Abstract
Sensory signals of widely differing dynamic range and intensity are transformed into a common firing rate code by thalamocortical neurons. While a great deal is known about the ionic currents, far less is known about the specific channel subtypes regulating thalamic firing rates. We hypothesized that different K+ and Ca2+ channel subtypes control different stimulus–response curve properties. To define the channels, we measured firing rate while pharmacologically or genetically modulating specific channel subtypes. Inhibiting Kv3.2 K+ channels strongly suppressed maximum firing rate by impairing membrane potential repolarization, while playing no role in the firing response to threshold stimuli. By contrast, inhibiting Kv1 channels with α-dendrotoxin or maurotoxin strongly increased firing rates to threshold stimuli by reducing the membrane potential where action potentials fire (Vth). Inhibiting SK Ca2+-activated K+ channels with apamin robustly increased gain (slope of the stimulus–response curve) and maximum firing rate, with minimum effects on threshold responses. Inhibiting N-type Ca2+ channels with ω-conotoxin GVIA or ω-conotoxin MVIIC partially mimicked apamin, while inhibiting L-type and P/Q-type Ca2+ channels had small or no effects. EPSC-like current injections closely mimicked the results from tonic currents. Our results show that Kv3.2, Kv1, SK potassium and N-type calcium channels strongly regulate thalamic relay neuron sensory transmission and that each channel subtype controls a different stimulus–response curve property. Differential regulation of threshold, gain and maximum firing rate may help vary the stimulus–response properties across and within thalamic nuclei, normalize responses to diverse sensory inputs, and underlie sensory perception disorders.
A great diversity of signals with widely different dynamic ranges and intensities are first transformed into a thalamocortical (TC) neuron firing rate code and then transmitted to forebrain circuits (cerebral cortex, amygdala, and striatum) for integration. To understand the regulation of this transform function of TC neurons, we sought to define the ion channels that regulate the stimulus–response firing rate curve in a thalamic nucleus containing a relatively homogeneous population of calbindin-positive relay neurons, the lateral dorsal nucleus (Jones, 2001).
Understanding the ion channels that regulate thalamic sensory relay neurons is important since inherited mutations of these ion channels could underlie aberrant sensory function in some human neurological diseases. Thalamus-based sensory perception disorders could include the low threshold for sensory arousal that occurs in certain forms of insomnia (Anderson et al. 2005), the sensory auras that occur in certain forms of epilepsy (Kalachikov et al. 2002), the sensory hallucinations that occur in schizophrenia (Sim et al. 2006), and the sensory hyperresponsiveness found in fragile X and autism (Miller et al. 1999).
Thalamic relay neurons fire continuously in response to various sensory stimuli (e.g. Calton & Taube, 2005) and during reward anticipation following conditioning (Komura et al. 2001). In vitro, firing rate is continuous and non-accommodating at rates proportional to the depolarizing current. In vivo, firing would be initiated by continuous depolarizing currents generated by metabotropic glutamate receptors (Hughes et al. 2002) and neuromodulatory hormones (e.g. orexin, Bayer et al. 2002) and by phasic depolarizing currents generated by ionotropic glutamate receptor synaptic transmission.
The use of dissociated and young thalamic relay neurons is necessary to study ionic current since this method minimizes dendritic and axonal arbors and facilitates voltage-clamp experiments. Using this approach, a variety of K+ currents that could potentially regulate firing have been defined in thalamic relay neurons (Huguenard et al. 1991; Huguenard & Prince, 1991). A low threshold K+ current was further shown to regulate tonic firing (McCormick, 1991). However, such studies predated the development of targeted gene deletion and high affinity peptide toxins that can link specific channel subtypes to the regulation of thalamic relay neuron firing. Interestingly, a recent study did provide evidence that ryanodine-receptor-mediated calcium release inhibits action potential firing in thalamic relay neurons through an unidentified, calcium-regulated K+ channel subtype (Budde et al. 2000).
Our goal is to define and compare the relative role of specific K+ and Ca2+ channel subtypes in regulating thalamic relay neuron firing using highly selective peptide toxins, gene deletion techniques, and other pharmacological tools. Thalamocortical neurons are of particular interest because of their central role in sensory transmission and their distinctiveness as a glutamatergic projection neuron with an extensive dendritic and axonal arbor yet which fire continuously at very high rates exceeding 150 Hz in vivo, similar to fast spiking GABAergic interneurons (Komura et al. 2001). Importantly, certain ion channels are only expressed in thalamus beyond 3 weeks of age (e.g. Kv3.2 Hernandez-Pineda et al. 1999). Consequently, we developed methods to record fast firing in adult (3–7 month old) thalamocortical neurons.
We report that in adult thalamocortical neurons, firing responses to threshold current injections are strongly inhibited by a Kv1 low-threshold K+ channel, and moderately inhibited by an M-type, a low [Ba2+]-sensitive, a BK Ca2+-activated, and a K-ATP K+ channel subtype. Gain, the maximum slope of the current stimulus–firing rate curve, is strongly limited by the N-type Ca2+ channel and SK Ca2+-activated K+ channel beyond 10–30 Hz, while P/Q-type, L-type and T-type Ca2+ channels had limited roles in regulating firing. Maximum firing rate, the highest rate achieved with saturating current injections, was altered by many of the channels listed above, but was prominently enhanced by activity of the high threshold K+ channel Kv3.2.
Methods
Slice preparation and electrophysiological recordings
All procedures were performed in accordance with animal experimental protocols approved by the Harvard University Office for Research Subject Protection, an agency accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC). The procedures used in these experiments were similar to those previously described (Anderson et al. 2005). Both Cav3.1 (α1G) and Kv3.2 knockout mice were studied in a C57BL6 genetic background and were genotyped as previously described (Anderson et al. 2005; Lau et al. 2000). Controls were wild-type littermate mice. Adult (3–7 months olds) or immature (15 days old) C57/BL6 mice were deeply anaesthetized with isofluorane and decapitated. Recordings from male and female mice were not significantly different and therefore the results were combined. The brain was quickly removed and placed in ice cold, oxygenated (5% CO2) sucrose cutting solution containing (mm): 234 sucrose, 5 KCl, 5 MgSO4, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO2 and 10 glucose. Coronal brain slices (230 μm) were cut using a Vibratome 3000 tissue slicer in zero Z-axis vibration mode. Slices were transferred to oxygenated (5% CO2) artificial cerebrospinal fluid (ACSF) for 30–60 min at 37°C and then stored at room temperature for 1–9 h before recording. ACSF contained (mm): 124 NaCl, 3.5 KCl, 1 MgCl2, 1.2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 25 glucose.
Thalamocortical relay neurons were visually identified for recording in the lateral dorsal thalamic nucleus at 400× magnification using infrared DIC optics on an upright Olympus BX-51WI microscope (Olympus, Tokyo, Japan). Recording pipettes were pulled from 1.5 mm OD capillary tubing (A-M Systems, Carlsborg, WA, USA) using a Flaming/Brown P-97 pipette puller (Sutter Instruments, Novato, CA, USA) and had tip resistances of 3–5 MΩ when filled with internal solution. For current-clamp recordings, internal solution contained (mm): 135 potassium methanesulphonate, 10 Hepes, 4 KCl, 2 NaCl, 4 Mg-ATP, 0.3 Tris-GTP, 7 phosphocreatine, 1 EGTA, 0.1 CaCl2 (pH 7.25, osmolarity ∼290 mosmol l−1). In experiments where EGTA concentration was reduced to 100 μm, CaCl2 concentration was reduced to 10 μm. For voltage-clamp recordings, internal solution contained (mm): 125 caesium methanesulphonate, 10 Hepes, 2 MgCl2, 4 Mg-ATP, 0.3 Tris-GTP, 7 phosphocreatine, 10 EGTA, 1 CaCl2, 2 QX-314 (pH 7.25, osmolarity ∼290 mosmol l−1).
All recordings were made using a HEKA triple EPC-10 patch-clamp amplifier utilizing Patchmaster 2 software (HEKA Instruments, Southboro, MA, USA) at ∼35°C in oxygenated (5% CO2) ACSF. After achieving a gigaohm seal, a stable recording was obtained by holding the cell in the whole-cell, voltage-clamp configuration at Vc=−70 mV. Recordings were then performed in current-clamp mode and accepted for cells with a resting Vm between −60 and −82 mV, with most cells in the range of −72 to −80 mV and a series resistance < 20 MΩ. Continuous lines mark 0 mV values within the inset traces. Series resistance was 80% compensated and the measured −7 mV junction potential was corrected. Data were unfiltered and sampled at 10–50 kHz.
Action potential analysis
All firing curves were obtained with incremental current steps of 20 pA up to 400 pA and with steps of 100 pA from 400 pA to 1 nA. Current pulses were 1 s in duration with a 5 s interpulse interval. To evaluate the role of K+ and Ca2+ channels in the firing response to excitatory postsynaptic currents (EPSCs), we examined sample EPSCs in the LDn thalamocortical neuron induced by application of 4-AP (100 μm, M. R. Kasten and M. P. Anderson, data not shown) and developed a current injection protocol that mimicked the time course of the EPSC. The EPSC-like current injection consisted of a 0.5 ms linear rise to maximum, a 1 ms linear decay to 40% of maximum and a further 5 ms linear decay to baseline (see example in Fig. 8C, below). Action potentials were identified and time-stamped utilizing MiniAnalysis software (Synaptosoft, Fort Lee, NJ, USA). Action potential properties were analysed using custom software written in MATLAB. Rheobase (or threshold current to tonic firing) was defined as the current necessary to induce tonic firing (2 or more action potentials beyond the burst distributed across the 1 s pulse). Firing gain is defined as the greatest increase of instantaneous action potential firing rate over a 100 pA current injection window for each individual cell. Maximum firing rate was the maximum number of action potentials achieved in 1 s in response to current injections up to 1 nA. Data are plotted to 700 pA since firing ceased beyond this value in some conditions.
