Abstract
We have reported that [Arg8]-vasopressin-stimulated insulin release is blunted in islet cells isolated from V1b receptor-deficient (V1bR−/−) mice. In this study, we used V1bR−/− mice to examine the physiological role of the V1b receptor in regulating blood glucose levels in vivo, and we found that the fasting plasma glucose, insulin and glucagon levels were lower in V1bR−/− mice than in wild-type (V1bR+/+) mice. Next, we evaluated glucose tolerance by performing an intraperitoneal glucose tolerance test (GTT). The plasma glucose and insulin levels during the GTT were lower in V1bR−/− mice than in V1bR+/+ mice. An insulin tolerance test (ITT) revealed that, after insulin administration, plasma glucose levels were lower in V1bR−/− mice than in V1bR+/+ mice. In addition, a hyperinsulinaemic–euglycaemic clamp study showed that the glucose infusion rate was increased in V1bR−/− mice, indicating that insulin sensitivity was enhanced at the in vivo level in V1bR−/− mice. Furthermore, we found that the V1b receptor was expressed in white adipose tissue and that insulin-stimulated phosphorylation of Akt as an important signaling molecule was increased in adipocytes isolated from V1bR−/− mice. Thus, the blockade of the V1b receptor could result, at least in part, in enhanced insulin sensitivity by altering insulin signalling in adipocytes.
The neurohypophysial hormone [Arg8]-vasopressin (AVP) is known to promote vascular smooth muscle cell contraction, water reabsorption in the kidney and adrenocorticotropic hormone (ACTH) release from the anterior pituitary gland (Altura & Altura, 1977; Gillies et al. 1982; Schrier et al. 1993; Nielsen et al. 1995). AVP also regulates the blood glucose level by stimulating gluconeogenesis and glycogenolysis in the liver (Hems & Whitton, 1973; Michell et al. 1979) and by stimulating insulin and glucagon secretion in the pancreas (Dunning et al. 1984a,b; Mineo et al. 1997; Yibchok-Anun et al. 1999; Abu-Basha et al. 2002). These diverse functions of AVP are mediated by specific G protein-coupled receptors. AVP has a high affinity for the oxytocin receptor and three vasopressin-receptor subtypes: the V1a, V1b and V2 receptors. One of these, the V1b receptor, is mainly expressed in the pituitary gland, adrenal gland and pancreas (Ventura et al. 1999; Oshikawa et al. 2004).
In in vitro and ex vivo experiments using several AVP-receptor agonists and antagonists, it was demonstrated that AVP-induced insulin and glucagon secretion is mediated via the V1b receptor (Lee et al. 1995; Richardson et al. 1995; Yibchok-anun & Hsu, 1998; Yibchok-Anun et al. 1999; Folny et al. 2003). These AVP-induced insulin and glucagon releases are affected by changes in glucose concentrations (Gao et al. 1990; Abu-Basha et al. 2002). That is, AVP increases glucagon release at low glucose concentrations (5.6 mm or below), while AVP enhances insulin release at high glucose concentrations (more than 7 mm) (Gao et al. 1990, 1992; Abu-Basha et al. 2002). In addition, intravenous AVP administration increases plasma insulin and glucagon levels in rat and sheep (Dunning et al. 1984a; Spruce et al. 1985; Mineo et al. 1997) and plasma glucagon levels in human (Spruce et al. 1985). However, little is known about the effects of AVP mediated via the V1b receptor on glucose homeostasis in vivo.
In our previous study, we generated V1b receptor-deficient (V1bR−/−) mice and demonstrated that the V1b receptor plays a crucial role in maintaining basal ACTH secretion as well as in regulating hypothalamic–pituitary–adrenal (HPA) axis activity (Tanoue et al. 2004). In addition to the distinct phenotype in the HPA axis, our in vitro study with pancreatic islets showed that AVP caused insulin release from the islet cells of wild-type (V1bR+/+) mice, while its effect was completely lost in the islet cells of V1bR−/− mice (Oshikawa et al. 2004). In pharmacological studies, SSR149415, a selective antagonist for the V1b receptor, inhibited AVP-induced insulin release from islet cells of V1bR+/+ mice, whereas OPC-21268, a V1a receptor antagonist, had no effect (Oshikawa et al. 2004). Thus, studies of V1bR−/− mice have provided convincing evidence that the V1b receptor participates in AVP-induced insulin release from pancreatic islets, and this finding in the in vitro study using V1bR−/− mice led us to investigate the influence of V1b receptor deficiency on the regulation of insulin release and consequent glucose homeostasis in vivo.
Methods
Materials
Human insulin used for the glucose tolerance test was purchased from Novo Nordisk (Novolin R; Bagsvared, Denmark). Ascensia Dexter ZII, used for measuring blood glucose in the hyperinsulinaemic–euglycaemic clamp test, was from Bayer Medical (Tokyo, Japan). SuperScript III reverse transcriptase and DMEM culture medium were from Invitrogen (Carlsbad, CA, USA). ISOGEN was from Nippon Gene (Tokyo, Japan) and Ex Taq polymerase was from Takara (Tokyo, Japan). Anti-Akt monoclonal antibody was obtained from Santa Cruz Biotechnology (CA, USA), and anti-phospho-Akt (Ser473) monoclonal antibody was from Cell Signalling Technology (Danvers, MA, USA). Human insulin used for culture was from Sigma-Aldrich (St Louis, MO, USA). The insulin ELISA kit was from Morinaga (Tokyo, Japan). The glucose CII-test kit, glucagon ELISA kit, and all other reagents were from Wako Pure Chemicals (Tokyo, Japan).
Animals
The generation of V1b receptor-deficient (V1bR−/−) mice was previously described (Tanoue et al. 2004). Briefly, by homologous recombination, we disrupted exon 1, which contains the translation initiation codon. The non-V1b receptor-deficient littermates (V1bR+/+) were used as age-matched control subjects for V1bR−/− mice. The genetic background of the animals used for all experiments was a mixture of 129Sv and C57BL/6J. In some sets of experiments, we analysed V1bR−/− on the congenic C57BL/6J background (V1bR(C57BL/6J)−/−), which was generated by backcrossing with C57BL/6J mice more than six times, to evaluate the fasting plasma glucose and insulin levels. Animals were housed in micro-isolator cages in a pathogen-free barrier facility and were placed on a 12 h light–dark cycle with ad libitum access to food and water except when an experimental protocol was specified. All data presented here were obtained from male mice. All experiments were performed under the approved guidelines for the Care and Use of Laboratory Animals of the National Research Institute for Child Health and Development.
