Abstract
Cortical excitability can be reliably assessed by means of paired-pulse stimulation techniques. Recent studies demonstrated particularly for motor and visual cortex that cortical excitability is systematically altered following the induction of learning processes or during the development of pathological symptoms. A recent tactile coactivation protocol developed by Godde and coworkers showed that improvement of tactile performance in humans can be achieved also without training through passive stimulation on a time scale of a few hours. Tactile coactivation evokes plastic changes in somatosensory cortical areas as measured by blood oxygenation level-dependent (BOLD) activation in fMRI or SEP-dipole localization, which correlated with the individual gain in performance. To demonstrate changes in excitability of somatosensory cortex after tactile coactivation, we combined assessment of tactile performance with recordings of paired-pulse SEPs after electrical median nerve stimulation of both the right coactivated and left control hand at ISIs of 30 and 100 ms before, 3 h after and 24 h after tactile coactivation. Amplitudes and latencies of the first and second cortical N20/P25 response components were calculated. For the coactivated hand, we found significantly lowered discrimination thresholds and significantly reduced paired-pulse ratios (second N20/P25 response/first N20/P25 response) at an ISI of 30 ms after tactile coactivation indicating enhanced cortical excitability. No changes in paired-pulse behaviour were observed for ISIs of 100 ms. Both psychophysical and cortical effects recovered to baseline 24 h after tactile coactivation. The individual increase of excitability correlated with the individual gain in discrimination performance. For the left control hand we found no effects of tactile coactivation on paired-pulse behaviour and discrimination threshold. Our results indicate that changes in cortical excitability are modified by tactile coactivation and were scaled with the degree of improvement of the individual perceptual learning. Conceivably, changes of cortical excitability seem to constitute an additional important marker and mechanism underlying plastic reorganization.
Training and learning are well-documented measures to induce changes in cortical representation parallel to an improvement of perceptual or behavioural performance (Weinberger, 1995; Buonomano & Merzenich, 1998; Sanes & Donoghue, 2000; Dinse & Merzenich, 2002). The outcome of use- and experience-dependent cortical plasticity can be demonstrated by non-invasive neurophysiological and fMRI techniques and is often described in terms of changes of cortical representational maps. More recently, bidirectional alteration of neuronal excitability has been advocated as a complementary mechanism underlying plastic changes (Bi & Poo, 2001; Sjostrom et al. 2001; Wolters et al. 2003).
Facilitatory and inhibitory effects on cortical excitability have been investigated by recording evoked potentials following paired-pulse stimulation techniques in visual and somatosensory systems (Klostermann et al. 2000; Ragert et al. 2004; Sparing et al. 2005).
Studies in motor systems have shown that task-induced MEP facilitation measured by changes in MEP amplitudes and paired-pulse behaviour is positively correlated with improvements in performance (Muellbacher et al. 2001; Ziemann et al. 2001), which has been suggested to reflect the early consolidation stage of motor skill acquisition in primary motor cortex (Muellbacher et al. 2002). In the somatosensory system, paired-pulse SEPs were used to demonstrate changes in excitability or to investigate recovery functions of the nervous system (Schwartz & Shagass, 1964; Shagass & Schwartz, 1964; Ragert et al. 2004) in particular in patients with neurological diseases (Nakashima et al. 1992; Ugawa et al. 1996; Mochizuki et al. 2001). More recently, a close connection has been demonstrated between changes of human tactile performance (Tegenthoff et al. 2005) and an increase of cortical excitability (Ragert et al. 2004) evoked by 5 Hz TMS.
A recent tactile coactivation protocol developed by Godde and coworkers showed that improvement of tactile performance in humans can be also achieved without training through passive stimulation on a time scale of only a few hours (Godde et al. 1996, 2000; Pleger et al. 2001, 2003; Dinse et al. 2003a,b, 2005, 2006; Hodzic et al. 2004; Seitz & Dinse, 2007).
Tactile coactivation is a task-free, passive stimulation protocol independent of cognitive factors like attention or reinforcement. The basic idea behind this design was to coactivate a large number of receptive fields on the tip of the index finger (IF) in a Hebbian manner in order to strengthen their mutual interconnectedness. In a combined assessment measuring the performance of a spatial tactile discrimination task and the cortical reorganization by SEP recording, it was found that the change in discrimination abilities could be predicted by the changes of the SEP-dipole localizations (Pleger et al. 2001; Dinse et al. 2003b) or by changes in the cortical activation as measured as a blood oxygenation level-dependent (BOLD) signal using fMRI (Pleger et al. 2003). In all cases, the amount of perceptual gain resulting from this procedure linearly correlated with the amount of cortical reorganization suggesting a causal relation (Pleger et al. 2001, 2003; Dinse et al. 2003a, 2005).
