Abstract
The pre-Bötzinger Complex (preBötC) inspiratory centre remains active in isolated brainstem–spinal cords and brainstem slices. The extent to which findings in these models depend on their dimensions or superfusate [K+] and [Ca2+] (both of which determine neuronal excitability) is not clear. We report here that inspiratory-related rhythms in newborn rat slices and brainstem–spinal cords with defined boundaries were basically similar in physiological Ca2+ (1.2 mm) and K+ (3 mm). Hypoglossal nerve rhythm was 1 : 1-coupled to preBötC activity in slices and to cervical nerve bursts in en bloc preparations lacking the facial motonucleus (VII). Hypoglossal rhythm was depressed in brainstems containing (portions of) VII, while pre/postinspiratory lumbar nerve bursting was present only in preparations with > 79% VII. preBötC-related slice rhythms were inhibited in 1.5 mm Ca2+ solution, whereas their longevity and burst rate were substantially augmented in 1 mm Ca2+. Ca2+ depression of slice rhythms was antagonized by raising superfusate K+ to 8–10 mm. This strong extracellular Ca2+/K+ antagonism of inspiratory (motor) rhythms was also revealed in brainstem–spinal cords without VII, while the inhibition was progressively attenuated with increasing amount of rostral tissue. We hypothesize that depression of hypoglossal rhythm and decreased Ca2+ sensitivity of preBötC rhythm are probably not related to an increased content of rostral respiratory structures, but rather to larger brainstem dimensions resulting in interstitial gradients for neuromodulator(s) and K+, respectively. We discuss whether block of pre/postinspiratory activity in preparations with < 79% VII is due to impairment of the pathway from preinspiratory interneurons to abdominal muscles
The neural control of breathing is studied in reduced preparations such as isolated newborn rat brainstem–spinal cords (Smith et al. 1990; Ballanyi et al. 1999). Microsectioning of this in vitro model showed that neurons of the pre-Bötzinger Complex (preBötC) inspiratory centre remain active in a transverse brainstem slice (Smith et al. 1991). In both, slice and en bloc models, rhythmogenic preBötC networks and preBötC-driven motor circuits are studied under quite divergent experimental conditions. preBötC slice thickness ranges from 200 to 1000 μm, and rostrocaudal boundaries of slices and brainstem–spinal cords vary notably (Rekling et al. 1996; Ballanyi et al. 1999; Koshiya & Smith, 1999; Ruangkittisakul et al. 2006). This may affect fictive inspiratory rhythms as the preBötC is neighboured by other ventral respiratory column structures such as the more rostral Bötzinger complex (BötC) and the parafacial respiratory group (pFRG), which is presumably closely associated with the retrotrapezoid nucleus (RTN) (Monnier et al. 2003; Feldman & Del Negro, 2006). While differences in the content of ventral respiratory column structures may modulate preBötC rhythms specifically, unspecific modulation may arise from interstitial accumulation or, conversely, washout of neuromodulator(s), which both depend on physical dimensions of isolated preparations (Ballanyi, 1999; Ruangkittisakul et al. 2006). preBötC activity may also be affected by superfusate constituents such as Ca2+ and K+ that modulate excitability in other neuronal systems (Hille, 2001; Somjen, 2002). The most common values of interstitial [Ca2+] and [K+] in diverse mammalian brain regions in vivo are 1.2 mm and 3 mm, respectively (Somjen, 2002). This contrasts with a wide range of superfusate Ca2+ (0.8–2.4 mm) and K+ (3–11 mm) in studies on isolated respiratory networks (Suzue, 1984; Smith et al. 1991; Johnson et al. 1996; Rekling et al. 1996; Ruangkittisakul et al. 2006).
Differences in dimensions of preparations and/or superfusate composition may be responsible for discrepant findings, e.g. regarding the capability of the isolated preBötC to generate rhythm. The above transection study (Smith et al. 1991) showed that the rate of inspiratory-related cervical nerve bursting is not affected by transection between preBötC and facial motonucleus (VII) in 1.5 mm Ca2+ solution. Other studies on this model showed that such transection depressed inspiratory burst rate in 2 mm Ca2+ (McLean & Remmers, 1994), whereas cervical rhythm was abolished following transection in ‘Suzue-type’ solution with 2.4 mm Ca2+ (Onimaru & Homma, 1987). We hypothesize that effects of brainstem transection on inspiratory-related rhythms depend critically on superfusate Ca2+.
The constancy of extensions of respiratory brainstem marker nuclei in early postnatal rats enables ‘online’ histology for generation of preBötC slices with defined boundaries that are capable of stable rhythm in 3 mm K+ (Ruangkittisakul et al. 2006). Our finding here that cranial nerves and blood vessels are ventral brainstem surface landmarks with a constant anatomical relation to the marker nuclei allowed generation of brainstem–spinal cords with defined rostral boundary. As one major aim of our study, we quantified in these en bloc preparations in physiological (1.2/3 mm) Ca2+/K+ the relation of their boundaries to the rate and duration of inspiratory cervical/hypoglossal nerve bursts and longevity of these rhythms, and compared the findings with those in the above slices. We then studied the Ca2+ dependence of preBötC (related) rhythms in both models. Finally, we elucidated the dependence on the rostral brainstem boundary of pre/postinspiratory lumbar nerve activity driving expiratory muscles (Janczewski et al. 2002; Janczewski & Feldman, 2006).
We found that preBötC (related) rhythms in 1.2 mm Ca2+ and 3 mm K+ were basically similar in slices and en bloc preparations. Hypoglossal rhythm was stable and 1 : 1-coupled to preBötC population activity in slices and to cervical bursting in brainstems without VII. In contrast, hypoglossal rhythm was depressed in en bloc preparations with more rostral boundaries, while pre/postinspiratory lumbar nerve bursting was only present in brainstems with a major portion of VII. Most importantly, preBötC bursting and associated motor rhythms in slices and en bloc medullas without VII were greatly depressed upon Ca2+ elevation by only 0.5 and 0.6 mm, respectively, and were reactivated by 8–10 mm K+. Conversely, in 1 mm instead of 1.2 mm Ca2+ solution, slice rhythms had a substantially greater longevity (> 3.5 h vs.∼1.5 h) and higher long-term burst rate. After spontaneous arrest of rhythm in 3 mm K+ and 1–1.2 mm Ca2+ in (slice) preparations with exposed preBötC, similar rhythm was reevoked by 6–7 mm K+ and cAMP elevation by rolipram.
We discuss whether lack of pre/postinspiratory activity in preparations with < 79% VII is due to impairment of the pathway from preinspiratory (pFRG) interneurons to abdominal expiratory muscles. We hypothesize that depression of hypoglossal rhythm and attenuated Ca2+ sensitivity of preBötC rhythm in less-reduced en bloc medullas is probably not related to increased content of rostral respiratory structures, but rather due to larger dimensions creating interstitial gradients for inhibitory neuromodulator(s) and K+. The strong dependence of inspiratory rhythms on an extracellular Ca2+/K+ antagonism suggests use of 1 mm Ca2+ for analysing isolated preBötC functions in 3 mm K+.
Methods
Ethical approval
The University of Alberta Animal Care and Ethics Committee approved all procedures and provided governance for the animal care.
Preparations and solutions
Brainstem–spinal cord preparations were generated from 57 Sprague–Dawley (SD) and 54 Wistar (W) rats between postnatal day 0 (P0) and P4. Animals were anaesthetized with 2–3% isoflurane and rapidly decerebrated after the paw withdrawal reflex disappeared. The neuraxis was isolated at 19–22°C in superfusate (for composition, see below) (Brockhaus & Ballanyi, 1998, 2000). The brainstem was transected rostral to the trigeminal nerve (Fig. 1), and the spinal cord cut at the cervical level (C6−8), except for one series of experiments in which the entire spinal cord was isolated for recording from ventral lumbar (L1−2) nerve roots. To obtain preparations with a defined boundary, rostral brainstem tissue including the pons was manually sectioned with a razor blade based on the constancy of ventral surface anatomical landmarks that we have revealed here (Fig. 1; see also online supplemental material Fig. S1). The desired rostrocaudal sectioning level was histologically verified after the electrophysiological experiments (supplemental Figs S1–S3). Preparations were fixed with the ventral side up at the spinal cord to the bottom silicone layer of the superfusion chamber with insect pins (volume 1.5 ml). Further stabilization was achieved by suction electrodes used to record nerve activities (see below). Superfusate was administered at a flow rate of 5 ml min−1 via a peristaltic pump (Watson-Marlow Alitea-AB, Sin-Can, Calgary, Alberta, Canada). Temperature in the recording chamber was kept at 25–27°C (TC-324B, Harvard Apparatus, Saint-Laurent, Quebec, Canada).
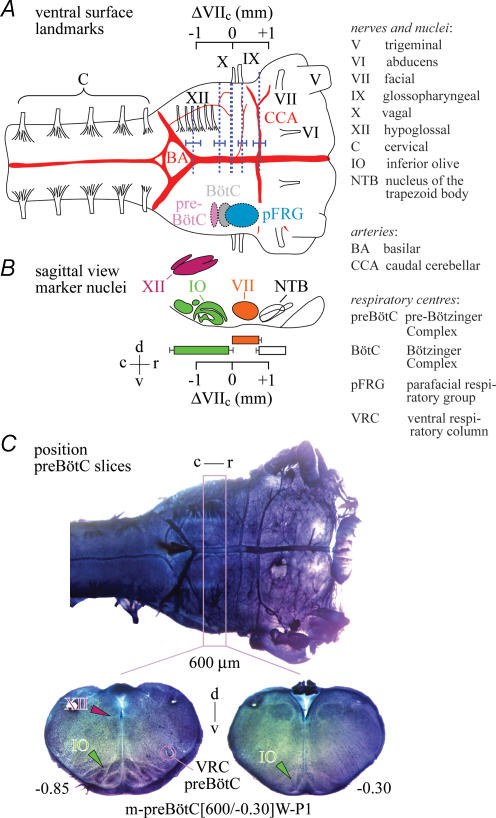
Figure 1. Regions of the ventral respiratory column and their ventral brainstem surface/nuclei landmarks in newborn rat brainstem–spinal cords.
