Abstract
Blood vessels are capable of structural changes in a dynamic process called ‘vascular remodelling’, which involves cell growth, death, phenotypic change and migration, as well as extracellular matrix synthesis and degradation. An integrated view of the interrelationships of the different elements of the arterial wall is made possible by fluorescence confocal microscopy which enables collection of serial optical sections of relatively thick specimens without the need to cut them as with conventional histology. With the aid of image analysis software, these serial sections can be further reconstructed to obtain 3-D images, where the structures of interest are localized and quantified. Confocal microscopy can be combined with pressure myography to obtain, simultaneously, information on vascular function and 3-D structure at near-to-physiological conditions. There are a vast number of fluorescent compounds useful for imaging vessel structure and function. Nuclear dyes allow the identification of the different types of vascular cells and the quantification of their number, shape and orientation. The speed of confocal image acquisition and processing makes it possible to scan entire intact arteries stained with fluorescent kits or antibodies to locate infrequent events such as cell apoptosis, proliferation or migration. Confocal microscopy is not only useful for imaging vascular wall structure, but also to visualize and quantify, by the intensity of fluorescence, the generation of vascular cell factors such as nitric oxide or superoxide anion. In conclusion, confocal microscopy and image analysis software provide insight into vascular wall structure and function and the active process of vascular remodelling in physiological and pathological situations.
Vascular wall structure and the process of remodeling
Blood vessels are dynamic structures, capable of structural changes in a process called vascular remodelling. This process of vessel restructuring might constitute a physiological adaptation in response to changes in environmental conditions or tissue demands, as occurs during pregnancy (Hilgers et al. 2003) or endurance training (Weber, 2000). However, vascular remodelling also occurs in association with certain pathological conditions – atherosclerosis, hypertension, diabetes – where it becomes a reactive process and actively participates in the development of cardiovascular accidents (Rizzoni et al. 2003).
Given the importance of vascular remodelling in cardiovascular pathology, the structural alterations of blood vessels have been the subject of study with several methodologies and from different points of view: physiological methods (wire or pressure myography), classical histology, biochemical and molecular biology techniques, cell cultures, etc. Each of these methods has their advantages and pitfalls. Physiological methods allow measurement of gross vessel structure at near-to-physiological conditions, but they lack the detail that can be obtained using histological techniques. As a disadvantage, histological techniques involve several processes – embedding, dehydration, sectioning – which produce a certain degree of distortion, and 3-dimensional (3-D) visualization of the vascular wall cannot be achieved. We have developed a method that allows the study of vascular wall structure in intact vessels under near-to-physiological conditions, with minimal 3-D distortion and with a detailed analysis of the different cellular and extracellular elements. The first problem we deal with is the need to visualize an intact blood vessel, because even small arteries are relatively thick specimens for conventional microscopy. However, this difficulty can be overcome with the use of confocal microscopy, which can produce optical sections throughout relatively thick tissues without the need for cutting thin slices. Confocal microscopy also eliminates blur and flare from out-of-focus planes in an object, and axial resolution is greatly improved (Pawley, 1989). We have combined pressure myography with confocal microscopy to study the vascular wall at the cellular level in intact arteries maintained at their physiological shape and level of pressurization (Arribas et al. 1999b). We named this technique ‘confocal myography’. The technique is straightforward and it involves five main steps: vessel pressurization, staining, image capturing and 3-D reconstruction and quantification. The simplicity of vessel processing, the speed of confocal microscopes for image acquisition and the development of image analysis software for visualization and quantification, enables the collection of image data from all the layers of the vascular wall in a relatively short time (Arribas et al. 2007).