The aim of this study was to explore whether the passive tactile coactivation also induces changes in SI excitability in parallel with an improvement of discrimination performance. To this end, we combined the tactile coactivation protocol with SEP recordings using paired-pulse stimulation of the median nerve and assessment of tactile performance. Furthermore, we asked whether there are correlations between changes in spatial tactile performance and paired-pulse behaviour analogous to findings in practice-dependent plasticity in the human motor system.
Methods
Subjects
We tested 14 healthy right-handed subjects (5 female, 9 male; mean age 23.7 years). All participants gave their written informed consent and underwent clinical neurological examinations to exclude somatic illness before their participation. The study was approved by the Ethics Committee of the Ruhr-University of Bochum and was performed in accordance with the Declaration of Helsinki.
Psychophysiological tests
The measurement of discrimination thresholds served as a marker of perceptual improvement induced by tactile coactivation. Tactile two-point discrimination on the fingers was assessed using the method of constant stimuli as previously described (Godde et al. 2000; Pleger et al. 2001, 2003; Dinse et al. 2003a,b, 2006; Tegenthoff et al. 2005; Bliem et al. 2007). To overcome problems in the use of two-point measurements associated with hand held probes, we used a specifically designed apparatus that allows a standardized and objective form of testing. To extract thresholds, we obtain psychometric curves based on many repeated stimulus presentations. According to previous studies, test–retest reliability is in the region of 0.9 (Cronbach's α) (Dinse et al. 2006; Ragert et al. submitted).
In brief, seven pairs of rounded needle-probes were used (diameter 200 μm) with separation distances between 0.7 and 2.5 mm in 0.3 mm steps. For control, zero distance was tested with only a single needle-probe. The number of single-needle presentations was 1/8, i.e. eight presentations in one session. Inspection of the data showed that false alarms were zero under each condition. The probes were mounted on a rotatable disc that allowed switching rapidly between distances. To accomplish a rather uniform and standardized type of stimulation, the disc was installed in front of a plate that was movable up and down. The arm and fingers of the subjects were fixed on the plate and the subjects were then asked to move the arm down. The down-movement was arrested by a stopper at a fixed position above the probes. The test finger was held in a hollow containing a small hole through which the distal phalanx of the finger came to touch the probes approximately at the same indentations in each trial. The probes were always presented parallel to the fingertip. Each distance was presented 8 times in randomized order resulting in 64 single trials per session. Subjects were aware that in some trials single needle-probes were presented, but not how often. The subject had to decide immediately after touching the probes if he or she had the sensation of one or two tips by answering ‘one’ or ‘two’. After each session individual discrimination thresholds were calculated. The summed subject's responses (‘one’ for one tip and ‘two’ for two tips) were plotted against the tip distance as a psychometric function and were fitted with a logistic regression method (SPSS v. 10.01). The needle-probe distance yielding 50% correct responses was considered the perceptual threshold as a marker for individual tactile performance. To provide further evidence that a change in discrimination sensitivity is unlikely to be due to changes in the response criterion, in previous studies (Pleger et al. 2001; Bliem et al. submitted) we have calculated the false alarm as well as the hit rates and the discrimination index (d′ value) (Wickens, 2002), which showed an increase of d′ after tactile coactivation on the coactivated finger only.
Paired-pulse stimulation
To assess excitability changes, we applied a paired-pulse protocol consisting of paired electrical stimulation of the median nerve with interstimulus interval (ISI) of 30 and 100 ms in combination with recordings of the somatosensory evoked potentials.
