Abstract
Renshaw cell properties have been studied extensively for over 50 years, making them a uniquely well-defined class of spinal interneuron. Recent work has revealed novel ways to identify Renshaw cells in situ and this in turn has promoted a range of studies that have determined their ontogeny and organization of synaptic inputs in unprecedented detail. In this review we illustrate how mature Renshaw cell properties and connectivity arise through a combination of activity-dependent and genetically specified mechanisms. These new insights should aid the development of experimental strategies to manipulate Renshaw cells in spinal circuits and clarify their role in modulating motor output.
‘…they may appropriately be given the distinguishing title of “Renshaw cells”’ (Eccles et al. 1954). For more than 50 years there has been sustained interest in the special class of interneurons – Renshaw cells – that mediate recurrent inhibition of spinal α-motoneurons in the ventral horn of mammals and were named in honour of their discoverer, Birdsey Renshaw (Renshaw, 1941, 1946). Renshaw cells are capable of firing a high-frequency burst discharge in response to activation via motoneuron axon collaterals, and this key feature has been central to their physiological identification and analysis by traditional electrophysiological approaches in vivo. Early studies of recurrent inhibition and ‘direct’ inhibition by Eccles, Curtis, and their colleagues, were critical in establishing the nature of chemical inhibitory neurotransmission and the organization of inhibitory circuits in the central nervous system (CNS) (Curtis & Andersen, 2001; Burke, 2006). These early studies also contributed to defining the excitatory effects on central neurons of acetylcholine, glutamate and aspartate, and the inhibitory actions of GABA and glycine. Moreover, their analysis of the cholinergic synapses between motor axon collaterals and Renshaw cells provided the first experimental proof of Dale's principle on the identity of neurotransmitter release at all branches of individual axons (see historical review of Eccles' life and work by Curtis & Andersen, 2001). Throughout, Renshaw cell accessibility and unambiguous identification was of vital importance in these classical studies that contributed so many novel insights into basic neurobiological principles.
Despite the detailed information generated in the 1950s and 1960s, there remained controversy over the existence of Renshaw cells and their role in recurrent inhibition. The questions at the time prompted Willis (1971) to review, and support convincingly, the evidence that recurrent inhibition of motoneurons is mediated by activity of Renshaw cells following their excitation by recurrent collaterals of motor axons. This view, based largely on studies of the cat spinal cord, is retained to the present time and is integral to our understanding of motor control. Although it is no longer necessary, as it was for Willis (1971), to ‘make a case’ for the Renshaw cell, a strong case may be made now for continuing study of this fascinating interneuron because of the fundamental insights that can be obtained concerning mechanisms of circuit and synapse development and regulation of interneuron function in the CNS. A broad set of key identification criteria can now be applied to Renshaw cells and include electrophysiological, pharmacological, anatomical and genetic characteristics. These have enabled new critical analyses and stimulated new research that is advancing at a fast pace compared with other spinal interneurons. In this context, the title of this paper paraphrases the title of Willis' original review, and reflects our belief that the Renshaw cell remains a rare and valuable commodity for analysis of spinal interneuronal circuits and their development.
Although Renshaw cells participate in a relatively ‘simple’ local recurrent inhibitory circuit, and much is known about their physiology and morphology, it is humbling to recognize that there is as yet no definitive functional hypothesis regarding their contributions to motor control and behaviour. It is known that Renshaw cells synapse directly on Ia inhibitory interneurons, ventral spinocerebellar neurons and other Renshaw cells, as well as on α-motoneurons. Potentially, as well as mediating motoneuron recurrent inhibition, Renshaw cells can modulate both recurrent inhibition produced by other Renshaw cells and Ia reciprocal inhibition between antagonist motor pools. Further complexity arises because motoneurons themselves receive different levels of recurrent inhibition depending on their motor unit characteristics. Experiments in anaesthetized cats, as well as modelling studies, suggested that recurrent inhibition mediated by Renshaw cells can influence motoneuron recruitment/de-recruitment, modulate the activity in synergist and antagonist motoneuron pools, and produce decorrelation and/or synchronization of motor output (reviewed extensively in: Hultborn et al. 1979; Baldissera et al. 1981; Windhorst, 1990; Jankowska, 1992; Maltenfort et al. 1998; Mattei et al. 2003). In decerebrate cats, and in recent models of spinal locomotor circuitry, Renshaw cells exhibit rhythmic activity during fictive locomotion in the absence of sensory feedback (see Rybak et al. 2006a,b). Various models and experimental data over the last 35 years thus suggest a variety of potential physiological roles for Renshaw cell inhibition in locomotor control, at least in the adult. We suggest that Renshaw cells have distinct functional roles during different stages of development and maturation of spinal circuitry. In the adult spinal cord there is no consensus, yet, on the functional meaning of ‘recurrent inhibition’ although it could be predicted that there should be alterations in force generation/maintenance as well as rigidity, spasticity or tremor as a consequence of Renshaw cell dysfunction. However, it has been difficult to directly test these and related hypotheses because of the lack of experimental tools to selectively antagonize/delete/knockout Renshaw cells or monitor their behaviour in freely moving non-anaesthetized animals (see also Fetcho, 2001). The new understanding of the cellular, synaptic and genetic properties of Renshaw cells reviewed below should aid the development of experimental strategies to manipulate Renshaw cells in vivo and clarify their functional role in modulation of motor output.
Anatomical identification of Renshaw cells – expression of calcium binding proteins
Early studies identified a ‘Renshaw cell area’ in ventral lamina VII by marking the positions from where population or single unit Renshaw cell-like responses were recorded (Thomas & Wilson, 1965; Willis, 1971). More accurate anatomical definition followed the successful application of intracellular labelling techniques (Jankowska & Lindstrom, 1971; Van Keulen, 1979; Lagerback & Kellerth, 1985a,b; Fyffe, 1990, 1991a,b; see also Fig. 1), and 3D morphometric data from single identified Renshaw cells are now available permitting the creation of detailed computational models (Alvarez et al. 1997; Bui et al. 2003, 2005; Ascoli, 2006). Combination of intracellular recording/labelling with immunolabelling confirmed the glycinergic/GABAergic nature of Renshaw cell-mediated neurotransmission in recurrent inhibition (e.g. Fyffe, 1991b; Schneider & Fyffe, 1992; also, Cullheim & Kellerth, 1981). Most importantly, the combined approaches led to validation of criteria useful for the morphological identification of Renshaw cells (Alvarez et al. 1997; Carr et al. 1998).
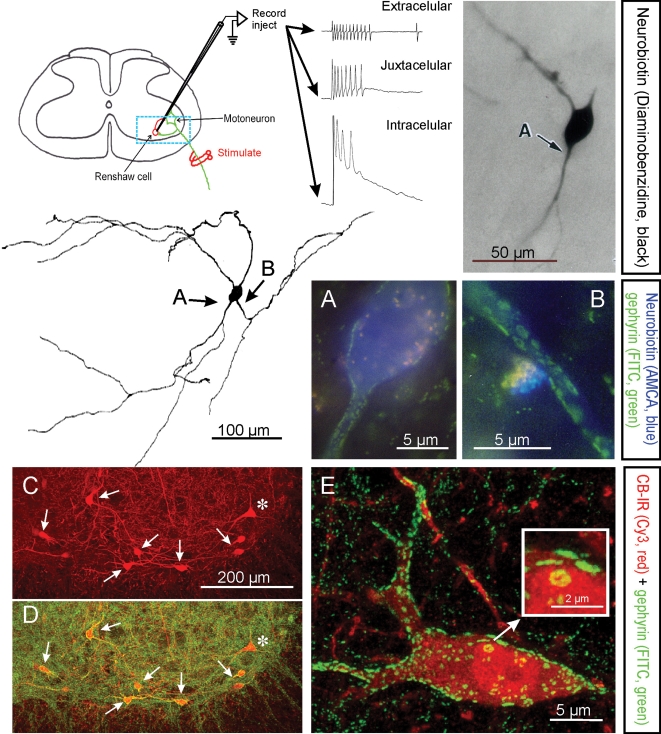
Figure 1. Electrophysiological, morphological and neurochemical characteristics of Renshaw cells.