Imaging vascular cells
Blood vessels are composed of different cells, endothelial, smooth muscle (SMC), fibroblasts and other, less characterized, cell types. Each of them is subjected to different chemical and haemodynamic stimuli and they have various growth/death rates. Therefore, endothelial, SMC and adventitial cells are likely to contribute in various ways to the process of vascular remodellng. Nuclear dyes that intercalate with DNA, such as DAPI, propidium iodide or Hoescht 33342, are useful in studying the organization and relationship between the different types of cells in the vascular wall (Arribas et al. 1999b). With the aid of nuclear stains and confocal myography we have identified cellular alterations in number, density, size and shape in intact resistance arteries from several rat models of hypertension (Arribas et al. 1997a,b) and in critical limb ischaemia in humans (Coats et al. 2003). The possibility to study intact blood vessels without 3-D distortion also enabled us to determine alterations in cellular orientation in the X, Y (Arribas et al. 1996, 1999a) and Z axis, which might represent migratory processes (Arribas et al. 1997b).
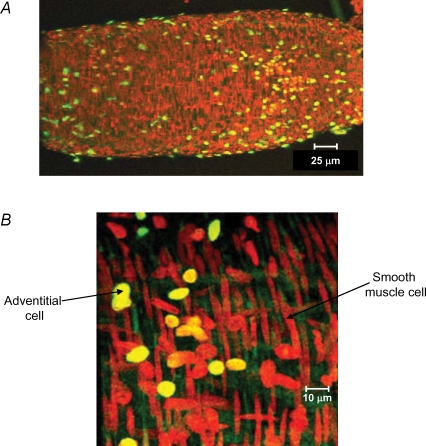
Cell death and proliferation are part of the remodelling process. However, they are not easy to detect as they are not frequent and occur at discrete time points. Therefore, these processes have been mostly studied in cells in culture incubated under certain conditions – growth factors, serum deprivation, apoptotic stimuli, among others – rather than in intact blood vessels. The development of fluorescence kits and antibodies for proliferation (i.e. ‘in vivo’ Bromodeoxyuridine (BrDu) incorporation; ‘in vitro’ anti-Proliferating cell nuclear antigen (PCNA) antibody) or death markers (i.e. fluorescent Tunel kits; anti-caspases antibodies or fluorescence kits), together with confocal microscopy – which permits the scanning of a large amount of arterial tissue in a short time – makes possible the detection of cell death and proliferation events in intact arteries. Moreover, with the aid of these tools it is possible to identify the contribution of the different layers of the vascular wall to the process of remodelling in terms of cell proliferation/death. For example, we have found that the adventitia seems to be the most active layer in terms of cell turnover. In intact rat cerebral arteries stained with a fluorescent derivative of Tunel, it was found that the majority of Tunel-positive cells are located in the adventitia (Fig. 1). (Online Supplemental material shows a video of a 3-D reconstruction of Fig. 1)
Figure 1. Detection of apoptosis in intact resistance arteries.
Extended focus reconstruction obtained with Metamorph image analysis software of serial optical sections taken with a Leica TCS SP2 confocal microscope from a rat cerebral artery stained with propidium iodide (red) and Fluorescein-Tunel (green). A, low magnification (×20) showing the entire artery. B, high magnification (×63) showing the different types of cells in the vascular wall (round nuclei, adventitial cells; elongated nuclei, smooth muscle cell). The majority of Tunel-positive cells are located in the adventitial layer.
