Abstract
Neurotransmitter receptors on taste bud cells (TBCs) and taste nerve fibres are likely to contribute to taste transduction by mediating the interaction among TBCs and that between TBCs and taste nerve fibres. We investigated the functional expression of P2 receptor subtypes on TBCs of mouse fungiform papillae. Electrophysiological studies showed that 100 μm ATP applied to their basolateral membranes either depolarized or hyperpolarized a few cells per taste bud. Ca2+ imaging showed that similarly applied 1 μm ATP, 30 μm BzATP (a P2X7 agonist), or 1 μm 2MeSATP (a P2Y1 and P2Y11 agonist) increased intracellular Ca2+ concentration, but 100 μm UTP (a P2Y2 and P2Y4 agonist) and α,β-meATP (a P2X agonist except for P2X2, P2X4 and P2X7) did not. RT-PCR suggested the expression of P2X2, P2X4, P2X7, P2Y1, P2Y13 and P2Y14 among the seven P2X subtypes and seven P2Y subtypes examined. Immunohistostaining confirmed the expression of P2X2. The exposure of the basolateral membranes to 3 mm ATP for 30 min caused the uptake of Lucifer Yellow CH in a few TBCs per taste bud. This was antagonized by 100 μm PPADS (a non-selective P2 blocker) and 1 μm KN-62 (a P2X7 blocker). These results showed for the first time the functional expression of P2X2 and P2X7 on TBCs. The roles of P2 receptor subtypes in the taste transduction, and the renewal of TBCs, are discussed.
Heterogeneous taste bud cells (TBCs) and sensory nerve fibres form mammalian taste buds, and their interaction through chemical signalling is suggested to trigger not only taste sensation but also the turnover of TBCs. In fact, TBCs release serotonin (Huang et al. 2005) and ATP (Finger et al. 2005) in response to taste stimuli, and release ATP on depolarization through specific channels (Romanov et al. 2007). Recently, it has been shown that TBCs release ATP in response to taste stimulation via pannexin 1 hemichannels and that the released ATP causes the release of serotonin from other TBCs in mouse tongues (Huang et al. 2007). Also, TBCs and associated nerve fibres express a variety of receptors for neurotransmitters, including cholecystokinin (Herness et al. 2002b), serotonin (Kaya et al. 2004), adrenaline (Herness et al. 2002a), acetylcholine (Ogura, 2002) and ATP (Bo et al. 1999; Kim et al. 2000; Rong et al. 2000; Kataoka et al. 2004). As the first step to test for such chemical signalling, we used several methods to investigate the functional expression of P2 receptors, receptors for ATP, on TBCs in mouse fungiform papillae.
TBCs are classified into four cell types, type I to type IV. Type I cells are assumed to be supportive. Type II cells are taste receptor cells, but they have no chemical synapses with taste nerve fibres. Only type III cells form chemical synapses with taste nerve fibres (Seta & Toyoshima, 1995). Type II cells thus may function via novel type chemical synapses (Clapp et al. 2004), or paracrine signalling systems (Kaya et al. 2004) to transmit taste information to type III cells or to taste nerve endings.
On the other hand, these TBCs are short lived. Their lifetime is estimated to be about 9 days (Beidler & Smallman, 1965), although a subset of type II cells lives longer (Farbman, 1980; Cho et al. 1998). It is suggested that older TBCs are eliminated by apoptotic pathways (Zeng & Oakley, 1999; Zeng et al. 2000) and are replaced with newborn TBCs derived from type IV cells, precursor cells for all cell types. It appears that P2 receptor subtypes contribute to the apoptosis.
Mammalian P2 receptors consist of seven P2X subtypes (ion channels) and eight P2Y subtypes (G-protein-coupled receptors). The expression of P2X2 and P2X3 was shown on rat taste nerve fibres immunohistochemically (Bo et al. 1999), and that of P2X3 was suggested electrophysiologically on trigeminal afferents (Rong et al. 2000). P2Y subtype expression was shown pharmacologically in mouse TBCs (Kim et al. 2000; Baryshnikov et al. 2003), and later identified as P2Y1 in rat TBCs (Kataoka et al. 2004), and as P2Y2 and P2Y4, and probably P2Y1 and P2Y6, in mouse TBCs in foliate and circumvallate papillae (Bystrova et al. 2006). However, the expression of other P2 receptor subtypes, typically that of P2X subtypes, remains to be identified.
In the present study, we show the expression of P2X2 and P2X7, in addition to P2Y1, on the basolateral membranes of mouse fungiform TBCs, with a variety of techniques including voltage- and current-clamping, reverse transcriptase-mediated polymerase chain reactions (RT-PCR), immunohistostaining, Ca2+ imaging, and dye uptake. Also we show the cell-type-dependent expression of P2 subtypes. The roles of these purinergic receptors are discussed in terms of TBC networks and TBC turnover.
Methods
Preparation of peeled epithelia
Mouse lingual epithelia were prepared as described in our previous papers (Ohtubo et al. 2001; Higure et al. 2003). In brief, ddY-strain mice were killed by decapitation after being anaesthetized with ether. We hypodermically injected an elastase solution (see ‘Solutions’ for the composition of this and following solutions) into the tongues, incubated them at 25°C for 10 min, and then peeled off the lingual epithelia with forceps. We mounted an epithelium on a recording platform with the basolateral membrane side of TBCs (the opposite side of the tongue surface) facing upward (and receptor membranes facing inside the recording platform), and placed the recording platform under a microscope equipped with a water-immersion objective. The basolateral membrane was always irrigated with either a physiological saline or stimulating solutions, to minimize the hydrolysis of ATP in the stimulating solutions. The time constant of the exchange of solutions estimated with Rhodamine B, a fluorescent dye, was 3.5 s. The receptor membrane was acclimated to the physiological saline. All experimental protocols were conducted in compliance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences approved by the Council of the Physiological Society of Japan.
Electrophysiology
Electrophysiological studies under cell-attached and whole-cell-clamp conditions were carried out according to our previous papers (Ohtubo et al. 2001; Higure et al. 2003).
In brief, electrical responses were recorded with electrodes (∼5 MΩ) filled with a KCl electrode solution, amplified with a voltage-clamp amplifier (Axopatch 200B, Axon Instruments, Union City, CA, USA), filtered at 10 kHz, digitized with an A/D converter (Digidata 1200, Axon Instruments), and stored using pCLAMP data acquisition and analysis software (ver. 9.0, Axon Instruments) on a personal computer. The liquid junction potential of ∼5 mV between recording and reference electrodes was neglected.
Ca2+ imaging
After mounting on a recording platform, we soaked the basolateral membrane side of the peeled epithelium in a fura-2 AM (acetoxymethyl ester form of the Ca2+-selective fluorescent dye, fura-2) solution for 20 min at 37°C, washed with the physiological saline for 10 min, and then placed under a fluorescence microscope equipped with a water-immersion objective. The basolateral membrane was irrigated similarly in the whole-cell-clamp experiments, except that the Ca2+ concentration was decreased to 1 mm (lowered-Ca2+ saline) to prevent the decrease of Ca2+ responses during the repeated application of ATP. The receptor membrane was acclimated to the physiological saline.
