Abstract
Trafficking of leukocytes to sites of inflammation is an important step in the establishment of an immune response. Chemokines are critical regulators of leukocyte trafficking and are widely studied molecules for their important role in disease and for their potential as new therapeutic targets. The ability of chemokines to induce leukocyte recruitment has been mainly measured by in vitro chemotaxis assays, which lack many components of the complex biological process of leukocyte migration and therefore provide incomplete information about chemokine function in vivo. In vivo assays to study the activity of chemokines to induce leukocyte recruitment have been difficult to establish. We describe here the development of a robust in vivo recruitment assay for CD8+ and CD4+ T lymphocytes induced by the CXCR3 ligands IP-10 (CXCL10) and I-TAC (CXCL11). For this assay, in vitro activated T lymphocytes were adoptively transferred into the peritoneum of naïve mice. Homing of these transferred T lymphocytes into the airways was measured following intratracheal instillation of chemokines. High recruitment indices were achieved that were dependent on chemokine concentration and CXCR3 expression on the transferred lymphocytes. Recruitment was also inhibited by antibodies to the chemokine. The assay models the natural condition of chemokine-mediated lymphocyte migration into the airways as chemokines are expressed in the airways during inflammation. The nature of this model allows flexibility to study wildtype and mutant chemokines and chemokine receptors and the ability to evaluate chemokine antagonists and antibodies in vivo. This assay will therefore help elucidate a deeper understanding of the chemokine system in vivo.
Keywords: Chemokines, cell trafficking, chemotaxis
1. Introduction
Chemokines are a superfamily of chemotactic cytokines, which play important roles in the generation and delivery of immune and inflammatory responses. To date, more than fifty chemokines have been identified, which are grouped into two main sub-families based on the position of the first two cysteine residues, the CXC and CC chemokine subfamilies, and two smaller families (C and CX3C), each with essentially only one member (reviewed by (Luster, 1998; Charo and Ransohoff, 2006). Chemokines are involved in many, if not most, diseases ranging from viral infections, asthma, rheumatoid arthritis and cancer by orchestrating the movement of leukocytes and other cells, which express their seven-transmembrane spanning G protein-coupled receptors. In addition to binding to their high affinity receptors, chemokines also interact with proteoglycans, a process shown to be important for the retention and sequestration of chemokines on the endothelium and extracellular matrix and for maintenance of haptotactic gradients that are thought to direct the movement of leukocytes in vivo (Tanaka et al., 1993; Luster et al., 1995; Hoogewerf et al., 1997; Middleton et al., 1997).
One of the earliest identified chemokines is IP-10 (Interferon-induced protein of 10kDa), or CXCL10 (Luster et al., 1985), which directs the trafficking of activated T lymphocytes and other effector lymphocytes, such as NK and NKT cells (Taub et al., 1993) (Taub et al., 1995; Weng et al., 1998). It does so by binding to its high affinity receptor CXCR3, which it shares with two other ligands, interferon-inducible T cell-α chemoattractant (I-TAC/CXCL11) and monokine-induced by γ-interferon (Mig/CXCL9). The CXCR3 ligands have been shown to be upregulated in a number of human diseases, such as atherosclerosis (Mach et al., 1999), multiple sclerosis (Balashov et al., 1999; Sorensen et al., 1999), allograft rejection (Melter et al., 2001; Zhao et al., 2002), viral hepatitis (Narumi et al., 1997) and others.
The recruitment of leukocytes to sites of inflammation is a complex, multiple step process, which has been explored for many years. The multi-step cascade (Butcher, 1991; Springer, 1994) begins with rolling or tethering of the leukocytes along the endothelium, and is mediated by selectin molecules on endothelial cells interacting with their carbohydrate ligands on leukocytes. Subsequently, chemokines are presented by endothelial glycosaminoglycans to chemokine receptors on rolling leukocyte. This induces integrin activation in the leukocyte, which leads to leukocyte firm arrest and subsequent diapedesis/extravasation through the endothelium into the tissue.
Chemokine-induced trafficking of leukocytes or other chemokine receptor-bearing cells plays crucial roles in inflammation, yet few experimental systems exist to study this recruitment process in vivo. The most commonly used methods to study chemokine recruitment activity are in vitro chemotaxis assays. For these assays, the chemokine and the chemokine receptor-expressing cells are generally placed on opposite sides of a membrane with varying pore sizes. One widely used device for measuring chemotaxis is the Boyden chamber, where the chemokine is added to the bottom wells, a membrane is laid over the wells and the cells are placed on top of the membrane and allowed to migrate at 37° C for a set amount of time. The cells that migrated through the membrane are enumerated and compared to the number of cells that migrated without addition of chemokine.
Although in vitro chemotaxis assays are widely used and have yielded valuable information in the study of the chemokine system, they clearly do not recapitulate all of the components of the in vivo trafficking cascade. The deficiencies of the in vitro chemotaxis assay include lack of chemokine gradient, as well as lack of physiological flow, matrix components and endothelial cells. Some of these deficiencies have been addressed by for example coating the membrane with extracellular matrix proteins, such as collagen, fibronectin, or matrigel. In other modification of the assay, endothelial or epithelial cells are grown on the membrane before the chemotaxis assay to mimic the transmigration process. Chemotaxis chambers, such as the Zigmond (Zigmond, 1977) or Dunn (Zicha et al., 1991) chamber, also try to simulate a chemokine gradient. However, each system only addresses some of the complex components of the in vivo recruitment process.
We describe here the development of a new, physiologically relevant in vivo lymphocyte recruitment assay that fully captures the complex in vivo trafficking cascade, which can be used to study the chemokine family and other chemoattractants.
2. Materials and methods
2.1 Materials and mice
Chemically synthesized IP-10 and I-TAC were obtained from the University of British Columbia, Vancouver. The anti-IP-10 antibody was purified as described (Khan et al., 2000). C57Bl/6 mice were purchased from National Cancer Institute. The OT-I TCR mice in the C57Bl/6 background were obtained from Jackson Immunoresearch Laboratories and crossed with Thy1.1 mice and with CXCR3 knockout (KO) mice. CXCR3 KO mice (Hancock et al., 2000) backcrossed >9 generations were a kind gift of Craig Gerard (Children's Hospital, Boston). All mice were bred and used at Massachusetts General Hospital under protocols approved by the Subcommittee on Research and Animal Care.