A, schematic drawing of ventral brainstem surface indicating the proposed locations of the pre-Bötzinger complex (preBötC), the Bötzinger complex (BötC) and the parafacial respiratory group (pFRG) of the ventral respiratory column (Alheid et al. 2004; Feldman & Del Negro, 2006). Vertical dotted lines and attached horizontal bars show means ±s.d. of the positions of surface structures in Sprague–Dawley (SD) and Wistar (W) rats of postnatal days (P) 0–4, specifically cranial nerves (IX, X, XII) and arteries (BA, CCA). The positions of BA, CCA, IX and XII were quantified (in mm) in reference to dotted line at X. The centre of the ∼0.2 mm spanning preBötC is presumably located 0.5 mm caudal to the caudal end of VII nucleus (VIIc) (+ and − signs indicating the location of structures rostral or caudal to VIIc, respectively), and the VII nucleus extends 0.76 mm rostrocaudally (Smith et al. 1991; Ruangkittisakul et al. 2006; supplemental Figs S1 and S2). The pFRG extends presumably from the rostral boundary of VII to ∼0.2 mm caudal to VIIc (Onimaru & Homma, 2003, 2006; Onimaru et al. 2006). We found here that the caudal boundary of X nerve is located at VIIc, whereas the position of the CCA, at −0.74, matches well with the rostral end of VII. Furthermore, the proximal rostral boundary of the most rostral XII root coincides with the rostral preBötC border. The ΔVIIc scale in A is positioned based on findings from cuts at levels including the BA, XII and X lines (supplemental Fig. S1). B, position projected onto a sagittal brainstem view of the respiratory marker nuclei IO, VII, XII (Ruangkittisakul et al. 2006) and NTB (supplemental Fig. S2). Scales in A and B have identical dimensions and positions, thus allowing comparison of respiratory structures relative to VII, XII, IO and NTB. C, most surface structures of A are seen in a fixed and thionin-stained SD–P2 brainstem. The pink box indicates the mean position of 600 μm thick ‘m-preBötC slices’ with the preBötC in the middle. The slice was labelled ‘m-preBötC[600/−0.30]W–P1’ (m, middle; 600, slice thickness in μm; −0.30, rostral boundary in mm; W–P1, rat strain and age). Here, we classified slices according to their rostral instead of caudal boundary in contrast to Ruangkittisakul et al. (2006) to allow for comparison with rostral boundaries of brainstem–spinal cord preparations. Other abbreviations: r, rostral; c, caudal; d, dorsal; v, ventral.
Superfusate with [Ca2+] (Heinemann et al. 1977; Nicholson et al. 1978; Hansen, 1985; Richter & Acker, 1989; Trippenbach et al. 1990; Nilsson et al. 1993; Puka-Sundvall et al. 1994; Somjen, 2002; Brown et al. 2004) and [K+] (Leusen, 1972; Hansen, 1985; Somjen, 2002; Brown et al. 2004) resembling most common values in the extracellular space of brain tissue in vivo, i.e. 1.2 mm and 3 mm, respectively, contained (mm): 120 NaCl, 3 KCl, 1.2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3 and 30 d-glucose (pH adjusted to 7.4 by gassing with 95% O2, 5% CO2).
preBötC-related rhythms in brainstem–spinal cords in standard solution were compared with those in solution of elevated Ca2+, K+ and Mg2+ (2.4, 6.2 and 1.3 mm, respectively), very similar to that in the first study (Suzue, 1984) and numerous follow-up reports on this and other isolated respiratory networks (e.g. Oshima et al. 2000; Herlenius et al. 2002; Iizuka, 2004; St. John et al. 2005; Kuwana et al. 2006; Onimaru et al. 2006). This ‘Suzue-type’ solution contained (mm): 120 NaCl, 6.2 KCl, 2.4 CaCl2, 1.3 MgCl2, 1.25 NaH2PO4, 26 NaHCO3 and 30 d-glucose (pH adjusted to 7.4 by gassing with 95% O2, 5% CO2). In a related series of experiments, Ca2+ and K+ in the standard solution were varied between 1.2 and 6 mm, and 3 and 10 mm, respectively.
preBötC-related rhythms were also studied in 600 μm thick ‘m-preBötC slices’ with the preBötC in the middle (Ruangkittisakul et al. 2006). After removal of the cerebellum and sectioning close to the caudal cerebellar artery (De Araujo & Campos, 2005) (Fig. 1), the brainstem of P0–4 SD (n= 8) or W rats (n= 28) was glued rostral side down to a metal plate and transferred to a vibrating microtome (Leica VT1000S, Leica Microsystems, Richmond Hill, Ontario, Canada). Serial transverse sectioning (100 μm) was stopped, and one ‘rhythmic’ slice was cut, when specific landmarks were reached, in particular subregions of the inferior olive (Fig. 1 and supplemental Fig. S1). Slices were fixed caudal side up with insect pins in the recording chamber and superfused with standard saline in which Ca2+ and K+ were varied between 1 and 1.8 mm and 3 and 10 mm, respectively, at either 1 or 2 mm Mg2+ (Ruangkittisakul et al. 2006).
Rolipram (Sigma-Aldrich, Canada), a phosphodi-esterase-4 blocker (O'Donnell & Zhang, 2004) stimulating inspiratory rhythm (Ruangkittisakul & Ballanyi, 2006; Ruangkittisakul et al. 2006), was kept frozen in stock solution (1 mm in dimethyl sulfoxide). Chemicals for salines were purchased from Fisher Scientific (Oakville, Ottawa, Ontario, Canada).
Electrophysiological recording
Discharge of preBötC-driven motoneurons providing inspiratory motor output in brainstem–spinal cord preparations (Smith et al. 1990; Ballanyi et al. 1999) was recorded from ventral spinal (C3–4) and cranial (hypoglossal) nerve roots with suction electrodes (outer diameter 80–250 μm) filled with standard superfusate. In brainstem preparations with a complete spinal cord, activity was recorded simultaneously from C3–4 and ventral L1−2 roots, the latter containing axons of pre/postinspiratory active motoneurons that appear to be driven by the pFRG and innervate expiratory abdominal muscles (Janczewski et al. 2002; Iizuka, 2004). Neuronal population activity was recorded with a differential amplifier (×10 000, DAM 50, World Precision Instruments, Sarasota, FL, USA), bandpass-filtered (0.3–3 kHz) and integrated. In the slices, suction electrode recording was done at the caudal surface from the ventral respiratory column containing the preBötC (Alheid et al. 2004), in most cases combined with recording from one hypoglossal nerve root. Signals were fed into a computer (sampling rate 1 kHz) via a digital recording system (Powerlab/8SP, ADInstruments, Colorado Springs, CO, USA).
Data analysis
Respiratory-related activity of brainstem–spinal cord preparations and slices was continuously recorded and quantified by measuring every 20 min over 2 min time periods the frequency of rhythm and single burst duration. Longevity of rhythms was defined as the time from start of recording until the time when the interval between consecutive bursts exceeded 1 min. Burst duration was defined, using Clampfit software (Molecular Devices, Union City, CA, USA), as the time interval from when the signal increased above and decreased below a threshold set at 10% of the peak amplitude value for that burst. Rhythms modified by changing superfusate cation composition or adding rolipram were analysed over a time period of 2 min at steady-state. Values are reported as means ±s.e.m. except for histological analyses where means ±s.d. were determined. Significance of values was determined by Student's one-sample t test using SigmaPlot (Systat software, Point Richmond, CA, USA). Significance was defined as *P < 0.05 and **P < 0.01.
Histology
For histological analysis after the experiments, both aspects of transected brainstem–spinal cords were fixed in phosphate buffer (1 : 2 mixture of 0.1 m NaH2PO4+ 0.1 m Na2HPO4 in H2O, pH 7.2) containing 4% paraformaldehyde (all agents from Sigma). For staining after > 15 min of fixation, preparations were incubated in phosphate buffer for at least 2 min and subsequently immersed 90 s for en bloc brainstems and slices and 45 s for 200–250 μm thick sagittal brainstem–pons slices in a solution consisting of 1% thionin acetate (Sigma) in a mixture of 0.1 m sodium acetate trihydrate and 0.1 m acetic acid (Fisher). After staining, preparations were first ‘washed’ with phosphate buffer (2 min) and then with 50% ethanol (4 min) before returning to phosphate buffer for 2 min. Subsequently, preparations were transferred to a Petri dish and photographed (PL-A686 6.6 megapixel camera, Capture-SE software, PixeLINK, Ottawa, Ontario, Canada) in phosphate buffer under a stereo microscope (Zeiss-SR15, magnification ×32; Carl Zeiss, Jena, Germany).
Defined sectioning of brainstem–spinal cords
Brainstem–spinal cords with defined rostral boundary were generated using ventral brainstem surface landmarks (Fig. 1 and supplemental Fig. S1). Specifically, we found that the vagal nerve is located 0.06 ± 0.05 mm (n= 5) caudal to the caudal end of VII (VIIc) used as reference, whereas the location of the caudal cerebellar artery matches well with the rostral border of VII, i.e. 0.74 ± 0.16 mm (n= 14) vs. 0.76 ± 0.07 mm (n= 16) rostral to VIIc, respectively. The area covered by the most rostral hypoglossal nerve root spans the proposed (Smith et al. 1991; Ruangkittisakul et al. 2006) extension of the preBötC between 0.4 and 0.6 mm caudal to VIIc, with its rostral end located 0.41 ± 0.13 mm (n= 5) caudal to VIIc (Fig. 1 and supplemental Fig. S1).
preBötC slice boundaries can be determined ‘online’ by comparing respiratory marker nuclei in (pre)rhythmic slices with those in a reference brainstem atlas (Ruangkittisakul et al. 2006). The rostrocaudal locations of the marker structures, and thus slice boundaries, are referred to their distance from VIIc. Here, we used the atlas to identify the rostral boundary of transected brainstem–spinal cords (Fig. 1 and supplemental Figs S1–S3). This method was not suited to determine the boundary of preparations transected between VIIc and the inferior olive due to lack of marker structures. In these cases, the boundary was determined by measuring the distance in sagittal slices between VIIc and the caudal surface of the rostral aspects of the sectioned preparations (supplemental Figs S2 and S3). Analysis of the sagittal sections revealed a distance between inferior olive and VIIc of 0.16 ± 0.03 mm (n= 5), similar to our previous value (0.13 ± 0.03 mm, n= 26) based on 50 μm transverse serial sections (Ruangkittisakul et al. 2006).
Ventral respiratory column structures in newborn rat brainstem–spinal cords
As one major aim, we investigated the dependence of fictive respiratory rhythms in brainstem–spinal cords on their rostral boundary. We restricted our analysis to preparations without pons as influences of this structure on respiratory activity in this model were previously studied (Monteau et al. 1989; Errchidi et al. 1991; see also Alheid et al. 2004).
In the original voltage-sensitive dye imaging study on newborn rat brainstem–spinal cords, the pFRG was defined as a pre/postinspiratory active neuronal group, located ventrolateral to VII and close to the ventral surface (Onimaru & Homma, 2003). More recently, Onimaru et al. (2006) proposed that the pFRG may include regions up to ∼0.2 mm caudal to VIIc (Fig. 1). Moreover, cells with pFRG-like activity pattern are also distributed caudal to that area (Arata et al. 1990; Smith et al. 1990; Ballanyi et al. 1999; Onimaru et al. 2003).
Compared to the RTN/pFRG, the preBötC was proposed to have a more defined rostrocaudal extension of ∼0.2 mm, centred ∼0.5 mm caudal to VIIc (Smith et al. 1991; Ruangkittisakul et al. 2006) (Fig. 1). Inspiratory neurons seem to be by far the major respiratory neuron class in thin preBötC slices. However, in less-reduced brainstem preparations a variety of respiratory neuron classes including pFRG-like cells are active within the region spanned by the preBötC (Arata et al. 1990; Smith et al. 1990; Schwarzacher et al. 1995; Sun et al. 1998; Onimaru et al. 2003; Paton et al. 2006). The area between preBötC and VIIc contains the BötC (Fig. 1) with a large number of expiratory neurons in addition to other types of respiratory cells (Feldman, 1986; Sun et al. 1998). Due to this overlap in the distribution of distinct respiratory neuron classes within the rostral ventrolateral respiratory column and other reasons, results from transection experiments should be interpreted with caution (Wilson et al. 2006).