Measurement of plasma glucose, insulin and glucagon
After male mice aged 8–10 weeks had fasted for 18 h (19:00–13:00 h), blood samples were collected from the tail vein in the conscious state in a rodent restrainer (Harvard Apparatus, Inc., MA, USA). For the measurement of plasma glucose levels, blood samples (10 μl) were immediately mixed with 10 volumes of 0.33 m HClO4 to inhibit the utilization of glucose before measurement. The mixtures were centrifuged at 800g for 5 min, the supernatants (10 μl) were taken up, and the glucose concentrations were determined by the glucose oxidase method (Glucose CII-test kit) with an identically treated standard. For the measurement of plasma insulin and glucagon levels, blood samples were centrifuged for 10 min at 800g and 4°C, and the supernatants were collected and stored at −80°C until use. Plasma insulin and glucagon levels were measured by ELISA assay.
Intraperitoneal glucose tolerance test (GTT)
Male mice aged 8–10 weeks were fasted for 18 h before receiving intraperitoneal (i.p.) administration of 1.5 g glucose (kg body weight)−1 in saline (0.9% NaCl). Blood samples of conscious mice were taken from the tail vein in heparinized microcapillary tubes at 0, 10, 30, 60 and 120 min after glucose loading, and the plasma glucose and insulin levels were determined.
Intraperitoneal insulin tolerance test (ITT)
The insulin-tolerance test was performed with male mice aged 8–10 weeks after a 4 h fast. The animals were intraperitoneally injected with 1.0 U (kg body weight)−1 of insulin. Blood samples (10 μl) of conscious mice were drawn from the tail vein for the measurement of plasma glucose levels at 0, 10, 30, 60 and 120 min after the insulin injection.
Hyperinsulinaemic–euglycaemic clamp test
The hyperinsulinaemic–euglycaemic clamp test was carried out in non-anaesthetized male mice aged 8–10 weeks according to the method reported previously (Kim et al. 2000; Voshol et al. 2001). In brief, at least 2 days before the experiments, 10-week-old mice were anaesthetized with sodium pentobarbital (40 mg kg−1, i.p.), and an indwelling catheter was inserted in the left jugular vein as described in previously (Tanoue et al. 2002; Aoyagi et al. 2007). The catheters were externalized through an incision in the skin flap behind the head, and the animals were returned to individual cages after surgery. The mice showed normal behaviour after 2 days of surgery. Experiments were started after a 16 h fast. A 120 min hyperinsulinaemic condition was conducted with a continuous infusion of insulin at a rate of 15 mU kg−1 min−1 after 100 mU kg−1 bolus administration. Blood samples (20 μl) were collected at 5 min intervals for the immediate measurement of blood glucose concentrations using Ascensia Dexter ZII, and 20% glucose in saline (0.9% NaCl) was infused at variable rates to maintain the blood glucose in a range from 80 to 100 mg dl−1. After the clamp test, mice were then anaesthetized with ether and killed by cervical dislocation. The glucose infusion rate (GIR) was determined during the final 20 min.
Plasma glucose and insulin response to AVP
In male mice aged 8–10 weeks, an aliquot of 100 ng kg−1 of AVP was injected into the tail vein, as described by Mineo et al. (1997) with some modifications. Food was withdrawn during assay. Blood (100 μl) samples of conscious mice were collected from the tail vein before and 15 min after the AVP injection. Plasma glucose and insulin levels were measured by the ELISA method.
RT-PCR
Mice aged 8–10 weeks were anaesthetized with ether and killed by cervical dislocation. Then, total RNA was isolated from heart, liver, femoral muscle, epididymal white adipose tissue (WAT) and brown adipose tissue (BAT) of mice using ISOGEN. First-strand cDNA was synthesized from 5 μg of DNase-digested total RNA by SuperScript III reverse transcriptase in 20 μl of a reaction volume. The primers were designed as follows: 5′-CTCTGCTGGACACCTTTCTTCATCG-3′ upstream and 5′-CTGATGGACTTTGAAGATTTAGGTG-3′ downstream for the V1a receptor; 5′-ACAGGTGCTCAGCATGTTTG-3′ upstream and 5′-CATCTTCACGGTTCGGATCT-3′ downstream for the V1b receptor; 5′-GTCTCCTCGGAGTTGCGTAG-3′ upstream and 5′-CTGGGAGTCTTCCTCACCTG-3′ downstream for the V2 receptor; 5′-TTCTTCGTGCAGATGTGGAG-3′ upstream and 5′-AGGACGAAGGTGGAGGAGTT-3′ downstream for the oxytocin receptor; and 5′-GCTTCTGCCAAGACCTTCAC-3′ upstream and 5′-CAGTAGGGTTCCCACCTCAA-3′ downstream for the insulin receptor. The GAPDH primers were 5′-CCATCACCATCTTCCAGGAG-3′ upstream and 5′-TCCAGCTCTGGGATGACCTT-3′ downstream as a standard. The PCR reactions were carried out with Ex Taq polymerase. After the reaction mixture was treated at 95°C for 5 min, 30 cycles of amplification were performed as follows: 30 s at 95°C, 30 s at 65°C, and 1 min at 72°C. The final extension step was performed at 72°C for 10 min. The PCR products were electrophoresed and visualized on a 1.5% agarose gel stained with ethidium bromide.
Primary culture of isolated adipocytes
Isolation of adipocytes was carried out as described previously (Hiroyama et al. 2007). WAT was isolated from surrounding epididymus, femoral and subcutaneous upper back areas of 3-week-old male mice, which were anaesthetized with ether and killed by cervical dislocation, and was minced finely. Samples were incubated in 2 mg ml−1 collagenase in Krebs–Ringer buffer (120 mm NaCl, 1.27 mm CaCl2, 1.2 mm MgCl2, 4.75 mm KCl, and 1.2 mm KH2PO4) containing 15 mm sodium bicarbonate, 10 mm Hepes (pH 7.4), 2 mm sodium pyruvate and 1% BSA for 60 min at 37°C with shaking (100 r.p.m. min−1). After incubation, samples were filtered through a nylon mesh (70 μm) and centrifuged at 80g for 5 min at room temperature. A precipitate including immature adipocytes was washed twice with the same buffer. Cells were suspended in DMEM containing 10% fetal bovine serum, 50 U ml−1 penicillin and 50 μg ml−1 streptomycin. Cells were then seeded in a 12-well dish at a concentration of 1.2–1.8 × 104 cells per well. At the confluent condition, adherent preadipocytes were cultured in differentiation-inducing DMEM medium containing 10% fetal bovine serum, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin, 20 nm insulin, 1 nm triiodothyronine, 0.5 mm isobutylmethylxanthine, 0.5 μm dexamethazone and 0.125 mm indomethacin for 3 days. The differentiation to mature adipocytes was checked with Oil-Red O staining. Assays were carried out using cells differentiated to mature adipocytes with lipid droplets.