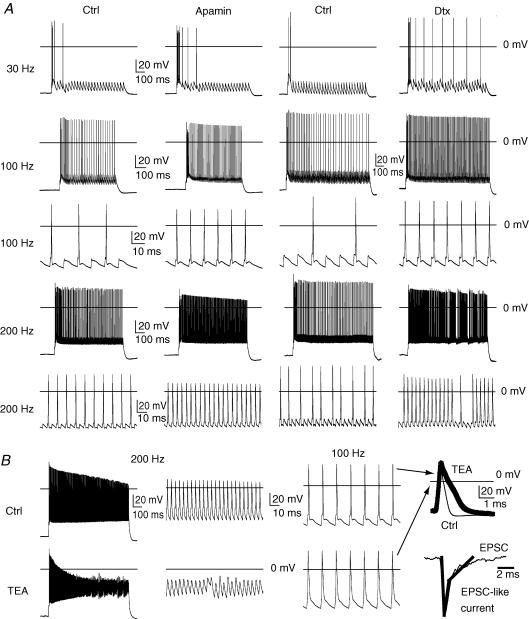
Figure 8. Role of Kv1, SK and Kv3.2 in the firing response to excitatory postsynaptic currents (EPSCs).
EPSC stimuli were designed based on the kinetics of EPSC events observed in these neurons during whole-cell voltage clamp (−70 mV, Fig. 8B bottom right). The current pulse used to model an EPSC stimulus is shown in the thicker tracing overlay with peak current reached in 0.5 ms and decaying to 40% of peak value in 1 ms. A slower component decays to baseline over a 5 ms period. A, EPSC-like stimuli at 30 Hz (1000 pA peak current) caused an initial burst of action potentials and then largely failed to evoke firing in controls (Ctrl). Washing in apamin (300 nm) to inhibit SK Ca2+-activated K+ channels in this same control neuron increased the number of spikes within the burst and improved coupling over the first few EPSC-like stimuli. Washing in α-dendrotoxin (100 nm) to block Kv1 K+ channels increased the number of spikes within the burst, but also increased EPSC-spike coupling. Bath application of apamin and α-dendrotoxin increased EPSC-spike coupling at 100 Hz (900 pA peak) and 200 Hz (700 pA peak). B, washing in the non-selective Kv3.2 channel blocker (TEA, 1 mm) depolarized the interstimulus membrane potential and attenuated action potential height with 200 Hz EPSC-like stimuli (900 pA, peak). At 100 Hz (900 pA, peak), action potentials at 100 Hz were widened, but only weakly attenuated, by 1 mm TEA (far right, thick trace versus Ctrl, thin trace, 1 ms and 20 mV scale).
To determine the properties in Table 1 and listed in the text, action potentials were analysed during low tonic firing rates (< 10 Hz). To examine action potential properties at higher firing rates for Figs 2 and 7, action potentials were analysed at the current injection where firing rate first exceeded 80 Hz or the highest firing rate achieved (if less than 80 Hz). Action potential threshold (Vth) was determined as the voltage at which the slope of the action potential reached ≥ 20 V s−1 (Bekkers & Delaney, 2001). Action potential slopes (up and down) were measured as maximum slope over a 30 mV window during the action potential. Half-width was determined as the duration of the action potential at the voltage halfway between threshold and the action potential peak. The fast after-hyperpolarization (fAHP) was the minimum voltage within 2 ms following the action potential peak subtracted from Vth. Input resistance (Vm/Iinj) was measured using a 1 s, −20 pA, −100 pA, or +20 pA current injection from rest (Table 1). In drug application experiments, firing rate curves were recorded after ≥ 7 min incubation. Multiple firing rate curves were recorded from individual cells and showed very little variation, so single firing rate curves were utilized for each cell in each condition.
Table 1.
Membrane and action potential properties
| Ctrl (n= 82) | Kv3.2 KO (n= 16) | TEA (n= 8) | d15 (n= 6) | DTX 100 nm (n= 7) | MTX 10 nm (n= 5) | Ba2+ 100 μm (n= 12) | Ba2+ 10 μm (n= 6) | Glib (n= 9) | Diaz (n= 8) | XE 991 10 μm (n= 7) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vm (mV) | −76.4 ± | −76.8 ± | −76.3 ± | − 69.8 ± | −72.7 ± | −73.5 ± | −64.8 ± | −74.8 ± | −78.0 ± | −77.6 ± | −75.3 ± |
| 0.3 | 0.7 | 1.1 | 1.2* | 2.0 | 2.4 | 1.4** | 1.0 | 0.9 | 0.7 | 0.7 | |
| Rin (MΩ) | 136.4 ± | 102.9 ± | 148.2 ± | 383.3 ± | 153.6 ± | 102.7 ± | 274.8 ± | 128.0 ± | 145.6 ± | 94.1 ± | 156.4 ± |
| (−20 pA) | 6.0 | 5.8** | 15.5 | 52.3* | 27.0 | 6.8* | 48.7* | 9.0 | 30.7 | 7.6** | 9.1 |
| Rin (MΩ) | 157.0 ± | 117.1 ± | 162.2 ± | 645.0 ± | 231.6 ± | 133.7 ± | 322.3 ± | 127.3 ± | 164.9 ± | 104.7 ± | 179.6 ± |
| (+20 pA) | 8.0 | 7.4** | 16.0 | 49.6** | 65.1 | 17.1* | 81.2 | 10.2 | 39.9 | 8.2** | 29.6 |
| Rin (MΩ) | 98.4 ± | 83.6 ± | 102.9 ± | 281.3 ± | 111.9 ± | 81.0 ± | 166.0 ± | 93.5 ± | 100.9 ± | 81.8 ± | 110.7 ± |
| (−100 pA) | 3.1 | 2.8** | 8.4 | 16.7** | 14.0 | 3.4 | 20.6* | 7.1 | 11.5 | 7.8 | 3.9* |
| Vth (mV) | −45.8 ± | −45.8 ± | −45.0 ± | −36.8 ± | −51.0 ± | −46.6 ± | −47.8 ± | −52.5 ± | −48.4 ± | −43.4 ± | −45.7 ± |
| 0.5 | 1.2 | 1.4* | 1.3** | 0.8** | 2.4 | 1.3 | 1.5* | 1.5 | 1.6 | 1.5 | |
| AP up-slope | 336.2 ± | 314.1 ± | 350.0 ± | 147.4 ± | 414.1 ± | 257.3 ± | 339.5 ± | 393.5 ± | 331.6 ± | 380.2 ± | 375.2 ± |
| (V s−1) | 3.7 | 14.5 | 25.9 | 28.3** | 19.4* | 32.6* | 16.7 | 44.4 | 22.3 | 30.9 | 31.6 |
| AP down-slope | 168.9 ± | 137.7 ± | 94.6 ± | 44.1 ± | 223.2 ± | 179.4 ± | 135.5 ± | 189.5 ± | 145.6 ± | 165.7 ± | 172.4 ± |
| (V s−1) | 3.7 | 4.4** | 7.6** | 6.3** | 17.0* | 19.1* | 6.9** | 9.7 | 7.3* | 9.4 | 7.3 |
| AP half- | 415.3 ± | 447.2 ± | 556.9 ± | 987.0 ± | 384.1 ± | 439.7 ± | 453.5 ± | 381.6 ± | 478.8 ± | 391.9 ± | 389.6 ± |
| width (μs) | 7.2 | 13.0* | 28.6** | 104.8* | 16.2 | 29.0 | 13.7* | 17.9 | 20.1* | 11.7 | 9.2* |
| fAHP (mV) | 16.7 ± | 13.1 ± | 6.5 ± | 10.4 ± | 14.9 ± | 14.4 ± | 10.2 ± | 13.3 ± | 15.7 ± | 18.8 ± | 17.9 ± |
| 0.3 | 0.8 | 1.6** | 1.2* | 0.5 | 1.9 | 1.0** | 1.5 | 1.1 | 1.5 | 0.6 | |
| mAHP (mV) | 13.1 ± | 13.7 ± | 11.5 ± | 17.7 ± | 9.1 ± | 8.4 ± | 10.8 ± | 10.1 ± | 14.2 ± | 14.0 ± | 14.1 ± |
| 0.3 | 1.0 | 0.9* | 1.4* | 0.4** | 1.1 | 0.6* | 0.8* | 1.0 | 1.0 | 0.7 | |
| Ctrl (n= 30) | Pax (n= 7) | Apamin (n= 7) | Ni (n= 11) | Nife (n= 8) | CTX MVIIC (n= 6) | CTX GVIA (n= 12) | AGA IVA (n= 9) | GKO (n= 12) | |||
| Vm (mV) | −74.8 ± | −74.7 ± | −74.4 ± | −73.6 ± | −75.0 ± | −74.0 ± | −75.6 ± | −75.4 ± | −77.6 ± | ||
| 0.3 | 3.2 | 0.3 | 0.7 | 1.3 | 1.9 | 0.6 | 0.6 | 0.6** | |||
| Rin (MΩ) | 118.8 ± | 121.7 ± | 136.9 ± | 120.9 ± | 120.9 ± | 104.2 ± | 128.3 ± | 98.4 ± | 107.8 ± | ||
| (−20 pA) | 8.3 | 10.2 | 0.3 | 10.0 | 7.1 | 14.6 | 12.4 | 7.0 | 8.4 | ||
| Rin (MΩ) | 126.3 ± | 127.5 ± | 137.1 ± | 138.2 ± | 125.3 ± | 102.1 ± | 107.7 ± | 95.3 ± | 135.8 ± | ||
| (+20 pA) | 8.5 | 11.6 | 0.3 | 14.4 | 12.9 | 13.0 | 10.4 | 11.1 | 10.4 | ||
| Rin (MΩ) | 90.0 ± | 100.0 ± | 102.8 ± | 97.3 ± | 95.6 ± | 71.0 ± | 91.9 ± | 72.1 ± | 80.7 ± | ||
| (−100 pA) | 5.1 | 2.5 | 0.3 | 9.2 | 9.4 | 10.7 | 6.1 | 7.0 | 6.6 | ||
| Vth (mV) | −46.7 ± | −49.4 ± | −45.7 ± | −47.5 ± | −48.8 ± | −49.6 ± | −46.9 ± | −46.2 ± | −45.9 ± | ||
| 0.8 | 1.2 | 0.3 | 1.1 | 1.8 | 2.7 | 1.0 | 1.1 | 1.0 | |||
| AP up-slope | 310.5 ± | 334.8 ± | 304.0 ± | 277.7 ± | 316.6 ± | 307.2 ± | 394.0 ± | 377.2 ± | 367.5 ± | ||
| (V s−1) | 13.9 | 25.8 | 0.3 | 25.2 | 19.7 | 43.1 | 25.8** | 17.7* | 40.3 | ||
| AP down-slope | 159.2 ± | 129.0 ± | 148.4 ± | 158.5 ± | 157.0 ± | 161.9 ± | 204.8 ± | 173.9 ± | 191.1 ± | ||
| (V s−1) | 3.9 | 9.6* | 0.3 | 7.9 | 6.9 | 14.0 | 12.6** | 5.6* | 13.9* | ||
| AP half- | 439.3 ± | 485.0 ± | 466.4 ± | 444.1 ± | 450.2 ± | 424.4 ± | 340.7 ± | 387.2 ± | 375.9 ± | ||
| width (μs) | 10.5 | 20.8 | 0.3 | 18.3 | 8.1 | 21.6 | 11.3** | 13.0* | 29.8 | ||
| fAHP (mV) | 16.2 ± | 11.8 ± | 17.7 ± | 13.5 ± | 14.9 ± | 13.1 ± | 16.6 ± | 16.7 ± | 19.9 ± | ||
| 0.6 | 0.7* | 0.3 | 1.1** | 0.8 | 0.8* | 0.9 | 0.7 | 1.2* | |||
| mAHP (mV) | 12.7 ± | 8.5 ± | 12.2 ± | 9.8 ± | 13.4 ± | 7.1 ± | 10.4 ± | 12.5 ± | 13.9 ± | ||
| 0.5 | 1.4* | 0.3* | 1.1** | 1.3 | 1.7* | 0.8* | 0.6 | 0.7 | |||
P < 0.05
P < 0.001, mean ±s.e.m., pipette: 100 μm EGTA (above) and 1 mm EGTA (below).