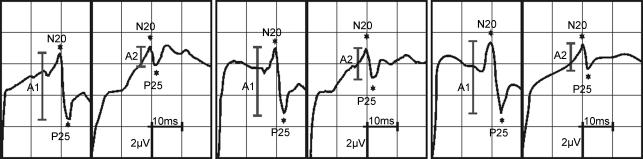
Nerve stimulation was performed with a block electrode placed on the wrist (pulse duration 0.2 ms, repetitive rate of the paired stimuli 2 Hz). Stimulation of the median nerve was chosen to establish a link between SEP recordings and the cortical representation of the right index finger (IF) selected for the tactile coactivation. Subjects had to report a prickling sensation in the thumb, index and middle finger of the stimulated hand to verify correct positioning of the stimulating block electrode. In all participants, the chosen stimulation intensity induced a small muscular twitch in the thenar muscles. During median nerve stimulation and SEP recordings, subjects were seated in a comfortable chair and were instructed to relax but stay awake with closed eyes. SEPs were recorded and stored for offline analysis with conventional Neuropack 8 equipment (Nihon Kohden, bandpass filter 2–2000 Hz, sensitivity 2 μV per division). Paired-pulse SEP recordings were made using a three-electrode array. Two electrodes (C3′ and C4′) were located over the left and right SI, 2 cm posterior to C3 and C4 according to the international 10–20 system (American Electroencephalographic Society, 1994). A reference electrode was placed over the midfront (FZ) position. The electrical potentials were recorded in epochs from 0 to 200 ms after the stimulus. A total number of 200 stimulus-related epochs were recorded for each ISI. Peak-to-peak amplitudes of the cortical N20–P25 response component were analysed and compared before and after tactile coactivation of the right IF. In addition to an analysis of the raw amplitude data, paired-pulse suppression was expressed as a ratio (A2/A1) of the amplitudes of the second (A2) and the first (A1) N20–P25 peak (Fig. 1).
Figure 1. Cortical responses to paired-pulse stimulation of one subject at an ISI of 30 ms before (left), 3 h after (middle) and 24 h after (right) tactile coactivation.
The N20–P25 amplitudes of the first (A1) and second (A2) response are marked by grey bars. Note the reduction of paired pulse inhibition after tactile coactivation, which recovers to baseline 24 h later.
Tactile coactivation
The coactivation protocol was the same as in our previous studies (Godde et al. 1996, 2000, 2003; Pleger et al. 2001, 2003; Hodzic et al. 2004; Dinse et al. 2006; Bliem et al. 2007). Coactivation stimuli were drawn from a Poisson process at different interstimulus intervals between 100 and 3000 ms; average frequency was 1 Hz, pulse duration was 10 ms. Pulses were digitally recorded and were played back on a portable MP3 player allowing unrestrained mobility of the subject during tactile coactivation. To apply tactile coactivation, a device (diameter 20 mm) consisting of two small stimulators was taped of the right index finger and transmitted the tactile stimuli of the coactivation protocol to the skin via two needles (diameter 0.8 mm each) seperated by a distance of 6 mm; mean stimulation duration was 3 h. Subjects were instructed not to pay attention to the stimulation and not to sleep, but to resume their daily routine: some read, some took a walk, some worked on a computer keyboard. In a recent study we showed that focusing or distracting attention has no effect on the amount of coactivation-induced discrimination improvement (B. Bliem & H. R. Dinse, submitted). Amplitude of the probe movement was in the range of 200 μm. To demonstrate the Hebbian nature of coactivation, we have recently shown that stimulating a very small skin area with only a single needle caused neither changes of thresholds nor changes in cortical activation, implying that ‘co’-activation is indeed crucial (Pleger et al. 2003).
Experimental schedule
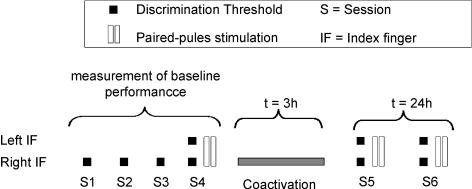
We combined measurement of somatosensory evoked potentials as a marker of cortical excitability and two-point-discrimination thresholds as a marker of tactile perception for the index fingers of both hands. Paired-pulse SEPs with an ISI of 30 and 100 ms were recorded from the left (control) and right median nerve before and after tactile coactivation. Two-point-discrimination of both index fingers was also performed before and after tactile coactivation. Twenty-four hours after termination of tactile coactivation we repeated the SEP and discrimination experiments.
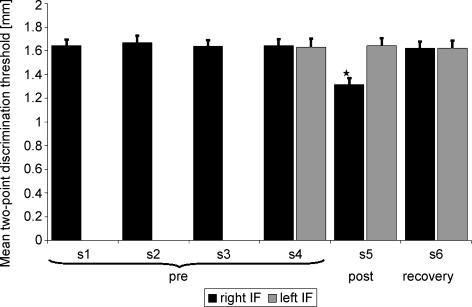
The tactile discrimination thresholds for all subjects were tested on six consecutive sessions (S1 to S4 before tactile coactivation, S5 immediate after, and S6 24 h after tactile coactivation) on the right index finger in order to obtain a stable baseline performance (Fig. 2).
Figure 2. Experimental schedule: measurement of baseline performance of the right IF consisting of four sessions, one session post-tactile coactivation (S5) and one session 24 h after tactile coactivation (S6).
Performance of the left IF was tested in session 4, 5 and 8. SEPs were recorded in session 4, 5 and 6.