Western blotting
The cultured adipocytes were incubated in DMEM (low glucose) containing 0.3% BSA for 3 h at 37°C and stimulated with insulin at a final concentration of 50 nm for 5 min at 37°C. After incubation, the dishes were placed on ice and washed with ice-cold PBS. Cells were collected in lysis buffer (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 1% NP-40, 2 mm Na3VO4, 10 mm NaF, 10 mm Na4P2O7, 1 mm EDTA, 1 mm EGTA, 5 μg ml−1 leupeptin, 5 μg ml−1 aprotinin and 1 mm PMSF) and disrupted using a Branson sonicator (Sonifier 250; Branson, Danbury, CT, USA) at setting 5 for 5 s. Protein concentrations were measured using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA), and 20 μg of protein was applied. SDS-PAGE and protein transfer were carried out according to the procedures previously described (Shibata et al. 2003) and processed using Enhanced Chemiluminescence (ECL) plus reagents (GE Healthcare, Buckinghamshire, UK) for detection with specific antibodies against Akt (diluted 1 : 1000 in TBS-T buffer; 10 mm Tris-HCl pH 7.5, 150 mm NaCl and 0.1% Tween 20) or phospho-Akt (diluted 1 : 1000 in TBS-T buffer containing 5% milk).
Statistical analysis
Data are expressed as the means ±s.e.m. Statistical analyses were performed using the unpaired Student's t test and ANOVA followed by a post hoc comparison with Fisher's PLSD using Statview software, version 5.0 (Concepts, Inc., Berkeley, CA, USA). Differences between groups were considered statistically significant at the level of P < 0.05.
Results
Fasting plasma glucose, insulin and glucagon levels in V1bR−/− mice
To determine the alteration of the glucose metabolism in V1bR−/− mice, we assessed the fasting plasma glucose and insulin levels. After 18 h fasting, the plasma glucose and insulin levels of V1bR−/− mice were significantly lower than those of V1bR+/+ mice. Plasma glucagon levels were also decreased in V1bR−/− mice compared with those in V1bR+/+ mice (Table 1).
Table 1.
Plasma glucose, insulin, and glucagon, levels in V1bR+/+ and V1bR−/− mice
| V1bR+/+ | V1bR−/− | |
|---|---|---|
| 4 h fasting | ||
| Glucose (mg dl−1) | 118.3 ± 4.8 (n = 16) | 90.5 ± 4.1 (n = 15)*** |
| 18 h fasting | ||
| Glucose (mg dl−1) | 66.3 ± 2.3 (n = 16) | 49.6 ± 3.8 (n = 16)** |
| Insulin (pg ml−1) | 431.4 ± 54.7 (n = 16) | 249.8 ± 56.8 (n = 16)* |
| Glucagon (pg ml−1) | 830.8 ± 71.9 (n = 7) | 372.5 ± 25.3 (n = 5)*** |
Values are the means ±s.e.m.*P < 0.05, **P < 0.01 and ***P < 0.001 versus V1bR+/+ mice by the unpaired Student's t test.
Intraperitoneal glucose tolerance test (GTT)
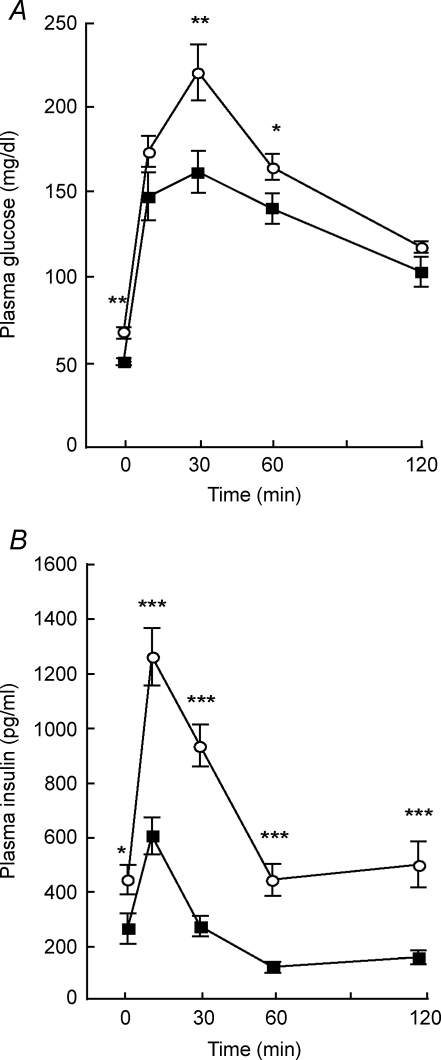
Since the fasting plasma glucose and insulin levels were lower in V1bR−/− mice, we performed a GTT to investigate whether the glucose tolerance was altered in V1bR−/− mice. After an i.p. injection with 1.5 g (kg body weight)−1 of glucose, the plasma glucose levels were lower in V1bR−/− mice than in V1bR+/+ mice, particularly at 30 and 60 min after the glucose injection (Fig. 1A). In addition, the plasma insulin levels were significantly lower in V1bR−/− mice than in V1bR+/+ mice at all points (Fig. 1B). We also evaluated the difference in plasma glucose curves by two-way ANOVA. The plasma glucose curves of V1bR−/− mice tended to be lower than those of V1bR+/+ mice (genotype × time, P= 0.08 by two-way ANOVA). The plasma insulin curves of V1bR−/− mice were significantly different from those of V1bR+/+ mice (genotype × time, P < 0.001 by two-way ANOVA). These findings suggested that insulin sensitivity could be enhanced in V1bR−/− mice because the plasma glucose levels were lower in V1bR−/− mice than in V1bR+/+ mice despite the lower plasma insulin levels during the GTT.
Figure 1. Intraperitoneal glucose tolerance test (GTT) for V1bR−/− mice.
Plasma glucose (A) and insulin levels (B) during GTT in V1bR+/+ (○) and V1bR−/− (▪) mice (n= 16 of each). Values are the means ± S.E.M. *P < 0.05, **P < 0.01 and ***P < 0.001 versus V1bR+/+ mice by post hoc comparison with Fisher's PLSD.
