Abstract
The cytokine interleukin-15 (IL-15) has been demonstrated to have anabolic effects in cell culture systems. We tested the hypothesis that IL-15 is predominantly expressed by type 2 skeletal muscle fibres, and that resistance exercise regulates IL-15 expression in muscle. Triceps brachii, vastus lateralis quadriceps and soleus muscle biopsies were obtained from normally physically active, healthy, young male volunteers (n= 14), because these muscles are characterized by having different fibre-type compositions. In addition, healthy, normally physically active male subjects (n= 8) not involved in any kind of resistance exercise underwent a heavy resistance exercise protocol that stimulated the vastus lateralis muscle and biopsies were obtained from this muscle pre-exercise as well as 6, 24 and 48 h post-exercise. IL-15 mRNA levels were twofold higher in the triceps (type 2 fibre dominance) compared with the soleus muscle (type 1 fibre dominance), but Western blotting and immunohistochemistry revealed that muscle IL-15 protein content did not differ between triceps brachii, quadriceps and soleus muscles. Following resistance exercise, IL-15 mRNA levels were up-regulated twofold at 24 h of recovery without any changes in muscle IL-15 protein content or plasma IL-15 at any of the investigated time points. In conclusion, IL-15 mRNA level is enhanced in skeletal muscles dominated by type 2 fibres and resistance exercise induces increased muscular IL-15 mRNA levels. IL-15 mRNA levels in skeletal muscle were not paralleled by similar changes in muscular IL-15 protein expression suggesting that muscle IL-15 may exist in a translationally inactive pool.
The cytokine interleukin-15 (IL-15) is a recently discovered growth factor, which is expressed in skeletal muscle (Grabstein et al. 1994) and has been suggested to play a role in muscle–adipose tissue interaction (Argiles et al. 2005). In human skeletal muscle cell cultures, IL-15 induces an accumulation of myosin heavy chain (MHC) protein in differentiated myotubes, suggesting IL-15 as an anabolic factor in muscle growth (Furmanczyk & Quinn, 2003). Early skeletal muscle cell culture studies indicate that IL-15 may stimulate differentiation in conditions under which the strong differentiating effects of the insulin-like growth factors (IGFs) are inhibited (Quinn et al. 1997). Later studies showed that IL-15 not only exerts its effects independently of IGF-1, but in contrast to IGF-1, IL-15 has effects on fully differentiated myotubes (Quinn et al. 2002) and the hypertrophic action of IL-15 on skeletal muscle cells does not involve stimulation of skeletal myoblast proliferation or differentiation (Quinn et al. 2002). Quantitative real-time PCR shows that IL-15 is expressed by C2C12 myoblasts and that IL-15 mRNA levels are up-regulated more than tenfold in differentiated myotubes compared with undifferentiated myoblasts (Quinn et al. 2005). Furthermore, in contrast to IGF-I, which stimulated only protein synthesis under these culture conditions, IL-15 both stimulated protein synthesis and inhibited protein degradation in cultured skeletal myotubes (Quinn et al. 2002).
Studies of isolated rat skeletal muscles suggest that the main mechanism involved in the anabolic effects of IL-15 relies on a decrease in the proteolytic rate, as incubation of isolated rat muscle in the presence of human recombinant IL-15 resulted in decreased proteolytic rate, while it had no effect on total protein synthesis as measured by the incorporation of 14C-phenylalanine into muscle protein (Busquets et al. 2005). The potential therapeutic effect of IL-15 was demonstrated in an in vivo model, which showed that IL-15 was able to antagonize the enhanced muscle protein breakdown in a cancer cachexia model. Indeed, IL-15 treatment partly inhibited skeletal muscle wasting in tumour-bearing rats by decreasing (eightfold) protein degradative rates to values even lower than those observed in non-tumour-bearing animals. IL-15 did not modify the plasma levels of corticosterone and insulin in the tumour-bearing rats (Carbo et al. 2000). A follow-up study by the same group suggested that IL-15 may decrease muscle fibre apoptosis by affecting tumour necrosis factor (TNF)-alpha signalling (Figueras et al. 2004).