2.2 CD8+ Th1 cell polarization and culture
CD8+ polarized Th1 OT-I cells were prepared and cultured as described (Grabie et al., 2003; Medoff et al., 2005). Spleen and peripheral lymph nodes of OT-I TCR (wt or CXCR3 KO) mice were removed and passed through a 70 μm mesh filter to obtain a single cell suspension. After red blood cells lysis (Sigma-Aldrich), cells were incubated with MACS anti-CD8α microbeads (Miltenyi Biotec Inc.) for 20 minutes at 14°C, and passed over a MACS Midi LS+ column. CD8+ selected cells were cultured at a 1:10 ratio with irradiated antigen-presenting cells prepared from spleens of C57Bl/6 mice pre-incubated with OVA SIINFEKL peptide, anti-CD28 (BD-Pharmingen), IL-2 (PeproTech ), IL-12 (R&D Systems) in complete RPMI supplemented with 10 % heat inactivated FCS. Cells were fed with IL-2 (5−10 U/ml) on day 3 and 5. Cells were harvested on day 6 with Lympholyte (Cedarlane). Activation status was analyzed by FACS analysis.
2.3 Chemotaxis
Chemokine dilutions in RPMI media supplemented with 1% low endotoxin BSA (Sigma Aldrich) were added to the bottom well of a 96-well chemotaxis plate (Neuroprobe). Activated OT-I cells (day 8−11 in culture with IL-2) were washed into the same buffer at a concentration of 5×105 cells/ml and 50 μl of cells were added on top of the membrane (5 μm pore size, polycarbonate filters). The chemotaxis plate was incubated at 37° C for two hours and transferred to 4° C for 10 minutes before removing the membrane. Cells in the bottom wells were counted under a microscope. Results are presented as chemotactic indices by dividing the number of cells in the bottom well in response to the chemokine by the average number of cells in the bottom well without the addition of chemokines. Each experiment was performed in duplicate and repeated a minimum of two times.
2.4 Internalization of CXCR
Internalization of murine CXCR3 was measured as previously described (Sauty et al., 2001). Briefly, polarized CD8+ T cells were resuspended in complete RPMI, 10% FCS, and various concentrations of I-TAC and IP-10 were added to the cell suspension and incubated for 30 min at 37° C. The cells were washed and stained with anti-mCXCR3 antibody conjugated to PE (R&D Systems), or an IgG control and analyzed by FACS.
2.5 In vivo OT-I recruitment
OT-I cells (5−7×106 in 0.5 ml HBSS) were injected intraperitoneally into male C57Bl/6 mice (6−10 weeks of age). After 48 hr, mice were sedated with Ketamine (80 mg/kg) and Xylazine (12 mg/kg) given by intraperitoneal injection. The trachea was exposed in a sterile fashion and 50 μl PBS, I-TAC or IP-10 (0.5−50 μg in PBS) were injected intratracheally with a 28g bent needle. After 18 hr, mice were sedated with Ketamine and Xylazine and exsanguinated by cutting the renal artery. Bronchial alveolar lavages (BAL) were performed with six aliquots of 0.5 ml PBS containing 0.6 mM EDTA. Red blood cells were lysed with RBC lysis buffer (Sigma-Aldrich) after which total cells in the BAL were counted with a hemocytometer. Cells were incubated for 10 minutes with 2.4G2 anti-FcαIII/II receptor (BD Pharmingen) and were then stained with FITC-conjugated anti-murine CD3, PE-conjugated anti-murine CD4, or PE-conjugated anti-murine CD90.1 and APC-conjugated anti-murine CD8 at 4°C for 20 minutes. Cells were fixed with 1% paraformaldehyde, and cytofluorimetry was performed using a FACS Caliber Cytometer (Becton-Dickinson) and analyze using CellQuest software. The number of T lymphocytes was calculated after gating on the lymphocyte subpopulation as determined by the forward side scatter. All experiments were repeated two or three times, and had three to four mice per group, unless otherwise stated.
2.6 Inhibition of recruitment by anti IP-10 antibody
Anti-IP-10 antibody or control hamster IgG was injected intraperitoneally into mice as indicated in 0.5 ml of PBS four hours before OT-I cell transfer. Forty-eight hours later mice were injected intratracheally with IP-10 or PBS as described above, and 2 hours later were injected with a second dose of anti-IP-10 or control antibody, before proceeding with the assay as described.
2.7 CD4+ Th1 cell polarization and culture
CD4+ T cells were purified by removing the spleen and peripheral lymph nodes of OT-II TCR, Thy1.1 mice and passing them through a 70 μm mesh filter to obtain single cell suspension. After red blood cells lysis, cells were incubated with CD4 Dynabeads (Dynal Biotech) according to protocol. Purified CD4+ T cells (3 × 105 cells/ml) were activated on anti-CD3 coated 24-well plates with anti-CD28, IL-12, and anti-IL-4 (11B11). Cells were fed with IL-2 initially on day 2, 4 and 5 before use on day 6. The experiment was performed twice with three mice per group.
2.8 Statistical analysis
Statistical significance was determined by two-tailed student t-test. A p-value of less then 0.05 was considered statistically significant. All bar graphs represent the average of each group of mice +/− standard deviation from one experiment.