Intraperitoneal insulin tolerance test (ITT) and hyperinsulinaemic–euglycaemic clamp test
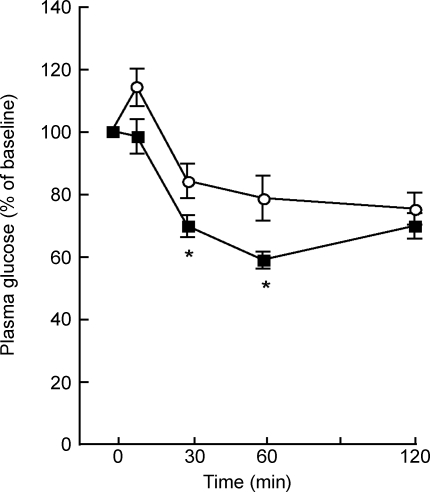
To further assess in vivo insulin sensitivity in V1bR−/− mice, we performed an ITT and a hyperinsulinaemic–euglycaemic clamp test. Mice fasted for 4 h were used for the ITT. The plasma glucose-lowering effect by insulin administration was evaluated in mice. The plasma glucose levels after 4 h fasting were significantly lower in V1bR−/− mice than in V1bR+/+ mice (Table 1). During the ITT, the decreases in the plasma glucose levels at 30 and 60 min in V1bR−/− mice were significantly greater than those in V1bR+/+ mice (Fig. 2). Although the plasma glucose curves during the ITT were not significantly different between two groups (Fig. 2, genotype × time, P= 0.17 by two-way ANOVA), the decreases in the plasma glucose levels in V1bR−/− mice after the insulin administration tended to be greater than those in V1bR+/+ mice, suggesting that the plasma glucose-lowering effect of insulin was increased in V1bR−/− mice.
Figure 2. Intraperitoneal insulin tolerance test (ITT) for V1bR−/− mice.
Relative plasma glucose levels during ITT in V1bR+/+ (○, n= 16) and V1bR−/− (▪, n= 15) mice. Values are the means ± S.E.M. *P < 0.05 versus V1bR+/+ mice by post hoc comparison with Fisher's PLSD.
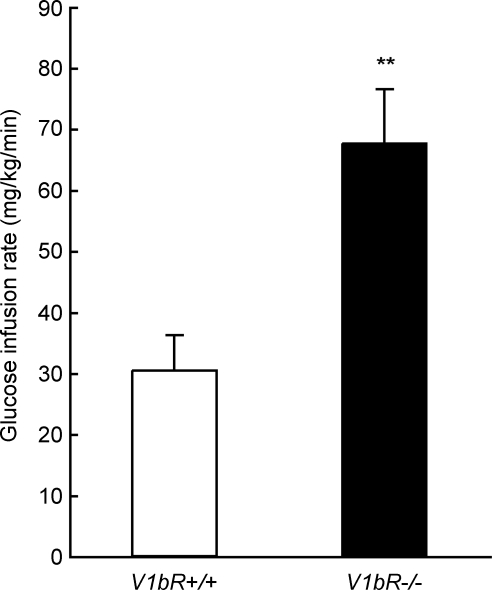
Next, we evaluated whole-body glucose utilization under the hyperinsulinaemic condition. We measured the glucose infusion rate (GIR) during the hyperinsulinaemic–euglycaemic clamp test in awake V1bR+/+ and V1bR−/− mice. The GIR in V1bR−/− mice was about two times higher than that in V1b+/+ (67.4 ± 8.92 mg kg−1 min−1 in V1bR−/− mice, n= 11, versus 30.4 ± 5.77 mg kg−1 min−1 in V1b+/+ mice, n= 8, P < 0.01) (Fig. 3). These data indicated that the insulin-stimulated whole-body glucose uptake was increased in V1bR−/− mice, which implied that insulin sensitivity was enhanced in V1bR−/− mice.
Figure 3. GIR measured by analysis with the hyperinsulinaemic–euglycaemic clamp test.
Insulin-mediated whole-body glucose uptake was determined in awake V1bR+/+ (open bar, n= 8) and V1bR−/− mice (filled bar, n= 11). Values are the means ± S.E.M. **P < 0.01 versus V1bR+/+ mice by the unpaired Student's t test.
Insulin sensitivity of V1b receptor-deficient mice with a genetic background of C57BL/6J
To exclude the modification of insulin sensitivity by genetic background, we analysed fasting plasma levels of glucose and insulin and performed the ITT in male V1b receptor-deficient mice (8–10 weeks old) with a C57BL/6J genetic background (V1bR(C57BL/6J)−/−). After 18 h fasting, the plasma insulin levels were lower in V1bR(C57BL/6J)−/− mice than in control mice (C57BL/6J mice) (947.5 ± 102.0 pg ml−1 in control mice, n= 4, versus 585.0 ± 14.4 pg ml−1 in V1bR(C57BL/6J)−/− mice, n= 4, P < 0.05). In addition, the fasting plasma glucose levels were lower in V1bR(C57BL/6J)−/− mice than in control C57BL/6J mice (148.9 ± 4.3 mg dl−1 in control mice, n= 12, versus 106.9 ± 9.8 mg dl−1 in V1bR(C57BL/6J)−/− mice, n= 10, P < 0.01). In addition, the plasma glucose levels were significantly decreased in V1bR(C57BL/6J)−/− mice 30 min after the insulin administration (53.3% decrease in control mice, n= 4, versus 64.2% decrease in V1bR(C57BL/6J)−/− mice, n = 3). These results suggested that insulin sensitivity was enhanced in V1bR(C57BL/6J)−/− mice.
Plasma glucose and insulin response to AVP administration
In order to evaluate whether AVP was involved in insulin release in vivo, we measured the insulin levels after an intravenous (i.v.) administration of AVP. Fifteen minutes after AVP administration, the plasma glucose levels were increased in both V1bR+/+ and V1bR−/− mice (153.1 ± 5.9 to 193.8 ± 17.1 mg dl−1 in control mice, n= 3, versus 146.2 ± 15.7 to 173.7 ± 33.4 mg dl−1 in V1bR−/− mice, n= 3). The plasma insulin levels were increased in V1bR−/− mice as well as in V1bR+/+ mice (441.7 ± 28.9 to 536.7 ± 78.8 pg ml−1 in control mice, n= 3, versus 410.0 ± 22.9 to 461.7 ± 56.4 pg ml−1 in V1bR−/− mice, n= 3). However, there was no significant difference in the increase in plasma glucose and insulin levels between V1bR+/+ and V1bR−/− mice.