During the past few years, skeletal muscle has been acknowledged as a cytokine-producing organ. It has been demonstrated that skeletal muscles produce and express cytokines belonging to distinct different families. Thus, skeletal muscles have the capacity to express, e.g. TNF-alpha, IL-6, IL-8, IL-15 and IL-18 (Nieman et al. 2003; Chan et al. 2004). However, whereas expression of these cytokines in skeletal muscle is very low and of unknown physiological significance, it has recently been demonstrated that the expression of some cytokines is markedly enhanced by muscle contractions. Among these cytokines, solid evidence exists that IL-6 (Pedersen et al. 2003a,b; Febbraio & Pedersen, 2002, 2005) and IL-8 (Nieman et al. 2003; Chan et al. 2004; Akerstrom et al. 2005) are regulated by muscle contractions – both at the mRNA and the protein level. Recently, we reported that resting healthy human muscles express cytokines in a fibre type-specific manner. Immunohistochemistry demonstrated that TNF-alpha and IL-18 were expressed by type 2 fibres, whereas the expression of IL-6 was more prominent in type 1 compared with type 2 fibres (Plomgaard et al. 2005). The latter finding is, however, uncertain as a study by Hiscock et al. (2004) reported higher expression of IL-6 in type 2 fibres compared with type 1 fibres.
The regulatory role of muscle contraction in regard to IL-15 is not clear. Previous human studies have reported that skeletal muscle IL-15 mRNA levels were not changed immediately after a 3 h run (Nieman et al. 2003) and that plasma IL-15 (measured up to 6 h into recovery) did not change in response to 2.5 h of treadmill running (Ostrowski et al. 1998). Skeletal muscle IL-15 mRNA levels, measured immediately after a 2 h weight training bout, did not differ from baseline (Nieman et al. 2004), whereas plasma IL-15 protein was increased immediately after acute resistance exercise in one study (Riechman et al. 2004).
Given that IL-15 has been characterized as an anabolic factor, we tested the hypothesis that type 2 skeletal muscle fibres predominantly express IL-15. Triceps brachii, quadriceps pars vastus lateralis and soleus muscle biopsies were obtained from normally physically active healthy young male volunteers, because these muscles are characterized by different fibre type compositions, and the expression of IL-15 on both mRNA and protein levels was determined. In addition, we studied the effect of an acute heavy resistance exercise bout on IL-15 mRNA and protein expression in skeletal muscle.
Methods
Subjects
In Study 1, 14 healthy male subjects participated. They underwent a medical examination and a standard set of blood tests. The subjects were normally physically active, but did not participate in any competitive sports. In study 2, eight healthy normally physically active male subjects not involved in any kind of resistance exercise participated. Subject characteristics are listed in Table 1 as mean ±s.e.m. In both studies, subjects were informed both orally and in writing about risks and discomfort associated with the experimental protocol. The protocol was approved by the Municipal Ethical Committee for Copenhagen and Frederiksberg (KF: 01-034/03) and was in accordance with the Declaration of Helsinki.
Table 1.
Subject characteristics
| Study 1 | Study 2 | |
|---|---|---|
| Number of subjects | 14 | 8 |
| Age (years) | 24 ± 1 | 25 ± 1 |
| Height (cm) | 183 ± 2 | 186 ± 2 |
| Body mass (kg) | 79 ± 2 | 85 ± 3 |
| BMI (kg m−2) | 24 ± 1 | 25 ± 1 |
Study 1
The subjects were instructed not to perform any vigorous exercise 24 h prior to the experiment and to report at the laboratory after an overnight fast. Biopsies were obtained from three different muscle groups: triceps brachii caput medialis, quadriceps pars vastus laterelis and triceps surae pars soleus using a Bergström biopsy needle (Bergstrom, 1975). First the skin and the muscle fascia were anaesthetized using Lidocain (20 mg ml−1; SAD, Copenhagen, Denmark). A 5–7 mm incision was made and the Bergström needle was introduced into the muscle tissue, suction was applied and three to five cuts were made. The biopsy was split into two parts. Approximately 50 mg of the biopsy was used for RNA isolation. Any superficial blood was quickly removed and the biopsy was frozen in liquid nitrogen. The other part of the biopsy was prepared for histochemical analysis by mounting a small muscle piece in Tissue-Tek (Sakura Finetek, Zoeterwoude, the Netherlands) followed by freezing in 2-methyl-butane (Acros Organics, NJ, USA) pre-cooled in liquid nitrogen. Both samples were stored at −80°C until analysed.