Fura-2-stained TBCs and non-taste lingual epithelial cells were excited at 340 and 380 nm with a spectroscope-type high-speed wavelength changer (C7773, Hamamatsu Photonics, Hamamatsu, Japan). Images of fura-2 fluorescence were acquired every 2.5 s with an intensified CCD camera through a water-immersion objective (Fluor-40X, Olympus, Tokyo, Japan), stored in a computer, and analysed with AQUACOSMOS software (version 2.0, Hamamatsu Photonics). Averaged Ca2+ levels over respective cell areas were sequentially plotted as a ratio of F340/F380. Whenever the ratio obtained from given TBCs or non-taste lingual epithelial cells increased by 0.2 or more, in a time-locked manner with the stimulus, we concluded that these cells displayed Ca2+ responses to ATP or its analogues.
RT-PCR
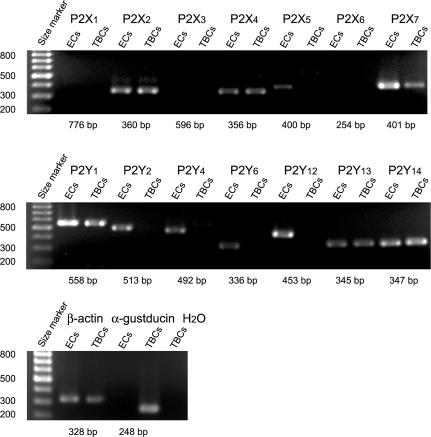
The expression of P2 receptor subtypes on TBCs and non-taste lingual epithelial cells was tested with RT-PCR experiments using oligonucleotide primers specific for 14 different P2 receptor subtypes (Table 1). In order to obtain the total mRNA of TBCs, we liberated taste buds by puffing a Ca2+- and Mg2+-nominally-free solution onto the cleft between taste buds and surrounding epithelial cells in the physiological saline (Fig. 1). The liberated taste buds were collected with another micropipette, transferred into Isogen (Wako Pure Chemical Industries, Ltd, Osaka, Japan), homogenized and stored at −80°C. Lingual epithelia containing no taste buds among fungiform papillae (non-taste lingual epithelia) were immediately homogenized in Isogen, and stored at −80°C. Total mouse brain mRNA similarly extracted and stored was used as a control to test the primer sets.
Table 1.
Summary of gene-specific primer sets employed for varying ATP receptors
| Subtypes | Primer sequence 5′-sense-3′ 5′-antisense-3′ | Expected product size | Position | Reference |
|---|---|---|---|---|
| P2X1 | 5′-CATTGTGCAGAGAACCCAGAA-3′ | 776 bp | 530–550 | (Coutinho-Silva et al. 1999) |
| 5′-ATGTCCTCCGCATACTTGAAC-3′ | 1285–1305 | |||
| P2X2 | 5′-ACGTTCATGAACAAAAACAAG-3′ | 360 bp | 1355–1375 | (Ren et al. 2003) |
| 5′-TCAAAGTTGGGCCAAACCTTTGG-3′ | 1692–1714 | |||
| P2X3 | 5′-CTGTATATCAGACTTCTTCACCTACGA-3′ | 596 bp | 194–220 | (Ren et al. 2003) |
| 5′-TTATGTCCTTGTCGGTGAGGTTAG-3′ | 766–789 | |||
| P2X4 | 5′-GAGAATGACGCTGGTGTGCC-3′ | 356 bp | 533–552 | (Dai et al. 2001) |
| 5′-TTGGTGAGTGTGCGTTGCTC-3′ | 869–888 | |||
| P2X5 | 5′-TCCACCAATCTCTACTGC-3′ | 400 bp | 743–760 | (Dai et al. 2001) |
| 5′-CCAGGTCACAGAAGAAAG-3′ | 1125–1142 | |||
| P2X6 | 5′-TACGTACTAACAGACGCA-3′ | 254 bp | 7–24 | (Dai et al. 2001) |
| 5′-ATATCAGGGTTCTTTGGG-3′ | 243–260 | |||
| P2X7 | 5′-AAGTCTCTGCCTGGTGTC-3′ | 401 bp | 499–516 | (Dai et al. 2001) |
| 5′-GGCATATCTGAAGTTGTAGC-3′ | 880–899 | |||
| P2Y1 | 5′-TGGCGTGGTGTACCCTCTCAAGTC-3′ | 558 bp | 1101–1124 | (Shariatmadari et al. 2003) |
| 5′-CGGGACAGTCTCCTTCTGAATGTA-3′ | 1635–1658 | |||
| P2Y2 | 5′-CTGGAACCCTGGAATAGCAC-3′ | 513 bp | 489–508 | (Fischer et al. 2001) |
| 5′-GCTGGTGGTGACGAAGTAGA-3′ | 982–1001 | |||
| P2Y4 | 5′-AGCCCAAGTTCTGGAGATGGTG-3′ | 492 bp | 341–362 | (Suarez-Huerta et al. 2001) |
| 5′-GGTGGTTCCATTGGCATTGG-3′ | 813–832 | |||
| P2Y6 | 5′-CACCTGTGATTTGGCAACTG-3′ | 336 bp | 1538–1557 | BC027331 |
| 5′-TCTTGGCAAATGGATGTGAA-3′ | 1854–1873 | |||
| P2Y12 | 5′-CACCTCAGCCAATACCACCT-3′ | 453 bp | 445–464 | NM_027571 |
| 5′-AACATGAAGGCCCAGATGAC-3′ | 878–897 | |||
| P2Y13 | 5′-GAAGAGAGGCACATGCAACA-3′ | 345 bp | 1656–1675 | NM_028808 |
| 5′-TTACTAATGCCAGGCCAACC-3′ | 1981–2000 | |||
| P2Y14 | 5′-CAGTGCATGGAGCTCAAAAA-3′ | 347 bp | 895–914 | NM_133200 |
| 5′-GCAGCCGAGAGTAGCAGAGT-3′ | 1222–1241 | |||
| β-actin | 5′-CACCCTGTGCTGCTCACC-3′ | 328 bp | 381–398 | NM_007393 |
| 5′-GCACGATTTCCCTCTCAG-3′ | 691–708 | |||
| α-gustducin | 5′-CTCTTCCAGGAGAAAGTGGC –3′ | 248 bp | 924–943 | XM_144196 |
| 5′-TCAGAAGAGCCCACAGTCTT –3′ | 1153–1172 |
Figure 1. Collection of fungiform taste buds.
Each taste bud in peeled lingual epithelium (A) is liberated by puffing the Ca2+- and Mg2+-nominally-free solution onto the cleft between taste buds and surrounding epithelial cells in the physiological saline (B), and brought to another micropipette (C) that sucked the taste bud. Perigemmal cells surrounding the taste bud remained in the epithelium (D). Scale bar, 10 μm.
