Abstract
Endocannabinoids are released from postsynaptic neurons, activate presynaptic cannabinoid receptors and cause various forms of short-term and long-term synaptic plasticity throughout the brain. Using hippocampal and cerebellar neurons, we have revealed that endocannabinoid release can be induced through two different pathways. One is independent of phospholipase Cβ (PLCβ) and driven by Ca2+ elevation alone (Ca2+-driven endocannabinoid release, CaER), and the other is PLCβ-dependent and driven by activation of Gq/11-coupled receptors (receptor-driven endocannabinoid release, RER). CaER is induced by activation of either voltage-gated Ca2+ channels or NMDA receptors. RER is functional even at resting Ca2+ levels (basal RER), but markedly enhanced by a small Ca2+ elevation (Ca2+-assisted RER). In Ca2+-assisted RER, PLCβ serves as a coincidence detector of receptor activation and Ca2+ elevation. We have also demonstrated that Ca2+-assisted RER is essential for the endocannabinoid release triggered by synaptic activity. Our anatomical data show that a set of receptors and enzymes required for RER are well organized so that the excitatory input can trigger RER effectively. Certain forms of spike-timing-dependent plasticity (STDP) are reported to depend on endocannabinoid signalling. The NMDA receptor and PLCβ might play key roles in the endocannabinoid-dependent forms of STDP as coincidence detectors with different timing dependences.
Our knowledge of the endocannabinoid system has been rapidly expanded in the last several years. In 2001, it was first demonstrated that endocannabinoids work as a retrograde messenger in the CNS and contribute to activity-dependent modulation of synaptic transmission (Kreitzer & Regehr, 2001; Maejima et al. 2001; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001). Since then, the endocannabinoid-mediated retrograde modulation has been reported in various brain regions (Chevaleyre et al. 2006; Hashimotodani et al. 2007b). It is now known that the endocannabinoid signal contributes to many forms of short-term and long-term synaptic plasticity throughout the brain (Gerdeman & Lovinger, 2003; Chevaleyre et al. 2006; Hashimotodani et al. 2007b). In this report, we focus on molecular mechanisms underlying endocannabinoid release induced by synaptic activities.
Induction of endocannabinoid release
Endocannabinoids are produced on demand and released from postsynaptic neurons. The released endocannabinoids travel backward across the synapse, activate presynaptic CB1 cannabinoid receptors (CB1Rs) and cause suppression of neurotransmitter release. Endocannabinoid release can be induced through two different pathways. One is driven by Ca2+ elevation alone (Ca2+-driven endocannabinoid release, CaER). The other is driven by activation of Gq/11-coupled receptors (receptor-driven endocannabinoid release, RER).
CaER contributes to depolarization-induced transient suppression of synaptic transmission, which is known as DSI for inhibitory synapse (Llano et al. 1991; Pitler & Alger, 1992) and DSE for excitatory synapse (Kreitzer & Regehr, 2001). DSI/DSE is induced postsynaptically by strong depolarization (e.g. to 0 mV for 5 s), and expressed presynaptically as a transient reduction of transmitter release. Therefore, involvement of some retrograde signal was proposed. In 2001, it was revealed that endocannabinoids serve as a retrograde messenger in DSI/DSE (Kreitzer & Regehr, 2001; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001). Induction of ‘pure’ DSI/DSE, which is induced by depolarization alone without any simultaneous activation of Gq/11-coupled receptors, requires a large increase in intracellular Ca2+ concentration (to a micromolar range) (Brenowitz & Regehr, 2003; Maejima et al. 2005), which is primarily caused by Ca2+ influx through voltage-gated Ca2+ channels. Later studies demonstrated that the ‘pure’ DSI/DSE is phospholipase Cβ (PLCβ) independent (Hashimotodani et al. 2005; Maejima et al. 2005). In addition to depolarization, activation of NMDA-type glutamate receptors also induces a transient suppression of synaptic transmission in a Ca2+- and CB1R-dependent manner (Ohno-Shosaku et al. 2007). We showed that this suppression is caused by Ca2+ inflow into postsynaptic neurons through NMDA receptors and not through voltage-gated Ca2+ channels activated secondarily by NMDA receptor-induced local depolarization (Ohno-Shosaku et al. 2007). Therefore, NMDA receptors can take the place of voltage-gated Ca2+ channels for CaER. Molecular mechanisms of CaER still remain to be elucidated.
