Abstract
The properties of the human motor cortex can be studied non-invasively using transcranial magnetic stimulation (TMS). Stimulation at high intensity excites corticospinal cells with fast conducting axons that make direct connections to motoneurones of human upper limb muscles, while low-intensity stimulation can suppress ongoing EMG. To assess whether these cells are used in normal voluntary contractions, we used TMS at very low intensities to suppress the firing of single motor units in biceps brachii (n= 14) and first dorsal interosseous (FDI, n= 6). Their discharge was recorded with intramuscular electrodes and cortical stimulation was delivered at multiple intensities at appropriate times during sustained voluntary firing at ∼10 Hz. For biceps, high-intensity stimulation produced facilitation at 17.1 ± 2.1 ms (lasting 2.4 ± 0.9 ms), while low-intensity stimulation (below motor threshold) produced suppression (without facilitation) at 20.2 ± 2.1 ms (lasting 7.6 ± 2.2 ms). For FDI, high-intensity stimulation produced facilitation at 23.3 ± 1.2 ms (lasting 1.8 ± 0.4 ms), with suppression produced by low-intensity stimulation at 25.2 ± 2.6 ms (lasting 7.5 ± 2.6 ms). The difference between the onsets of facilitation and suppression was short: 3.1 ± 1.2 ms for biceps and 2.0 ± 1.5 ms for FDI. This latency difference is much less than that previously reported using surface EMG recordings (∼10 ms). These data suggest that low-intensity cortical stimulation inhibits ongoing activity in fast-conducting corticospinal axons through an oligosynaptic (possibly disynaptic) path, and that this activity is normally contributing to drive the motoneurones during voluntary contractions.
The control of human voluntary movement involves activation of both excitatory and inhibitory connections in the motor cortex. The activity of these cortical circuits may be investigated by direct recordings from cortical cells during voluntary tasks in non-human primates (e.g. Evarts, 1965; Lemon et al. 1976; Fetz & Cheney, 1980; for review see Phillips & Porter, 1977). In humans, the rapidly conducting component of the corticospinal pathway and its possible involvement in voluntary movements has been investigated indirectly with magnetoencephalography (e.g. Salenius et al. 1997), electroencephalography (e.g. Macefield & Gandevia, 1991; for review see Colebatch, 2007), and stimulation of the motor cortex (e.g. Day et al. 1989; Palmer & Ashby, 1992; Nielsen et al. 1993).
Magnetic or electrical stimulation of the motor cortex is a non-invasive method to assess the excitability of the motor pathways in awake human subjects in which the stimuli and evoked responses can be timed precisely (for reviews see Rothwell et al. 1991; Petersen et al. 2003). These studies have usually focused on the evoked excitatory output from corticospinal cells. This output evokes a short-latency response with a central conduction time of ∼5 ms for upper limb muscles (Merton & Morton, 1980; Rothwell et al. 1991). Central conduction times measured to motor nuclei at many spinal levels are consistent with a rapidly conducting projection at ∼70 m s−1 (e.g. Gandevia & Plassman, 1988). However, stimulation at intensities subthreshold for evoking motor potentials can inhibit the output to a second cortical stimulus (Kujirai et al. 1993). When delivered alone, very low-intensity TMS can reduce the ongoing electromyographic activity during a voluntary contraction (Davey et al. 1994) and during walking (Petersen et al. 2001). This is believed to occur by suppression of ongoing corticospinal excitation to motoneurones by activation of intracortical inhibitory circuits. If the low-intensity TMS activates intracortical inhibitory cells with only one or a few synapses to the motor cortical output cell (e.g. Kujirai et al. 1993; Fisher et al. 2002), the suppression would occur at a latency not much longer than that of the short-latency facilitation evoked by higher stimulus intensities. However, the latency of the suppression of the surface EMG normally occurs ∼10 ms after the short-latency facilitation (Davey et al. 1994; Petersen et al. 2001). One interpretation of this long delay is that the cortical stimulus suppresses the output of corticospinal cells with slowly conducting axons (or indirect paths), and that it is these cells that drive the voluntary contraction. The present study was designed to resolve this paradox.