Expression of the AVP receptor genes in insulin-sensitive tissues
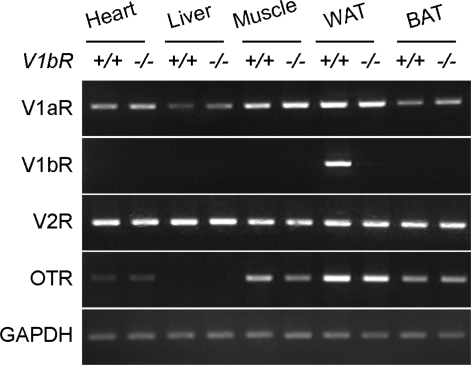
We examined whether V1b receptor mRNA was expressed in insulin-sensitive tissues such as heart, liver, muscle, WAT and BAT. In V1bR+/+ mice, the V1b receptor transcript was detected only in WAT, not in heart, liver, muscle or BAT (Fig. 4). On the other hand, the V1b receptor transcript was not detectable in all tested tissues, including WAT, from V1bR−/− mice (Fig. 4). We also examined the expression levels of other AVP-receptor members such as the V1a, V2 and oxytocin receptors in the insulin-sensitive tissues. The V1a and V2 receptors were expressed in all tested tissues of V1bR−/− and V1bR+/+ mice, whereas the oxytocin receptors were detected in all tissues except the liver. The expression levels of these receptors were not altered in insulin-sensitive tissues of V1bR−/− mice, suggesting that there was no obvious up-regulation of other receptors in V1bR−/− mice. In addition, we analysed the expression levels of the insulin receptor in these tissues but found that there was no difference in the mRNA expression levels of V1bR−/− and V1bR+/+ mice (data not shown).
Figure 4. Expression of the vasopressin receptor family.
Total RNAs were purified from the heart, liver, muscle, WAT and BAT. RT-PCR was then performed to detect vasopressin receptor gene expression using specific primer sets as described in Methods. The RT-PCR for GAPDH was performed as a control. +/+ and −/− show V1bR+/+ and V1bR−/− mice, respectively. OTR, oxytocin receptor.
Insulin-stimulated phosphorylation of Akt in adipocytes of V1bR−/− mice
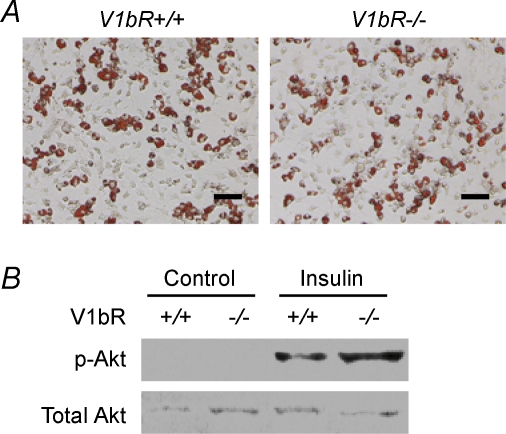
In order to address underlying mechanisms of enhanced insulin sensitivity, we analysed insulin signalling in adipocytes isolated from WAT, an insulin-sensitive tissue, of V1bR−/− mice. As Akt is implicated in insulin signalling and is phsophorylated in response to insulin, we investigated the phosphorylation of Akt with the adipocytes. In Oil-Red O staining, there was no remarkable difference between the adipocytes of V1bR+/+ and V1bR−/− mice. After insulin stimulation, while the total amount of Akt detected by Western blotting with the anti-Akt antibody did not differ between V1bR−/− and V1bR+/+ mice, insulin-stimulated phosphorylation of Akt was higher in V1bR−/− mice than in V1bR+/+ mice (Fig. 5).
Figure 5. Effect on phosphorylation of Akt by insulin stimulation.
Phosphorylation of Akt was detected using isolated and derived adipocytes from WAT in the absence and presence of insulin. A, adipocytes stained with Oil-Red O. The bars indicate 100 μm. B, immunoblots of the phosphorylation of Akt and total Akt. +/+ and −/− show V1bR+/+ and V1bR−/− mice, respectively. p-Akt, phosphorylated Akt; Akt, total Akt.
Discussion
Several ex vivo or in vitro studies have shown that AVP stimulates insulin or glucagon secretion depending on the glucose concentrations in mouse islets and rat pancreas (Gao et al. 1990, 1992; Abu-Basha et al. 2002). In our previous study, we also found that AVP stimulates insulin release from mouse pancreatic islet cells and that AVP-induced insulin release from pancreatic islet cells was blunted in V1b receptor-deficient mice (Oshikawa et al. 2004), suggesting that AVP/V1b signalling could be involved in regulating glucose homeostasis by affecting in vivo insulin release from mouse pancreatic islet cells. Therefore, in this study, we investigated the effect of AVP on regulating blood glucose levels in V1b receptor-deficient mice. V1bR−/− mice showed lower plasma glucose levels accompanied by decreased plasma insulin levels under the fasting condition. Using studies with the GTT, ITT and hyperinsulinaemic–euglycaemic clamp test, we found that insulin sensitivity was increased at the in vivo level in V1bR−/− mice. In addition, Akt phosphorylation by insulin stimulation was enhanced in cultured adipocytes of V1bR−/− mice, suggesting that enhanced insulin signalling could contribute to increased insulin sensitivity. This phenotype of altered insulin sensitivity was also observed in V1b receptor-deficient mice with the C57BL/6J genetic background (V1bR(C57BL/6J)−/−) as well as in mutant mice with the mixed genetic background of 129Sv and C57BL/6J, indicating that a deficiency of the V1b receptor caused insulin hypersensitivity and this phenotype was not influenced by genetic background.
Since in vitro AVP-induced insulin release was impaired in V1bR−/− mice, we had expected that these mice would show lower plasma insulin and higher plasma glucose levels. As demonstrated in the present study, the lower plasma insulin levels in V1bR−/− mice were concordant with the finding of our previous in vitro study (Oshikawa et al. 2004). However, V1bR−/− mice unexpectedly exhibited lower plasma glucose levels under the fasting condition, even after glucose loading. The following are possible explanations for the lower plasma glucose in V1bR−/− mice. First, the lower levels of plasma glucose in V1bR−/− mice observed under the fasting condition and after glucose loading could have been caused by increased insulin sensitivity. Indeed, other genetically modified mice in which insulin hypersensitivity was observed also had lower levels of plasma glucose (Terauchi et al. 1999; Ueki et al. 2002; Duttaroy et al. 2004). Second, reduced glucagon secretion may have contributed to lower levels of plasma glucose, particularly under the fasting condition. AVP was reported to stimulate glucagon release in vitro (Gao et al. 1992; Yibchok-anun & Hsu, 1998; Folny et al. 2003), and we recently reported that AVP stimulated glucagon release via the V1b and oxytocin receptors in vitro (Fujiwara et al. 2007). However, the oxytocin receptor can contribute to AVP-induced glucagon release only at a high ligand concentration, e.g. 10−8m, which is over the physiological range, and AVP-induced glucagon release was mainly mediated through the V1b receptors in the physiological range of AVP in vivo (Yibchok-Anun et al. 1999; Fujiwara et al. 2007). Therefore, the V1b receptor deficiency could have led to reduced glucagon secretion, resulting in reduced plasma glucose levels. In addition to the two above-mentioned possible explanations for insulin hypersensitivity and reduced glucagon secretion, glucocorticoid hormones could have affected glucose homeostasis in V1bR−/− mice. In our previous study, we found that plasma ACTH and corticosterone levels were lower in V1bR−/− mice than in V1bR+/+ mice (Tanoue et al. 2004). In rodents, corticosterone is known as a key hormone that causes gluconeogenesis in the liver and increases plasma glucose levels (Golf et al. 1984; Sistare & Haynes, 1985). Several studies have shown that plasma glucose levels are decreased in adrenalectomized animals or patients with chronic adrenal insufficiency (Addison's disease) (Serrano-Rios et al. 1974; Haluzik et al. 2002; Long et al. 2003). Thus, at least in part, decreased corticosterone levels could have caused lower plasma glucose levels in the V1bR−/− mice, although the reduction in the basal corticosterone levels was somewhat trivial in the V1bR−/− mice. The reduced plasma glucose levels observed in V1bR−/− mice could have been due to enhanced insulin sensitivity, reduced glucagon and/or reduced corticosterone levels.
