Abstract
The δ2 glutamate receptor (GluRδ2) belongs to the ionotropic glutamate receptor (iGluR) family and plays a crucial role in the induction of cerebellar long-term depression (LTD), a form of synaptic plasticity underlying motor learning. Nevertheless, the mechanisms by which GluRδ2 regulates cerebellar LTD have remained elusive. Because a mutation occurring in lurcher mice causes continuous GluRδ2 channel activity that can be abolished by 1-naphtylacetylspermine (NASP), a channel blocker for Ca2+-permeable iGluRs, GluRδ2 is thought to function as an ion channel. Here, we introduced a mutant GluRδ2 transgene, in which the putative channel pore was disrupted, into GluRδ2-null Purkinje cells using a virus vector. Surprisingly and similar to the effect of the wild-type GluRδ2 transgene, the mutant GluRδ2 completely rescued the abrogated LTD in GluRδ2-null mice. Furthermore, NASP did not block LTD induction in wild-type cerebellar slices. These results indicate that GluRδ2, a member of the iGluR family, does not serve as a channel in the regulation of LTD induction.
Glutamate receptors come in two types: ionotropic and metabotropic. Ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels that mediate fast excitatory neurotransmission in the mammalian central nervous system. They are subdivided into four subfamilies: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, kainate receptors, N-methyl-d-aspartate (NMDA) receptors, and δ glutamate receptors (Hollmann & Heinemann, 1994). The δ2 glutamate receptor (GluRδ2) (Araki et al. 1993; Lomeli et al. 1993), which is predominantly expressed at the postsynaptic sites of parallel fibre (PF)–Purkinje cell synapses (Landsend et al. 1997), plays a crucial role in cerebellar functions: GluRδ2-null mice display ataxia and synapse malformation, such as a reduced number of PF–Purkinje cell synapses and the sustained innervation of Purkinje cells by multiple climbing fibres (CFs). In addition, long-term depression (LTD), a form of synaptic plasticity thought to underlie motor coordination and information storage (Ito, 1989), is abrogated at PF–Purkinje cell synapses in GluRδ2-null cerebella (Kashiwabuchi et al. 1995). Despite its importance, GluRδ2's role in cerebellar function has remained elusive – mainly because of the lack of specific agonists for GluRδ2 (Yuzaki, 2004). For example, although currents have never been evoked using wild-type GluRδ2, this situation might be due to a lack of information regarding GluRδ2's agonists or the conditions necessary to induce currents. Thus, whether GluRδ2 functions as an ion channel has been a long-standing question.
A point mutation at the end of the third transmembrane region of GluRδ2, which was originally identified in ataxic mutant lurcher mice (Zuo et al. 1997), causes the continuous activation of its channels (GluRδ2Lc). Like AMPA and kainate receptors, GluRδ2Lc exhibited a rectified current–voltage relationship, was sensitive to the polyamine antagonist 1-naphtylacetylspermine (NASP), and exhibited moderate Ca2+ permeability (Kohda et al. 2000; Wollmuth et al. 2000). In addition, similar to wild-type AMPA receptors, the Ca2+-permeability of GluRδ2Lc was abolished by the replacement of glutamine (Q) by arginine (R) at the putative channel pore region (the Q/R site; Fig. 1A and B). Although these findings suggested that endogenous GluRδ2 may serve as a Ca2+-permeable channel during LTD-inducing stimulation (Wollmuth et al. 2000), we recently used a GluRδ2 transgene containing a mutation at the Q/R site to demonstrate that the Ca2+ permeability of GluRδ2 was not essential for its functions (Kakegawa et al. 2007). Nevertheless, a fundamental question regarding the ion channel activity of wild-type GluRδ2 has remained unanswered. If GluRδ2 functions as a channel under certain conditions, it may activate voltage-gated Ca2+ channels and contribute to the Ca2+ signals necessary for LTD induction.
