Abstract
Previous studies have shown that catecholamine secretion from the adrenal medulla plays a critical role in chronic intermittent hypoxia (CIH)-induced alterations in cardiovascular function. In the present study we examined the cellular mechanisms associated with the effects of CIH on adrenal chromaffin cell catecholamine secretion. Experiments were performed on adult male mice (C57/BL6) that were exposed to 1–4 days of CIH or to normoxia. Perforated patch electrical capacitance recordings were performed on freshly prepared adrenal medullary slices that permit separating the chromaffin cell secretion from sympathetic input. CIH resulted in a significant increase in the readily releasable pool (RRP) of secretory granules, and decreased stimulus-evoked Ca2+ influx. Continuous hypoxia (CH) either for 2.5 h (equivalent to hypoxic duration accumulated over 4 days of CIH) or for 4 days were ineffective in evoking changes in the RRP and Ca2+ influx. CIH activated PKC in adrenal medullae as evidenced by increased phosphorylation of PKC at Thr514 and PKC inhibitors prevented CIH-induced increases in the RRP and restored stimulus-evoked attenuation of Ca2+ influx. CIH resulted in elevated thio-barbituric acid reactive substances (TBARSs, an index of oxidized proteins) and an antioxidant prevented CIH-induced changes in the RRP, suggesting the involvement of reactive oxygen species (ROS). These results demonstrate that CIH increases the RRP in adrenal chromaffin cells via ROS-mediated activation of PKC and suggest that CIH can directly affect the secretory capacity of chromaffin cells and contribute, in part, to elevated catecholamine levels.
Humans with sleep-disordered breathing manifested as recurrent apnoeas exhibit considerable cardiovascular morbidity. A major advance in the field of apnoea research is the discovery that chronic intermittent hypoxia (CIH) rather than chronic intermittent hypercapnia is a major contributing factor for evoking the cardiovascular morbidity (Prabhakar et al. 2005). Studies in both humans and experimental models have shown that CIH leads to hypertension as well as increased plasma and urinary catecholamines (CAs) (Fletcher, 1997; Fletcher et al. 1999; Phillips & Somers, 2000). It has been further shown that adrenalectomy prevents CIH-induced elevations of serum CAs as well as hypertension. These data suggest that CA secretion from the adrenal medulla plays a critical role in CIH-induced cardiovascular pathologies (Bao et al. 1997). We recently reported that catecholamine secretion from adrenal medullae in response to acute hypoxia is markedly enhanced by prior exposure to CIH. This facilitation involves reactive oxygen species (ROS)-mediated signalling (Kumar et al. 2006). The cellular mechanism for the effects of CIH on chromaffin cell secretion, however, is not known.
Chromaffin cells in the adrenal medulla release catecholamines through the Ca2+-dependent fusion of large dense core secretory granules. The amount of catecholamine released is in part regulated by the number of fusion-competent secretory granules that make up the readily releasable granule pool (RRP) (Heinemann et al. 1993). Thus, regulation of the number of granules that comprise the RRP represents a major control point for regulating the catecholamine secretory capacity from chromaffin cells (Smith, 1999). Previous studies have shown that the number of granules in the RRP is regulated by several separate mechanisms, including the direct Ca2+-mediated as well as protein kinase C (PKC)-dependent increase in the rate at which granules are recruited to the RRP, thus increasing its size (von Rüden & Neher, 1993; Smith et al. 1998). Here we examine whether CIH increases the RRP of chromaffin cells, and if so, by what mechanism(s). Our results show that in mouse adrenal tissue slices, CIH but not continuous hypoxia, increases the number of catecholamine-containing secretory granules in the RRP. This increased secretory capacity involves ROS-mediated protein kinase C (PKC) activity.