Renshaw cells are classically identified by their high-frequency discharge in response to antidromic motor axon action potentials recorded either extracellulary, juxtacellularly or intracellularly. The diagram shows the basic experimental set-up and resulting recordings. The typical high-frequency burst of action potentials recorded extracellularly is diminished following impalement of the cell but a long EPSP is revealed. The blue box indicates the approximate region of neuropil represented in C and D. Electrophysiologically identified Renshaw cells were injected with neurobiotin (Alvarez et al. 1997), which was initially revealed with α-methyl-coumarin (AMCA) to allow visualization of the pattern of gephyrin immunolabelling (FITC fluorochrome) on the recorded cell. Subsequent processing with diaminobenzidine permitted full reconstruction of the dendritic trees of the same cells. In this manner it was found that Renshaw cells are characterized by very large patches of gephyrin immunoreactivity in their proximal dendritic regions and somatic membrane; the frequency of these clusters drops markedly on more distal dendrites (Alvarez et al. 1997). A and B show at high magnification gephyrin-immunoreactive clusters in the regions indicated with arrows in the reconstructed neuron. C and D, Renshaw cells are also strongly immunoreactive (IR) for calbindin and the large majority of calbindin-IR cells in the ventral spinal cord display the large gephyrin clusters characteristic of Renshaw cells. In the confocal images shown in C and D only one calbindin-IR cell (*) did not display large gephyrin-IR clusters and was probably not a Renshaw cell. Non-Renshaw calbindin-IR cells tend to have larger cell bodies and do not receive strong inputs from cholinergic boutons. E, 2D projection of a 3D reconstructed calbindin-IR Renshaw cell displaying the characteristic large gephyrin clusters (note much larger clusters on the calbindin-IR cells than in the adjacent neuropil). One large cluster in the soma is shown magnified in the inset. Gephyrin-IR clusters represent inhibitory postsynaptic densities and the ones on Renshaw cells are among the larger found in the spinal cord.
Immunohistochemical analysis of the distribution of gephyrin (Fig. 1), an abundant scaffolding protein of inhibitory postsynaptic densities (PSDs), along dendritic arbors of spinal neurons revealed that Renshaw cells uniquely display a high density of proximal inhibitory synapses with uncommonly large PSDs (Alvarez et al. 1997). In rat Renshaw cells, the mean PSD areas identified by gephyrin immunoreactivity gradually increase with age after birth, and in the adult range from 0.09 to 6.11 μm2, with a mean gephyrin cluster size in adult rats of 0.56 ± 0.02 μm2 (mean ±s.e.m.; Geiman et al. 2000). These PSDs are one to two orders of magnitude larger than typical inhibitory PSDs in other spinal neurons (Alvarez et al. 1997) or elsewhere in the CNS (e.g. Nusser et al. 1998). Critically, the characteristic large gephyrin clusters/inhibitory PSDs were displayed by all electrophysiologically identified Renshaw cells and were not present on other types of spinal neurons. The Renshaw cell-specific gephyrin signature was then observed in cells located in the ‘Renshaw cell area’ in all mammalian species studied so far. Subsequent studies using this identification criterion confirmed that rodent Renshaw cells express high levels of the calcium-buffering protein calbindin Dk28 (Carr et al. 1998; Geiman et al. 2000), a suggestion first made in the primate spinal cord (Arvidsson et al. 1992). Calbindin immunoreactivity, combined with anatomical criteria such as location and cell size (Renshaw cells in adult cat and rat have mean soma diameters of around 20 to 25 μm; Fyffe, 1990; Geiman et al. 2000), makes the Renshaw cell pool easily identifiable in histological sections of adult, neonatal and embryonic rat and mouse spinal cords (see Geiman et al. 2000; Mentis et al. 2006). However, this approach should be used with caution because Renshaw cells are not the only calbindin-expressing cells in the spinal cord (Fig. 1C, asterisk). The expression of calbindin in non-Renshaw cell spinal interneurons changes during postnatal development, with an overall decrease in the level of expression and number of calbindin-positive neurons (Zhang et al. 1990; Smith et al. 2005). More recent studies indicated that rodent Renshaw cells also express parvalbumin and calretinin, in addition to calbindin, but expression of the former proteins is weaker and not uniform and many other cells express them at higher levels; therefore, they are not reliable markers for Renshaw cell identification. Renshaw cells in the cat, the species in which they were first physiologically identified, express little calbindin immunoreactivity (Carr et al. 1998; Mentis et al. 2006).
Immunocytochemical identification of Renshaw cells provided the first estimates of Renshaw cell abundance. Approximately 750 Renshaw cells were estimated in the sixth lumbar (L6) segment of the adult cat spinal cord, and most are located in the ventral part of lamina VII (Carr et al. 1998). In experiments to count the number of Renshaw cells in the comparable L4 and L5 segments of mouse/rat spinal cord, it was found that the Renshaw cell population accounts for about 2–3% of all ventral interneurons, with a predicted Renshaw cell:motoneuron ratio of about 1 : 5 (FitzSimons et al. 2006). In addition, Renshaw cells are of small size compared with other ventral interneurons and extend small dendritic arbors. Their axons make local arborizations and extend rostro-caudally only a few ipsilateral spinal segments (McCurdy & Hamm, 1994; Lagerback & Kellerth, 1985a; Fyffe, 1991a,b; Alvarez et al. 1997; Bui et al. 2003). Thus, in agreement with the functional organization of recurrent inhibition (Eccles et al. 1961; Hamm, 1990; Turkin et al. 1998) the anatomical substrate also suggests large convergence of motor inputs on a small population of Renshaw cells that then send a highly divergent output to several motor pools within a restricted rostro-caudal domain limited by the extension of the Renshaw cell axon. There are also subdvisions in the Renshaw cell pool related to particular motor actions, such as extensor versus flexor coupled Renshaw cells (Noga et al. 1987; Nishimaru et al. 2006). Moreover, recurrent inhibition is more intense over motor pools involved with more stereotyped motor output (e.g. proximal muscles) than on motoneurons that require a high degree of independent control to generate fine movement (Hamm, 1990; Turkin et al. 1998). Viewed in this manner the electrophysiological and anatomical data suggest that Renshaw cells provide a limited set of parallel inhibitory pathways that modulate simultaneously the activity of motoneuron ensembles related to broad motor synergies.
Synaptic architecture of Renshaw cells
The distribution of various synaptic inputs over the soma–dendritic membrane is a key factor in defining a postsynaptic neuron's integrative properties. The availability of specific markers for Renshaw cells and for identification of diverse populations of pre- and postsynaptic elements recently permitted the study of their synaptic organization with unprecedented detail, expanding on early ultrastructural synaptological analyses (Lagerback & Ronnevi, 1982a,b; Lagerback, 1983). The studies reviewed here also revealed some of the developmental processes that refine synaptic input specificity on these interneurons, and have led to challenges of long-held and commonly accepted views on the nature of the synaptic drive to Renshaw cells. Current evidence indicates that Renshaw cells receive the majority of their monosynaptic synaptic inputs from segmental (sensory, interneuronal and motoneuron) sources and very few direct inputs from descending systems. Synapses from motor axons and other segmental sources can be identified by immunolabelling against transporters present in cholinergic and glutamatergic terminals, namely, the vesicular acetylcholine transporter (VAChT, in motor axons) and vesicular glutamate transporter isoforms 1 and 2 (VGLUT1, in sensory synapses; VGLUT2, in spinal interneurons; Fig. 2). Excitatory synapses identified by these markers preferentially target dendrites (80% of all excitatory synaptic inputs are on dendrites) and their density increases at mid-dendritic regions (∼75–300 μm distal from the cell body: Alvarez et al. 1999; Mentis et al. 2006). In contrast, > 90% of the proximal synaptic coverage is inhibitory. Renshaw cells therefore display a striking proximo-distal segregation of inhibitory versus excitatory inputs.
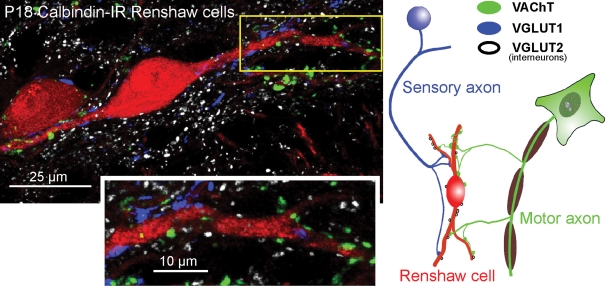
Figure 2. Distribution of excitatory synapses on Renshaw cells identified by the synaptic markers VGLUT1, VGLUT2 and VAChT.