Study 2
We chose to study the effects of strength training of m. quadriceps as multiple biopsies can be obtained from vastus lateralis without major side-effects. A pre-test was completed 1–2 weeks prior to the study to determine the workload to be used during the first set in the experiment. The pre-test was performed on a leg press and a knee extensor kicking machine and the load was gradually increased until the subjects could only just complete between six and eight repetitions. The subjects were allowed to rest as much as they wanted between the sets to avoid muscle fatigue. The subjects were specifically asked not to participate in any heavy exercise 48 h prior to the experiment. On the experimental day, a heavy-resistance training protocol that stimulated the vastus lateralis muscle was used. The exercise protocol contained four sets of 6–8, 6–8, 10–14 and 10–14 repetitions and was carried out on a leg press machine and then on a knee extensor machine. The intention was to reach total exhaustion in each set. The load was adjusted during the training so that the predetermined repetitions could be completed. Subjects were encouraged to lower the weights slowly (the eccentric phase) and to push them up as fast as possible (the concentric phase). There was a 90 s rest period between each set and a 3 min rest period between the two machines. The complete exercise routine (a total of eight sets) was completed in ∼20 min (Psilander et al. 2003).
Muscle biopsies were obtained from the vastus lateralis muscle using a biopsy needle (Bergstrom, 1975) with suction before exercise and 6, 24 and 48 h after the end of exercise. The pre-biopsy was obtained from the left leg and the 6, 24 and 48 h biopsies were obtained from the right leg. Incisions were made, at minimum, 3 cm apart, and only one biopsy was obtained from each incision site (Psilander et al. 2003). The biopsies were quickly frozen in liquid nitrogen and stored at −80°C until analysed.
Blood analyses
Blood samples were drawn from an antecubital vein. Plasma was obtained by drawing blood samples into glass tubes containing EDTA. The tubes were immediately spun at 3500 g for 15 min at 4°C and the supernatant was isolated and stored at −20°C until analyses were performed. Plasma levels of cholesterol, glucose and insulin were measured using routine laboratory methods. Plasma samples were assayed for concentrations of IL-15 using a Quantikine human IL-15 Immunoassay Kit (R & D Systems, Minneapolis, MN, USA). To optimize the IL-15 assay, 100 μl of sample were used instead of 50 μl, both for subject samples and for the standard curve. The dynamic range of the standard curve was 0.95–59.6 pg ml−1, where a double logarithmic scale showed a linear relationship with r2= 0.999. All samples were within the range of the standard curve. The detection limit was calculated to be 0.23 pg ml−1. The intra- and interassay coefficients of variation were validated within our work and were 2.7% and 7.7%, respectively.
RNA isolation, reverse transcription and real-time PCR
In both studies, total RNA was extracted from ∼50 mg muscle tissue using TRIzol Reagent (Invitrogen, Carlsbad, USA) following the manufacture's instruction or from ∼25 mg of muscle tissue by a modified guanidinium thiocyanate (GT)–phenol–chloroform extraction method as previously described (Pilegaard et al. 2000). The RNA concentration was determined spectrophotometrically and 2 μg total RNA was reverse transcribed either in a total volume of 100 μl using Taqman Reverse Transcription Kit (Applied Biosystems, NJ, USA) and random hexamers as primers or using the Superscript II RNase H− system (Invitrogen, CA, USA) and oligo dT as previously described (Pilegaard et al. 2000). Real-time PCR was performed using an ABI 7900 sequence detection system (Applied Biosystems). Primers and probes for IL-15 and the endogenous controls 18S rRNA, β-actin and GAPDH mRNA were amplified using pre-developed assay reagents (Applied Biosystems). The PCR conditions followed the procedure recommended by the manufacturer with a 10 μl reaction volume and each sample run in triplicates with 50 cycles for IL-15 and 40 cycles for the endogenous controls. The mRNA content of IL-15 and endogenous control genes was calculated from the cycle threshold (Ct) values, using a standard curve constructed from a serial dilution of aliquots of cDNA pooled from all the samples. Of the three endogenous controls measured, only β-actin mRNA content was independent of muscle type and β-actin was therefore suitable as an endogenous control and the IL-15 mRNA content was related to the β-actin mRNA content and the ratio is presented. To ensure normalization, the cDNA content was determined using OliGreen reagent (Molecular Probes, the Netherlands) as previously described (Lundby et al. 2005), and the IL-15 mRNA content was related to total cDNA content and the ratio is presented.