Methods
General methods
Experimental protocols were approved by the institutional animal care and use committee (IACUC) of Case Western Reserve University. Studies were performed on male mice (C57/BL6; 6–10 weeks old) that were exposed to either (1) 1–4 days of CIH, i.e. alternating episodes of hypoxia (5% O2 nadir for 15 s) and normoxia (21% O2 for 5 min), nine episodes per hour for 8 h per day as previously described (Peng & Prabhakar, 2003); (2) chronic hypoxia (CH), i.e. 2.5 h or 4 days of hypobaric hypoxia (0.4 ATM); or (3) 1–4 days of room air (normoxia), serving as controls. In experiments where the effect of reactive oxygen species was examined, mice were given manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP; Alexis Biochemicals, CA, USA) at 5 mg kg−1 day−1i.p. MnTMPyP is a membrane-permeant superoxide dismutase mimetic that traps superoxide anion radicals without generating H2O2. Control and experimental mice were injected with vehicle (saline) or MnTMPyP every day during the 4 days of CIH exposure. All experiments were performed within 8 h of terminating the final day of CIH exposure.
Adrenal medulla slice preparation and electrophysiological recording
Animals were anaesthetized by isoflurane (Abbott Laboratories, Abbott Park, IL, USA) and killed by decapitation. Adrenal glands were removed and 200 μm thick slices were prepared for experimentation as previously described (Chan & Smith, 2003). Adrenal tissue slices were superfused at 1 ml min−1 with normal-Ca2+ bicarbonate-buffered saline (BBS) containing (mm): 140 NaCl, 2 KCl2, 3 CaCl2, 2 MgCl2, 26 NaHCO3, 10 glucose and gassed with 95% O2–5% CO2. Electrophysiological and capacitance measurements were performed as previously described (Chan et al. 2005).
Western blot analysis of PKC levels in adrenal medulla
Adrenal glands were collected from control, CIH-, or CH-treated mice. The medulla was dissected from the cortex and placed in 150 μl protein extraction reagent (Pierce, Rockford IL, USA) with 1.5 μl protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and 0.150 μl phosphatase inhibitor cocktail (Calbiochem, La Jolla CA, USA), homogenized and centrifuged at 10 000 g for 5 min. Protein concentration of the homogenate was determined against BSA standards (Bio-Rad, Hercules, CA, USA). Protein was subjected to gel electrophoresis (12% Tris-glycine polyduramide gels; Cambrex, Rockland, ME, USA) and transferred to nitrocellulose membrane. Membranes were blocked in Tris-buffered saline (TBS; 20 mm Tris, pH 7.6) containing 4% BSA, and probed with a polyclonal anti-phospho-pan-PKC antibody (Cell Signaling Technology Inc., Danvers, MA, USA) or with a monoclonal anti β-actin antibody in TBS containing 4% BSA at 25°C overnight. Protein bands were visualized by enhanced chemiluminescence (Peirce, Rockford, IL, USA).
Measurements of thiobarbituric acid reactive substances (TBARSs)
Medullary tissue was homogenized in 10 volumes of 20 mm phosphate buffer (pH 7.4) at 4°C and centrifuged at 500 g for 10 min at 4°C. TBARSs were analysed in supernatant as previously described (Ramanathan et al. 2005; Kumar et al. 2006). Briefly, 100 μl of sample or the calibration standard was added to 50 μl of 8.1% (w/v) SDS, 375 μl of 20% (v/v) acetic acid and 375 μl of 0.8% (w/v) thiobarbituric acid. The samples were heated for 60 min followed by incubation on ice for 10 min and centrifuged at 3000 g for 15 min. The supernatant was removed and the absorbance of the solution was monitored at 532 nm. Malondialdehyde (MDA) was used as a standard, and the level of TBARSs was reported in nmol of MDA formed per mg of protein.
Data analysis
All data are expressed as means ±s.e.m. Statistical significance was evaluated by Student's unpaired t test or one-way ANOVA for repeated measures. P-values less than 0.05 were considered significant.
