Abstract
Endocannabinoids are released from neurons in activity-dependent manners, act retrogradely on presynaptic CB1 cannabinoid receptors, and induce short-term or long-term suppression of transmitter release. The endocannabinoid release is triggered by postsynaptic activation of voltage-gated Ca2+ channels and/or Gq-coupled receptors such as group I metabotropic glutamate receptors (I-mGluRs) and M1/M3 muscarinic receptors. However, the roles of NMDA receptors, which provide another pathway for Ca2+ entry into neurons, in endocannabinoid signalling have been poorly understood. In the present study, we investigated the possible contribution of NMDA receptors in endocannabinoid production by recording IPSCs in cultured hippocampal neurons. Under the conditions minimizing the activation of voltage-gated Ca2+ channels, local application of NMDA (200 μm) transiently suppressed cannabinoid-sensitive IPSCs, but not cannabinoid-insensitive IPSCs. This NMDA-induced suppression was abolished by blocking NMDA receptors, CB1 receptors and diacylglycerol lipase, but not by inhibiting voltage-gated Ca2+ channels. When the postsynaptic neuron was dialysed with 30 mm BAPTA, the NMDA-induced suppression was reduced significantly. A lower dose of NMDA (20 μm) exerted little effect when applied alone, but markedly enhanced the cannabinoid-dependent suppression driven by muscarinic receptors or I-mGluRs. These data clearly indicate that the activation of NMDA receptors facilitates the endocannabinoid release either alone or in concert with the Gq-coupled receptors.
Activity-dependent change in synaptic strength is thought to be a fundamental process that underlies higher brain functions including learning and memory. NMDA-type glutamate receptors are known to play crucial roles in induction of synaptic plasticity in various regions of the CNS (Malenka & Nicoll, 1999). Recent studies have revealed that the endocannabinoid system also contributes to activity-dependent synaptic modulation in the CNS (Chevaleyre et al. 2006). Endocannabinoids are bioactive lipids and mediate a retrograde signal in neural tissues (Alger, 2002). They are released from postsynaptic neurons and activate presynaptic CB1 cannabinoid receptors, thereby inducing short-term or long-term suppression of transmitter release. Endocannabinoid-mediated synaptic plasticity has been identified in various brain regions including the hippocampus, cerebellum, amygdala, basal ganglia and neocortex (Chevaleyre et al. 2006; Hashimotodani et al. 2007b).
As the mechanisms of endocannabinoid production, two main intracellular pathways have been reported (Ohno-Shosaku et al. 2005). One is a phospholipase Cβ (PLCβ)-dependent pathway, which is driven by Gq-coupled receptors including group I metabotropic glutamate receptors (I-mGluRs) (Maejima et al. 2001; Varma et al. 2001; Ohno-Shosaku et al. 2002) and M1/M3 muscarinic receptors (Ohno-Shosaku et al. 2003; Fukudome et al. 2004). These receptors stimulate PLCβ, which yields diacylglycerol (DAG) and inositol-1,4,5-trisphosphate. DAG is converted to the major endocannabinoid 2-arachidonolyglycerol (2-AG) by DAG lipase (Piomelli, 2003). 2-AG is then released from neurons and retrogradely activates presynaptic CB1 receptors. The receptor-driven endocannabinoid release is enhanced by a small Ca2+ elevation to submicromolar levels (Maejima et al. 2005) that is attributable to Ca2+-dependent enhancement of PLCβ activity (Hashimotodani et al. 2005). The other pathway is independent of PLCβ (Hashimotodani et al. 2005; Maejima et al. 2005) and is driven by a large Ca2+ elevation to micromolar levels (Brenowitz & Regehr, 2003). This pathway is responsible for depolarization-induced suppression of inhibition (DSI) (Kreitzer & Regehr, 2001a; Ohno-Shosaku et al. 2001; Wilson & Nicoll, 2001) or excitation (DSE) (Kreitzer & Regehr, 2001b). Postsynaptic depolarization activates voltage-gated Ca2+ channels and causes a large Ca2+ elevation, thereby inducing endocannabinoid release. The released endocannabinoids then suppress the inhibitory or excitatory transmission (DSI/DSE). It has been suggested that DSI/DSE is also mediated by 2-AG (Kim & Alger, 2004; Straiker & Mackie, 2005; Szabo et al. 2006; Hashimotodani et al. 2007c; Uchigashima et al. 2007), although the molecular identities of enzymes involved in this pathway are still unclear.
Thus, it is well established that an intracellular Ca2+ signal is crucial for induction of endocannabinoid release through both the PLCβ-dependent and PLCβ-independent pathways. In most of the previous studies, Ca2+ elevation for triggering endocannabinoid release was caused by activation of voltage-gated Ca2+ channels. Under physiological conditions, however, Ca2+ elevation can be induced through multiple pathways. Although NMDA receptors constitute a major calcium entry pathway into central neurons, it is not well understood how NMDA receptors are involved in endocannabinoid signalling. In the present study, we used cannabinoid-sensitive IPSCs in cultured hippocampal neurons, and examined whether NMDA receptors can contribute to the endocannabinoid release through the PLCβ-dependent and PLCβ-independent pathways. Our data demonstrate that NMDA receptors provide a novel Ca2+ entry pathway for endocannabinoid production in hippocampal neurons.