Muscle lysate and Western blotting
Muscle tissue was freeze-dried and dissected free of visual blood, fat and connective tissues. Depending on weight, muscle lysate was then prepared by the addition of either 0.4, 0.8 or 1.2 ml of a 0.1 m sodium phosphate buffer, pH 7.7, containing 1 μg ml−1 pepstatin A, 1 mm sodium orthovanadate, 1 mm sodium fluoride and complete protease inhibitor cocktail (Roche, Basel, Switzerland) to the freeze-dried muscle tissue. The muscle tissue was then homogenized using cooled racks in a Tissuelyser (Qiagen, Valencia, CA, USA) for 1 min at 30 Hz followed by 15 min incubation on ice. The homogenization and incubation on ice was repeated two or three times depending on the degree of homogenization of the tissue. Homogenates were then rotated end over end for 1 h at 4°C and centrifuged at 16 000 g at 4°C for 1 h. The supernatant protein concentrations were determined using the Bio-Rad DC kit (Biorad, Hercules, CA, USA) using BSA as standard. All determinations were done in triplicates. Muscle protein lysate per lane were diluted in 5 × sample buffer (167mm, 0.5 M dithiothreitol, 30% Glycerol, 10% SDS (w/v), 0.05%), boiled and separated on 12% Bis-Tris gels (Invitrogen, Taastrup, Denmark) and transferred to PVDF membranes (Hybond-P, GE Healthcare, Little Chalfont, UK). Membranes were then blocked for 1 h at room temperature in blocking buffer (Tris-buffered saline with 0.1% Tween-20 and 5% Top-Block (Sigma-Aldrich, St Louis, MO, USA), washed 3 times for 5 min in wash buffer (Tris-buffered saline with 0.1% Tween-20) and cut into pieces to determine both IL-15 and β-actin levels in the same samples. The membranes were then incubated overnight at 4°C in blocking buffer containing a primary antibody against either human IL-15 (AF315, R & D Systems) at a final concentration of 0.2 μg ml−1 or against actin (A3853, Sigma) at a final concentration of 0.3 μg ml−1. The membranes were then washed 3 times in wash buffer and incubated for 1 h at room temperature with rabbit anti-goat horseradish peroxidase (HRP) (P0449) or rabbit anti-mouse HRP (P0260) (Dako, Glostrup, Denmark) secondary antibody at 1: 2000 dilutions in blocking buffer, followed by 3 times 5 min washing in wash buffer. Following detection using Supersignal West Femto (Pierce, Rockford, IL, USA; IL-15) or ECL (GE Healthcare; actin) and quantification using a CCD image sensor (ChemiDoc XRS, Biorad) and software (Quantity One, Biorad), the IL-15 protein content was expressed as arbitrary units relative to actin protein content. It should be noted that the the IL-15 species measured using Western blot were the ∼19 kDa cell associated form (Bamford et al. 1998; Neely et al. 2001), whereas we were unable to detect the 15 kDa mature form of IL-15.
Histology and immunohistochemistry
For identification of muscle fibre composition, frozen biopsies of the triceps, vastus lateralis and soleus muscles were cut in 6 μm consecutive, transverse sections on a cryostat at −20°C. All sections were immediately collected on glass slides and processed for histology and histochemical analyses. Routine ATPase histochemistry was performed after pre-incubation at pH 10.30 allowing identification of different fibre types (fibre types 1 and 2) counting in average ∼450 fibres per biopsy. For epitope retrieval, sections were pre-incubated overnight in tris-EGTA (TEG) buffer (1.211 g Tris, 0.95 g EGTA, 1 l distilled H2O) at 60°C and afterwards in 0.5% H2O2 in Tris-buffered saline (TBS) (N6507, Sigma-Aldrich) for 15 min at room temperature to quench endogenous peroxidase. Afterwards, sections were incubated sequentially with 0.01–0.1% avidin (A9390, Sigma-Aldrich) followed by 0.001–0.01% biotin (B4501, Sigma-Aldrich), each step for 20 min, in order to block endogenous biotin.