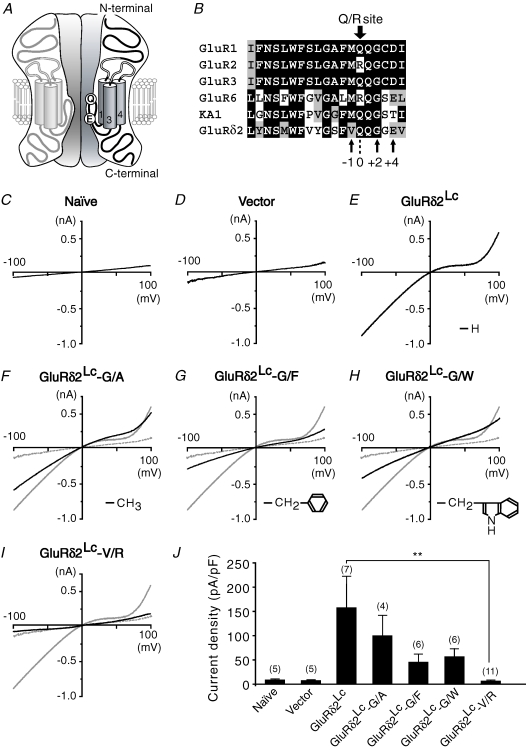
Figure 1. Mutations known to disrupt the channel pores of AMPA/kainate receptors effectively suppressed the current flow through GluRδ2Lc channels in HEK 293 cells.
A, presumed membrane topology of GluRδ2. Glutamine (Q) at the Q/R site and a negatively charged glutamate (E) are shown, which are presumed to be exposed to the channel pore and to determine its sensitivity to spermine analogues. B, alignment of the amino acid sequences of the channel pore regions of representative iGluRs. GluRδ2 has a Q at the Q/R site (position 0). The conserved glycine (G) residue at position +2 from the Q/R site contributes to the narrow constriction of the iGluR channel. Letters are shaded according to the similarity of the amino acids at each position. C–I, representative current–voltage relationships recorded from naïve HEK 293 cells (Naïve; panel C), or HEK 293 cells expressing an empty vector (Vector; panel D), GluRδ2Lc (panel E), GluRδ2Lc in which alanine (G/A, panel F), phenylalanine (G/F, panel G) or tryptophan (G/W, panel H) were substituted for glycine (G) at position +2, or GluRδ2Lc in which arginine was substituted for valine (V) at position −1 (V/R, panel I). The thin traces shown in panels F–I indicate the current–voltage relationship of cells expressing GluRδ2Lc (taken from panel E); the thin dashed traces indicate the current–voltage relationship of cells expressing empty vector (taken from panel D). Insets of panel E–H: side-chain structures of the replaced amino acids. J, bar graph showing the mean current density at −80 mV. The number of experiments is given in parentheses. **P < 0.01.
To address this long-standing question, we exploited the fact that the putative channel pore region of GluRδ2 shares considerable similarity with that of AMPA and kainate receptors (Fig. 1A and B). We demonstrated that a mutation in the GluRδ2's putative channel pore domain did not interfere with GluRδ2's function to induce LTD, while similar mutations completely blocked the channel activities of GluRδ2Lc and wild-type AMPA/kainate receptors. Furthermore, although currents passing through GluRδ2Lc channels and glial AMPA receptors, both of which are composed of subunits containing Q at the Q/R site, were potently blocked by NASP, LTD was normally induced in cerebellar slices in the presence of NASP. Therefore, we propose that GluRδ2 does not serve as an ion channel in the regulation of LTD induction.
Methods
Clones and transfection
Site-directed mutagenesis was accomplished by overlap extension using polymerase chain reaction (PCR) with Pfu DNA polymerase (Stratagene, La Jolla, CA, USA). The nucleotide sequences of the amplified open reading frames were confirmed using bidirectional sequencing. GluRδ2 cDNAs were cloned into the expression vector pTracer-enhanced green fluorescent protein (Invitrogen, Carlsbad, CA, USA), and 4 μg of the plasmid was transfected into human embryonic kidney 293 (HEK 293) cells using the CellPhect transfection kit (Amersham Biosciences, Piscataway, NJ, USA). The incubation of the cells, the processing of the samples, and the cell-surface biotinylation assay were performed as previously described (Matsuda et al. 2003).