Taste buds were obtained from 21 mice, non-taste lingual epithelia from seven mice, and brains from two mice. Total mRNA was extracted as a water-soluble fraction, precipitated in 37.5% isopropanol supplemented with Dr. GenTLE precipitation carrier (Takara BIO, Tokyo, Japan), washed by ethanol, and then treated with DNase (Takara BIO, Japan) following the manufacturer's protocol. RT-PCR was carried out with a QIAGEN OneStep RT-PCR kit (QIAGEN GmbH, Hilden, Germany).
Reverse transcription was performed at 55°C for 30 min. PCR cycles consisted of an initial step of 94°C for 7.5 min and 45 subsequent cycles of 94°C for 30 s (denaturation), 50–62°C for 1 min (annealing), 72°C for 3 min (extension) and final extension step of 72°C for 10 min. Annealing temperatures were: 50°C for P2X6 and P2X7, 54°C for P2X3, P2X4, P2X5, P2Y1, P2Y6 and α-gustducin, 58°C for P2X2, P2Y12, P2Y14 and β-actin, 62°C for P2X1, P2Y2, P2Y4 and P2Y13. PCR products were analysed by 1.5% agarose gel electrophoresis, stained with ethidium bromide (0.5 μg ml−1), and visualized by UV illumination.
Optimum annealing temperatures were obtained by preliminary PCR experiments on brain mRNA; we tested a range of annealing temperatures from 50°C to 62°C in 4°C steps, and took an annealing temperature that yielded a clear single band with a correct size on agarose gels for each primer set. Controls for DNA contamination and PCR carryover were performed by exclusion of RNA from the RT-PCR reaction. Amplification of housekeeping genes, β-actin and of α-gustducin, a G-protein that specifically expressed in TBCs, was used as a positive control.
Immunohistochemistry
Immunohistostaining of P2 receptor subtypes and cell marker proteins was observed in optical slices obtained with confocal laser scanning microscopy (TCS-SL, Leica Microsystems CMS GmbH, Wetzlar, Germany). For serotonin immunohistostaining, mice were injected intraperitoneally with 5-hydroxytryptophan (80 mg kg−1) 1 h before being killed as described in a previous paper (Clapp et al. 2004). We fixed peeled epithelia overnight with a fixation solution, washed with six 10-min changes of a phosphate-buffered solution (PBS), and incubated the epithelia in 10 mm citrate buffer (pH 6.0) for 20 min at 85°C. After a brief wash with the PBS, the epithelia were incubated in a blocking solution for 4 h.
The epithelia were then probed using indirect immunofluorescence. Two antibodies to P2X2 (1 : 1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1 : 300, Alomone Laboratories, Israel), an antibody to P2X4 (1 : 300, Calbiochem, Darmstadt, Germany), and two antibodies to P2X7 (1 : 50, Santa Cruz Biotechnology; 1 : 200, Alomone Laboratories) served as the primary antibodies. Alexa 488-conjugated donkey anti-rabbit IgG (1 : 400, Molecular Probes, Eugene, OR, USA), Alexa 555-conjugated donkey anti-mouse IgG (1 : 400, Molecular Probes), and Alexa 633-conjugated donkey anti-goat IgG (1 : 200, Molecular Probes) served as secondary antibodies. Each antibody was diluted with the blocking solution to the concentration indicated, respectively, above. The specificities of these secondary antibodies were determined in the absence of respective primary antibodies. For negative control, 1 μg of Alomone's P2X2 antibody was pre-incubated with 1 μg of its corresponding antigen peptide for 1 h at room temperature according to the manufacturer's instructions.
The cell types of TBCs were identified with cell-type-specific marker proteins: IP3R3 (Clapp et al. 2001) and PLCβ2 (DeFazio et al. 2006) for type II cells, SNAP-25 and serotonin for type III cells (Yang et al. 2000; Clapp et al. 2004; DeFazio et al. 2006). We thus took anti-SNAP-25 mouse monoclonal antibody (1 : 1000, Sigma, St Louis, MO, USA), anti-SNAP-25 goat polyclonal antibody (1 : 10, Santa Cruz Biotechnology), anti-serotonin mouse monoclonal antibody (1 : 50, Dako A/S, Denmark), anti-IP3R3 mouse monoclonal antibody (1 : 50, BD, Franklin Lakes, NJ, USA), and anti-PLCβ2 rabbit antibody (1 : 100, Santa Cruz Biotechnology) as primary antibodies, and the respective antibodies mentioned above as the secondary antibody. Although electron microscopy is essential for the precise identifications of cell types, it is known that identifications with antibodies against IP3R3 (Clapp et al. 2004) and SNAP-25 (Yang et al. 2000) agree with electron microscopic identifications.
Lucifer Yellow CH uptake
We examined the uptake of Lucifer Yellow CH (LY) through P2X7 by irrigating the basolateral membranes of TBCs mounted on the recording platform with a LY solution or a low divalent cation LY solution in the presence and absence of 3 mm ATP for 30 min at 37°C. The flow rate was 0.5 ml min−1. Also, 100 μm pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) or 1 μm 1-(N,O-bis[5-isoquinolinesulphonyl]-N-methyl-l-tyrosyl)-4-phenylpiperazine (KN-62) was added to the low divalent cation LY solution in several experiments.
Solutions
All solutions were prepared with deionized water and the components were expressed in millimolar concentrations, unless otherwise noted. The KCl electrode solution comprised: 150 KCl, 5 MgCl2, 10 EGTA, 5 Na2ATP, 0.3 Na3GTP, and 10 Hepes-KOH, pH 7.2. The physiological saline comprised: 150 NaCl, 5 KCl, 2 CaCl2, 0.5 MgCl2, 10 glucose, and 5 Hepes-NaOH, pH 7.4. The elastase solution comprised: 1 mg ml−1 elastase dissolved in the physiological saline. The low Ca2+ saline comprised: 150 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 5 Hepes-NaOH, pH 7.4. The Ca2+-nominally-free solution comprised: 150 NaCl, 5 KCl, 2 MgCl2, 10 glucose, and 5 Hepes-NaOH, pH 7.4. The Ca2+- and Mg2+-nominally-free solution comprised: 140 NaCl, 5 KCl, 2 EGTA, 10 glucose and 10 Hepes, pH 7.4. The PBS comprised: 137 NaCl, 2.67 KCl, 8.09 Na2HPO4, and 1.47 KH2PO4. The low divalent cation saline comprised: 155 NaCl, 5 KCl, 0.5 CaCl2, 10 glucose, and 5 Hepes-NaOH, pH 7.4. The fixation solution was the mixture of fresh 4% paraformaldehyde and Zambonic solution, both of which were prepared with the phosphate buffer. The blocking solution comprised: 3% normal donkey serum dissolved in the PBS and supplemented with 1% bovine serum albumin and 0.3% Triton X. Fura-2 AM solution comprised: 10 μm fura-2 AM dissolved in the physiological saline supplemented with 0.25% pluronic F-127. The LY solution was prepared by dissolving 0.5 mg ml−1 LY in the physiological saline. The low divalent cation LY solution was prepared by dissolving 0.5 mg ml−1 LY in the low divalent cation saline.