Methods
Electrophysiology
All experiments were performed according to the guidelines laid down by the animal welfare committees of Kanazawa University and Osaka University. Cultured hippocampal neurons were prepared from newborn rats as previously described (Ohno-Shosaku et al. 2001). Briefly, rats were anaesthetized with ether and decapitated. Hippocampi were rapidly removed and cells were mechanically dissociated. The cultures were kept at 36°C in 5% CO2 for 10–15 days before use. The neurons were whole-cell clamped with patch pipettes, and the current responses were recorded with a patch-clamp amplifier (EPC9/3 or EPC10/2, HEKA, Lambrecht/Pfalz, Germany). For recording IPSCs, the presynaptic neuron was stimulated by applying voltage pulses (from −80 mV to 0 mV, 2 ms) at 0.5 Hz, and the evoked IPSCs were recorded from the postsynaptic neuron at −80 mV, unless otherwise indicated. The magnitude of IPSC suppression was calculated from mean amplitudes of 10–12 consecutive IPSCs before treatment and seven consecutive IPSCs obtained between 4 s and 18 s after the end of local puff application. In some cases, electrical stimulation failed to excite action potentials in the presynaptic neurons for a short period of time after the end of puff application of a Na+-free solution. Such failure traces were not included in the calculation. Voltage-gated Ca2+ currents were evoked by applying depolarizing voltage pulses (from −80 mV to −10 mV, 50 ms), and the amplitude of inward currents was measured. The voltage-gated Ca2+ current was assumed to be blocked completely by 0.1 mm CdCl2. All experiments were performed at room temperature (24–26°C).
Solutions
A standard external solution contained (mm): 140 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 10 Hepes, 10 glucose and 0.01 CNQX (pH 7.3, adjusted with NaOH). The bath was continuously perfused with this solution at a flow rate of 1–3 ml min−1. WIN55,212-2, AM281, 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP), and voltage-gated Ca2+ channel blockers were bath-applied. For induction of endocannabinoid release, NMDA, oxotremorine M (oxo-M) and 3,5-dihydroxyphenylglycine (DHPG) were locally applied via a 250 μm wide puff pipette located near the neurons, by using a perfusion valve controller (VC-6M, Warner Instruments, Hamden, CT, USA). The solution used for this local application contained (mm): 145 N-methyl-d-glucamine (NMDG), 135 HCl, 2.5 KCl, 3 CaCl2, 10 Hepes and 0.01 CNQX (pH 7.3, adjusted with HCl), unless otherwise indicated. In the experiments shown in Fig. 5, NMDA was locally applied by using a solution that contained (mm): 145 NMDG, 135 HCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes and 0.01 CNQX (pH 7.3, adjusted with HCl). For measurements of Ca2+ currents, the external solution contained (mm): 130 NaCl, 10 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 10 Hepes, 10 glucose and 1 4-aminopyridine (pH 7.3, adjusted with NaOH). Patch pipettes were filled with a solution containing (mm): 130 potassium gluconate, 15 KCl, 6 MgCl2, 0.2 EGTA, 10 Hepes, 5 Na2ATP and 0.2 Na2GTP (pH 7.3 adjusted with KOH). In some experiments (Fig. 1), the pipettes were filled with a Cs+-based solution, which contained (mm): 120 CsCl, 5 MgCl2, 1 CaCl2, 10 EGTA, 10 Hepes, 30 CsOH, 5 Na2ATP and 0.2 Na2GTP (pH 7.3 adjusted with CsOH). In the experiments shown in Fig. 5, solutions containing 5 mm EGTA or 30 mm BAPTA were used. The EGTA solutions contained (mm): 120 potassium gluconate, 15 KCl, 6 MgCl2, 5 EGTA, 10 Hepes, 5 Na2ATP and 0.2 Na2GTP (pH 7.3 adjusted with KOH). The BAPTA solution contained (mm): 72 potassium gluconate, 15 KCl, 6 MgCl2, 3 CaCl2, 30 BAPTA, 10 Hepes and 5 Na2ATP (pH 7.3 adjusted with KOH). The electrode resistance ranged from 2 to 5 MΩ.
Figure 5. Effects of postsynaptic BAPTA injection on the NMDA-induced suppression of IPSCs.
A and B, representative experiment showing the effects of NMDA application (200 μm, 5 s) in the presence of Mg2+. IPSCs were recorded at the holding potential of −80 mV (left) and 0 mV (right) in the postsynaptic neuron dialysed with the pipette solution containing 5 mm EGTA (A) or 30 mm BAPTA (B). C, averaged data for the NMDA-induced suppression of IPSCs at these four recording conditions.
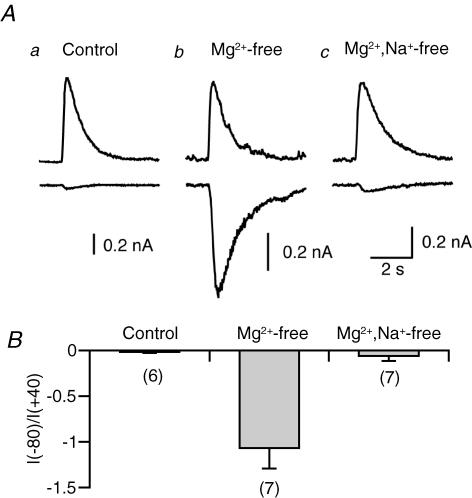
Figure 1. NMDA-induced whole-cell currents under three different conditions.