Recombinant Sindbis virus and in vivo injection
The recombinant Sindbis virus for the expression of GluRδ2 in combination with a yellow fluorescent protein containing a nuclear localization signal (nYFP) in the same neuron was constructed as previously described (Matsuda et al. 2003). Postnatal day 21–36 GluRδ2-null mice (ho5J mice; Jackson Laboratory, Bar Harbor, ME, USA) were anaesthetized using an intraperitoneal injection of ketamine/xylazine (80/20 mg kg−1; Sigma, St Louis, MO, USA), and 2 μl of the solution containing the recombinant Sindbis virus (titre, 1.0 × 109 TU ml−1) was injected into the vermis of cerebellar lobules V–VIII using a glass pipette (30 μm in diameter) and a microinjector (Nanoliter; World Precision Instruments, Sarasota, FL, USA). Parasagittal slices (100 μm) were prepared and subjected to immunohistochemical analysis under a confocal laser-scanning microscope (Fluoview; Olympus, Tokyo, Japan), as previously described (Kohda et al. 2007). All procedures relating to the care and treatment of the animals were performed in accordance with NIH guidelines.
Electrophysiology
Patch-clamp recordings were made from HEK 293 cells and cerebellar slices using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA) and the pCLAMP system (version 9.2, Axon Instruments), as previously described (Kohda et al. 2000; Kakegawa et al. 2007). The detailed procedure is described in the Supplementary information. In some experiments, spermine (100 μm, Sigma) was added to the internal solution to maintain the voltage-dependent blockage of Ca2+-permeable AMPA/kainate receptors during the recordings (Bowie & Mayer, 1995). A synthetic peptide that mimicked the C-terminus of GluRδ2 (pep-GluRδ2CT7: PDRGTSI) or a control peptide in which the sequence of pep-GluRδ2CT7 was scrambled (pep-GluRδ2SCR: RIPTDSG) was also included in the internal solution in some experiments, as previously described (Kohda et al. 2007). In the pharmacological experiments, NASP (Sigma) was extracellularly applied to block Ca2+-permeable AMPA/kainate receptors and GluRδ2. For the statistical analysis, the amplitudes of the PF-evoked excitatory postsynaptic currents (PF-EPSCs) were averaged every 1 min and normalized according to a baseline measurement performed for 1 min immediately before the conjunctive stimulation.
Data analysis and statistics
Data are presented as the mean ±s.e.m., and statistical significance was defined as P < 0.05, as determined using the Mann–Whitney U-test.
Results
Common mutations disrupting iGluR channel pores
GluRδ2 contains a glutamine (Q) residue at the Q/R site (position 0; Fig. 1A and B) in the putative channel pore domain; this residue determines the Ca2+ permeability of AMPA and kainate receptors (Burnashev et al. 1992). In addition, the +4 position of GluRδ2 contains a negatively charged glutamate (E; Fig. 1A and B) that contributes to the inward rectification of AMPA and kainate receptor currents (Panchenko et al. 1999). Indeed, GluRδ2Lc channels exhibited moderate Ca2+ permeability and a rectified current–voltage relationship (Kohda et al. 2000; Wollmuth et al. 2000) in HEK 293 cells. Therefore, we expected that mutations rendering AMPA and kainate receptor channels non-conductive would also disrupt the GluRδ2 channel pores.