Subsequently, sections were pre-treated in 10% normal goat serum (04009-1B, In Vitro, Denmark) in TBS for 30 min at room temperature in order to block non-specific binding before the immunohistochemistry was performed. The sections were incubated overnight at 4°C with primary, monoclonal IgG antibodies raised against human IL-15 (MAB647, R & D Systems), which was detected by using biotinylated goat anti-mouse IgG diluted 1: 200 (B8774, Sigma-Aldrich), followed by streptavidin–biotin–peroxidase complex (StreptABComplex/HRP) (K377, DakoCytomation, Denmark) prepared at the manufacturer's recommended dilutions for 30 min at room temperature. The immunoreaction was visualized using 0.015% H2O2 in 3,3-diaminobenzidine tetrahydrochloride (DAB) in TBS for 10 min at room temperature. The sections stained by immunohistochemistry were always processed simultaneously and under the same laboratory conditions. In order to evaluate the extent of non-specific binding in the immunohistochemical experiments, control sections were incubated in the absence of primary or secondary antibody. Results were considered only if these controls were negative. To control the IL-15 specificity of the antibody, we pre-absorbed the primary antibody with its corresponding antigenic protein. For this purpose, we used human IL-15 protein (PHC9154, Biosource, Germany). Results were considered only if this pre-absorbtion of the anti-IL-15 antibody resulted in negative immunostainings. For the simultaneous examination and recording of the stainings, a Zeiss Axioplan 2 light microscope was used.
Statistics
Data are presented as mean ±s.e.m. If data were not normally distributed, logarithmical transformation was performed and data presented as geometric means ±s.e.m. In study 1 the difference between mRNA and protein expression in the three muscle groups was tested using a one-way ANOVA calculated in Excel 2000 (Microsoft). In study 2 the effect of resistance exercise on mRNA and protein expression was tested using a one-way ANOVA for repeated measures (SigmaStat, USA). If the ANOVA showed a significant difference, a Student's paired t test with Bonferroni correction was used as post hoc test in study 1 and Student–Neuman–Keuls post hoc test was used in study 2 to locate differences. P < 0.05 was considered significant.
Results
Study 1. IL-15 expression in triceps, vastus lateralis and soleus muscle
Fibre type composition
Soleus consisted of 68–83%, vastus lateralis of 40–56% and triceps only of 20–33% type 1 fibres. MHC IIa mRNA content correlated negatively (P < 0.05) with the percentage occurrence of type 1 fibres (data not shown) (n= 7).
IL-15 mRNA and protein expression
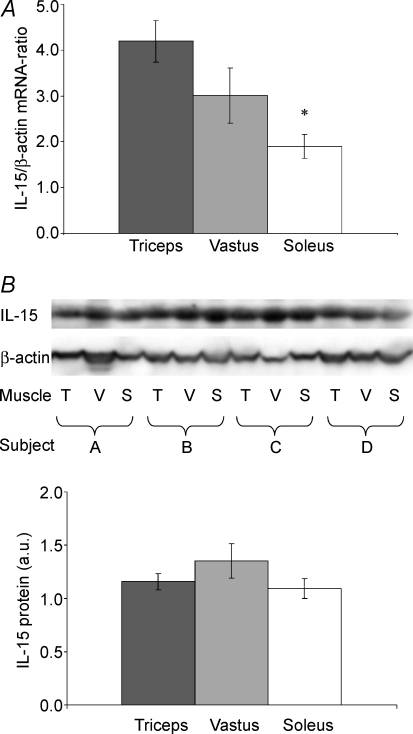
The highest level of IL-15 mRNA was found in triceps muscle with a twofold higher level than in soleus (P < 0.001) (Fig. 1A). The level of IL-15 mRNA tended to be lower in vastus lateralis than in triceps (P= 0.07) (Fig. 1A) (n= 14). No differences were apparent in IL-15 protein content in triceps, vastus lateralis and soleus as visualized by Western blot (n= 14) (Fig. 1B).