3. Results
3.1 In vitro activation of CD8+ T lymphocytes
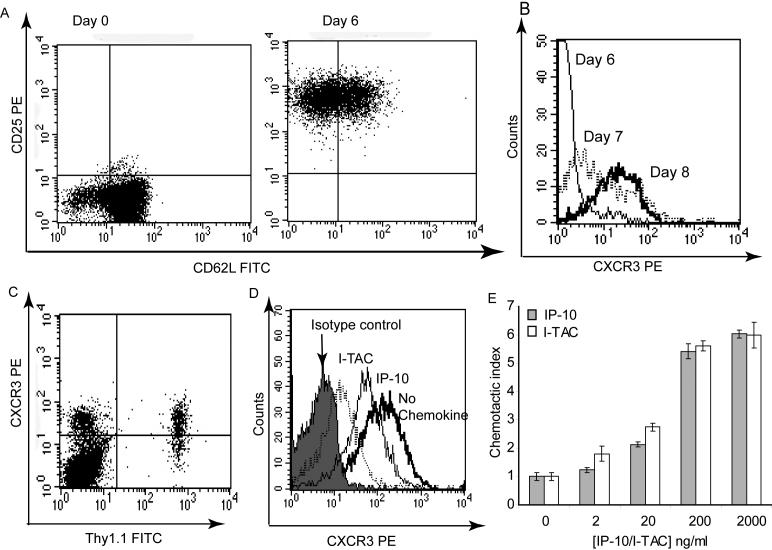
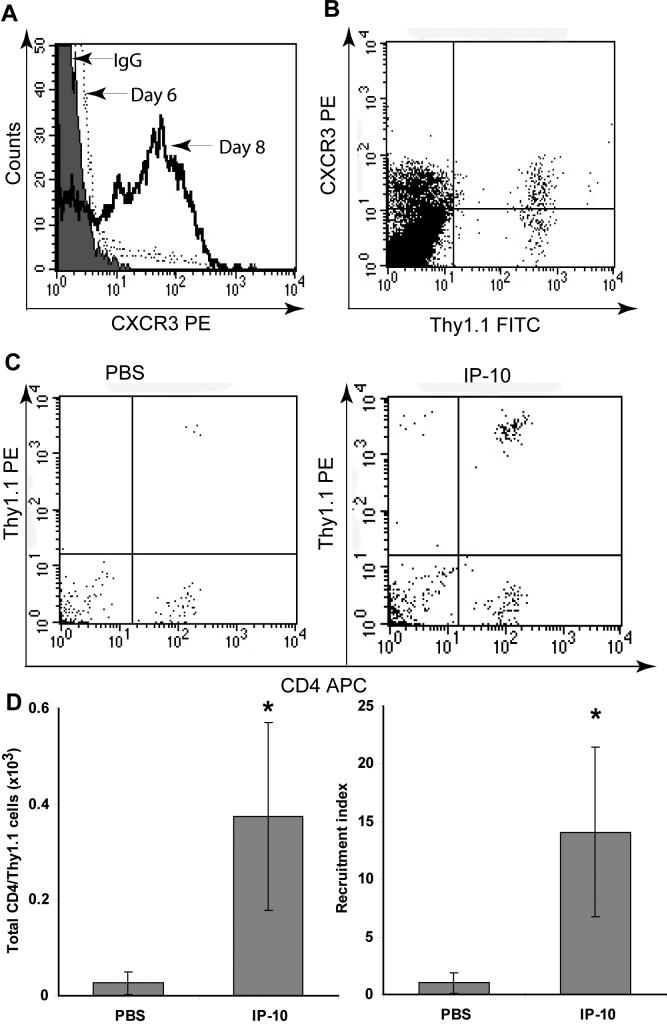
Functionally activated CD8+ T lymphocytes were used for the development of our in vivo recruitment assay. We used ovalbumin specific CD8+ T cells (OT-I cells) from a T cell receptor (TCR)-transgenic mouse that were cultured for six days with antigen, IL-2 and IL-12. To evaluate the activation status of CD8+ T cells prior to adoptive transfer into mice, their expression of CD62L and CD25 was analyzed by flow cytometry. After six days in culture, the cells were confirmed to have an activated phenotype with downregulated CD62L and upregulated CD25 (Fig 1A). Once the activation status of the cells was confirmed, they were adoptively transferred into mice by intraperitoneal injection. Although at the time of adoptive transfer CXCR3 expression was still low (Fig 1B), its expression increased when cells were cultured for two more days with IL-2. This mirrored the development of the cells in vivo, as the cells displayed high CXCR3 expression in the spleen at the time of harvest (Fig 1C), three days after adoptive transfer.
Figure 1. Characterization of in vitro activated CD8+ T lymphocytes.
A) Expression of activation markers CD62L and CD25 on Day 0 and 6 of culture, as determined by Flow cytometry. B) Expression of CXCR3 on different days of in vitro culture. C) Expression of CXCR3 on Thy1.1+ T lymphocytes in the spleen three days after adoptive transfer of cells. D) CXCR3 internalization induced by 10 nM I-TAC or IP-10 E) Chemotaxis induced by I-TAC and IP-10. Each bar presents mean chemotactic index +/− STD. Experiments in D and E were performed with activated CD8+ T lymphocytes after eight days in culture.
Activated CD8+ T lymphocytes were analyzed for their responsiveness to CXCR3 ligands after eight days in culture, when they displayed high levels of CXCR3. The two CXCR3 ligands I-TAC and IP-10 induced internalization of CXCR3 within 20 minutes (Fig 1D). As previously reported, I-TAC induced more internalization than IP-10 (Sauty et al., 2001; Colvin et al., 2004). I-TAC and IP-10 both induced robust in vitro chemotaxis of activated CD8+ T lymphocytes (Fig 1E), confirming that these cells were responsive to the chemokines of interest.