Rather than recording surface EMG, we have studied the change in firing probability of single motor units evoked by TMS to obtain an accurate comparison of the latency of the initial facilitation with that of pure suppression (in the absence of facilitation). We hypothesized that the latency difference between the facilitation evoked by high-intensity stimulation and the suppression evoked by low-intensity stimulation would be short. This would be consistent with activation of oligosynaptic intracortical inhibitory circuits, which reduce output of the rapidly conducting corticospinal cells during the voluntary contraction.
Methods
Experiments were performed on six healthy adult subjects with no history of neurological disorders. They were studied on multiple occasions. In one subject, only multiunit studies were performed. Subjects were seated comfortably in a chair. All procedures were approved by the local ethics committee and conformed to the Declaration of Helsinki. Subjects gave informed written consent to participate in the study.
EMG recordings
EMG activity was recorded from right biceps brachii or right first dorsal interosseus (FDI) using a concentric needle electrode (13R05 Dantec Medical, Denmark). The electrode was positioned such that activity from a single motor unit could be clearly identified during a weak contraction (< 5% maximal contraction strength). In some studies, multiunit activity was recorded from the needle electrode. Auditory feedback of the EMG activity was provided to help the subject to maintain a continuous stable contraction throughout the experiment. EMG signals were amplified, filtered (25 Hz – 3 kHz), sampled (10 kHz) and stored to computer via an analog-to-digital interface (micro 1401 and Spike2 software, Cambridge Electronic Design, UK).
To allow on-line analysis of single motor unit firing, the EMG signal was passed through a window discriminator (Digitimer D130 spike processor, Welwyn Garden City, UK), and a running histogram of interspike intervals was displayed.
Transcranial magnetic stimulation of the motor cortex
During the sustained weak contraction of the identified single motor unit, transcranial magnetic stimuli (TMS) were delivered over the contralateral motor cortex with either a round (13 cm outside diameter coil, the current running in the clockwise direction) or a figure-of-eight prototype coil (diameter of the wing 9 cm, handle orientated postero-laterally) and a Magstim Rapid stimulator (Magstim Company Ltd, Dyfed, UK). During previous studies of suppression of EMG by cortical stimulation, both a figure-of-eight coil and round coil have been used (Davey et al. 1994; Petersen et al. 2001). No obvious differences in the evoked suppression have been noted (also confirmed here for single unit recordings). Similarly, the rapid rate stimulator produces a biphasic stimulus pulse. Although the effect of such a stimulus may differ from the standard monophasic stimulus pulse, the study by Petersen et al. (2001) used both a rapid rate stimulator and a standard Magstim 200. In that study no difference in the suppression of the EMG was seen. In the present study, a rather high stimulus rate was optimal because the recording time for the the units was limited.
Stimuli (20–65% maximal stimulator output) were delivered at ∼1.1 s intervals with four different conditions. These included: no stimulus (0%) as a control, a subthreshold stimulus that was intended to produce suppression but no initial facilitation, a slightly higher intensity stimulus (by ∼5%) that would also produce suppression, and a higher intensity stimulus (by ∼10%) that would produce initial facilitation. Note that the high-intensity stimulus does not evoke an overt motorevoked potential and, in the conventional sense, when referring to excitation of the muscle it is ‘subthreshold’. To emphasize the low intensity of stimulation used in this study, we define ‘very low’ intensity stimulation as the lowest level of ‘subthreshold’ stimulation which produces pure short-latency suppression. The four stimulus conditions were delivered in random order. Stable recording sessions over at least 10 min were required to obtain sufficient stimuli (> 100) in each condition to evaluate the effect of TMS in the single motor units. Throughout the text the intensities are given as the percentage output on the stimulator.