Figure 1. IL-15 in triceps, vastus lateralis and soleus.
IL-15 mRNA levels (RT-PCR) (A) and IL-15 protein levels (Western blotting) (B) are shown for triceps (T), vastus lateralis (V) and soleus (S) muscles (n= 14). Means ±s.e.m. are shown; * shows significant difference between triceps and soleus (P < 0.001).
IL-15 immunohistochemistry
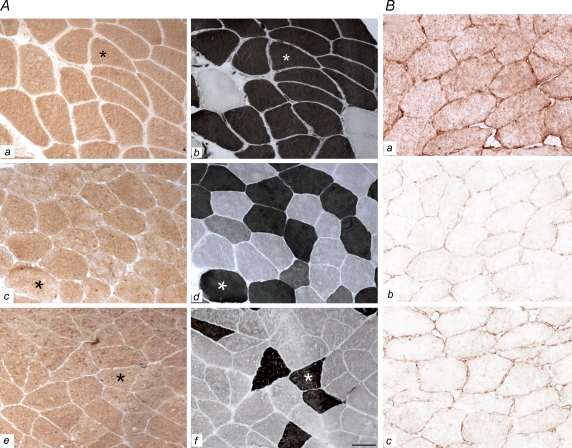
IL-15 protein expression as measured by immunohistochemistry (IHC) is shown in Fig. 2. The IL-15 is expressed homogenously within the fibres without any fibre type difference as judged by comparing IL-15 immunostainings with neighbouring muscle fibres stained for myofibrillar ATPase. Also, IL-15 protein expression was comparable in the examined muscle groups: the triceps, vastus lateralis and soleus muscle (Fig. 2a). The extent of non-specific (false positive) binding in the immunohistochemistry was evaluated by applying control sections. These were incubated in the absence of an essential part of the IHC (such as omitting the primary or secondary antibody during the IHC) or by pre-absorbtion of the primary antibody with its corresponding antigenic protein. Results were considered only if these controls were negative (Fig. 2B).
Figure 2. IL-15 expression.
A, IL-15 expression is shown for triceps (a), vastus lateralis (c) and soleus (e) muscles. Myofibrillar ATPase staining is shown in neighbouring tissue sections of the triceps (b), vastus lateralis (d) and soleus (f) muscles (b, d and f are consecutive sections to those shown in a, c and e). Fibre types are distinguished by ATPase staining and fibres appearing black are type 2 fibres. The asterisks depict matching fibres for each muscle group (n= 7). As displayed, IL-15 protein expression is comparable in muscle fibre types and in the different muscles of triceps, vastus lateralis and soleus. Scale bar: 89 μm. B, IL-15 expression as defined by our standard IL-15 IHC is shown in a, while negative control sections are shown in b and c. Bb, negative control section as seen after the primary antibody was pre-absorbed with its corresponding antigenic protein, thereby preventing the following IHC binding of primary IL-15 antibodies to endogenous IL-15 proteins of the muscle. Bc, negative control section after incubation in the absence of primary IL-15 antibodies, whereby the IHC results in negative immunostaining. Scale bar: 47 μm
Study 2. The effect of resistance exercise on muscle IL-15 expression and plasma IL-15
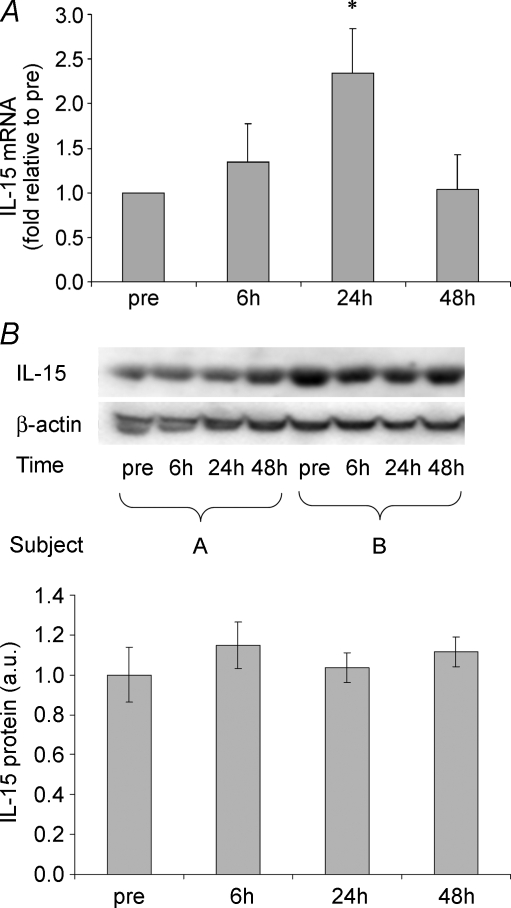
The heavy resistance exercise session elicited a twofold up-regulation of IL-15 mRNA at 24 h of recovery. The IL-15 mRNA levels had returned to pre-exercise levels 48 h after the end of exercise (Fig. 3A). Western blot revealed no change in IL-15 protein levels at the investigated time points after resistance exercise (Fig. 3B). It appears that IL-15 protein levels did not change with exercise. Plasma IL-15 concentrations (mean ±s.e.m.) were 1.99 ± 0.15, 1.84 ± 0.13, 1.90 ± 0.12 and 1.88 ± 0.17 pg ml−1 when measured before and 6, 24 and 48 h post-exercise, respectively, with no significant effect of resistance exercise.
Figure 3. IL-15 in resistance exercise.