Sessions were statistically analysed for stability (repeated measures and univariate analysis of variance (ANOVA) or Student's t test). In the fourth session, the thresholds of the left IF were additionally measured. After the fourth session the coactivation protocol was applied to the right IF for 3 h. Reassessment of tactile discrimination thresholds of the right and left IF (S5) was performed approximately 15 min after termination of tactile coactivation. The left IF was tested to confirm previous findings about the local specificity of tactile coactivation induced changes (Godde et al. 1996, 2000, 2003; Pleger et al. 2001, 2003; Ragert et al. 2003; Hodzic et al. 2004; Dinse et al. 2006; Bliem et al. 2007).
After 3 h of tactile coactivation, SEP measurements were repeated in order to study possible intracortical excitability changes in terms of changes of paired-pulse behaviour. During tactile coactivation application, electrodes for median nerve stimulation were removed; however, exact electrode positions were marked on the wrist before removal. In a recovery measurement we repeated the testing of the discrimination threshold and the paired-pulse stimulation 24 h after the termination of the tactile coactivation (S6) on both test (right) and control (left) IF.
Results
Paired-pulse stimulation
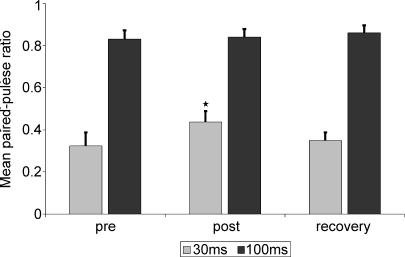
Peak-to-peak amplitudes of the N20/P25 response component generated in S1 were measured and compared before and after tactile coactivation. Paired pulse behaviour was expressed as a ratio (A2/A1) of the second (A2) to the first response (A1). The influence of tactile coactivation on paired-pulse behaviour at an ISI of 30 ms after stimulation of the right median nerve as assessed by one-way ANOVA for repeated measurements (with inner subject factor time) yielded a significant effect with P < 0.05 at F2,24= 3.673. In the precondition, at an ISI of 30 ms the average paired-pulse ratio after right median nerve stimulation was 0.323 ± 0.06. Reassessment of the paired-pulse behaviour 3 h after tactile coactivation application revealed a suppression of the paired-pulse inhibition recorded in the contralateral SI. The A2/A1 ratio was significantly increased to 0.437 ± 0.05 (P= 0.006, paired t test). Twenty-four hours after termination of tactile coactivation the effect was completely reversed. The paired-pulse ratio significantly decreased to 0.352 ± 0.04 (P= 0.033, paired t test) and was statistically not different from the precoactivation measurement (P= 0.814) (see Fig. 3).
Figure 3. Mean paired-pulse ratio at ISIs of 30 (grey bars) and 100 ms (black bars) of the test side (right median nerve stimulation).
Significantly reduced suppression of paired-pulse ratio after tactile coactivation (post) at ISIs of 30 ms; the effect recovered 24 h after tactile coactivation (recovery). There was no significant influence of coactivation on the paired-pulse ratio at ISIs of 100 ms.
As assessed by one-way ANOVA for repeated measurements (with inner subject factor time), we found no significant influence of tactile coactivation on paired-pulse ratios at ISIs of 100 ms (P= 0.421 at F2,24= 0.896).
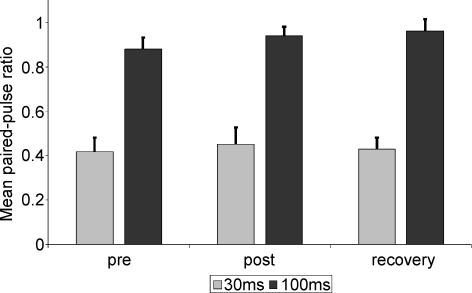
As a control, we also assessed cortical excitability in the right hemisphere after stimulation of the left median nerve. We could not find a significant influence on paired-pulse ratio at either ISI 30 ms (one-way ANOVA for repeated measurements: P= 0.726 at F2,24= 0.324) and 100 ms (P= 0.346 at F2,24= 1.111; see Fig. 4).
Figure 4. Mean paired-pulse ratio at ISIs of 30 (grey bars) and 100 ms (black bars) of the control side (left median nerve stimulation).
We found no significant effect of the contralateral tactile coactivation on the paired-pulse behaviour of the left control side.
Additionally the latencies of the first and the second response peak were analysed. In no condition did we found a significant effect of tactile coactivation on latencies of the second response according to one-way ANOVA for repeated measurements.
