Abstract
Recent data suggest novel functional roles for cerebellar involvement in a number of neurologic diseases. Function of cerebellar neurons is known to be modulated by norepinephrine and adrenergic receptors. The distribution of adrenergic receptor subtypes has been described in experimental animals, but corroboration of such studies in the human cerebellum, necessary for drug treatment, is still lacking. In the present work we studied cell-specific localizations of α1 adrenergic receptor subtype mRNA (α1a, α1b, α1d), and α2 adrenergic receptor subtype mRNA (α2a, α2b, α2c) by in situ hybridization on cryostat sections of human cerebellum (cortical layers and dentate nucleus). We observed unique neuron-specific α1 adrenergic receptor and α2 adrenergic receptor subtype distribution in human cerebellum. The cerebellar cortex expresses mRNA encoding all six α adrenergic receptor subtypes, whereas dentate nucleus neurons express all subtype mRNAs, except α2a adrenergic receptor mRNA. All Purkinje cells label strongly for α2a and α2b adrenergic receptor mRNA. Additionally, Purkinje cells of the anterior lobe vermis (lobules I to V) and uvula/tonsil (lobules IX/HIX) express α1a and α2c subtypes, and Purkinje cells in the ansiform lobule (lobule HVII) and uvula/tonsil express α1b and α2c adrenergic receptor subtypes. Basket cells show a strong signal for α1a, moderate signal for α2a and light label for α2b adrenergic receptor mRNA. In stellate cells, besides a strong label of α2a adrenergic receptor mRNA in all and moderate label of α2b message in select stellate cells, the inner stellate cells are also moderately positive for α1b adrenergic receptor mRNA. Granule and Golgi cells express high levels of α2a and α2b adrenergic receptor mRNAs. These data contribute new information regarding specific location of adrenergic receptor subtypes in human cerebellar neurons. We discuss our observations in terms of possible modulatory roles of adrenergic receptor subtypes in cerebellar neurons responding to sensory and autonomic input signals, and review species differences in cerebellar adrenergic receptor expression.
Keywords: receptors, alpha-adrenergic, norepinephrine, in situ hybridization, cerebellum, locus coeruleus
The cerebellar cortex receives three classes of afferents: mossy, climbing and multilayered fibers (Haines, 2002). The first two classes use glutamate to transmit sensorimotor, timing, error and error corrections signals, which are modified by GABAergic interneurons and fast chemical synapses (Ito, 1984). The third class uses catecholamines, including norepinephrine (NE), and slow volume transmission to modulate the response of the fast synapses (Abbot and Sotelo, 2000). NE axons from locus coeruleus (LC) and subcoeruleus innervate all layers of the cerebellar cortex (Moore and Card, 1984), transmitting phasic or tonic impulses (Aston-Jones et al., 1999) to activate specific adrenergic receptors (ARs).
ARs are G protein-coupled receptors with nine distinct AR subtypes (α1a, α1b, α1d, α2a, α2b, α2c, β1, β2 and β3). ARs regulate neurotransmitter release in the CNS and play a vital role in various processes, including autonomic, somatosensory, motor, cognitive, nociceptive and endocrine functions, and the control of alertness, temperature, blood pressure and shivering (Kamibayashi and Maze, 2000). Distribution of AR subtypes in animal and human tissues has been described using both RNA (Northern, RNase protection, in situ hybridization) and protein (immunohistochemical, autoradiographic) approaches (Palacios et al., 1987; Jones and Palacios, 1991; Pascual et al., 1992; Nicholas et al., 1993; Aoki et al., 1994; Berkowitz et al., 1994; Pieribone et al., 1994; Price et al., 1994; Scheinin et al., 1994; Stafford Smith et al., 1995, 1999; Tavares et al., 1996; Wang et al., 1996; Day et al., 1997) that indicates some species differences making extrapolation from animal studies to human difficult. To our knowledge, no other reports on the distribution of αAR subtype mRNAs in human cerebellum are currently available.
NE involvement in signaling of cerebellar neurons has been documented (Moises et al., 1979; Yeh and Woodward, 1983; Basile and Dunwiddie, 1984; Bickford-Wimer et al., 1991; Woodward et al., 1991; Kondo and Marty, 1998), and abnormal levels of NE or αAR densities have been noted in cerebella of patients with olivocerebellar atrophy, dementia with Lewy bodies, Parkinson’s and Alzheimer’s diseases (Kish et al., 1984a,b; Shimohama et al., 1986; Meana et al., 1992; Grijalba et al., 1994; Russo-Neustadt and Cotman, 1997; Leverenz et al., 2001). Additionally, animal studies have shown that NE is essential for normal cerebellar development (Sievers and Klemm, 1982; Luthman et al., 1990; Podkletnova and Alho, 1998), and human studies indicate cerebellar involvement in neurodevelopmental disorders, such as autism, fetal alcohol, Joubert, Down’s and fragile X syndromes, attention deficit hyperactivity disorder, and posterior fossa malformations (Holroyd et al., 1991; Reiss et al., 1991; Sowell et al., 1996; Courchesne, 1997; Berquin et al., 1998).
Given this involvement of NE and ARs in cerebellar function, development and disease, we investigated neuron specific expression of α1 and α2AR mRNAs in human cerebellum. We demonstrate distinct cell-specific distribution of α1 and α2AR mRNAs in cerebellar neurons, and show expression in some neurons where αAR subtypes were not previously known to exist. We discuss our findings in terms of species differences and functional activity of αAR subtypes in cerebellar neurons. These findings will enhance our understanding of noradrenergic actions in human cerebellum and should lead to emerging strategies of pharmacological interventions designed to treat neurological diseases of the cerebellum.
EXPERIMENTAL PROCEDURES
Human cerebellum
After institutional approval and family consent, human cerebellar tissue was obtained within 1–2 h postmortem from four patients (age range 74–86 years) without history of premorbid CNS disease who were enrolled in the Duke Rapid Autopsy Program. A neuropathologist grossly identified regions and areas of interest of the cerebellum at the time of tissue harvest; each cerebellar area was later confirmed by examination of tissue architecture using light microscopy. Tissue blocks were chosen from the anterior lobe vermis (lobules I to V), the ansiform lobule (lobule HVII) and the uvula/tonsil (lobules IX/HIX) in order to represent tissue from anterior and posterior lobes, as well as spinocerebellum (vermis and paravermis) and pontocerebellum (lateral hemispheres). The tissue was placed immediately into ice cold 4% paraformaldehyde for 16 –24 h, then briefly rinsed in phosphate-buffered saline (PBS), and transferred to ice cold 20% sucrose (in PBS) until the tissue sank (to prevent freezing artifacts; at least 24–36 h). Thereafter, cerebellar samples were covered with embedding media (OCT©; Baxter Scientific; Deerfield, IL, USA) and slowly frozen in liquid nitrogen (3–5 min per tissue), and stored at −70 °C for future use.
αAR subtype selective cDNA probe constructs
Human α1AR and α2AR subtype cDNA constructs (α1a, α1b, α1d, α2a, α2b, and α2c) were used as templates for the generation of control (sense) and specific (antisense) RNA probes. Human α1AR cDNA constructs consisted of the following: α1aAR, 0.326 kb (kb) PvuII/HindIII fragment in pGEM-4Z (Promega Corporation; Madison, WI, USA), nucleotides 958–1283 (GenBank #L31774); α1bAR, 0.673kb XhoI/BamHI fragment in pGEM-4Z (Promega), nucleotides 94–766 (GenBank #L31773); and α1dAR, 0.377kb EcoRI/PstI fragment, nucleotides 520–896 (GenBank #L31772). Human α2AR cDNA constructs consisted of the following: α2aAR, 0.324 kb BglII/AccI fragment in pSP72 (Promega), nucleotides 318–642 (GenBank #M18415); α2bAR 0.333kb PstI/BamHI fragment in pGEM-3Z (Promega), nucleotides 1395–1728 (Genbank #M34041); and α2cAR, 0.348kb SacI/AccI fragment in pGEM-3Z (Promega), nucleotides 377–725 (Genbank #J03853). The control β-actin probe consisted of a 104-bp fragment of the human β-actin gene cloned into the AccI/HindII sites of the vector pGEM-4Z (Promega). Radiolabeled single strand sense and antisense RNA probes were made using linearized cDNA constructs, [35S]-αUTP (DuPont, NEN, Boston, MA, USA), and either SP6 or T7 RNA polymerase, as previously described (Titus, 1992; Wilson et al., 1997). Control RNA probes were slightly larger than antisense probes due to increased amounts of polylinker transcribed with sense strand RNA.
In situ hybridization
In situ hybridization in human tissues is often difficult due to lack of pristine tissue conditions resulting from inevitable delays in tissue harvesting. In order to produce the best signal-to-noise ratio, we used cerebellar tissue from one of the human brains to optimize the in situ hybridization methods previously used with rat tissue (Scheinin et al., 1994; Schambra et al., 1994). We reported in detail the protocol to accommodate to different conditions of human tissue processing, which included elimination of some procedural steps and decreases post fixation time and RNase levels (see Wilson et al., 1997). Therefore, in situ hybridization methods are presented only briefly below.