The confocal image (projection of 4 confocal optical planes; z-step = 0.5 μm) shows calbindin-IR (Cy3, red) Renshaw cells contacted by VAChT-IR (fluorescein-isothiocyanate (FITC), green), VGLUT1-IR (Alexa 405, blue) and VGLUT2-IR (Cy5, white) varicosities, summarized in the diagram at right. Individual confocal images were obtained using an Olympus FV1000 confocal microscope with excitation lines at 405, 488, 568 and 635 nm, and a 60 × oil (N.A. = 1.4) objective digitally zoomed 2 ×. Presumed contacts between varicosities and apposed dendrites are identified where varicosity immunofluorescence partially overlaps with dendrite calbindin immunofluorescence in a single optical plane. Most VGLUT1 and VAChT-IR inputs target dendrites, and the indicated, relatively proximal, dendritic segment (boxed area) is shown at higher magnification in the inset below. The density of VGLUT1 and VACHT-IR contacts increases distally (not shown; but see Fig. 3 for more distal contacts). Synaptic surface space on somata and most proximal dendrites is dominated by inhibitory synapses (see Fig. 1). VAChT and VGLUT1-IR varicosities are usually of relatively large size (see also Alvarez et al. 1999; Mentis et al. 2006), whereas VGLUT2-IR contacts tend to be of smaller size. Relative abundance of each type of excitatory input on Renshaw cells is on average VAChT > VGLUT2 > VGLUT1.
Motor axon synapses
VAChT-IR motor axon synapses are present at high density on adult Renshaw cell dendrites; each Renshaw cell receives inputs from multiple motoneurons and the overall density of these cholinergic synapses is two to four times greater than for VGLUT1- or VGLUT2-IR contacts. Structurally, strings of VAChT-IR synaptic varicosities arising from each presynaptic motor axon wrap around one or more dendritic segments of individual Renshaw cells (Fig. 3C), an organization that probably results in each collateral establishing large numbers of release sites on the postsynaptic cell (Alvarez et al. 1999). This type of connectivity is rare for the more sparsely distributed VGLUT1- and VGLUT2-IR contacts. As at the neuromuscular junction (NMJ), the structural relationships between motor axon synapses and postsynaptic Renshaw cell dendrites probably reflect a relatively high probability of release and quantal content, and thus contribute to forming cholinergic synapses of maximal strength and high safety factor. The profuse cholinergic synapses from motor axons are paralleled by a relatively high postsynaptic sensitivity to acetylcholine as revealed by acetylcholine-evoked whole-cell currents (Fig. 3B). α4 and α2 nicotinic receptor subunits are expressed by Renshaw cells, but while α4 subunits are expressed also by other spinal interneurons, α2 subunits appear restricted to Renshaw cells (Dourado & Sargent, 2002; Ishii et al. 2005). Thus, α2 subunit expression correlates with the cells' high postsynaptic sensitivity to acetylcholine. The strength of motor axon synapses on Renshaw cells is evident by the high-frequency burst of action potentials evoked reliably even by single action potentials traveling in single motor axons (Eccles et al. 1954; Van Keulen, 1981). Long-duration EPSPs (> 50 ms) underlie these synaptically evoked bursts of action potentials (Eccles et al. 1961; Walmsley & Tracey, 1981). However, the synaptic currents responsible for these depolarizations are not yet well explained since unitary nicotinic EPSCs in Renshaw cells are of relatively short duration (time constant ∼5 ms), at least in neonates (Dourado & Sargent, 2002). Active membrane properties and/or further receptor mechanisms might contribute to the long duration of the motor axon EPSP.
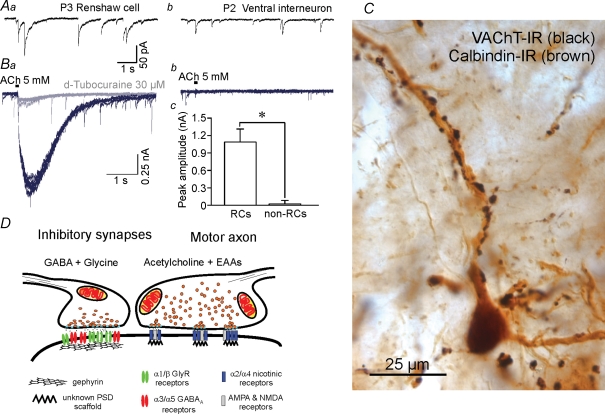
Figure 3. Renshaw cells receive inhibitory and cholinergic inputs with specific functional and morphological characteristics.
A, comparison of inhibitory currents in Renshaw cells and other spinal interneurons. Inhibitory mPSCs isolated pharmacologically with blockers of nicotinic, AMPA and NMDA receptors and recorded in the presence of tetradotoxin (see Gonzalez-Forero & Alvarez, 2005) display biphasic glycinergic (fast) and GABAergic (slow) components (Aa). Peak amplitudes are higher than in other interneurons (Ab) and dominated by the opening of glycine receptors. The decays have long GABAA-dependent components that correspond with high expression of α3/α5-containing GABAA receptors. Other ventral spinal interneurons generally lack these long GABAergic decays (Ab). B, Renshaw cells also display high postsynaptic sensitivity to acetylcholine (Ba and Bc) while other ventral interneurons are relatively insensitive (Bb and Bc) (D. Gonzalez-Forero and F. J. Alvarez, unpublished observations). Traces show responses to 30 ms pulses of 5 mm acetylcholine (ACh) from the same neurons from which records Aa and Ab were obtained before nicotinic receptor block. Recordings were performed on cells voltage-clamped at −75 mV in 0 Ca2+ and 0 Mg2+. All neurons with long GABAAR mPSCs exhibited ACh-evoked postsynaptic responses, whereas those with faster or no GABAergic events showed either small-amplitude or no responses (Bc). Postsynaptic responsiveness to ACh in Renshaw cells was effectively abolished with 30 μm d-tubocurarine (grey trace). C, motor axons imunolabelled with diamunobenzidine(DAB)–silver intensified VAChT immunoreactivity (black in the image) establish a large number of synapses around adult calbindin-IR Renshaw cell dendrites (brown in the image). Ultrastructurally each bouton displays multiple small active zones (F.J. Alvarez unpublished observations, not shown) and co-release acetylcholine and EAAs on PSDs that presumably co-localize nicotinic, AMPA and NMDA receptors (Mentis et al. 2005). These characteristics result in quite secure synapses with long EPSPs. D, the specific structural characteristics of each input on Renshaw cells correlate with their physiological properties. Inhibitory presynaptic boutons frequently co-release glycine and GABA and are opposite to large gephyrin clusters that accumulate large numbers of glycine and GABAA receptors in relatively large PSDs. It was concluded that the presence of strong and long-lasting excitatory motor axon inputs on Renshaw cells modulate the development of strong and long-lasting inhibitory synapses on these cells; the origin(s) of these inhibitory synapses have not yet been characterized (Geiman et al. 2000; Gonzalez-Forero et al. 2005).
Two recent studies using whole-cell recordings of Renshaw cells in neonates described some surprising findings that point to unexpected complexity at the motor axon synapses on Renshaw cells. Cocktails of cholinergic blockers (muscarinic and nicotinic) did not completely block either the monosynaptic compound EPSP elicited from the ventral root or the unitary EPSC elicited from single motoneuron axons (Mentis et al. 2005; Nishimaru et al. 2005). However, the remaining EPSP (20–30% of the total) was abolished by NMDA and AMPA receptor antagonists. Acetylcholine exhibited no cross-talk with NMDA or AMPA receptors (Mentis et al. 2005), suggesting that excitatory aminoacids (EAAs) and acetylcholine might be co-released from motor axons on Renshaw cells. The specific EAA and EAA vesicular transporters at intraspinal motor axon terminals, and whether co-release of acetylcholine and an EAA occurs from all motor axon collateral branches and on all postsynaptic targets, are at present undetermined (Shupliakov et al. 1993; Herzog et al. 2004; Mentis et al. 2005; Nishimaru et al. 2005). Co-release of several neurotransmitters might explain earlier failures using cholinergic antagonists alone to completely block synaptic excitation of adult Renshaw cells from motor axons in vivo in the cat (Eccles et al. 1954; Curtis & Ryall, 1966a,b) or recurrent inhibition in the rat in vitro spinal cord (Schneider & Fyffe, 1992). NMDA components to the synaptic current could also contribute to the unexplained long time course of the motor axon EPSP. The conclusion that EAAs contribute to the excitation of Renshaw cells by motoneurons challenges the long-held notion that mammalian motoneurons are purely cholinergic, a concept that was based on the effective block of synaptic transmission at the NMJ by cholinergic antagonists (Fatt & Katz, 1952). Other recent evidence suggests that EAAs are also co-released at the NMJ and are involved in presynaptic short-term plasticity, without direct postsynaptic action (Waerhaug & Ottersen, 1993; Pinard et al. 2003). In contrast to the NMJ, the postsynaptic localization of NMDA and AMPA receptors at excitatory synapses on Renshaw cells suggests that expression of these receptors serves to mediate direct postsynaptic responses to EAA release from intraspinal motor axons.