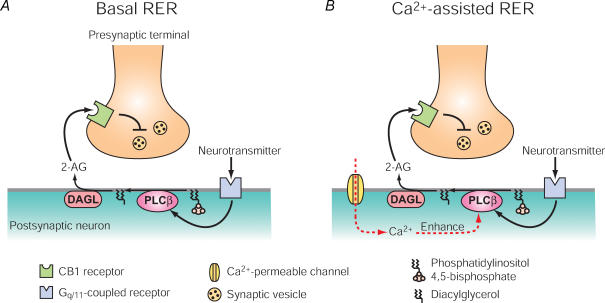
RER was first reported in a study showing that postsynaptic activation of type 1 metabotropic glutamate receptor (mGluR1) induced CB1R-dependent retrograde suppression of synaptic transmission in the cerebellum (Maejima et al. 2001). Since this discovery, RER was found to be induced in various brain regions by activation of Gq/11-coupled receptors, such as group I mGluRs (mGluR1/5) (Varma et al. 2001; Ohno-Shosaku et al. 2002; Galante & Diana, 2004; Kushmerick et al. 2004; Narushima et al. 2006), M1 and/or M3 muscarinic acetylcholine receptors (Kim et al. 2002; Fukudome et al. 2004; Narushima et al. 2007; Newman et al. 2007), orexin receptor (Haj-Dahmane & Shen, 2005) and oxytocin receptor (Oliet et al. 2007). Molecular mechanisms of RER have been elucidated by using pharmacological and genetic tools. Figure 1 shows a current model for RER. Activation of Gq/11-coupled receptors stimulates PLCβ and yields diacylglycerol (DAG). DAG is then converted by DAG lipase (DAGL) to 2-arachidonoylglycerol (2-AG), one of the two major endocannabinoids. This model is supported by studies showing prevention of RER by pharmacological inhibition of PLC or DAGL (Melis et al. 2004; Haj-Dahmane & Shen, 2005; Maejima et al. 2005; Safo & Regehr, 2005; Hashimotodani et al. 2007c; Newman et al. 2007; Straiker & Mackie, 2007; Uchigashima et al. 2007) and absence of RER in PLCβ1- or PLCβ4-deficient mice (Hashimotodani et al. 2005; Maejima et al. 2005). RER was originally thought to be Ca2+ independent, because RER was functional even under the conditions that prevent Ca2+ elevation (‘basal RER’, Fig. 1A) (Maejima et al. 2001). However, our later studies have revealed that RER is highly sensitive to the Ca2+ level of the postsynaptic neuron and is markedly enhanced by a small Ca2+ elevation to a physiological (submicromolar) range (‘Ca2+-assisted RER’, Fig. 1B) (Hashimotodani et al. 2005; Maejima et al. 2005). We have also demonstrated that PLCβ plays a key role as a Ca2+ sensor for ‘Ca2+-assisted RER’ (Hashimotodani et al. 2005; Maejima et al. 2005), which is briefly described in the following section.
Figure 1. Models of basal receptor-driven endocannabinoid release (RER) and Ca2+-assisted RER.
A, at basal Ca2+ levels, strong activation of Gq/11-coupled receptors (e.g. mGluR1/5, M1/M3) stimulates PLCβ through Gαq/11. PLCβ hydrolyses phosphatidylinositol 4,5-bisphosphate into diacylglycerol and inositol 1,4,5-trisphosphate. Diacylglycerol is then hydrolysed to 2-arachidonoylglycerol (2-AG) by diacylglycerol lipase (DAGL). 2-AG is released and activates presynaptic CB1 cannabinoid receptors, leading to the suppression of neurotransmitter release. B, when activation of Gq/11-coupled receptors coincides with Ca2+ increase through Ca2+-permeable channels (e.g. voltage-gated Ca2+ channel, NMDA receptor), PLCβ activation is enhanced. In this condition, 2-AG production can be induced even by weak activation of Gq/11-coupled receptors, which is subthreshold for basal RER.