Results
Intermittent hypoxia increases the RRP in chromaffin cells
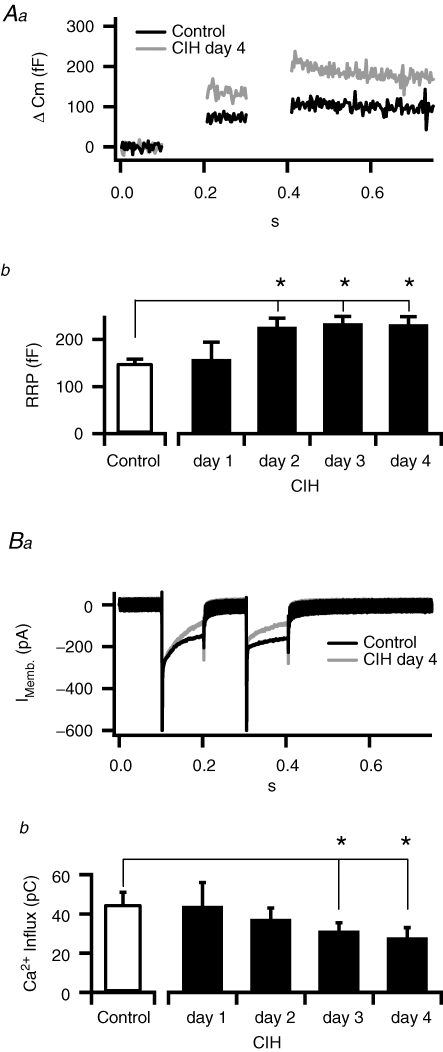
All electrophysiological experiments were performed in freshly prepared adrenal medullary slices because they allow measurements of chromaffin cell function in the native cell context (including cell morphology and cell–cell contacts). Slices also permit separation of chromaffin cell secretion from sympathetic input. Regulation of the readily releasable pool (RRP) of secretory granules represents a key control point for setting the overall transmitter release from chromaffin cells. The RRP dual-pulse depression protocol is based on a previous study in isolated chromaffin cells (Gillis et al. 1996) and has been used extensively to quantify the number of granules resident at defined steps along the secretion path (Smith et al. 1998; Smith, 1999; Voets et al. 1999; Yang et al. 2002). Briefly, two 100 ms square pulse stimuli were delivered at a 100 ms interval (Fig. 1Aa). Cell membrane capacitance was monitored to provide an index of granule fusion. The evoked capacitance response for each pulse was measured and their magnitudes were used to calculate the total number of release-ready granules. The first pulse causes granule fusion and depletion of the RRP, leaving fewer granules for release during the second pulse. This results in a depletion-dependent secretory depression in response to the second pulse. By measuring the ratio of the second response to the first, the total size of the RRP can be calculated (see Fig. 1 legend and Gillis et al. (1996) for a full description of the technique). In CIH exposed cells, the magnitude of the readily releasable pool was greater than that of the control chromaffin cells (Fig. 1A). Average data for the time course of CIH revealed that the RRP increased to a significant level by day 2 and remained elevated on the fourth day of CIH exposure (P < 0.05). In contrast, CIH exposure decreased the magnitude of depolarization-evoked Ca2+ influx (Fig. 1B), with significance reached after 3 days of CIH exposure.
Figure 1. Intermittent hypoxia leads to an increased RRP.
Aa. Example capacitance records from control (black trace) and 4 day CIH-exposed mice (grey trace). Evoked responses from CIH-exposed cells are larger than those recorded from control cells. Cells were voltage clamped at −80 mV and stimulated with a pair of 100 ms depolarizations. Cell capacitance (Cm) is proportional to the cell surface area, which increases with granule fusion and catecholamine release. Thus stimulus-evoked jumps in Cm provide an index of the number of granules fusing. In the example recording provided, the first pulse resulted in a greater capacitance increase than the second pulse (Cm1 and Cm2, respectively). Formally, the RRP can thus be quantified as RRP =S/(1 −R2) where S is the sum of Cm1 and Cm2 and R is the ratio of Cm2 to Cm1. Only cells that exhibit strong depression (Cm2 is small compared to Cm1) provide an accurate estimate of the releasable pool size by the dual pulse method. For this reason only cells that exhibited a depression ratio (Cm2/Cm1) of 0.7 or less were included for further analysis. Ab, data pooled from control and mice exposed to CIH for 1–4 days (n= 5, 7, 9 and 12, respectively) showing that the CIH-evoked increase in the RRP reaches significance (*P < 0.02) within 2 days. Ba, representative evoked currents during the dual-pulse protocol showing that CIH treatment causes decreased Ca2+ influx. Bb, pooled data (n= 12) showing the time course of CIH exposure on evoked Ca2+ influx and demonstrating that the decrease reaches significance (*P < 0.02) by day 3.