Results
Electrophysiological studies
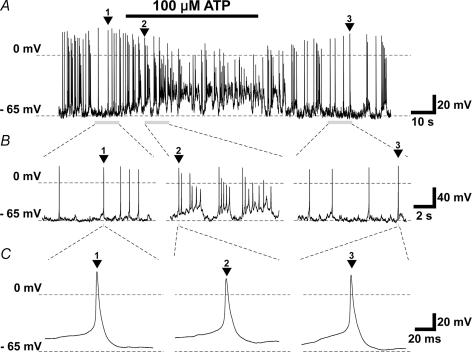
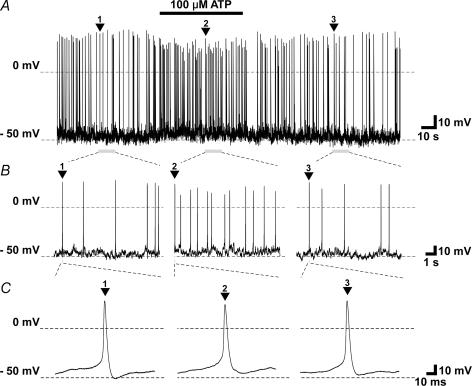
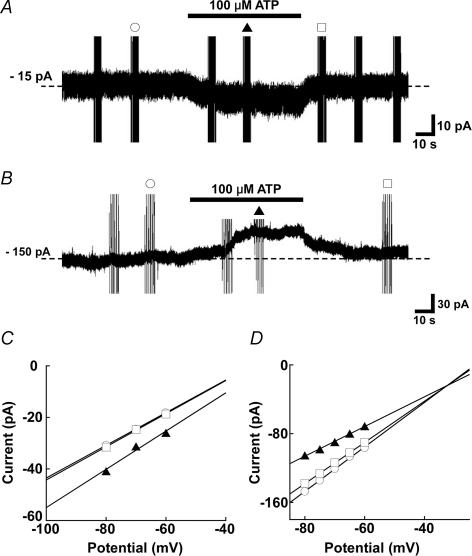
A subset of TBCs responded to 100 μm ATP applied to their basolateral membranes in cell-attached and whole-cell electrophysiological recording conditions. In cell-attached recording, 3 cells out of 10 increased their firing frequency, and one cell decreased its frequency (Fig. 2). In whole-cell current-clamp mode, three cells out of 12 gave generator potentials, and increased firing frequency (Figs 3 and 4). The generator potentials often comprised discrete subthreshold depolarizing events which appeared to sum and lead to spiking (Fig. 3). Under the whole-cell voltage-clamp mode, 3 cells out of 12 increased membrane conductance, and generated an inward current (Fig. 5). The estimated reversal potential was ∼−10 mV. In contrast, one cell decreased inward current magnitude by decreasing membrane conductance. The estimated reversal potential was ∼−32 mV.
Figure 2.
Increasing (A) and decreasing (B) firing frequencies of different taste bud cells (TBCs) recorded under the cell-attached mode in response to 100 μm ATP. Bars over traces show the duration of the application of ATP to the basolateral membranes of TBCs, in this and following figures.
Figure 3.
Oscillating, depolarizing receptor potential with action potentials in response to 100 μm ATP (A), time-expanded sections labelled with numbered arrowheads (B and C), obtained under whole-cell current-clamp conditions. Holding currents, −20 pA.
Figure 4.
Non-oscillating depolarizing receptor potential with increasing firing frequency in response to 100 μm ATP (A), and time-expanded sections labelled with numbered arrowheads (B and C), obtained under zero-current whole-cell current-clamp mode.
Figure 5. Inward (A) and outward (B) currents of different TBCs, elicited by 100 μM ATP applied to the basolateral membrane of TBCs recorded under a whole-cell voltage-clamp mode, and their respective current–voltage relations (C and D).
C and D, plotted data are before (^), during (▴), and after (□) the application of ATP, as respective symbols label on current noises in A and B show. The reversal potential of ∼−10 mV for A was estimated by extrapolating the relations obtained with test potentials of −80 mV to −60 mV in 10-mV steps from a holding potential of −70 mV (C). That of −32 mV for B was similarly estimated with test potentials of −80 mV to −60 mV in 5-mV steps from the holding potential (D). A in this figure and traces in Fig. 3 were obtained from the same cell.
Ca2+ imaging
The averaged intracellular calcium concentration ([Ca2+]i) of single taste buds was increased in response to the application of 1 μm ATP to their basolateral membrane side (Fig. 6). Ca2+ responses consisted of a phasic and a following tonic component. Also, Ca2+ responses occurred in non-taste lingual epithelial cells including perigemmal cells, cells surrounding TBCs, and epithelial cells lining lingual epithelia among fungiform papillae.
Figure 6. ATP-induced Ca2+ imaging of a single taste bud (A–C) and single TBCs (D).
A, time-lapse images. Frame numbers are identical to numerals on B except the frame labelled with 0, a differential interference contrast (DIC) image of a taste bud examined in this figure. Dashed lines, the outline of the taste bud. The outline of typical TBCs (a and b) and non-taste epithelial cells (c and d) are shown with continuous lines. B, averaged fluorescence ratios of the whole taste bud. C, frame 5 overlaid on frame 0 in A. D, fluorescence ratios of labelled cells in A. Horizontal scale, seconds. Vertical scale, a ratio of F340/F380.
A subset of TBCs, mostly on the peripheral part of taste buds, substantially increased [Ca2+]i upon ATP application. Their response patterns were similar to that of the whole taste bud, though some cells yielded little tonic component. Also, the onset timing of Ca2+ responses differed among these cells, even though they were simultaneously exposed to ATP.
Successive applications of ATP decreased the response magnitude, suggesting that responding P2 receptors were desensitized during stimulation (Baryshnikov et al. 2003). In order to reduce the desensitization, we decreased the Ca2+ concentration in the physiological saline to 1 mm (lowered-Ca2+ saline). Although the lowered-Ca2+ saline partially reversed the desensitization, Ca2+ responses were still decreased with successive applications, which was remarkable in a few cells (Fig. 7A). In the following Ca2+ imaging, we used the lowered-Ca2+ saline unless otherwise noted.
Figure 7.
Fluorescence ratios of single TBCs (A–G) and a single non-taste lingual epithelial cell (H) in response to ATP or ATP agonists under indicated conditions.
The threshold concentration of ATP was between 0.01 μm and 0.1 μm, and the magnitude of the Ca2+ responses increased with increasing ATP concentration (Fig. 7B). The number of ATP-sensitive TBCs responding within a taste bud increased with increasing ATP concentration, and reached a saturation level of ∼5 cells per taste bud at ATP concentration higher than 100 μm (Table 2).
Table 2.