3.2 Intratracheally injected chemokines induce recruitment of CD8+ cells into the airways
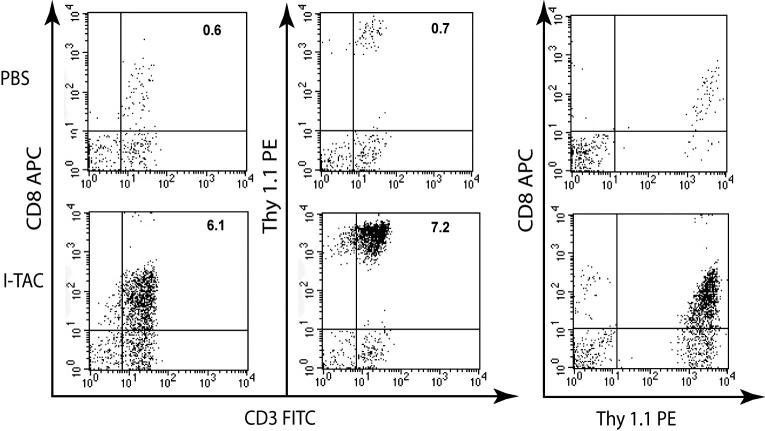
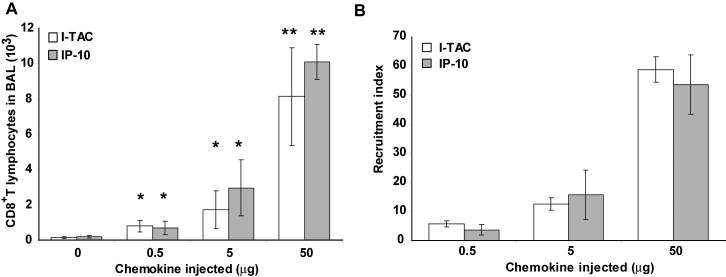
Forty-eight hours after adoptive transfer of activated CD8+ T lymphocytes, the CXCR3 ligand I-TAC was instilled intratracheally into the airways of mice. Eighteen hours later, the airways were lavaged and infiltrating T lymphocytes were measured by flow cytometry. After instillation of only PBS, very few CD8+ T lymphocytes were recovered from the airways (Fig 2). In contrast, after instillation of 5 μg I-TAC, a large population of CD8+ T lymphocytes had infiltrated into the airways. We used the Thy1.1 allele to definitively identify transferred T cells since we transferred Thy1.1 OT-I cells into Thy1.2 C57Bl/6 mice. Use of Thy1.1 staining demonstrated that nearly all of the infiltrated CD8+ cells in the airways were adoptively transferred cells. Different amounts of I-TAC were instilled intratracheally into the airways, with even the lowest amount of I-TAC (0.5 μg) resulting in statistically significant recruitment of CD8+ cells into the airways with a recruitment index of 5.8 (Fig. 3). Higher amounts of I-TAC led to increased recruitment, with 5 and 50 μg I-TAC resulting in a recruitment index of 12.5 and 58.8, respectively. Instillation of the CXCR3 ligand IP-10 induced a similar recruitment of CD8+ T lymphocytes, with similar potency.
Figure 2. Flow cytometric analysis of CD8+ T lymphocyte recruitment into the airways induced by I-TAC.
I-TAC (5 μg) was injected intratracheally after adoptive transfer of activated CD8+ T lymphocytes 48 hr prior. The BAL was harvested 18 hr later, and CD8+ T lymphocytes were analyzed by flow cytometry after gating on the lymphocyte subpopulation. The percentages of CD8+/CD3+ T cells of total events are shown in the upper right corners.
Figure 3. Dose response of I-TAC and IP-10 induced recruitment of activated CD8+ T lymphocytes in vivo.
PBS, I-TAC or IP-10 was injected intratracheally at the indicated amount. The BAL was harvested 18 hr later, and CD8+ T lymphocytes were analyzed by flow cytometry. A) Total CD8+ T lymphocytes in BAL. B) Recruitment index was calculated in comparison to intratracheal injection of PBS. * p < 0.05, ** p < 0.001 compared to PBS injection.
3.3 Optimizing conditions for the recruitment assay
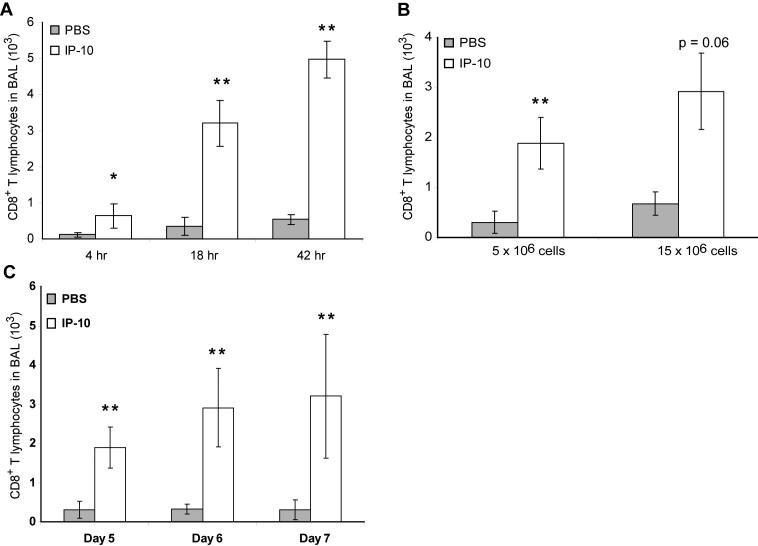
In order to define the conditions of the recruitment assay in more detail, different parameters were varied. The timing of the lymphocyte recruitment after instillation of chemokine was investigated by performing the airway lavage after 4, 18 and 42 hr. As seen in Figure 4A, 4 hr after instillation of 5 μg IP-10, a small, yet statistically significant number of CD8+ cells had infiltrated into the airways, with a recruitment index of 5.7. Eighteen hours after chemokine instillation, a larger number of Thy1.1 positive cells were recovered from the airways, leading to a recruitment index of 9.3. Performing the BAL 42 hr after chemokine instillation resulted in a similar recruitment index of 9.2. The 18 hr time point was therefore chosen as the standard for all further experiments.
Figure 4. Variation of assay parameters.
A) Timing of BAL: PBS or IP-10 (5 μg) was injected intratracheally after adoptive transfer of activated CD8+ T lymphocytes 48 hr prior. The BAL was harvested at the indicated time points, and CD8+ T lymphocytes were analyzed by flow cytometry. B) Number of CD8+ T lymphocytes adoptively transferred. PBS or IP-10 (5 μg) was injected intratracheally after adoptive transfer of activated CD8+ T lymphocytes as indicated 48 hr prior. The BAL was harvested after 18 hr, and CD8+ T lymphocytes were analyzed by flow cytometry. 2 mice per group were used for the 15×106 cells groups. C) Timing of in vitro culture: CD8+ T lymphocytes were activated in vitro for the indicated number of days and then adoptively transferred into mice. PBS or IP-10 (5μg) was injected intratracheally 48 hr later. The BAL was harvested after 18 hr, and CD8+ T lymphocytes were analyzed by flow cytometry. * p < 0.05, ** p < 0.01 each compared to PBS injection under the same conditions.