Mechanisms of Ca2+-assisted RER
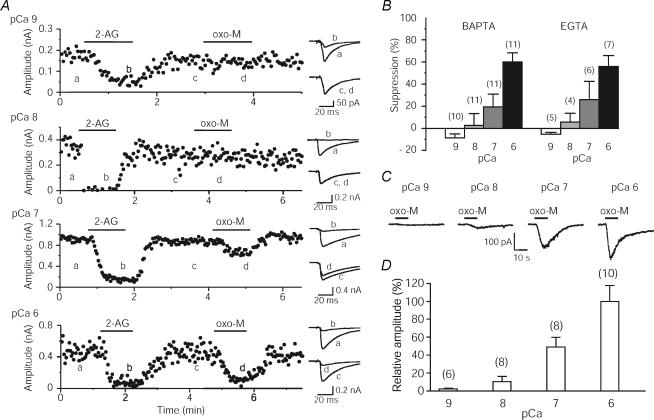
To determine the role of PLCβ in endocannabinoid release, we used cultured hippocampal neurons and monitored the endocannabinoid release by measuring cannabinoid-sensitive synaptic currents (Hashimotodani et al. 2005). We found that RER mediated by muscarinic receptors or group I mGluRs (I-mGluRs) was absent in mutant mice lacking PLCβ1, which is the predominant isoform in the forebrain including hippocampus (Watanabe et al. 1998) among the four isoforms of the PLCβ family (PLCβ1–4). This PLCβ1-dependent RER was augmented by increasing the postsynaptic Ca2+ concentration. We measured PLCβ1 activity in intact neurons by using the exogenous TRPC6 channel, a member of the canonical transient receptor potential family. Because the TRPC6 channel is activated by intracellular DAG, we used it as a biosensor for the PLC product DAG. Large inward currents were induced by activation of the Gq/11-coupled receptors in hippocampal neurons expressing TRPC6 channels, but not in control neurons. These currents were also negligible in TRPC6-expressing neurons from the PLCβ1-deficient mice, indicating that the receptor-driven TRPC6 current reflects PLCβ1 activity. By measuring the TRPC6 current at different Ca2+ levels, we found that the receptor-driven PLCβ1 activation showed a similar Ca2+ dependence to that of the receptor-driven endocannabinoid release (Fig. 2). These results indicate that PLCβ1 is most effectively activated when Ca2+ elevation and Gq/11-coupled receptor activation coincide, and contributes to ‘Ca2+-assisted RER’.
Figure 2. Intracellular Ca2+ dependence of RER and PLCβ1-mediated TRPC6 activation driven by muscarinic activation.
A, simultaneous whole-cell voltage clamp recordings were made from neuron pairs in rat hippocampal culture. The presynaptic neurons were stimulated and cannabinoid-sensitive IPSCs were recorded from the postsynaptic neurons dialysed with the solutions containing 30 mm BAPTA with the indicated pCa levels. Examples representing the effects of 2-AG (30 nm) and oxotremorine-M (oxo-M, 0.3 μm) at the four different pCa levels. IPSC traces acquired at the indicated time points are shown on the right. B, averaged data for oxo-M-induced suppression of IPSC at four different pCa levels buffered with 10–30 mm BAPTA or 10 mm EGTA. C and D, rat cultured hippocampal neurons expressing exogenous TRPC6 were dialysed with 10 mm BAPTA-containing solutions with the indicated pCa levels. Sample traces (C) and averaged amplitudes (D) of oxo-M (3 μm)-induced currents at the four different pCa levels. In the summary bar graphs (D), the amplitudes were normalized to the values for pCa 6. (Modified from Hashimotodani et al. 2005, with permission.)