As described above, plasma insulin levels were lower in V1bR−/− mice than in V1bR+/+ mice under the fasting condition as well as during glucose loading. Insulin secretion from pancreatic islets is mainly regulated by glucose, which stimulates an increase in the intracellular Ca2+ concentrations in pancreatic β-cells and causes insulin secretion (Henquin, 2004). AVP can work as a positive modulator for glucose-stimulated insulin release by acting on this signal (Gao et al. 1990; Lu et al. 1993). There are two possible explanations for the lower plasma insulin levels in V1bR−/− mice: (i) a primary disturbed effect on modulating insulin release by deficient AVP/V1b-receptor signalling, leading to impaired insulin secretion from pancreatic β-cells, and (ii) secondary effects of decreased plasma glucose levels. Our in vitro study with V1bR−/− mice clearly demonstrated that AVP can stimulate insulin release from pancreatic β-cells via the V1b receptor (Oshikawa et al. 2004), but the effect of AVP on plasma insulin levels remains unclear. It was reported that the i.v. administration of AVP increased the plasma insulin and glucagon levels in rat and sheep, suggesting that AVP may regulate glucose homeostasis by influencing pancreatic hormone secretion in vivo as well as in vitro (Dunning et al. 1984a; Mineo et al. 1997). In contrast, i.v. administration of AVP does not affect human plasma insulin levels (Spruce et al. 1985). In order to evaluate whether AVP could primarily affect insulin release in vivo, we measured the glucose and insulin levels after the i.v. administration of AVP in V1bR+/+ and V1bR−/− mice. In our present experiments, 100 ng kg−1 of AVP administration increased plasma glucose and insulin levels in both V1bR+/+ and V1bR−/− mice. The alteration of the plasma glucose levels by AVP administration may result from direct stimulation of the liver following gluconeogenesis and glycogenolysis (Hems & Whitton, 1973). As there was no significant difference in the increase in plasma insulin levels between V1bR+/+ and V1bR−/− mice, the insulin response could have been due to the secondary stimulus effect of increased plasma glucose levels rather than the primary stimulus effect of AVP on the V1b receptors in pancreatic β-cells. Thus, as the secondary effect, it is possible that the lower plasma glucose levels in V1bR−/− mice led to the suppression of glucose-dependent insulin secretion, resulting in reduced plasma insulin levels.
The mechanisms of increased insulin sensitivity in V1bR−/− mice are not fully explained by the results of our study. The V1b receptor was expressed in WAT, where the phosphorylation of Akt involved in insulin signalling was increased in V1bR−/− mice. We hypothesized that not only the glucose metabolism but also adipose tissue lipolysis could be altered in V1bR−/− mice, which would result in increased insulin signalling in WAT. Therefore, we examined the serum glycerol level to evaluate adipose tissue lipolysis in V1bR−/− mice, but we found that there was no significant difference between V1bR+/+ and V1bR−/− mice (serum glycerol, 155.5 ± 17.0 μmol l−1 in V1bR+/+ mice, n= 5, and 154.5 ± 7.4 μmol l−1 in V1bR−/− mice, n= 5, P= 0.96 by Student's t test). This result suggests that there is no difference in adipose tissue lipolysis between V1bR+/+ and V1bR−/− mice. Thus, the glucose metabolism, but not the lipid metabolism, was altered in V1bR−/− mice, and enhanced insulin signalling in WAT could lead to increased glucose uptake, resulting in insulin hypersensitivity. In addition to the direct modification of insulin sensitivity in adipose tissue, insulin sensitivity could be altered in other insulin-sensitive tissues, such as liver, heart and muscle, because the GIR assessed by the clamp test was two times higher in V1bR−/− mice than in V1bR+/+ mice. Especially, muscle is one of the major tissues for the uptake and utilization of glucose in response to insulin stimulation (Klip & Paquet, 1990). However, several reports have indicated that the altered glucose metabolism in both muscle and adipose tissue could affect the whole-body glucose metabolism and insulin sensitivity (Shepherd et al. 1993; Abel et al. 2001; Carvalho et al. 2005). For instance, the whole-body glucose metabolism and insulin sensitivity are altered in mice in which glucose transporter 4 is modified by an adipose tissue-specific-transgenic or knockout technique (Shepherd et al. 1993; Abel et al. 2001). Similarly, our finding suggests that the alteration of the adipose tissue glucose metabolism could contribute to the whole-body glucose metabolism and insulin sensitivity in V1bR−/− mice.
Since the V1b receptor is not detected in muscle, heart or liver, the modification of insulin sensitivity does not appear to depend directly on the V1b receptor but could be an indirect effect of it. Similar findings were observed in M3 muscarinic acetylcholine receptor-deficient mice (Duttaroy et al. 2004). In these mice, muscarinic stimulation of pancreatic insulin and glucagon release was abolished. These findings imply increased insulin sensitivity in M3 receptor-deficient mice, although the M3 receptor has no direct effect on insulin sensitivity (Duttaroy et al. 2004). One of the reasons for hypersensitivity to insulin is the considerable negative feedback of the insulin receptor. Several reports have suggested that insulin sensitivity is controlled by downstream signalling molecules of the insulin receptor, such as the insulin receptor substrate (IRS), phosphatidylinositol 3-kinase (PI3K) and protein kinase B/Akt (PKB/Akt) (Summers et al. 1999; Watson et al. 2004). Usually, insulin activates the insulin receptor and promotes the entry of glucose into tissues; however, a high density of insulin down-regulates the activated insulin receptor by the negative feedback of activated PI3K and PKB/Akt (Hirashima et al. 2003). In addition, reciprocal feedback regulation causes the dissociation of the insulin receptor and IRS with PI3K in primary adipocytes (Hers et al. 2002). Thus, it is possible that the low plasma insulin level in V1bR−/− mice may reduce the negative feedback of the insulin receptor, probably resulting in hypersensitivity to insulin.