Only after a large number of stimuli were obtained could we get an indication of the success of the initial choice of stimulus intensities. Occasionally we needed to do an extra run to get a measure of the suppression or of the facilitation. It has been reported that TMS at an interstimulus interval of about 1 Hz may produce effects on cortical excitability in hand muscles (Chen et al. 1997) although this does not occur for elbow flexor muscles (Martin et al. 2006). For experiments involving single motor unit recording a high stimulus rate is preferred. Nevertheless, a possible effect of the interstimulus interval would be the same for all the intensities as they were delivered in random order. Furthermore, we found no indication of a strong effect of the magnetic stimulus itself when we compared the results to the first and second half of the recordings, although a small progressive effect on overall excitability cannot be ruled out.
Cortical stimuli were not delivered at random times, but were given at specific intervals after a discharge of the motor unit and these intervals were set depending on the firing rate of the unit. In previous studies which focused on excitation of single units, the interval following the ‘trigger’ spike was short so as to permit the excitatory stimulus to activate the motor unit and increase its firing probability at a short latency (before the next spontaneous firing) (e.g. Hultborn et al. 1987; Nielsen et al. 1993). In the current study, stimuli were triggered from the discharge of the identified motor unit with a long delay (70–100 ms; Fig. 1, right panels) set so that the effect of the stimulus at the motoneurones was timed to arrive close to the spontaneous discharge of the single unit (determined from the on-line analysis of firing intervals during the sustained voluntary contraction, Fig. 1A and B). This procedure improved the likelihood that the stimulus would delay the firing of the motor unit and it reduced the number of trials required to measure evoked changes in the probability of firing (e.g. Fournier et al. 1986). For some units, additional runs were needed in which a shorter delay was set (< 70 ms) to produce a spontaneous firing probability that would favour observation of facilitation by a high-intensity cortical stimulus (Fig. 1, left panels).
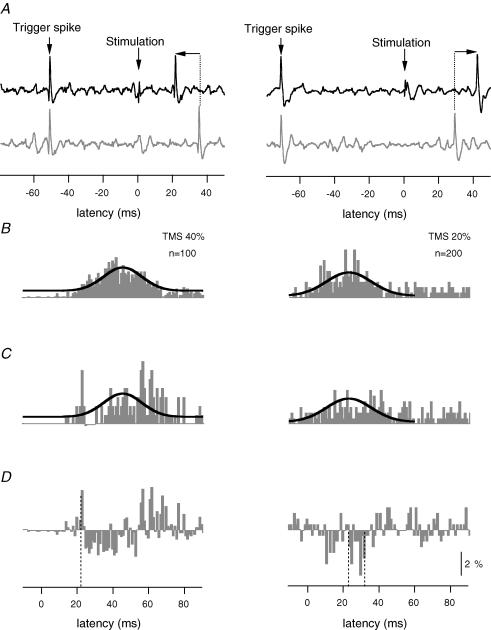
Figure 1. Illustration of the method to evoke and analyse short-latency facilitation (left panels) with high-intensity TMS and short-latency suppression (right panels) with low-intensity TMS.
A, left and right panels, single trials of motor unit activity in a stimulated (black trace) and a control (grey trace) condition. Both conditions are triggered from the previous spike (Trigger spike) with a delay (50 ms left panel and 70 ms right panel) set so that the effect of the stimulus would occur prior to the time (left panels) or at about the time (right panels) that the firing probability of the unit was highest. Note that after the cortical stimulus (indicated by a vertical arrow at time zero) the firing of the motor unit is advanced (left panel) or delayed (right panel) in the stimulated condition relative to the control condition (shown by the horizontal arrows). B, left and right panels, poststimulus time histogram (PSTH) of the firing of the motor unit triggered from a Trigger spike without stimulation. A Gaussian curve has been fitted to the histogram. This curve is also superimposed on the PSTH in C, which shows the firing probability of the motor unit after high-intensity (40%, left panel) or very low-intensity (20%, right panel) stimulation of the motor cortex. For very low-intensity TMS, the timing of the stimulation was set to reduce the firing probability of the motor unit around the time of the highest probability of firing in the control condition (B). The histograms in the left and right panels are produced from 100 and 200 trigger spikes, respectively. D, left and right panels, subtracted histograms derived from the difference between histograms C and B. Vertical dashed lines indicate facilitation (left panel) and suppression (right panel). For panels B, C and D the vertical calibration bar represents a firing probability of 2%.