A, examples of NMDA-induced currents recorded at +40 mV (upper traces) and −80 mV (lower traces). NMDA (200 μm) was locally applied for 2 s. NMDA was dissolved in a standard extracellular solution (a), an Mg2+-free standard solution (b) or an Mg2+–Na+-free solution (c). The patch pipettes were filled with a Cs+-based solution. B, averaged data for relative amplitudes of NMDA-induced currents at −80 mV compared to those recorded at +40 mV in the three experimental conditions. The number of experiments is indicated in parentheses for this and subsequent figures.
Drugs
WIN55,212-2, AM281, CPP, DHPG and oxo-M, were purchased from Tocris Cookson (Bristol, UK). SNX-482, ω-agatoxin IVA (AgTX), and ω-conotoxin GVIA (CgTX) were from Peptide Institute (Osaka, Japan). CNQX, NMDA and tetrahydrolipstatin (THL) were from Sigma-Aldrich (St Louis, MO, USA). Nifedipine was from Nacalai Tesque (Kyoto, Japan).
Statistics
Averaged data from different experiments are presented as means ±s.e.m. Statistical significance was assessed by Student's t test for paired or unpaired data. One, two and three asterisks indicate P < 0.05, P < 0.01 and P < 0.001, respectively.
Results
Condition for NMDA receptor activation without apparent depolarization
We looked for suitable conditions in which NMDA receptors of recorded neurons were sufficiently activated without apparent depolarization. When NMDA (200 μm) was dissolved in a standard NaCl-based solution and locally applied for 2 s, a large outward current was induced at + 40 mV (Fig. 1Aa, upper trace). In the same neuron, NMDA application induced only a small inward current at −80 mV (Fig. 1Aa, lower trace), as expected from the ‘Mg2+ block’ of NMDA receptors. The relative amplitude of NMDA-induced currents at −80 mV compared to that recorded at + 40 mV was −0.02 ± 0.01 (n= 6) (Fig. 1B, left). When Mg2+ was omitted from the NMDA-containing solution, NMDA application induced a large inward current even at −80 mV (Fig. 1Ab, lower trace). The relative amplitude was −1.07 ± 0.22 (n= 7) (Fig. 1B, middle). Such a large inward current could induce local depolarization at distal dendrites, and thereby might activate voltage-gated Ca2+ channels. To minimize NMDA-induced local depolarization, Na+ in the Mg2+-free NMDA-containing solution was replaced with a membrane-impermeant cation, NMDG. With this solution, the NMDA-induced inward current at −80 mV was markedly reduced (−0.06 ± 0.05, n= 7, relative to the current at +40 mV) (Fig. 1A and B, right). Under this condition, sufficient NMDA receptor activation was expected to be achieved without activation of voltage-gated Ca2+ channels. Therefore, this NMDG-based solution was used for NMDA application in the following experiments.
NMDA-induced endocannabinoid release
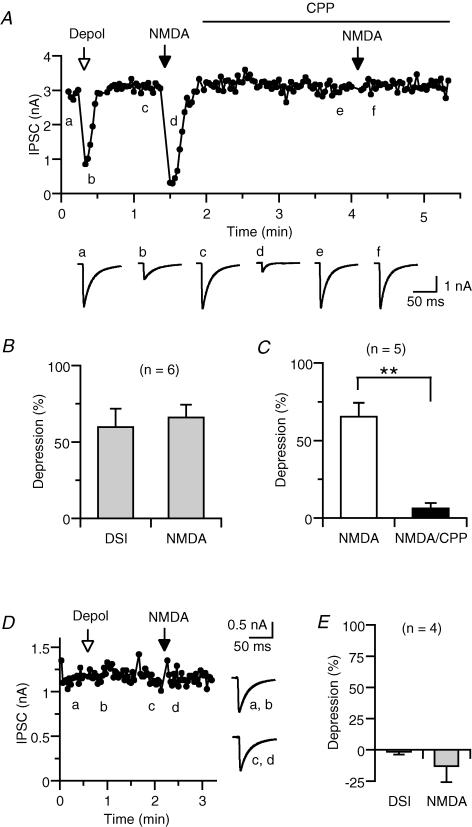
We first examined whether activation of NMDA receptors causes endocannabinoid release, by monitoring the amplitude of IPSCs. IPSCs in hippocampal neurons are classified into two categories, namely cannabinoid sensitive and cannabinoid insensitive (Ohno-Shosaku et al. 2001). Their cannabinoid sensitivities can be checked easily by applying a depolarizing voltage pulse to the postsynaptic neuron (0 mV, 3–5 s). Cannabinoid-sensitive IPSCs display a transient suppression following depolarization, a phenomenon known as depolarization-induced suppression of inhibition (DSI) (Fig. 2A) (Ohno-Shosaku et al. 2001). By contrast, cannabinoid-insensitive IPSCs do not exhibit DSI (Fig. 2E). We compared effects of NMDA application in these two distinct types of IPSCs. In cannabinoid-sensitive IPSCs, local application of NMDA (200 μm) for 5 s always induced transient suppression (Fig. 2A). On average, effects of depolarization (3 s) and NMDA application were comparable (Fig. 2B). The NMDA-induced suppression was blocked by an NMDA receptor antagonist, CPP (50 μm) (Fig. 2A and C). In contrast to cannabinoid-sensitive IPSCs, cannabinoid-insensitive IPSCs were not affected by NMDA application (Fig. 2D and E).