Imaging extracellular matrix
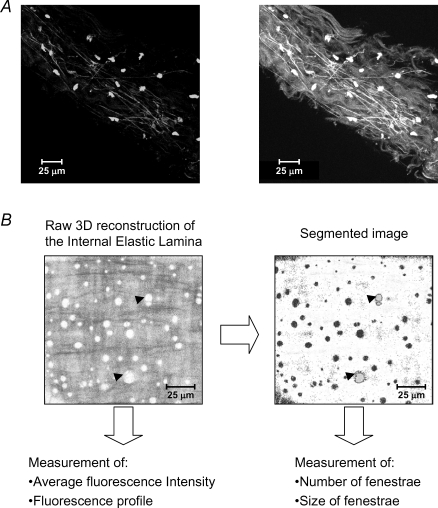
Some elements of the vascular wall have autofluorescent properties, which can be used for visualization and/or quantification. For example, the fibrous extracellular matrix proteins collagen and elastin exhibit autofluorescence in the band of 488/515 nm (Wong & Langille, 1996). Collagen-emitted fluorescence is much weaker than elastin and can only be detected only using a very high laser intensity profile (Fig. 2). Therefore, to study collagen distribution, the use of specific antibodies seems more appropriate. The autofluorescent properties of elastin can, however, be used to visualize and quantify the organization of elastic fibre network and lamina in the arterial wall, such as number and size of fenestrae, fluorescence intensity levels and wavelength profile (with the aid of spectral microscopes) (Fig. 2). This approach can be used in both intact small arteries (González et al. 2005) or even in large vessels cut open and mounted with the endothelial side facing up (Somoza et al. 2006). The combined study of vascular mechanical properties, with pressure myography, and elastic fibre network with confocal microscopy enabled demonstration of the relationship between altered elastic fibre organization and density with increased vessel stiffness in resistance arteries from spontaneously hypertensive rats (Briones et al. 2003; González et al. 2006). (Online Supplemental material includes two videos showing the 3-D organization of the internal elastic lamina of a hypertensive (SHR) and a normotensive (WKY) rat mesenteric artery.)
Figure 2. Imaging and quantification of extracellular matrix in intact resistance arteries.
A, extended focus reconstruction obtained with Metamorph image analysis software of serial optical sections taken from the adventitia of a human artery (×63; Leica TCS-SP2 spectral microscope). Left panel shows adventitial cells (round dots) stained with DAPI and autofluorescent elastic fibres. Right panel shows the same reconstruction where the images have been captured at higher laser intensity and brightness conditions, allowing visualization of collagen bundles. B, extended focus reconstruction of the internal elastic lamina of a rat mesenteric resistance artery and the measurements that can be obtained from the images. Left panel show the raw extended focus reconstruction (fenestrae in white) and right panel shows the segmented image where the number and size of fenestrae can be quantified. Arrowheads indicate examples of fenestrae in the raw and segmented images.
Imaging functional processes
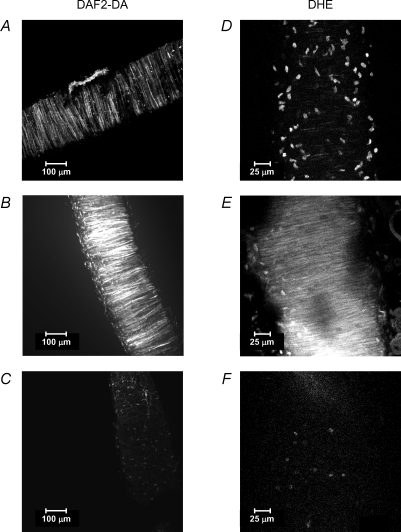
Vascular function is tightly regulated by vasoactive factors. Among others, nitric oxide (NO) and superoxide anion have a relevant role in the maintenance of a normal vascular structure and function and the imbalance between them is at the base of various pathological conditions (Paravicini & Touyz, 2006). NO and superoxide anion have usually been measured by indirect methods (vascular relaxation/contraction, activity or expression of the enzymatic synthetic machinery, detection of degradation products in plasma, among others). The development of dyes that bind to NO, such as DAF2-Diacetate (DAF2-DA; Kojima et al. 1998) or superoxide anion, like dihydroethidium (DHE; Suzuki et al. 1995) and produce fluorescent compounds, together with confocal microscopy enable visualization of the bioavailability of these compounds in the vascular wall and the relationship between them (Fig. 3). DAF2-DA enters the vascular cells where it is cleaved by vascular esterases to DAF2 which is then retained in the cell. The combination of DAF2 with NO, in the presence of oxygen, produces a highly fluorescent compound detected in the cytoplasm with the 488 nm/515 nm band and can be used for real-time bioimaging of NO with fine temporal and spatial resolution. On