Data analysis
Single motor unit activity was discriminated off-line using a template-matching algorithm (Spike2 version 4). Each triggered sweep was visually inspected (using custom software in MATLAB) to confirm that the motor unit was correctly identified. For each motor unit, post-stimulus time histograms (PSTHs) were constructed for each of the four stimulus conditions (1 ms bin width). The non-stimulated control condition produced a histogram that reflects the distribution of firing times of the single motor units when driven by volition (Fig. 1B). This is a near-Gaussian distribution (black line in Fig. 1B) because it is triggered from the previous firing of that unit (cf. Matthews, 1996). In the stimulated condition, the PSTH reflects the change in the distribution of firing times as a result of the TMS. The Gaussian curve from the control condition is superimposed in the stimulated condition in Fig. 1C, to help illustrate that there are evoked changes from the expected distribution. The decrease or increase in the discharge of the single motor units as a result of the TMS (Fig. 1D) was formally calculated by subtraction of the control PSTH (Fig. 1B) from the stimulated PSTHs (Fig. 1C). For each of these ‘difference’ histograms a cumulative sum was calculated. Each histogram was normalized to the number of trigger stimuli in each condition, to give a value of the probability of firing that could be compared across conditions.
Increased firing probability of each motor unit was identified by visual inspection as a peak in the difference PSTH constructed from the stimuli of high intensity. The cumulative sum was used to assist accurate measurement of the onset latency and the duration of the peak. Similarly, for low-intensity stimuli, we measured the onset latency and duration of the suppression from the PSTH. In addition, the size of the reduction in the probability of firing for each motor unit was measured from the number of negative counts in the time period of the suppression in the difference histogram.
For the multiunit recordings in which many motor units were discharging, PSTHs were constructed based on threshold crossings of the signal. Each time the multiunit signal crossed a user-defined threshold, an event was created that represented the firing of a motor unit. PSTHs were constructed using these events for each of the four stimulus conditions and analysed as described above. The measurements of facilitation and suppression were the same as those described for the single motor units.
Statistics
The evoked changes in probability of firing of the single motor units were assessed using a paired t test. The probability of firing of single motor units during the suppression was compared with the probability of firing during the non-stimulated condition. Data are presented as mean ±s.e.m. Statistical significance was set at the 5% level.
Results
Data were obtained from 24 motor units in biceps brachii and 13 motor units in FDI. The mean firing rate of the motor units in biceps was 10.7 ± 0.3 Hz and in FDI was 9.4 ± 0.5 Hz.
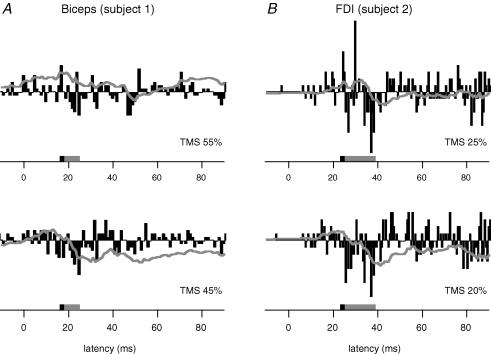
Several TMS intensities were used for each motor unit, and we were able to identify intensities at which a clear suppression occurred in 14 biceps units and 6 FDI units. A facilitation at short latency was evoked with higher intensities of stimulation. In the 17 remaining motor units (10 biceps, 7 FDI) we did not observe suppression without a preceding facilitation or we did not observe a short-facilitation with the standard run involving four intensities of stimulation. While we could not ‘isolate’ the suppression with the stimulus levels used, it would presumably be possible if more stimulus levels had been examined, and so failure to document suppression for these units does not imply that they cannot be suppressed by cortical disfacilitation. Hence, these units were not considered further. Figure 2 shows the difference PSTHs (derived from control and stimulated conditions) for a single motor unit in biceps (left panels) and FDI (right panels) from two subjects. The suppression of voluntary activity was clear in in both muscles (at 16 ms, firing probability reduced by 35% in biceps, and at 23 ms, firing probability reduced by 58% in FDI, Fig. 2). The stimulus intensities required to produce suppression with no facilitation ranged from 20 to 45% of stimulator output, and were usually lower for FDI than biceps. Evidence for suppression of single motor unit activity was seen in all five subjects.