Dorsal root primary afferent sensory synapses
Classical electrophysiological studies concluded that Renshaw cells lack direct monosynaptic inputs from primary afferents. However, recent evidence clearly demonstrated the existence of primary afferent synapses on neonatal and adult Renshaw cells (Mentis et al. 2006). Sensory synaptic inputs reliably evoked Renshaw cell firing in the neonatal in vitro rodent spinal cord preparation, and based on the ventral location of Renshaw cells and the known projections of sensory afferents it was speculated that this input probably derives from Ia sensory afferents. Thus, it may be inferred that neonatal Renshaw cells could mediate inhibitory actions from group Ia afferents, in addition to recurrent inhibition on motoneurons. This is, however, generally not believed to be the case in the adult in vivo cat spinal cord (Curtis & Ryall, 1966a; Ryall & Piercey, 1971; but see Frank & Fuortes, 1956), despite morphological evidence for the presence of sensory synapses on adult cat Renshaw cells (Mentis et al. 2006). Nevertheless, dorsal root activation evoked Renshaw cell firing in a lesser known in vivo study performed in fetal kittens (Naka, 1964). The apparent discrepancy between neonate and adult Renshaw cells may be best explained by a functional loss of this input during development.
Anatomical analyses throughout development suggest that this functional loss of input occurs without physical removal of the synapses, a process that was described as ‘functional de-selection’ (Mentis et al. 2006). Sensory synapses are first established on a few Renshaw cells at late embryonic stages and then increase in numbers and extend to the whole Renshaw cell pool during early postnatal development. In comparison, motor axon inputs are established earlier and continue to proliferate postnatally, in parallel with the sensory synapses. Motor axon synapses continue to be added after postnatal day 15 (P15), whilst the dendritic arbors continue to grow. In contrast, sensory synapses stop proliferating after this age and consequently their overall density gradually decreases towards adulthood. This is paralleled by a partial regression of the synaptic apparatus shown by smaller active zones and postsynaptic densities. Sensory afferent inputs on Renshaw cells are probably weakened by this process, and consequently there is a modification of the way in which Renshaw cells are functionally integrated in the motor circuit. Thus, having functionally lost synaptic drive from proprioceptors, Renshaw cells now become specialized in recurrent inhibition. Interestingly, P15 is just after the onset of weight-bearing locomotion, a period of changing locomotor performance accompanied by changes in proprioceptive inputs and the modification of interneuronal motor circuits and behaviour.
What is the function, if any, of the observed sensory afferent synapses on adult Renshaw cells? One possibility is that these synapses are ‘postsynaptically silent’ (Isaac, 2003; Voronin & Cherubini, 2004) having lost postsynaptic AMPA receptors during postnatal development. Classical pharmacological studies indicated that Renshaw cells are strongly excited by l-glutamate and l-aspartate (Curtis et al. 1960). Accordingly, adult Renshaw cells abundantly express postsynaptic GluR2 and GluR4 AMPA receptor subunits (F.J. Alvarez data unpublished, see Fig. 4). Thus, changes in receptor expression do not explain the diminished actions of glutamatergic primary afferent synapses on these cells. Alternatively the receptors might not be adequately positioned opposite to sensory afferent synaptic boutons. Another possibility is that one or several pre- or postsynaptic mechanisms result in sensory synapses that evoke weak synaptic currents on Renshaw cells and these are subthreshold at the cell body (‘whispering synapses’; Voronin & Cherubini, 2004). Weak synaptic actions could be shunted by the powerful inhibition that matures on Renshaw cells during the second postnatal week (Gonzalez-Forero & Alvarez, 2005; see below). It is not known whether ‘silent’ or ‘whispering’ proprioceptive synapses on adult Renshaw cells could be strengthened in certain physiological or pathological situations (e.g. spinal cord or peripheral nerve injury), thereby, altering the modulation of recurrent inhibition.
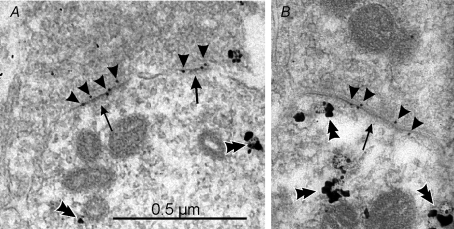
Figure 4. Renshaw cells express postsynaptic receptors that include GluR2 and GluR4 subunits in their composition.
The images show calbindin-IR dendrites sampled from the ventral Renshaw cell region, immunostained in pre-embedding with immuno-nanogold and amplified with silver (double arrowheads). A proportion of postsynaptic densities (arrows) at excitatory synapses can be labelled with post-embedding immunogold (10 nm, arrowheads) for GluR4 AMPA receptor subunits in A and B. Similar results were obtained with antibodies against GluR2/3 or specific for GluR2. Although the combined pre- and postembedding immunocytochemistry and cryosubstitution techniques necessary to reveal these receptor subunits results in slightly compromised ultrastructure, particularly vesicle size and shape, the PSDs are asymmetric and contain AMPA receptors; hence, they are highly likely to represent excitatory (Type 1) synapses. Antibodies against GluR1 did not immunolabel any synapses on Renshaw cells, but intensely immunolabelled synapses in the dorsal horn. The images were obtained from a P20 animal.
Interneuronal excitatory synapses
Less is known about other excitatory synapses on Renshaw cells. Interneuronal VGLUT2-positive synapses on Renshaw cells occur at higher densities than primary afferent (VGLUT1) synapses, but VGLUT2 boutons tend to be smaller than the primary afferent synapses. Whether their strength also changes with development is not known. Interneuronal inputs are believed to polysynaptically mediate the facilitatory actions of descending and sensory inputs on adult Renshaw-mediated recurrent inhibition. Preliminary data obtained by genetically labelling different spinal interneuronal populations revealed a significant input from V3 interneurons (Geiman et al. 2004). Interestingly, V3 interneurons extend ipsilaterally and contralaterally projecting axons and one report indicates modulation of Renshaw cell activity by commissural excitatory interneurons (Kiehn, 2006). These studies suggest a possible further role for Renshaw cells in mediating contralateral inhibition, at least in the neonate, analogous to the crossing contralateral inhibitory influences mediated by Ia inhibitory interneurons (Jankowska et al. 2005).
Descending inputs
On average only two to five contacts per cell were identified for 5-HT axons in rat and cat gephyrin-identified Renshaw cells (Carr et al. 1999). Further analysis of more distal dendritic locations on calbindin-identified rat Renshaw cells, and analysis of additional descending inputs (dopaminergic and noradrenergic) only marginally increased the original low estimates (Z. Deng and R. E. W. Fyffe, unpublished observations). It is unlikely that such a sparse input directly modulates Renshaw cell activity. More probably, the known actions of 5-HT and catecholamines on recurrent inhibition could be mediated by paracrine actions and affect Renshaw cell firing or synaptic function directly, or indirectly by acting on circuit elements that modulate Renshaw cells, including the motor axon recurrent collaterals. The possibility that non-monoaminergic descending inputs directly contact Renshaw cells has not yet been thoroughly investigated.