We drew a similar conclusion from a study on mouse cerebellar slices (Maejima et al. 2005). We made whole-cell recordings from Purkinje cells (PCs) and examined their excitatory synapses arising from climbing fibres (CFs) and parallel fibres (PFs). We sampled PCs from the rostral half of the cerebellum where PLCβ4 is the predominant isoform in PCs (Watanabe et al. 1998). We found that the mGluR1-driven endocannabinoid release depended on postsynaptic Ca2+ levels within a physiological range, and that the enhancement of endocannabinoid release by the combination of depolarization (Ca2+ elevation) and mGluR1 activation was abolished in PLCβ4-deficient mice. These results indicate that PLCβ4 plays a key role in Ca2+-assisted RER in the cerebellum.
It has been reported that endocannabinoid release is markedly facilitated when depolarization and Gq/11-coupled receptor activation is combined in various brain regions, including the hippocampus (Varma et al. 2001; Kim et al. 2002; Ohno-Shosaku et al. 2002, 2003), neocortex (Fortin et al. 2004), cerebellum (Maejima et al. 2005) and striatum (Narushima et al. 2007). This phenomenon has often been described as ‘enhancement of DSI/DSE by receptor activation’, which gives a false impression that the ‘pure’ DSI/DSE mechanism (CaER) is enhanced by receptor activation. However, there is no experimental evidence for the enhancement of CaER by receptor activation. Our results indicate rather that the enhanced DSI/DSE includes two distinct components, namely ‘pure’ DSI/DSE caused by CaER and the component caused by Ca2+-assisted RER (Maejima et al. 2005). The ‘enhancement of DSI/DSE by receptor activation’ can be explained largely, if not totally, by the addition of Ca2+-assisted RER to CaER.
Physiological significance of Ca2+-assisted RER
Using mouse cerebellar slices, we also examined the role of Ca2+-assisted RER in the endocannabinoid release triggered by synaptic activity. Repetitive stimulation of PFs (10 pulses at 100 Hz) induced a transient suppression of transmission at PF–PC synapses. This suppression was blocked by a selective mGluR1 antagonist, CB1R antagonists, and postsynaptic injection of 30 mm BAPTA. The suppression was also abolished in the slices prepared from PLCβ4-deficient mice. These results indicate that Ca2+-assisted RER involving the mGluR1–PLCβ4 cascade is essential for effective endocannabinoid release by PF activity. Furthermore, we demonstrated by biochemical analysis that combined weak mGluR1 activation and mild depolarization in PCs effectively produces 2-AG, whereas either stimulus alone does not produce detectable 2-AG. Several other studies also reported the data suggesting a major contribution of Ca2+-assisted RER to short-term retrograde suppression triggered by synaptic activities (Brown et al. 2003; Melis et al. 2004; Brenowitz & Regehr, 2005).
Ca2+-assisted RER might also play a role in integrating different synaptic inputs. We have reported recently that DSI is constantly enhanced by tonic activation of the M1 muscarinic receptor in the striatum (Narushima et al. 2007). In the striatal medium spiny (MS) neurons, the M1 receptor is tonically activated by ambient acetylcholine that is released from tonically active cholinergic interneurons. The tonic activation of M1 receptor–PLCβ cascade, which is insufficient to induce endocannabinoid release, makes the neurons ready to respond to upcoming depolarization, which will be caused by glutamatergic input under physiological conditions, and to release endocannabinoids. Therefore, it is expected that MS neurons can integrate glutamatergic and cholinergic inputs and regulate the function of cannabinoid-sensitive presynaptic terminals through Ca2+-assisted RER.