Results
First, we studied inspiratory cervical nerve rhythms in three groups of brainstem–spinal cords with the boundary (i) slightly rostral to VII (thus including the BötC and RTN/pFRG), (ii) between VII and preBötC, and (iii) at or into the rostral preBötC border (Fig. 1).
Inspiratory cervical rhythm in transected brainstem–spinal cords
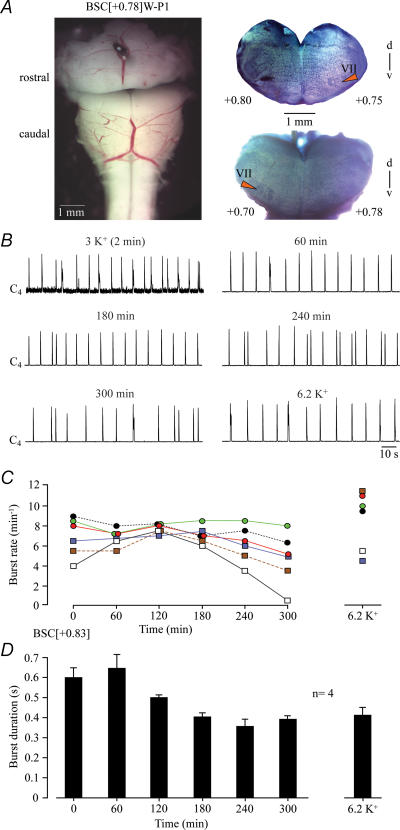
The mean boundary of six preparations with VII was 0.83 ± 0.08 mm rostral to VIIc (Fig. 2). In standard 1.2 mm Ca2+, 3 mm K+ solution, these brainstems generated cervical rhythm with an initial frequency of 6.9 ± 0.8 bursts min−1 and a burst duration of 0.60 ± 0.05 s (Fig. 2). After 3 h of recording, burst rate did not change (7.1 ± 0.35 bursts min−1), whereas burst duration decreased to 0.40 ± 0.02 s. Raising superfusate K+ from 3 to 6.2 mm (as in ‘Suzue-type’ solution) after the 5 h recording period reactivated rhythm, which stopped after 298 min in one preparation lacking ∼10% of VII, and increased mean burst rate above control (8.7 ± 1.2 bursts min−1, n= 6) with no stimulating effect on burst duration (Fig. 2).
Figure 2. Cervical rhythm in 1.2 mM Ca2+, 3 mM K+ solution in brainstem–spinal cords with VII.
A, left panel, transected brainstem–spinal cord (BSC) preparation from a P1 W rat with the rostral boundary 0.78 mm rostral to VIIc, thus a ‘BSC[+0.78]W–P1’, in standard saline after manual transection with a razor blade. Right panels, photographs of fixed and thionin-stained cut surfaces of corresponding rostral and caudal brainstem blocks after experiment. Numbers indicate transection level referred to VIIc (compare Fig. 1 and supplemental Fig. S1). The differing numbers on the left and right side of transection indicate a lateral tilt of the section plane. B, in the preparation of A, regular band-pass-filtered and integrated inspiratory-related bursting of the 4th ventral cervical (C4) nerve rootlet was monitored for 5 h before superfusate K+ level was raised to 6.2 mm. C, burst rates of 6 BSC with a mean boundary 0.83 rostral to VIIc (BSC[+0.83]). Note that rhythms slowed down slightly during the 4th hour of recording and stopped in one preparation shortly before 5 h (after 298 min). Raising K+ to 6.2 mm after 5 h recording increased burst rates in all but one brainstems. D, burst duration in 4 preparations of C. Bars represent means and s.e.m. Numbers in the left part of the abscissa indicate the time period after start of recording.
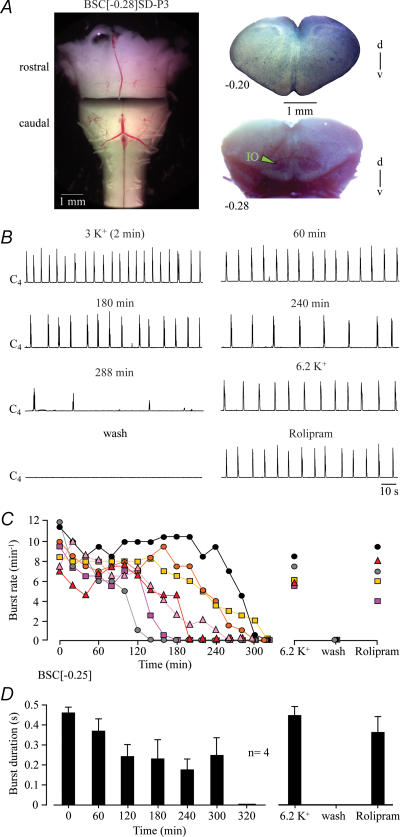
The mean boundary of seven medullas cut between VII and preBötC was 0.25 ± 0.04 mm caudal to VIIc (Fig. 3). The initial burst rate of 9.4 ± 0.7 bursts min−1 decreased to 8.1 ± 0.6 bursts min−1 within 20 min and remained rather stable for > 2 h in five cases. Burst duration decreased from initially 0.47 ± 0.02 s to 0.24 ± 0.06 s within 2 h, but remained stable thereafter (Fig. 3). In three preparations, rhythm stopped after 2–3 h, whereas four preparations showed rhythm for > 4 h (mean longevity 236 ± 26 min, n= 7). 6.2 mm K+ and the cAMP-elevating agent rolipram (1 μm) reactivated rhythms with rates (6.7 ± 0.6 vs. 6.9 ± 0.9 bursts min−1) and durations (0.45 ± 0.04 vs. 0.36 ± 0.08 s) very similar to control (Fig. 3).
Figure 3. Cervical rhythm in brainstem–spinal cords cut between preBötC and VII.
A, left panel, transected BSC[−0.28]SD–P3 preparation. Right panels, fixed and thionin-stained cut surfaces of corresponding rostral and caudal brainstem blocks. B, between 3 and 4 h of recording, inspiratory C4 nerve bursting slowed down notably before rhythm stopped spontaneously after 288 min (last bursts shown). Very similar rhythm was reactivated by raising superfusate K+ to 6.2 mm and, after return to 3 mm K+ saline, by bath-application of the cAMP-elevating agent rolipram (1 μm). C, burst rates of 7 BSC[−0.25]. Note that rhythm persisted in 5 preparations for > 3 h. D, burst duration in 4 preparations of C with very similar mean rostral boundary (BSC[−0.26]). Bars represent means and s.e.m. Numbers in the left part of the abscissa indicate the time period after start of recording.
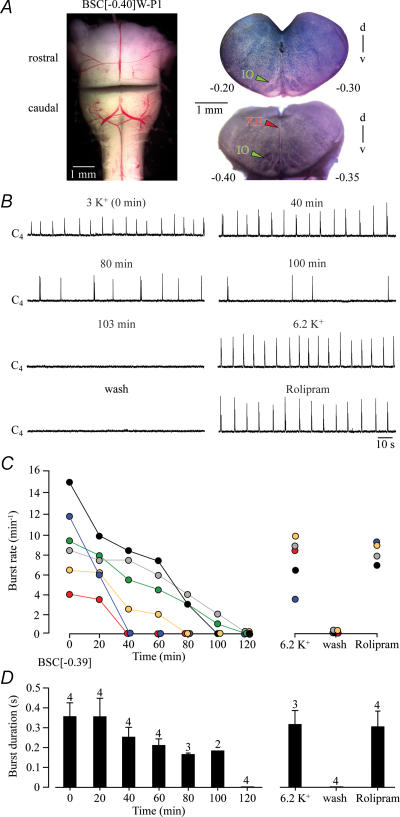
The mean boundary of six preparations with rostrally exposed preBötC was 0.39 ± 0.04 mm caudal to VIIc (Fig. 4). Initial burst rate was 9.3 ± 1.7 bursts min−1, but decreased monotonically until rhythm stopped spontaneously (mean longevity 71.5 ± 14.1 min, n= 6). In three preparations still active after 80 min of recording, burst rate at that time was 3.3 ± 0.3 bursts min−1 and burst duration was 0.17 ± 0 s (Fig. 4). K+ at 6.2 mm and rolipram (1 μm) reactivated rhythms with rates slightly lower than (7.5 ± 1.2 vs. 8.3 ± 0.5 bursts min−1) and durations similar to (0.32 ± 0.07 vs. 0.30 ± 0.08 s) the initial value (Fig. 4).
Figure 4. Cervical rhythm of brainstem–spinal cords with boundaries at or into the rostral preBötC border.
A, left panel, transected BSC[−0.40]W–P1 preparation. Right panels, fixed and thionin-stained cut surfaces of corresponding brainstem blocks. B, C4 bursting was revealed for > 1 h before rhythm stopped after 103 min. Very similar rhythm was reactivated by 6.2 mm K+ and, after return to 3 mm K+, bath-application of rolipram (1 μm). C, burst rates of 6 BSC[−0.39]. Note that rhythm stopped spontaneously after time periods of 40–120 min. D, burst duration in 4 preparations of C. Bars represent means and s.e.m. Digits above bars indicate number of (still active) preparations tested. Numbers in the left part of the abscissa indicate the time period after start of recording.
Four preparations with the rostral boundary ≥ 0.45 mm caudal to VIIc, and thus likely to be very close to the preBötC centre (Fig. 1), did not generate respiratory rhythm in either 3 mm or 6.2 mm K+. In three of these cases, elevated K+ induced < 0.3 s irregular cervical nerve bursts with > 15 events min−1 and a distinct activity consisting of a > 1 min massive discharge (supplemental Fig. S4).
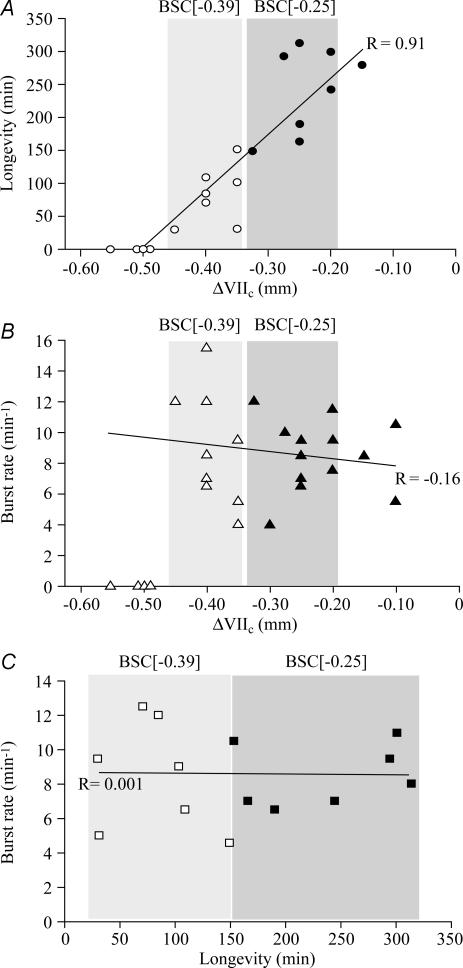
Analysis of pooled data from the above groups of brainstem–spinal cord preparations revealed a significant correlation between rostral boundary and longevity of rhythm, but not between boundary and burst rate, or initial frequency and longevity (Fig. 5).
Figure 5. Correlation between rostral boundary, longevity and burst rate of cervical rhythm in brainstem–spinal cords.