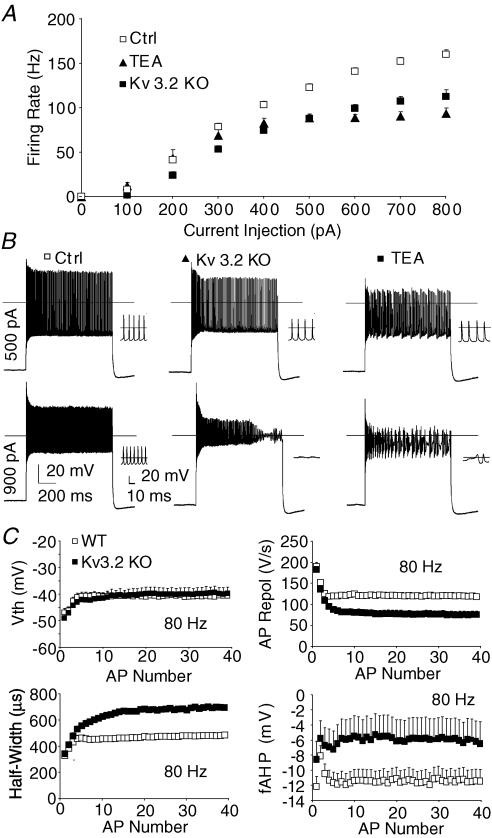
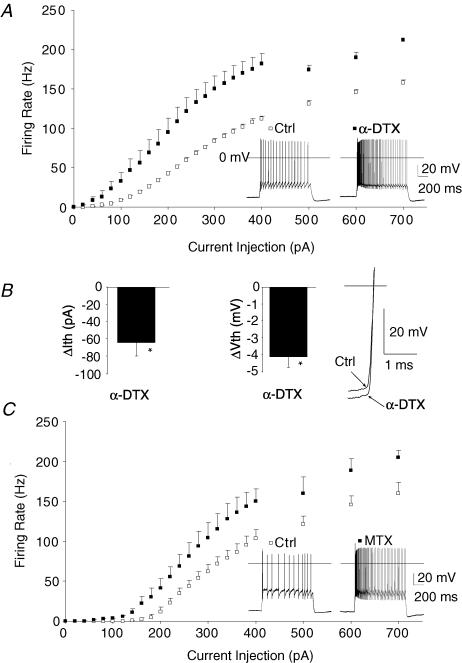
Figure 2. High threshold K+ channel KV3.2 is required to sustain high firing rates in adult thalamocortical neurons.
A, genetically deleting (Kv3.2 KO) or non-selectively inhibiting (TEA, 1 mm) Kv3.2 suppressed maximum firing of thalamocortical neurons (n= 47, control; n= 16, KV3.2 KO; n= 8, TEA). Statistical analyses by repeated-measures ANOVA: P < 0.001 (Ctrl versus TEA); P < 0.001 (Ctrl versus KV3.2 KO). B, at 500 pA, firing rate was suppressed when KV3.2 was inhibited by gene deletion (KV3.2 KO) or a non-selective blocker (TEA, 1 mm, bath) when compared to control (Ctrl). At 900 pA, tonic firing of action potentials failed during the current pulse in Kv3.2 KO. Insets show traces at a higher temporal resolution. C, during 80 Hz firing, Kv3.2 KO mice demonstrate normal action potential threshold, while half-width is elongated, action potential repolarization rate (AP Repol) is slowed, and fast after hyperpolarization (fAHP) is reduced.
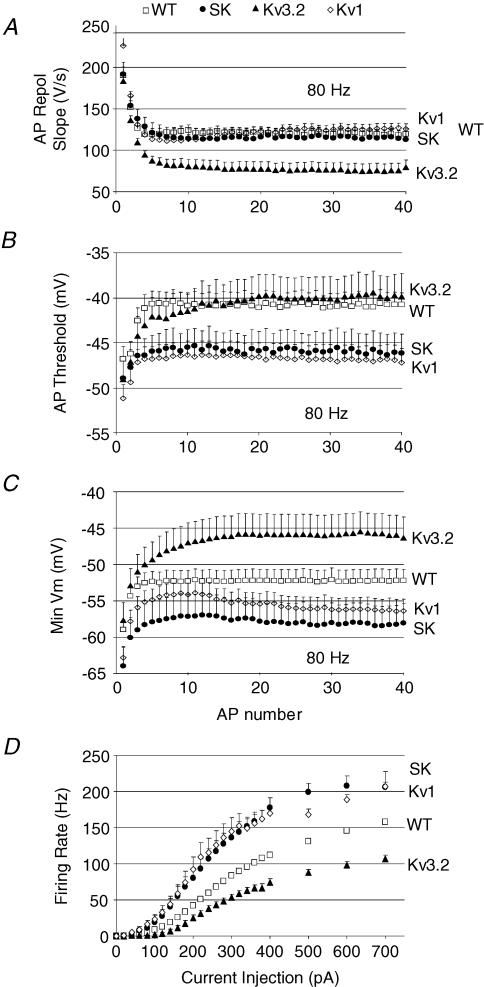
Figure 7. Differential contribution of K+ channels to action potential repolarization and threshold and membrane potential minimum while firing at high rates (80 Hz).
A, action potential repolarization is slowed when Kv3.2 is deleted (Kv3.2) and unaffected when SK or Kv1 channels are blocked compared to controls (WT). B, action potential threshold (Vth) is lowered when Kv1 or SK channels are blocked, but unaltered when Kv3.2 is deleted compared to controls (WT). C, minimum membrane potential (Min Vm) is more hyperpolarized when Kv1 and SK channels are blocked, but more depolarized when Kv3.2 is deleted compared to controls (WT). D, overlay of firing rate curves for control (WT), SK channel blocker, Kv1 channel blocker, and Kv3.2 gene deletion demonstrate different roles for these K+ channels in regulating firing.
Pharmacology and statistics
Drugs were bath applied using a gravity perfusion system in oxygenated (95% O2, 5% CO2) ACSF. All recordings of firing rate were performed in the presence of antagonists of the AMPA/kainate and GABA-A receptors, 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 μm, Tocris, Elleville, MO, USA) and picrotoxin (100 μm, Sigma, St Louis, MO, USA), respectively. The following drugs were used from Sigma: tetraethylammonium (TEA), diazoxide, glibenclamide, nifedipine, cadmium chloride, paxilline and barium chloride. The following drugs were used from Alomone Laboratories (Jerusalem, Israel): rtertiapin-Q, α-dendrotoxin, maurotoxin, ω-agatoxin IVA, ω-conotoxin GVIA, and ω-conotoxin MVIIC. The following drugs were used from Tocris Cookson (Ellisville, Missouri): XE 991, apamin, and 1-ethyl-2-benzimidazolinone (1-EBIO). Statistics were evaluated by Student's unpaired, t test for single measurements and by repeated-measures ANOVA for firing rate curves.
Results
Immature thalamocortical neurons display impaired peak firing rates
Many previous studies have examined the properties of thalamocortical neurons in young rodents (< 15 days old). We first asked whether neurons from young mice represent a good in vitro model for investigating action potential firing properties of adult thalamocortical neurons. Previous studies in cat suggested relatively late maturation of thalamocortical neuron tonic and burst action potential firing at 2–3 weeks after birth (Pirchio et al. 1997). Ramoa & McCormick (1994) also found delayed maturation of lateral dorsal geniculate neurons of ferret, but did not examine maximum firing rate. The postnatal time course of action potential firing maturation in thalamus could vary amongst species and therefore it is important to examine this property in the murine thalamus. In vivo, single unit recordings are largely only available for rat but not the mouse; in awake adult rats thalamocortical neurons peak firing rate exceeded 150 Hz (Komura et al. 2001).