Arrangement of endocannabinoid signalling molecules
Anatomical studies have elucidated the subcellular distributions of endocannabinoid signalling molecules including mGluR1α, mGluR5, M1 muscarinic receptor, Gαq/11, PLCβs, DAGLα, monoacylglycerol lipase (MAGL) and CB1R. In general, the elements involved in endocannabinoid production are co-localized on postsynaptic sites (Lujan et al. 1996; Tanaka et al. 2000; Yoshida et al. 2006; Narushima et al. 2007; Uchigashima et al. 2007), whereas CB1R, and MAGL which degrades 2-AG and terminates the retrograde endocannabinoid signal (Dinh et al. 2002; Hashimotodani et al. 2007c), are located on presynaptic terminals (Dinh et al. 2002; Gulyas et al. 2004). The fine arrangement of these elements is, however, unique to each brain region.
In hippocampal pyramidal neurons, mGluR5, Gαq/11 and DAGLα are distributed in postsynaptic spines (Lujan et al. 1996; Tanaka et al. 2000; Yoshida et al. 2006). CB1R is expressed at low levels on excitatory presynaptic terminals that face the DGLα-expressing spine heads (Katona et al. 2006; Yoshida et al. 2006), and expressed at high levels on cholecystokinin-positive inhibitory terminals that face the somatodendritic surface lacking DAGLα. In cerebellar PCs, mGluR1α, Gαq/11 and PLCβ4 are all concentrated at the perisynaptic region of spine heads (Baude et al. 1993; Tanaka et al. 2000; Nakamura et al. 2004). However, DAGLα is not found on spine heads and necks, and is highly concentrated at the base of spine neck and somatodendritic surface (Yoshida et al. 2006). Therefore, DAG should travel from spine heads to the bases of spine necks to be converted to 2-AG. This distance between PLCβ4 and DAGLα might be physiologically significant, considering that DAG also functions as an activator for PKCγ, which plays a crucial role in motor coordination and synapse elimination in PCs (Chen et al. 1995; Kano et al. 1995). CB1R is accumulated at the perisynaptic region of PF terminals and inhibitory presynaptic terminals (Kawamura et al. 2006; Yoshida et al. 2006). In striatal MS neurons, mGluR5 and DAGLα are widely expressed on the extrasynaptic somatodendritic surface, and their densities are highest in spines (Uchigashima et al. 2007). In contrast to mGluR5, the M1 receptor is sparse in spines and shows widespread somatodendritic distribution (Narushima et al. 2007). CB1R is expressed at high levels on inhibitory presynaptic terminals of parvalbumin-positive interneurons and MS neurons and at low levels on excitatory corticostriatal afferents (Uchigashima et al. 2007).
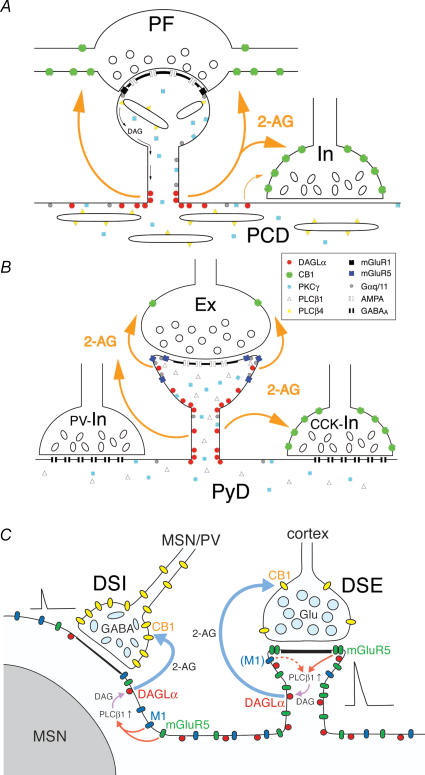
These anatomical data are largely consistent with our electrophysiological data. As a whole, a set of receptors and enzymes required for RER are closely localized so that the glutamatergic (and/or cholinergic) activity can trigger 2-AG production effectively (Fig. 3). 2-AG is then released and travels some distance to presynaptic terminals expressing CB1R. The density of CB1R is high at inhibitory and low at excitatory terminals, as if the spatial disadvantage of inhibitory terminals, which are located far from the 2-AG production site, is compensated by increasing the sensitivity to 2-AG (Fig. 3).