A, significant (P < 0.01) correlation between the longevity of cervical inspiratory rhythm and rostral boundary (R is correlation coefficient). In contrast, no significant correlations were revealed between burst rates (determined after 10 min of recording) and rostral boundaries (B) or longevity of rhythm (C). Grey areas indicate the two groups of preparations used for the analyses in Figs 3 and 4. Note that preparations with the rostral boundary at −0.50 or more caudal (thus caudal to the proposed preBötC centre) did not show respiratory rhythm (compare supplemental Fig. S4). A and C contain less data points compared to B as longevity was not tested in all preparations.
Pre/postinspiratory lumbar rhythm in transected brainstem–spinal cords
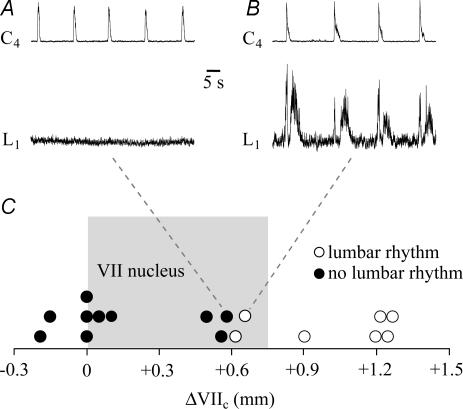
In a further approach, we studied the dependence of pre/postinspiratory lumbar nerve bursting (Janczewski et al. 2002) on the rostral boundary of en bloc brainstems with complete spinal cord. Ten preparations with boundaries < 0.6 mm rostral to VIIc, thus containing < 79% of VII, did not show pre/postinspiratory lumbar bursting, but generated regular cervical inspiratory rhythm (Fig. 6). On the contrary, seven preparations with boundaries > 0.6 mm rostral to VIIc showed both pre/postinspiratory lumbar and inspiratory cervical nerve bursting (Fig. 6).
Figure 6. Dependence of pre/postinspiratory lumbar nerve bursting on rostral boundary of brainstem–spinal cords.
A, a BSC[+0.58]W–P1 preparation generated stable inspiratory C4 bursting, but did not show respiratory-related activity in the first ventral lumbar (L1) root. B, pre/postinspiratory L1 bursting was revealed in a BSC[+0.67]W–P0. C, plot of the occurrence (^) or absence (•) of lumbar pre/postinspiratory rhythm vs. the rostral boundary. Grey area indicates the rostrocaudal extension of VII (compare Fig. 1).
Hypoglossal nerve activity in transected brainstem–spinal cords
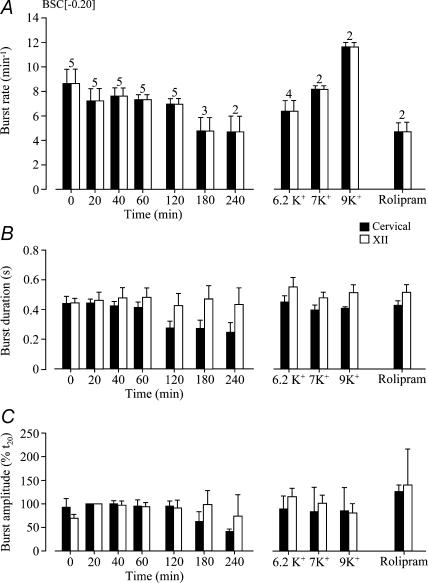
Next, we assessed whether inspiratory hypoglossal rhythms in en bloc preparations also depend on the transection level. In five preparations cut between preBötC and VIIc (mean boundary 0.20 ± 0.04 mm caudal to VIIc), burst rates in 3 mm K+ of synchronous rhythms in cervical and most rostral hypoglossal nerve roots were identical. After an initial time period of 8.7 ± 1.1 bursts min−1, burst rate stabilized at 7.1 and 7.7 bursts min−1 between 20 and 120 min, but decreased to 4.8 ± 1.0 and 4.8 ± 1.3 bursts min−1 at 180 and 240 min, respectively (Fig. 7). The amplitude of rostral hypoglossal root bursts was stable during recording periods > 4 h in some cases (Figs 7 and 8). Also their duration remained stable at ∼0.4 s, whereas cervical burst duration decreased from ∼0.4 s to < 0.3 s after 1 h of recording (Fig. 7). Hypoglossal burst duration was also similar to control when rhythm was reactivated after spontaneous arrest by elevated K+ (6.2, 7, 9 mm) or rolipram (1 μm), all of which restored cervical burst duration to control values (Fig. 7). Only 9 mm K+ increased the burst rate of re-evoked rhythms above initial control values. Under all these conditions, hypoglossal and cervical rhythms showed 1 : 1-coupling (Fig. 7). Neither K+ nor rolipram affected the amplitude of hypoglossal or cervical bursting. In 12 additional preparations with similar boundaries caudal to VIIc, the amplitude of bursting of rostral hypoglossal roots remained constant or increased during the first hour of recording (Fig. 8).
Figure 7. Statistical analysis of cervical and hypoglossal rhythms in brainstem–spinal cords without VII.
A, in 5 BSC[−0.20] preparations, rhythms in cervical roots (open bars) and most rostral hypoglossal (XII) roots (filled bars) had identical burst rates indicating a 1 : 1 coupling ratio. As evident from numbers above bars, rhythm stopped in 2 and 3 preparations before 3 and 4 h of recording, respectively. Identical burst rates were also revealed upon reactivation of rhythm with elevated K+ or rolipram (1 μm). B, XII burst duration was similar during both long-term recording and re-stimulation of rhythm. In contrast, cervical burst duration decreased during the first hour of recording, but was restored by raised K+ and rolipram. C, cervical burst amplitude decreased after 2 h of recording, but was re-increased by the stimulatory procedures. XII burst amplitude referred to the value at 20 min (t20) was stable under all conditions. Numbers of preparations above bars in A are identical to those in B and C.
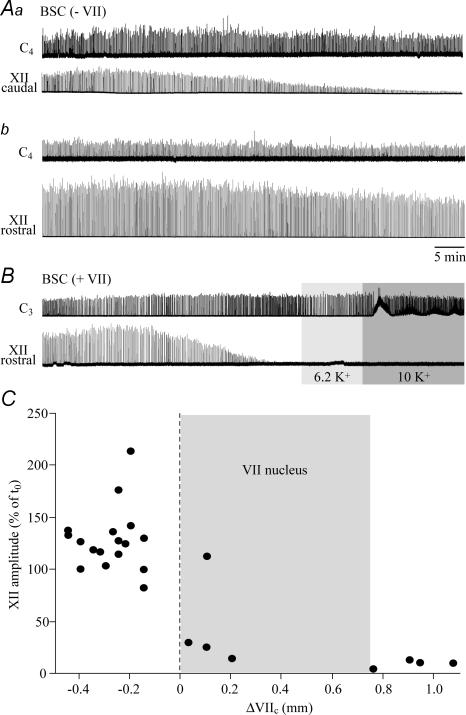
Figure 8. Dependence of XII rhythm on rostral boundary of brainstem–spinal cords.
A, in a BSC[−0.15]SD–P2 preparation, cervical rhythm monitored within < 2 min after transfer to the experimental chamber (Aa) was stable for > 1 h. In contrast, the amplitude of synchronous bursting in a caudal XII root started to decrease after ∼17 min and reached < 10% of the initial amplitude after 1 h. Ab, changing the suction electrode from the caudal to the most rostral XII root within < 5 min after end of the recording in Aa revealed robust bursting. The amplitude of most rostral XII root bursts remained at > 70% of the initial value for the illustrated time period and continued for > 2 h at similar levels afterwards. B, in a BSC[+1.1]SD–P1 preparation, cervical rhythm monitored within < 2 min after transfer to the experimental chamber was stable for > 1 h, whereas bursting of the most rostral XII root started to decrease in amplitude ∼17 min after start of the recording and disappeared after ∼35 min. XII rhythm could not be reactivated by raising superfusate K+ from 3 mm to 6.2 or 10 mm. Same time scale in A and B. C, plot of XII nerve burst amplitude after 1 h, referred to the value at the start of recording (t0) vs. rostral boundary. Grey area indicates the rostrocaudal extension of VII nucleus (compare Figs 1 and 6).
In contrast, burst amplitude, but not duration, decreased within 20–90 min after start of recording in caudal hypoglossal roots of brainstem–spinal cords without VII (Fig. 8). Bursting disappeared in 1 of 4 preparations and stabilized after ∼1 h in the other cases at 17 ± 6.9% of the initial value. In four preparations with VII (mean boundary 0.11 ± 0.07 mm rostral to VIIc), amplitude of rostral hypoglossal root bursts fell to 23 ± 4.7% of initial values after 20–50 min of recording. Furthermore, the depression was more pronounced (6.8 ± 2.8%) after similar time periods in four preparations with complete VII (Fig. 8).
Rhythm in preBötC slices
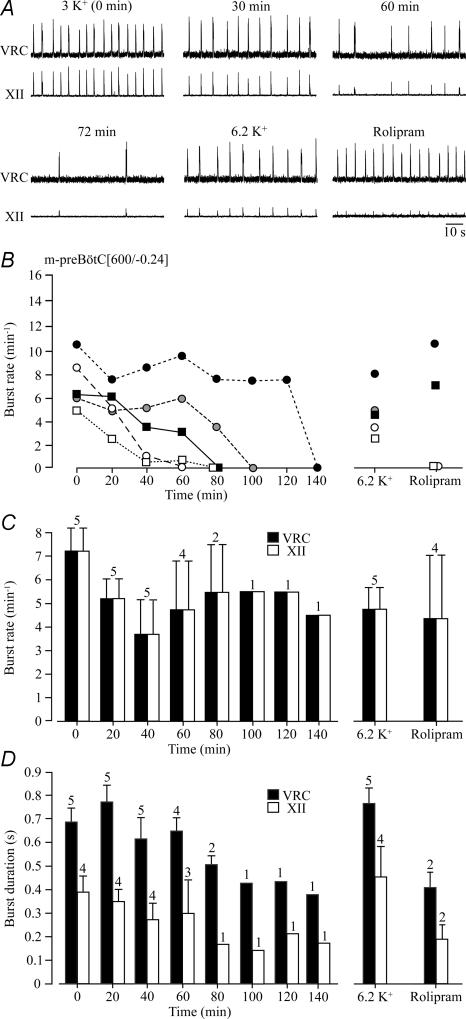
Next, we compared inspiratory-related rhythms in 1.2 mm Ca2+ and 3 mm K+ between en bloc medullas and 600 μm m-preBötC slices with a mean rostral boundary 0.24 ± 0.11 mm (n= 5) caudal to VIIc. The initial rate of bursting in the region of the ventral respiratory column containing the preBötC was 7.2 ± 1.0 bursts min−1, but decreased within 20 min to 5.2 ± 0.8 bursts min−1. Burst rate remained approximately at that value until rhythm stopped after 60–140 min (mean longevity 87.8 ± 16.8 min, n= 5) (Fig. 9). preBötC burst duration fluctuated between 0.5 and 0.8 s within the first 80 min of recording. As with cervical bursts in en bloc medullas, the rate of hypoglossal bursts was 1 : 1-coupled to preBötC activity, while their duration was shorter and decreased from an initial value of 0.39 ± 0.07 to 0.30 ± 0.14 after 1 h (Fig. 9). After spontaneous arrest, preBötC and hypoglossal rhythms were restored by 6.2 mm K+ or rolipram (1 μm) with rates comparable to those in 3 mm K+ 20–40 min after start of recording. While the duration of K+-induced preBötC and hypoglossal bursts was also similar to control, rolipram-induced bursts were shorter (Fig. 9).