To evaluate the effects of mutations on the GluRδ2 channel pore, we introduced various mutations into GluRδ2Lc, which exhibited continuous channel activity in the absence of added ligands. A glycine (G) residue at position +2 (Fig. 1B) is thought to form the narrow constriction of the iGluR channel pore (Kuner et al. 2003). Indeed, the replacement of glycine with amino acids containing bulkier side chains, such as alanine (A; GluRδ2Lc-G/A), phenylalanine (F; GluRδ2Lc-G/F) or tryptophan (W; GluRδ2Lc-G/W), reduced GluRδ2Lc currents in HEK 293 cells (Fig. 1E–H and J). Although these mutations substantially reduced the GluRδ2Lc currents, we decided to continue searching for mutations that could block the currents. Thus, we next replaced valine (V) at position −1 (Fig. 1B) with arginine (R; GluRδ2Lc-V/R); similar mutations have been reported to disrupt the channel pores of AMPA (Dingledine et al. 1992; Robert et al. 2002) and kainate receptors (Robert et al. 2002). We found that the currents in cells expressing GluRδ2Lc (158 ± 65 pA pF−1 at −80 mV, n= 7; Fig. 1E and J) were almost completely blocked by this mutation (7.9 ± 1.5 pA pF−1 at −80 mV, n= 11; P= 0.009; Fig. 1I and J); the remaining current levels were indistinguishable from those observed in naïve HEK 293 cells (9.0 ± 2.2 pA pF−1 at −80 mV, n= 5; P= 0.257; Fig. 1C and J) or those expressing empty vectors (7.7 ± 1.9 pA pF−1 at −80 mV, n= 5; P= 0.423; Fig. 1D and J). Because cell surface biotinylation assays revealed that GluRδ2Lc-V/R was transported to the cell surface in a manner similar to that of GluRδ2Lc (see online supplemental material, Supplemental Fig. 1), this mutation is likely to disrupt the channel pore. In addition, these findings strongly support the view that the putative channel pore region of GluRδ2 shares considerable structural similarity with that of AMPA and kainate receptors.
GluRδ2wt-V/R rescued LTD in GluRδ2-null Purkinje cells
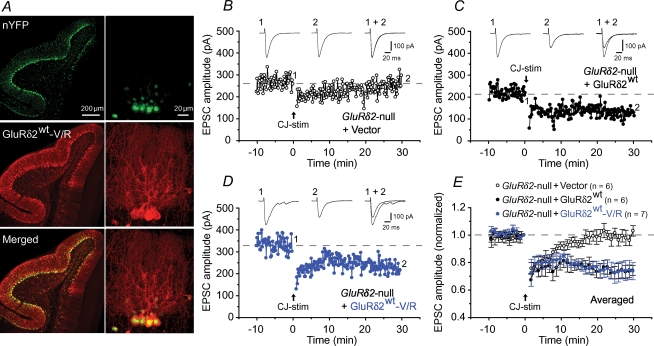
Since the V/R mutation probably disrupted the channel pore of GluRδ2Lc, we next introduced the V/R mutation into wild-type GluRδ2 (GluRδ2wt-V/R). To examine the effect of this mutation on the function of GluRδ2wt, we expressed GluRδ2wt-V/R together with nYFP in GluRδ2-null Purkinje cells using a Sindbis virus vector and performed whole-cell voltage-clamp recordings in acute cerebellar slices. Similar to a previous report (Kohda et al. 2007), we observed nYFP signals and GluRδ2 immunoreactivity in many Purkinje cells (Fig. 2A); Sindbis virus infection itself did not significantly affect the basic membrane properties of the Purkinje cells or the kinetics of PF–EPSCs in the Purkinje cells (data not shown). Next, we examined whether LTD could be restored in GluRδ2-null Purkinje cells transduced with GluRδ2wt, GluRδ2wt-V/R, or nYFP only. In GluRδ2-null Purkinje cells transduced with GluRδ2wt, the conjunctive stimulation, which consisted of 30 single PF stimuli together with a 200 ms depolarizing pulse, successfully induced LTD in PF-EPSCs (74 ± 4% at t= 30 min, n= 6; Fig. 2C and E), but it did not induce LTD in GluRδ2-null Purkinje cells expressing nYFP only (103 ± 4% at t= 30 min, n= 6; Fig. 2B and E); these results support the view that the impaired LTD in GluRδ2-null Purkinje cells was not caused by developmental deficits, but by a lack of GluRδ2 activity at the PF synapses (Yuzaki, 2004). Surprisingly, the expression of GluRδ2wt-V/R also rescued the abrogated LTD (74 ± 6% at t= 30 min, n= 7; P= 0.025 versus vector, and P= 0.999 with GluRδ2wt; Fig. 2D and E), a result suggesting that channel activities were not required for GluRδ2's role in the regulation of LTD induction.