Figure 2. Effects of NMDA application on IPSCs.
A, representative data showing NMDA-induced suppression of cannabinoid-sensitive IPSCs. The amplitude of IPSC evoked by presynaptic stimulation was plotted as a function of time. At the time indicated by the open arrow, the postsynaptic neuron was depolarized from −80 mV to 0 mV for 5 s. At the times indicated by the filled arrows, 200 μm NMDA was locally applied for 5 s. CPP (50 μm) was added to both the bath solution and the NMDA-containing solution for the duration indicated by the horizontal bar. IPSC traces acquired at the indicated time points (a–f) are shown on the lower panel. Each trace is the average of 3–5 consecutive IPSCs. B, averaged data for the IPSC suppression induced by depolarization (0 mV, 3 s) and NMDA application (200 μm NMDA, 5 s) from six neuron pairs. C, averaged data for the effects of CPP (50 μm) on NMDA-induced suppression. D and E, representative experiment (D) and averaged data (E) showing the effects of depolarization (0 mV, 3 s) and NMDA application (200 μm NMDA, 5 s) on cannabinoid-insensitive IPSCs.
The involvement of endocannabinoids was further confirmed by using the CB1 antagonist AM281. Effects of depolarization and NMDA application on cannabinoid-sensitive IPSCs were tested before and after the treatment with AM281 (Fig. 3A), the action of which is known to be almost irreversible. We usually applied the cannabinoid agonist WIN55,212-2 prior to AM281, in order to confirm the cannabinoid sensitivity of the IPSC and the antagonizing effect of AM281 on WIN55,212-2. After the treatment with AM281, both DSI and NMDA-induced suppression were completely abolished (Fig. 3A and B). Using the same preparation, we previously reported that the DAG lipase inhibitor THL blocked DSI without affecting the presynaptic cannabinoid sensitivity, suggesting that DSI is mediated by 2-AG (Hashimotodani et al. 2007c). Like DSI, the NMDA-induced suppression of IPSCs was found to be abolished in the THL-pretreated neurons (Fig. 3C and D). Taken together, these data indicate that NMDA receptor activation induces endocannabinoid release and causes a transient suppression of cannabinoid-sensitive IPSCs.
Figure 3. Cannabinoid dependence of NMDA-induced suppression of IPSCs.
A, representative data showing blockade of NMDA-induced IPSC suppression by the CB1 antagonist AM281. Effects of depolarization (0 mV, 3 s) and NMDA application (200 μm, 5 s) on IPSCs were examined before and after the sequential treatment with 0.3 μm WIN55,212-2 and 0.3 μm AM281. Each trace (lower panel) is the average of 5 consecutive IPSCs acquired at the indicated time point (a–j). B, averaged data for IPSC suppression induced by depolarization and NMDA application before and after the treatment with AM281. C and D, representative experiment (C) and averaged data (D) for the effects of treatment with THL (5 μm, 3–5 min) on NMDA-induced IPSC suppression.
Mechanisms of NMDA-induced endocannabinoid release
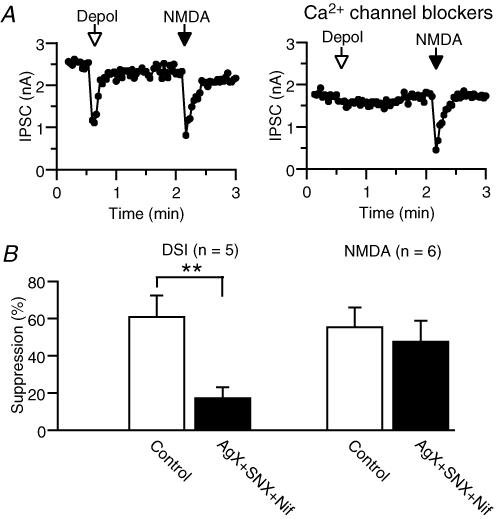
The mechanisms of NMDA-induced endocannabinoid release were further studied. A simple explanation is that Ca2+ influx through NMDA receptors directly stimulates endocannabinoid production. However, the possibility remains that the activation of NMDA receptors might cause local depolarization, which in turn activates voltage-gated Ca2+ channels and induces an endocannabinoid release. To test this possibility, we applied NMDA under the blockade of voltage-gated Ca2+ channels. In the first series of experiments, we used pharmacological tools. It is reported that N-type Ca2+ channels exclusively contribute to the transmitter release at cannabinoid-sensitive GABAergic terminals in the hippocampus (Wilson et al. 2001). Therefore, we tried to block voltage-gated Ca2+ channels other than N-type by using Ca2+ channel blockers for P/Q-type (AgTX), R-type (SNX-482) and L-type (nifedipine). Application of a mixture of 0.1 μm AgTX, 0.1 μm SNX-482 and 20 μm nifedipine decreased the total Ca2+ current by 69.4 ± 3.6% (n= 5). The treatment with these Ca2+ channel blockers markedly reduced DSI, but not the NMDA-induced suppression (Fig. 4). In the second series of experiments, we electrically inactivated voltage-gated Ca2+ channels. In this series of experiments, we used the Mg2+-containing solution for NMDA application. When NMDA was applied to the neurons held at −80 mV, IPSCs were not evidently suppressed (Fig. 5A and C). When the holding potential of the postsynaptic neuron was changed from −80 mV to 0 mV, a large outward current was induced and IPSCs were suppressed due to activation of voltage-gated Ca2+ channels. When the membrane potential was held at 0 mV for several minutes, the outward current decreased and IPSCs recovered gradually. At this steady-state condition, voltage-gated Ca2+ channels are expected to be inactivated. In these neurons held at 0 mV, NMDA application induced a robust suppression of IPSCs (Fig. 5A and C). The NMDA-induced suppression of IPSCs at 0 mV was less prominent in the neurons dialysed with 30 mm BAPTA (Fig. 5B and C, right; P < 0.01, unpaired t test). These results collectively indicate that voltage-gated Ca2+ channels make little contribution to the NMDA-induced suppression, and that the elevation of intracellular Ca2+ concentration caused by Ca2+ influx through NMDA receptors induces endocannabinoid release from hippocampal neurons.