IL-15 mRNA levels (A) and IL-15 protein levels (Western blotting) (B) are shown for vastus lateralis muscle biopsies obtained before, as well as 6, 24 and 48 h post one bout of resistance exercise. Means ±s.e.m. are shown; * shows significant difference from pre-exercise value (P < 0.05).
Discussion
The novel findings in the present study were that the level of IL-15 mRNA was higher in human skeletal muscles dominated by type 2 muscle fibres than in muscles dominated by type 1 muscle fibres, and that IL-15 mRNA content increased following a bout of resistance exercise. Previous studies in humans have not shown any change in IL-15 mRNA level following either resistance or endurance exercise (Nieman et al. 2003, 2004). The biopsies in these studies were obtained immediately after the end of exercise. We found an elevation of IL-15 mRNA 24 h after resistance exercise, which is not in contrast to previous findings. Despite an increase in IL-15 mRNA levels, we did not measure elevated levels of plasma IL-15 protein after a bout of resistance exercise. This is in contrast to Riechman et al. (2004), who previously found a small, but significant increase in plasma IL-15 protein following resistance exercise. Thus, Riechman et al. (2004) reported an increase in plasma IL-15 of approximately 5% in plasma obtained before and immediately after the end of a resistance exercise bout, but did not obtain any measurements at later time points. In general, our findings of a difference in IL-15 mRNA level, both after resistance exercise and between different muscle groups, were not paralleled by similar differences in muscular IL-15 protein expression, visualized by Western blot and immunohistochemistry.
It has been suggested that IL-15 may exist in a translationally inactive pool, which is stored in the cell and ready for translation (Bamford et al. 1998; Van & Grooten, 2005). We cannot extrapolate the findings in immune cells directly to our muscle data; however, the idea that IL-15 transcription may take place without the formation of IL-15 protein is compatible with our findings.
The expression of IL-15 mRNA has been found in many distinct tissues (Grabstein et al. 1994; Giri et al. 1995). The IL-15 mRNA was shown by Grabstein et al. (1994) to be expressed in human muscle. Nieman et al. (2003) have further shown that IL-15 is one of the most abundantly expressed cytokines at the mRNA level in human muscle, when comparing expression levels following the RT-PCR technique. Adding to this, several studies (Quinn et al. 1995, 2002; Carbo et al. 2000; Furmanczyk & Quinn, 2003) have shown that IL-15 has an anabolic effect on muscle cell culture and decreases the muscle degradation rate in a cachexia model, suggesting that IL-15 might be of importance in muscle growth.
We demonstrated a twofold increase in the level of IL-15 mRNA. In a study by Quinn et al. (2002), where the mouse C2 skeletal myogenic cell line was transduced with a retroviral expression vector for IL-15, it was shown that factors secreted from the transduced cells could induce increased myofibrillar protein accumulation in co-cultured myotubes, suggesting that IL-15 may act in a paracrine manner on adjacent muscle cells (Quinn et al. 2002). If this is the case, even very small changes in IL-15 levels may give rise to a considerable change in protein concentration with an effect on adjacent muscle cell growth.