Psychophysiological tests
In the psychophysical tests of the right index finger during the initial training period S1–S4, all subjects achieved a stable baseline performance (one-way ANOVA for repeated measurements: F3,39= 1.1, P= 0.361) indicating no differences in performance. The mean discrimination threshold of sessions 1–4 (before tactile coactivation) was 1.65 mm ± 0.05 (s.e.m.). However, average discrimination threshold tested on the right index finger in session S5 after tactile coactivation yielded a significant effect (P < 0.001 at F2,26= 66.846 as assessed by one-way ANOVA for repeated measurements, with inner subject factor time). The discrimination thresholds 3 h after tactile coactivation were significantly lowered to 1.32 mm ± 0.15 (P < 0.001, paired t test). Twenty-four hours after termination of tactile coactivation the improvement was abolished (mean discrimination threshold in session 6 was 1.64 ± 0.05 mm, P < 0.001, paired t test, S5–S6). As shown in Fig. 5 we found no significant difference between the mean precoactivation threshold and the threshold in the recovery measurement (P= 0.734, t test).
Figure 5. Mean two-point discrimination thresholds in mm in the different sessions.
Thresholds of right the index finger (IF) are shown in grey, thresholds of the left index finger in black. Thresholds of the right index finger were significantly lower after coactivation (post) in session 5. In the recovery measurement (recovery) 24 h after coactivation the threshold was back to baseline. There was no influence of coactivation on the test side (left IF) which was not coactivated.
In contrast, the left non-coactivated IF remained unchanged as assessed in a one-way ANOVA with P= 0.732 at F2,26= 0.315.
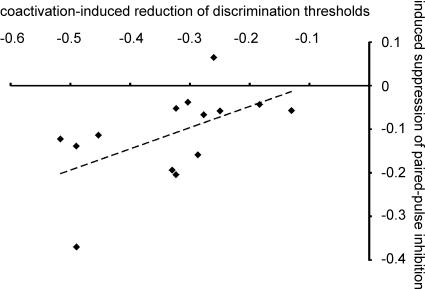
In order to study the relation between coactivation-induced gain in discrimination performance and the induced suppression of paired-pulse inhibition, we performed a linear bivariate correlation analysis (Pearson). This analysis showed a close link between individual gain in performance and the degree of excitability changes (r=−0.556; P= 0.038; n= 14). Little gain in spatial discrimination abilities was associated with small changes in excitability. On the other hand, those subjects who showed a large enhancement of cortical excitability also showed the largest improvement after tactile coactivation (see Fig. 6).
Figure 6.
Linear bivariate correlation analysis of coactivation-induced reduction of discrimination thresholds (x-axis) and the induced suppression of paired pulse inhibition (y-axis) with linear regression
Discussion
The aim of this study was to show that tactile coactivation increases cortical excitability in the representation of the coactivated finger in SI, as demonstrated by SEP recordings using paired-pulse median nerve stimulation. In accordance with previous studies (Godde et al. 1996, 2000, 2003; Pleger et al. 2001, 2003; Dinse et al. 2003a,b, 2005, 2006; Ragert et al. 2003; Hodzic et al. 2004; Bliem et al. 2007) we confirmed a significant improvement of spatial two-point-discrimination performance within a coactivation time of 3 h. In line with previous studies, the recovery measurement 24 h after tactile coactivation showed that the improvement of tactile discrimination was completely reversible (Godde et al. 1996, 2000, 2003; Pleger et al. 2001, 2003; Dinse et al. 2003a,b, 2005, 2006; Ragert et al. 2003; Hodzic et al. 2004; Bliem et al. 2007). In addition, as previously described, the coactivation-induced improvement of discrimination performance was restricted to the stimulated hand with no effects on the opposite left side, indicating a local specificity of the coactivation-induced improvement (Godde et al. 1996, 2000, 2003; Pleger et al. 2001, 2003; Dinse et al. 2003a,b, 2005; 2006; Ragert et al. 2003; Hodzic et al. 2004; Bliem et al. 2007). Evidence has accumulated from fMRI and SEP measurements that the underlying neural changes are occurring within an early representation that must contain well-ordered topographic maps, like in somatosensory S1, to allow for this spatial selectivity (Pleger et al. 2001, 2003; Dinse et al. 2003b). The important role of the somatosensory cortex for mediating plastic changes was discussed in previous studies and is supported by electrophysiological data (Recanzone et al. 1992; Godde et al. 1996).