Figure 4. Effects of voltage-gated Ca2+ channel blockers on DSI and NMDA-induced suppression of IPSCs.
A, representative experiment. Effects of depolarization (0 mV, 3 s) and NMDA application (200 μm, 5 s) on IPSCs were examined before (left) and after (right) the treatment with 0.1 μm AgTX, 0.1 μm SNX-482 and 20 μm nifedipine for more than 3.5 min. B, averaged data for the effects of voltage-gated Ca2+ channel blockers on DSI and NMDA-induced suppression of IPSCs.
NMDA-induced enhancement of receptor-driven endocannabinoid release
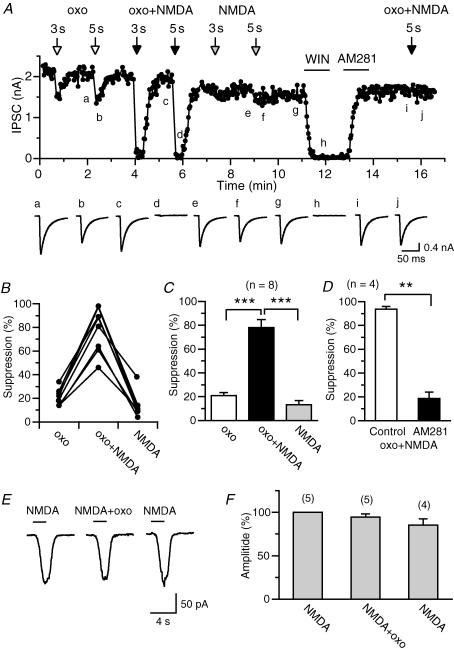
Next, we determined whether the activation of NMDA receptors can facilitate the endocannabinoid release through the receptor-driven pathway. When a low dose of the muscarinic agonist oxo-M (0.3 μm) was applied locally to the postsynaptic neuron for 1–5 s, IPSCs were suppressed slightly (Fig. 6A–C). Application of a low dose of NMDA (20 μm) caused only minor effects on IPSCs (Fig. 6A–C). When oxo-M (0.3 μm) and NMDA (20 μm) were applied simultaneously, IPSCs were markedly suppressed (Fig. 6A–C). Effects of oxo-M alone, NMDA alone and oxo-M plus NMDA were independent of the order of application, and were repeatable. The marked suppression induced by the coapplication of oxo-M and NMDA was antagonized by AM281 (Fig. 6A and D), indicating that the suppression is mediated by endocannabinoids. The enhancement of endocannabinoid release by coapplication of oxo-M and NMDA can be explained by the enhancement of PLCβ activity by NMDA-induced Ca2+ elevation, similar to the enhancement by depolarization-induced Ca2+ elevation (Hashimotodani et al. 2005). However, it is also possible that NMDA receptors might be potentiated by muscarinic activation (Harvey et al. 1993). To test this possibility, we measured inward currents induced by application of NMDA (20 μm) alone or coapplication of NMDA (20 μm) and oxo-M (0.3 μm). We obtained no evidence that shows potentiation of NMDA-induced currents by oxo-M under these experimental conditions (Fig. 6E and F).
Figure 6. Enhancement of muscarinic receptor-driven endocannabinoid release by NMDA application.