Figure 2. Virally mediated expression of GluRδ2 mutants with disrupted channel pores rescued abrogated cerebellar LTD in GluRδ2-null mice.
A, confocal images of the virus-infected GluRδ2-null cerebellum. Infected Purkinje cells were identified using nYFP fluorescence (top). Transduced GluRδ2wt-V/R was visualized using anti-GluRδ2 antibodies and Alexa 546-conjugated secondary antibodies (middle) and merged with the nYFP signals (bottom). Each right panel shows a magnified view. B–E, PF-EPSC amplitudes following LTD-inducing conjunctive stimulation (CJ-stim) recorded from GluRδ2-null Purkinje cells expressing empty vector (+ Vector; B), GluRδ2wt (+ GluRδ2wt; C), or GluRδ2wt-V/R (+ GluRδ2wt-V/R; D). Averaged data are shown in E. Inset traces: PF–EPSCs just before (1) and 30 min after (2) CJ-stim and their superimposition (1 + 2).
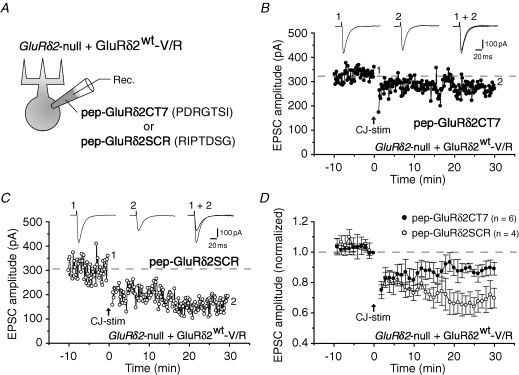
To further examine the function of GluRδ2wt-V/R, we introduced pep-GluRδ2CT7, a peptide that matched the C-terminal seven amino acids of GluRδ2, and pep-GluRδ2SCR, a peptide in which the sequence of pep-GluRδ2CT7 was scrambled, to GluRδ2-null Purkinje cells expressing GluRδ2wt-V/R (Fig. 3A). We previously showed that pep-GluRδ2CT7 interfered with LTD induction in wild-type Purkinje cells by interfering with PDZ proteins that bind to the C-terminal end of endogenous GluRδ2 (Kohda et al. 2007). Similarly, LTD induction was significantly inhibited by pep-GluRδ2CT7 (500 μm) but not by pep-GluRδ2SCR (500 μm) in GluRδ2-null Purkinje cells transduced with GluRδ2wt-V/R; the EPSC amplitudes at 30 min after conjunctive stimulation were 90 ± 4% (n= 6) in Purkinje cells perfused with pep-GluRδ2CT7 (Fig. 3B and D) and 70 ± 6% (n= 4) in cells perfused with pep-GluRδ2SCR (P= 0.033; Fig. 3C and D). These results suggest that the C-terminus of GluRδ2, rather than the channel domain, plays an essential role in LTD induction.
Figure 3. Suppression of LTD in GluRδ2-null Purkinje cells expressing GluRδ2wt-V/R by a peptide mimicking the C-terminus of GluRδ2.
A, schematic diagram of the experimental set-up. GluRδ2-null Purkinje cells transduced with GluRδ2wt-V/R were perfused with pep-GluRδ2CT7, a peptide that matched the C-terminal 7 amino acids of GluRδ2, and pep-GluRδ2SCR, a peptide in which the sequence of pep-GluRδ2CT7 was scrambled, via a recording (Rec.) patch pipette. B and C, representative LTD data recorded from GluRδ2-null Purkinje cell expressing GluRδ2wt-V/R perfused with pep-GluRδ2CT7 (500 μm; B) or pep-GluRδ2SCR (500 μm; C). Inset traces: PF-EPSCs recorded at times 1 and 2. D, averaged LTD data.