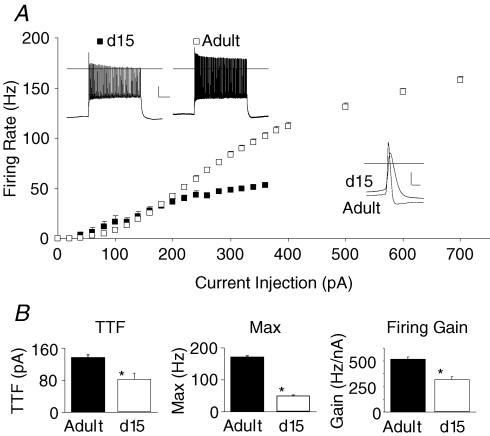
For an initial assessment of firing rate maturation in the murine thalamocortical neuron, we performed whole-cell recordings in coronal slices of lateral dorsal thalamic nucleus (LDn) of young (15 day old) and adult (3–7 months old) mice. A hyperpolarizing current injection evoked a low threshold calcium spike and action potential burst characteristic of the thalamocortical neuron in every neuron reported in this study (M. R. Kasten and M. P. Anderson, data not shown). Action potential firing responses to a 1 s tonic current injection ranging from 20 to 700 pA are plotted in Fig. 1A. In thalamocortical neurons from 15-day-old mice, rheobase (minimum current to fire) was lower (mean ±s.e.m., 80 ± 15 pA versus 139 ± 6 pA in adult, P < 0.001) and maximum firing rate was strongly reduced (48 ± 3 Hz versus 173 ± 3 Hz in adult, P < 0.001). Gain was also attenuated in 15-day-old mice (310 ± 31 Hz nA−1versus 501 ± 14 Hz nA−1 in adults; P < 0.01). Action potential half-width was much longer in 15-day-old mice (half-width = 987 ± 96 μs versus 415 ± 7 μs for adult, P < 0.001). While adult thalamocortical neurons displayed a relatively linear firing rate curve over a broad range of currents (0–700 pA), 15-day-old thalamocortical neurons failed to increase firing in response to current injections greater than 200 pA and stopped firing with current injections > 360 pA. The results indicate thalamocortical neurons of 15-day-old mice have attenuated firing responses when compared to adults. Based on these findings, all subsequent studies report the effects of ion channel blockers in the adult murine thalamus.
Figure 1. Age regulates peak action potential firing rate in lateral dorsal nucleus thalamocortical neurons.
A, adult neurons (3–7-month-old mice, n= 47) displayed a relatively linear increase of firing rate in response to increasing current injections. By contrast, immature neurons of day 15 old mice (d15, n= 6) displayed impaired firing responses reaching a plateau at ∼50 Hz. Representative voltage tracings (inset, top) demonstrate firing in response to a 300 pA current injection. Single action potentials at threshold current injections in 15 day and 3–7-month-old mice are also compared (inset, bottom). B, threshold current to initiate tonic firing (TTF), maximum firing rate (Max), and maximum firing gain were all significantly reduced in thalamic slices from d15 compared to adult mice. Statistical significance in B by t test (*P < 0.05). Calibration top: 20 mV, 200 ms, bottom: 20 mV, 1 ms.
Genetic and pharmacologic block of Kv3.2 channels reduces peak firing rate
Neurons capable of sustained firing at high frequencies generally express one of the Kv3 potassium channel subtypes. These high-voltage-activated, rapidly voltage-gated, delayed-rectifier channels are uniquely suited to support high action potential firing rates for extended periods of time (Rudy et al. 1999; Fernandez et al. 2005). Kv3.2, the major channel expressed in thalamocortical neurons, does not reach adult levels until the third postnatal week (Moreno et al. 1995). Other Kv3 subunits are only weakly expressed in dorsal thalamus (Weiser et al. 1994). Immunohistochemistry revealed that Kv3.2 protein localizes to axon termini and somatodendritic compartments in thalamocortical neurons (Moreno et al. 1995). Despite strong expression of Kv3.2 in thalamus, the function in thalamocortical neurons remains unknown.
To explore Kv3 channel function, we first blocked the channel acutely using tetraethylammonium (TEA, 1 mm, Fig. 2A). TEA (1 mm) blocks ∼80% of Kv3.2 and produces significant inhibition of only a few other known channels, including slo1-containing BK channels (Kd∼80–330 μm) and homomeric Kv1.1 channels (Kd∼500 μm; Coetzee et al. 1999). TEA strongly suppressed maximum firing rate (100 ± 4 Hz versus 173 ± 3 Hz in controls; P < 0.001) and moderately decreased gain (398 ± 26 Hz nA−1versus 501 ± 14 Hz nA−1 in controls; P < 0.01), but had minimal effects on rheobase (108 ± 17 pA versus 139 ± 6 pA in controls; P > 0.1). TEA had no effect on resting membrane potential, input resistance, or action potential threshold (Table 1). For a more definitive test, we examined firing properties in mice with a homozygous kcnc2 gene deletion (Kv3.2 KO, Lau et al. 2000).
As observed for TEA, maximum firing rate of thalamocortical neurons in Kv3.2 KO mice were strongly reduced when compared to controls (Fig. 2A and B; 111 ± 7 Hz versus 173 ± 3 Hz, respectively; P < 0.001). Like TEA (Table 1), Kv3.2 KO reduced action potential downward slope (132 ± 5 V s−1versus 169 ± 4 V s−1 in control; P < 0.001) and fAHP (13.1 ± 0.8 mV versus 16.8 ± 0.3 mV in control; P < 0.001) at firing rates of < 10 Hz. At higher firing rates (∼80 Hz), action potential half-width was greatly increased (Fig. 2C, 702 ± 60 μs versus 496 ± 22 μs in control; P < 0.01). Traces in Fig. 2B reveal that the membrane potential between action potentials becomes more depolarized in Kv3.2 KO compared to controls with 900 pA current injections causing action potential attenuation and failure. The results suggest Kv3.2 is required to maintain a hyperpolarized interspike membrane potential during high action potential firing rates to prevent sodium channel inactivation. Interestingly, during 80 Hz firing, action potential threshold (Vth) for Kv3.2 KO was not different from controls (Fig. 2C; ∼80 Hz). The data suggest Kv3.2 is necessary to sustain membrane hyperpolarization during strong current injections to achieve high maximum firing rates and to prevent voltage-gated sodium channel inactivation.
Kv1 K+ channel blockers strongly lower firing threshold
Blocking low voltage-activated, α-dendrotoxin-sensitive, K+ channels enhances firing responses in several other neuronal subtypes, including cortical layer V pyramidal neurons and hippocampal CA3 pyramidal neurons (Bekkers & Delaney, 2001; Mitterdorfer & Bean, 2002). Kv1.1 and Kv1.2 mRNA are strongly expressed in thalamocortical neurons (Wang et al. 1994), but thus far have only been linked to synaptic terminus regulation in thalamocortical neurons (Lambe & Aghajanian, 2001). Because of their low threshold for activation, we hypothesized that Kv1 K+ channels might control the response to threshold stimuli in thalamocortical (TC) neurons. To study Kv1 channels we examined the effects of the Kv1 toxin α-dendrotoxin (100 nm) or the Kv1.2 subtype-specific toxin maurotoxin (10 nm) on TC firing properties.
α-Dendrotoxin lowered the current necessary to initiate firing (Fig. 3A and B, ΔIth; 68 ± 9 pA versus 132 ± 17 pA for controls before α-dendrotoxin, P < 0.01; n= 5) and increased firing rate over the entire range of current injections (P < 0.001, repeated-measures ANOVA). Action potential threshold was also significantly lowered by α-dendrotoxin (Vth, Fig. 3B; Vth=−51.0 ± 0.8 mV versus−47.1 ± 1.1 mV, P < 0.01). Maurotoxin, which specifically blocks Kv1 channels containing a Kv1.2 subunit, produced a strong increase in firing rate (Fig. 3C; P < 0.001 by repeated-measures ANOVA). Maurotoxin also lowered threshold current to fire (124 ± 20 pA versus 184 ± 16 pA for controls before maurotoxin, P < 0.05). These results suggest Kv1 channels containing the Kv1.2 subunit regulate the threshold current necessary to initiate firing in thalamus.
Figure 3. Kv1 K+ channel-blocking toxins lower the threshold current required to initiate action potential firing in adult thalamocortical neurons.
A, α-dendrotoxin (α-DTX, 100 nm, n= 7) significantly decreased the current that initiated firing in thalamocortical neurons. Inset demonstrates voltage response to a 200 pA current injection before and after application of 100 nmα-DTX. B, α-DTX lowered the threshold current for firing (ΔIth) and significantly reduced the membrane potential where action potentials fire (ΔVth). Inset, trace demonstrating α-DTX effect on action potential threshold aligned at Vm= 0 mV (horizontal line). C, maurotoxin (MTX, 10 nm, n= 5), a Kv1.2 channel specific toxin, produced a similar reduction in the current initiating firing. Inset demonstrates voltage response to a 200 pA current injection before and after application of 10 nm MTX.
Inhibiting SK calcium-activated K+ channels strongly increases firing gain
SK channels (SK1-3) are small conductance Ca2+-activated K+ channels. These channels open in response to the binding of intracellular Ca2+ and decay with a time course of 50–200 ms. As such, these channels have been suggested to mediate the medium afterhyperpolarization (mAHP) in some systems (Bond et al. 2004; Villalobos et al. 2004; but see Yue & Yaari, 2006). SK channel blockade increases firing in a subset of neurons (Smith et al. 2002), but their function in thalamocortical neurons has never been examined. Immunohistochemistry reveals that SK1-3 family members are heavily expressed in thalamus (Sailer et al. 2004).
To evaluate SK channel function in TC neurons, firing rate curves were obtained while inhibiting all SK channel subtypes (SK1-3) with the peptide toxin apamin (300 nm). Because of the Ca2+ dependence of SK channels, we first tested apamin with minimal intracellular calcium buffering (100 μm EGTA with 10 μm CaCl2). Under these conditions, apamin strongly augmented firing rate (Fig. 4A). Both gain (Fig. 4C, 816 ± 114 Hz nA−1versus 427 ± 45 in control, P < 0.01) and maximum firing rate (218 ± 10 Hz versus 149 ± 24 Hz in control, P < 0.01) were markedly increased. In contrast to the effect of Kv1 blocker, threshold was unaffected (ΔIth, Fig. 4C). Resting membrane potential and input resistance were also unaffected (Table 1).