Inhibitory inputs
Inhibitory synapses are important modulators of the gain of the recurrent inhibitory circuit, although the source of the inhibitory inputs from ipsilateral (including other Renshaw cells) and/or contralateral interneurons is not yet clearly defined (see further discussion and references in Gonzalez-Forero & Alvarez, 2005). On adult Renshaw cells, most inhibitory synapses are proximally located and, as noted above, are characterized by very large PSDs and presynaptic active zones (Alvarez et al. 1997; Geiman et al. 2000). These large inhibitory PSDs (which are among the largest in the whole CNS) are visualized by their high level of expression of gephyrin, a protein that anchors glycine receptors (GlyRs) and is also involved in GABAA receptor clustering (Meier, 2003). Consequently the inhibitory PSDs on Renshaw cells have a high density of GlyRs and GABAA receptors. It was suggested that the size of the postsynaptic inhibitory receptor cluster, which is quite variable, is an important factor in regulating synaptic strength (Lim et al. 1999; Oleskevich et al. 1999). In addition, receptor subunit composition is important. GABAA receptors in Renshaw cells contain α3–5β2–3γ2 subunits, a relatively uncommon molecular composition, while adult GlyRs are of the typical mature form of α1-containing receptors (Geiman et al. 2002). In concurrence with the dual receptor content of the PSD, many presynaptic boutons at these synapses co-release GABA and glycine (Geiman et al. 2002; Gonzalez-Forero & Alvarez, 2005). The structural and molecular characteristics result in inhibitory synapses characterized by mixed IPSCs with a very large amplitude fast peak dominated by the opening of α1-containing (fast kinetics) GlyRs, followed by a very slow decay dominated by the activity of GABAA receptors (Gonzalez-Forero & Alvarez, 2005). The slow decays are significantly slower than at other GABAergic synapses because the presence of α3 and α5 subunits confers the receptor with particularly slow kinetics (Fig. 3A; see also Gingrich et al. 1995; and discussion in Gonzalez-Forero & Alvarez, 2005).
It was proposed that the structural and functional features of the inhibitory synaptic apparatus are adapted to efficiently modulate the time course and large amplitude of the motor axon synapse (Geiman et al. 2000, 2002; Gonzalez-Forero & Alvarez, 2005). The characteristics of inhibitory synapses develop after the first week postnatal and are firmly established by P20, corresponding with the maturation time-course of the motor axon input. Experimental up-regulation of motor firing during this developmental period resulted in increased gephyrin clustering and larger amplitudes of glycinergic and, to a lesser extent, GABAergic components of IPSCs. In contrast, down-regulation of motor firing decreased gephyrin clustering on Renshaw cells. It was concluded that postnatal motor axon activity heterosynaptically modulates receptor recruitment and structural enlargement of inhibitory synapses and this is reflected in their final strength (Gonzalez-Forero et al. 2005). This mechanism probably refines the ‘matching’ between excitatory and inhibitory inputs in Renshaw cells. It is also tempting to speculate that another consequence is increased shunting of weaker excitatory inputs converging onto Renshaw cells, like those from the sensory afferents (see above). In this case the motor axon synapses could represent a ‘master’ input that drives, through direct and indirect mechanisms, critical aspects of the overall postnatal maturation of the integrative properties of Renshaw cells.
However, not all of the characteristics of inhibitory synapses on Renshaw cells are shaped by activity-dependent mechanisms. The time courses of glycinergic and GABAergic inhibitory currents speed up during postnatal development and seem related to specific receptor subunit expression programs. Acceleration of glycinergic neurotransmission occurs because of a switch of GlyR subunits from ‘slower’ fetal forms (α2β) to ‘faster’ adult forms (α1β). This switch occurs in many other cell types as well, and it is independent of synaptic activity (Mangin et al. 2002). In contrast, the postnatal subunit composition of Renshaw cell GABAA receptors changes more subtly from neonate to adult, with no overt ‘switching’ as seen for glycine receptors (benzodiazepine sensitive α3 and α5 subunits are the main subunits contributing to Renshaw cell GABAA receptors throughout development). In the neonatal spinal cord, GABAA receptors incorporating β2/3 subunits may be subject to differential neurosteroid modulation because in postnatal, but not mature, Renshaw cells, the developmental acceleration of GABAergic IPSCs can be mimicked by inhibitors of neurosteroid action (e.g. finasteride, 50 μm for 1 h; Alvarez & Gonzalez-Forero, 2006). Experimental alteration of Renshaw cell activity in vivo results in changes to the amplitude of synaptic currents (related to release and receptor number), but the developmental regulation of their time courses is not altered, suggesting stability in subunit expression, composition and modulation of inhibitory receptors in developing Renshaw cells (Gonzalez-Forero et al. 2005).
Development of synaptic input/output properties and the changing functional role of Renshaw cells during development
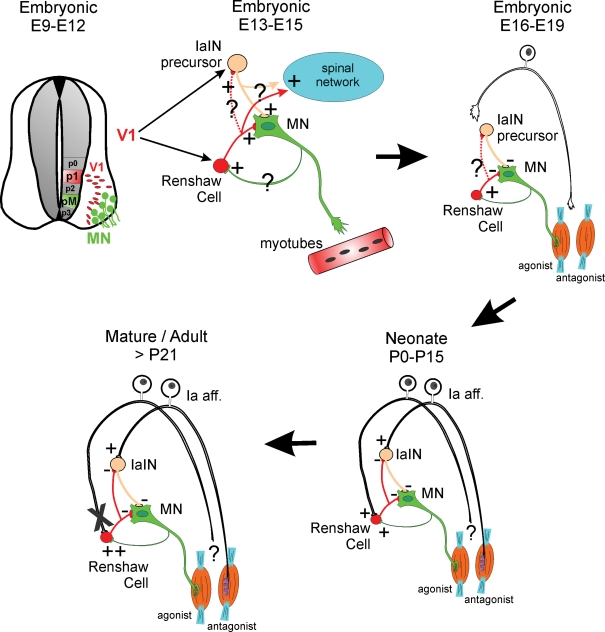
Taken together, the data on Renshaw cell neurotransmitter receptors lead to the hypothesis that the expression of particular receptor subunits at nicotinic, glycinergic, GABAergic and AMPAergic synapses is intrinsically specified in the Renshaw cell phenotype and shows striking differences with other ventral interneurons. Other properties determining the relative weights of different inputs on Renshaw cell synaptic integration and firing modulation (like synaptic densities, structure and receptor numbers, and the electrotonic structure of the neuron) are adjusted postnatally in concert with the activity patterns of developing spinal motor circuits. Changes in synaptic weights necessarily affect the information that Renshaw cells integrate and modify Renshaw cell function during postnatal development. Yet, Renshaw-like cells in the early embryonic spinal cord have a further distinct functional role. At this time inhibitory synapses exert depolarizing effects and therefore Renshaw cells enhance, rather than decrease, motoneuron firing. This contributes to spread to the spinal inteneuronal network early spontaneous activity episodes initiated in motoneurons (Wenner & O'Donavan, 1999, 2001; Hanson & Landmesser, 2003). This early episodic activity in embryos coincides with axon pathfinding and early synaptogenesis and is critical for the correct wiring of ventral spinal motor circuits and for the early development of neuronal and muscle properties (O'Donovan et al. 1998; Myers et al. 2005). It seems reasonable to conclude that developing interneurons, in this case Renshaw cells, acquire different functions adapted to the network needs at each developmental stage. This is accompanied by reorganization of their synaptic inputs (summary in Fig. 5).
Figure 5. The diagrams summarize the changing function and integration of Renshaw cells in ventral circuits during development.
Renshaw cells are derived from V1 interneurons that are generated from p1 progenitors in the early neural tube (top, left). V1 interneurons take a ventral–lateral migratory path and position themselves in close relationship to motoneurons with whom they start to interact synaptically. V1 interneurons give rise to several classes of ipsilaterally projecting GABAergic/glycinergic premotor interneurons that include Renshaw cells and IaIN precursors (top, middle). At mid-embryonic stages all early connectivity (primarily cholinergic and GABAergic and less glycinergic and glutamatergic) is excitatory (Hanson & Landmesser, 2003; Myers et al. 2005); however, morphological details of these early connections are largely unknown (question marks). It is believed that this early connectivity has important roles in transmitting motoneuron spontaneous activity to the whole spinal network and that this spontaneous activity is important during early axonal guidance and target recognition. An amplifier role for early motoneuron-initiated spontaneous activity could be accomplished by Renshaw cells directly or indirectly through connections with other interneurons like IaINs. The anatomical nature and organization of these connections remains speculative at present. Current studies are actively pursuing clarification of this early synaptology. At late embryonic stages (top, right), inhibitory synapses become hyperpolarizing and the Renshaw cell's role in transmitting excitation down-regulates in favour of exerting inhibitory influences over the motoneurons. Primary afferent axons commence to invade the ventral horn of the spinal cord in late embryos at the same time that they induce the differentiation of the sensory apparatus in the periphery (muscle spindles and Golgi tendon organs). By the time of birth (bottom, right) Renshaw cells are contacted by both sensory afferents and motor axons and in this sense they are a class of proprioceptive inhibitory interneuron that also has direct excitation from motoneurons. The source of the sensory input is as yet unknown. If it originates from antagonist muscles, then Renshaw cells could behave at this stage as a special class of IaIN. After maturation and onset of weight-bearing locomotion (bottom, left) this sensory input is functionally lost (indicated by an X), although the sensory inputs are still present structurally, and the Renshaw cell's role then becomes more focused to recurrent inhibition, as it is in the adult.