A, representative data showing enhanced endocannabinoid release by simultaneous activation of NMDA and muscarinic receptors. The amplitude of cannabinoid-sensitive IPSCs was plotted as a function of time. At the time points indicated by open, filled and grey arrows, 0.3 μm oxo-M, 0.3 μm oxo-M plus 20 μm NMDA, and 20 μm NMDA, respectively, were locally applied for 3 or 5 s. WIN55,212-2 (0.3 μm) and AM281 (0.3 μm) were bath-applied for the duration indicated by horizontal bars. Each IPSC trace (lower panel) is the average of 5–10 consecutive IPSCs acquired at the indicated time points a–j. B and C, individual (B) and averaged (C) data for the IPSC suppression by application of oxo-M, oxo-M + NMDA, and NMDA obtained from the experiments similar to A. The duration of agonist application ranged from 1 to 5 s. D, averaged data for the IPSC suppression induced by application of oxo-M + NMDA before and after the treatment with AM281. E and F, representative experiment (E) and averaged data (F) for inward currents induced by consecutive applications of NMDA (20 μm) alone, NMDA (20 μm) + oxo-M (0.3 μm), and NMDA (20 μm) alone.
Similar experiments were performed with the I-mGluR agonist DHPG. We found that a low dose of NMDA (20 μm) significantly enhanced the suppression of IPSCs induced by DHPG. Application of DHPG (5 μm) induced only a small suppression of IPSCs (Fig. 7A–C) but this DHPG-induced suppression was significantly enhanced by the simultaneous application of 20 μm NMDA (Fig. 7A–C). This enhanced suppression was blocked by AM281. Co-application of DHPG (5 μm) did not significantly enhance NMDA-induced inward currents (Fig. 7E and F). These results strongly suggest that mild activation of NMDA receptors, which by itself cannot induce endocannabinoid release, can enhance the endocannabinoid release driven by Gq-coupled receptor activation.
Figure 7. Enhancement of I-mGluR-driven endocannabinoid release by NMDA application.
A, representative data showing enhanced endocannabinoid release by simultaneous activation of NMDA receptors and I-mGluRs. The amplitude of cannabinoid-sensitive IPSCs was plotted as a function of time. At the time points indicated by open, closed and grey arrows, 5 μm DHPG, 5 μm DHPG + 20 μm NMDA, and 20 μm NMDA, respectively, were locally applied for 1 s. B and C, individual (B) and averaged (C) data for the IPSC suppression by application of DHPG, DHPG + NMDA, and NMDA obtained from the experiments similar to A. The duration of agonist application ranged from 1 to 10 s. Data connected by a line were obtained from the same neuron pair. D, averaged data for the IPSC suppression induced by application of DHPG + NMDA before and after the treatment with AM281. E and F, representative experiment (E) and averaged data (F) for inward currents induced by consecutive applications of NMDA (20 μm) alone, NMDA (20 μm) + DHPG (5 μm), and NMDA (20 μm) alone.
Discussion
The present study demonstrates that the activation of NMDA receptors can directly contribute to the generation of endocannabinoid signalling through elevating postsynaptic Ca2+ concentration. Strong activation of NMDA receptors alone induced endocannabinoid release, and suppressed the cannabinoid-sensitive synaptic transmission. Mild activation of NMDA receptors, which by itself caused little effect, enhanced the endocannabinoid release driven by muscarinic receptors or I-mGluRs. These functions of NMDA receptors are essentially the same as those of voltage-gated Ca2+ channels (Ohno-Shosaku et al. 2005; Chevaleyre et al. 2006). Because NMDA receptor activation may cause depolarization of neurons and thereby open voltage-gated Ca2+ channels, it is not easy to separate the effect resulting from NMDA receptor activation from secondary activation of voltage-gated Ca2+ channels. In the present study, we tried to separate these two, and demonstrate that the effect of NMDA is attributable to Ca2+ influx through NMDA receptors into hippocampal neurons. Under physiological conditions, however, NMDA receptors and voltage-gated Ca2+ channels may work in concert with each other. Cooperative activation of Ca2+ channels, NMDA receptors and Gq-coupled receptors might be physiologically important for the activity-dependent generation of endocannabinoid signal.
In the literature, there are several studies suggesting NMDA-induced endocannabinoid release. In a study with gas chromatography/mass spectrometry analysis, Stella & Piomelli (2001) measured the contents of endocannabinoids in cultured cortical neurons under various conditions. Incubation of neurons with 300 μm NMDA for 2.5 min caused a marked accumulation of 2-AG, which was enhanced by coincubation with the cholinergic agonist carbachol (1 mm). Although their experimental conditions are quite different from ours (i.e. a longer exposure time and higher agonist doses), these data are qualitatively consistent with those in the present study. In a electrophysiological study on hypoglossal motoneurons, Mukhtarov et al. (2005) investigated effects of postsynaptic Ca2+ elevation on glycinergic transmission. Although this study aimed to characterize depolarization-induced suppression of glycinergic transmission and Ca2+-dependent potentiation of glycine receptors, it additionally included the experiments with NMDA. Application of 200 μm NMDA dissolved in a standard external solution (2 mm Ca2+ and 1.3 mm Mg2+) for 5–10 s induced a suppression of glycinergic IPSCs at −70 mV, which was abolished by a CB1 antagonist. The details of this phenomenon were not described, and it is not clear how NMDA receptors were activated under these conditions (−70 mV, 1.3 mm Mg2+). It seems likely that NMDA application induced a small local depolarization, which further activated NMDA receptors and induced a further depolarization, resulting in regenerative activation of NMDA receptors and membrane depolarization. If this regenerative process was activated, the observed NMDA-induced suppression of glycinergic IPSCs might be caused, at least partly, by the DSI mechanism. In the present study, we used a Mg2+-free, NMDG-based solution for NMDA application, and minimized the local depolarization. Furthermore, we confirmed that the NMDA-induced suppression of IPSCs persisted under the pharmacological or electrical blockade of voltage-gated Ca2+ channels. Therefore, the present study is the first clear demonstration of the direct effect of NMDA receptor activation on generation of the endocannabinoid signal in neurons.