The numbers of responding taste bud cells in single taste buds
| Agonist | Mean ±s.d. | n |
|---|---|---|
| ATP (1 μm) | 2.0 ± 1.6 | 30 |
| ATP (100 μm) | 4.7 ± 0.7 | 12 |
| ATP (3 mm) | 5.3 ± 2.6 | 6 |
| 2MeSATP (1 μm) | 2.1 ± 0.6 | 10 |
| UTP (100 μm) | 0.0 ± 0.0 | 11 |
| BzATP (30 μm) | 2.2 ± 0.6 | 3 |
| α,β-meATP (100 μm) | 0.0 ± 0.0 | 3 |
The irrigation of basolateral membranes with the Ca2+-nominally-free solution suppressed the tonic component magnitude, and slightly reduced the phasic one (Fig. 7C), suggesting that Ca2+ influx from extracellular space causes the tonic component, and that Ca2+ released from intracellular stores, and desensitization during stimulation, cause the phasic component.
A variety of ATP agonists also elicited Ca2+ responses (Fig. 7D–G and Table 2). That is, the Ca2+ response to 1 μm 2-methylthio ATP (2MeSATP), a P2Y1 and P2Y11 agonist (Ralevic & Burnstock, 1998), and that to 10 μm (data not shown) and 30 μm 3′-O-(4-benzoyl)benzoyl ATP (BzATP), a P2X7 subtype agonist (Chessell et al. 1998) suggest the functional expression of the respective receptor subtypes. The failure to respond to 100 μm UTP, a P2Y2 and P2Y4 subtype agonist (Ralevic & Burnstock, 1998), suggests the absence of these receptor subtypes. Also the failure to respond to 10 and 100 μmα,β-methylene adenosine 5′-triphosphate (α,β-meATP), a P2X agonist except P2X2, P2X4 (North, 2002) and P2X7 (Chessell et al. 1997), suggests the absence of other receptor subtypes.
Non-taste lingual epithelial cells also responded to 1 μm 2MeSATP, and 10 μm and 30 μm BzATP (data not shown). In addition, they responded to 100 μm UTP (Fig. 7H), suggesting that they functionally expressed P2Y2 or P2Y4 subtypes in addition to the P2 subtypes of TBCs mentioned above.
RT-PCR
The pharmacological classification of P2 receptor subtypes is difficult because of the lack of specific ligands for respective receptor subtypes. Instead, we classified them, except P2Y11, by taking advantage of RT-PCR on TBCs excised from lingual epithelia with special care in eliminating non-taste lingual epithelial cells (see Methods). RT-PCR reactions with primers for P2 receptor subtypes yielded cDNA fragments of the correct size for 6 out of 14 tested primers sets (Fig. 8 and Table 1). These cDNA fragments showed that expressed P2 subtypes were P2X2, P2X4, P2X7, P2Y1, P2Y13 and P2Y14. RT-PCR reactions on non-taste lingual epithelial cells yielded cDNA fragments of the correct size for 11 out of 14 tested primers sets; P2X2, P2X4, P2X5, P2X7, P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, P2Y13 and P2Y14 (ECs in Fig. 8 and Table 1).
Figure 8. PCR-amplified products from cDNA reverse-transcribed from mRNA isolated from TBCs and non-taste lingual epithelial cells (ECs).
Oligonucleotide primers specific for 14 different P2 receptor subtypes were run in separate reactions and the products run on agarose gel electrophoresis. The expected product size of each reaction is listed below each lane. Oligonucleotide primers specific for β-actin and α-gustducin are similarly examined as positive controls. H2O used in RT-PCR experiments is also examined as a negative control.
These results suggested that mRNAs for P2X2, P2X4, P2X7, P2Y1, P2Y13 and P2Y14 subtypes occurred both in TBCs and in non-taste lingual epithelial cells, and that mRNAs for P2X5, P2Y2, P2Y4, P2Y6 and P2Y12 subtypes occurred only in non-taste lingual epithelial cells. The detection of P2Y2 and P2Y4 subtypes on non-taste lingual epithelial cells agreed with their Ca2+ responses to UTP. Neither TBCs nor non-taste lingual epithelial cells had mRNAs for P2X1, P2X3 and P2X6 though the primer sets for them yielded cDNA fragments of the correct sizes when tested on mouse brains (see Methods).
Immunohistostaining
In order to confirm the translation of mRNA for P2 receptor subtypes in TBCs, we immunostained their product proteins, except those for P2Y1, P2Y13 and P2Y14, because P2Y1 had already been identified in rat TBCs (Kataoka et al. 2004), and the antibodies against P2Y13 and P2Y14 were unavailable. In addition, we examined the dependence of their translation on cell types with cell-type-specific marker antibodies.
It was shown that IP3R3 (Clapp et al. 2001) and PLCβ2 (Clapp et al. 2004) were type II cell markers, and SNAP-25 and serotonin were type III markers (Yang et al. 2000). However, single-cell RT-PCR showed that 5 TBCs out of 11 expressing PCR products for SNAP-25 expressed those for IP3R3 in mouse circumvallate papillae (DeFazio et al. 2006). That is, the single-cell RT-PCR study suggests the coexistence of mRNA for IP3R3 and that for SNAP-25 in the same cells in circumvallate papillae. If this is the case in fungiform papillae, and if fungiform TBCs translate both mRNAs, IP3R3 would not be a cell-type-specific marker any more. Thus, we tested the overlap of immunoreactivity for these cell-type-specific marker proteins (Fig. 9A) before examining the cell-type-dependent expression of P2 receptor subtypes.
Figure 9. Immunohistochemical confocal images of P2X2 and cell-type-specific markers.
A, immunoreactivity for PLCβ2 (blue), IP3R3 (green), SNAP-25 (red), and their overlay. B, P2X2-immunoreactivity (green) within a taste bud outlined by a dashed circle on each focal plane apart from its taste pore as indicated distance, and overlaid on respective DIC images. Note that no perigemmal cells (cells surrounding the taste bud) are P2X2 immunoreactive. C, immunoreactivity for P2X2 (green), SNAP-25 (red), and their overlay. P2X2 immunoreactivity in the upper row is determined with a Santa Cruz's antibody, and other P2X2 immunoreactivity in this figure is determined with Alomone's antibody (see text). Both P2X2 antibodies show that P2X2 occurs on a type III cell and several SNAP-25-immunoreacitve nerve endings. D, immunoreactivity for P2X2 (green), serotonin (red), SNAP-25 (blue), and their overlay. White regions, the coexpression of these three antigens; yellow regions, that of P2X2 and serotonin; violet regions, that of serotonin and SNAP-25. E, immunoreactivity for P2X2 (green), IP3R3 (red), and their overlay. F, P2X2 immunoreactivity with a control antigen-blocking peptide and its DIC image. G, no primary antibody control for P2X2 and its DIC image. H, P2X2 immunoreactivity in lingual epithelial cells between filiform papillae, and its overlay on a DIC image. I, uptake of Lucifer Yellow CH (LY) in the presence of 3 mm ATP. Note that there may be ATP-independent LY uptake (see text). Ia, overlay of fluorescence micrograph of LY (green) at the soma level onto the DIC image of the same TBC. Ib, overlay of Ia onto IP3R3 (red) and SNAP-25 (blue) images. Ic, 3-D reconstruction of the LY-stained cells, a type II cell and a type III cell. The type II cell is stained with LY. Upper white ring shows the maximum outline of the taste bud, and lower one shows that of the taste duct of the taste bud. Another three cells outside the taste bud are stained with LY: two near the upper white ring and one near the lower ring. Id, top view of Ic. Scale bars, 10 μm.