These observations point to several principles that underlie the development of Renshaw cell properties and are perhaps also relevant during development of other spinal interneuron populations: (1) Neurotransmitter receptor expression and thus neuronal receptivity to particular inputs/neurotransmitters is cell-type specific; (2) Activity-dependent modification of synaptic input weights could be strongly patterned by major excitatory inputs; (3) Interneuronal function changes during development according to shifting input weights and circuit maturation; (4) Weakened and non-functional connectivity can be preserved and could be potentially significant in certain conditions; and (5) Activity-dependent and -independent mechanisms both contribute to shape the synaptic organization of Renshaw cells.
Ontogeny of Renshaw cells
The presence of activity-independent features intrinsic to the Renshaw cell phenotype, like strong motor axon inputs, receptor subunit expression, inhibitory phenotype and ipsilateral projections to motoneurons, suggest possible genetic determination. Until recently little was known about these mechanisms. New advances have shown that the embryonic spinal cord contains a small number of canonical neuronal classes, each derived from one of 11 progenitor areas distributed in distinct dorso-ventral regions of the early embryonic neural tube. Positional inductive signals acting on these dorso-ventral zones regulate the expression of characteristic combinations of transcription factors (see reviews in Jessell, 2000; Lee & Pfaff, 2001; Goulding et al. 2002). Each progenitor area is thus characterized by a different genetic program and gives rise to different classes of postmitotic neurons that specifically up-regulate unique complements of late-expressing transcription factors. These few early types of embryonic interneurons subsequently generate the larger number of adult interneuronal types. For example, most ventral horn interneurons are believed to be derived from just four embryonic subclasses referred to as V0, V1, V2 and V3. It is clear that later differentiation steps are required to further subdivide these classes into the diversity of adult spinal interneurons.
Renshaw cells specifically derive from engrailed-1-expressing V1-embryonic interneurons which are in turn generated by p1 progenitors characterized by the expression of Pax6, Dbx2 and Nkx6.2 transcription factors (Sapir et al. 2004). Deletion of Pax6 prevents the generation of embryonic V1-interneurons. In Pax6 mutants, Renshaw cells are not present and recurrent inhibition of motoneurons is lost (Sapir et al. 2004), demonstrating that V1-interneurons are necessary for developing Renshaw cells and the recurrent inhibitory circuit. In contrast, deletion of the late transcription factor engrailed-1 did not alter Renshaw cell differentiation or connectivity with motor axons, but led to path finding errors during early extension of V1-axons in the embryo (Saueressig et al. 1999) and a reduction in the numbers of synapses from Renshaw cells onto motoneurons (Sapir et al. 2004). Although V1-interneurons appear necessary to generate Renshaw cells, Renshaw cells constitute only 10% of the V1-generated interneurons in the ventral horn. Thus, a variety of other cell types also derive from the V1 background including interneurons with synaptic inputs characteristic of Ia inhibitory interneurons (IaINs) (Alvarez et al. 2005). Thus it was proposed that the V1-interneuron subgroup defines a large group of interneurons, or ‘metatype’, which share common features including an inhibitory phenotype, a ventro-lateral migration pattern in the embryonic spinal cord that positions them adjacent to or in between motor pools, and axonal projections that remain ipsilateral in the ventral horn. The V1 class also represents a phylogenetically conserved ‘canonical’ interneuron, being present in the spinal cords of zebrafish, Xenopus tadpoles and embryonic chicks (Wenner et al. 2000; Higashijima et al. 2004; Li et al. 2004). In teleost fish and premetamorphic amphibians these neurons constitute a single functional class but this is not the case in birds and mammals, suggesting cellular diversification during the evolutionary transition to the increasingly more complex terrestrial locomotion. It does appear then that mammalian IaINs and Renshaw cells are relatively close genetically and phylogenetically, and this could explain some of their common characteristics, their tightly coupled function in reflex pathways and the fact that, transiently during development, Renshaw cells may act very similarly to Ia inhibitory interneurons in mediating Ia afferent inhibition. However, these two cell types also display many clear differences. Whilst de-selection of functional Ia sensory inputs by Renshaw cells is an activity-dependent process, earlier genetic-induction mechanisms could determine other key characteristics of the Renshaw cell phenotype, such as their peculiar complement of nicotinic and inhibitory receptor subunit compositions and the corresponding unique organization of motor and inhibitory inputs.
Similar to the staggered process of motoneuron differentiation into classes, columns and pools (Shirasaki & Pfaff, 2002; Price & Briscoe, 2004), it is likely that embryonic interneuron classes sequentially subdivide into ever more restricted subgroups displaying increasingly limited sets of properties that finally give raise to individual types of adult interneurons. The data reviewed here argue in favour of a process in which embryonic origins define the cell's competencies for receiving and responding to synapses from specific afferent sources. However, the final relative strengths of functional synaptic inputs are sculpted through activity-dependent mechanisms and lead to mature functional integration of interneuron subclasses into spinal cord circuits.
Recently acquired knowledge and ongoing investigations are uncovering mechanisms that specify and differentiate Renshaw cell synaptic properties. In the future, the generality of these observations for interneuronal circuit development will need to be tested on other cell types. Also, importantly, this work is opening new avenues for Renshaw cell experimental manipulation. For example, knowledge of cell-type specific gene expression patterns opened the possibility for generating conditional mutants in which V1-derived neurons (and others) are deleted in embryo or silenced in neonates (Gosgnach et al. 2006). These genetic manipulations slowed down the locomotor rhythmic motor output because of diminished inhibitory modulation of motoneurons and the motor network. Although the resulting abnormal motor behaviours cannot be specifically ascribed to Renshaw cells, it is now only a matter of time until more specific targeting is implemented and permits the specific silencing/deletion of Renshaw cells in neonates and adult animals. These animal models should prove invaluable for answering old questions pertaining to Renshaw cell function, and will probably lead the way for similar analysis in other interneuron subclasses.
Acknowledgments
The authors would like to thank Dr David Gonzalez-Forero, Debbie Harrington, Ricardo Zerda and Zhihui (Joy) Deng for technical assistance and help in generating some of the unpublished material included in this review. We are also grateful to Dr Timothy Cope for his insightful comments on the manuscript. The authors' work summarized in this review was supported by grants from the National Institutes of Health (U.S.A.) to F.J.A. (NS047357) and R.E.W.F. (NS25547, NS40850) and from the National Science Foundation to F.J.A. (998441).