At a cellular level, the duration of plastic changes following long-term potentiation (LTP) protocols varies between minutes and days, implying the involvement of multiple mechanisms and mediating pathways (Abraham, 2003). One key variable that determines how long LTP in the dentate gyrus will persist is the number of high-frequency trains delivered. Repetition of trains on one day, or across consecutive days, increases the persistence of LTP. There is agreement that short-lasting forms of LTP are based on post-translational modifications (LTP form 1), while longer-lasting forms (LTP forms 2 and 3) require dendritic protein synthesis and gene transcription (Raymond, 2007). In fact, applying coactivation on three consecutive days had no effect on the magnitude of changes, but on the duration. Only on day 5 did the thresholds return to the original conditions (Godde et al. 2000). Coactivating all finger tips of a hand instead of a single finger resulted in much stronger and longer-lasting effects (Kalisch et al. 2007). Using high-frequency tactile stimuli mimicking LTP-like stimulation for only 20 min evoked tactile acuity improvements comparable in magnitude, which recovered to baseline only after 48 h (Ragert et al. submitted). Conceivably, combining repeated applications with other forms of coactivation protocols will lead to higher persistence of the evoked improvement.
In systematic studies of paired-pulse behaviour following stimulation of the median nerve in a healthy population (Shagass & Schwartz, 1964; Romani et al. 1995; Hoshiyama & Kakigi, 2001), a significant suppression of the second response has been shown for short ISIs of 20–40 ms, while at ISIs higher than 100 ms no significant suppression has been observed. In addition, no significant changes of the latency of the second response as a function of the respective ISI were found (Schwartz & Shagass, 1964; Hoshiyama & Kakigi, 2001). These findings are in accordance with our data using ISIs of 30 and 100 ms in the precoactivation baseline measurements. However, after tactile coactivation, we found a significant reduction of paired pulse suppression in the SEPs of the right index finger recorded in left SI. The observed changes were restricted to ISIs of 30 ms and recovered to baseline 24 after termination of tactile coactivation.
In previous studies of paired-pulse behaviour of somatosensory cortex, the mean latencies after which the second response occurred were reported to be lengthened at short but shortened at long ISIs (Allison, 1962). Shagass & Schwartz (1964) presented similar findings in a study using a double pulse stimulation of a peripheral nerve. We could not find a significant influence of tactile coactivation on the latencies of the second responses.
During the last years, paired-pulse stimulation has become a common tool to investigate cortical excitability and cortical plasticity to demonstrate intracortical inhibition or facilitation and changes in the balance of both (Kujirai et al. 1993; Ziemann et al. 1996). For the motor system, Kujirai et al. (1993) and Ziemann et al. (1996) proposed that the effect of suppression at shorter ISIs is produced by activation of the intracortical GABAergic inhibitory system. The hypothesis of GABAergic influence was supported in an experimental study on differences between I-waves using paired pulse stimulation of the human motor cortex (Hanajima et al. 1998). Excitability changes have been discussed in terms of an enhancement of intracortical glutamergic excitation (Castro-Alamancos & Connors, 1996) or a reduction of intracortical GABAergic inhibition (Hess et al. 1996), or a mixture of both. Dinse et al. (2003) showed that NMDA receptor activation is required to induce plasticity changes on the coactivated SI. The synchronized activation of NMDA receptors may cause the described long term potention (LTP)-like changes of cortical excitability seen in the experiment.
The fact that the second response to two stimuli given in short succession is strongly suppressed has been denoted as short-term plasticity to describe changes of neural behaviour resulting from prior activity (Zucker, 1989; Zucker & Regehr, 2002). Presynaptic depletion of releasable vesicles, postsynaptic receptor desensitization or other presynaptic mechanisms depressing vesicle release (Bellingham & Walmsley, 1999) seem to be involved. Neural refractoriness may play a role only at very short interstimulus intervals, < 2 ms (Ziemann et al. 1996; Dobrunz & Stevens, 1997; Bellingham & Walmsley, 1999; Hoshiyama & Kakigi, 2003). Hoshiyama & Kakigi (2003) investigated the role of depletion of synaptic transmitters during high frequency stimulation of the median nerve. They recorded somatosensory evoked cortical magnetic fields (SEF) using stimulations in trains at ISIs of 10, 20 and 30 ms. An attenuation of the components of the SEF was recognized after the second stimulus, but there was no significant attenuation with the third or later stimuli. From this observation they concluded that depletion was unlikely to be responsible for the attenuation of the SEF components during repetitive stimulations.