Figure 2. Typical data for a single motor unit in biceps and first dorsal interosseus (FDI).
Difference histograms to show the response to two levels of very low-intensity TMS in a motor unit from biceps (A, n= 400 triggers) and FDI (B, n= 200 triggers). Cortical stimulation was delivered at time zero. The time of the suppression is indicated by the grey box in each histogram and the time-window for the facilitation seen at high-intensity TMS (not illustrated) is indicated by a black box. The grey line indicates the cumulative sum for the difference histogram. At both intensities of stimulation there is a suppression of firing of the motor unit.
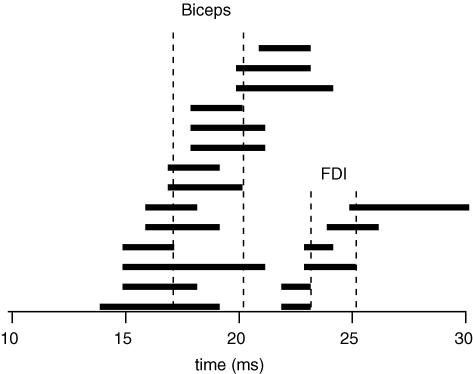
The onset latency of the suppression of activity by low-intensity TMS for the biceps units was 20.2 ± 2.1 ms with a duration of 7.6 ± 2.2 ms. The onset latency of the suppression for the FDI units was 25.2 ± 2.6 ms and the duration was 7.5 ± 2.6 ms. For these units, when there was facilitation with a higher TMS intensity, it occurred at 17.1 ± 2.1 ms with a duration of 2.4 ± 0.9 ms for biceps. For FDI the facilitation occurred at 23.2 ± 1.2 ms and lasted 1.8 ± 0.4 ms. The mean difference between the onset of the facilitation and the onset of the suppression when there was no facilitation was 3.1 ± 1.2 ms for biceps and 2.0 ± 1.5 ms for FDI. The latencies for facilitation and suppression are plotted for all 20 units in Fig. 3. With the available data, there was no evidence that the latency difference depended on the type of stimulating coil.
Figure 3. Differences in timing between the onset of facilitation and the onset of suppression after TMS for each motor unit.
Each horizontal line represents data from one motor unit and indicates the time difference between the onset of facilitation (left end) and the onset of the suppression (right end). Motor units are ordered by the onset time of the facilitation for biceps and FDI. Cortical stimulation was delivered at time zero.
The amplitude of the suppression was calculated from the change in probability of firing during the period of suppression in the control and stimulated conditions. Low-intensity TMS reduced the probability of firing of biceps single motor units from 17.7 ± 6.9% to 10.2 ± 4.2% (reduction of 41%, P < 0.001). For FDI motor units, the probability of firing was reduced from 13.4 ± 5.1% to 7.0 ± 4.1% during the suppression (reduction of 49%, P < 0.005).