Figure 9. preBötC and XII rhythms in preBötC slices in 1.2 mM Ca2+ and 3 mM K+.
A, bursting within the ventral respiratory column (VRC), neighbouring the preBötC and reflecting its activity, showed 1 : 1-coupling to XII nerve bursting in a m-preBötC[600/−0.10]W–P2 slice in standard solution. Following spontaneous arrest of rhythm after 72 min (last bursts shown), 1 : 1-coupled bursting was reactivated by raised K+ and, after return to 3 mm K+ solution, rolipram (1 μm). B, burst rates in 5 m-preBötC[600/−0.24] slices with mean rostral/caudal boundaries 0.24 ± 0.11/0.86 ± 0.05 mm caudal to VIIc show that rhythm lasted for > 80 min in only 2 slices. Rhythm was effectively reactivated in all slices by 6.2 mm K+, whereas rolipram (1 μm) was only effective in 2 of
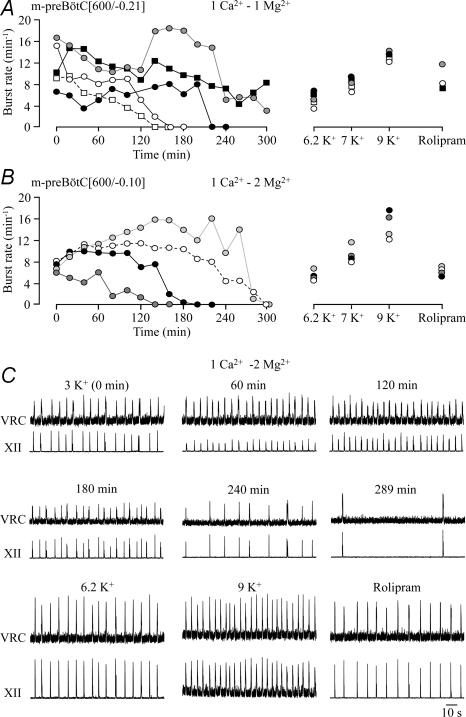
The limited longevity of slice rhythms in standard 1.2 mm Ca2+ and 3 mm K+ solution contrasted with ∼4 h longevity of rhythm in m-preBötC slices with similar boundaries in our previous study (Ruangkittisakul et al. 2006). Thus, we tested next whether this difference may be due to use of 1 mm instead of 1.2 mm Ca2+ in that report. Indeed, rhythm in five slices with the rostral boundary 0.21 ± 0.08 mm caudal to VIIc lasted > 2 h and even > 3 h in three of these cases in 1 mm Ca2+. Thus, longevity was significantly greater (211.4 ± 39.2 min, P < 0.05) in 1 mm Ca2+ compared to slices in 1.2 mm Ca2+ (Fig. 10). Also in 1 mm Ca2+, hypoglossal bursts showed 1 : 1-coupling to preBötC bursting, but their amplitude fluctuated by ∼40% with a period of 20–40 min in two slices. Rhythm was effectively restored after spontaneous arrest by both K+ (6–9 mm) and rolipram (1 μm) (Fig. 10).
Figure 10. preBötC and XII bursting in preBötC slices in 1 mM Ca2+ and 3 mM K+.
A, rhythm in 5 m-preBötC[600/−0.21] slices with mean rostral/caudal boundaries 0.21 ± 0.08/0.82 ± 0.04 mm caudal to VIIc kept in superfusate with 3 mm K+, 1 mm Ca2+ and 1 mm Mg2+ persisted for extended time periods, in 2 cases > 4 h. B, rhythm with similar burst rate and longevity was seen in 4 m-preBötC[600/−0.10] slices with mean rostral/caudal boundaries 0.10 ± 0.07/0.71 ± 0.06 mm caudal to VIIc in superfusate with 3 mm K+, 1 mm Ca2+ and 2 mm Mg2+. Rhythm was effectively reactivated after spontaneous arrest by 6.2, 7 and 9 mm K+ and rolipram (1 μm). C, the recording from a m-preBötC[600/−0.05]W–P2 slice included in B shows a 1 : 1-coupling of VRC/preBötC and XII rhythms during spontaneous and re-evoked activity. Note that the recording in rolipram was done 8 h after start of recording and that the amplitude of XII bursting fluctuated during the first 120 min after start of recording. 4 slices tested. C, mean values of burst rates in B show 1 : 1-coupling between XII and VRC/preBötC bursts under all conditions. D, mean burst durations reveal that VRC/preBötC bursts are notably longer than XII bursts.
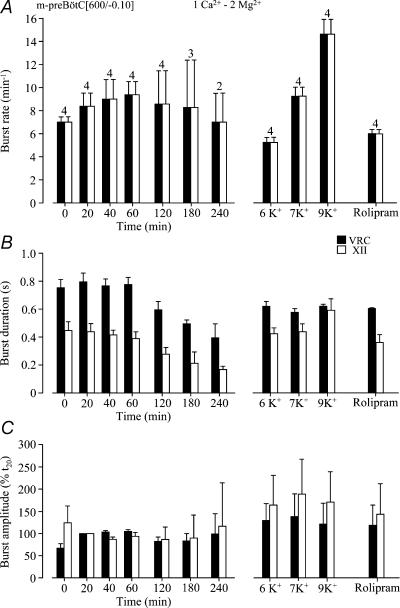
Next, we studied whether slice rhythms are modified by raising Mg2+ from 1 to 2 mm as was used by Ruangkittisakul et al. (2006). In four slices with mean rostral boundary 0.10 ± 0.07 mm caudal to VIIc, longevity of rhythm (216 ± 37.1 min, n= 4) was very similar in 1 mm Ca2+, 2 mm Mg2+ to that in 1 mm Ca2+, 1 mm Mg2+ slices and significantly (P < 0.05) greater than in 1.2 mm Ca2+, 1 mm Mg2+ slices. Also two of these slices showed fluctuations in hypoglossal burst amplitude (Fig. 10). In three slices, synchronous preBötC and hypoglossal rhythms were stable for > 3 h, in two cases for > 4 h (Figs 10 and 11). Burst rates during these time periods ranged from 7 to 9.4 bursts min−1, which was higher than in 1.2 mm Ca2+ (after 40–60 min). The duration of preBötC and hypoglossal bursts decreased from initially 0.75 ± 0.06 s to 0.49 ± 0.03 s and 0.45 ± 0.06 s to 0.21 ± 0.08 s after 3 h, respectively (Fig. 11). After spontaneous arrest, rhythm of similar burst duration was restored by K+ (6.2, 7, 9 mm) or rolipram (1 μm). K+ at 6.2 mm and 7 mm as well as rolipram restored bursting at rates similar to control, whereas 9 mm K+ induced a faster rhythm at ∼15 bursts min−1 (Fig. 11).
Figure 11. Statistical analysis of preBötC and XII rhythms in preBötC slices in 1 mM Ca2+ and 3 mM K+.
A, the 4 m-preBötC[600/−0.10] slices of Fig. 10B showed a 1 : 1-coupling of VRC/preBötC and XII nerve bursting. Note that burst rate slightly increased during the first hour of recording and that 9 mm K+ restored rhythm of ∼50% higher burst rate than the initial rhythm in 3 mm K+. B, the durations of both VRC/preBötC and XII bursts decreased following the first hour of recording. Both, K+ and rolipram (1 μm) re-stimulated burst durations. C, the amplitude of VRC/preBötC and XII bursts, referred to the value at 20 min (t20), was stable in control solution, whereas elevated K+ and rolipram had an augmenting effect. Numbers of slices shown above bars in A are identical to those in B and C.
Ca2+ sensitivity of inspiratory rhythms in transected brainstem–spinal cords
As one major finding, inspiratory-related rhythms in en bloc medullas with distinct rostral boundaries shared various features with those in preBötC slices. These features included a similar limited longevity of rhythms in brainstem–spinal cords with exposed preBötC and m-preBötC slices. In the slices, longevity and burst rate were greatly enhanced by lowering superfusate Ca2+ from 1.2 mm to 1 mm. This suggests that, conversely, raising Ca2+ above 1.2 mm may depress preBötC-related (motor) rhythms. In the Introduction, we hypothesized that raised Ca2+ may be responsible for block of cervical rhythms in transected brainstem–spinal cords. The studies reporting such block (Onimaru & Homma, 1987; Onimaru et al. 2006) used ‘Suzue-type’ solution containing 2.4 mm Ca2+ and also higher K+ (6.2 mm) and Mg2+ (1.3 mm) compared to the standard solution used here.
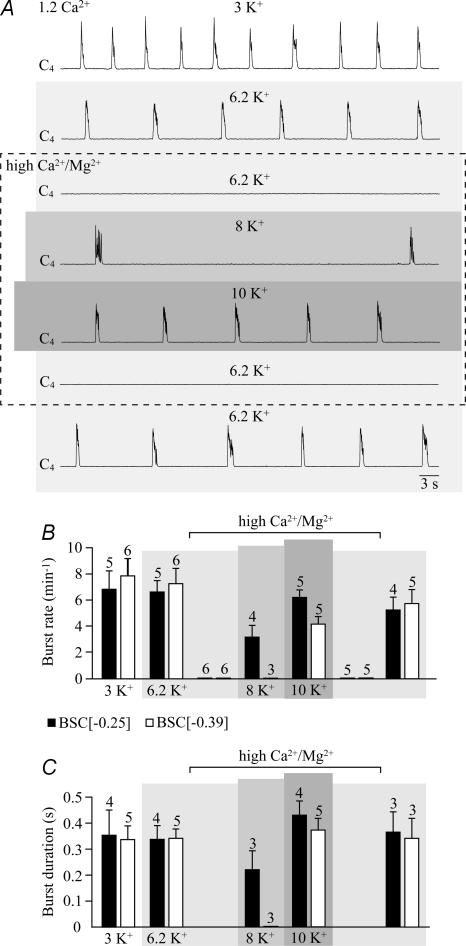
We tested the effects of this solution on five brainstem–spinal cord preparations that were transected between preBötC and VII. Raising first K+ from 3 to 6.2 mm changed neither the rate nor the duration of cervical bursts, whereas subsequent elevation of Ca2+ (from 1.2 to 2.4 mm) and Mg2+ (from 1 to 1.3 mm) blocked rhythm within < 10 min (Fig. 12). Rhythm with a rate and burst duration similar to control was reactivated in that solution by 10 mm K+, whereas 8 mm K+ was less effective (Fig. 12). Six preparations cut more caudally (at the rostral preBötC border) responded similarly. However, 10 mm K+ reactivated rhythm with a burst duration similar to controls, but at a lower rate, and 8 mm K+ did not restore bursting (Fig. 12). This high Ca2+/K+ (Mg2+) solution also modified non-respiratory activity in three preparations with boundaries ≥ 0.45 mm caudal to VIIc that did not generate respiratory rhythm (supplemental Fig. S4).
Figure 12. Block by high Ca2+/K+ (Mg2+) solution of cervical rhythm in brainstem–spinal cords without VII.