Analysis of electric sources following multichannel SEP recordings in human subjects after application of a similar tactile coactivation protocol revealed a significant shift in the localization and strength of the N20 dipole of the coactivated index finger indicating a coactivation-induced enlargement in primary somatosensory cortex (Pleger et al. 2001; Dinse et al. 2003b). These changes in dipole localization were significantly correlated to the two-point discrimination abilities. Similarly, in a study using fMRI measurements before and after tactile coactivation, a correlation between the increase of the strength of the BOLD signal and the perceptual improvement was found (Pleger et al. 2003). These results implied that the outcome of perceptual learning can be predicted by changes in activation of the finger representation of somatosensory cortex.
In rats, coactivation-induced effects included an enlargement of receptive fields at the level of SI accompanied by an increase of receptive field overlap and an enlargement of the representational maps implying a recruitment of processing resources (Godde et al. 1996; Dinse et al. 1997; Dinse & Merzenich, 2002). Our findings about the altered paired pulse behaviour after tactile coactivation indicate that in addition to the described changes, cortical excitability is also modified by tactile coactivation. Notably, we report that the improvement of spatial two-point discrimination ability and the individual reduction of paired-pulse suppression showed also a significant correlation after tactile coactivation. These results indicate that perceptual gain can be predicted not only from cortical map size, but also from the level of cortical excitability as assessed from paired-pulse stimulation techniques.
An increase in neural excitability is discussed as a possible mechanism underlying plastic changes. For example, tasked-induced MEP facilitation positively correlated with improvements in performance (Muellbacher et al. 2001; Ziemann et al. 2001) and was speculated to reflect the early consolidation stage of motor skills acquisition in primary motor cortex (Muellbacher et al. 2002). Garry et al. (2004) found a strong relationship between the changes in excitability measured by MEP and within-session improvement in performance of a motor practice. Changes in cortical facilitation and inhibition could be demonstrated in healthy subjects performing different learning tasks (Liepert et al. 1998) as well as in patients with various neurological disorders like migraine, poststroke or dystonia (Cohen et al. 1998; Ziemann et al. 1998; Liepert et al. 2000; Gerwig et al. 2005).
Accordingly, the analysis of cortical excitability may help us to understand the nature of learning processes and neurological disorders. Further studies are required to find out in how far these changes can be influenced by physical and pharmacological methods to establish new strategies of therapy.
References
- Abraham WC. How long will long-term potentiation last? Philos Trans R Soc Lond B Biol Sci. 2003;358:735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T. Recovery functions of somatosensory evoked responses in man. Electroencephalogr Clin Neurophysiol. 1962;14:331–343. doi: 10.1016/0013-4694(62)90110-4. [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society. Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–113. [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bliem B, Frombach E, Ragert P, Knossalla F, Woitalla D, Tegenthoff M, Dinse HR. Dopaminergic influences on changes in human tactile acuity induced by tactile coactivation. Exp Brain Res. 2007;181:131–137. doi: 10.1007/s00221-007-0912-5. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci U S A. 1996;93:1335–1339. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Ziemann U, Chen R, Classen J, Hallett M, Gerloff C, Butefisch C. Studies of neuroplasticity with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:305–324. doi: 10.1097/00004691-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Godde B, Hilger T, Haupt SS, Spengler F, Zepka R. Short-term functional plasticity of cortical and thalamic sensory representations and its implication for information processing. Adv Neurol. 1997;73:159–178. [PubMed] [Google Scholar]
- Dinse HR, Kalisch T, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Improving human haptic performance in normal and impaired human populations through unattended activation-based learning. Transaction Appl Perc. 2005;2:71–88. [Google Scholar]
- Dinse HR, Kleibel N, Kalisch T, Ragert P, Wilimzig C, Tegenthoff M. Tactile coactivation resets age-related decline of human tactile discrimination. Ann Neurol. 2006;60:88–94. doi: 10.1002/ana.20862. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Merzenich MM. Adaptation of inputs in the somatosensory system. In: Fahle M, Poggio T, editors. Perceptual Learning. Cambridge, MA, USA: MIT Press; 2002. pp. 19–42. [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. GABAergic mechanisms gate tactile discrimination learning. Neuroreport. 2003a;14:1747–1751. doi: 10.1097/00001756-200309150-00018. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003b;301:91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Garry MI, Kamen G, Nordstrom MA. Hemispheric differences in the relationship between corticomotor excitability changes following a fine-motor task and motor learning. J Neurophysiol. 2004;91:1570–1578. doi: 10.1152/jn.00595.2003. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Niehaus L, Kastrup O, Stude P, Diener HC. Visual cortex excitability in migraine evaluated by single and paired magnetic stimuli. Headache. 2005;45:1394–1399. doi: 10.1111/j.1526-4610.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Godde B, Ehrhardt J, Braun C. Behavioral significance of input-dependent plasticity of human somatosensory cortex. Neuroreport. 2003;14:543–546. doi: 10.1097/00001756-200303240-00002. [DOI] [PubMed] [Google Scholar]
- Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport. 1996;8:281–285. doi: 10.1097/00001756-199612200-00056. [DOI] [PubMed] [Google Scholar]
- Godde B, Stauffenberg B, Spengler F, Dinse HR. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci. 2000;20:1597–1604. doi: 10.1523/JNEUROSCI.20-04-01597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–1778. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hodzic A, Veit R, Karim AA, Erb M, Godde B. Improvement and decline in tactile discrimination behavior after cortical plasticity induced by passive tactile coactivation. J Neurosci. 2004;24:442–446. doi: 10.1523/JNEUROSCI.3731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R. Two evoked responses with different recovery functions in the primary somatosensory cortex in humans. Clin Neurophysiol. 2001;112:1334–1342. doi: 10.1016/s1388-2457(01)00564-8. [DOI] [PubMed] [Google Scholar]
- Hoshiyama M, Kakigi R. Changes in somatosensory evoked responses by repetition of the median nerve stimulation. Clin Neurophysiol. 2003;114:2251–2257. doi: 10.1016/s1388-2457(03)00285-2. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Tegenthoff M, Dinse HR. Differential effects of synchronous and asynchronous multifinger coactivation on human tactile performance. BMC Neurosci. 2007;8:58. doi: 10.1186/1471-2202-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann F, Funk T, Vesper J, Siedenberg R, Curio G. Double-pulse stimulation dissociates intrathalamic and cortical high-frequency (>400Hz) SEP components in man. Neuroreport. 2000;11:1295–1299. doi: 10.1097/00001756-200004270-00030. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Hanajima R, Kowa H, Motoyoshi Y, Ashida H, Kamakura K, Motoyoshi K, Ugawa Y. Somatosensory evoked potential recovery in myotonic dystrophy. Clin Neurophysiol. 2001;112:793–799. doi: 10.1016/s1388-2457(01)00512-0. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Nitta T, Takahashi K. Recovery functions of somatosensory evoked potentials in parkinsonian patients. J Neurol Sci. 1992;108:24–31. doi: 10.1016/0022-510x(92)90183-l. [DOI] [PubMed] [Google Scholar]
- Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci U S A. 2001;98:12255–12260. doi: 10.1073/pnas.191176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron. 2003;40:643–653. doi: 10.1016/s0896-6273(03)00677-9. [DOI] [PubMed] [Google Scholar]
- Ragert P, Becker M, Tegenthoff M, Pleger B, Dinse HR. Sustained increase of somatosensory cortex excitability by 5 Hz repetitive transcranial magnetic stimulation studied by paired median nerve stimulation in humans. Neurosci Lett. 2004;356:91–94. doi: 10.1016/j.neulet.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, Tegenthoff M. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci Lett. 2003;348:105–108. doi: 10.1016/s0304-3940(03)00745-6. [DOI] [PubMed] [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the ‘long’ in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Romani A, Bergamaschi R, Versino M, Callieco R, Calabrese G, Cosi V. Recovery functions of early cortical median nerve SSEP components: normative data. Electroencephalogr Clin Neurophysiol. 1995;96:475–478. doi: 10.1016/0168-5597(95)00145-i. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Shagass C. Recovery functions of human somatosensory and visual evoked potentials. Ann N Y Acad Sci. 1964;112:510–525. doi: 10.1111/j.1749-6632.1964.tb26765.x. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Dinse HR. A common framework for perceptual learning. Curr Opin Neurobiol. 2007;17:148–153. doi: 10.1016/j.conb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Shagass C, Schwartz M. Recovery functions of somatosensory peripheral nerve and cerebral evoked responses in man. Electroencephalogr Clin Neurophysiol. 1964;17:126–135. doi: 10.1016/0013-4694(64)90144-0. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/s0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Sparing R, Dambeck N, Stock K, Meister IG, Huetter D, Boroojerdi B. Investigation of the primary visual cortex using short-interval paired-pulse transcranial magnetic stimulation (TMS) Neurosci Lett. 2005;382:312–316. doi: 10.1016/j.neulet.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Forster AF, Nicolas V, Dinse HR. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Genba-Shimizu K, Kanazawa I. Somatosensory evoked potential recovery (SEP-R) in various neurological disorders. Electroencephalogr Clin Neurophysiol. 1996;100:62–67. doi: 10.1016/0168-5597(95)00195-6. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens TD. Elementary Signal Detection Theory. Oxford: Oxford University Press; 2002. [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]