Cerebellum hybridization and photographic emulsion conditions
Ten micrometer horizontal frozen sections of human cerebellum were cut on a cryostat (Kryostat 1720 digital; Leitz, Wetzlar, Germany) using a −20 °C block, thaw mounted onto silylated microscope slides (CEL Associates, Houston, TX, USA), and stored at −70 °C with desiccant until hybridization. To start hybridization, slides were warmed to room temperature using a hair dryer at cool setting (15–30 min), then rinsed twice for 5 min in 2× saline-sodium citrate buffer (SSC; 1× SSC=0.15 M NaCl, 0.04 M Na citrate, pH 7.2). No permeabilization or prehybridization steps were performed. Hybridization buffer (0.02 M DTT, 1× Denhart’s Solution [Sigma, St. Louis, MO, USA], 1 mg/ml salmon sperm DNA [Sigma] heated to 80 °C before use, 50 μg/ml transfer RNA [Sigma], 2× SSC, 50% formamide, 9% dextran sulfate), and 5000–7000 cpm/μl linearized radiolabeled probe were applied to the slides. The slides were then incubated at 50 °C overnight in sealed plastic containers lined with Whatman filter paper soaked with 50% formamide in 2× SSC buffer. To remove non-specific binding, slides were washed as follows: sequential immersion in 1 μl/ml β-mercaptoethanol solutions in 2× SSC (50 °C, brief dip) and 50% formamide in 2×SSC (50 °C, 10 min, then 20 min), followed by RNase treatment using 10 μg/ml RNase in 2× SSC (35 °C, 30 min). Subsequent washes at room temperature included the following: 2× SSC (5 min), 50% formamide in 2× SSC (50 °C, 5 min), and 2× SSC (10 min), followed by dehydration steps using 2 min immersions each in 0.3 M ammonium acetate solutions containing 50%, 70%, then 100% ethanol, followed by a brief wash in pure 100% ethanol. After air drying for 30–60 min, slides were dipped in warm (40 °C) autoradiography emulsion (Kodak NTB2; Rochester, NY, USA) in a darkroom illuminated with a Kodak safe-light #2, dried for several hours in the dark, then placed in light-sealed slide boxes with desiccant at 4 °C for 4 weeks. After warming for 90 min to room temperature, exposed slides were developed under safelight by sequential immersion in fresh D19 developer (Kodak) mixed 1:1 with distilled water (dH2O; 15 °C, 4 min), followed by room temperature immersions in dH2O (20 s), fixer (Kodak; 5 min), and dH2O rinses (3×5 min). Slides were counterstained with hematoxylin and eosin, dehydrated in an ascending ethanol and xylene series, and cover slipped. Dry slides were examined and photographed under bright and dark field microscopy (high power: Leitz DMRB; low power: WILD M420).
Control experiments
In order to ensure that the detected signal represented specific probe hybridization, several positive and negative controls were performed. Negative controls included sense probe experiments and demonstration of loss of signal in known positive samples exposed to excess (50 μg/ml) RNase before hybridization. An antisense human βactin probe was used as a positive control for experimental conditions and general neuronal anatomy. Controls for α1AR and α2AR subtype specificity of antisense probes included simultaneously performed in situ experiments in lines of transfected cells stably expressing individual human α1AR subtypes, inclusion of known positive and negative human non-cerebellar tissues, as well as RNase protection assays using mRNA derived from clonal α1AR subtype cell lines with the same probes.
Detection and analysis
Analysis of general and cell-specific αAR mRNA expression was performed by examining silver granules present in cerebellar tissue sections and signaling neurons under darkfield conditions, and the observation confirmed and photographed using bright field microscopy. Semiquantitative image analysis was performed using the MCID system (Brock University, St. Catharines, Ontario, Canada). Silver grains resulting from in situ hybridization experiments with αAR mRNA probes were counted from multiple samples from each patient. For each representative cell type within the cerebellum, a minimum of five high power darkfield grain counts were performed; variability of αAR mRNA expression was ≤10–15% for the vast majority of cell types at all cerebellar layers. Although cerebellum samples were harvested within 2 h postmortem, some degradation of mRNA is theoretically possible prior to tissue collection; hence, in order to ensure uniformity in reporting of results, samples for each patient were analyzed separately, αAR subtype mRNA expression compared for each patient, and then averaged. The abundant signal of either α2a or α2bAR mRNA expression in Purkinje cells was arbitrarily defined as 100%. It should be noted that, despite of efforts to normalize data, signal assessment for in situ experiments is semi-quantitative, since some experimental artifacts cannot be ruled out (e.g. different probes may not hybridize exactly equally). Therefore, for final reporting we utilized a more general scale as follows: +++ denotes heavy signal (71–100%); ++ moderate signal (31–70%); + light signal (5–30%); +/0 represents signal just above background and 0 that signal is undetectable. When we found signal in select neurons only, we denoted that with brackets.
RESULTS
General
It is well known that the cerebellar cortex contains what can be called major cells, in terms of their numbers and extensive distribution throughout the cortex, and minor cells that are much fewer in number and/or found mainly in more geographically restricted areas of the cortex. Examples of the former are the granule, Golgi, basket, stellate and Purkinje cells; these cortical neurons are the target of this investigation. Examples of the latter are the cells of Lugaro and the unipolar brush cells. Due to their fewer numbers and more restricted distribution, these cells were excluded from the present study.
Pilot experiments to determine appropriate in situ hybridization conditions (hybridization time, temperature, and RNase concentration) were performed in n=1 patient samples. For final analysis tissue samples from three human cerebellar regions were compared from n=3 patients. These selected cerebellar regions are shown in Fig. 1A. The corresponding tissue sections are shown as follows: anterior lobe vermis (lobules I to V; Fig. 1B), ansiform lobule (lobule HVII; Fig. 1C), and uvula/tonsil (lobules IX/HIX; Fig. 1D).
Fig. 1.

(A) Drawing of unfolded view of human cerebellar cortex indicating the regions analyzed in the current study. (B–D) Drawings of regions analyzed in this study; scale bar=0.5 cm: (B) Anterior lobe vermis (lobules I–V); (C) Ansiform lobule (lobule H VII); D: Uvula/tonsil (lobules IX/HIX).
Controls
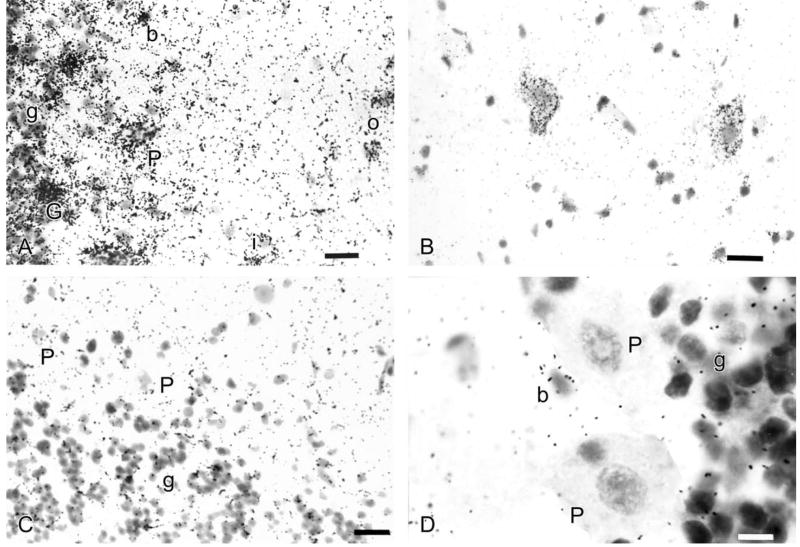
Control experiments confirmed sensitive and specific hybridization of α1AR and α2AR subtype mRNA probes, with no evidence of cross-hybridization between αAR subtypes or binding of sense probes. For example, sense probes produced no hybridization signal, whereas antisense probes hybridized specifically to cerebellar tissue and control cells (clonal cell lines stably expressed an individual human αAR subtype) without cross-hybridization to other subtypes. The β-actin antisense probe served both as positive control and as a marker to delineate the cerebellar cortex and its neurons (Fig. 2A), as well as dentate nucleus and its neurons (Fig. 2B). Negative controls included hybridization with appropriate sense probes, i.e. the α1aAR sense probe (Fig. 2C) and RNase treatment before hybridization (Fig. 2D).
Fig. 2.
Control experiments were performed in human cerebellar tissue to ensure reported signal represents specific αAR mRNA signal: (A) Positive control: β-actin antisense, showing (left to right) granule cells (g), Golgi cell (G), Purkinje cell (P), basket cell (b), inner stellate cell (i), and outer stellate cell (o). (B) Positive control: β-actin antisense, two dentate nucleus neurons; ansiform lobule. (C) Negative control: α1aAR sense probe. (D) Negative control: RNase treatment before hybridization. Note (left to right) unlabeled basket cells (b), Purkinje cells (P) and granule cells (g); bright field. Magnification: A–C: 40×, scale bar=125 μm; D: 100×, scale bar=10 μm.
General α1 versus α2AR mRNA expression in cerebellum
Both α1AR and α2AR mRNAs were present in the cerebellum in every patient, with α2AR mRNA expression greater than α1AR. Overall, the Purkinje cell layer contained the highest abundance of both α1AR and α2AR subtype mRNA in human cerebellum. Some subtype specificity was observed in the other two major layers of the cerebellar cortex, with signal intensity in the molecular layer> granule cell layer for α1AR mRNA, and granule cell layer>molecular cell layer for α2AR mRNA. In addition to general α1AR versus α2AR selectivity, unique αAR subtype specificity was evident, varying by cell layer and cell body location (Table 1).
Table 1.
Subtype-specific cellular distribution of α1 and α2AR mRNA in human cerebellum
| Location | α1a | α1b | α1d | α2a | α2b | α2c |
|---|---|---|---|---|---|---|
| Molecular layer | ||||||
| Outer stellate cells | 0 | (+) | 0 | +++ | (++) | 0 |
| Inner stellate cells | 0 | ++ | 0 | +++ | (++) | 0 |
| Basket cells | +++ | 0 | 0 | ++ | + | 0 |
| Purkinje cell layer | ||||||
| Purkinje cells | (++)1,3 | (++)2,3 | (+++)2,3 | +++ | +++ | (++)1,3 |
| Granule cell layer | ||||||
| Golgi cells | 0 | 0 | 0 | +++ | +++ | 0 |
| Granule cells | 0 | 0 | 0 | +++ | +++ | 0 |
| Cerebellar nuclei | ||||||
| Dentate nucleus | ++ | + | +++ | 0 | +++ | +/0 |
Symbols represent relative levels of mRNA signal: +++ denotes heavy signal (71–100%); ++ moderate signal (31–70%); + light signal (5–30%); +/0 signal just above background; 0 signal undetectable. Brackets denote select cells, and specifically: 1=anterior lobe vermis, 2=ansiform lobule, 3=uvula/tonsil.