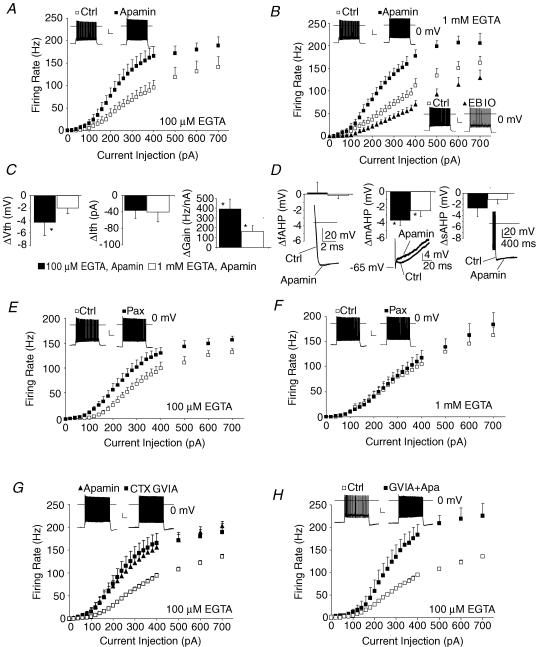
Figure 4. SK and BK Ca2+-activated K+ channels inhibit firing in adult thalamocortical neurons.
A, apamin (300 nm), an SK1–3 subunit channel blocker, enhanced thalamocortical firing with 100 μm EGTA internal solution. B, apamin (300 nm) enhanced, while 1-EBIO (200 μm), an SK channel activator, suppressed firing with 1 mm EGTA internal solution. C, apamin decreased action potential firing threshold (ΔVth) at low firing rates (in 100 μm EGTA only), but strongly increased gain (ΔGain) under both conditions. Threshold current to initiate firing (ΔIth) was not significantly altered by apamin. D, apamin inhibited the mAHP without significantly altering fAHP or sAHP. Inset, traces demonstrating fAHP, mAHP and sAHP before (thin trace) and after apamin (thick trace). E and F, paxilline (2 μm), a BK channel blocker, enhanced firing of thalamocortical neurons with 100 μm EGTA internal solution, but failed to alter firing with 1 mm EGTA internal solution. G, N-type calcium channel blocker ω-CTX GVIA (200 nm, preincubation) enhanced firing responses with 100 μm EGTA internal solution. Apamin is shown in the same plot to demonstrate the similarity between the two antagonists. H, concurrently blocking both SK and N-type Ca2+ channels enhances firing responses more than either blocker alone. Insets in each plot show voltage traces under control and drug conditions in response to a 200 pA current injection. All statistical analysis by repeated-measures ANOVA: P < 0.001 for all curves (A, B, E, G and H) but paxilline in 1 mm EGTA (F, P > 0.05). Statistical analysis: *P < 0.05 by t test for all bar graphs (C and D). n= 5–12 each condition.
In heterologous systems, several Ca2+ channel subtypes are in close physical association with Ca2+-activated K+ channels (Berkefeld et al. 2006), making them resistant to calcium buffering. Consistent with this notion, we found that moderately strong Ca2+ buffering (1 mm EGTA with 100 μm CaCl2) failed to occlude the effects of SK blocker (Fig. 4B). This result contrast to the effects of the BK Ca2+-activated K+ channel blocker paxilline (Fig. 4E and F, see below). For the SK channel blocker apamin gain (722 ± 65 Hz nA−1versus 564 ± 48 in control, P < 0.05) and maximum firing rate (199 ± 14 Hz versus 167 ± 12 Hz in control, P < 0.01) were still strongly increased. The SK channel activator 1-EBIO (200 μm) produced the opposite effect of apamin, strongly suppressing the firing rate curve (Fig. 4B), decreasing gain (336 ± 45 Hz nA−1versus 454 ± 40 in control, P < 0.05) and lowering peak firing rate (142 ± 20 Hz versus 170 ± 15 Hz in control, P < 0.05) without altering resting membrane potential or input resistance (Table 1). At higher concentrations (1 mm), 1-EBIO abolished tonic firing, even with strong current injections (n= 2, not shown).
Previous studies implicated SK channels in the medium after-hyperpolarization potential (mAHP, Bond et al. 2004; Villalobos et al. 2004). Consistent with this notion, apamin suppressed mAHP, but not the fast or the slow AHP (Fig. 4D). Apamin did not significantly affect other properties such as action potential threshold, half-width, or depolarization or repolarization rate at firing rates < 10 Hz (Table 1). Importantly, apamin did lower action potential threshold at firing rates of 80 Hz (Fig. 7B). The data suggest SK channels are active only at firing rates beyond 10 Hz and act primarily to increase interspike interval and raise action potential threshold. Regulation of gain results from the relationship between firing rate and intracellular [Ca2+]; as firing rate increases, intracellular [Ca2+] will increase and activate more SK current causing greater firing rate suppression. This property of SK channels prevents saturation at moderate stimulus intensities (firing rate saturated at ∼400 pA with apamin, Fig. 4A) preserving a relatively linear firing rate response across a broad stimulus range (100–700 pA).
BK calcium-activated K+ channel blocker lowers firing threshold
BK channels (slo1-containing channels) are large-conductance Ca2+-activated K+ channels. While Type I BK channels have been suggested to support high firing rates much like Kv3 channels due to their role in rapid repolarization of the action potential, the Type II BK channels have been suggested to inhibit firing by inducing an mAHP similar to that induced by the SK channel (Brenner et al. 2005).
To determine whether BK channels regulate firing of TC neurons, firing curves were obtained in the presence of the Type I and Type II BK inhibitor paxilline. While 2 μm paxilline did not affect resting membrane potential or input resistance (Table 1), it did enhance firing (Figs 4E, P < 0.001 by repeated-measures ANOVA). Application of paxilline lowered rheobase (Table 1), and increased gain (667 ± 80 Hz nA−1versus 434 ± 33 in control, P < 0.05) and maximum firing rate (167 ± 6 Hz versus 147 ± 7 Hz in control, P < 0.05). In these experiments, low Ca2+ buffering was used (100 μm EGTA with 10 μm CaCl2). Since BK channel binding affinity for Ca2+ is weak without extreme depolarization (Horrigan & Aldrich, 2002), we suspected that moderate Ca2+ buffering (1 mm EGTA with 100 μm CaCl2) which failed to alter the effects of SK blockers might occlude the effects of the BK blocker. Consistent with this notion, paxilline failed to augment firing under these conditions (Fig. 4F). The data suggest BK Ca2+-sensitive K+ channels moderately inhibit firing of thalamocortical neurons.
N-type Ca2+ channel blockers increase gain
Having determined that firing rate properties of thalamocortical neurons are strongly regulated by SK and moderately regulated by BK channels, it is important to identify potential routes of Ca2+ entry into the cell that could mediate these prominent effects. Thalamocortical neurons possess mRNA for all known Ca2+ channel α-subunits except the R-type (Cav2.3) and two T-type (Cav3.2 and Cav3.3) channels (Talley et al. 1999; Ludwig et al. 1997).
P/Q- and N-type Ca2+ channels are HVA Ca2+ channels which open and close very rapidly, but only in response to substantial depolarization (Vm > −30 mV), and as such, should activate only as action potentials are firing (Patil et al. 1998), suggesting that either of these Ca2+ channel might be the source of Ca2+ that activates SK K+ channels to regulate gain, while having no effect on rheobase. We utilized the P/Q- and N-type Ca2+ channel toxin ω-conotoxin MVIIC (1 μm) as an initial test of this hypothesis.
Consistent with our hypothesis, ω-conotoxin MVIIC significantly enhanced firing of thalamocortical neurons (Fig. 5B, P < 0.001 by repeated-measures ANOVA), acting entirely through increases of firing gain (933 ± 103 Hz nA−1versus 457 ± 66 Hz nA−1 in control; P < 0.001) with no effect on rheobase (140 ± 25 pA versus 137 ± 26 pA in control; P > 0.2).
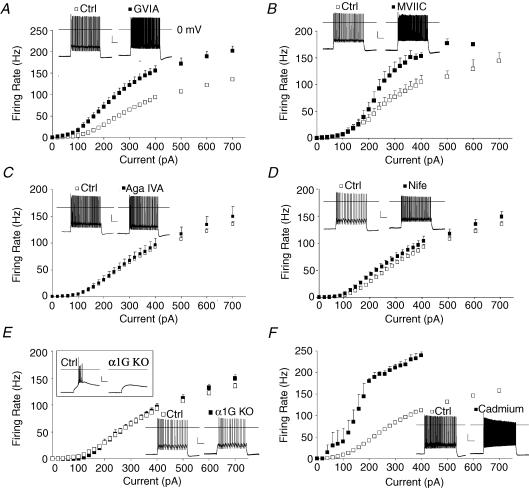
Figure 5. N-type calcium channels inhibit firing in adult thalamocortical neurons.
A, ω-conotoxin GVIA (200 nm, preincubation > 1 h), an N-type Ca2+ channel blocker, enhanced firing. B, ω-conotoxin MVIIC (1 μm, wash in), a P/Q and N-type Ca2+ channel blocker, enhanced firing. C, ω-agatoxin IVA (100 nm, preincubation > 1 h), a P/Q-type Ca2+ channel blocker, did not alter firing. D, nifedipine (10 μm), an L-type Ca2+ channel blocker, had minimal effects on firing. E, genetic deletion of the Cav3.1 T-type Ca2+ channel (α1G KO), abolished burst firing (1 s, −200 pA current injection, inset), but did not alter tonic firing. F, cadmium (200 μm), a broad-spectrum Ca2+ channel blocker, greatly enhanced firing. Statistical analysis by repeated-measures ANOVA: P < 0.001 for cadmium, ω-CTX, and MVIIC; P > 0.05 for Aga IVA and α1G KO; P= 0.03 for nifedipine; *P < 0.05 by t test. n= 5–12 each condition. Insets in each plot show voltage traces under control and drug conditions in response to a 200 pA current injection.
To determine whether the increased firing gain was caused by inhibition of P/Q-type or N-type Ca2+ channels, we used the N-type specific blocker ω-conotoxin GVIA (Fig. 5A, 200 nm). The N-type blocker strongly enhanced firing, increasing both gain (672 ± 37 Hz nA−1versus 412 ± 18 Hz nA−1; P < 0.001) and maximum firing rate (215 ± 15 Hz versus 156 ± 5 Hz; P < 0.01). Somewhat surprisingly, rheobase was also lowered following preincubation with CTX GVIA (113 ± 15 pA versus 162 ± 9 pA; P < 0.05). CTX GVIA did not significantly alter resting membrane potential or input resistance, but did accelerate action potential up-slope and down-slope and decrease mAHP (Table 1).