Continuous hypoxia increases the RRP in chromaffin cells
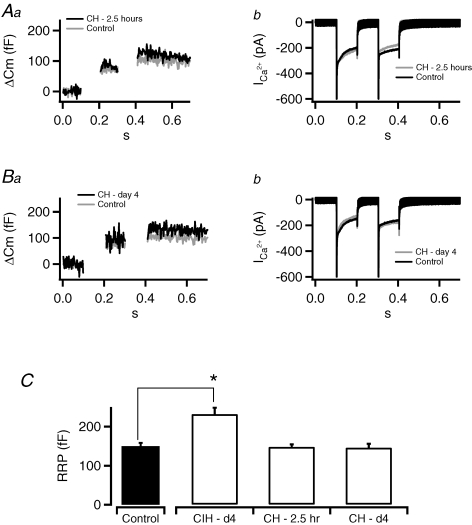
To determine whether a comparable cumulative duration of continuous hypoxia (CH) also increases RRP, mice were exposed to 2.5 h of hypobaric hypoxia (0.4 atm), equivalent to the cumulative duration of hypoxia experienced during 4 days of CIH treatment. As shown in Fig. 2, exposure to 2.5 h of CH affected neither cell capacitance (Fig. 2Aa) nor Ca2+ influx (Fig. 2Ab). It is possible that a single exposure to CH may not be adequate to evoke changes in RRP. Therefore, to test whether multiple exposures to CH can induce hypoxic sensitivity, another group of mice were exposed to 4 days of CH. Cells taken from mice exposed to CH for 4 days also failed to show any difference in secretory capacity (RRP) or Ca2+ influx. These results suggest that a comparable cumulative duration of CH of either 2.5 h or 4 days was ineffective in evoking changes in secretory behaviour as well as Ca2+ influx in chromaffin cells (pooled data did not show significance and are not shown).
Figure 2. Continuous hypoxia does not lead to an increased RRP.
Mice were exposed to continuous hypoxia to either match the accumulated total time of exposure under CIH (CH–2.5 h) or match the total days of exposure (CH–day 4). Tissue slices were prepared and the RRP was measured by dual-pulse excitation. A, representative examples of capacitance recordings (a) and calcium currents (b) evoked from CH–2.5 h treatment are provided and do not exhibit the enhanced capacitance response or decreased Ca2+ influx measured under the CIH–day 4 condition. B, likewise, evoked capacitance jumps (a) and calcium currents (b) under CH–day 4 also failed to display the facilitation measured in the CIH-treated mice. C, data pooled for all conditions (n= 12, 15, 7, 9) showing that only cells taken from mice treated with intermittent hypoxia (CIH–day 4) show a significant difference in the size of the RRP as assayed by dual pulse stimulation (*P < 0.02).