Neuron-specific expression of α1 and α2AR mRNA
All Purkinje cells showed strong α2aAR (Fig. 3A; Table 1) and α2bAR mRNA expression (Fig. 3B; Table 1). Purkinje cells of the anterior lobe vermis and uvula/tonsil expressed moderate levels of α2cAR mRNA (Fig. 3C; Table 1). In contrast, expression of the three α1AR subtypes was variable and specific to select Purkinje cells in cerebellar regions (Table 1). Thus, Purkinje cells of the anterior lobe vermis and uvula/tonsil expressed moderate levels of α1aAR (Table 1), whereas Purkinje cells of the ansiform lobule and uvula/tonsil showed a moderate signal for α1bAR mRNA (Table 1), and a strong label for α1dAR mRNA (Fig. 3D; Table 1). Granule cells labeled intensely for α2aAR (Fig. 4A; Table 1) and α2bAR mRNA (Table 1), but not for the remaining AR subtypes (Fig. 4B shows α2cAR; Table 1). Golgi cells also expressed high levels of α2a (Fig. 4C; Table 1) and α2bAR mRNA (Table 1), but none of the other subtypes. Basket cells showed a strong α1aAR mRNA signal (Fig. 4D; Table 1), moderate levels of α2aAR mRNA and a weaker signal for α2bAR mRNA (Table 1). Inner stellate cells showed high levels of α2aAR (Table 1) and moderate levels of α1bAR mRNA in the inner half of the molecular layer (Fig. 4E; Table 1). Outer stellate cells expressed strong signal for α2aAR mRNA (Fig. 4F; Table 1) and moderate signal for α2bAR mRNA in select cells (Table 1). Dentate nucleus neurons expressed moderate levels of α1aAR mRNA (Fig. 5A;Table 1), high levels of α1dAR (Fig. 5B; Table 1) and α2bAR mRNA (Fig. 5C; Table 1), light levels of α1bAR mRNA (Table 1), and low or background signal of α2cAR mRNA (Fig. 5D;Table 1), while α2aAR mRNA was absent.
Fig. 3.
Representative expression of αAR subtypes in Purkinje cells. (A) Strong expression of α2aAR mRNA in three Purkinje cells. (B) Similar high expression levels of α2bAR mRNA in two Purkinje cells. (C) Significantly lower abundance of α2cAR mRNA in two Purkinje cells. (D) Moderate expression of α1dAR mRNA in two select Purkinje cells. Magnification: 100×, scale bar=10 μm.
Fig. 4.

Cell specific expression of α1AR and α2AR mRNAs in human cerebellar interneurons. (A) α2aAR mRNA is abundant in granule cells. (B) Granule cells do not express α2cAR mRNA above background label (grains may represent pooling artifacts). (C) Strong expression of α2aAR mRNA in a Golgi cell (G) and granule cells (g). (D) Basket cells (b), close to a Purkinje cell (P), express abundant α1aAR mRNA. (E) Inner stellate cells (i) express moderate levels of α1bAR mRNA in the inner half of the molecular layer. (F) Outer stellate cells (o) abundantly express α2aAR mRNA. Magnification: A–D: 100×; E, F: 40×, scale bar=125 μm, scale bar=10 μm.
Fig. 5.
Dentate nucleus neurons of human ansiform lobule. (A) Moderate expression of α1aAR mRNA. (B) Abundant α1dAR mRNA expression in two dentate nucleus neurons. (C) High abundance of α2bAR mRNA in a dentate nucleus neuron. (D) α2cAR mRNA expression in dentate nucleus neurons is just above background. Magnification: 100×, scale bar=10 μm.
DISCUSSION
Principal findings
This study provides unique information on subtype-specific mRNA expression of six α-ARs in human cerebellum, taken from anterior lobe vermis (lobules I–V), ansiform lobule (lobule HVII), and uvula/tonsil (lobules IX/HIX). AR subtype mRNAs are differentially expressed in cerebellar neurons (Table 1). The α2aAR and α2bAR mRNAs are present in all neurons of the cerebellar cortex, but α2aAR mRNA is not found in dentate nucleus neurons. Message of the α1a, α1b, α1d and α2cAR subtypes also is observed in region-specific Purkinje cells. In addition, α1aAR mRNA is expressed specifically by basket cells and dentate nucleus neurons, α1bAR by inner stellate cells, and α1dAR by dentate nucleus neurons.
Species-specific differences
Species-specific differences in distribution of α1 and α2AR subtypes have been described for CNS and peripheral tissues (Palacios et al., 1987; Pazos et al., 1988; Jones and Palacios, 1991; Schwinn et al., 1991; Go et al., 1993; Pascual et al., 1992; Van Liefde et al., 1993; Zilles et al., 1993; Aoki et al., 1994; Berkowitz et al., 1994; Price et al., 1994). The present in situ hybridization results contribute to our knowledge of interspecies differences in expression of αARs in cerebellum, and add important new information on their localization in human. In addition, while earlier animal studies mostly described αAR subtypes in different cerebellar layers (Tables 2–4), our study identified cell-type specific expression of αAR transcripts. Finally, we optimized our in situ hybridization conditions previously described in rat (Scheinin et al., 1994; Schambra et al., 1994), to control for variations in postmortem human tissue and to allow detection of rare or fragile mRNAs (Wilson et al., 1997).
Table 2.
Subtype-specific distribution of α1 and α2AR mRNA in cerebella of experimental animals
Symbols represent relative levels of mRNA signal from individual studies as follows: +++ denotes heavy signal; ++ moderate signal; + light signal; +/0 signal just above background; 0 signal undetectable;
denotes promoter reporter transgenic model. Brackets denote select cells within a cerebellar layer.
Table 4.
Subtype-specific distribution of α1 and α2ARs in cerebella of experimental animals demonstrated by autoradiography and ligand binding
Ligands used include the following: α1AR:
= [3H]prazosin,
= [125I]HEAT,
= [125I]BE2254,
=BMY7378,
=methoxamine,
= (+)-niguldipine,
=noradrenaline,
=SB 216469,
=tamsulosin,
= [125I]IBE,
=WB4101,
=chloroethylclonidine. α2AR:
= [3H]para-aminoclonidine ([3H]PAC),
= [3H]yohimbine,
= [3H]idazoxan,
= [3H]rauwolscine,
= [3H]UK 14304,
= [3H]RX821002,
=oxymetazoline,
=ARC-239,
= [3H]bromoxidine ([3H]UK-1434),
= [3H]clonidine,
=BRL-44408,
=prazosin[3H]RX821002,
= [125I]para-iodoclonidine ([125I]PIC). Curved brackets denote label only in lobules 9 and 10. Square brackets denote presence of α2cAR in neonatal rat (P7–P17) but absence in adult rats.
Inner 1/3 of molecular layer.
Species-specific differences of α2ARs in cerebellum
A comparison of our α2AR subtype expression data in human cerebellum (Table 1) with the expression, immunocytochemical and autoradiographic data in cerebella of experimental animals (Tables 2– 4) confirms ubiquitous localization of the α2aAR subtype. However, localization of α2bAR and α2cARs in human differs from that in animal cerebella. Our observations of moderate and widespread expression of α2bAR mRNA in human cerebellum are reflected in some previous rodent studies (Tavares et al., 1996; Wang et al., 1996, 2002; Winzer-Serhan and Leslie, 1997a), but not in others (Table 2). In contrast, while we observed α2c expression only in select human Purkinje cells, strong expression and immunocytochemical signals of this subtype were reported for the three cerebellar layers and cerebellar nuclei of rat (Nicholas et al., 1993; Rosin et al., 1996). These discrepancies might theoretically be due to differences in probe preparation, i.e. our using probes against sequences encoding subtype-specific intracellular loops of the subtype genes versus others using full length clones which cross-hybridize unless more stringent conditions are used (Wang et al., 2002), or may be due to technical difficulties, such as sensitivity of α2bAR mRNA to degradation after poor fixation (Winzer-Serhan and Leslie, 1997a). We observed similar technical problems in preliminary experiments, but optimized fixation time, RNase concentration and buffer stringency to overcome these issues (Wilson et al., 1997). More likely, true species difference may exist between primate and rodent α2b and α2cAR expression patterns. In support of this interpretation, Go et al. (1993) observed α2bAR protein in Purkinje cells of monkey but not rat; further divergence of the promoter regions between rodent and human α2bAR was recently reported (Cayla et al., 2004). Thus, in parallel with the expansion of both cerebrum and cerebellum in primates and the addition of fine motor control, cognitive and language functions (Petersen et al., 1989; Leiner et al., 1991, 1993; Desmond and Fiez, 1998; Thach 1998; Wickelgren, 1998; Schmahmann, 1997; Ito, 2000) to the control of posture, movement and reflexes (Ito, 1984; Jueptner and Weiller, 1998), the α2bAR may have gained functional importance and replaced the α2cAR.
Species-specific differences of α1ARs in cerebellum
Expression of α1AR mRNAs in human cerebellum was more limited than that of the α2AR mRNAs and specific to certain cerebellar neurons (Table 1). Because the animal data are still scant (Tables 2–4), only partial comparisons are possible. Overall, our finding of relatively low abundance of α1ARs in human cerebellum agrees with the animal data. However, our most striking findings, expression of α1aAR in basket cells, α1bAR in inner stellate cells and α1dAR in dentate nucleus neurons, have not been reported previously. These three subtypes were also present in select Purkinje cells, as was noted in rat (Day et al., 1997), and our finding of moderate levels of α1aAR mRNA in dentate neurons confirms an earlier report in rat (Domyancic and Morilak, 1997). Using α1AR ligands, species differences were noted in one study, such that the α1AR was observed in mouse, rat and cat, but not guinea-pig cerebella (Palacios et al., 1987). Also, the α1bAR subtype may be more abundant in rodent than human cerebellar cortex (Wilson and Minneman, 1989; Yang et al., 1998; Acosta-Martinez et al., 1999).