The firing curve for the conotoxins appeared quite similar to that for apamin (Fig. 4G), suggesting the Ca2+ and K+ channels might strongly couple. However, we failed to establish exclusive coupling of N-type Ca2+ and SK-type K+ channels since simultaneously blocking both channel types produced a larger effect than apamin alone (Fig. 4H).
P/Q-type Ca2+ channel blockade does not alter firing gain or threshold
To determine whether P/Q channels regulate firing in thalamocortical neurons, we examined firing of thalamocortical neurons after preincubation in the P/Q Ca2+ channel blocker ω-agatoxin IVA (100 nm, > 1 h).
ω-Agatoxin IVA preincubation did not significantly alter firing in thalamocortical neurons (Fig. 5C). Firing gain (402 ± 30 Hz nA−1versus 418 ± 19 Hz nA−1 in controls; P > 0.2), maximum firing rate (173 Hz ± 15 Hz versus 156 ± 5 Hz in controls; P > 0.2), or firing threshold (158 ± 18 pA versus 161 ± 9 pA in controls; P > 0.2) were all unchanged following preincubation with ω-agatoxin IVA. These results suggest P/Q-type Ca2+ channels do not regulate firing of thalamocortical neurons.
L-type Ca2+ channel blockers weakly lower firing threshold
L-type channels demonstrate significant activation at more negative membrane potentials than N- or P/Q-type HVA calcium channels (Harkins et al. 2003). Based on the effects of SK and BK Ca2+-sensitive K+ channel blockers, we speculated that the L-type Ca2+ channel blocker nifedipine might also enhance firing.
Nifedipine produced only a small increase of firing in thalamocortical neurons (Fig. 5D, P < 0.01 by repeated-measures ANOVA) by reducing rheobase (118 ± 9 pA versus 162 ± 9 pA; P < 0.01), while having no significant effect on gain (391 ± 28 Hz nA−1versus 412 ± 18 Hz nA−1; P > 0.2) or maximum firing rate (165 ± 8 Hz versus 156 ± 5 Hz; P > 0.2). Nimodipine, a different L-type Ca2+ channel blocker, also enhanced firing (data not shown). These results suggest L-type Ca2+ channels weakly regulate firing threshold.
Genetic deletion of Cav3.1 T-type Ca2+ channel inhibits burst, but not tonic firing
Thalamocortical neurons demonstrate a rebound burst in response to transient hyperpolarization. This burst results from activation (following hyperpolarizing de-inactivation) of the low-voltage-activated (LVA) T-type Ca2+ channel and results in a wide depolarizing peak that activates several Na+ channel-dependent action potentials (4–8 per burst). Cav3.1 is the major T-type calcium channel transcript in thalamocortical neurons. These channels are largely inactivated at normal resting potential in thalamocortical neurons (∼80% inactivated at −77 mV), but are still capable of passing enough Ca2+ to induce a high frequency burst with depolarization from resting potentials even below ∼−72 mV (M. R. Kasten and M. P. Anderson, personal observation). T-type Ca2+ channels have been proposed to generate a tonic window current at the point where the steady-state voltage-dependent inactivation and activation curves overlap (Perez-Reyes, 2003). Loss of window current could in principle reduce tonic Ca2+ influx at rest to alter threshold firing responses.
Although genetic deletion of Cav3.1 (α1G KO) completely eliminated action potential burst firing following hyperpolarization (Fig. 5E, inset), it failed to significantly alter tonic action potential firing in response to depolarizing current inputs (Fig. 5E). Loss of Cav3.1 did not alter rheobase, gain, or maximum firing rate, or action potential properties but did significantly hyperpolarize resting membrane potential (Table 1). The latter result suggests tonic T-type Ca2+ window current might control resting intracellular [Ca2+] to regulate resting membrane potential.
Complete HVA Ca2+ channel blockade increases all firing properties
To concurrently block all Ca2+ channels, we applied the broad-spectrum Ca2+ channel blocker cadmium (200 μm). Cadmium strongly increased evoked firing (Fig. 5F, P < 0.001 by repeated-measures ANOVA). Cadmium markedly lowered rheobase (85 ± 20 pA versus 139 ± 6 pA in ctrl, P < 0.05), and increased gain (1390 ± 214 Hz nA−1versus 501 ± 14 Hz nA−1 in ctrl, P < 0.05) and maximum firing rate (224 ± 14 Hz versus 173 ± 3 in ctrl, P < 0.05). T-type Ca2+ channel currents are only weakly inhibited by this concentration of cadmium (M. R. Kasten and M. P. Anderson, data not shown). The results suggest calcium influx through a cadmium-sensitive Ca2+ channel strongly regulates not only gain and maximum firing rate as observed with N-type and P/Q-type Ca2+ channel blockers, but also rheobase.
Multiple K+ channels moderately regulate firing threshold
Having determined that SK channels and Kv1 channels strongly modulate firing gain and threshold, we next compared the effects of these channels to those of other K+ channels previously suggested to regulate the firing in other neuron types. Monomeric Kir2.2 is strongly inhibited by 10 μm Ba2+, while monomeric Kir2.1 and Kir2.3 channels are much less sensitive (Schram et al. 2003). Ba2+ also inhibits GIRK channels and leak K+ currents, but typically the latter requires higher concentrations (Coetzee et al. 1999). We found that 10 μm Ba2+ increased thalamocortical neuron firing rate (Fig. 6C, repeated-measures ANOVA, P < 0.001), reduced threshold (83 ± 12 pA versus 139 ± 6 pA in control; P < 0.01), and lowered action potential threshold (−52.5 ± 1.5 mV versus−45.3 ± 0.7 mV; P < 0.01), without significantly altering resting membrane potential (Vm−74.8 ± 1.0 versus−76.4 ± 0.3 in control; P > 0.1) or input resistance (128 ± 9 MΩversus 136 ± 6 MΩ in control; P > 0.1). The latter results indicate that 10 μm Ba2+ does not increase firing rate through effects on resting membrane potential or leak conductances. Ba2+ at 10 μm also did not significantly change maximum firing rate (203 ± 23 Hz versus 172 ± 4 Hz in control; P > 0.1) or gain (528 ± 28 Hz nA−1versus 473 ± 12 Hz nA−1 in control; P > 0.1). These results suggest 10 μm Ba2+-sensitive K+ channels moderately regulates threshold current necessary to induce firing.
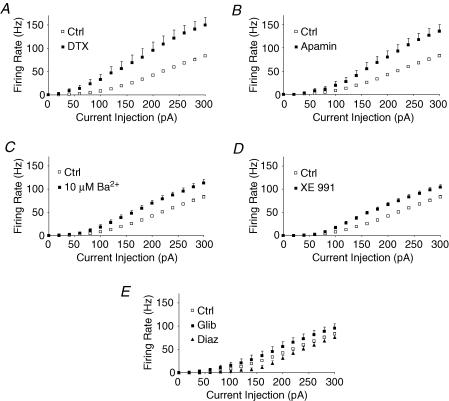
Figure 6. Comparison of the relative role of various low-threshold and inwardly rectifying K+ channels on firing in adult thalamocortical neurons.
A, α-DTX markedly increased firing responses to threshold current injections. B, apamin had minimal effects on firing at threshold, but markedly increased firing responses to current injections once firing at 10 Hz and beyond. C, 10 μm barium, which inhibits inward-rectifier K+ channels, caused a small increase of firing rate. D, KCNQ (M-type) K+ channel blocker XE 991 (10 μm) modestly increased the firing rate. E, K-ATP inward-rectifier K+ channel (Kir6) activator, diazoxide (Diaz, 100 μm), inhibited firing rate near threshold, while the Kir6 blocker, glibenclamide (Glib, 30 μm), weakly increased firing rates. Statistical analysis by repeated-measures ANOVA: P < 0.001 for each of Ctrl versus XE 991 (n= 7) and 10 μm Ba2+ (n= 6); and P= 0.03 for Ctrl versus Glib (n= 9); and P= 0.006 for Ctrl versus Diaz (n= 7). For control data, s.e.m. bars are within the symbols.
KCNQ channel blocker XE 991 moderately lowered firing threshold
KCNQ (Kv7) channels are slowly activating, non-inactivating K+ channels that are present in a wide range of neurons. KCNQ channels underlie the M-type current, and at least three of the five KCNQ subtypes are present in LDn thalamus (KCNQ2, KCNQ3 and KCNQ5; Schroeder et al. 2000; Saganich et al. 2001). KCNQ channels limit excitability in many neuronal subtypes and are reported to support the mAHP in hippocampal neurons (Shen et al. 2005; Yue & Yaari, 2006). These observations suggested KCNQ channels might regulate thalamocortical neuron excitability.
The KCNQ channel blocker XE 991 (10 μm) moderately enhanced firing rate (Fig. 6D, repeated-measures ANOVA, P < 0.001), moderately lowered threshold (86 ± 8 pA versus 135 ± 8 pA in control; P < 0.001) and mildly decreased peak firing rate (143 ± 7 Hz versus 172 ± 4 Hz in control; P < 0.01). Gain was not affected (514 ± 23 Hz nA−1versus 473 ± 12 Hz nA−1 in control; P > 0.1). Other action potential properties were similarly unaffected. These results suggest KCNQ K+ channels have a relatively small role in regulating firing threshold in thalamocortical neurons.
KATP activators increase firing threshold
KATP channels (Kir6) are weakly inwardly rectifying K+ channels which open in response to reduced intracellular ATP and elevated intracellular ADP (Gribble et al. 1997). KATP channel subunits are widely expressed throughout the brain and are thought to regulate neurons during conditions of high activity (Allen & Brown, 2004).