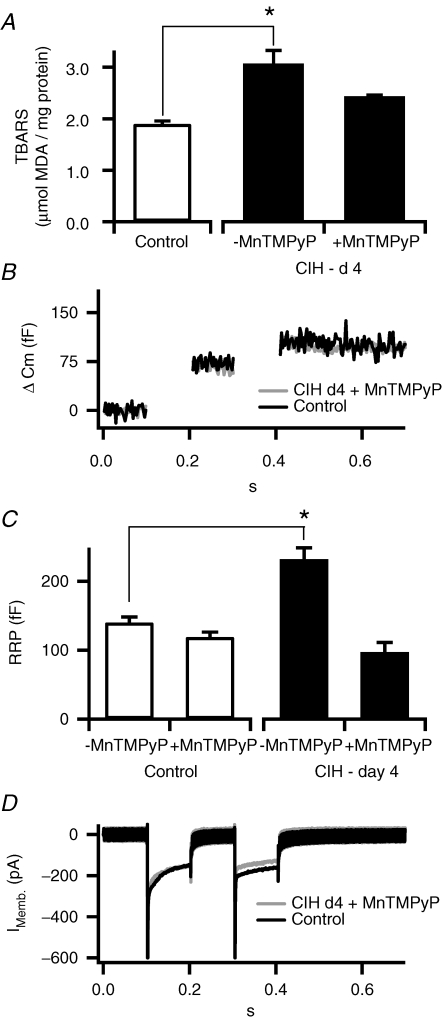
Reactive oxygen species (ROS) scavenger prevents the CIH-induced increase in the readily releasable pool. CIH increases reactive oxygen species (ROS), measured as thiobarbituric acid reactive substances (TBARSs; Ramanathan et al. 2005), an index of increased protein oxidation. A recent study suggests that ROS-mediated signalling plays a critical role in CIH-induced changes in adrenal medullary secretion (Kumar et al. 2006). To assess the potential role of ROS in CIH-evoked changes in chromaffin cell secretory capacity, we monitored the TBARS levels in adrenal medullae harvested from CIH and normoxia exposed mice. As shown in Fig. 3A, TBARS levels were significantly elevated in CIH adrenal medullae compared to controls. Systemic administration of MnTMPyP (5 mg kg−1 day−1i.p.), a scavenger of ·O2−, prevented elevations in TBARSs in CIH-exposed adrenal medulla. MnTMPyP tends to decrease the size of the RRP in control cells (P > 0.05), whereas it completely blocked CIH-induced increase in the RRP as well as the decrease in evoked Ca2+ influx (Fig. 3D; pooled data did not show significant difference from control and are not shown). These results taken together suggest that CIH elevates ROS in chromaffin cells and ROS-mediated signalling plays a role in CIH-evoked increase in RRP.
Figure 3. Superoxide dismutase mimetic blocks the CIH-dependent increase in RRP.
General oxidative status of cells was determined by a TBARS assay. A, TBARSs were measured in control adrenal medullary tissue and in CIH–day 4 tissues with and without concurrent injection with superoxide dismutase mimetic (MnTMPyP). Pooled data (n= 3, 5 and 5) show that administration of MnTMPyP blocks the increased oxidative stress observed in CIH–day 4 treated mice. B, representative recordings showing likewise that co-treatment with MnTMPyP blocked the CIH–day 4-mediated increase in evoked capacitance jumps. C, pooled data showing that MnTMPyP treatment decreased the RRP measured in control cells. Though this failed to reach significance, it may indicate a small basal level of ROS-mediated signalling even in the control recording condition. MnTMPyP cotreatment did abolish the significant CIH-dependent increase in RRP from CIH–day 4 cells (n= 7, 5, 7 and 6, respectively). D, MnTMPyP treatment also reversed the CIH-dependent decrease in evoked calcium influx.