Additionally, the number of adoptively transferred cells was varied from 5×106 to 15×106, and did not significantly change the recruitment index (Figure 4B). Therefore, 5−7×106 cells were usually injected intraperitoneally for the recruitment assay. Next, the timing of the in vitro activation of the CD8+ cells was investigated. Cells were grown in culture for 5, 6 or 7 days before being adoptively transferred. Each of the time points resulted in a statistically significant recruitment of transferred cells in response to IP-10 (Figure 4C). The best recruitment indices were obtained after six days of culture, which was subsequently chosen as the standard in vitro activation time.
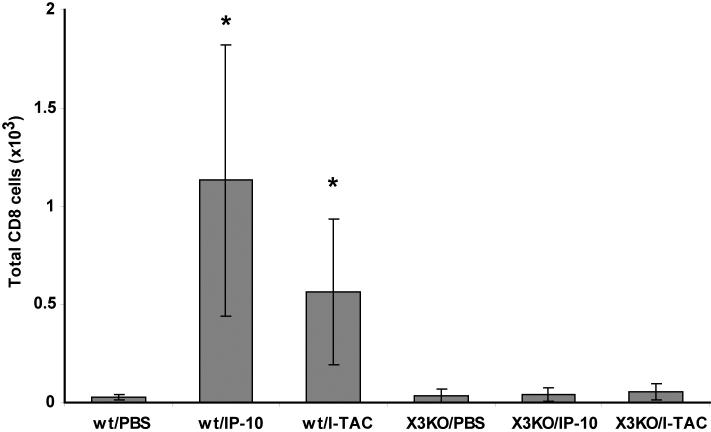
3.4 CXCR3 dependency of assay
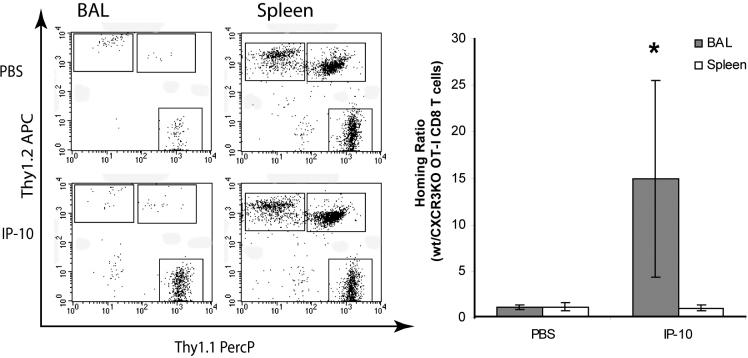
In order to confirm that the recruitment induced by instillation of IP-10 and I-TAC was mediated by CXCR3, we cultured both wild-type and CXCR3-deficient OT-I CD8+ T cells for six days as described above and injected them intraperitoneally into wild-type mice. Both cell populations were similarly activated as determined by CD25 and CD62L expression (data not shown). Two days after adoptive cell transfer, PBS or 5 μg of either IP-10 or I-TAC were instilled intratracheally into mice, and recruitment measured 18 hours later by BAL. Instillation of IP-10 or I-TAC two days after adoptive cell transfer resulted in robust recruitment of WT CD8+ T cells into the airways but not CXCR3-deficient cells (Figure 5A). This clearly demonstrates the direct dependence of the CXCR3 ligand-induced T cell recruitment on CXCR3. As an additional demonstration of the dependence of IP-10- and I-TAC-induced recruitment on CXCR3, we performed competitive adoptive transfer experiments. In these experiments, to directly compare the influence of CXCR3 on the efficiency of T cell trafficking into the airways in the same mouse, wt Thy1.1 OT-I T cells and CXCR3-deficient (knockout, KO) Thy1.2 OT-I cells were co-injected into the same Thy1.l×Thy1.2 mouse. Two days later, PBS, IP-10 or I-TAC were instilled into the airways and 18 hours later recruitment into the airways and spleens was measured. The ratio of wt OT-I cells:CXCR KO OT-I cells recovered from an anatomical site (spleen or BAL) is reported as the homing ratio. As can be seen from the primary FACS plots, after PBS injection a very low number of wt and CXCR3 KO OT-I cells were present in the airways, with the homing ratio of wt:CXCR3 KO cells close to one (Figure 6). After IP-10 instillation, wt OT-I cells were recruited to the airways, with the homing ratio increasing from 1.1 after PBS injection to 15.0 after IP-10 injection. In contrast, in the spleen the ratio of wt to CXCR3 KO OT-I cells was close to one both after PBS and IP-10 instillation, demonstrating that both cells types were similarly present in the mouse, but only CXCR3 expressing cells migrated to the airways after instillation of IP-10.
Figure 5. Transfer of wt or CXCR deficient activated CD8+ T cells.
Activated wt or CXCR3 KO OT-I cells were adoptively transferred into mice. Forty-eight hours later, PBS, 5 μg I-TAC or IP-10 was injected intratracheally. The BAL was harvested 18 hr later, and CD8+ T lymphocytes were analyzed by flow cytometry. * p < 0.05 compared to PBS injection.
Figure 6. Co-transfer of wt and CXCR3 KO activated CD8+ T cells.
Activated wt (Thy1.1) and CXCR3 KO (Thy1.2) OT-I cells (5×106 each) were adoptively transferred into the same Thy1.1×Thy1.2 mice. Forty-eight hours later, PBS or 5 μg IP-10 was injected intratracheally. The BAL and spleen were harvested 18 hr later, and Thy1.1 and Thy1.2 single positive cells were analyzed by flow cytometry as shown after gating on CD3+/CD8+ T lymphocytes. The homing ratio was calculated as wt/CXCR3 KO OT-I CD8+ T lymphocytes. * p < 0.05 compared to PBS injection.