In a recent study with V1b vasopressin receptor-deficient mice, Lolait et al. (2007) reported that there was no significant difference in the glucose-lowering effect of insulin administration in control and mutant mice, although the plasma glucose levels after the insulin administration tended to be lower in the mutant mice. The different results in the study of Lolait et al. and ours in terms of the glucose-lowering effect by insulin with V1b receptor-deficient mice could be in part due to different experimental protocols, such as fasting periods. While we performed the ITT with insulin at a concentration of 1 U kg−1 after short-term (4 h) fasting, Lolait et al. performed the ITT with insulin at a concentration of 0.75 or 3 U kg−1 after long-term (12 h) fasting. It is well known that the fasting period is critical to the assessment of the hypoglycaemic effect of insulin (Randle, 1998). In fact, we observed no significant difference in the glucose-lowering effect between V1bR+/+ and V1bR−/− mice evaluated by ITT at all measuring points under the same fasting condition as that used in the GTT experiment (18 h fasting) (data not shown). Thus, increased insulin sensitivity was observed during the ITT with V1bR−/− mice after only 4 h fasting, and this phenotype was further confirmed by the clamp test. Furthermore, another study with V1b receptor-deficient mice congenic for C57BL/6J mice for the glucose-lowering effect by insulin showed that a deficiency of the V1b receptor resulted in increased insulin sensitivity. These results indicated that insulin hypersensitivity by V1b-receptor deficiency was not influenced by genetic background.
In conclusion, our study with V1b receptor-deficient mice revealed that a deficiency of the V1b receptor resulted in a phenotype with lower plasma insulin and glucagon concentrations and hypersensitivity to insulin. We found that the glucose metabolism in the adipose tissue was altered in V1bR−/− mice, while lipolysis was not altered. The altered adipose tissue glucose metabolism could contribute to whole-body insulin sensitivity. Thus, AVP/V1b receptor signalling is an important pathway to modulate insulin sensitivity. These findings indicate that the AVP/V1b receptor pathway plays a crucial role in regulating glucose homeostasis by affecting insulin and glucagon secretion and that long-term antagonism for the V1b receptor could result in increased insulin sensitivity.
Acknowledgments
We thank Drs Kamohara and Momose for their participation in helpful discussions and Ms Kubota for animal maintenance. This work was supported in part by research grants from the Scientific Fund of the Ministry of Education, Science and Culture of Japan, the Ministry of Human Health and Welfare of Japan, Japan Health Sciences, the Novaratis Foundation, and the Takeda Science Foundation.
References
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- Abu-Basha EA, Yibchok-Anun S, Hsu WH. Glucose dependency of arginine vasopressin-induced insulin and glucagon release from the perfused rat pancreas. Metabolism. 2002;51:1184–1190. doi: 10.1053/meta.2002.34052. [DOI] [PubMed] [Google Scholar]
- Altura BM, Altura BT. Vascular smooth muscle and neurohypophyseal hormones. Fed Proc. 1977;36:1853–1860. [PubMed] [Google Scholar]
- Aoyagi T, Birumachi J, Hiroyama M, Fujiwara Y, Sanbe A, Yamauchi J, Tanoue A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology. 2007;148:2075–2084. doi: 10.1210/en.2006-1315. [DOI] [PubMed] [Google Scholar]
- Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab. 2005;289:E551–E561. doi: 10.1152/ajpendo.00116.2005. [DOI] [PubMed] [Google Scholar]
- Dunning BE, Moltz JH, Fawcett CP. Actions of neurohypophysial peptides on pancreatic hormone release. Am J Physiol Endocrinol Metab. 1984a;246:E108–E114. doi: 10.1152/ajpendo.1984.246.1.E108. [DOI] [PubMed] [Google Scholar]
- Dunning BE, Moltz JH, Fawcett CP. Modulation of insulin and glucagon secretion from the perfused rat pancreas by the neurohypophysial hormones and by desamino-D-arginine vasopressin (DDAVP) Peptides. 1984b;5:871–875. doi: 10.1016/0196-9781(84)90109-8. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53:1714–1720. doi: 10.2337/diabetes.53.7.1714. [DOI] [PubMed] [Google Scholar]
- Folny V, Raufaste D, Lukovic L, Pouzet B, Rochard P, Pascal M, Serradeil-Le Gal C. Pancreatic vasopressin V1b receptors: characterization in In-R1-G9 cells and localization in human pancreas. Am J Physiol Endocrinol Metab. 2003;285:E566–E576. doi: 10.1152/ajpendo.00148.2003. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Hiroyama M, Sanbe A, Yamauchi J, Tsujimoto G, Tanoue A. Mutual regulation of vasopressin- and oxytocin-induced glucagon secretion in V1b vasopressin receptor knockout mice. J Endocrinol. 2007;192:361–369. doi: 10.1677/joe.1.06864. [DOI] [PubMed] [Google Scholar]
- Gao ZY, Drews G, Nenquin M, Plant TD, Henquin JC. Mechanisms of the stimulation of insulin release by arginine-vasopressin in normal mouse islets. J Biol Chem. 1990;265:15724–15730. [PubMed] [Google Scholar]
- Gao ZY, Gerard M, Henquin JC. Glucose- and concentration-dependence of vasopressin-induced hormone release by mouse pancreatic islets. Regul Pept. 1992;38:89–98. doi: 10.1016/0167-0115(92)90075-6. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Golf SW, Bepperling F, Graef V. Effect of 5 alpha-dihydrocorticoids on enzymes of gluconeogenesis in rat liver. Steroids. 1984;43:85–91. doi: 10.1016/0039-128x(84)90060-6. [DOI] [PubMed] [Google Scholar]
- Haluzik M, Dietz KR, Kim JK, Marcus-Samuels B, Shulman GI, Gavrilova O, Reitman ML. Adrenalectomy improves diabetes in A-ZIP/F-1 lipoatrophic mice by increasing both liver and muscle insulin sensitivity. Diabetes. 2002;51:2113–2118. doi: 10.2337/diabetes.51.7.2113. [DOI] [PubMed] [Google Scholar]
- Hems DA, Whitton PD. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973;136:705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC. Pathways in beta-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004;53(Suppl. 3):S48–S58. doi: 10.2337/diabetes.53.suppl_3.s48. [DOI] [PubMed] [Google Scholar]