Effects of NE on cerebellar function
NE axons from LC and subcoeruleus innervate all cells of the cerebellar cortex and cerebellar nuclei (Bloom et al., 1971; Landis et al., 1975; Kimoto et al., 1981; Moore and Card, 1984; Powers et al., 1989; Nelson et al., 1997). NE is released by volume transmission in a paracrine fashion from varicosities of thin axons and diffuses over more or less extended distances to activate specific AR subtypes in non-synaptic junctions (Abbot and Sotelo, 2000). The synchronized release of NE by LC neurons throughout the neuraxis can be either tonic (steady, low to high levels of NE, depending on state of vigilance), or phasic (additional bursts of high levels of NE during stimulus-evoked activity; Aston-Jones et al., 1991, 1999). LC stimulation and iontophoretical applications of NE to rat cerebellar slices or cells increase spontaneous Purkinje cell firing at low concentrations (0.5–10 μM), increase the frequency of spontaneous firing of stellate cells at > 16 μM, resulting in depression of Purkinje cell spontaneous activity, and cause consistent depression of Purkinje cell spontaneous activity at higher concentrations (25–100 μM; Basile and Dunwiddie, 1984; Mori-Okamoto and Tatsuno, 1988; Kondo and Marty, 1998). Furthermore, 10 mM NE has been reported to potentiate the inhibitory activity of stellate and basket cells (Llano and Gerschenfeld, 1993; Saitow and Konishi, 2000), while application of 100 mM NE for 30 min causes both a short-term enhancement of GABAergic inhibition and prolonged inhibition lasting more than 2 h. This is suggestive of the long-term depression involved in motor learning that occurs after simultaneous stimulation of climbing fibers (CF) and parallel fibers (PF) (Mitoma and Konishi, 1999; Gao et al., 2003). Thus, NE enhances stimulus-evoked signals, i.e. increases the “signal-to-noise ratio” of firing for both glutamatergic excitatory and GABAergic inhibitory synapses of cerebellar interneurons (Freedman et al., 1977; Moises et al., 1979, 1990; Mori-Okamoto and Tatsuno, 1988; Woodward et al., 1991). In contrast, depletion of cerebellar NE impairs motor performance (Watson and McElligott, 1984). Activation of specific ARs has been reported for some NE concentrations: α2aAR for > 16 μM NE, αAR below that range, α2AR at 10 mM and α1AR at 0.5 M (Yeh and Woodward, 1983; Basile and Dunwiddie, 1984; Mori-Okamoto and Tatsuno, 1988; Kondo and Marty, 1998). Thus, modulation of cerebellar neuron activity appears to be regulated by specific AR subtypes that selectively respond to certain levels or exposure times of NE.
Properties of α2AR subtypes
Although the α2AR subtypes are similar in structure, binding properties and coupling to G proteins, differences have been observed in subcellular localization, second messenger coupling, desensitization and trafficking properties in various transfection systems (Jones et al., 1991; Pepperl and Regan, 1993; Wozniak and Limbird, 1998; Olli-Lähdesmäki et al., 1999). For instance, when α2a and α2bARs are co-expressed in a rat pheochromocytoma (PC12) cell line (Olli-Lähdesmäki et al., 1999) or mouse embryonic spinal cord neurons in culture (Wozniak and Limbird, 1998), they both appear dispersed over the plasma membrane and concentrated at the tips of neurites. However, when α2bAR is expressed alone, it is evenly distributed in the plasma membrane of the cell body (Olli-Lähdesmäki et al., 1999). If these findings also hold true for cerebellar neurons, and future studies reveal comparable receptor locations in human cerebellar neurons, then the α2a and α2bARs co-expressed in the present study in human cortical neurons could act both as pre- and postsynaptic receptors on axons and dendrites. In contrast, the α2bAR expressed without the α2aAR in human dentate nucleus neurons may instead reflect its function as a postsynaptic receptor, located on cell bodies and dendrites. Different from the α2a and α2bARs, the α2cAR subtype is thought to be located mainly intracellularly (Wozniak and Limbird, 1998; Olli-Lähdesmäki et al., 1999; Hurt et al., 2000).
In assessing α2AR subtype-specific desensitization in PC12 cells, Olli-Lähdesmäki et al. (1999) found that the α2aAR was rapidly internalized (with some receptors remaining at the tips of the neurites), but the α2bAR was more extensively internalized after agonist-induced activation (NE; 10 μM, 30min). No change was observed for α2cAR. In human choriocarcinoma (JEG-3) cells, the α2aAR responded to nanomolar concentrations of agonist with inhibition of reporter gene activity, while micromolar concentrations reversed the inhibition and caused potentiation (Pepperl and Regan, 1993). In contrast, the α2bAR responded only with stimulation of activity to either concentration, and the α2cAR only with inhibition. Also, signaling and trafficking of α2AR subtypes is regulated in many ways by specific kinases, arrestins and spinophilin. For instance, arrestin-3 (β-arrestin-2) promotes internalization of α2b and α2cAR subtypes, but not α2aARs, and arrestin-2 (β-arrestin-1) selectively promotes internalization of α2bARs only (DeGraff et al., 2002; Wang et al., 2004). Further distinguishing features of α2AR subtypes are their response to stimulation frequencies and their affinity to agonist. Thus, Hein et al. (1999) observed in neurons from AR subtype knock-out mice that the α2aAR inhibits neurotransmitter release with higher speed and at higher action potential frequencies than the α2cAR, but that NE had a higher affinity for α2cARs than α2aARs, and the latter were deactivated faster after agonist stimulation (Bünemann et al., 2001).
If agonist response of cerebellar α2ARs is similar, it could be reasoned that the α2aAR inhibits neurotransmission during tonic and low levels of stimulation from the LC. However, when external signals produce LC phasic stimulation, both the α2a and α2bARs may activate cerebellar neurons and potentiate their firing at inhibitory and excitatory synapses, respectively. Due to the limited expression of the α2cAR subtype, we speculate that this AR is involved in slow inhibition of transmitter release from dentate nucleus neurons and select Purkinje cells in humans, possibly similar to what was observed in mouse brain, where α2aAR inhibited dopamine release at higher speed than α2cAR, and α2cAR signaling was attenuated after prolonged agonist exposure (Bücheler et al., 2002).
Potential roles of α2ARs in cerebellar function
To clarify the role of NE and its receptors, it is important to know where specific AR subtypes are located. For instance, are they located on the pre- and/or postsynaptic membrane, on cell bodies, dendrites or axons, proximal or distal to classic synapses, and preferentially associated with excitatory or inhibitory synapses? Several studies of other brain regions, using combined light and electron microscopy with dual immunocytochemical labels for the α2aAR and tyrosine hydroxylase (catecholamine synthesizing enzyme) or dopamine beta-hydroxylase have shown that α2aARs can be located presynaptically on catecholaminergic and non-catecholaminergic axons and terminals, postsynaptically near catecholaminergic terminals on dendritic shafts and spines of various non-adrenergic neurons, and on some glial processes (Aoki et al., 1998; Milner et al., 1998; Glass et al., 2001). Similar to these findings, our observation of abundant α2a and α2bAR message in non-catecholaminergic cells may represent not only postsynaptic heteroreceptors located on dendrites, but also presynaptic receptors located near GABAergic and/or glutamatergic axon terminals.
It is interesting that we observed a similar abundance of both α2a and α2bAR subtype mRNAs in neurons of the cerebellar cortex, but no α2aAR mRNA in dentate nucleus neurons. Both cortical and dentate nucleus neurons receive glutamatergic excitatory afferents that originate in pontine and spinal cord nuclei and terminate as mossy fibers (MF), as well as feed-back and timing information through CF from the inferior olives (Blumenfeld, 2002; Haines, 2002). These inputs are much more numerous in primates than in rodents, no doubt reflecting the expansion of the cerebrum and cerebellum during evolution (Leiner et al., 1991, 1993). We speculate that the α2bAR subtype is associated with glutamatergic synapses related to this expansion and therefore more abundant in primates than in rodents. The absence of α2aAR mRNA in dentate nucleus neurons may on the other hand point to a role of this subtype in states of vigilance (Aston-Jones et al., 1991, 1999). We suggest that cerebellar cortical neurons need to be able to respond to changes in vigilance, i.e. reducing their activity during sleep or increasing activity during stages of high alertness. This response may be regulated, depending on the levels of NE transmitted from the LC, by αARs. In contrast, the lack of the α2a and α2aAR subtype in dentate nucleus neurons may allow independence of these neurons from states of vigilance.
We observed that both granule and Golgi cells abundantly express α2aAR and α2bAR subtypes. Granule cells, the principal excitatory neurons of the cerebellar cortex, receive excitatory MF inputs which they propagate to synapses of their ascending axon segment with Purkinje cell dendrites and, after bifurcation of their axons and formation of PF, to additional Purkinje, Golgi, basket and stellate cell dendrites (Eccles et al., 1967; Gundappa-Sulur et al., 1999; Cohen and Yarom, 2000; Haines, 2002). Golgi cells, inhibitory interneurons, are located in the outer part of the granule cell layer and extend their dendrites throughout all layers of the cerebellar cortex. These dendrites are oriented in all planes to receive excitatory MF input at their bases in the granule cell layer, and from PF and CF collaterals at their apices in the molecular layer. They are inhibited by recurrent axon collaterals of Purkinje cells. Golgi cell axons arborize profusely in the granule cell layer to terminate on granule cell dendrites in cerebellar glomeruli, where they exert GABAergic feedback inhibition (Eccles et al., 1967; Haines, 2002). Granule and Golgi cells are reciprocally innervated and fire synchronously and rhythmically after MF stimulation, but the firing level can vary according to strength of incoming MF signal (Maex and Schutter, 1998). It is, therefore, possible that the α2aAR subtype modulates activity of these cells according to states of vigilance, while the α2bAR subtype provides modulation appropriate to the strength of glutamatergic input.
Properties of α1AR subtypes
Several non-neuronal transfection systems have been used to define differences between α1AR subtypes with similar results, but confirmation in neuronal systems is lacking. In these transfection studies, α1aARs are localized on the cell surface and intracellularly, α1bARs mainly on the cell surface, and α1dARs predominantly intracellularly. Both α1a and α1bARs undergo rapid agonist-mediated phosphorylation, desensitization and internalization, apparently via common GRK2/β-arrestin pathways, but faster and at lower agonist concentrations for α1bARs than α1aARs (Fonseca et al., 1995; Yang et al., 1999; McCune et al., 2000; Chalothorn et al., 2002; Price et al., 2002; Morris et al., 2004). The α1dAR subtype also undergoes agonist-mediated phosphorylation and desensitization; however, internalization appears to be primarily constitutive and may result from direct activation of protein kinase C, or cross-talk with other receptors endogenously expressed in the same cell (McCune et al., 2000; García-Sáinz et al., 2001).