We optically and horizontally sliced each taste bud along their longitudinal axes with laser confocal microscopy, and counted the number of immunoreactive cell bodies. The confocal microscopy showed no overlaps between SNAP-25-immunoreactive cells (107 cells) and IP3R3-immunoreactive cells (520 cells) in 56 taste buds. We similarly examined 12 taste buds for the overlap of IP3R3 and PLCβ2 immunoreactivity. The immunoreactivity for both IP3R3 and PLCβ2 occurred in 154 cells, that for IP3R3 alone in 3 cells, and that for PLCβ2 alone in 11 cells. These results agreed with previous studies (Yang et al. 2000), and showed that IP3R3 was a type II cell marker and SNAP-25 a type III cell marker in mouse fungiform TBCs. Type I and IV cells were identified as non-immunoreactive cells.
P2X2 immunoreactivity occurred within taste buds but not in perigemmal cells (Fig. 9B). Within taste buds, some P2X2-immunoreactive cells were immunoreactive to SNAP-25 (Fig. 9C) and serotonin (Fig. 9D) but not immunoreactive to IP3R3 (Fig. 9E). Triple-immuno-histostaining showed that SNAP-25-immunoreactivity outlined serotonin-immunoreactive TBCs (a subset of type III cells), where P2X2- and serotonin-immunoreactivity coexpressed on the fraction of the cell surface. These results showed that the subset of type III cells express P2X2.
The antigen peptide for Alomone's P2X2 antibody suppressed the immunoreactivity (Fig. 9F). Also, controls in which the primary antibody was omitted showed no specific labelling (Fig. 9G). Perigemmal cells were non-immunoreactive to P2X2 (Fig. 9B), but a few non-taste lingual epithelial cells were immunoreactive to P2X2 (Fig. 9H).
No immunoreactivity for P2X4 was observed. P2X7 immunoreactivity on TBCs was unclear. Although an antibody obtained from Santa Cruz immunostained a few TBCs immunoreactive to IP3R3, its antigen peptide, the sequence of 264 amino acid residues, was unavailable. In order to perform absorption experiments, we tested another P2X7 antibody, the antigen peptide for which was available. However, this P2X7 antibody hardly immunohistostained any TBCs. Therefore, we tested the expression of P2X7 by monitoring the uptake of Lucifer Yellow CH, as shown in the next section.
Uptake of Lucifer Yellow CH
In the LY solution, a few TBCs showed uptake of Lucifer Yellow CH (LY), a 457 Da fluorescent dye, in response to the application of 3 mm ATP on their basolateral membranes, while other TBCs showed LY uptake independent of ATP stimulation (Figs 9I and 10). The number of LY-stained cells per taste bud was 2.1 ± 0.9 (mean ±s.d., n= 16) including a mean of ATP-independently stained cells of 1.0 ± 0.8 cells per taste bud (n= 8). The difference gives ∼1 cell per taste bud showing ATP-dependent LY uptake. A few non-taste lingual epithelial cells also stained with LY both ATP dependently and ATP independently.
Figure 10. Numbers of LY-stained TBCs per taste bud under different conditions.
The basolateral membranes of TBCs were irrigated with 0 mm or 3 mm ATP for 30 min with or without a P2 blocker, PPADS and a P2X7-selective blocker, KN-62. Numerals in parentheses show the numbers of taste buds examined, and horizontal bars, s.d.*P < 0.05 (two-tailed t test). **P < 0.003 (one-way ANOVA followed by Scheffé's multiple comparison).
The number of ATP-dependently stained cells per taste bud increased to approximately three in the low divalent cation LY solution on the basolateral membrane side, whereas that of ATP-independently stained cells remained unchanged (Fig. 10). The addition of either 100 μm PPADS, a non-selective blocker against P2 receptors, or 1 μm KN-62, a selective blocker against P2X7, to the 3 mm ATP stimulating solution decreased the number of stained TBCs, and the number was comparable to that of ATP-independently stained ones. These results suggest that the ATP-dependent uptake results from the activation of P2X7, showing that a few TBCs, the cell types of which are unidentified, express P2X7. Also it is shown that ATP-independent uptake did not result from the activation of purinergic receptors by spontaneously released ATP. Both ATP-independently stained TBCs and ATP-dependently stained TBCs excluded Trypan Blue, showing that these TBCs were vital and that their cell membranes were intact. Further study is needed to identify the cell type that expressed P2X7 and to elucidate the ATP-independent uptake mechanism.
Discussion
The present study showed for the first time that TBCs functionally expressed P2X2 and P2X7 in mice. Although RT-PCR studies suggested the expression of P2X4, P2Y1, P2Y13 and P2Y14, and though Ca2+ responses to 2MeSATP suggested the expression of P2Y1 or P2Y11, further studies are needed to confirm their expressions.
Electrophysiological studies suggested that ATP increased the open probability of one channel type with a reversal potential of ∼−10 mV, and decreased that of another channel type with a reversal potential of −32 mV (Fig. 5C and D). None of the ions involved in the present experimental conditions had equilibrium potentials equal to these reversal potentials. Thus it is likely that the ATP-opened channel is a non-selective cation channel which agrees with the facts that P2X2, P2X4 and P2X7 open non-selective cation channels. The ATP-closed channel could be a non-selective cation channel as well, but more permeable to K+ than the ATP-opened channels.
ATP is hydrolysed into adenosine by endogenous ATPase, and adenosine depolarizes diaphragm muscle fibres (Esau, 1994) and carotid body cells (Xu et al. 2006), or hyperpolarized neural cells (Pan et al. 1995). However, we believe that ATP, not adenosine, produced the present results, because the basolateral membranes were directly exposed to running solutions of either the physiological saline or stimulating ATP solution, which washed away adenosine produced by the hydrolysis and continuously supplied ATP. Also, the present results showed that both ATP and non-hydrolysed ATP agonists elicited Ca2+ responses, and that ATP caused LY uptake which was blocked by ATP receptor antagonists. We thus conclude that ATP was a primary agonist for the present results, though we do not exclude the functional expression of receptors for adenosine on TBCs.
In a previous study, we showed with a voltage-sensitive dye that a mixture of taste substances depolarized a group of TBCs and hyperpolarized another group within single taste buds of mouse fungiform papillae (Ohtubo et al. 2001). We suggested that some responses of TBCs were secondary, and that they were responses to transmitters released from TBCs responding to taste substances. If one of the released neurotransmitters was ATP (Finger et al. 2005), the present study suggests that it would activate both the depolarizing and hyperpolarizing groups of TBCs, as we reported in the previous paper.