CIH increases the RRP through protein kinase C (PKC)
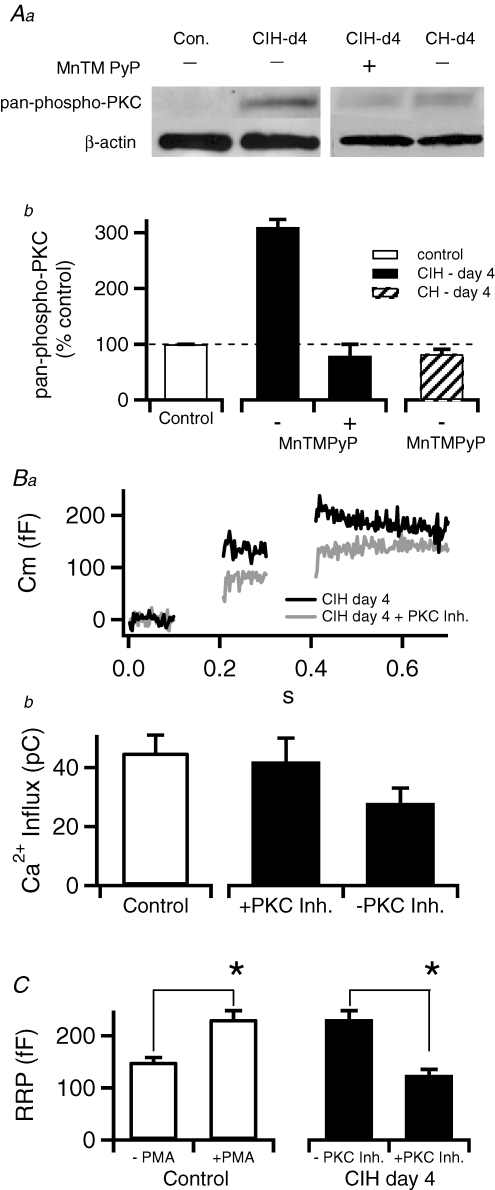
It has been shown that PKC represents a key regulatory element in setting the secretory capacity in chromaffin cells (Gillis et al. 1996). Furthermore, ROS have been shown to activate PKC (Dada et al. 2003). We monitored phosphorylation of PKC at Thr514, which denotes catalytically active enzyme (Keranen et al. 1995), as an index of PKC activity. A representative Western blot and pooled data are presented in Fig. 4A. CIH increased phospho-PKC levels in adrenal medulla relative to control by 210% (P < 0.01). The CIH-induced elevations in phospho-PKC were abolished by co-treatment with MnTMPyP (Fig. 4Ab). In contrast, CH for 4 days had no effect on the phosphorylation status of PKC (Fig. 4Ab). Thus, these results suggest CIH leads to increased PKC activation.
Figure 4. The CIH–day 4-mediated increase in the RRP is due to an increased PKC phosphorylation.
Aa, adrenal tissue collected from control and CIH–day 4 mice was evaluated for relative levels of phospho-PKC with a pan-phospho-PKC polyclonal antibody. β-Actin served as a control for protein loading. Ab, phospho-PKC levels were quantified from 3 blots by densitometry and show that CIH–day 4 treatment resulted in an approximate 210% increase in total PKC phosphorylation over control levels whereas the Phospho-PKC levels in CIH–day 4 + MnTMPyP and CH showed no change. Ba, representative recordings show that treatment with protein kinase inhibitors blocks the CIH–day 4-mediated augmentation of evoked-capacitance jumps. Bb, pooled data from control and CIH-exposed cells with and without 5 min bath applied pretreatment with PKC inhibitors (n= 5, 12 and 7, respectively) showing that block of PKC restores Ca2+ influx to control levels. C, quantified data (n= 5, 5, 12 and 7, respectively) showing that treating control cells with PMA (100 nm) causes the RRP to grow in magnitude to match that observed in CIH–day 4-treated chromaffin cells (*P < 0.02). Likewise, block of PKC in CIH–day 4 cells causes the RRP to shrink in size to match that measured in control cells.
Next, we examined whether PKC inhibition had an impact on the CIH-induced increase in chromaffin cell RRP. We tested both Ro-31-8220 (100 nm) and Gö 6983 (100 nm), members of the bisindolylmaleimide family of PKC blockers. Both PKC inhibitors provided identical results and therefore the data were pooled. As shown in Fig. 4Ba, pharmacological blockade of PKC resulted in smaller evoked capacitance responses in CIH-treated cells. Likewise, PKC inhibitors restored Ca2+ influx in CIH-treated cells to control levels. Furthermore, phorbol 12-myristate 13-acetate (PMA; 100 nm), a potent activator of PKC, increased the RRP in control chromaffin cells to a level comparable with that seen in CIH-exposed cells (Fig. 4Bb). Taken together, these data indicate that CIH treatment alters stimulus–secretion functioning in chromaffin cells through a ROS-mediated regulation of PKC.