Potential roles of α1AR subtypes in cerebellar function
Localization of α1AR messages is more limited than that of the α2AR transcripts and lacking in Golgi and granule cells. Only dentate nucleus neurons express all three α1AR subtypes with abundant α1dAR, moderate α1aAR and low α1bAR levels. This co-expression may be functionally significant, because α1a and α1bARs can form heterodimers which may alter their protein expression and trafficking (Stanasila et al., 2003; Uberti et al., 2003). These dentate neurons send cerebellar output to nuclei in the thalamus and red nucleus, are excited by MF and CF collaterals, and are either inhibited by Purkinje cells, or released from this inhibition when the Purkinje cells themselves are inhibited by basket and stellate cells (Haines, 2002). It is interesting that these dentate neurons express abundant signal for both α2bAR and α1dAR transcripts. As discussed above, we suggest that the α2bAR is related to the incoming glutamatergic signals from MF or CF, and speculate that α1a and α1bARs may play a role in the timing and receipt of the inhibitory signals from Purkinje cells, while α1dARs are constitutively active in association with other endogenous receptors to modulate the activity of dentate nucleus neurons.
It is well known that the cerebellum influences movement by synaptic mechanisms and circuits that utilize spatial and temporal timing. The limited or expansive domain of the dendritic arbor, the number of synaptic delays within a given circuit, and the constant flow of inputs from various body parts all combine to produce muscle synergy. The results of the present study, showing a differential preference for certain AR subtypes on functionally different cerebellar cell populations, suggest that timing most likely also has a molecular component. Basket cells express abundant α1aAR mRNA and inner stellate cells moderate levels of α1bAR mRNA. Basket cells, located in the inner molecular layer near Purkinje cell bodies, receive excitatory PF (axosomatic) and CF input. Basket cell dendrites arborize in the inner 2/3 of the molecular layer, and their axons extend along the Purkinje cell layer, where axon collaterals wrap around numerous Purkinje cell bodies to form the characteristic baskets. PF excitation of basket cell dendrites produces “off beam” GABAergic inhibition of Purkinje cells lateral to those excited by the same signal, which can be sustained up to 1 min and emphasizes the zone of Purkinje cell activity (Eccles et al., 1967; Palay and Chan-Palay, 1974; Haines, 2002). Inner stellate cells are located near basket cells in the inner molecular layer and some of their axons participate in the formation of baskets around Purkinje cell soma. In contrast, the short axons of outer stellate cells contact nearby Purkinje cell dendrites. Excitation of stellate cells though PF produces GABAergic inhibition of Purkinje cells, which narrows the spatial extent of Purkinje cell excitation in a “patchy” manner (Eccles et al., 1967; Cohen and Yarom, 1998, 2000; Blumenfeld, 2002; Haines, 2002). If the α1aAR in basket cells and the α1bAR in inner stellate cells function similarly as in non-neuronal transfected cells (Chalothorn et al., 2002), then the ability of the α1bAR subtype to react faster and to lower levels of NE may be important in the selective inhibition by stellate cells of specific populations of Purkinje cells. This inhibition might be followed by basket cell inhibition, modulated by α1aARs at a slightly slower speed, but requiring a more intense NE signal, resulting in a focused incoming excitatory signal from PF to select groups of Purkinje cells.
Purkinje cells express six αAR subtypes
While we observed an abundance of both α2aAR and α2bAR mRNA in all Purkinje cells, the remaining four AR subtypes are located in Purkinje cells of specific cerebellar lobules as follows: α1aAR and α2cAR in the anterior lobe vermis (lobules I to V); α1bAR and α1dAR in the ansiform lobule (lobule HVII); and α2cAR, α1aAR, α1bAR and α1dAR messages in the uvula/tonsil (lobule IX/HIX). This uneven location of α1 and α2AR subtype mRNAs may be related to the organization of Purkinje cells into sagittal microzones or functional compartments (Oberdick et al., 1998). Purkinje cells, their cell bodies located in a sheet between the granular and molecular layers, receive excitatory input from granule cell PF and inferior olive CF in their dendritic trees in the molecular layer. As the only output neurons of the cerebellar cortex, they inhibit cerebellar and vestibular nuclei. Their output, in part, is modulated by feed-forward inhibition from granule and stellate cells, as well as from Purkinje cells (Eccles et al., 1967; Gundappa-Sulur et al., 1999; Bower, 2002; Haines, 2002).
The abundance of α2aAR and α2bAR mRNA in all Purkinje cells may again be related to a role of these receptors in modulating Purkinje cell activity in response to states of vigilance, controlling signal-to-noise ratios and supporting glutamatergic synapses, as discussed above. In contrast, it is interesting that we observed α1bAR and α1dAR messages only in the two posterior lobe regions, the ansiform lobule and uvula/tonsil. The posterior lobe is phylogenetically the youngest part of the cerebellum and greatly expanded in primates (Leiner et al., 1991; Schmahmann, 1991). It is also interesting that in the two regions which are part of the spinocerebellum, the anterior lobe vermis and the uvula/tonsil, Purkinje cells are positive for α1aAR and α2cAR mRNA, while Purkinje cells of the ansiform lobule, part of the cerebrocerebellum, are not. The spinocerebellum receives spinal, trigeminal and vestibular inputs and is involved in motor execution, whereas the cerebrocerebellum receives corticopontine inputs and functions in motor planning. It is possible, therefore, that α1aAR and α2cAR subtypes play a role in motor execution. In support of this idea, in rat, α2cAR transcripts have been found prominently in basal ganglia, nuclei important in motor function (Scheinin et al., 1994; Winzer-Serhan et al., 1997c).
CONCLUSIONS
Recent neuroimaging, neuroanatomical and lesion-behavioral studies have expanded our understanding of cerebellar function and diseases. No longer is the cerebellum thought to only control posture, voluntary movement and certain reflexes (Ito, 1984; Thach et al., 1992; Jueptner and Weiller, 1998), but also to control aspects of behavior, visceral responses, and cognitive processing that include language and forms of learning (Petersen et al., 1989; Haines et al., 1984, 1990; Leiner et al., 1991; Desmond and Fiez, 1998; Thach, 1998; Wickelgren, 1998; Schmahmann, 1991, 1997; Ito, 2000). Localized or global, pre- or postnatal cerebellar damage often is reflected in a variety of cerebellar dysfunctions which include ataxia, autism, Joubert syndrome and cerebellar cognitive affective syndrome (Holroyd et al., 1991; Schmahmann, 1991; Kemper and Bauman, 1993; Bastian et al., 1996; Courchesne, 1997; Schmahmann and Sherman, 1998). Also in some diseases, such as Alzheimer’s and Parkinson’s disease, olivocerebellar atrophy and dementia with Lewy bodies, alterations of NE projections to the cerebellum and ARs have been documented (Kish et al., 1984a,b; Shimohama et al., 1986; Meana et al., 1992; Grijalba et al., 1994; Russo-Neustadt and Cotman, 1997; Leverenz et al., 2001. It is, therefore, important to fully know the location and understand the function of NE modulation by AR subtypes in the cerebellum and to be cognizant of possible species differences. Thus, the present study demonstrates the heterogeneous distribution of α1 and α2AR subtype mRNAs in human cerebellum, reports previously unknown expression of subtypes in certain cerebellar neurons and points out important species differences. This information should promote our understanding of the modulatory roles in cerebellar signaling exerted by the autonomic nervous and reticular activating systems through NE and αARs, cerebellar function in normal and disease states, and be suggestive of possible novel pharmacologic therapies.
Table 3.
Subtype-specific distribution of α1 and α2ARs in cerebella of experimental animals demonstrated by immunocytochemistry
| Location | α1a | α1b | α1d | α2a | α2b | α2c | Species | References |
|---|---|---|---|---|---|---|---|---|
| Molecular layer | ||||||||
| +/0 | Monkey | Aoki et al., 1994 | ||||||
| +++ | Rat | Rosin et al., 1996 | ||||||
| (++) | Rat | Talley et al., 1996 | ||||||
| Purkinje cell layer | ||||||||
| Purkinje cells | 0 | Rat | Go et al., 1993 | |||||
| ++ | Monkey | Go et al., 1993 | ||||||
| + | Rat | Aoki et al., 1994 | ||||||
| ++ | Monkey | Aoki et al., 1994 | ||||||
| 0 | Rat | Rosin et al., 1996 | ||||||
| ++ | Rat | Talley et al., 1996 | ||||||
| ++ | Rat | Acosta-Martinez et al., 1999 | ||||||
| Granule cell layer | ||||||||
| Granule cells | +++ | Rat | Aoki et al., 1994 | |||||
| +++ | Monkey | Aoki et al., 1994 | ||||||
| +++ | Rat | Rosin et al., 1996 | ||||||
| +++ | Rat | Talley et al., 1996 | ||||||
| Cerebellar nuclei | ||||||||
| Dentate | +++ | Rat | Rosin et al., 1996 | |||||
| Interposed | +++ | Rat | Rosin et al., 1996 | |||||
| ++ | Rat | Acosta-Martinez et al., 1999 | ||||||
| Medial | +++ | Rat | Acosta-Martinez et al., 1999 | |||||
| Glia | ||||||||
| Oligodendrocytes | ++ | Rat | Talley et al., 1996 | |||||
Symbols represent relative levels of mRNA signal from individual studies as follows: +++ denotes heavy signal; ++ moderate signal; + light signal; +/0 signal just above background; 0 signal undetectable. Brackets denote select cells within a cerebellar layer.
Acknowledgments
We are very grateful to and thank Dr. Jean Lauder for critical reading and advice in preparation of this manuscript, Dr. Joanne M. Pyper for providing the β-actin cDNA, Sarah Owen and Stella Page for their technical advice and Bobbie Connelly for her assistance in manuscript preparation. This work was funded in part by NIH grant HL49103. Organizations facilitating human tissue collection include Duke Rapid Autopsy Program (NIH-AG05128 and Glaxo Wellcome), Duke General Clinical Research Center (NIH-M01RR30), NDRI (NIH-RR06042), and IIAM.