A, in a BSC[−0.28]SD–P3, elevating superfusate Ca2+ from 1.2 to 2.4 mm and Mg2+ from 1.0 to 1.3 mm (3rd trace from top) abolished the inspiratory rhythm which was previously stable in both 3 mm (top trace) and 6.2 mm K+ (2nd trace from top). Rhythm very similar to control was reactivated in 2.4 mm Ca2+ and 1.3 mm Mg2+ by raising K+ further, to 10 mm (5th trace from top), whereas 8 mm K+ was less effective (4th trace from top). Rhythm was blocked again after return to 6.2 mm K+, 2.4 mm Ca2+ and 1.3 mm Mg2+ solution (2nd trace from bottom) and recovered effectively in 6.2 mm K+, 1.2 mm Ca2+ and 1 mm Mg2+ (bottom trace). B and C, mean rates (B) and durations (C) of cervical bursts in two sets of preparations with different mean rostral boundaries, specifically BSC[−0.25] and BSC[−0.39]. Digits above bars indicate the number of preparations tested. Only a subset of preparations in B was used for the analysis in C. Bars represent means and s.e.m.
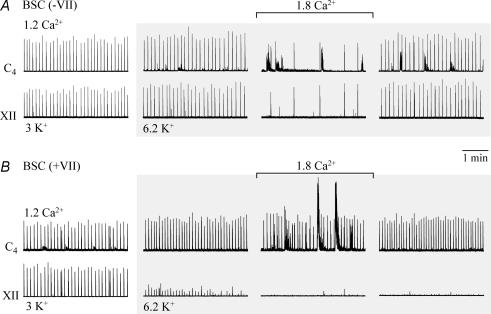
The fact that Ca2+ was doubled, whereas Mg2+ was raised by only 30%, suggests that Ca2+ is responsible for depression of cervical rhythm. To test this, eight preparations with exposed preBötC were kept in 6.2 mm K+ to maintain stable rhythm and were superfused with 1.8 mm Ca2+ at constant Mg2+ (1 mm). This modest Ca2+ rise by 0.6 mm decreased burst rate within < 10 min from 5.5 ± 0.9 to 1.1 ± 0.4 bursts min−1 (P < 0.01; block of rhythm in 3 cases) (Fig. 13). The inhibition by Ca2+ of cervical rhythm was accompanied in > 50% of cases by non-respiratory bursting that was absent, or less pronounced, in hypoglossal recordings (Fig. 13). Thus, Ca2+ effects were quantified by analysing only synchronous cervical and hypoglossal bursts, which were 1 : 1-coupled during both control and raised Ca2+ (Fig. 13).
Figure 13. Different sensitivity to raised superfusate Ca2+ of inspiratory rhythms in brainstem–spinal cords without or with VII.
A, in a BSC[−0.25]SD–P3 without VII, raising Ca2+ from 1.2 to 1.8 mm after changing from 3 to 6.2 mm K+ reversibly depressed both cervical and XII inspiratory rhythms. Note the occurrence of pronounced non-respiratory activity in the cervical, but not the XII recording during 1.8 mm Ca2+. B, 1.8 mm Ca2+ did not depress cervical rhythm in a BSC[+0.75]W–P1 preparation with complete VII, but induced large amplitude non-respiratory activity. Note that XII rhythm was stable in A, whereas it disappeared in B during the time period of raising K+. Same time scale in A and B.
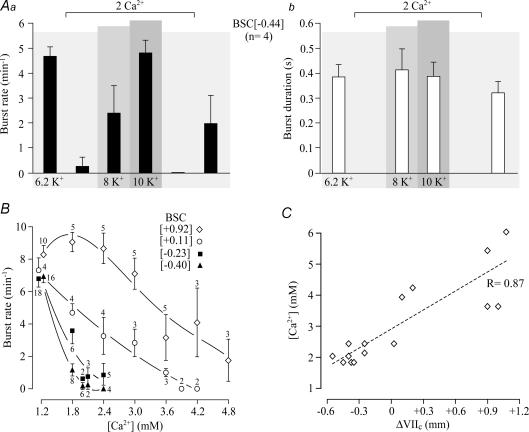
In four of the preparations exposed to 1.8 mm Ca2+, a subsequent Ca2+ rise to 2 mm depressed rhythm further to 0.3 ± 0.3 bursts min−1 (P < 0.01; block of rhythm in 3 cases) (Fig. 14). Rhythm reappeared at 4.8 ± 0.5 bursts min−1 with burst duration similar to control in 10 mm K+ after block by 2 mm Ca2+, whereas 8 mm K+ was less effective (2.4 ± 1.1 bursts min−1).
Figure 14. Dependence of Ca2+-induced depression of cervical rhythm on rostral boundary in brainstem–spinal cords.
A, effects of raising Ca2+ from 1.2 to 2 mm on mean rate (Aa) and duration (Ab) of cervical bursts in 4 BSC[−0.44] without VII. B, effects of increased Ca2+ on cervical burst rates in 4 groups of brainstem–spinal cords with different mean rostral boundaries referred to VIIc indicated in brackets, specifically +0.92 ± 0.15 (n= 10), +0.11 ± 0.07 (n= 4), −0.23 ± 0.07 (n= 18) and −0.40 ± 0.05 (n= 16). Note that 1.8–2 mm Ca2+ had a major depressing effect on rhythm in preparations without VII (filled symbols), whereas > 3 mm Ca2+ was necessary for a notable depression of burst rate in preparations with (portions of) VII (open symbols). Digits next to symbols indicate the number of preparations tested. C, significant (P < 0.01) relation between superfusate Ca2+ necessary to abolish preBötC rhythm and the rostral boundary of brainstem–spinal cords referred to their distance from VIIc (ΔVIIc). Note that considerably higher Ca2+ levels were necessary to block rhythm in preparations with complete VII. Values represent means and s.e.m.
In contrast to preparations transected between VII and preBötC, cervical rhythm in 6.2 mm K+ was not blocked by 1.8–2.4 mm Ca2+ in preparations with (portions of) VII (Figs 13 and 14). The [Ca2+] to effectively depress or abolish rhythm increased significantly with increasing amount of tissue rostral to the preBötC (Fig. 14).
Ca2+ block of preBötC slice rhythm
The latter results in brainstem–spinal cord preparations suggested that the capability of the preBötC to generate rhythm in moderately elevated K+ is substantially impaired by raising Ca2+ by only 0.6 mm. In a final approach, we investigated whether preBötC bursting and inspiratory motor rhythm in slices with robust longevity in 1 mm Ca2+ and 3 mm K+ have a similarly high sensitivity to acutely elevated superfusate Ca2+.
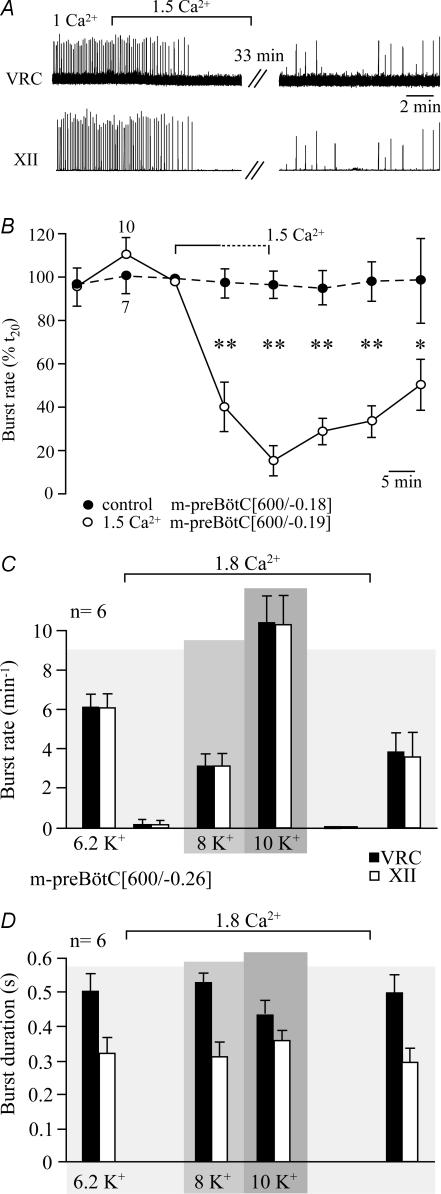
In 10 slices with a mean rostral boundary 0.19 ± 0.10 caudal to VIIc, raising Ca2+ from 1 to 1.5 mm depressed within 10–15 min the rate of 1 : 1-coupled preBötC and hypoglossal bursting from 8.1 ± 0.8 to 1.2 ± 0.4 bursts min−1 (P < 0.01; block of rhythm in 4 cases) (Fig. 15). Both rhythms started to recover within 10–20 min after return to 1 mm Ca2+, but mean recovery was < 50% of control even after 30 min of washout of 1.5 mm Ca2+. The incomplete recovery of burst rates was not caused by time-dependent spontaneous slowing of rhythm in 3 mm K+ as inspiratory frequency was stable during comparable time periods in seven control slices with a mean rostral boundary 0.18 ± 0.11 mm caudal to VIIc (Fig. 15).
Figure 15. Block by Ca2+ of preBötC and XII rhythm in preBötC slices.
A, synchronous inspiratory bursting of XII nerve and VRC/preBötC in a m-preBötC[600/−0.30]W-P1 slice was abolished by raising superfusate Ca2+ from 1 to 1.5 mm (in 3 mm K+ and 2 mm Mg2+). Note that rhythm did not fully recover within > 40 min after return to 1 mm Ca2+. B, plot of burst rate of 10 m-preBötC[600/−0.19] slices with mean boundaries 0.19 ± 0.10/0.82 ± 0.05 mm caudal to VIIc (^) in response to 1.5 mm Ca2+ (in 3 mm K+ and 2 mm Mg2+). The dotted line in bracket indicating the application of elevated [Ca2+] symbolizes that administration periods varied between 10 and 20 min depending on when the inhibitory effect reached steady-state. Filled circles indicate burst rates in 7 control m-preBötC[600/−0.18] slices with mean boundaries 0.18 ± 0.11/0.78 ± 0.10 mm caudal to VIIc in 1 mm Ca2+, 3 mm K+ and 2 mm Mg2+ solution. Asterisks show significant (*P < 0.05; **P < 0.01) difference between controls and the group treated with 1.5 mm Ca2+. C and D, mean rate (C) and duration (D) of VRC/preBötC (▪) and XII bursts (□) in 6 m-preBötC[600/−0.26] slices with mean boundaries 0.26 ± 0.12/0.86 ± 0.03 mm caudal to VIIc during variation of superfusate Ca2+ and K+. In control, slices were kept in 6.2 mm K+, 1 mm Ca2+ and 2 mm Mg2+. Subsequently, rhythm was blocked upon elevation of Ca2+ to 1.8 mm and reactivated by raising K+, first to 8 mm, then to 10 mm. Rhythm was blocked again upon return to 1.8 mm Ca2+ in 6.2 mm K+ and recovered to values close to control following lowering of Ca2+ to 1 mm.
For comparison with our findings in brainstem–spinal cords, the effects of 1.8 mm Ca2+ in 6.2 mm K+ were tested in six m-preBötC slices with a mean rostral boundary 0.26 ± 0.12 mm caudal to VIIc. Synchronous preBötC and hypoglossal burst rates were depressed by 1.8 mm Ca2+ from 6.1 ± 0.8 to 0.2 ± 0.2 bursts min−1 (P < 0.01; block of rhythm in 5 cases). Elevating K+ to 8 mm and 10 mm reactivated both types of rhythms at 3.2 ± 0.7 and 10.1 ± 1.9 bursts min−1, respectively, with burst durations similar to control (Fig. 15).