Discussion
The amount of catecholamine release from adrenal medullae is, in part, set by regulating the number of release-competent granules, a population that comprises the ‘ready-releasable pool’ (RRP; Heinemann et al. 1993). The present results demonstrate that CIH increases the RRP in adrenal chromaffin cells. The effects of CIH on RRP were time dependent in that 1 day exposure was ineffective and required a minimum of 2 days of CIH. Previous work demonstrated that PKC plays a fundamental role in regulation of secretory capacity by increasing the rate of granule recruitment to the RRP (Smith et al. 1998). The following lines of evidence suggest CIH-evoked increase in the RRP requires PKC activation: (1) CIH increased the phosphorylation of PKC at Thr514, which generates catalytically active enzyme (Keranen et al. 1995), and (2) PKC inhibitors prevented the effects of CIH on the RRP. In striking contrast, continuous hypoxia (CH) either for 2.5 h (equivalent to hypoxic duration accumulated over 4 days of CIH) or for 4 days did not increase the RRP. These findings are consistent with a previous report that CIH, but not CH, augments catecholamine secretion from adrenal medulla in rats (Kumar et al. 2006). Unlike CIH, CH did not result in increased PKC activation as evidenced by the probe of PKC phosphorylation at Thr514. Therefore, the absence of RRP increase by CH is conceivably due to its inability to activate PKC. Several isoforms of PKC have been identified. However, which of the PKC isoforms are affected by CIH and how they contribute to alteration in the RRP requires further investigation.
Our results provide evidence for the involvement of ROS-mediated signalling in CIH-evoked increase in the secretory capacity of adrenal chromaffin cells. First, MnTMPyP, a scavenger of O2− anions, prevented CIH-evoked increase in RRP. Second, CIH increased TBARS levels in the adrenal medulla. Third, MnTMPyP, a membrane-permeant scavenger of O2− anions, prevented CIH-induced increases in TBARSs, suggesting that elevated ROS leads to changes in TBARSs. How might ROS contribute to increases in RRP by CIH? ROS is a potent activator of PKC (Konishi et al. 1997; Yamamoto et al. 2000). Because CIH resulted in PKC activation and PKC inhibitors prevented increases in CIH-evoked increases in RRP, we suggest that ROS mediates CIH-induced PKC activation. Further studies, however, are needed to establish whether CIH-induced increase in PKC is a result of direct activation of PKC by ROS or concomitant inhibition of phosphatases, or both.
Chromaffin cells of the adrenal medulla release catecholamines through Ca2+-mediated fusion of secretory granules with the cell surface. Several groups have documented that PKC activation decreases depolarization-evoked Ca2+ influx in chromaffin cells (Sena et al. 1995; Gillis et al. 1996). This is further demonstrated in our data showing that CIH attenuated the stimulus-evoked Ca2+ currents in chromaffin cells. PKC inhibitors restored the CIH-dependent decrease in stimulus-evoked Ca2+ influx. Ca2+ plays multiple roles in the regulation of secretion from chromaffin cells. Not only does it act to trigger the final step of granule fusion and transmitter release, it also regulates steps upstream from the final fusion event. In order to sustain prolonged catecholamine release, chromaffin cells must recruit reserve granules to the RRP. This recruitment has been shown to be dependent on Ca2+ (von Rüden & Neher, 1993) through PKC-dependent and -independent mechanisms (Smith et al. 1998), and thus any perturbation to the influx of Ca2+ may act to alter secretory granule recruitment to the RRP. It is likely that CIH acts through a mechanism that is either insensitive to decreased Ca2+ influx or overcomes this potential limitation. Alternatively, CIH may act to increase the RRP by elevating cytosolic Ca2+ levels independent of voltage-gated calcium entry. For example, CIH may act to increase cytosolic Ca2+ by decreasing the extrusion of Ca2+ through Na+/Ca2+ exchange. Further experimentation is required to determine the effects of CIH on cell calcium mobilization and to determine its potential influence on the stimulus–secretion function of the adrenal medulla.