Abbreviations
- AR
adrenergic receptor
- CF
climbing fibers
- dH2O
distilled water
- LC
locus coeruleus
- MF
mossy fibers
- NE
norepinephrine
- PBS
phosphate-buffered saline
- PF
parallel fibers
- SSC
saline-sodium phosphate
References
- Abbot LC, Sotelo C. Ultrastructural analysis of catecholaminergic innervation in weaver and normal mouse cerebellar cortices. J Comp Neurol. 2000;426(2):316–329. [PubMed] [Google Scholar]
- Acosta-Martinez M, Fiber JM, Brown RD, Etgen AM. Localization of α1B-adrenergic receptor in female rat brain regions involved in stress and neuroendocrine function. Neurochem Int. 1999;35:383–391. doi: 10.1016/s0197-0186(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Bylund DB, Pundt LL, Wamsley JK. α2-Agonist binding sites in brain: [125I]para-iodoclonidine versus [3H]para-aminoclonidine. Brain Res Bull. 1993;32:97–102. doi: 10.1016/0361-9230(93)90062-g. [DOI] [PubMed] [Google Scholar]
- Aoki C, Go C-G, Venkatesan C, Kurose H. Perikaryal and synaptic localization of α2A-adrenergic receptor-like immunoreactivity. Brain Res. 1994;650:181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go C-G, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Basile AS, Dunwiddie TV. Norepinephrine elicits both excitatory and inhibitory responses from Purkinje cells in the in vitro rat cerebellar slice. Brain Res. 1984;296:15–25. doi: 10.1016/0006-8993(84)90507-9. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Berkowitz DE, Price DT, Bello EA, Page SO, Schwinn DA. Localization of messenger RNA for three distinct α2-adrenergic receptor subtypes in human tissue. Evidence for species heterogeneity and implication for human pharmacology. Anesthesiology. 1994;81:1235–1244. doi: 10.1097/00000542-199411000-00018. [DOI] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, Castellanos FX. Cerebellum in attention-deficit hyperactivity disorder: a morphometric MRI study. Neurology. 1998;50(4):1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer P, Pang K, Rose GM, Gerhardt GA. Electrically-evoked release of norepinephrine in the rat cerebellum: an in vivo electrochemical and electrophysiological study. Brain Res. 1991;558(2):305–311. doi: 10.1016/0006-8993(91)90782-q. [DOI] [PubMed] [Google Scholar]
- Bloom FE, Hoffer BJ, Siggins GR. Studies on norepinephrine-containing afferents to Purkinje cells in rat cerebellum. I. Localization of the fibers and their synapses. Brain Res. 1971;25:501–521. doi: 10.1016/0006-8993(71)90457-4. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Neuroanatomy through clinical cases. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Bower JM. The organization of cerebellar cortical circuitry revisited. Implications for function. N Y Acad Sci. 2002;978:135–155. doi: 10.1111/j.1749-6632.2002.tb07562.x. [DOI] [PubMed] [Google Scholar]
- Boyajian CL, Loughlin SE, Leslie FM. Anatomical evidence for alpha-2 adrenoceptor heterogeneity: differential autoradiographic distributions of [3H]rauwolscine and [3H]idazoxan in rat brain. J Pharmacol Exp Ther. 1987;241:1079–1091. [PubMed] [Google Scholar]
- Brüning G, Kaulen P, Baumgarten HG. Quantitative autoradiographic localization of α2-antagonist binding sites in rat brain using [3H]idazoxan. Neurosci Lett. 1987;83:333–337. doi: 10.1016/0304-3940(87)90110-8. [DOI] [PubMed] [Google Scholar]
- Bücheler MM, Hadamek K, Hein L. Two α2-adrenergic receptor subtypes, α2A and α2c, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–826. doi: 10.1016/s0306-4522(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Bünemann M, Bücheler MM, Philipp M, Lohse MJ, Hein L. Activation and deactivation kinetics of α2A and α2Cadrenergic receptor-activated G protein-activated inwardly rectifying K+ channel currents. J Biochem Chem. 2001;276:47512–47517. doi: 10.1074/jbc.M108652200. [DOI] [PubMed] [Google Scholar]
- Cayla C, Heinonen P, Viikari L, Schaak S, Snapir A, Bouloumie A, Karvonen MK, Pesonen U, Scheinin M, Paris H. Cloning, characterization and identification of several polymorphisms in the promoter region of the human α2B-adrenergic receptor gene. Biochem Pharmacol. 2004;67:469–478. doi: 10.1016/j.bcp.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Chalothorn D, McCune DF, Edelmann SE, García-Cazarín ML, Tsujimoto G, Piascik MT. Differences in the cellular localization and agonist-mediated internalization properties of the α1-adrenoceptor subtypes. Mol Pharmacol. 2002;61:1008–1016. doi: 10.1124/mol.61.5.1008. [DOI] [PubMed] [Google Scholar]
- Cohen D, Yarom Y. Patches of synchronized activity in the cerebellar cortex evoked by mossy-fiber stimulation: Questioning the role of parallel fibers. Proc Natl Acad Sci U S A. 1998;95:15032–15036. doi: 10.1073/pnas.95.25.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Yarom Y. Cerebellar on-beam and lateral inhibition: two functional distinct circuits. J Neurophysiol. 2000;83(4):1932–1940. doi: 10.1152/jn.2000.83.4.1932. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997;7:269–278. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Day HEW, Campeau S, Watson SJ, Jr, Akil H. Distribution of α1a-, α1b- and α1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- DeGraff JL, Gurevich VV, Benovic JL. The third intracellular loop of α2-adrenergic receptors determines subtype specificity of arrestin interaction. J Biol Chem. 2002;277:43247–43252. doi: 10.1074/jbc.M207495200. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trend Cogn Sci. 1998;2(9):355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- De Vos H, Convents A, De Keyser J, De Backer JP, Van Megen IJB, Ebinger G, Vauquelin G. Autoradiographic distribution of α2 adrenoceptors, NAIBS, and 5-HT1A receptors in human brain using [3H]idazoxan and [3H]rauwolscine. Brain Res. 1991;566:13–20. doi: 10.1016/0006-8993(91)91675-q. [DOI] [PubMed] [Google Scholar]
- De Vos H, Vauquelin G, De Keyser J, De Backer JP, Van Liefde I. Regional distribution of α2A- and α2B-adrenoceptor subtypes in postmortem human brain. J Neurochem. 1992;58:1555–1560. doi: 10.1111/j.1471-4159.1992.tb11378.x. [DOI] [PubMed] [Google Scholar]
- De Vos H, Bricca G, De Keyser J, De Backer J-P, Bousquet P, Vauquelin G. Imidazoline receptors, non-adrenergic idazoxan binding sites and the α2-adrenoceptors in the human central nervous system. Neuroscience. 1994;59:589–598. doi: 10.1016/0306-4522(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Domyancic AV, Morilak DA. Distribution of α1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol. 1997;386:358–378. doi: 10.1002/(sici)1096-9861(19970929)386:3<358::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The cerebellum as neuronal machine. New York: Springer; 1967. [Google Scholar]
- Fernández-López A, del Arco C, González AM, Gómez T, Calvo P, Pazos A. Autoradiographic localization of α2-adrenoceptors in chick brain. Neurosci Lett. 1990;120:97–100. doi: 10.1016/0304-3940(90)90177-b. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Button DC, Brown RD. Agonist regulation of α1B-adrenergic receptor subcellular distribution and function. J Biol Chem. 1995;270:8902–8909. doi: 10.1074/jbc.270.15.8902. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hoffer BJ, Woodward DJ, Puro D. Interaction of norepinephrine with cerebellar activity evoked by mossy and climbing fibers. Exp Neurol. 1977;55:269–288. doi: 10.1016/0014-4886(77)90175-3. [DOI] [PubMed] [Google Scholar]
- Gao W, Dunbar RL, Chen G, Reinert KC, Oberdick J, Ebner TJ. Optical imaging of long-term depression in the mouse cerebellar cortex in vivo. J Neurosci. 2003;23(5):1859–1866. doi: 10.1523/JNEUROSCI.23-05-01859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sáinz JA, Vázquez-Cuevas FG, Romero-Avila MT. Phosphorylation and desensitization of α1d-adrenergic receptors. Biochem J. 2001;353:603–610. doi: 10.1042/0264-6021:3530603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Aicher SA, Milner TA, Pickel VM. Subcellular localization of α2A-adrenergic receptors in the rat medial nucleus tractus solitarius: regional targeting and relationship with catecholamine neurons. J Comp Neurol. 2001;433:193–207. doi: 10.1002/cne.1135. [DOI] [PubMed] [Google Scholar]
- Go C-G, Aoki CJ, Venkatesan C, Kurose H. α2A-Adrenergic receptors: pre- and postsynaptic. Soc Neurosci Abstr. 1993;19:1788. [Google Scholar]
- Grijalba B, Berciano J, Anciones B, Pazos A, Pascual J. Adrenergic receptors in the cerebellum of olivocerebellar atrophy. J Neural Transm Gen Sect. 1994;96(2):135–142. doi: 10.1007/BF01277935. [DOI] [PubMed] [Google Scholar]
- Grijalba B, Callado LF, Meana JJ, García-Sevilla JA, Pazos A. α2-Adrenoceptor subtypes in the human brain: a pharmacological delineation of [3H]RX-821002 binding to membranes and tissue sections. Eur J Pharmacol. 1996;310:83–93. doi: 10.1016/0014-2999(96)00381-0. [DOI] [PubMed] [Google Scholar]
- Gundappa-Sulur G, De Schutter E, Bower JM. Ascending granule cell axon: An important component of cerebellar cortical circuitry. J Comp Neurol. 1999;408:580–596. doi: 10.1002/(sici)1096-9861(19990614)408:4<580::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Haines DE, Dietrichs E, Sowa TE. Hypothalamocerebellar and cerebello-hypothalamic pathways: A review and hypothesis concerning cerebellar circuits which may influence autonomic centers and affective behavior. Brain Behav Evol. 1984;24:198–220. doi: 10.1159/000121317. [DOI] [PubMed] [Google Scholar]
- Haines DE, May PJ, Dietrichs E. Neuronal connections between the cerebellar nuclei and hypothalamus in Macaca fascicularis: Cerebello-visceral circuits. J Comp Neurol. 1990;299:106–122. doi: 10.1002/cne.902990108. [DOI] [PubMed] [Google Scholar]