A GluRδ2Lc channel blocker, NASP, did not affect LTD induction
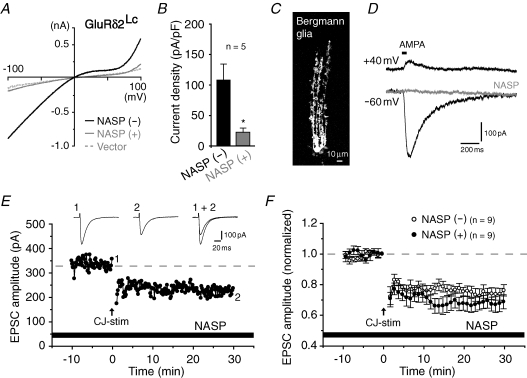
The lurcher mutation may allosterically affect the pore domain structure in a manner such that the V/R mutation does not disrupt the channel pore of wild-type GluRδ2. To exclude this possibility, we employed a complimentary pharmacological approach using a spermine analogue, NASP, that selectively blocks the channel pores of Ca2+-permeable AMPA/kainate receptors composed of subunits containing Q at the Q/R site (Koike et al. 1997). As expected from its amino acid sequence at the Q/R site (Fig. 1A and B), GluRδ2Lc-invoked currents were significantly blocked by 10 μm of NASP (109 ± 25 pA pF−1 without NASP and 23 ± 7 pA pF−1 with NASP; holding potential, −80 mV; n= 5; P= 0.015; Fig. 4A and B). Thus, wild-type GluRδ2, which exists as a homomeric receptor containing Q at the Q/R site in vivo (Kohda et al. 2000), is likely to be blocked by NASP if it functions as a channel. Indeed, both wild-type and lurcher-type AMPA receptors were effectively blocked by NASP (Kohda et al. 2000). Taking diffusion through the tissues into account, we used a higher concentration of NASP in the cerebellar slice preparations; we confirmed that 100 μm of NASP effectively blocked the AMPA-induced currents in Bergmann glial cells, which express Ca2+-permeable AMPA receptors (Müller et al. 1992; Iino et al. 2001) (Fig. 4C and D). Nevertheless, conjunctive stimulation elicited robust LTD in wild-type cerebellar slices in the presence of 100 μm NASP (69 ± 5% at t= 30 min, n= 9; Fig. 4E and F), similar to its effect in the absence of NASP (76 ± 4% at t= 30 min, n= 9, P= 0.233; Fig. 4F); thus, this result strongly supports the view that wild-type GluRδ2 does not function as a channel in the LTD-induction process. In addition, it suggests that AMPA receptors in Bergmann glial cells do not participate in LTD induction.
Figure 4. Channel blockers of GluRδ2 did not hamper LTD induction in wild-type cerebellum.
A, current–voltage curves recorded from HEK 293 cells expressing GluRδ2Lc before (−) and after (+) the application of NASP (10 μm). The thin dashed trace indicates the representative current–voltage relationship of cells expressing an empty vector (taken from Fig. 1D) for comparison. B, bar graph showing the mean current density at −80 mV; *P < 0.05. C, confocal image of Bergmann glia visualized by the inclusion of Alexa 488-conjugated dextran in a patch pipette. D, AMPA-induced current responses from Bergmann glia before (thick traces; holding potential at +40 mV and −60 mV) and after (thin trace; holding potential at −60 mV) the application of 100 μm of NASP. AMPA currents were evoked by puffing (RS)-AMPA (10 mm) to the soma of Bergmann glia in the presence of cyclothiazide (50 μm). E and F, representative (E) and summarized (F) LTD data recorded from wild-type cerebellar slices in the presence of NASP (100 μm). Inset traces: PF-EPSCs recorded at times 1 and 2.