References
- Alvarez FJ, Dewey DE, Harrington DA, Fyffe RE. Cell-type specific organization of glycine receptor clusters in the mammalian spinal cord. J Comp Neurol. 1997;379:150–170. [PubMed] [Google Scholar]
- Alvarez FJ, Dewey DE, McMillin P, Fyffe RE. Distribution of cholinergic contacts on Renshaw cells in the rat spinal cord: a light microscopic study. J Physiol. 1999;515:787–797. doi: 10.1111/j.1469-7793.1999.787ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Gonzalez-Forero D. Maturation of GABAA synapses on Renshaw cells. Abstr Soc Neurosci. 2006:716–717. [Google Scholar]
- Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J Comp Neurol. 2005;493:177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Ulfhake B, Cullheim S, Ramirez V, Shupliakov O, Hokfelt T. Distribution of calbindin D28k-like immunoreactivity (LI) in the monkey ventral horn: do Renshaw cells contain calbindin D28k-LI? J Neurosci. 1992;12:718–728. doi: 10.1523/JNEUROSCI.12-03-00718.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat Rev Neurosci. 2006;7:318–324. doi: 10.1038/nrn1885. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neural systems. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, Vol. II, Motor Control, Part I. Bethesda, MD, USA: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Bui TV, Cushing S, Dewey D, Fyffe RE, Rose PK. Comparison of the morphological and electrotonic properties of Renshaw cells, Ia inhibitory interneurons, and motoneurons in the cat. J Neurophysiol. 2003;90:2900–2918. doi: 10.1152/jn.00533.2003. [DOI] [PubMed] [Google Scholar]
- Bui TV, Dewey DE, Fyffe RE, Rose PK. Comparison of the inhibition of Renshaw cells during subthreshold and suprathreshold conditions using anatomically and physiologically realistic models. J Neurophysiol. 2005;94:1688–1698. doi: 10.1152/jn.00284.2005. [DOI] [PubMed] [Google Scholar]
- Burke RE. John Eccles' pioneering role in understanding central synaptic transmission. Prog Neurobiol. 2006;78:173–188. doi: 10.1016/j.pneurobio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Carr PA, Alvarez FJ, Leman EA, Fyffe RE. Calbindin D28k expression in immunohistochemically identified Renshaw cells. Neuroreport. 1998;9:2657–2661. doi: 10.1097/00001756-199808030-00043. [DOI] [PubMed] [Google Scholar]
- Carr PA, Pearson JC, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on immunohistochemically-identified Renshaw cells in cat and rat lumbar spinal cord. Brain Res. 1999;823:198–201. doi: 10.1016/s0006-8993(98)01210-4. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Kellerth JO. Two kinds of recurrent inhibition of cat spinal α-motoneurones as differentiated pharmacologically. J Physiol. 1981;312:209–224. doi: 10.1113/jphysiol.1981.sp013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Andersen P. Sir John Carew Eccles, A.C. Biogr Mem Fellows R Soc. 2001;47:159–187. doi: 10.1098/rsbm.2001.0010. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, Watkins JC. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Ryall RW. The acetylcholine receptors of Renshaw cells. Exp Brain Res. 1966a;2:66–80. doi: 10.1007/BF00234361. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Ryall RW. The synaptic excitation of Renshaw cells. Exp Brain Res. 1966b;2:81–96. doi: 10.1007/BF00234362. [DOI] [PubMed] [Google Scholar]
- Dourado M, Sargent PB. Properties of nicotinic receptors underlying Renshaw cell excitation by α-motor neurons in neonatal rat spinal cord. J Neurophysiol. 2002;87:3117–3125. doi: 10.1152/jn.2002.87.6.3117. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Iggo A, Ito M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. The electric activity of the motor end-plate. Proc R Soc Lond B Biol Sci. 1952;140:183–186. doi: 10.1098/rspb.1952.0055. [DOI] [PubMed] [Google Scholar]
- Fetcho JR. Optical and genetic approaches towards understanding spinal circuits. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. FL, USA: CRC Press, Boca de Raton; 2001. pp. 3–20. [Google Scholar]
- FitzSimons E, Van Zundert B, Constaine-Paton M, Brown RH, Jr, Alvarez FJ. Alterations in the Renshaw cell recurrent inhibitory circuit in the G93A SOD1 mouse model ALS. Abstr Soc Neurosci. 2006:678–679. [Google Scholar]
- Frank K, Fuortes MG. Unitary activity of spinal interneurones of cats. J Physiol. 1956;131:424–435. doi: 10.1113/jphysiol.1956.sp005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE. Evidence for separate morphological classes of Renshaw cells in the cat's spinal cord. Brain Res. 1990;536:301–304. doi: 10.1016/0006-8993(90)90038-d. [DOI] [PubMed] [Google Scholar]
- Fyffe RE. Glycine-like immunoreactivity in synaptic boutons of identified inhibitory interneurons in the mammalian spinal cord. Brain Res. 1991a;547:175–179. doi: 10.1016/0006-8993(91)90590-r. [DOI] [PubMed] [Google Scholar]
- Fyffe RE. Spatial distribution of recurrent inhibitory synapses on spinal motoneurons in the cat. J Neurophysiol. 1991b;65:1134–1149. doi: 10.1152/jn.1991.65.5.1134. [DOI] [PubMed] [Google Scholar]
- Geiman EJ, Knox MC, Alvarez FJ. Postnatal maturation of gephyrin/glycine receptor clusters on developing Renshaw cells. J Comp Neurol. 2000;426:130–142. doi: 10.1002/1096-9861(20001009)426:1<130::aid-cne9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Geiman EJ, Narayan S, Goulding M. Synaptic inputs from ventral horn interneurons to Renshaw cells. Abstr Soc Neurosci. 2004:838–835. [Google Scholar]
- Geiman EJ, Zheng W, Fritschy JM, Alvarez FJ. Glycine and GABAA receptor subunits on Renshaw cells: relationship with presynaptic neurotransmitters and postsynaptic gephyrin clusters. J Comp Neurol. 2002;444:275–289. doi: 10.1002/cne.10148. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: implications for structure–function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Alvarez FJ. Differential postnatal maturation of GABAA, glycine receptor, and mixed synaptic currents in Renshaw cells and ventral spinal interneurons. J Neurosci. 2005;25:2010–2023. doi: 10.1523/JNEUROSCI.2383-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Pastor AM, Geiman EJ, Benitez-Temino B, Alvarez FJ. Regulation of gephyrin cluster size and inhibitory synaptic currents on Renshaw cells by motor axon excitatory inputs. J Neurosci. 2005;25:417–429. doi: 10.1523/JNEUROSCI.3725-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lanuza G, Sapir T, Narayan S. The formation of sensorimotor circuits. Curr Opin Neurobiol. 2002;12:508–515. doi: 10.1016/s0959-4388(02)00371-9. [DOI] [PubMed] [Google Scholar]
- Hamm TM. Recurrent inhibition to and from motoneurons innervating the flexor digitorum and flexor hallucis longus muscles of the cat. J Neurophysiol. 1990;63:395–403. doi: 10.1152/jn.1990.63.3.395. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Landry M, Buhler E, Bouali-Benazzouz R, Legay C, Henderson CE, Nagy F, Dreyfus P, Giros B, El Mestikawy S. Expression of vesicular glutamate transporters, VGLUT1 and VGLUT2, in cholinergic spinal motoneurons. Eur J Neurosci. 2004;20:1752–1760. doi: 10.1111/j.1460-9568.2004.03628.x. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Masino MA, Mandel G, Fetcho JR. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004;24:5827–5839. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Lindstrom S, Wigstrom H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res. 1979;37:399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Isaac JT. Postsynaptic silent synapses: evidence and mechanisms. Neuropharmacology. 2003;45:450–460. doi: 10.1016/s0028-3908(03)00229-6. [DOI] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. Comparison of α2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J Comp Neurol. 2005;493:241–260. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Matsuyama K. Relative contribution of Ia inhibitory interneurones to inhibition of feline contralateral motoneurones evoked via commissural interneurones. J Physiol. 2005;568:617–628. doi: 10.1113/jphysiol.2005.088351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Lindstrom S. Morphological identification of Renshaw cells. Acta Physiol Scand. 1971;81:428–430. doi: 10.1111/j.1748-1716.1971.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Lagerback PA. An ultrastructural study of serially sectioned Renshaw cells. III. Quantitative distribution of synaptic boutons. Brain Res. 1983;264:215–223. doi: 10.1016/0006-8993(83)90819-3. [DOI] [PubMed] [Google Scholar]
- Lagerback PA, Kellerth JO. Light microscopic observations on cat Renshaw cells after intracellular staining with horseradish peroxidase. I. The axonal systems. J Comp Neurol. 1985a;240:359–367. doi: 10.1002/cne.902400404. [DOI] [PubMed] [Google Scholar]
- Lagerback PA, Kellerth JO. Light microscopic observations on cat Renshaw cells after intracellular staining with horseradish peroxidase. II. The cell bodies and dendrites. J Comp Neurol. 1985b;240:368–376. doi: 10.1002/cne.902400405. [DOI] [PubMed] [Google Scholar]