In summary, the present study demonstrates that CIH increases the RRP in adrenal chromaffin cells via ROS-mediated activation of PKC. Previous studies on rodents have shown that CIH results in elevated circulating catecholamines, which involves increased sympathetic activity and subsequent secretion from adrenal medulla (Bao et al. 1997). The present results suggest that CIH can directly affect the secretory capacity of chromaffin cells and contribute, in part, to the sustained elevated catecholamine levels, independent of sympathetic stimulation.
Acknowledgments
The research is supported by grants from National Institutes of Health (HL-25830 and HL-076537 to NRP; NS-052123 to CS) and the National Science Foundation (IBN-0344768 to CS). BK was supported by the NIH (TS HL07653).
Author's present address
S. A. Khan and N. R. Prabhakar: Centre for Systems Biology, Department of Medicine, University of Chicago, Chicago IL 60637, USA.
References
- Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- Chan SA, Polo-Parada L, Landmesser LT, Smith C. Adrenal chromaffin cells exhibit impaired granule trafficking in NCAM knockout mice. J Neurophysiol. 2005;94:1037–1047. doi: 10.1152/jn.01213.2004. [DOI] [PubMed] [Google Scholar]
- Chan SA, Smith C. Low frequency stimulation of mouse adrenal slices reveals a clathrin-independent, protein kinase C-mediated endocytic mechanism. J Physiol. 2003;553:707–717. doi: 10.1113/jphysiol.2003.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC. Sympathetic activity and blood pressure in the sleep apnea syndrome. Respiration. 1997;64(Suppl. 1):22–28. doi: 10.1159/000196732. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- Heinemann C, von Ruden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Current Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol. 2003;94:2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- Phillips BG, Somers VK. Neural and humoral mechanisms mediating cardiovascular responses to obstructive sleep apnea. Respir Physiol. 2000;119:181–187. doi: 10.1016/s0034-5687(99)00113-9. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol. 2005;32:447–449. doi: 10.1111/j.1440-1681.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena CM, Tomé AR, Santos RM, Rosário LM. Protein kinase C activator inhibits voltage-sensitive Ca2+ channels and catecholamine secretion in adrenal chromaffin cells. FEBS Lett. 1995;359:137–141. doi: 10.1016/0014-5793(95)00026-6. [DOI] [PubMed] [Google Scholar]
- Smith C. A persistent activity-dependent facilitation in chromaffin cells is caused by Ca2+ activation of protein kinase C. J Neurosci. 1999;19:589–598. doi: 10.1523/JNEUROSCI.19-02-00589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20:1243–1253. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- Voets T, Neher E, Moser T. Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron. 1999;23:607–615. doi: 10.1016/s0896-6273(00)80812-0. [DOI] [PubMed] [Google Scholar]
- von Rüden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuzaki H, Konishi H, Ono Y, Kikkawa U. H2O2-induced tyrosine phosphorylation of protein kinase Cδ by a mechanism independent of inhibition of protein-tyrosine phosphatase in CHO and COS-7 Cells. Biochem Biophys Res Commun. 2000;273:960–966. doi: 10.1006/bbrc.2000.3048. [DOI] [PubMed] [Google Scholar]
- Yang Y, Udayasankar S, Dunning J, Chen P, Gillis KD. A highly Ca2+-sensitive pool of vesicles is regulated by protein kinase C in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 2002;99:17060–17065. doi: 10.1073/pnas.242624699. [DOI] [PMC free article] [PubMed] [Google Scholar]