Multiunit activity
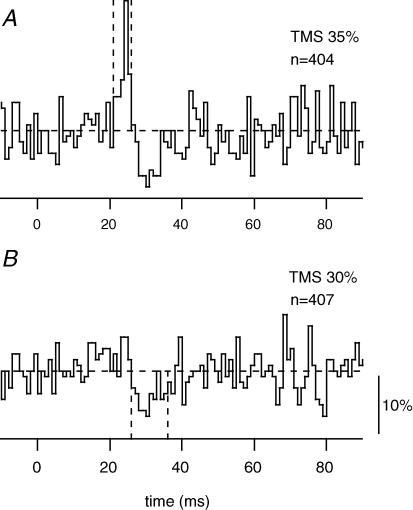
For biceps, the PSTHs based on threshold-triggered multiunit EMG (n= 7) showed a difference in latency between the facilitation and suppression of 6.6 ± 0.9 ms. This difference is double that for single biceps units, but closer to that seen with the surface EMG recordings (Davey et al. 1994; Petersen et al. 2001; confirmed here). The duration of the facilitation was also prolonged (5.0 ± 0.4 ms). This might partly account for the increase in the latency difference. The results were similar for two multiunit recordings in FDI, one of which is illustrated in Fig. 4. Facilitation was observed at 22 ms (duration ∼4 ms) with a stimulus intensity of 35%. At a reduced stimulus intensity (30%), suppression with no facilitation began at 26 ms, so that the latency difference in this example was 4 ms.
Figure 4. An example of multiunit EMG response to two levels of TMS.
PSTHs derived from threshold-triggered multiunit EMG activity from FDI in one subject. A, response to high-intensity TMS. B, response to very low-intensity TMS. The threshold-trigger level was set to include activity from ∼3 motor units. The horizontal dashed line indicates the mean level for the unstimulated control trials. The timing of the facilitation and the suppression are indicated by vertical dashed lines. The vertical calibration bar represents a firing probability of 10%.
Discussion
Our results show that activation of intracortical inhibitory circuits by very low-intensity, subthreshold TMS can reduce the firing probability of voluntarily activated motor units. By comparison with the initial excitatory response evoked by high-intensity stimulation, this reduction occurs at short latency. This suggests that activity in fast-conducting corticospinal axons is driving the motoneurones during the voluntary motor task.
The mean latency to the initial facilitation seen here for biceps and first dorsal interosseous (Fig. 3) was similar to that observed previously (e.g. Day et al. 1989; Palmer & Ashby, 1992; de Noordhout et al. 1999). This suggests that we have made comparisons of the latency of suppression with an appropriately fast conducting, descending excitatory pathway. The spread of the onset latencies of both the facilitation and the suppression between units is likely to reflect in part the precise recording location along the muscle fibres of the motor unit. The short duration of the facilitatory peaks (< 2.5 ms, Palmer & Ashby, 1992) in the current study are consistent with a fast rising excitatory postsynaptic potential (e.g. Fetz & Gustafsson, 1983) originating from monosynaptic corticomotoneuronal projections. Other studies using electrical stimulation of the corticospinal axons have shown peaks of slightly shorter duration (e.g. Day et al. 1989; Nielsen et al. 1995; Petersen et al. 2002). The slightly wider peaks seen here and elsewhere with TMS (Palmer & Ashby, 1992) may reflect the activation of multiple descending volleys (I waves) evoked by the magnetic stimulus (Burke et al. 1993; Di Lazzaro et al. 1998a; see also Edgley et al. 1997). When studied during a voluntary contraction, at least the earliest latency of the facilitation is likely to represent activation of the most direct pathway from the motor cortex to the motoneurones. The suppression of low-threshold motor units by low-intensity TMS was observed on average only 2–3 ms after the onset latency of the facilitation produced by higher stimulus intensities in the same motor unit. Because this difference in timing is short, the low-intensity TMS is also likely to affect activity in the same cortical cells that project to the motoneurones via rapidly conducting corticospinal paths. Therefore, such cells are likely to be contributing to the ongoing excitatory drive received by the motoneurones in a voluntary contraction.