- Lagerback PA, Ronnevi LO. An ultrastructural study of serially sectioned Renshaw cells. I. Architecture of the cell body, axon hillock, initial axon segment and proximal dendrites. Brain Res. 1982a;235:1–15. doi: 10.1016/0006-8993(82)90192-5. [DOI] [PubMed] [Google Scholar]
- Lagerback PA, Ronnevi LO. An ultrastructural study of serially sectioned Renshaw cells. II. Synaptic types. Brain Res. 1982b;246:181–192. doi: 10.1016/0006-8993(82)91166-0. [DOI] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat Neurosci. 2001;4:1183–1191. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- Li WC, Higashijima S, Parry DM, Roberts A, Soffe SR. Primitive roles for inhibitory interneurons in developing frog spinal cord. J Neurosci. 2004;24:5840–5848. doi: 10.1523/JNEUROSCI.1633-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Alvarez FJ, Walmsley B. Quantal size is correlated with receptor cluster area at glycinergic synapses in the rat brainstem. J Physiol. 1999;516:505–512. doi: 10.1111/j.1469-7793.1999.0505v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy ML, Hamm TM. Topography of recurrent inhibitory postsynaptic potentials between individual motoneurons in the cat. J Neurophysiol. 1994;72:214–226. doi: 10.1152/jn.1994.72.1.214. [DOI] [PubMed] [Google Scholar]
- Maltenfort MG, Heckman CJ, Rymer WZ. Decorrelating actions of Renshaw interneurons on the firing of spinal motoneurons within a motor nucleus: a simulation study. J Neurophysiol. 1998;80:309–323. doi: 10.1152/jn.1998.80.1.309. [DOI] [PubMed] [Google Scholar]
- Mangin JM, Guyon A, Eugène D, Paupardin-Tritsch D, Legendre P. Functional glycine receptor maturation in the absence of glycinergic input in dopaminergic neurones of the rat substantia nigra. J Physiol. 2002;542:685–697. doi: 10.1113/jphysiol.2002.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei B, Schmied A, Mazzocchio R, Decchi B, Rossi A, Vedel JP. Pharmacologically induced enhancement of recurrent inhibition in humans: effects on motoneurone discharge patterns. J Physiol. 2003;548:615–629. doi: 10.1113/jphysiol.2002.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. The enigma of transmitter-selective receptor accumulation at developing inhibitory synapses. Cell Tissue Res. 2003;311:271–276. doi: 10.1007/s00441-002-0694-9. [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O'Donovan MJ. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci U S A. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O'Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CP, Lewcock JW, Hanson MG, Gosgnach S, Aimone JB, Gage FH, Lee KF, Landmesser LT, Pfaff SL. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron. 2005;46:37–49. doi: 10.1016/j.neuron.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Naka KI. Electrophysiology of the fetal spinal cord. II. Interaction among peripheral inputs and recurrent inhibition. J Gen Physiol. 1964;47:1023–1038. doi: 10.1085/jgp.47.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Kiehn O. Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. J Neurosci. 2006;26:5320–5328. doi: 10.1523/JNEUROSCI.5127-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga BR, Shefchyk SJ, Jamal J, Jordan LM. The role of Renshaw cells in locomotion: antagonism of their excitation from motor axon collaterals with intravenous mecamylamine. Exp Brain Res. 1987;66:99–105. doi: 10.1007/BF00236206. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1998;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Chub N, Wenner P. Mechanisms of spontaneous activity in developing spinal networks. J Neurobiol. 1998;37:131–145. doi: 10.1002/(sici)1097-4695(199810)37:1<131::aid-neu10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Alvarez FJ, Walmsley B. Glycinergic miniature synaptic currents and receptor cluster sizes differ between spinal cord interneurons. J Neurophysiol. 1999;82:312–319. doi: 10.1152/jn.1999.82.1.312. [DOI] [PubMed] [Google Scholar]
- Pinard A, Levesque S, Vallee J, Robitaille R. Glutamatergic modulation of synaptic plasticity at a PNS vertebrate cholinergic synapse. Eur J Neurosci. 2003;18:3241–3250. doi: 10.1111/j.1460-9568.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- Price SR, Briscoe J. The generation and diversification of spinal motor neurons: signals and responses. Mech Dev. 2004;121:1103–1115. doi: 10.1016/j.mod.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Renshaw B. Influence of the discharge of motoneurons upon exciation of neighboring motoneurons. J Neurophysiol. 1941;4:167–183. [Google Scholar]
- Renshaw B. Central effects of centripetal impulses in axons of spinal ventral roots. J Neurophysiol. 1946;9:191–204. doi: 10.1152/jn.1946.9.3.191. [DOI] [PubMed] [Google Scholar]
- Ryall RW, Piercey MF. Excitation and inhibition of Renshaw cells by impulses in peripheral afferent nerve fibers. J Neurophysiol. 1971;34:242–251. doi: 10.1152/jn.1971.34.2.242. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol. 2006a;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Stecina K, Shevtsova NA, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from the effects of affeent stimulation. J Physiol. 2006b;577:641–658. doi: 10.1113/jphysiol.2006.118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Pax6 and engrailed 1 regulate two distinct aspects of Renshaw cell development. J Neurosci. 2004;24:1255–1264. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saueressig H, Burrill J, Goulding M. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development. 1999;126:4201–4212. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- Schneider SP, Fyffe RE. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J Neurophysiol. 1992;68:397–406. doi: 10.1152/jn.1992.68.2.397. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Ornung G, Brodin L, Ulfhake B, Ottersen OP, Storm-Mathisen J, Cullheim S. Immunocytochemical localization of amino acid neurotransmitter candidates in the ventral horn of the cat spinal cord: a light microscopic study. Exp Brain Res. 1993;96:404–418. doi: 10.1007/BF00234109. [DOI] [PubMed] [Google Scholar]
- Smith C, Siembab VC, Berrocal MC, Goulding M, Alvarez F. Postnatal diversification of V1-derived interneurons. Abstr Soc Neurosci. 2005:603–615. [Google Scholar]
- Thomas RC, Wilson VJ. Precise localization of Renshaw cells with a new marking technique. Nature. 1965;206:211–213. doi: 10.1038/206211b0. [DOI] [PubMed] [Google Scholar]
- Turkin VV, Monroe KS, Hamm TM. Organization of recurrent inhibition and facilitation in motor nuclei innervating ankle muscles of the cat. J Neurophysiol. 1998;79:778–790. doi: 10.1152/jn.1998.79.2.778. [DOI] [PubMed] [Google Scholar]
- Van Keulen LC. Axon trajectories of Renshaw cells in the lumbar spinal cord of the cat, as reconstructed after intracellular staining with horseradish peroxidase. Brain Res. 1979;167:157–162. doi: 10.1016/0006-8993(79)90270-1. [DOI] [PubMed] [Google Scholar]
- Van Keulen L. Autogenetic recurrent inhibition of individual spinal motoneurones of the cat. Neurosci Lett. 1981;21:297–300. doi: 10.1016/0304-3940(81)90220-2. [DOI] [PubMed] [Google Scholar]
- Voronin LL, Cherubini E. ‘Deaf, mute and whispering’ silent synapses: their role in synaptic plasticity. J Physiol. 2004;557:3–12. doi: 10.1113/jphysiol.2003.058966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waerhaug O, Ottersen OP. Demonstration of glutamate-like immunoreactivity at rat neuromuscular junctions by quantitative electron microscopic immunocytochemistry. Anat Embryol (Berl) 1993;188:501–513. doi: 10.1007/BF00190144. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Tracey DJ. An intracellular study of Renshaw cells. Brain Res. 1981;223:170–175. doi: 10.1016/0006-8993(81)90818-0. [DOI] [PubMed] [Google Scholar]
- Wenner P, O'Donovan MJ. Identification of an interneuronal population that mediates recurrent inhibition of motoneurons in the developing chick spinal cord. J Neurosci. 1999;19:7557–7567. doi: 10.1523/JNEUROSCI.19-17-07557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner P, O'Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol. 2001;86:1481–1498. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- Wenner P, O'Donovan MJ, Matise MP. Topographical and physiological characterization of interneurons that express engrailed-1 in the embryonic chick spinal cord. J Neurophysiol. 2000;84:2651–2657. doi: 10.1152/jn.2000.84.5.2651. [DOI] [PubMed] [Google Scholar]
- Willis WD. The case for the Renshaw cell. Brain Behav Evol. 1971;4:5–52. doi: 10.1159/000125422. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Activation of Renshaw cells. Prog Neurobiol. 1990;35:135–179. doi: 10.1016/0301-0082(90)90020-h. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Morita Y, Hironaka T, Emson PC, Tohyama M. Ontological study of calbindin-D28k-like and parvalbumin-like immunoreactivities in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1990;302:715–728. doi: 10.1002/cne.903020404. [DOI] [PubMed] [Google Scholar]