The threshold concentration of Ca2+ responses was between 0.01 μm and 0.1 μm, suggesting that the responsible P2 receptor subtypes have low EC50 values. Our RT-PCR study suggested the expression of P2X2, P2X4, P2X7, P2Y1, P2Y13 and P2Y14. Published EC50 values for these mouse P2 receptor subtypes include: P2X2, 123 μm (Zhong et al. 2000); P2X4, 2.3 μm (Jones et al. 2000); P2X7, 298 μm (Chessell et al. 1998). Although the EC50 of mouse P2Y1 is unavailable, that of human P2Y1 is 304 nm (Palmer et al. 1998). EC50 values for P2Y13 and P2Y14 are unknown in any species. Within this limitation, we assume that P2X4 and P2Y1 are responsible for Ca2+ responses induced by 1 μm ATP, and that these two, plus P2X2 and P2X7 contribute to the generation of the electrophysiological responses to 100 μm ATP.
ATP-evoked Ca2+ responses in mouse TBCs were inhibited by the PLC inhibitor U73122, suggesting they were mediated solely by the activation of P2Y receptors (Baryshnikov et al. 2003). However, the concentration of ATP used for the inhibition experiments was 10 μm (Figs 4 and 5 of their paper), which was much lower than the EC50 of P2X2, 123 μm (Zhong et al. 2000). Therefore, there were no conflict between the previous result and our present result on the expression of P2X2. On the other hand, the concentration of 10 μm was close to the EC50 of P2X4, 2.3 μm (Jones et al. 2000). Our present result that P2X4 immunoreactivity was undetectable in taste buds agrees with the previous result (Baryshnikov et al. 2003), suggesting that P2X4 is less important in TBCs.
ATP caused LY uptake in a few TBCs per taste bud, and PPADS and KN-62 blocked the uptake. P2X7 is shown to form large pore channels in response to prolonged exposure to ATP, and these large pore channels are permeable to LY (North, 2002; Faria et al. 2005). In addition, it was shown that the ATP stimulation in solutions containing low concentrations of extracellular divalent cations created a much larger aqueous pore (Chessell et al. 1997). These results agree with the present results in that the number of TBCs stained with LY increased in the low divalent cation solution. ATP-independent uptake of LY may be caused by carrier-mediated mechanisms as shown in skates and rats (Ballatori et al. 1999).
P2X7 is involved in apoptosis of various cells (Wang et al. 2004; Bulanova et al. 2005). Mammalian TBCs are short-lived and a rapid turnover of TBCs occurs in each taste bud. For example, the lifetime of rat TBCs was ∼9 days (Beidler & Smallman, 1965) though a subset of type II cells expressing α-gustducin was shown to live longer (Farbman, 1980; Cho et al. 1998). It is suggested that old TBCs of mice employ p53, a tumour-suppressor protein linked to apoptosis, Bax, a bcl-2 family death factor, and a protease, caspase-2, as part of their apoptotic pathway (Zeng & Oakley, 1999; Zeng et al. 2000). In cultured mesangial cells of the renal glomerulus, the activation of P2X7 in response to ATP increased p53 in protein levels and caused apoptotic DNA fragmentation (Schulze-Lohoff et al. 1998). Therefore, we assume that P2X7 may contribute to the renewal of TBCs.
The present RT-PCR and Ca2+-imaging experiments showed no expression of P2Y2 nor P2Y4 receptor subtypes on TBCs in fungiform papillae, but they were expressed on non-taste lingual epithelial cells among fungiform papillae. In circumvallate and foliate papillae, on the other hand, immunohistostaining showed that isolated TBCs and isolated taste buds expressed P2Y2 and P2Y4 (Bystrova et al. 2006). This discrepancy may result from the differences among taste papillae and their innervation; the chorda tympani, a branch of cranial nerve VII, innervates TBCs in fungiform papillae and some TBCs in foliate papillae, while the glossopharyngeal nerve, cranial nerve IX, innervates the remainder of TBCs in foliate papillae and all TBCs in circumvallate papillae. The glossopharyngeal nerve may affect the translation of P2Y2 and P2Y4. Also it is equally possible that the epithelium in the two regions of the tongue is specified differently during development.
The present results suggest that these P2 receptor subtypes link the constituents of taste buds. It was shown that taste nerve fibres expressed P2X2 and P2X3 (Bo et al. 1999) and that taste stimulation released ATP from TBCs (Finger et al. 2005). Recent studies showed that type II cells released ATP through voltage-gated pannexin hemichannels in response to a variety of taste substances (Huang et al. 2007; Romanov et al. 2007) and released ATP caused serotonin release from other TBCs, probably type III cells (Huang et al. 2007). It is thus likely that P2X2 found on the subset of type III cells in the present study mediates the signal transduction between TBCs. Also, P2Y1 functionally expressed on rat type II and type III cells (Kataoka et al. 2004) may contribute to the signal transduction.
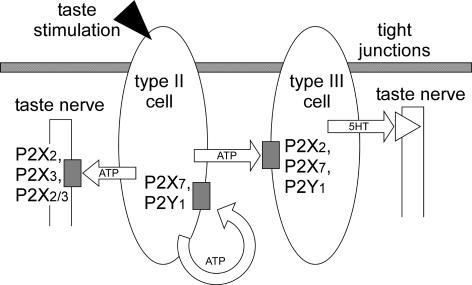
Therefore, we assume the following hypothetical roles of P2 subtypes within taste buds (Fig. 11). Type II cells release ATP as a paracrine signal (white arrows) in response to taste stimulation (arrowhead), and that released ATP stimulates P2X2, P2X3, or their heteromultimer on taste nerve fibres, and P2X2, P2X7, or P2Y1 (shaded boxes) on type III cells. Released serotonin stimulates serotonin receptors (triangle) on taste nerve endings. Self-(circular arrow) or paracrine-stimulation of P2X7 (shaded box) on TBCs triggers the apoptotic pathway, though intensive ATP release is essential for the high EC50 of P2X7. Also, this self- or paracrine-stimulation of P2X2 or P2Y1 on TBCs contributes to TBC–TBC interactions.
Figure 11. Hypothetical TBC networks linked with P2 receptor subtypes.
Taste stimulation (arrowhead) releases ATP from type II cells as a paracrine signal (white arrows). Released ATP stimulates P2 receptor subtypes (shaded boxes) on type III cells or taste nerve fibres. Stimulated type III cells release serotonin (5-HT), which activates serotonin receptors (triangle) on taste nerve endings. Self-stimulation (circular arrow) or paracrine stimulation of P2X7 leads the type II and type III cells to apoptosis. Note that the present results showed P2X2 was expressed on type III but not on type II cells. The cell type that expresses P2X7 remains to be investigated, though it is shown in type II and III cells.
When P2X4, P2Y13 and P2Y14 (not shown in the figure) were functionally expressed on TBCs, they would contribute to these TBC–TBC interactions as well as other P2 subtypes expressed on TBCs. The role of P2 receptor subtypes on non-immunoreactive TBCs, type I or IV cells, may be to regulate their supportive functions.