We believe it is unlikely that the very low-intensity TMS produces the suppression via spinal networks. Petersen et al. (2001), in studies of the lower limb, failed to demonstrate a similar suppression to that evoked with TMS with transcranial electrical stimulation. The output from transcranial electrical stimulation is much less affected by the excitability of the cortical cells and acts on the proximal axon rather than the soma (e.g. Di Lazzaro et al. 1998a; see also Burke et al. 1993). In addition, responses to TMS had a lower threshold in tibialis anterior than in soleus. One pathway that could mediate the suppression is that causing reciprocal inhibition. A similar argument may be used for the biceps muscle as the threshold for the triceps brachii is higher than biceps. As the intrinsic hand muscles have a low threshold for cortical stimulation (e.g. Merton & Morton, 1980; Gandevia & Rothwell, 1987; Rothwell et al. 1991; Palmer & Ashby, 1992), it is unlikely that another pathway could be activated with weak magnetic stimulus and produce suppression of the motoneurones at short latency. Finally, direct recordings from the spinal cord have not been able to demonstrate any descending volleys to subthreshold TMS (Di Lazzaro et al. 1998b).
Paired-pulse stimulation introduced by Kujirai et al. (1993) is a technique used to study intracortical excitability. In this protocol, stimulus at an intensity subthreshold for an excitatory response in motoneurones inhibits the response to a subsequent suprathreshold stimulus when the interval between the two pulses is ∼2 ms (e.g. Kujirai et al. 1993; Fisher et al. 2002). This interval is similar to the latency difference between the onset latency of the facilitation and the suppression in the current study. This similarity in timing using comparably low-intensity stimuli suggests that the same intracortical circuits that inhibit fast-conducting corticospinal cells may be involved. Studies using the paired-pulse protocol indicate that the inhibition is mediated via oligosynaptic or even disynaptic actions that reduce the excitability of the cortical output cells (e.g. Kujirai et al. 1993: Ziemann et al. 1996; Nakamura et al. 1997; Di Lazzaro et al. 1998b).
The latency of the suppression in this study is shorter than previously reported in studies using surface recordings of EMG (Davey et al. 1994; Petersen et al. 2001). These studies reported a 10 ms difference in latency between the onset of the facilitation and the suppression. This comparatively late suppression may suggest that indirect (non-monosynaptic) or slowly conducting descending pathways are inhibited by the low-intensity TMS. However, our studies of single motor units suggest that this is not the case. Artefactual delays in the onset latency of EMG inhibition or disfacilitation can occur with surface EMG recordings due to alignment of the repolarization phase of the EMG potentials for many motor units that, when rectified and averaged, appear as a false EMG peak or as an apparent delay in the onset of any inhibition (Widmer & Lund, 1989; Poliakov & Miles, 1992). Under such conditions the extent of the artefactual delay can be ∼10 ms (see Fig. 2 in Poliakov & Miles, 1992). In addition, the different conduction velocities of motor axons among the motor units that comprise the surface EMG will result in dispersion of the timing of the muscle action potentials and will blur the facilitation and suppression. One way to illustrate this is to construct PSTHs from event triggering of multiunit EMG made with intramuscular electrodes. This procedure minimizes the artefact due to the synchronized repolarization phase of the EMG potentials but still shows the effects of the different conduction times of the active motor units. We found that the facilitation peak, in multiunit PSTHs, was ∼5 ms in duration, presumably due not only to the different conduction velocities of the motor axons, but also to the location of the recording electrode. The longer duration of the facilitatory peak consequently delays the appearance of suppression at least in those motor units that are driven by faster conducting corticospinal axons.
The use of very low-intensity TMS to suppress voluntary activity, particularly when assessed from single motor units, offers a method to investigate the excitability of cortical cells that are actively involved in the current motor task. The short latency of the suppression in single motor units suggests that the cortical cells involved in the motor tasks project to the motoneurones via fast conducting axons including those with monosynaptic connections. Not withstanding the difficulties in interpretation of PSTHs derived from multiunit recordings, the findings based on single motor units may help with interpretation of studies with surface recording of EMG. Such recordings allow investigation of the cortical involvement in different motor tasks that can be performed naturally without perturbations, such as walking and breathing. When a reduction in the rectified surface EMG has a long delay from the earliest onset of excitation to cortical stimulation (∼10 ms), it is reasonable to conclude that there has been cortical disfacilitation of the voluntarily active motoneurones (Petersen et al. 2001). However, to infer that this disfacilitation involves rapidly conducting corticospinal paths requires additional studies of the latencies of the excitatory and disfacilitatory effects in single motor units. Alternatively, improved methods are needed to remove some of the inevitable artefacts due to rectification and synchronization in surface EMG recordings (Poliakov & Miles, 1992).