The KATP channel activator diazoxide (100 μm, Fig. 6E) strongly decreased input resistance (Rin= 94 ± 7 MΩversus 143 ± 8 MΩ in control; P < 0.01) and suppressed the action potential firing rate curve when compared to controls (repeated-measures ANOVA; P= 0.006). Firing rate curves were also obtained before or after addition of the KATP channel inhibitor glibenclamide, which revealed a small but significant increase of firing (30 μm; Fig. 6E; repeated-measures ANOVA; P= 0.03). No effect on resting membrane potential or input resistance was observed (Table 1). The results suggest thalamocortical neurons contain KATP channels that can suppress firing and that these channels may serve to regulate excitability during metabolic challenge.
Figure 6 also compares the relative impact of blocking Kv1, SK, M-type, and K-ATP and 10 μm Ba2+-sensitive channels on threshold of the firing response curve. The results suggest Kv1 plays the greatest role in regulating firing responses to threshold stimuli (Fig. 6A).
Action potential and membrane properties at high firing rates
Blocking N-type Ca2+ channels, SK Ca2+-activated K+ channels, or Kv1 K+ channels strongly increased, while deleting Kv3.2 K+ channels markedly suppressed, peak firing rates (Figs 2–5). To compare the effects of these channels, we examined their influence on membrane and action potential properties while firing action potentials at a moderately high rate.
To examine the details of how these channels regulate firing, their influence on firing properties were compared at 80 Hz, since nearly all cells (even Kv3.2 KO) were capable of firing at this rate. The kinetics of action potential repolarization did not correlate well with the ability to fire at high rates: while Kv3.2 slowed repolarization rate, SK and Kv1 blockers, which both enhanced maximum firing rate, had no effect on repolarization rate compared to controls (Fig. 7A). Similarly, while both SK and Kv1 blockers lowered action potential threshold, Kv3.2 deletion had no effect compared to controls (Fig. 7B). While these different channels could promote or inhibit peak firing rate through entirely different mechanisms, we did observe that the minimum membrane potential reached between spikes (Min Vm, Fig. 7C) responded to each channel blocker in a way that correlates well with their effects on peak firing rate; blocking SK or Kv1 channels resulted in a more hyperpolarized Min Vm and higher peak firing rate than controls, while blocking Kv3.2 channels caused a more depolarized Min Vm and attenuated peak firing rate (Fig. 7D).
EPSC-action potential coupling is also regulated by Kv1, SK, and Kv3.2 K+ channels
To determine whether these K+ channels that strongly regulated the firing response to continuous currents also influenced the response to phasic synaptic currents, we applied excitatory postsynaptic currents (EPSC)-like stimuli to LDn neurons. Traces in Fig. 8 (lower right corner) show the EPSC-like stimulus overlaid on an EPSC recorded from the LDn neuron. Comparing the firing response to EPSC-like currents at varying interpulse frequencies revealed that acute inhibition of Kv1 K+ channels with α-dendrotoxin (100 nm, bath) increased EPSC–action potential coupling during the initial stimulus (burst firing) and during the continuous low frequency EPSC stimuli at 30 Hz (Fig. 8A). By contrast, acutely blocking the SK K+ channels with apamin (300 nm, bath) increased EPSC–action potential coupling during the initial stimulus (burst firing), but caused only transient increases of the firing response to continuous low frequency EPSC stimuli at 30 Hz (Fig. 8A). Both Kv1 and SK channel blockers increased EPSC–action potential coupling in response to continuous EPSC stimuli of higher frequencies (100 Hz and 200 Hz, Fig. 8A). While blocking these channels enhanced coupling even at high frequencies, acutely blocking Kv3.2 K+ channels with the non-selective blocker TEA (1 mm, bath) markedly attenuated action potential height and depolarized the interpulse membrane potential during 200 Hz EPSC-like stimulus (Fig. 8B). At 100 Hz stimulus, TEA widened (Fig. 8B, right traces, 20 mV, 1 ms scale), but did not attenuate action potentials (Fig. 8B, 20 mV, 10 ms scale). The results are consistent with the firing response to continuous depolarizing currents, but reveal an even greater separation of Kv1 and SK K+ channels, the former affecting threshold and the latter affecting the response only when firing beyond a critical rate, SK channel inhibition of higher firing rates being explained by the cumulative increase of intracellular [Ca2+] as firing rate increases.
Discussion
This study of the K+ and Ca2+ channels that regulate action potential firing in thalamocortical neurons identified the major and minor channel subtypes controlling thalamic signal transmission through the lateral dorsal nucleus. Somewhat distinct effects on the stimulus–response curve were observed for each channel. Kv1, but not SK or Kv3.2, channels regulated threshold. N-type Ca2+ channels, SK Ca2+-activated and Kv1 K+ channels all regulated gain. L-type Ca2+ channels, and BK Ca2+-activated, M-type, Ba2+-sensitive, and K-ATP K+ channels represent minor regulators of the firing rate curve. Finally, Kv3.2 channels enhanced, while Kv1 and SK channels suppressed, peak firing rate.
A meaningful study of thalamocortical action potential firing in vitro should utilize intact and fully mature neurons that fire at rates comparable to those observed in vivo. Rates of 100–200 Hz are achieved in the thalamus under natural conditions. For example, non-primary auditory thalamic relay nuclei neurons fire at rates exceeding 100 Hz for multiple seconds in anticipation of a sucrose reward (Komura et al. 2001), ventral somatosensory thalamus neurons exceed 150 Hz for many seconds during somatic pain stimuli (Lee et al. 1999), and anterior dorsal thalamus head-direction cells approach 200 Hz during centripetal motion (Bassett et al. 2005). By studying the adult thalamic slice, we were able to record neurons that fired at rates exceeding 200 Hz.
Kv3.2 supports high-frequency firing in thalamocortical neurons
Thalamocortical neurons demonstrate remarkably rapid action potentials for large projection neurons. Half-width for control neurons tested was ∼400 μs (Table 1). Fast action potentials in several cell types are attributed to fast-activating, ultra-rapidly de-activating Kv3 channels (Lau et al. 2000; Kaczmarek et al. 2005). It is known that Kv3 channels activate rapidly at supra-threshold voltages (V1/2=−20 to +40 mV) and de-activate in microseconds upon repolarization, ideal properties to promote fast spiking (Rudy & McBain, 2001). We now show that the thalamus-enriched Kv3.2 channel subtype is necessary to achieve high peak firing rates in thalamocortical neurons. Kv3.2 contributes to the fAHP even at low action potential firing rates (< 10 Hz). When firing at moderate rates, the fAHP and action potential (AP) repolarization became increasingly dependent on Kv3.2 causing a widening of action potential half-width in Kv3.2 deleted neurons (Fig. 2C). The latter observation could be explained if other potassium channels contributing to action potential repolarization at low firing rates become inactivated at depolarized membrane potentials. This widened action potential will increase the activation of N-type Ca2+ channels, causing calcium influx and SK K+ channel activation, which we show potently inhibits action potential firing (Figs 4 and 5). Kv3.2 is also necessary to maintain a hyperpolarized membrane potential between spikes. When larger currents drive high firing rates, the membrane potential becomes depolarized and action potential height progressively decreases when Kv3.2 is missing (Fig. 2B). The findings suggest that fast after-hyperpolarizations and effective repolarization of the membrane potential are critical to de-inactivating Na+ channels during fast firing rates.
The data suggest that signalling pathways which inhibit Kv3.2 channel activity (e.g. PKA phosphorylation, Atzori et al. 2000) will introduce a low-pass filter limiting the transmission of high firing rates as might occur during a seizure or intense (aversive) sensory stimulus.
SK Ca2+-activated K+ and N-type Ca2+ channels couple to reduce gain and preserve a linear firing response across a broad range of input intensities
Blocking SK Ca2+-activated K+ channels (with apamin) strongly increased firing gain. SK channels open directly in response to binding intracellular Ca2+ (Xia et al. 1998). Previous studies reported that SK channels support the medium afterhyperpolarization (mAHP), a 5–100 min period following a spike when membrane potential remains below threshold (Abel et al. 2004; Bond et al. 2004; Villalobos et al. 2004). Our data further support this conclusion. Apamin decreased mAHP, but not fAHP or sAHP. SK blocker effects were not attenuated when the concentration of intracellular Ca2+ chelator was increased (Fig. 4A and B) suggesting strong coupling between the SK K+ channels and the Ca2+ influx pathway. Inhibiting N-type Ca2+ channels mimicked and partially occluded the effects of the SK blocker suggesting this HVA Ca2+ channel represents a major source of intracellular Ca2+ activating SK channels in the thalamic relay neuron.
Kv1 is the major low-voltage activated K+ channel regulating firing threshold
Blocking Kv1, Kv7 (KCNQ), and low [Ba2+]-sensitive (possibly Kir2) low-threshold K+ channels enhanced firing responses at low current injections, but the effects of Kv1 blockers vastly exceeded the effects of all the other channel blockers. The channels raised threshold current by two distinct mechanisms, either by decreasing input resistance or increasing action potential threshold.
Inward rectifier K+ channels typically inhibit excitability by decreasing input resistance and hyperpolarizing the cell. Recent studies demonstrated that inward rectifier channels also activate hyperpolarization-activated currents that further decrease input resistance and shunt excitatory currents (Ih; Day et al. 2005). In this study, major changes in resting membrane potential and input resistance were not observed with the low [Ba2+]-sensitive (possibly Kir2) K+ channel blocker. While the inward-rectifier channels Kir2 and Kir6 display rectification, outward currents are still rather pronounced with a peak at approximately −60 mV for Kir2, well below Vth (Panama & Lopatin, 2006). This rectification property could allow Kir2 to influence action potential threshold without strongly influencing resting membrane properties. Kir6 ATP-regulated K+ channels are heavily expressed in the thalamus (Kir6.2, Thomzig et al. 2005). The Kir6 channel activator diazoxide decreased input resistance and suppressed firing at threshold, suggesting Kir6 channels may have profound effects on thalamic firing when intracellular ATP is depleted (e.g. ischaemia/anoxia or seizures).
By contrast, Kv1 and Kv7 channels are generally closed near resting membrane potentials and activate with depolarization below the threshold for action potential firing. These channels introduce a shunting current, increasing the amount of current necessary to reach threshold for spike initiation (activation between Vm=−60 mV and −40 mV, Coetzee et al. 1999).