- Hers I, Bell CJ, Poole AW, Jiang D, Denton RM, Schaefer E, Tavare JM. Reciprocal feedback regulation of insulin receptor and insulin receptor substrate tyrosine phosphorylation by phosphoinositide 3-kinase in primary adipocytes. Biochem J. 2002;368:875–884. doi: 10.1042/BJ20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima Y, Tsuruzoe K, Kodama S, Igata M, Toyonaga T, Ueki K, Kahn CR, Araki E. Insulin down-regulates insulin receptor substrate-2 expression through the phosphatidylinositol 3-kinase/Akt pathway. J Endocrinol. 2003;179:253–266. doi: 10.1677/joe.0.1790253. [DOI] [PubMed] [Google Scholar]
- Hiroyama M, Aoyagi T, Fujiwara Y, Birumachi J, Shigematsu Y, Kiwaki K, Tasaki R, Endo F, Tanoue A. Hypermetabolism of fat in V1a vasopressin receptor knockout mice. Mol Endocrinol. 2007;21:247–258. doi: 10.1210/me.2006-0069. [DOI] [PubMed] [Google Scholar]
- Kim JK, Michael MD, Previs SF, Peroni OD, Mauvais-Jarvis F, Neschen S, Kahn BB, Kahn CR, Shulman GI. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest. 2000;105:1791–1797. doi: 10.1172/JCI8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A, Paquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- Lee B, Yang C, Chen TH, al-Azawi N, Hsu WH. Effect of AVP and oxytocin on insulin release: involvement of V1b receptors. Am J Physiol Endocrinol Metab. 1995;269:E1095–E1100. doi: 10.1152/ajpendo.1995.269.6.E1095. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, Young WS, 3rd, O'Carroll AM. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148:849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long W, Barrett EJ, Wei L, Liu Z. Adrenalectomy enhances the insulin sensitivity of muscle protein synthesis. Am J Physiol Endocrinol Metab. 2003;284:E102–E109. doi: 10.1152/ajpendo.00028.2002. [DOI] [PubMed] [Google Scholar]
- Lu M, Soltoff SP, Yaney GC, Boyd AE., 3rd The mechanisms underlying the glucose dependence of arginine vasopressin-induced insulin secretion in beta-cells. Endocrinology. 1993;132:2141–2148. doi: 10.1210/endo.132.5.8386610. [DOI] [PubMed] [Google Scholar]
- Michell RH, Kirk CJ, Billah MM. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979;7:861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Mineo H, Ito M, Muto H, Kamita H, Hyun HS, Onaga T, Yanaihara N. Effects of oxytocin, arginine-vasopressin and lysine-vasopressin on insulin and glucagon secretion in sheep. Res Vet Sci. 1997;62:105–110. doi: 10.1016/s0034-5288(97)90129-6. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshikawa S, Tanoue A, Koshimizu TA, Kitagawa Y, Tsujimoto G. Vasopressin stimulates insulin release from islet cells through V1b receptors: a combined pharmacological/knockout approach. Mol Pharmacol. 2004;65:623–629. doi: 10.1124/mol.65.3.623. [DOI] [PubMed] [Google Scholar]
- Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Richardson SB, Laya T, VanOoy M. Similarities between hamster pancreatic islet beta (HIT) cell vasopressin receptors and V1b receptors. J Endocrinol. 1995;147:59–65. doi: 10.1677/joe.0.1470059. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Briner V, Caramelo C. Cellular action and interactions of arginine vasopressin in vascular smooth muscle: mechanisms and clinical implications. J Am Soc Nephrol. 1993;4:2–11. doi: 10.1681/ASN.V412. [DOI] [PubMed] [Google Scholar]
- Serrano-Rios M, Hawkins FG, Escobar F, Mato JM, Larrodera L, De Oya M, Rodriguez-Minon JL. Insulin secretion in Addison's disease: effect of hydrocortisone treatment. Horm Metab Res. 1974;6:17–21. doi: 10.1055/s-0028-1093896. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- Shibata K, Katsuma S, Koshimizu T, Shinoura H, Hirasawa A, Tanoue A, Tsujimoto G. α1-Adrenergic receptor subtypes differentially control the cell cycle of transfected CHO cells through a cAMP-dependent mechanism involving p27Kip1. J Biol Chem. 2003;278:672–678. doi: 10.1074/jbc.M201375200. [DOI] [PubMed] [Google Scholar]
- Sistare FD, Haynes RC., Jr Acute stimulation by glucocorticoids of gluconeogenesis from lactate/pyruvate in isolated hepatocytes from normal and adrenalectomized rats. J Biol Chem. 1985;260:12754–12760. [PubMed] [Google Scholar]
- Spruce BA, McCulloch AJ, Burd J, Orskov H, Heaton A, Baylis PH, Alberti KG. The effect of vasopressin infusion on glucose metabolism in man. Clin Endocrinol (Oxf) 1985;22:463–468. doi: 10.1111/j.1365-2265.1985.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Summers SA, Yin VP, Whiteman EL, Garza LA, Cho H, Tuttle RL, Birnbaum MJ. Signaling pathways mediating insulin-stimulated glucose transport. Ann N Y Acad Sci. 1999;892:169–186. doi: 10.1111/j.1749-6632.1999.tb07795.x. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu TA, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85α subunit of phosphoinositide 3-kinase. Nat Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- Ueki K, Yballe CM, Brachmann SM, Vicent D, Watt JM, Kahn CR, Cantley LC. Increased insulin sensitivity in mice lacking p85β subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2002;99:419–424. doi: 10.1073/pnas.012581799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura MA, Rene P, de Keyzer Y, Bertagna X, Clauser E. Gene and cDNA cloning and characterization of the mouse V3/V1b pituitary vasopressin receptor. J Mol Endocrinol. 1999;22:251–260. doi: 10.1677/jme.0.0220251. [DOI] [PubMed] [Google Scholar]
- Voshol PJ, Jong MC, Dahlmans VE, Kratky D, Levak-Frank S, Zechner R, Romijn JA, Havekes LM. In muscle-specific lipoprotein lipase-overexpressing mice, muscle triglyceride content is increased without inhibition of insulin-stimulated whole-body and muscle-specific glucose uptake. Diabetes. 2001;50:2585–2590. doi: 10.2337/diabetes.50.11.2585. [DOI] [PubMed] [Google Scholar]
- Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- Yibchok-Anun S, Cheng H, Heine PA, Hsu WH. Characterization of receptors mediating AVP- and OT-induced glucagon release from the rat pancreas. Am J Physiol Endocrinol Metab. 1999;277:E56–E62. doi: 10.1152/ajpendo.1999.277.1.E56. [DOI] [PubMed] [Google Scholar]
- Yibchok-anun S, Hsu WH. Effects of arginine vasopressin and oxytocin on glucagon release from clonal alpha-cell line In-R1-G9: involvement of V1b receptors. Life Sci. 1998;63:1871–1878. doi: 10.1016/s0024-3205(98)00463-9. [DOI] [PubMed] [Google Scholar]