In summary, we report that appropriately timed stimulation of the human motor cortex can produce pure suppression of the firing of single motor units in two upper limb muscles, including an intrinsic muscle of the hand. The difference in latency between the onset of facilitation produced by high-intensity stimulation of the motor cortex and suppression produced by very low-intensity stimulation is short. Our data are consistent with the view that very low-intensity cortical stimulation activates intracortical circuits which, via an oligosynaptic path, inhibit the output of corticospinal cells with short-latency connections to motoneurones. An important corollary of this view is that activity in the fast-conducting corticospinal axons is probably responsible for driving motoneurones in volitional contractions.
Acknowledgments
This work was supported by the Novo Nordic Foundation and the National Health and Medical Research Council of Australia.
References
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Colebatch JG. Bereitschaftspotential and movementrelated potentials: Origin, significance, and application in disorders of human movement. Mov Disord. 2007;22:601–610. doi: 10.1002/mds.21323. [DOI] [PubMed] [Google Scholar]
- Davey NJ, Romaiguere P, Maskill DW, Ellaway PH. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. J Physiol. 1994;477:223–235. doi: 10.1113/jphysiol.1994.sp020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Noordhout AM, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ. Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain. 1999;122:1327–1340. doi: 10.1093/brain/122.7.1327. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998a;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998b;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120:839–853. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of discharge frequency to conduction velocity in pyramidal tract neurons. J Neurophysiol. 1965;28:216–218. doi: 10.1152/jn.1965.28.2.216. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M. Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol. 1986;377:143–169. doi: 10.1113/jphysiol.1986.sp016179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Plassman BL. Responses in human intercostal and truncal muscles to motor cortical and spinal stimulation. Respir Physiol. 1988;73:325–337. doi: 10.1016/0034-5687(88)90054-0. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987;110:1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of I a fibres: a study in man and the cat. J Physiol. 1987;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN, Hanby JA, Porter R. Relationship between the activity of precentral neurones during active and passive movements in conscious monkeys. Proc R Soc Lond B Biol Sci. 1976;194:341–373. doi: 10.1098/rspb.1976.0083. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. J Physiol. 1991;439:545–558. doi: 10.1113/jphysiol.1991.sp018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Theta burst stimulation does not reliably depress all regions of the human motor cortex. Clin Neurophysiol. 2006;117:2684–2690. doi: 10.1016/j.clinph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Ballegaard M. Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. J Physiol. 1995;484:791–802. doi: 10.1113/jphysiol.1995.sp020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Deuschl G, Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol. 1993;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Butler JE, Marchand-Pauvert V, Fisher R, Ledebt A, Pyndt HS, Hansen NL, Nielsen JB. Suppression of EMG activity by transcranial magnetic stimulation in human subjects during walking. J Physiol. 2001;537:651–656. doi: 10.1111/j.1469-7793.2001.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Pyndt HS, Nielsen JB. Investigating human motor control by transcranial magnetic stimulation. Exp Brain Res. 2003;152:1–16. doi: 10.1007/s00221-003-1537-y. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Gandevia SC. The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. J Physiol. 2002;544:277–284. doi: 10.1113/jphysiol.2002.024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Porter R. Corticospinal Neurones. Their Role in Movements. Vol. 34. London: Academic Press; 1977. Monographs of the Physiological Society. [PubMed] [Google Scholar]
- Poliakov AV, Miles TS. Quantitative analysis of reflex responses in the averaged surface electromyogram. J Neurosci Meth. 1992;43:195–200. doi: 10.1016/0165-0270(92)90029-d. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Lund JP. Evidence that peaks in EMG averages can sometimes be caused by inhibition of motoneurons. J Neurophysiol. 1989;62:212–219. doi: 10.1152/jn.1989.62.1.212. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]