the other hand, DHE permeates the cell and is directly oxidized by oxygen radicals (being specially sensitive to superoxide anion) to form ethidium bromide, which combines with DNA and is trapped in the nucleus, emitting fluorescence in the 590 nm wavelength (Suzuki et al. 1995). Obviously DAF2-DA and DHE have to be incubated in intact, non-fixed blood vessels, but survive 4% paraformaldehyde post-fixation and embedding in optimum cutting temperature (OCT), compound with no alteration in their fluorescent properties, even if stored for long periods (Fig. 3). Quantification of the bioavailability of NO can be performed with conventional image analysis software based on fluorescent intensity levels of DAF2-DA which increase in proportion to the amount of NO released by the vascular cells in basal or stimulated conditions (Fig. 3). The reaction is specific as it is blocked by the NO synthase inhibitor l-NAME (Fig. 3). Superoxide anion availability can be quantified by the number of stained cells per unit length (Suzuki et al. 1995). We have observed that under stimulated conditions – as with the superoxide anion donor pirogallol – the amount of superoxide produced is so high that fluorescence is located in all vascular cells and even released in the cytoplasm (Fig. 3). For quantification purposes, it is important to establish a profile of laser power, brightness and contrast for all the experimental groups. It is also advisable to study, whenever possible, control and experimental groups simultaneously to avoid variability caused by day-to-day laser fluctuations of the confocal microscope.
Figure 3. Imaging of nitric oxide and superoxide anion availability in intact resistance arteries.
Extended focus reconstructions obtained with Metamorph image analysis software of serial optical sections taken from a rat mesenteric resistance artery stained with DAF2-DA (left panels, ×20 air objective) or dihydroethidium (DHE) (right panels, ×63 oil immersion objective) and fixed after staining. A, DAF2 emitted fluorescence in non-stimulated conditions (basal NO). B, DAF2 emitted fluorescence after stimulation with 10−6m acetylcholine (stimulated NO). C, DAF2 emitted fluorescence after pre-incubation with the NO synthase inhibitor l-NAME (negative control). D, ethidium bromide emitted fluorescence in non-stimulated conditions (basal superoxide anion). E, ethidium bromide emitted fluorescence in an artery stimulated with 10−6m pyrogallol (stimulated superoxide anion). F, ethidium bromide emitted fluorescence in an artery pre-incubated with superoxide dismutase (negative control).
In summary, the use of fluorescent compounds and confocal microscopy permit visualization of the vascular wall 3-D organization in intact arteries and the changes that occur in the cellular and extracellular matrix components during vascular remodelling. This process can be performed in vessels maintained at physiological distending pressures with the aid of pressure myography, enabling the visualization of the vascular wall in 3-D and the study of the structure–function relationship. The ability to scan a large proportion of vessel in a short time also provides a useful tool to quantify unusual or scarce events such as apoptosis or migration. In addition to the information that can be gained from vascular structure with confocal microscopy, it is also possible to visualize and quantify with image analysis software bioavailability of some vascular factors, such as NO and superoxide anion. The development of new fluorescent compounds and more sophisticated and specific image analysis software will broaden the study of the vascular wall structure and function in physiological and pathological situations.
Acknowledgments
This work has been funded by European Union (QLG1-1999-00084); Ministerio de Educacion & Ciencia (BFU2004-04148) and Comunidad Autónoma de Madrid (UAMBio-0514 2006).