EPSC-like stimulation
Thalamic relay neurons fire both continuously in response to various metabotropic receptor signals (e.g. orexin, glutamate), but also relay signals transmitted as phasic stimuli with brief excitatory postsynaptic currents (EPSC) due to ionotropic glutamate receptors. To evaluate the role of these K+ channels in transmitting this pattern of stimuli we used EPSC-like stimuli and examined the responses before and after exposure to K+ channel blockers. The results suggest Kv1 but not SK K+ channels regulate the transmission of low frequency (30 Hz) EPSC inputs. Both Kv1 and SK K+ channels were found to limit the transmission of higher frequency (100 Hz and 200 Hz) EPSC inputs. As suggested from the studies of firing with continous stimuli, Kv3.2 was necessary to transmit high frequency EPSC inputs; inhibiting this channel will low-pass filter very high frequency EPSC events.
Functional implications
This study evaluates the relative role of K+ and Ca2+ channels in adult thalamocortical neuron firing properties. The experimental approach of directly comparing the effects of many K+ and Ca2+ channels enabled us to determine the relative importance of each channel subtype.
Thalamocortical neurons are located at a critical node within the brain where information from peripheral sensory organs, subcortical systems (striatum and cerebellum), and distinct cortical layers V and VI are relayed into the cerebral cortex and other forebrain grey matter structures. Thalamocortical neurons are conserved amongst all vertebrate organisms and display a characteristic response to external sensory signals. Consequently, this cell type represents an ideal system in which to study the principles of how ion channels govern the neuron's stimulus–response curve. Given the wide range of K+ channels we found that modulate the firing dynamics of thalamocortical neurons and the vast array of neuromodulatory inputs projecting to these cells (e.g. dopamine, serotonin, noradrenaline, histamine, acetylcholine, orexin), specific firing response patterns may be achieved depending on the demands of the specific behavioural condition. Each channel played a somewhat different role, regulating various components of the firing rate curve (threshold, gain and maximum). Kv1 channels played the largest role in regulating threshold and are inhibited by numerous neuromodulators (Fadool & Levitan, 1998). Kv3.2 channels were necessary to support high firing rates and are inhibited by PKA phosphorylation (Atzori et al. 2000); our studies indicate that this signalling pathway in the thalamus could strongly suppress peak firing rates. KCNQ (Kv7) channels are blocked by cholinergic stimuli (Yue & Yaari, 2006) and inhibiting these channels would modestly enhance tonic firing. In addition to direct SK channel modulation, the calcium channels that govern the influx of calcium are strongly regulated (Dolphin, 2003). Neuromodulatory inhibition of N-type channels in the thalamus will strongly enhance sensory response gain (Dolphin, 2003; Tedford & Zamponi, 2006). Calcium-induced calcium release through ryanodine receptors was also shown to regulate firing in thalamic relay neurons (Budde et al. 2000); our data suggest this may occur through SK or BK K+ channels. Modulating these various K+ and Ca2+ channels is expected to influence the fidelity of sensory processing during various natural awake and sleep states (Anderson et al. 2005) and during various disease states characterized by abnormal sensory processing.
Acknowledgments
This work was supported by the National Institute of Mental Health, the National Institute of Neurologic Disease and Stroke and the Burroughs Wellcome Fund. We thank Melissa W. Anderson for editing the manuscript and for administrative support, Robert E. Kasten for writing the MATLAB analysis program, and Elda Arrigoni, Nancy Chamberlin and Andrew Strassman for helpful comments on the manuscript. There are no conflicts to disclose.
References
- Abel HJ, Lee JCF, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramial neurons. J Neurophysiol. 2004;91:324–335. doi: 10.1152/jn.00583.2003. [DOI] [PubMed] [Google Scholar]
- Allen TG, Brown DA. Modulation of the excitability of cholinergic basal forebrain neurones by K-ATP channels. J Physiol. 2004;554:353–370. doi: 10.1113/jphysiol.2003.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MP, Mochizuki T, Xie J, Fischler W, Manger JP, Talley EM, Scammell TE, Tonegawa S. Thalamic Cav3.1 T-type Ca2+ channel plays a crucial role in stabilizing sleep. Proc Natl Acad Sci U S A. 2005;102:1743–1748. doi: 10.1073/pnas.0409644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M, Lau D, Tansey EP, Chow A, Ozaita A, Rudy B, McBain CJ. H2 histamine receptor-phosphorylation of Kv3.2 modulates interneuron fast spiking. Nat Neurosci. 2000;3:791–798. doi: 10.1038/77693. [DOI] [PubMed] [Google Scholar]
- Bassett JP, Zugaro MB, Muir GM, Golob EJ, Muller RU, Taube JS. Passive movements of the head do not abolish anticipatory firing properties of head direction cells. J Neurophysiol. 2005;93:1304–1316. doi: 10.1152/jn.00490.2004. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, Serafin M. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22:7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Delaney AJ. Modulation of excitability by α-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J Neurosci. 2001;21:6553–6560. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Sailer CA, Bildl W, Hohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-CaV channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Budde T, Sieg F, Braunewell KH, Gundelfinger ED, Pape HC. Ca2+-induced Ca2+ release supports the relay mode of activity in thalamocortical cells. Neuron. 2000;26:483–492. doi: 10.1016/s0896-6273(00)81180-0. [DOI] [PubMed] [Google Scholar]
- Calton JL, Taube JS. Degradation of head direction cell activity during inverted locomotion. J Neurosci. 2005;25:2420–2428. doi: 10.1523/JNEUROSCI.3511-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Levitan IB. Modulation of olfactory bulb neuron potassium current by tyrosine phosphorylation. J Neurosci. 1998;18:6126–6137. doi: 10.1523/JNEUROSCI.18-16-06126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez FR, Mehaffey WH, Molineux ML, Turner RW. High-threshold K+ current increases gain by offsetting a frequency-dependent increase in low-threshold K+ current. J Neurosci. 2005;25:363–371. doi: 10.1523/JNEUROSCI.3950-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins AB, Cahill AL, Powers JF, Tischler AS, Fox AP. Expression of recombinant calcium channels support secretion in a mouse pheochromocytoma cell line. J Neurophysiol. 2003;90:2325–2333. doi: 10.1152/jn.00425.2003. [DOI] [PubMed] [Google Scholar]
- Hernandez-Pineda R, Chow A, Amarillo Y, Moreno H, Saganich M, Vega-Saenz de Miera EC, Hernandez-Cruz A, Rudy B. Kv3.1-Kv3.2 channels underlie a high-voltage-activating component of the delayed rectifier K+ current in projecting neurons from the globus pallidus. J Neurophysiol. 1999;82:1512–1528. doi: 10.1152/jn.1999.82.3.1512. [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Coulter DA, Prince DA. A fast transient potassium current in thalamic relay neurons: kinetics of activation and inactivation. J Neurophysiol. 1991;66:1304–1315. doi: 10.1152/jn.1991.66.4.1304. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Slow inactivation of a TEA-sensitive K current in acutely isolated rat thalamic relay neurons. J Neurophysiol. 1991;66:1316–1328. doi: 10.1152/jn.1991.66.4.1316. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Bhattacharjee A, Desai R, Gan L, Song P, von Hehn CA, Whim MD, Yang B. Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels. Hear Res. 2005;206:133–145. doi: 10.1016/j.heares.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, Martinelli Boneschi F, Choi C, Morozov P, Das K, Teplitskaya E, Yu A, Cayanis E, Penchaszadeh G, Kottmann AH, Pedley TA, Hauser WA, Ottman R, Gilliam TC. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;30:335–341. doi: 10.1038/ng832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura Y, Tamura R, Uwano T, Nishijo H, Kaga K, Ono T. Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature. 2001;412:546–549. doi: 10.1038/35087595. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, Rudy B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–9085. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Dougherty PM, Antezana D, Lenz FA. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. J Comp Neurol. 1999;410:541–555. doi: 10.1002/(sici)1096-9861(19990809)410:4<541::aid-cne3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol. 1991;66:1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 1999;83:268–279. [PubMed] [Google Scholar]
- Mitterdorfer J, Bean BP. Potassium currents during the action potential of hippocampal CA3 neurons. J Neurosci. 2002;22:10106–10115. doi: 10.1523/JNEUROSCI.22-23-10106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno H, Kentros C, Bueno E, Weiser M, Hernandez A, Vega-Saenz de Miera E, Ponce A, Thornhill W, Rudy B. Thalamocortical projections have a K+ channel that is phosphorylated and modulated by cAMP-dependent protein kinase. J Neurosci. 1995;15:5486–5501. doi: 10.1523/JNEUROSCI.15-08-05486.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panama BK, Lopatin AN. Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol. 2006;571:287–302. doi: 10.1113/jphysiol.2005.097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil PG, Brody DL, Yue DT. Preferential closed-state inactivation of neuronal calcium channels. Neuron. 1998;20:1027–1038. doi: 10.1016/s0896-6273(00)80483-3. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Pirchio M, Turner JP, Williams SR, Asprodini E, Crunelli V. Postnatal development of membrane properties and delta oscillations in thalamocortical neurons of the cat dorsal lateral geniculate nucleus. J Neurosci. 1997;17:5428–5444. doi: 10.1523/JNEUROSCI.17-14-05428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E. Contributions of Kv3 channels to neuronal excitability. Ann N Y Acad Sci. 1999;868:304–343. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci. 2004;26:458–469. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Schram G, Pourrier M, Wang Z, White M, Nattel S. Barium block of Kir2 and human cardiac inward rectifier currents: evidence for subunit-heteromeric contribution to native channels. Cardiovasc Res. 2003;59:328–338. doi: 10.1016/s0008-6363(03)00366-3. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim K, Cullen T, Ongur D, Heckers S. Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm. 2006;113:907–928. doi: 10.1007/s00702-005-0363-8. [DOI] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- Thomzig A, Laube G, Pruss H, Veh RW. Pore-forming subunits of K-ATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. J Comp Neurol. 2005;484:313–330. doi: 10.1002/cne.20469. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci. 2004;24:3537–3542. doi: 10.1523/JNEUROSCI.0380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006;95:3480–3495. doi: 10.1152/jn.01333.2005. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]