In conclusion, the present study demonstrated that the level of IL-15 mRNA was enhanced in muscle groups dominated by type 2 fibres and that a bout of resistance exercise induced increased muscular IL-15 mRNA levels 24 h post-exercise.
Acknowledgments
Ruth Rousing, Hanne Villumsen and Bettina Starup Mentz are acknowledged for their technical assistance. The Centre of Inflammation and Metabolism is supported by a grant from the Danish National Research Foundation (no. 02-512-55). The study was further supported by the Danish Medical Research Council (nos 22-01-0019 and 271-06-0527), the Lundbeck Foundation and the Novo Nordisk Foundation. The Copenhagen Muscle Research Centre is supported by grants from the Copenhagen Hospital Corporation, the University of Copenhagen and the Faculties of Science and of Health Sciences at this University.
References
- Akerstrom TC, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK. Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol. 2005;563:507–516. doi: 10.1113/jphysiol.2004.077610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Argiles JM, Lopez-Soriano J, Almendro V, Busquets S, Lopez-Soriano FJ. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev. 2005;25:49–65. doi: 10.1002/med.20010. [DOI] [PubMed] [Google Scholar]
- Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Laboratory Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Busquets S, Figueras MT, Meijsing S, Carbo N, Quinn LS, Almendro V, Argiles JM, Lopez-Soriano FJ. Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol Med. 2005;16:471–476. [PubMed] [Google Scholar]
- Carbo N, Lopez-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer. 2000;83:526–531. doi: 10.1054/bjoc.2000.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol. 2004;287:R322–R327. doi: 10.1152/ajpregu.00030.2004. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Figueras M, Busquets S, Carbo N, Barreiro E, Almendro V, Argiles JM, Lopez-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2004;569:201–206. doi: 10.1016/j.febslet.2004.05.066. [DOI] [PubMed] [Google Scholar]
- Furmanczyk PS, Quinn LS. Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biol Int. 2003;27:845–851. doi: 10.1016/s1065-6995(03)00172-0. [DOI] [PubMed] [Google Scholar]
- Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–994. doi: 10.1096/fj.03-1259fje. [DOI] [PubMed] [Google Scholar]
- Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD, Pilegaard H. Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol. 2005;95:351–360. doi: 10.1007/s00421-005-0022-7. [DOI] [PubMed] [Google Scholar]
- Neely GG, Robbins SM, Amankwah EK, Epelman S, Wong H, Spurrell JC, Jandu KK, Zhu W, Fogg DK, Brown CB, Mody CH. Lipopolysaccharide-stimulated or granulocyte-macrophage colony-stimulating factor-stimulated monocytes rapidly express biologically active IL-15 on their cell surface independent of new protein synthesis. J Immunol. 2001;167:5011–5017. doi: 10.4049/jimmunol.167.9.5011. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs MF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol. 2004;96:1292–1298. doi: 10.1152/japplphysiol.01064.2003. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513:889–894. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003a;24:113–119. doi: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Keller P, Keller C, Fischer C, Hiscock N, Hall Gv, Plomgaard P, Febbraio MA. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003b;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Penkowa M, Pedersen BK. Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc Immunol Rev. 2005;11:53–63. [PubMed] [Google Scholar]
- Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol. 2003;95:1038–1044. doi: 10.1152/japplphysiol.00903.2002. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002;280:55–63. doi: 10.1006/excr.2002.5624. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Haugk KL, Damon SE. Interleukin-15 stimulates C2 skeletal myoblast differentiation. Biochem Biophys Res Commun. 1997;239:6–10. doi: 10.1006/bbrc.1997.7414. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Strait-Bodey L, Anderson BG, Argiles JM, Havel PJ. Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int. 2005;29:449–457. doi: 10.1016/j.cellbi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol. 2004;97:2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- Van Belle T, Grooten J. IL-15 and IL-15Ralpha in CD4+T cell immunity. Arch Immunol Ther Exp (Warsz) 2005;53:115–126. [PubMed] [Google Scholar]