Discussion
Inspiratory-related rhythms in newborn rat brainstem–spinal cords containing the preBötC inspiratory centre (plus more rostral respiratory regions) and preBötC slices were similar in physiological Ca2+/K+ solution. The reduced longevity of inspiratory rhythms in en bloc preparations with exposed preBötC and slices is caused by a strong extracellular Ca2+/K+ antagonism. We discuss whether the absence of pre/postinspiratory rhythms in brainstems lacking a major portion of VII may be due to reduction of the RTN/pFRG, whereas block of hypoglossal bursting in preparations with (portions of) VII may possibly result from interstitial accumulation of inhibitory neuromodulator(s).
Implications from defined sectioning of isolated brainstem–spinal cords
In early postnatal rats, the constancy of rostrocaudal extensions of marker brainstem nuclei enables the generation of preBötC slices with defined boundaries (Ruangkittisakul et al. 2006). Based on a similar constancy of ventral brainstem surface landmarks, we generated here brainstem–spinal cords with a defined rostral boundary. Such preparations with rostrally exposed preBötC allow the study of how the preBötC drives hypoglossal and spinal motor networks with as little as possible interference from the more rostral BötC and RTN/pFRG. Also preBötC interactions with the RTN/pFRG and/or BötC should be studied in defined preparations where the extension of the rostral ventral respiratory column can be determined within a few tens of micrometres. The finding of stable cervical rhythm in en bloc preparations cut between VII and preBötC is very similar to our previous report (Smith et al. 1991). As discussed below, use of 2–2.4 mm Ca2+ (instead of 1.5 mm Ca2+ in the latter study) explains depression/block of rhythm observed by others upon similar transection (Onimaru & Homma, 1987; McLean & Remmers, 1994; Onimaru et al. 2006).
Despite caveats (Wilson et al. 2006), sectioning experiments can increase understanding of structure–function relationships of respiratory networks. For example, the RTN/pFRG in the rostral aspect of transected newborn rat medullas remained functional without the preBötC (Onimaru et al. 2006). Furthermore, transection close to VIIc abolished expiration in juvenile rats in vivo (Janczewski & Feldman, 2006). Those authors hypothesized that breathing is generated by anatomically separate rhythm generators, one generating active expiration located close to VII in the region of the RTN/pFRG, the other generating inspiration located more caudally in the preBötC. The latter study supported the conclusion from a previous combined in vivo/in vitro report that the pFRG ultimately drives expiratory abdominal muscles in vivo via ipsilateral medullary premotoneurons located caudal to the preBötC that project to contralateral pre/postinspiratory lumbar motoneurons (Janczewski et al. 2002). The findings of Janczewski & Feldman (2006) also support the hypothesis, based on a differential sensitivity in vivo and in vitro of preBötC- and pFRG-related rhythms to opioids, that these rhythmogenic respiratory groups form a functionally coupled dual respiratory centre (Mellen et al. 2003). We found that the brainstem level critical for pre/postinspiratory lumbar bursting is close to the rostral and not caudal end of VII, at least in isolated neonatal rat brainstems. It cannot be concluded though that pFRG networks, which presumably generate this expiratory motor behaviour, are only located rostral to that sectioning level. For example, if the axons of caudal RTN/pFRG interneurons or premotoneurons project rostrally first, cutting would affect this pathway.
Modulation of preBötC rhythms by neighbouring brainstem regions
Burst rates of cervical rhythm in physiological Ca2+/K+ did not substantially differ between en bloc medullas with VII and those cut close to the rostral preBötC boundary. This suggests that rhythmogenic preBötC networks are not subjected to a major specific frequency modulation by rostrally neighbouring portions of the BötC or RTN/pFRG, in line with our findings in en bloc medullas (Smith et al. 1991) and, more recently, in preBötC slices (Ruangkittisakul et al. 2006). In extension of these studies, we show that the longevity of cervical and hypoglossal rhythms is limited to < 1.5 h, when the transection is at, or removes some portion of, the rostral preBötC. As one explanation, long-term preBötC rhythm in en bloc medullas may depend on drive from more rostral respiratory networks (Feldman & Janczewski, 2006; Onimaru & Homma, 2006). However, rhythm in 1.2 mm Ca2+ and 3 mm K+ lasted for considerably shorter time periods in 600 μm m-preBötC slices (∼1.5 h) than in en bloc medullas (∼4 h) with similar rostral boundaries between preBötC and VIIc. This would suggest that caudal structures also drive the preBötC. But, since longevity of rhythms is greater in 600 μm compared to 500 μm m-preBötC slices (Ruangkittisakul et al. 2006), it is more likely that the amount of neighbouring tissue, rather than a specific rostral or caudal (respiratory) structure, determines the strength of drive to the preBötC. In that regard, one of several possible explanations for spontaneous arrest of rhythm in 3 mm K+ is a washout of excitatory neuromodulator(s), which would occur more rapidly in preparations with exposed preBötC (Ruangkittisakul et al. 2006).
Sectioning at the preBötC border removes distal dendrites of preBötC neurons that extend 0.3–0.4 mm rostrocaudally (S. W. Schwarzacher & K. Ballanyi, unpublished observations). Sectioning thus reduces the connectivity and recurrent excitation within and between the bilaterally organized preBötC regions, which is presumably pivotal for rhythm generation at physiological K+ (Ramirez et al. 2002; Del Negro et al. 2005). This would explain why rhythm stops after shorter time periods in highly reduced en bloc preparations. Inspiratory rhythms were reactivated by raised K+ or the blocker of cAMP-specific phosphodiesterase-4 rolipram. The stimulatory action of rolipram supports previous assumptions of the pivotal role of cAMP for maintaining respiratory rhythm (Ballanyi et al. 1997, 1999; Richter et al. 1997; Ruangkittisakul et al. 2006). In addition to the spontaneous arrest of preBötC rhythm in physiological Ca2+/K+ solution, we showed a time-dependent decrease in the duration of preBötC and cervical/hypoglossal nerve bursts. Such ‘rundown’ of burst duration (which was moderate for hypoglossal rhythm in en bloc preparations) was reversed by raised K+ (and in most cases by rolipram) as a further indication of a proposed washout of endo-genous excitatory neurostimulator(s). This phenomenon has to be taken into account for studies analysing neuromodulator effects on the strength of inspiratory activities in slice and en bloc models. Elevating superfusate K+ for a stable burst duration may not be adequate as this would eventually decrease the sensitivity of preBötC networks to neuroactive agents such as opioids (Onimaru et al. 2006; Ruangkittisakul et al. 2006).
Ca2+/K+ antagonism of preBötC rhythms
One major finding of this study explains the limited longevity in physiological Ca2+/K+ of rhythms generated by the preBötC after rostral exposure (brainstem–spinal cords) or isolation (slices). In highly reduced en bloc medullas, raising Ca2+ by only 0.6 mm from 1.2 mm greatly depressed cervical and hypoglossal rhythms. This Ca2+ sensitivity of preBötC-driven motor systems is likely to be even higher as these preparations were kept at 6.2 mm instead of 3 mm K+ for stabilization of rhythm, while further elevation of K+ to 8–10 mm reversed the Ca2+ block. In line with that view, both the longevity and long-term burst rate of preBötC slice rhythms in 3 mm K+ were substantially augmented in 1 mm instead 1.2 mm Ca2+, whereas raising Ca2+ from 1 to 1.5 mm in 3 mm K+ depressed burst rate to < 20% of control. This supports our hypothesis that raised superfusate Ca2+ is responsible for depressed cervical rhythms in transected brainstem–spinal cords (Onimaru & Homma, 1987; McLean & Remmers, 1994; Onimaru et al. 2006). First, our findings suggest that preBötC slices are not active in 2.4 mm Ca2+‘Suzue-type’ superfusate used by numerous groups (see Methods for references). Also, we propose that robust slice rhythm in 3 mm K+ in our recent study (Ruangkittisakul et al. 2006) is, at least partly, related to use of 1 mm superfusate Ca2+. As a third implication, longevity of rhythms in en bloc preparations with exposed preBötC will likely be substantially greater in 1 mm instead of 1.2 mm Ca2+.
The finding that the Ca2+ block of rhythm is also revealed in preBötC recordings in the slices excludes the possibility that raised Ca2+ primarily affects (pre)motor circuits. The Ca2+ block of primary preBötC rhythm can, nevertheless, be indirect as suggested by the finding that block of slice rhythm by bath-application of persistent Na+ channel blockers is mimicked by local injection of the agents into the raphe, but not the preBötC (Pace et al. 2007). Performing a similar test was beyond the scope of this study, as was to elaborate the mechanism of Ca2+ block. As one possibility, Ca2+ may inhibit preBötC cells or tonic neurons driving the preBötC via a more pronounced stimulatory effect on (tonic) inhibitory than on excitatory synapses (Jefferys, 1995). In fact, GABAA and glycine receptors depress inspiratory bursting in newborn rat brainstem–spinal cords (Onimaru et al. 1990; Brockhaus & Ballanyi, 1998, 2000). Ca2+-induced block of rhythm could also be caused by Ca2+ screening of negative membrane surface charges (Hille, 2001; Somjen, 2002). However, if this was the main mechanism, raising Mg2+ from 1 to 2 mm should have a similar blocking effect on slice rhythms, while returning to normal Ca2+ should have reversed the depression. Alternatively, raised Ca2+ may have long-term effects on Ca2+ homeostasis in respiratory (drive) neurons with low Ca2+ buffering capacity (Alheid et al. 2002).
In line with our findings, early in vivo studies showed that Ca2+ injection into the ventriculo-cisternal space depresses breathing (Berndt et al. 1969; Leusen, 1972; Berkenbosch & Adan, 1974). In that regard, a Ca2+/K+ antagonism was proposed to compensate for respiratory depression by Ca2+ when K+ was simultaneously injected (Leusen, 1972). Moreover, our results show that isolated preBötC rhythms at physiological K+ are stimulated by decreasing superfusate Ca2+. We chose 1.2 mm for our standard solution as a major number of in vivo studies reported values of 1.1–1.3 mm for interstitial Ca2+ in diverse brain tissue, including the ventral respiratory column (Heinemann et al. 1977; Nicholson et al. 1978; Richter & Acker, 1989; Trippenbach et al. 1990; Nilsson et al. 1993; Puka-Sundvall et al. 1994; Somjen, 2002). Respiratory rhythm was also revealed in 0.8 mm Ca2+ and 6.2 mm K+ superfusate in ∼1 mm thick mouse brainstem slices (Rekling et al. 1996), while hypothalamic slices generate in vivo-like spontaneous activity in 0.75 mm, but not > 1 mm Ca2+ (Pittman et al. 1981). Accordingly, ‘real’in vivo extracellular Ca2+ levels may be close to, or slightly less than, 1 mm. However, there may be a critical minimal Ca2+ level as ≤ 0.5 mm superfusate Ca2+ is a common epilepsy model (Konnerth et al. 1986; Jefferys, 1995). We propose that 1 mm Ca2+ is best suited to study isolated preBötC functions in 3 mm K+.