- Haines DE, editor. Fundamental neuroscience. New York: Churchill Livingston; 2002. [Google Scholar]
- Happe HK, Coulter CL, Gerety ME, Sanders JD, O’Rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Holmberg M, Fagerholm V, Scheinin M. Regional distribution of α2C-adrenoceptors in brain and spinal cord of control mice and transgenic mice overexpressing the α2C-subtype: an autoradiographic study with [3H]RX821002 and [3H]rauwolscine. Neuroscience. 2003;117:875–898. doi: 10.1016/s0306-4522(02)00966-1. [DOI] [PubMed] [Google Scholar]
- Holroyd S, Reiss AL, Bryan RN. Autistic features in Joubert syndrome: a genetic disorder with agenesis of the cerebellar vermis. Biol Psychiatry. 1991;29:287–294. doi: 10.1016/0006-3223(91)91291-x. [DOI] [PubMed] [Google Scholar]
- Hurt CM, Feng FY, Kobilka B. Cell-type specific targeting of the α2c-adrenoceptor. J Biochem Chem. 2000;275:35424–35431. doi: 10.1074/jbc.M006241200. [DOI] [PubMed] [Google Scholar]
- Ito M. The cerebellum and neural control. New York: Raven Press; 1984. [Google Scholar]
- Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN. Anatomy of brain alpha1-adrenergic receptors: in vitro autoradiography with [125I]-heat. J Comp Neurol. 1985;231:190–208. doi: 10.1002/cne.902310207. [DOI] [PubMed] [Google Scholar]
- Jones CR, Palacios JM. Autoradiography of adrenoceptors in rat and human brain: α-adrenoceptor and idazoxan binding sites. In: Barnes CD, Pompeiano O, editors. Progress in brain research. Chapter 21. Vol. 88. Amsterdam: Elsevier; 1991. pp. 271–291. [DOI] [PubMed] [Google Scholar]
- Jones SB, Halenda SP, Bylund DB. Alpha 2-adrenergic receptor stimulation of phospholipase A2 and of adenylate cyclase in transfected Chinese hamster ovary cells is mediated by different mechanisms. Mol Pharmacol. 1991;39:239–245. [PubMed] [Google Scholar]
- Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movement as revealed by functional imaging studies. Brain. 1998;121:1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- Kamibayashi T, Maze M. Clinical uses of α2-adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Behav Neurol. 1993;11:175–187. [PubMed] [Google Scholar]
- Kimoto Y, Tohyama M, Satoh K, Sakumoto T, Takahashi Y, Shimizu N. Fine structure of rat cerebellar noradrenaline terminals as visualized by potassium permanganate “in situ perfusion” fixation method. Neuroscience. 1981;6:47–58. doi: 10.1016/0306-4522(81)90242-6. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak KS, Rajput AH, Gilbert JJ, Hornykiewicz O. Cerebellar norepinephrine in patients with Parkinson’s disease and control subjects. Arch Neurol. 1984a;41:612–614. doi: 10.1001/archneur.1984.04210080020007. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak KS, Hornykiewicz O. Reduction of noradrenaline in cerebellum of patients with olivopontocerebellar atrophy. J Neurochem. 1984b;42:1476–1478. doi: 10.1111/j.1471-4159.1984.tb02813.x. [DOI] [PubMed] [Google Scholar]
- Kondo S, Marty A. Differential effects of noradrenaline on evoked, spontaneous and miniature IPSCs in rat cerebellar stellate cells. J Physiol. 1998;509:233–243. doi: 10.1111/j.1469-7793.1998.233bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC, Shoemaker WJ, Schlumpf M, Bloom FE. Catecholamines in mutant mouse cerebellum: fluorescence microscopy and chemical studies. Brain Res. 1975;93:253–266. doi: 10.1016/0006-8993(75)90349-2. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44:113–128. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. Cognitive and language function of human cerebellum. Trends Neurosci. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- Leverenz JB, Miller MA, Dobie DJ, Peskind ER, Raskind MA. Increased alpha 2-adrenergic receptor binding in locus coeruleus projection areas in dementia with Lewy bodies. Neurobiol Aging. 2001;22:555–561. doi: 10.1016/s0197-4580(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Llano I, Gerschenfeld HM. β-Adrenergic enhancement of inhibitory synaptic activity in rat cerebellar stellate and Purkinje cells. J Physiol. 1993;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman J, Eriksdotter-Nilsson M, Jonsson G. Structural and neurochemical effects in mouse cerebellum following neonatal methylazoxymethanol and 6-hydroxydopamine treatment. Int J Dev Neurosci. 1990;8(1):107–118. doi: 10.1016/0736-5748(90)90027-y. [DOI] [PubMed] [Google Scholar]
- Maex R, Schutter ED. Synchronization of Golgi and granule cell firing in a detailed network model of the cerebellar granule cell layer. J Neurophysiol. 1998;80:2521–2537. doi: 10.1152/jn.1998.80.5.2521. [DOI] [PubMed] [Google Scholar]
- McCune DF, Edelmann SE, Olges JR, Post GR, Waldrop BA, Waugh DJJ, Perez DM, Piascik MT. Regulation of the cellular localization and signaling properties of the α1B- and α1D-adrenoceptors by agonists and inverse agonists. Mol Pharmacol. 2000;57:659–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- McCune SK, Voigt MM, Hill JM. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience. 1993;57:143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Barturen F, García-Sevilla JA. Characterization and regional distribution of α2-adrenoceptors in postmortem human brain using the full agonist [3H]UK 14304. J Neurochem. 1989;52:1210–1217. doi: 10.1111/j.1471-4159.1989.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Barturen F, Garro MA, Garcia-Sevilla JA, Fontan A, Zarranz JJ. Decreased density of presynaptic α2-adrenoceptors in postmortem brains of patients with Alzheimer’s disease. J Neurochem. 1992;58:1896–1904. doi: 10.1111/j.1471-4159.1992.tb10067.x. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lee A, Aicher SA, Rosin DL. Hippocampal α2A-adrenergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J Comp Neurol. 1998;395:310–327. [PubMed] [Google Scholar]
- Mitoma H, Konishi S. Monoaminergic long-term facilitation of GABA-mediated inhibitory transmission at cerebellar synapses. Neuroscience. 1999;88:871–883. doi: 10.1016/s0306-4522(98)00260-7. [DOI] [PubMed] [Google Scholar]
- Moises HC, Burne RA, Woodward DJ. Modification of the visual response properties of cerebellar neurons by norepinephrine. Brain Res. 1990;514:250–275. doi: 10.1016/0006-8993(90)91421-c. [DOI] [PubMed] [Google Scholar]
- Moises HC, Woodward DJ, Hoffer BJ, Freedman R. Interactions of norepinephrine with Purkinje cell responses to putative amino acid neurotransmitters applied by microiontophoresis. Exp Neurol. 1979;64:493–515. doi: 10.1016/0014-4886(79)90227-9. [DOI] [PubMed] [Google Scholar]
- Moore RY, Card JP. Noradrenaline-containing neuron systems. In: Björklund A, Hökfelt T, editors. Handbook of chemical neuroanatomy, Vol 2: Classical transmitters in the CNS, part I. Chapter IV. Elsevier Sciences Publisher B.V; 1984. pp. 157–276. [Google Scholar]
- Mori-Okamoto J, Tatsuno J. Effects of noradrenaline on the responsiveness of cultured cerebellar neurons to excitatory amino acids. Brain Res. 1988;448:259–271. doi: 10.1016/0006-8993(88)91263-2. [DOI] [PubMed] [Google Scholar]
- Morris DP, Price RR, Smith MP, Lei B, Schwinn DA. Cellular trafficking of human α1a-adrenergic receptors is both continuous and primarily agonist-dependent. Mol Pharm. 2004;66:843–854. doi: 10.1124/mol.104.000430. [DOI] [PubMed] [Google Scholar]
- Nelson TE, King JS, Bishop GA. Distribution of tyrosine hydroxylase-immunoreactive afferents to the cerebellum differs between species. J Comp Neurol. 1997;379:443–454. doi: 10.1002/(sici)1096-9861(19970317)379:3<443::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Hökfelt T. Distribution of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: An in situ hybridization study. J Comp Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Oberdick J, Baader SL, Schilling K. From zebra stripes to postal zones: deciphering patterns of gene expression in the cerebellum. Trends Neurosci. 1998;21:383–390. doi: 10.1016/s0166-2236(98)01325-3. [DOI] [PubMed] [Google Scholar]
- Olli-Lähdesmäki Kallio J, Scheinin M. Receptor subtype-induced targeting and subtype-specific internalization of human α2-adrenoceptors in PC12 cells. J Neurosci. 1999;19:9281–9288. doi: 10.1523/JNEUROSCI.19-21-09281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway GA, Jaconetta SM, Halaris AE. Characterization of subtypes of alpha-2 adrenoceptors in the human brain. J Pharmacol Exp Ther. 1993;264:967–976. [PubMed] [Google Scholar]
- Palacios JM, Hoyer D, Cortés R. α1-Adrenoceptors in the mammalian brain: similar pharmacology but different distribution in rodents and primates. Brain Res. 1987;419:65–75. doi: 10.1016/0006-8993(87)90569-5. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. Springer Verlag; 1974. [Google Scholar]
- Pascual J, del Arco C, Gonzalez AM, Pazos A. Quantitative light microscopic autoradiographic localization of α2-adrenoceptors in human brain. Brain Res. 1992;585:116–127. doi: 10.1016/0006-8993(92)91196-l. [DOI] [PubMed] [Google Scholar]
- Pazos A, González AM, Pascual J, Meana JJ, Barturen F, García-Sevilla JA. α2-Adrenoceptors in human forebrain: autoradiographic visualization and biochemical parameters using the agonist [3H]UK-14304. Brain Res. 1988;475:361–365. doi: 10.1016/0006-8993(88)90626-9. [DOI] [PubMed] [Google Scholar]
- Pepperl DJ, Regan JW. Selective coupling of α2-adrenergic receptor subtypes to cyclic AMP-dependent receptor gene expression in transiently transfected JEG-3 cells. Mol Pharmacol. 1993;44:802–809. [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hökfelt Distribution of in situ hybridization α1-adrenoceptors in rat brain revealed by experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkletnova I, Alho H. Neonatal noradrenaline depletion prevents the transition of Bergmann glia in the developing cerebellum. J Chem Neuroanat. 1998;14:167–173. doi: 10.1016/s0891-0618(98)00006-4. [DOI] [PubMed] [Google Scholar]