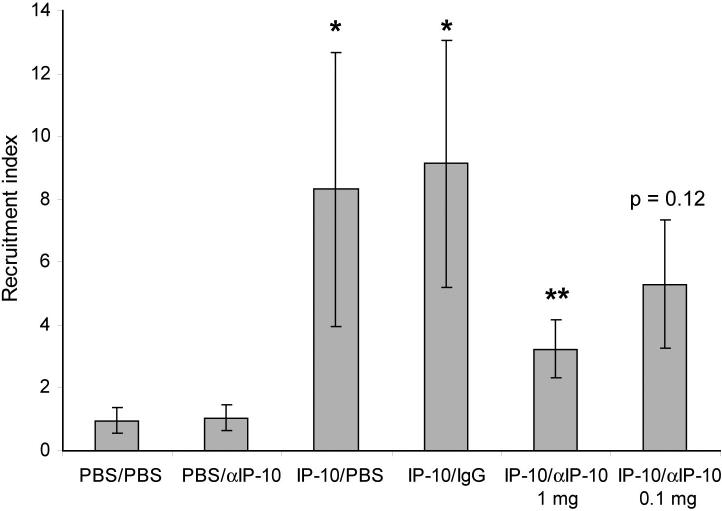
3.5 Inhibition with antibodies
The specificity of the induced recruitment in response to IP-10 was further confirmed by injecting a monoclonal antibody against IP-10 or a control antibody intraperitoneally 4 hr before the CD8+ cells were adoptively transferred, and again two hours after intratracheal instillation of IP-10. As seen in Figure 7, administration of 1 mg anti-IP-10 antibody (a 10-fold molar excess compared to IP-10), reduced the recruitment index from 9.1 for IP-10 with control antibody to 3.2 for IP-10 with anti-IP-10 antibody. Administration of 100 μg antibody, which is equivalent to a 1:1 molar ratio, reduced the recruitment index from 9.1 to 5.3, which was a not statistically significant (p = 0.12). These data demonstrate that the recruitment induced by IP-10 can be dramatically reduced by a ten-fold molar excess of systemically administered anti-IP-10 antibody.
Figure 7. Inhibition of lymphocyte recruitment by antibody against IP-10.
Anti-IP-10 or control antibody was injected i.p. into mice as indicated four hours before adoptive transfer of CD8+ T lymphocytes, and two hours after intratracheal injection of PBS or IP-10 (5 μg). The rest of the assay was performed as described for figure 2. * p < 0.01 compared to PBS injection, ** p < 0.02 compared to IP-10 instillation (with or without control antibody).
3.6 Alternative methods
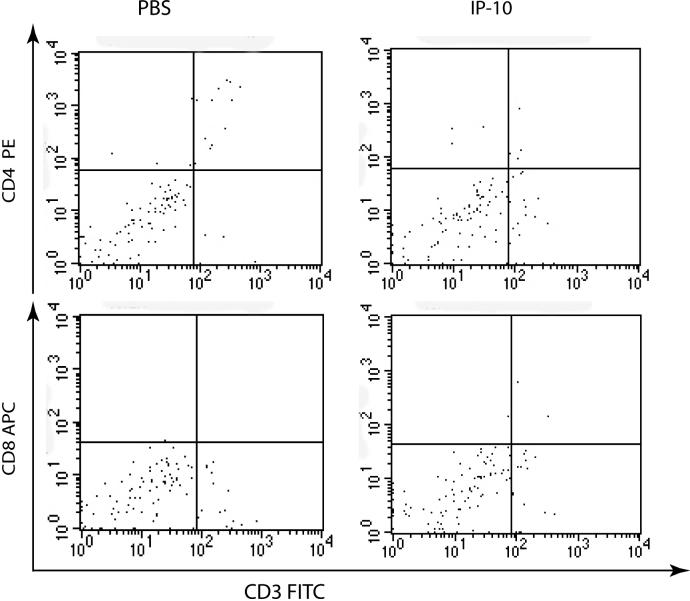
In order to investigate whether the assay can be further simplified, two alternative methods were tested. First, the assay was performed without adoptive cell transfer of activated T cells. For this, 5 μg of IP-10 was instilled intratracheally into unmanipulated mice and airway lavage performed 24 hours later. T lymphocyte infiltration into the airways was not observed when activated T cells were not adoptively transferred before the instillation of chemokines (Fig 8). Next, intratracheal chemokine instillation was compared with intranasal administration after adoptive transfer of activated CD8+ T cells. Although intranasal administration of 5 μg IP-10 induced a statistically significant recruitment of CD8+ T lymphocytes, the recruitment indices were much lower than for intratracheally instilled IP-10 (Figure 9). Indeed, it appears as if intranasal administration requires a ten-fold higher amount of chemokine compared to intratracheal instillation.
Figure 8. Recruitment of lymphocytes without adoptive cell transfer.
PBS or IP-10 (5μg) was injected intratracheally into mice. The BAL was harvested after 18 hr, and CD8+ and CD4+ T cells were analyzed by flow cytometry as indicated.
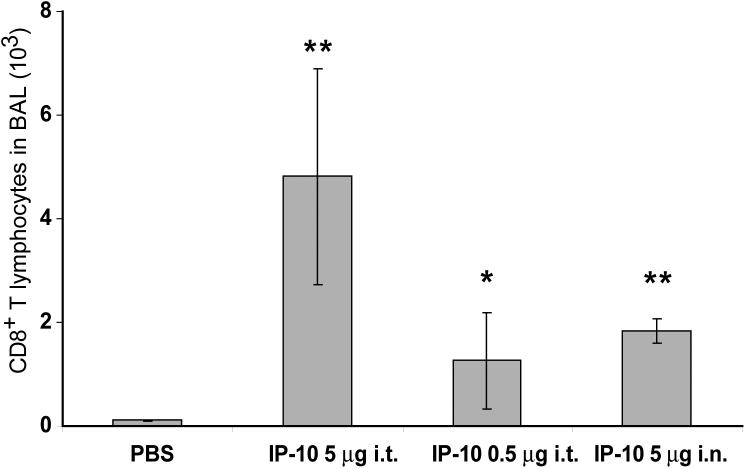
Figure 9. Intratracheal or intranasal instillation of chemokine.
IP-10 was injected intratracheally or intranasally into mice as indicated 48 hours after adoptive transfer of activated CD8+ T lymphocytes. The BAL was harvested 18 hr later and analyzed for CD8+ T lymphocytes by flow cytometry. * p < 0.05, ** p < 0.005 compared to PBS injection.
