bbamcr.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard EC, Niforatos W, Van Biesen T, Lynch KJ, Touma E, Metzger RE, Kowaluk EA, Jarvis MF. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J Neurophysiol. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li GW, Wang C, Gu Y, Huang LY. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Cherfils J, Melancon P. On the action of Brefeldin A on Sec7-stimulated membrane-recruitment and GDP/GTP exchange of Arf proteins. Biochem Soc Trans. 2005;33:635–638. doi: 10.1042/BST0330635. [DOI] [PubMed] [Google Scholar]
- Chow YW, Wang HL. Functional modulation of P2X2 receptors by cyclic AMP-dependent protein kinase. J Neurochem. 1998;70:2606–2612. doi: 10.1046/j.1471-4159.1998.70062606.x. [DOI] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Dina OA, McCarter GC, De Coupade C, Levine JD. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron. 2003;39:613–624. doi: 10.1016/s0896-6273(03)00473-2. [DOI] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, De Rooij J, Van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG. P2X receptor-mediated modulation of sensory transmission to the spinal cord dorsal horn. Neuroscientist. 2003;9:370–378. doi: 10.1177/1073858403252788. [DOI] [PubMed] [Google Scholar]
- Guo H, Huang LY. Alteration in the voltage dependence of NMDA receptor channels in rat dorsal horn neurones following peripheral inflammation. J Physiol. 2001;537:115–123. doi: 10.1111/j.1469-7793.2001.0115k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol. 1999;126:326–332. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RWT. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Presynaptic mechanism underlying cAMP-induced synaptic potentiation in medial prefrontal cortex pyramidal neurons. Mol Pharmacol. 2006;69:846–856. doi: 10.1124/mol.105.018093. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4+ neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase Cɛ mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. PGE2 increases release of substance P from renal sensory nerves by activating the cAMP-PKA transduction cascade. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1618–R1627. doi: 10.1152/ajpregu.00701.2001. [DOI] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oida H, Namba T, Sugimoto Y, Ushikubi F, Ohishi H, Ichikawa A, Narumiya S. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–2837. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCɛ second messenger pathways. Pain. 2005;113:185–190. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cɛ can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, Grunder S. Inflammatory mediators potentiate ATP-gated channels through the P2X3 subunit. J Biol Chem. 2001;276:21077–21082. doi: 10.1074/jbc.M101465200. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, Von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Shin HW, Nakayama K. Guanine nucleotide-exchange factors for arf GTPases: their diverse functions in membrane traffic. J Biochem (Tokyo) 2004;136:761–767. doi: 10.1093/jb/mvh185. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Capsaicin-induced sensitization of primate spinothalamic tract cells is prevented by a protein kinase C inhibitor. Brain Res. 1997;772:82–86. doi: 10.1016/s0006-8993(97)00876-7. [DOI] [PubMed] [Google Scholar]
- Southall MD, Bolyard LA, Vasko MR. Twenty-four hour exposure to prostaglandin downregulates prostanoid receptor binding but does not alter PGE2-mediated sensitization of rat sensory neurons. Pain. 2002;96:285–296. doi: 10.1016/S0304-3959(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem. 2001;276:16083–16091. doi: 10.1074/jbc.M011408200. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J Neurosci. 2000;20:RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Roberts JA, Evans RJ. Molecular properties of ATP-gated P2X receptor ion channels. Trends Pharmacol Sci. 2004a;25:487–493. doi: 10.1016/j.tips.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Vial C, Tobin AB, Evans RJ. G-protein-coupled receptor regulation of P2X1 receptors does not involve direct channel phosphorylation. Biochem J. 2004b;382:101–110. doi: 10.1042/BJ20031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Li GW, Huang LY. Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain. 2007;3:22. doi: 10.1186/1744-8069-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Stanchev D, Koles L, Klebingat M, Dihazi H, Flehmig G, Vial C, Evans RJ, Furst S, Mager PP, Eschrich K, Illes P. Regulation of human recombinant P2X3 receptors by ecto-protein kinase C. J Neurosci. 2005;25:7734–7742. doi: 10.1523/JNEUROSCI.2028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Su G, Ma L, Zhang X, Lei Y, Li J, Lin Q, Fang L. Protein kinases mediate increment of the phosphorylation of cyclic AMP-responsive element binding protein in spinal cord of rats following capsaicin injection. Mol Pain. 2005;1:26. doi: 10.1186/1744-8069-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Huang LY. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci U S A. 2004;101:11868–11873. doi: 10.1073/pnas.0401490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharowski K, Olbrich A, Thiemermann C. Reduction of myocardial injury by the EP3 receptor agonist TEI-3356. Role of protein kinase C and of KATP-channels. Eur J Pharmacol. 1999;367:33–39. doi: 10.1016/s0014-2999(98)00963-7. [DOI] [PubMed] [Google Scholar]
- Zeeh JC, Zeghouf M, Grauffel C, Guibert B, Martin E, Dejaegere A, Cherfils J. Dual specificity of the interfacial inhibitor brefeldin a for arf proteins and sec7 domains. J Biol Chem. 2006;281:11805–11814. doi: 10.1074/jbc.M600149200. [DOI] [PubMed] [Google Scholar]
- Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]