Figure 3. Schematic drawings showing the molecular, anatomical and physiological organization for endocannabinoid signalling of the three cell types in the brain.
A, in the Purkinje cell, diacylglycerol lipase-α (DAGLα) is densely concentrated at the base of spine neck. DAG produced at spine head diffuses to the base of spine neck and is converted to 2-arachidonoylglycerol (2-AG) by DAGLα. Released 2-AG activates CB1 cannabinoid receptors (CB1R) located on perisynaptic region of the parallel fibre (PF) terminal or nearby inhibitory terminal (In). B, in the hippocampal CA1 pyramidal cell, DAGLα is distributed in the spine head and neck. At this site, 2-AG is produced and travels to activate CB1R located on both excitatory (Ex) and cholecystokinin-positive inhibitory (CCK-In) terminals. The density of CB1R is low at the excitatory terminal, and high at the CCK-positive inhibitory terminal. PCD, Purkinje cell dendrite; PyD, pyramidal cell dendrite; PV-In, parvalbumin-positive inhibitory terminal. C, in the striatal medium spiny neuron, coincidental depolarization and mGluR5 activation are essential for PLCβ1/DAGLα-mediated production of 2-AG to induce retrograde suppression of corticostriatal synapse, because of low CB1 levels in excitatory corticostriatal afferents. This mGluR5-driven Ca2+-assisted RER is further facilitated with the aid of M1, while M1 activation alone fails to trigger 2-AG production because of its sparse distribution in the spines. This modulation will lead to the suppression of the hyperactivity of the medium spiny neuron (MSN). At MSN–MSN and parvalbumin (PV) interneuron–MSN synapses, which show high density of CB1R, both mGluR5 and M1 can induce RER because of their widespread somatodendritic distributions. This retrograde suppression will lead to the increase of the excitability and striatal output of the MSN. (Modified from Yoshida et al. 2006 and Uchigashima et al. 2007, with permission.)
Roles of two coincidence detectors, PLCβ and NMDA receptor
Based on our experimental data, we can speculate on the mechanisms of endocannabinoid-mediated synaptic plasticity. In Ca2+-assisted RER, PLCβ functions as a coincidence detector of Gq/11-coupled receptor activation and Ca2+ elevation (Hashimotodani et al. 2007a). If firing of presynaptic neurons activates postsynaptic I-mGluR and postsynaptic spikes induce Ca2+ elevation through voltage-gated Ca2+ channels, PLCβ can detect a certain timing of presynaptic and postsynaptic spikes. Indeed, involvement of the endocannabinoid signal in spike-timing-dependent plasticity (STDP) has been suggested at several types of synapses in the sensory cortex (Sjostrom et al. 2003; Bender et al. 2006; Nevian & Sakmann, 2006). At these synapses, long-term potentiation (LTP) is induced when presynaptic firing repeatedly precedes postsynaptic firing by 0–20 ms (pre-post timing), whereas long-term depression (LTD) is induced by their repeated firings in an inverse order (post-pre timing). This post-pre timing-dependent LTD (LTDpost-pre) was reported to depend on CB1R, I-mGluR and voltage-gated Ca2+ channels but not on postsynaptic NMDA receptors (Bender et al. 2006; Nevian & Sakmann, 2006). These data suggest the possibility that PLCβ might function as a post-pre timing detector in these endocannabinoid-mediated forms of LTDpost-pre.
Recently, we demonstrated that NMDA receptors can take the place of voltage-gated Ca2+ channels in both CaER and Ca2+-assisted RER by using cultured hippocampal neurons (Ohno-Shosaku et al. 2007). The NMDA receptor is the best known coincidence detector, and is generally thought to be able to detect a pre-post timing. Interestingly, it was recently reported in interneurons of dorsal cochlear nucleus that LTD was induced by a pre-post timing protocol (Tzounopoulos et al. 2007), which usually induces LTP in the sensory cortex. This pre-post timing-dependent LTD (LTDpre-post) was shown to be expressed presynaptically, and depend on postsynaptic Ca2+ elevation, postsynaptic NMDA receptors and presynaptic CB1R, but not on I-mGluRs (Tzounopoulos et al. 2007). These data suggest that the NMDA receptor might contribute to LTDpre-post by functioning as a pre-post timing detector and inducing CaER.