3.7 CD4+ lymphocyte recruitment
We next tested whether this in vivo recruitment assay could be utilized to measure the trafficking of activated CD4+ T cells in response to chemokines. For this, ovalbumin specific CD4+ T lymphocytes (OT-II cells; Thy1.1) were activated for 6 days in vitro. Similar to the CD8+ T lymphocytes, after six days in culture, CXCR3 expression on the CD4+ T lymphocytes was still low but increased after two further days of in vitro culture with IL-2 (Fig 10A). This mirrored the CXCR3 expression in vivo, where two days after adoptive transfer, most of the splenic CD4+/Thy1.1 cells were found to be CXCR3 positive (Fig 10B).
Figure 10. Adoptive transfer of activated CD4+ T lymphocytes.
A) CXCR3 expression of CD4+ T lymphocytes after six or eight days in culture. B) CXCR3 expression of Thy1.1+ CD4+ T lymphocytes in the spleen three days after adoptive transfer. C) PBS or IP-10 (5 μg) was injected intratracheally into mice as indicated 48 hours after adoptive transfer of activated CD4+ T lymphocytes. The BAL was harvested 18 hr later and analyzed for Thy1.1/CD4+ T lymphocytes by flow cytometry. D) Quantitative representation of experiment performed in C). Recruitment indices were calculated in comparison to intratracheal injection of PBS. * p < 0.05 compared to PBS injection.
Two days after adoptive transfer of activated CD4+ T cells into Thy1.2 C57Bl/6 mice, 5 μg IP-10 was intratracheally instilled. The airways were lavaged 18 hours later, and recruitment of CD4+/Thy1.1 cells was measured by flow cytometry (Fig 10C). Although the total cell numbers were lower than for CD8+ T lymphocytes, there was a statistically significant infiltration in response to 5 μg IP-10 with a recruitment index of 14.1 (Fig 10D), similar to what was normally seen for CD8+ T lymphocytes. These results demonstrate that the in vivo recruitment assay that we have described can be extended to activated CD4+ as well as CD8+ T cells.
4. Discussion
Lymphocyte homing into tissue is a tightly regulated process, which is important for immune surveillance and for the delivery of an adaptive immune response to sites of inflammation. Chemokines are critical mediators of lymphocyte trafficking and therefore have received much attention for their role in regulating the immune response and their potential as pharmaceutical targets. The study of chemokine-induced recruitment of leukocytes over the last twenty years has relied mostly on in vitro studies that do not fully reflect the complex phenomenon occurring in the body. In order to fully recapitulate cell migration in vivo, we have developed a reliable and physiologically relevant in vivo T lymphocyte recruitment assay that is widely applicable for chemokine research.
The recruitment assay utilizes in vitro activated T lymphocytes, which are the main target cells of our chemokines of interest. Use of the OT-I or OT-II mouse lines allowed for the efficient expansion of activated antigen-specific effector T cells, but other methods of in vitro activation might also be possible. Although at the time of adoptive transfer the expression of the chemokine receptor CXCR3 was still low, further in vitro activation led to an increase in CXCR3 expression and concomitantly to responsiveness to the two CXCR3 ligands I-TAC and IP-10, as demonstrated by receptor internalization and in vitro chemotaxis. It is important to note, that this increase in CXCR3 expression in culture also occurred in vivo for adoptively transferred CD8+ and CD4+ T cells, which at the time of harvest 18 hr after chemokine instillation, also had high CXCR3 expression, an important pre-requisite for CXCR3 ligand-induced recruitment.
The adoptive transfer of activated target cells, shown to be responsive to the chemokine of interest, provides the assay with a cell population able to migrate, without systemic activation as would be the case with the use of adjuvants or other systemically given stimulants. Whereas most other recruitment assays rely on quantification of total cells, or sometimes differential cell counts, this assay employs flow cytometry for the precise analysis of the cells recovered from the airways. Since activated target cells are adoptively transferred without systemic activation, the number of target cells in the tissue of interest is very low without instillation of chemokine. After instillation of chemokine, the recruitment of the target cells can be precisely analyzed by markers specific to the transferred cells, such as Thy1.1 cells transferred into Thy1.2 mice. This method achieves a low background with very high recruitment indices with both high sensitivity and specificity.
The anatomical compartment chosen for this recruitment assay is the airways, as both IP-10 and I-TAC, as well as other chemokines, have been shown to be expressed by bronchial epithelial cells especially during various inflammatory diseases, such as tuberculosis (Sauty et al., 1999), chronic obstructive pulmonary disease (COPD) (Saetta et al., 2002), and rhinovirus infections (Spurrell et al., 2005), resulting in the recruitment of CXCR3+ lymphocytes into the airways. By instilling I-TAC or IP-10 directly into the airways, this assay models the natural environment in which these chemokines are normally expressed during inflammatory processes. In contrast, other in vivo recruitment assays that have been used to measure chemokine activity inject chemokines into an induced air pouch in the skin (Wang et al., 2005; Gladue et al., 2006) or into the peritoneum (Xie et al., 1999; Proudfoot et al., 2003), which may be less biologically relevant to actual chemokine biology. In addition, in our hands, the airway recruitment assay we describe here resulted in much higher recruitment indices than the peritoneal assay that has been used to evaluate lymphocyte recruitment induced by chemokines, allowing for easier comparison of chemokine mutants and antagonists.
Instillation into the airways also localizes the chemokine in a distinct anatomical compartment where it is retained over an extended period of time, as we have shown previously by in vivo imaging of mice at different time points after intratracheal injection of radiolabeled IP-10 (Campanella et al., 2006). These imaging studies revealed that at time points between 15 minutes up to 5 days post instillation, 70 − 90 % of the chemokine was localized in the lung. A small percentage of the chemokine was found in the kidneys and livers, from where it presumably gets excreted at a slow rate, with an estimated 75 % of IP-10 still in the mice after 24 hours. The retention of the instilled chemokine in a defined tissue compartment over an extended period of time provides a sustained gradient for the directional recruitment into the airways observed in this assay.
Different experimental conditions were tested for this recruitment assay, showing it is very robust and works over a wide range of parameters. First, the chemokine amount was varied over three orders of magnitude from 0.5 μg to 50 μg for both I-TAC and IP-10. Even instillation of 0.5 μg of chemokine resulted in a statistically significant recruitment of adoptively transferred cells, with higher chemokine amounts leading to increased recruitment. In addition, we varied the in vitro culture time of the adoptively transferred cells as well as the number of cells transferred, and found that the assay was flexible over a range of values for both parameters. Similarly, we varied the time between chemokine instillation and bronchial lavage, and found good recruitment between four and forty-eight hours after chemokine instillation, which fits in well with the observed localization of IP-10 in the lung for at least 120 hours after instillation. The recruitment assay is therefore very robust over a wide range of parameters.