In the present study, we used cannabinoid-sensitive IPSCs to monitor the endocannabinoid release. In our culture conditions, we found that pharmacological activation of NMDA receptors induced the endocannabinoid release and suppressed IPSCs. In physiological conditions, however, synaptically driven endocannabinoid release through activation of NMDA receptors would be restricted around excitatory postsynaptic sites of dendrites, and the released endocannabinoids may hardly act on somatic inhibitory synapses. Therefore, the retrograde modulation of inhibitory transmission observed in the present study may be limited to, if any, the dendritic inhibitory synapses that are located close to excitatory synapses. Alternatively, synaptic activation of NMDA receptors might also occur at inhibitory synapses. At a selected population of inhibitory synapses on CA1 pyramidal cell soma, it has been demonstrated that aspartate, an agonist of NMDA receptors, is present on presynaptic terminals, whereas NMDA receptors were concentrated on postsynaptic membranes (Gundersen et al. 2004). Interestingly, it has been reported that vesicular glutamate transporter type 3 (vGluT3) is selectively expressed at cholecystokinin (CCK)-positive inhibitory terminals, which are known to be cannabinoid sensitive, in the hippocampus (Somogyi et al. 2004). It might be possible that aspartate is packed into synaptic vesicles by vGluT3, released together with GABA from cannabinoid-sensitive inhibitory terminals, and activates postsynaptic NMDA receptors. Further investigation using acute slice preparations is needed to evaluate the physiological relevance of the NMDA-receptor-mediated endocannabinoid release in the modulation of inhibitory transmission.
Recently, the contribution of NMDA receptors to synaptically driven endocannabinoid release has been reported at excitatory synapses in several brain regions (Beierlein & Regehr, 2006; Crozier et al. 2007; Tzounopoulos et al. 2007). In stellate cells (SCs) of the cerebellum, a brief parallel fibre burst induced a CB1-dependent transient suppression, which was prevented by postsynaptic BAPTA injection. This Ca2+-dependent endocannabinoid release was not altered by blocking either mGluR1 alone or NMDA receptors alone, but was prevented by blocking both types of receptors, indicating that the synaptically driven endocannabinoid release from SCs requires activation of NMDA receptors under the blockade of mGluR1 (Beierlein & Regehr, 2006). In pyramidal neurons of visual cortical layer 2/3, pairing stimulation (layer 4 stimulation with postsynaptic depolarization from −65 to −45 mV) at 1 Hz for 5–10 min induced CB1-dependent long-term depression (LTD). This LTD was blocked by intracellular loading of the NMDA receptor antagonist MK801, indicating that postsynaptic NMDA receptors are involved in pairing stimulation-induced endocannabinoid release (Crozier et al. 2007). In interneurons of dorsal cochlear nucleus, LTD was induced by pre-post timing protocol in which a presynaptic input was followed by a postsynaptic spike, which is usually used to induce LTP in the sensory cortex. This timing-dependent LTD was expressed presynaptically, and depended on postsynaptic Ca2+ elevation, postsynaptic NMDA receptors and presynaptic CB1 receptors, but not on I-mGluRs (Tzounopoulos et al. 2007). These data strongly suggest that the pairing stimulation could induce the endocannabinoid release through activation of NMDA receptors. In these studies, however, it is unclear whether Ca2+ influx through NMDA receptors was sufficient to induce the NMDA receptor-dependent endocannabinoid release or whether voltage-gated Ca2+ channels were also involved. Nevertheless, these studies have clearly demonstrated that synaptically activated NMDA receptors can contribute to the generation of the endocannabinoid signal.
Coincidence detection of two distinct signals is important for various aspects of neural functions. It is generally accepted that NMDA receptors function as a coincidence detector of postsynaptic depolarization and presynaptic glutamate release, which play crucial roles in induction of long-term potentiation and depression in various synapses in the CNS (Cotman et al. 1988; Bliss & Collingridge, 1993; Malenka & Nicoll, 1999). Another example is PLCβ. This enzyme is activated effectively when activation of Gq-coupled receptors coincides with Ca2+ elevation (Hashimotodani et al. 2007a). Thus, it can detect the coincidence of presynaptic transmitter release and postsynaptic depolarization (Ca2+ elevation). It is noteworthy that these two coincidence detectors are involved in generation of the endocannabinoid signal. The involvement of endocannabinoids in induction of synaptic plasticity has been reported in various brain regions (Chevaleyre et al. 2006; Hashimotodani et al. 2007b). It is interesting to investigate roles of these coincidence detectors in the cannabinoid-dependent forms of synaptic plasticity.