Acknowledgments
This work is partly supported by the 21st Centre of Excellence Program at the Kyushu Institute of Technology, and a Grants-in-Aid for Scientific Research (no. 16300094) from the Japan Society for the Promotion of Science (JSPS).
References
- Ballatori N, Hager DN, Nundy S, Miller DS, Boyer JL. Carrier-mediated uptake of lucifer yellow in skate and rat hepatocytes: a fluid-phase marker revisited. Am J Physiol Gastrointest Liver Physiol. 1999;277:G896–G904. doi: 10.1152/ajpgi.1999.277.4.G896. [DOI] [PubMed] [Google Scholar]
- Baryshnikov SG, Rogachevskaja OA, Kolesnikov SS. Calcium signaling mediated by P2Y receptors in mouse taste cells. J Neurophysiol. 2003;90:3283–3294. doi: 10.1152/jn.00312.2003. [DOI] [PubMed] [Google Scholar]
- Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–1111. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, Thon L, Adam D, Bulfone-Paus S. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174:3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- Bystrova MF, Yatzenko YE, Fedorov IV, Rogachevskaja OA, Kolesnikov SS. P2Y isoforms operative in mouse taste cells. Cell Tissue Res. 2006;323:377–382. doi: 10.1007/s00441-005-0098-8. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Michel AD, Humphrey PP. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br J Pharmacol. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Simon J, Hibell AD, Michel AD, Barnard EA, Humphrey PP. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998;439:26–30. doi: 10.1016/s0014-5793(98)01332-5. [DOI] [PubMed] [Google Scholar]
- Cho YK, Farbman AI, Smith DV. The timing of alpha-gustducin expression during cell renewal in rat vallate taste buds. Chem Senses. 1998;23:735–742. doi: 10.1093/chemse/23.6.735. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, Kanellopoulos JM, Motta-Ly I, Dautry-Varsat A, Ojcius DM. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol Cell Physiol. 1999;276:C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- Dai LJ, Kang HS, Kerstan D, Ritchie G, Quamme GA. ATP inhibits Mg2+ uptake in MDCT cells via P2X purinoceptors. Am J Physiol Renal Physiol. 2001;281:F833–F840. doi: 10.1152/ajprenal.0349.2000. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau SA. Role of adenosine in the depolarization of hypoxic hamster diaphragm membrane in vitro. Am J Respir Crit Care Med. 1994;149:910–914. doi: 10.1164/ajrccm.149.4.8143055. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Faria RX, Defarias FP, Alves LA. Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol Cell Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Fischer KG, Saueressig U, Jacobshagen C, Wichelmann A, Pavenstadt H. Extracellular nucleotides regulate cellular functions of podocytes in culture. Am J Physiol Renal Physiol. 2001;281:F1075–F1081. doi: 10.1152/ajprenal.2001.281.6.F1075. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Kaya N, Lu SG, Shen T, Sun XD. Adrenergic signalling between rat taste receptor cells. J Physiol. 2002a;543:601–614. doi: 10.1113/jphysiol.2002.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002b;22:10018–10029. doi: 10.1523/JNEUROSCI.22-22-10018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higure Y, Katayama Y, Takeuchi K, Ohtubo Y, Yoshii K. Lucifer Yellow slows voltage-gated Na+ current inactivation in a light-dependent manner in mice. J Physiol. 2003;550:159–167. doi: 10.1113/jphysiol.2003.040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, Humphrey PP. Functional characterization of the P2X4 receptor orthologues. Br J Pharmacol. 2000;129:388–394. doi: 10.1038/sj.bjp.0703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Toyono T, Seta Y, Ogura T, Toyoshima K. Expression of P2Y1 receptors in rat taste buds. Histochem Cell Biol. 2004;121:419–426. doi: 10.1007/s00418-004-0647-3. [DOI] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 2004;286:R649–R658. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- Kim YV, Bobkov YV, Kolesnikov SS. Adenosine triphosphate mobilizes cytosolic calcium and modulates ionic currents in mouse taste receptor cells. Neurosci Lett. 2000;290:165–168. doi: 10.1016/s0304-3940(00)01342-2. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ogura T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol. 2002;87:2643–2649. doi: 10.1152/jn.2002.87.6.2643. [DOI] [PubMed] [Google Scholar]
- Ohtubo Y, Suemitsu T, Shiobara S, Matsumoto T, Kumazawa T, Yoshii K. Optical recordings of taste responses from fungiform papillae of mouse in situ. J Physiol. 2001;530:287–293. doi: 10.1111/j.1469-7793.2001.0287l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Pan WJ, Osmanovic SS, Shefner SA. Characterization of the adenosine A1 receptor-activated potassium current in rat locus ceruleus neurons. J Pharmacol Exp Ther. 1995;273:537–544. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ren J, Bian X, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ. P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J Physiol. 2003;552:809–821. doi: 10.1113/jphysiol.2003.047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Burnstock G, Spyer KM. P2X purinoceptor-mediated excitation of trigeminal lingual nerve terminals in an in vitro intra-arterially perfused rat tongue preparation. J Physiol. 2000;524:891–902. doi: 10.1111/j.1469-7793.2000.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol Renal Physiol. 1998;275:F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- Seta Y, Toyoshima K. Three-dimensional structure of the gustatory cell in the mouse fungiform taste buds: a computer-assisted reconstruction from serial ultrathin sections. Anat Embryol (Berl) 1995;191:83–88. doi: 10.1007/BF00186781. [DOI] [PubMed] [Google Scholar]
- Shariatmadari R, Sipila P, Vierula M, Tornquist K, Huhtaniemi I, Poutanen M. Adenosine triphosphate induces Ca2+ signal in epithelial cells of the mouse caput epididymis through activation of P2X and P2Y purinergic receptors. Biol Reprod. 2003;68:1185–1192. doi: 10.1095/biolreprod.102.007419. [DOI] [PubMed] [Google Scholar]
- Suarez-Huerta N, Pouillon V, Boeynaems J, Robaye B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur J Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol. 2004;287:C1349–C1358. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- Xu F, Xu J, Tse FW, Tse A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol. 2006;290:C1592–C1598. doi: 10.1152/ajpcell.00546.2005. [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Kwan A, Oakley B. Gustatory innervation and bax-dependent caspase-2: participants in the life and death pathways of mouse taste receptor cells. J Comp Neurol. 2000;424:640–650. doi: 10.1002/1096-9861(20000904)424:4<640::aid-cne6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Oakley B. p53 and Bax: putative death factors in taste cell turnover. J Comp Neurol. 1999;413:168–180. doi: 10.1002/(sici)1096-9861(19991011)413:1<168::aid-cne12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Burnstock G. Pharmacological comparison of P2X receptors on rat coeliac, mouse coeliac and mouse pelvic ganglion neurons. Neuropharmacology. 2000;39:172–180. doi: 10.1016/s0028-3908(99)00145-8. [DOI] [PubMed] [Google Scholar]