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.138198/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol. 2007.138198
References
- Arribas SM, Costa R, Salomone S, Morel N, Godfraind T, McGrath JC. Functional reduction and associated cellular rearrangement in SHRSP rat basilar arteries are affected by salt load and calcium antagonist treatment. J Cereb Blood Flow Metab. 1999a;19:517–527. doi: 10.1097/00004647-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Arribas SM, Daly CJ, McGrath JC. Confocal Microscopy (a Volume of Methods in Enzymology) Vol. 307. San Diego: Academic Press; 1999b. Measurement of vascular remodeling by confocal microscopy; pp. 246–273. [DOI] [PubMed] [Google Scholar]
- Arribas SM, González JM, Briones AM, Somoza B, Daly CJ, Vila E, González MC, McGrath JC. Confocal myography for the study of hypertensive vascular remodelling. Clin Hemorheol Microcirc. 2007;37:205–210. [PubMed] [Google Scholar]
- Arribas SM, González C, Graham D, Dominiczak AF, McGrath JC. Cellular changes induced by chronic nitric oxide inhibition in intact rat basilar arteries revealed by confocal microscopy. J Hypertens. 1997a;15:1685–1693. doi: 10.1097/00004872-199715120-00073. [DOI] [PubMed] [Google Scholar]
- Arribas SM, Gordon JF, Daly C, Dominiczak AF, McGrath JC. Confocal microscopic characterization of a lesion in the cerebral vessel of the stroke-prone spontaneously hypertensive rat. Stroke. 1996;27:1118–1123. doi: 10.1161/01.str.27.6.1118. [DOI] [PubMed] [Google Scholar]
- Arribas SM, Hillier C, González C, McGrory S, Dominiczak AF, McGrath JC. Cellular aspects of vascular remodelling in hypertension revealed by confocal microscopy. Hypertension. 1997b;30:1455–1464. doi: 10.1161/01.hyp.30.6.1455. [DOI] [PubMed] [Google Scholar]
- Briones AM, González JM, Somoza B, Giraldo J, Daly CJ, Vila E, González MC, McGrath JC, Arribas SM. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J Physiol. 2003;552:185–195. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats P, Jarajapu YP, Hillier C, McGrath JC, Daly C. The use of fluorescent nuclear dyes and laser scanning confocal microscopy to study the cellular aspects of arterial remodelling in human subjects with critical limb ischaemia. Exp Physiol. 2003;88:547–554. doi: 10.1113/eph8802552. [DOI] [PubMed] [Google Scholar]
- González JM, Briones AM, Somoza B, Daly CJ, Vila E, Starcher B, McGrath JC, González MC, Arribas SM. Postnatal alterations in elastic fiber organization precede resistance artery narrowing in SHR. Am J Physiol Heart Circ Physiol. 2006;291:H804–H812. doi: 10.1152/ajpheart.01262.2005. [DOI] [PubMed] [Google Scholar]
- González JM, Briones AM, Starcher B, Conde MV, Somoza B, Daly C, Vila E, McGrath I, González MC, Arribas SM. Influence of elastin on rat small artery mechanical properties. Exp Physiol. 2005;90:463–468. doi: 10.1113/expphysiol.2005.030056. [DOI] [PubMed] [Google Scholar]
- Hilgers RH, Bergaya S, Schiffers PM, Meneton P, Boulanger CM, Henrion D, Levy BI, De Mey JG. Uterine artery structural and functional changes during pregnancy in tissue kallikrein-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1826–1832. doi: 10.1161/01.ATV.0000090672.07568.60. [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Pagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Pawley J. The Handbook of Biological Confocal Microscopy. Madison, WI, USA: IMR Press; 1989. [Google Scholar]
- Rizzoni D, Porteri E, Boari GEM, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- Somoza B, Abderrahim F, Gonzalez JM, Conde MV, Arribas SM, Starcher B, Regadera J, Fernandez-Alfonso MS, Diaz-Gil JJ, Gonzalez MC. Short-term treatment of spontaneously hypertensive rats with liver growth factor reduces carotid artery fibrosis, improves vascular function, and lowers blood pressure. Cardiovasc Res. 2006;69:764–771. doi: 10.1016/j.cardiores.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Swei A, Zweifach BW, Schmid-Schonbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol. 2000;15:264–272. doi: 10.1097/00001573-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Wong LC, Langille BL. Developmental remodeling of the internal elastic lamina of rabbit arteries: effect of blood flow. Circ Res. 1996;78:799–805. doi: 10.1161/01.res.78.5.799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.