In the hippocampus, it has been demonstrated that LTD of inhibitory transmission (LTDi) triggered by high-frequency stimulation is mediated by endocannabinoids (Chevaleyre & Castillo, 2003, 2004). This cannabinoid-dependent form of LTDi was shown to require the activation of I-mGluRs, but not NMDA receptors. By contrast, several examples of LTDi that require activation of postsynaptic NMDA receptors for induction have been reported (Stelzer et al. 1987; McLean et al. 1996; Wang & Stelzer, 1996; Caillard et al. 1999; Lu et al. 2000). This NMDA-dependent form of LTDi has been assumed to be caused by down-regulation of postsynaptic GABAA receptor functions. The present study, however, suggests the possibility that the endocannabinoid signal triggered by postsynaptic NMDA receptor activation might also contribute to LTDi.
In the present study, we did not determine the precise mechanisms of the effects of NMDA receptor activation on endocannabinoid release. However, it is reasonable to assume that the Ca2+ elevation caused by NMDA receptor activation and that by voltage-gated Ca2+ channel activation trigger the same processes. Namely, a large Ca2+ elevation by strong activation of NMDA receptors induces the endocannabinoid release through a PLCβ-independent pathway, whereas a small Ca2+ elevation by mild activation of NMDA receptors enhances the receptor-driven 2-AG release through Ca2+-dependent enhancement of PLCβ activation and the subsequent DAG lipase activity. Although fundamental intracellular mechanisms for endocannabinoid production appear to be similar, activation of voltage-gated Ca2+ channels and that of NMDA receptors may lead to different consequences in synaptic modulation. The Ca2+ elevation driven by voltage-gated Ca2+ channels is generally global, whereas that by NMDA receptors is restricted to around postsynaptic sites. Therefore, the endocannainoid signal driven by NMDA receptors can contribute to spatially limited synaptic modulation. At hippocampal excitatory synapses, NMDA receptors, mGluR5, and DAG lipase α, which are involved in 2-AG production, are all located on the postsynaptic membranes close to each other (Takumi et al. 1999; Racca et al. 2000; Yoshida et al. 2006). The CB1 receptor is located at excitatory synaptic terminals facing postsynaptic dendritic spines (Katona et al. 2006; Kawamura et al. 2006). The synaptic localization of these signalling molecules suggests the possibility that endocannabinoids are released from restricted regions of postsynaptic neurons in activity-dependent manners, modulate transmitter release from a small number of presynaptic terminals, and cause a pathway-selective plasticity.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research 18-08582 (Y.H.), 17650112, 17024021 and 18022016 (T.O.-S.), 17023021 and 17100004 (M.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Y.H. is a recipient of Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
References
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG. Local interneurons regulate synaptic strength by retrograde release of endocannabinoids. J Neurosci. 2006;26:9935–9943. doi: 10.1523/JNEUROSCI.0958-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23:6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillard O, Ben-Ari Y, Gaiarsa JL. Mechanisms of induction and expression of long-term depression at GABAergic synapses in the neonatal rat hippocampus. J Neurosci. 1999;19:7568–7577. doi: 10.1523/JNEUROSCI.19-17-07568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivationinduced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci U S A. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signaling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Gundersen V, Holten AT, Storm-Mathisen J. GABAergic synapses in hippocampus exocytose aspartate on to NMDA receptors: quantitative immunogold evidence for co-transmission. Mol Cell Neurosci. 2004;26:156–165. doi: 10.1016/j.mcn.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Harvey J, Balasubramaniam R, Collingridge GL. Carbachol can potentiate N-methyl-D-aspartate responses in the rat hippocampus by a staurosporine and thapsigargininsensitive mechanism. Neurosci Lett. 1993;162:165–168. doi: 10.1016/0304-3940(93)90586-a. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca2+-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007a;17:360–365. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007b;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007c;27:1211–1219. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarizationinduced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001a;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001b;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurinmediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- McLean HA, Caillard O, Ben-Ari Y, Gaiarsa JL. Bidirectional plasticity expressed by GABAergic synapses in the neonatal rat hippocampus. J Physiol. 1996;496:471–477. doi: 10.1113/jphysiol.1996.sp021699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtarov M, Ragozzino D, Bregestovski P. Dual Ca2+ modulation of glycinergic synaptic currents in rodent hypoglossal motoneurones. J Physiol. 2005;569:817–831. doi: 10.1113/jphysiol.2005.094862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Hashimotodani Y, Maejima T, Kano M. Calcium signaling and synaptic modulation: regulation of endocannabinoid-mediated synaptic modulation by calcium. Cell Calcium. 2005;38:369–374. doi: 10.1016/j.ceca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi J, Baude A, Omori Y, Shimizu H, El Mestikawy S, Fukaya M, Shigemoto R, Watanabe M, Somogyi P. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- Stelzer A, Slater NT, ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987;326:698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Urbanski MJ, Bisogno T, Di Marzo V, Mendiguren A, Bar W, Freiman I. Depolarization-induced retrograde synaptic inhibition in the cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol. 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Matsubara A, Rinvik E, Ottersen OP. The arrangement of glutamate receptors in excitatory synapses. Ann N Y Acad Sci. 1999;868:474–482. doi: 10.1111/j.1749-6632.1999.tb11316.x. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Stelzer A. Shared calcium signaling pathways in the induction of long-term potentiation and synaptic disinhibition in CA1 pyramidal cell dendrites. J Neurophysiol. 1996;75:1687–1702. doi: 10.1152/jn.1996.75.4.1687. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]