Utilization of an in vivo recruitment assay can reveal properties of chemokines or their mutants that might not be apparent from in vitro chemotactic assays, as we recently demonstrated with a monomeric mutant variant of IP-10 (Campanella et al., 2006). This monomeric IP-10 displayed full potency in chemotactic assays at ten-fold higher concentration than wild type IP-10. However, employing the in vivo airway recruitment assay described here, we found that monomeric IP-10 was unable to induce recruitment of activated CD8+ T lymphocytes, even at 100-fold higher concentration than wild type IP-10. This deficiency was due to monomeric IP-10's inability to bind to and be presented by endothelial cells. In vivo activity assays are therefore critical to fully understand the biological behavior of chemokines.
The development of a biologically relevant, robust, and easy to perform in vivo recruitment assay is an important step for chemokine research. It can be employed to measure and compare the activity of various chemokines and chemokine mutants, including possible agonistic or antagonistic chemokine variants. In addition, this assay could be very useful in exploring the in vivo effect of chemokine receptor mutations, which so far have only been investigated in vitro, as this assay is fully dependent on CXCR3 function in T cells in response to IP-10 or I-TAC. For this, chemokine receptor mutants could be transfected into either primary cells or cell lines, which then could be adoptively transferred to determine their ability to mediate in vivo recruitment. Furthermore, this assay allows for the study of the in vivo efficacy of chemokine and chemokine receptor antibodies or small molecule chemokine receptor inhibitors. As we have shown above, a monoclonal antibody against IP-10 was able to inhibit lymphocyte recruitment to the airways, demonstrating that this assay can be used for the study of chemokine inhibitors.
In conclusion, we describe the development of an in vivo chemokine-induced lymphocyte homing assay that measures the recruitment of adoptively transferred effector CD4+ and CD8+ T cells into the airways after intratracheal instillation of chemokines. This assay is biologically relevant as it models the natural conditions of lymphocyte recruitment induced by chemokines, which are expressed in bronchial epithelial cells during inflammatory conditions. The assay has very high recruitment indices with low background and is very robust over a wide range of parameters and will therefore be of use to research on chemokine function in vivo.
Acknowledgments
This work was supported by NIH grant RO1-CA69212 to ADL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96:6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Campanella GS, Grimm J, Manice LA, Colvin RA, Medoff BD, Wojtkiewicz GR, Weissleder R, Luster AD. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177:6991–8. doi: 10.4049/jimmunol.177.10.6991. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–27. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- Gladue RP, Cole SH, Roach ML, Tylaska LA, Nelson RT, Shepard RM, McNeish JD, Ogborne KT, Neote KS. The human specific CCR1 antagonist CP-481,715 inhibits cell infiltration and inflammatory responses in human CCR1 transgenic mice. J Immunol. 2006;176:3141–8. doi: 10.4049/jimmunol.176.5.3141. [DOI] [PubMed] [Google Scholar]
- Grabie N, Delfs MW, Westrich JR, Love VA, Stavrakis G, Ahmad F, Seidman CE, Seidman JG, Lichtman AH. IL-12 is required for differentiation of pathogenic CD8+ T cell effectors that cause myocarditis. J Clin Invest. 2003;111:671–80. doi: 10.1172/JCI16867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogewerf AJ, Kuschert GS, Proudfoot AE, Borlat F, Clark-Lewis I, Power CA, Wells TN. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–8. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- Khan IA, MacLean JA, Lee FS, Casciotti L, DeHaan E, Schwartzman JD, Luster AD. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–94. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. New England Journal of Medicine. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. Journal of Experimental Medicine. 1995;182:219–31. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–6. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. Journal of Clinical Investigation. 1999;104:1041–50. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff BD, Seung E, Wain JC, Means TK, Campanella GS, Islam SA, Thomas SY, Ginns LC, Grabie N, Lichtman AH, Tager AM, Luster AD. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med. 2005;202:97–110. doi: 10.1084/jem.20042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, Hancock WW, Briscoe DM. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–64. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and Surface Presentation of IL-8 by Venular Endothelial Cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997;158:5536–44. [PubMed] [Google Scholar]
- Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–90. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, Sinigaglia F, Fabbri LM. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–9. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11). J Immunol. 2001;167:7084–93. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. Journal of Immunology. 1999;162:3549–58. [PubMed] [Google Scholar]
- Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. Journal of Clinical Investigation. 1999;103:807–15. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Spurrell JC, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol. 2005;289:L85–95. doi: 10.1152/ajplung.00397.2004. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–88. [PubMed] [Google Scholar]
- Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–10. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- Weng Y, Siciliano SJ, Waldburger KE, Sirotina-Meisher A, Staruch MJ, Daugherty BL, Gould SL, Springer MS, DeMartino JA. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. Journal of Biological Chemistry. 1998;273:18288–91. doi: 10.1074/jbc.273.29.18288. [DOI] [PubMed] [Google Scholar]
- Xie H, Lim YC, Luscinskas FW, Lichtman AH. Acquisition of selectin binding and peripheral homing properties by CD4(+) and CD8(+) T cells. Journal of Experimental Medicine. 1999;189:1765–76. doi: 10.1084/jem.189.11.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DX, Hu Y, Miller GG, Luster AD, Mitchell RN, Libby P. Differential expression of the IFN-gamma-inducible CXCR3-binding chemokines, IFN-inducible protein 10, monokine induced by IFN, and IFN-inducible T cell alpha chemoattractant in human cardiac allografts: association with cardiac allograft vasculopathy and acute rejection. J Immunol. 2002;169:1556–60. doi: 10.4049/jimmunol.169.3.1556. [DOI] [PubMed] [Google Scholar]
- Zicha D, Dunn GA, Brown AF. A new direct-viewing chemotaxis chamber. J Cell Sci. 1991;99(Pt 4):769–75. doi: 10.1242/jcs.99.4.769. [DOI] [PubMed] [Google Scholar]
- Zigmond SH. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977;75:606–16. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]