Discussion
Whether GluRδ2 functions as an ion channel has been a long-standing question, but the lack of specific agonists has precluded an unequivocal conclusion. Thus, although we previously suggested that GluRδ2 does not function as a Ca2+-permeable channel (Kakegawa et al. 2007), we were unable to examine its ion channel activities directly. In this study, we demonstrated that a V/R mutation in GluRδ2's putative channel pore domain did not interfere with its role in the induction of LTD (Fig. 2), while a V/R mutation and similar mutations at position −1 almost completely blocked the channel activities of GluRδ2Lc (Fig. 1) and wild-type AMPA/kainate receptors (Dingledine et al. 1992; Robert et al. 2002). Furthermore, although currents passing through the GluRδ2Lc channels (Fig. 4A and B) and glial AMPA receptors (Fig. 4C and D), both of which are composed of subunits containing Q at the Q/R site, were potently blocked by NASP, LTD was normally induced in cerebellar slices in the presence of NASP (Fig. 4E and F). Therefore, although we cannot rule out the possibility that GluRδ2 may function as an ion channel in other phenotypes observed in GluRδ2-null mice, we propose that GluRδ2, which belongs to the ‘ionotropic’ glutamate receptor family, does not serve as an ion channel in the regulation of LTD induction.
The roles of GluRδ2 in regulating the number of postsynaptic AMPA receptors (Hirai et al. 2003) are consistent with the view that GluRδ2 may rather serve as a non-ionotropic receptor, which mediates signals by interacting with intracellular molecules. Many PDZ proteins, like PSD-93 (Roche et al. 1999), PTPMEG (Hironaka et al. 2000), S-SCAM (Yap et al. 2003) and delphilin (Miyagi et al. 2002), have been reported to interact with the C-terminal end of GluRδ2. We demonstrated that the decoy peptide that hampered the interaction of GluRδ2 with these PDZ proteins interfered with LTD induction not only in wild-type Purkinje cells (Kohda et al. 2007), but also in GluRδ2-null Purkinje cells expressing GluRδ2wt-V/R (Fig. 3). Interestingly, such non-ionotropic functions, which challenge classical thinking about ligand-gated ion channels, are not completely unprecedented; for example, the GluR2 subunit of AMPA receptors is reported to regulate dendritic spine formation in cultured hippocampus neurons independently of its ion channel activities (Passafaro et al. 2003). AMPA receptors have also been shown to regulate Lyn tyrosine kinase in the absence of channel activities (Hayashi et al. 1999). Similarly, kainate receptors have been reported to inhibit postspike potassium currents to increase the excitability of hippocampal CA1 pyramidal neurons, independently of their ion channel activities (Melyan et al. 2002). Thus, we speculate that iGluRs may generally have both ionotropic and non-ionotropic functions; GluRδ2 is unique in that it may have lost its ionotropic functions during evolution. Therefore, further studies on the mechanisms by which GluRδ2 exerts its functions should provide insights into the non-ionotropic functions of iGluRs.
Acknowledgments
This research was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (W.K., K.K. and M.Y.), the Takeda Science Foundation (W.K.), and the Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects (M.Y.).