- Powers RE, O’Connor DT, Price DL. Noradrenergic systems in human cerebellum. Brain Res. 1989;481:194–199. doi: 10.1016/0006-8993(89)90504-0. [DOI] [PubMed] [Google Scholar]
- Price DT, Lefkowitz RJ, Caron MG, Berkowitz D, Schwinn DA. Localization of mRNA for three distinct α1-adrenergic receptor subtypes in human tissue: implication for human α-adrenergic physiology. Mol Pharmacol. 1994;45:171–175. [PubMed] [Google Scholar]
- Price RR, Morris DP, Biswas G, Smith MP, Schwinn DA. Acute agonist-mediated desensitization of the human α1a-adrenergic receptor is primarily independent of carboxyl terminus regulation. Implication for regulation of α1aAR splice variants. J Biol Chem. 2002;277:9570–9579. doi: 10.1074/jbc.M111762200. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Aylward E, Freund LS, Joshi PK, Bryan RN. Neuroanatomy of fragile X syndrome: the posterior fossa. Ann Neurol. 1991;29:26–32. doi: 10.1002/ana.410290107. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of α2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt A, Cotman CW. Adrenergic receptors in Alzheimer’s disease brain: selective increases in the cerebella of aggressive patients. J Neurosci. 1997;17(14):5573–5580. doi: 10.1523/JNEUROSCI.17-14-05573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitow F, Konishi S. Excitability increase induced by β-adrenergic receptor-mediated activation of hyperpolarization-activated cation channels in rat cerebellar basket cells. J Neurophysiol. 2000;84:2026–2034. doi: 10.1152/jn.2000.84.4.2026. [DOI] [PubMed] [Google Scholar]
- Sastre M, García-Sevilla JA. α2-Adrenoceptor subtypes identified by [3H]RX821002 binding in the human brain: the agonist guanoxabenz does not discriminate different forms of the predominant α2A subtype. J Neurochem. 1994;63:1077–1085. doi: 10.1046/j.1471-4159.1994.63031077.x. [DOI] [PubMed] [Google Scholar]
- Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau RT., Jr Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of α2-adrenergic receptor subtypes gene expression in rat brain. Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. International review of neurobiology. Vol. 41. San Diego, CA: Academic Press; 1997. The cerebellum and cognition. [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schwinn DA, Page SO, Middleton JP, Lorenz W, Liggett SB, Yamamoto K, Lapetina EG, Caron MG, Lefkowitz RJ, Cotecchia S. The α1C-adrenergic receptor: Characterization of signal transduction pathways and mammalian tissue heterogeneity. Mol Pharmacol. 1991;40:619–626. [PubMed] [Google Scholar]
- Shimohama S, Taniguchi T, Fujiwara M, Kameyama M. Biochemical characterization of alpha-adrenergic receptors in human brain and changes in Alzheimer-type dementia. J Neurochem. 1986;47(4):1295–1301. [PubMed] [Google Scholar]
- Sievers J, Klemm HP. Locus coeruleus-cerebellum: interaction during development. Bibl Anat. 1982;23:56–75. [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DR, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reduction in lobules I-V. Alcohol Clin Exp Res. 1996;20(1):31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Stafford Smith M, Schambra UB, Wilson KH, Page SO, Hulett C, Light AR, Schwinn DA. α2-Adrenergic receptors in human spinal cord: specific localized expression of mRNA encoding α2-adrenergic receptor subtypes at four distinct levels. Mol Brain Res. 1995;34:109–117. doi: 10.1016/0169-328x(95)00148-l. [DOI] [PubMed] [Google Scholar]
- Stafford Smith M, Schambra UB, Wilson KH, Page SO, Schwinn DA. α1-Adrenergic receptors in human spinal cord: specific localized expression of mRNA encoding α1-adrenergic receptor subtypes at four distinct levels. Mol Brain Res. 1999;63:254–261. doi: 10.1016/s0169-328x(98)00287-3. [DOI] [PubMed] [Google Scholar]
- Stanasila L, Perez J-B, Vogel H, Cotecchia S. Oligomerization of the α1a- and α1b-adrenergic receptor subtypes. Potential implication in receptor internalization. J Biol Chem. 2003;278:40239–40251. doi: 10.1074/jbc.M306085200. [DOI] [PubMed] [Google Scholar]
- Strazielle C, Lalonde R, He̊bert C, Reader TA. Regional brain distribution of noradrenaline uptake sites, and of α1-, α2- and α-adrenergic receptors in PCD mutant mice: a quantitative autoradiographic study. Neuroscience. 1999;94:287–304. doi: 10.1016/s0306-4522(99)00321-8. [DOI] [PubMed] [Google Scholar]
- Talley EM, Rosin DL, Lee A, Guyenet PG, Lynch KR. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tavares A, Handy DE, Bogdanova NN, Rosene DL, Gavras H. Localization of alpha 2A- and alpha2B-adrenergic receptor subtypes in brain. Hypertension. 1996;27:449–455. doi: 10.1161/01.hyp.27.3.449. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goddkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Thach WT. What is the role of the cerebellum in motor learning and cognition? Trends Cogn Sci. 1998;2(9):331–337. doi: 10.1016/s1364-6613(98)01223-6. [DOI] [PubMed] [Google Scholar]
- Titus DE. RNA transcription in vitro. In: Titus DE, editor. Promega protocols and application guide. Vol. 298. Madison, WI: Promega; 1992. pp. 59–61. [Google Scholar]
- Uberti MA, Hall RA, Minneman KP. Subtype-specific dimerization of α1-adrenoceptors: effects on receptor expression and pharmacological properties. Mol Pharmacol. 2003;64:1379–1390. doi: 10.1124/mol.64.6.1379. [DOI] [PubMed] [Google Scholar]
- Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of α2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates on the pharmacologic effects of clonidine and related adrenergic agents. Brain Res Rev. 1984;7:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- Van Liefde I, Vauquelin G, De Keyser J, De Backer JP, De Vos H. α2A-Adrenoceptors and non-adrenergic idazoxan binding sites in calf brain and retina are distinct from those of human brain. Neurochem Int. 1993;22:501–509. doi: 10.1016/0197-0186(93)90046-8. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Alburges ME, Hunt MAE, Bylund DB. Differential localization of α2-adrenergic receptor subtypes in brain. Pharmacol Biochem Behav. 1992;41:267–273. doi: 10.1016/0091-3057(92)90097-y. [DOI] [PubMed] [Google Scholar]
- Wang R, Macmillan LB, Fremeau RT, Jr, Magnuson MA, Lindner J, Limbird LE. Expression of α2-adrenergic receptor subtypes in the mouse brain: Evaluation of spatial and temporal information imparted by 3 kb of 5′ regulatory sequence for the α2AAR-receptor gene in transgenic animals. Neuroscience. 1996;74:199–218. doi: 10.1016/0306-4522(96)00116-9. [DOI] [PubMed] [Google Scholar]
- Wang G-S, Chang N-C, Wu S-C, Chang AC. Regulated expression of α 2B adrenoceptor during development. Dev Dyn. 2002;225:142–152. doi: 10.1002/dvdy.10141. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Watson M, McElligott JG. Cerebellar norepinephrine depletion and impaired acquisition of specific locomotor tasks in rats. Brain Res. 1984;296:129–138. doi: 10.1016/0006-8993(84)90518-3. [DOI] [PubMed] [Google Scholar]
- Wickelgren I. The cerebellum: the brain’s engine to agility. Science. 1998;281:1588–1590. doi: 10.1126/science.281.5383.1588. [DOI] [PubMed] [Google Scholar]
- Wilson KM, Minneman KP. Regional variations of α1-adrenergic receptor subtypes in rat brain. J Neurochem. 1989;53:1782–1786. doi: 10.1111/j.1471-4159.1989.tb09243.x. [DOI] [PubMed] [Google Scholar]
- Wilson KH, Schambra UB, Smith MS, Page SO, Richardson CD, Fremeau RT, Jr, Schwinn DA. In situ hybridization: identification of rare mRNAs in human tissues. Brain Res Protoc. 1997;1:175–185. doi: 10.1016/s1385-299x(96)00028-1. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. α2B Adrenoceptor mRNA expression during rat brain development. Dev Brain Res. 1997a;100:90–100. doi: 10.1016/s0165-3806(97)00035-7. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoceptors during rat brain development, I. Alpha 2A messenger RNA. Neuroscience. 1997b;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoceptors during rat brain development, II. α2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1997c;76:261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Woodward DJ, Moises HC, Waterhouse BD, Yeh HH, Cheun JE. The cerebellar norepinephrine system: inhibition, modulation, and gating. Prog Brain Res. 1991;88:331–341. doi: 10.1016/s0079-6123(08)63820-0. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Limbird LE. Trafficking itineraries of G protein-coupled receptors in epithelial cells do not predict receptor localization in neurons. Brain Res. 1998;780:311–322. doi: 10.1016/s0006-8993(97)01216-x. [DOI] [PubMed] [Google Scholar]
- Yang M, Reese J, Cotecchia S, Michel MC. Murine alpha1-adrenoceptor subtypes. I. radioligand binding studies. J Pharmacol Exp Ther. 1998;286:841–847. [PubMed] [Google Scholar]
- Yang M, Ruan J, Voller M, Schalken J, Michel MC. Differential regulation of human α1-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:439–446. doi: 10.1007/pl00005373. [DOI] [PubMed] [Google Scholar]
- Yeh HH, Woodward DJ. Beta-1 adrenergic receptors mediate noradrenergic facilitation of Purkinje cell responses to gamma-aminobutyric acid in cerebellum of rat. Neuropharmacology. 1983;22:629–639. doi: 10.1016/0028-3908(83)90155-7. [DOI] [PubMed] [Google Scholar]
- Zilles K, Qu M, Schleicher A. Regional distribution and heterogeneity of α-adrenoceptors in rat and human central nervous system. J Hirnforsch. 1993;34:123–132. [PubMed] [Google Scholar]