These results suggest that PLCβ and the NMDA receptor may function differently in different central synapses as a coincidence detector for activity-dependent endocannabinoid release.
Conclusions
The endocannabinoid signal plays an essential role in many forms of synaptic plasticity. Generation of the endocannabinoid signal is finely regulated by neural activities through multiple mechanisms, which involve voltage-gated Ca2+ channels, NMDA receptors, I-mGluRs, M1/M3 muscarinic receptors, PLCβs and DAGL. These molecules are well organized so that neural activities can trigger the endocannabinoid release effectively. Among these signalling molecules, NMDA receptors and PLCβ can function as coincidence detectors of presynaptic and postsynaptic activities. Recent studies suggest that these molecules might play a key role as timing detectors in endocannabinoid-dependent STDP. Under physiological conditions, the endocannabinoid release might be induced by more complicated patterns of neural activities. The physiological significance of these two coincidence detectors in various forms of synaptic plasticity remains to be elucidated.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research 18-08582 (Y.H.), 17650112, 17024021, and 18022016 (T.O.-S.), 17023001 (M.W.), 17023021, and 17100004 (M.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Y.H. is a recipient of Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6:1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Chen C, Kano M, Abeliovich A, Chen L, Bao S, Kim JJ, Hashimoto K, Thompson RF, Tonegawa S. Impaired motor coordination correlates with persistent multiple climbing fiber innervation in PKCγ mutant mice. Cell. 1995;83:1233–1242. doi: 10.1016/0092-8674(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Trettel J, Levine ES. Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol. 2004;92:2105–2112. doi: 10.1152/jn.00351.2004. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca2+-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007a;17:360–365. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007b;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007c;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S. Impaired synapse elimination during cerebellar development in PKC γ mutant mice. Cell. 1995;83:1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kushmerick C, Price GD, Taschenberger H, Puente N, Renden R, Wadiche JI, Duvoisin RM, Grandes P, von Gersdorff H. Retroinhibition of presynaptic Ca2+ currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse. J Neurosci. 2004;24:5955–5965. doi: 10.1523/JNEUROSCI.0768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cβ4 with metabotropic glutamate receptor type 1α and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Narushima M, Hashimoto K, Kano M. Endocannabinoid-mediated short-term suppression of excitatory synaptic transmission to medium spiny neurons in the striatum. Neurosci Res. 2006;54:159–164. doi: 10.1016/j.neures.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman Z, Malik P, Wu TY, Ochoa C, Watsa N, Lindgren C. Endocannabinoids mediate muscarine-induced synaptic depression at the vertebrate neuromuscular junction. Eur J Neurosci. 2007;25:1619–1630. doi: 10.1111/j.1460-9568.2007.05422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Ano M, Takeda S, Tsubokawa H, Kano M. Endocannabinoid signaling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons. J Physiol. 2007 doi: 10.1113/jphysiol.2007.137505. DOI 10.1113/jphysiol.2007.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol. 2007;578:773–785. doi: 10.1113/jphysiol.2006.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Nakagawa S, Kushiya E, Yamasaki M, Fukaya M, Iwanaga T, Simon MI, Sakimura K, Kano M, Watanabe M. Gq protein alpha subunits Gαq and Gα11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur J Neurosci. 2000;12:781–792. doi: 10.1046/j.1460-9568.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Nakamura M, Sato K, Kano M, Simon MI, Inoue Y. Patterns of expression for the mRNA corresponding to the four isoforms of phospholipase Cβ in mouse brain. Eur J Neurosci. 1998;10:2016–2025. doi: 10.1046/j.1460-9568.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]