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.141291/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.141291
References
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel δ2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Hume RI, Heinemann SF. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992;12:4080–4087. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- Hirai H, Launey T, Mikawa S, Torashima T, Yanagihara D, Kasaura T, Miyamoto A, Yuzaki M. New role of δ2 glutamate receptors in AMPA receptor trafficking and cerebellar function. Nat Neurosci. 2003;6:869–876. doi: 10.1038/nn1086. [DOI] [PubMed] [Google Scholar]
- Hironaka K, Umemori H, Tezuka T, Mishina M, Yamamoto T. The protein-tyrosine phosphatase PTPMEG interacts with glutamate receptor δ2 and ε subunits. J Biol Chem. 2000;275:16167–16173. doi: 10.1074/jbc.M909302199. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia–synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Miyazaki T, Hirai H, Motohashi J, Mishina M, Watanabe M, Yuzaki M. Ca2+ permeability of the channel pore is not essential for the δ2 glutamate receptor to regulate synaptic plasticity and motor coordination. J Physiol. 2007;579:729–735. doi: 10.1113/jphysiol.2006.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluRδ2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Kohda K, Kakegawa W, Matsuda S, Nakagami R, Kakiya N, Yuzaki M. The extreme C-terminus of GluRδ2 is essential for induction of long-term depression in cerebellar slices. Eur J Neurosci. 2007;25:1357–1362. doi: 10.1111/j.1460-9568.2007.05412.x. [DOI] [PubMed] [Google Scholar]
- Kohda K, Wang Y, Yuzaki M. Mutation of a glutamate receptor motif reveals its role in gating and δ2 receptor channel properties. Nat Neurosci. 2000;3:315–322. doi: 10.1038/73877. [DOI] [PubMed] [Google Scholar]
- Koike M, Iino M, Ozawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neurosci Res. 1997;29:27–36. doi: 10.1016/s0168-0102(97)00067-9. [DOI] [PubMed] [Google Scholar]
- Kuner T, Seeburg PH, Guy HR. A common architecture for K+ channels and ionotropic glutamate receptors? Trends Neurosci. 2003;26:27–32. doi: 10.1016/s0166-2236(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Landsend AS, Amiry-Moghaddam M, Matsubara A, Bergersen L, Usami S, Wenthold RJ, Ottersen OP. Differential localization of δ glutamate receptors in the rat cerebellum: coexpression with AMPA receptors in parallel fiber-spine synapses and absence from climbing fiber-spine synapses. J Neurosci. 1997;17:834–842. doi: 10.1523/JNEUROSCI.17-02-00834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie DJ, Kohr G, Herb A, Seeburg PH, Wisden W. The rat δ1 and δ2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Fletcher M, Kamiya Y, Yuzaki M. Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. J Neurosci. 2003;23:10064–10073. doi: 10.1523/JNEUROSCI.23-31-10064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- Miyagi Y, Yamashita T, Fukaya M, Sonoda T, Okuno T, Yamada K, Watanabe M, Nagashima Y, Aoki I, Okuda K, Mishina M, Kawamoto S. Delphilin: a novel PDZ and formin homology domain-containing protein that synaptically colocalizes and interacts with glutamate receptor δ2 subunit. J Neurosci. 2002;22:803–814. doi: 10.1523/JNEUROSCI.22-03-00803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Möller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992;256:1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Panchenko VA, Glasser CR, Partin KM, Mayer ML. Amino acid substitutions in the pore of rat glutamate receptors at sites influencing block by polyamines. J Physiol. 1999;520:337–357. doi: 10.1111/j.1469-7793.1999.t01-1-00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- Robert A, Hyde R, Hughes TE, Howe JR. The expression of dominant-negative subunits selectively suppresses neuronal AMPA and kainate receptors. Neuroscience. 2002;115:1199–1210. doi: 10.1016/s0306-4522(02)00534-1. [DOI] [PubMed] [Google Scholar]
- Roche KW, Ly CD, Petralia RS, Wang YX, McGee AW, Bredt DS, Wenthold RJ. Postsynaptic density-93 interacts with the δ2 glutamate receptor subunit at parallel fiber synapses. J Neurosci. 1999;19:3926–3934. doi: 10.1523/JNEUROSCI.19-10-03926.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Jatzke C, Seeburg PH, Heintz N, Zuo J. The Lurcher mutation identifies δ2 as an AMPA/kainate receptor-like channel that is potentiated by Ca2+ J Neurosci. 2000;20:5973–5980. doi: 10.1523/JNEUROSCI.20-16-05973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Muto Y, Kishida H, Hashikawa T, Yano R. PKC regulates the δ2 glutamate receptor interaction with S-SCAM/MAGI-2 protein. Biochem Biophys Res Commun. 2003;301:1122–1128. doi: 10.1016/s0006-291x(03)00070-6. [DOI] [PubMed] [Google Scholar]
- Yuzaki M. The δ2 glutamate receptor: a key molecule controlling synaptic plasticity and structure in Purkinje cells. Cerebellum. 2004;3:89–93. doi: 10.1080/14734220410028921. [DOI] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in δ2 glutamate receptor gene. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.