Relation of hypoglossal bursting with cervical and preBötC rhythms
Simultaneous recordings revealed 1 : 1-coupling of hypoglossal bursting with either cervical nerve activity (in en bloc medullas) or preBötC population activity (in slices). This argues against concerns that hypoglossal recordings may not necessarily be indicative of respiratory activity in vitro, in particular at hypothermia (St-John et al. 2004). However, the amplitude of hypoglossal bursts fluctuated periodically in 3 mm K+, 1 mm Ca2+ and 1–2 mm Mg2+ in some slices. Elevating K+ to 6.2–9 mm stabilized, and sometimes increased, the amplitude of hypoglossal (and preBötC) bursts and restored their durations to, or even beyond, the values during the initial phase of recordings. In contrast to a stable hypoglossal rhythm in the slices and (in rostral roots of) en bloc preparations transected close to or into the preBötC, rhythm was greatly depressed in all hypoglossal roots within ∼1 h in preparations containing (portions of) VII (for reasons, see below). This finding is important for studies devoted to the analysis of the function of hypoglossal neurons in en bloc medullas. For example, it was proposed that serotonin depresses hypoglossal, but not phrenic, motor output in brainstem–spinal cords (Monteau et al. 1990). In such studies, it is pivotal to test first for stable control hypoglossal nerve amplitudes.
Influence of restricted diffusion on respiratory activities in en bloc medullas
Notably higher superfusate Ca2+ levels were necessary to block rhythm in less-reduced en bloc preparations, e.g. ≥ 3.6 mm in brainstems with complete VII. This is in line with the observation that 4 mm Ca2+ depressed cervical bursting in such preparations (Kuwana et al. 1998). As one explanation for this phenomenon, (respiratory) structures in the rostral medulla may be less sensitive to Ca2+, thus being capable of driving the preBötC in high Ca2+. Alternatively, the decreased Ca2+ sensitivity of inspiratory rhythms may result from the larger dimension of en bloc preparations, constituting a diffusion barrier for build-up, e.g. of a modest interstitial K+ gradient (and a pH gradient) by an anoxic core that does not, however, appear to contain pivotal O2- or pH-sensitive inspiratory structures (Ballanyi et al. 1992, 1999; Brockhaus et al. 1993; Ballanyi, 2004). It may, though, be argued that the pre/postinspiratory activity pattern of newborn rat pFRG neurons in vitro results from hypoxia/anoxia in en bloc preparations with more rostral tissue as evoked hypoxia activated preinspiratory bursting in postinspiratory ventral respiratory column neurons of adult rats (Schwarzacher et al. 1991). It may further be speculated that transection 0.6 mm or less rostral to VIIc reduces the anoxic core, inactivates these neurons and, consequently, silences lumbar pre/postinspiratory bursting. However, several findings argue against this hypothetical scenario. First, preinspiratory activity was also induced in postinspiratory neurons of the latter in vivo study by prolonged lung inflation or deflation during normoxia. Second, pre/postinspiratory abdominal muscle activity is seen in normoxic juvenile rats in vivo (Janczewski & Feldman, 2006). Furthermore, experimentally induced anoxia blocks the preinspiratory and greatly augments the postinspiratory component of pFRG-related activities (Ballanyi, 2004), opposite to the above in vivo findings by Schwarzacher et al. (1991). Finally, rostral pFRG neurons are located within < 300 μm from the ventral brainstem surface (Onimaru & Homma, 2003), whereas the anoxic core is restricted to tissue depths > 700 μm (Brockhaus et al. 1993).
In preparations with a major amount of rostral tissue, extracellular [K+] is several millimolar higher in the vicinity of preBötC neurons than in the superfusate, due to the modest K+ gradient (Brockhaus et al. 1993; Okada et al. 2005). These elevated interstitial K+ levels may antagonize the depressing action of elevated superfusate Ca2+ as suggested by the finding that preBötC rhythm persisted in ‘Suzue-type’ solution after transection of brainstem–spinal cords between the preBötC and VII, but was abolished upon subsequent removal of the transected rostral aspect of the preparation (Onimaru et al. 2006). Consequently, lower K+ (e.g. 2 mm), may be used instead of 3 mm in en bloc preparations with more rostral tissue to compensate for changes in the interstitial Ca2+/K+ ratio. Hampered diffusion of raised Ca2+ into the tissue could also explain the attenuated blocking effect. However, this is not likely as millimolar superfusate Ca2+ concentrations equilibrate within minutes in this en bloc preparation, which does not show an interstitial Ca2+ gradient in control (Völker et al. 1995; Ballanyi et al. 1996; Kuwana et al. 1998). Also the longevity of hypoglossal rhythms depended on the rostrocaudal extension of en bloc medullas. We hypothesize that arrest of hypoglossal bursting in preparations with VII is caused by accumulation of inhibitory neuromodulator(s) and/or metabolite(s) depressing hypoglossal (pre)motoneurons, but not preBötC interneurons and cervical premotoneurons.
Conclusions
The isolated inspiratory centre generates robust rhythm in physiological cation solution. preBötC rhythms are blocked by minor extracellular Ca2+ rises, but can be restored by additional notable elevation of extracellular K+. The dependence of inspiratory-related rhythms on this strong Ca2+/K+ antagonism decreases in en bloc preparations with increasing amount of tissue rostral to the preBötC. Based on these findings, we recommend the use of superfusate Ca2+ levels at the lower end of the physiological spectrum, i.e. 1 mm, for the study of functions of the isolated preBötC in physiological K+. In addition, one may consider the use of reduced superfusate K+ (e.g. 2 mm) for studies of respiratory rhythms in en bloc preparations with extended rostral tissue. It should be considered further that hypoglossal rhythm is blocked in such less-reduced brainstem–spinal cords, whereas pre/postinspiratory lumbar bursting is absent in more reduced en bloc medullas. Our findings suggest that discrepant results using en bloc preparations with varying rostral boundaries may be partly explained by different dimensions, affecting diffusion of endogenous neuromodulators, rather than changes in specific structures present in more rostral respiratory regions. We hypothesize that the preBötC is more sensitive in such solutions to CO2/H+, hypoxia/anoxia or neuromodulators such as (endogenous) opiates or substance P, all of which determine respiratory rhythm in vivo.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR), the Canadian Foundation for Innovation (CFI) and the Alberta Heritage Foundation for Medical Research (AHFMR). K.B. is an AHFMR Scientist. A.R. has been awarded a CIHR studentship (MFN training grant). We thank Dr G. F. Alheid for critical comments on the histological sections of the manuscript.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.142760/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.142760
References
- Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol. 2002;31:693–717. doi: 10.1023/a:1025799830302. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. doi: 10.1016/j.resp.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Arata A, Onimaru H, Homma I. Respiration-related neurons in the ventral medulla of newborn rats in vitro. Brain Res Bull. 1990;24:599–604. doi: 10.1016/0361-9230(90)90165-v. [DOI] [PubMed] [Google Scholar]
- Ballanyi K. Isolated tissues. In vitro preparations. In: Windhorst U, Johansson H, editors. Modern Techniques in Neuroscience Research. Heidelberg: Springer; 1999. pp. 307–326. [Google Scholar]
- Ballanyi K. Neuromodulation of the perinatal respiratory network. Curr Neuropharmacol. 2004;2:221–243. doi: 10.2174/1570159043476828. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Kuwana S, Völker A, Morawietz G, Richter DW. Developmental changes in the hypoxia tolerance of the in vitro respiratory network of rats. Neurosci Lett. 1992;148:141–144. doi: 10.1016/0304-3940(92)90824-q. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Lalley PM, Hoch B, Richter DW. cAMP-dependent reversal of opioid- and prostaglandin-mediated depression of the isolated respiratory network in newborn rats. J Physiol. 1997;504:127–134. doi: 10.1111/j.1469-7793.1997.127bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progr Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Völker A, Richter DW. Functional relevance of anaerobic metabolism in the isolated respiratory network of newborn rats. Pflugers Arch. 1996;432:741–748. doi: 10.1007/s004240050193. [DOI] [PubMed] [Google Scholar]
- Berkenbosch A, Adan AJ. Influence of CSF calcium concentration on the ventilatory response to CO2 and O2. Pflugers Arch. 1974;348:33–50. doi: 10.1007/BF00587737. [DOI] [PubMed] [Google Scholar]
- Berndt J, Fenner A, Berger K. Influence of calcium and magnesium on the respiratory response to changes in CSF pH. Respir Physiol. 1969;7:216–229. doi: 10.1016/0034-5687(69)90007-3. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K. Anticonvulsant adenosine A1 receptor-mediated adenosine action on neuronal networks in the brainstem-spinal cord of newborn rats. Neuroscience. 2000;96:359–371. doi: 10.1016/s0306-4522(99)00544-8. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. J Physiol. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–970. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araujo AC, Campos R. A systematic study of the brain base arteries and their blood supply sources in the chinchilla (Chinchilla lanigeria– Molina 1782) Braz J Morph Sci. 2005;22:221–232. [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errchidi S, Monteau R, Hilaire G. Noradrenergic modulation of the medullary respiratory rhythm generator in the newborn rat: an in vitro study. J Physiol. 1991;443:477–498. doi: 10.1113/jphysiol.1991.sp018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Mountcastle VB, editor. Handbook of Physiology, section 1, The Nervous System, vol. IV, Intrinsic Regulatory Systems in the Brain. Bethesda: American Physiological Society; 1986. pp. 463–524. Chap 9. [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Janczewski WA. Point : Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Counterpoint: the preBotC is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100:2096–2097. doi: 10.1152/japplphysiol.00119.2006. Discussion 2097–2098, 2103–2108. [DOI] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977;27:237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- Herlenius E, Aden U, Tang LQ, Lagercrantz H. Perinatal respiratory control and its modulation by adenosine and caffeine in the rat. Pediatr Res. 2002;51:4–12. doi: 10.1203/00006450-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 2001. [Google Scholar]
- Iizuka M. Rostrocaudal distribution of spinal respiratory motor activity in an in vitro neonatal rat preparation. Neurosci Res. 2004;50:263–269. doi: 10.1016/j.neures.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Heinemann U, Yaari Y. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. I. Development of seizurelike activity in low extracellular calcium. J Neurophysiol. 1986;56:409–423. doi: 10.1152/jn.1986.56.2.409. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Kuwana S, Okada Y, Natsui T. Effects of extracellular calcium and magnesium on central respiratory control in the brainstem-spinal cord of neonatal rat. Brain Res. 1998;786:194–204. doi: 10.1016/s0006-8993(97)01476-5. [DOI] [PubMed] [Google Scholar]
- Kuwana S, Tsunekawa N, Yanagawa Y, Okada Y, Kuribayashi J, Obata K. Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Botzinger complex. Eur J Neurosci. 2006;23:667–674. doi: 10.1111/j.1460-9568.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- Leusen I. Regulation of cerebrospinal fluid composition with reference to breathing. Physiol Rev. 1972;52:1–56. doi: 10.1152/physrev.1972.52.1.1. [DOI] [PubMed] [Google Scholar]
- McLean HA, Remmers JE. Respiratory motor output of the sectioned medulla of the neonatal rat. Respir Physiol. 1994;96:49–60. doi: 10.1016/0034-5687(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier A, Alheid GF, McCrimmon DR. Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J Physiol. 2003;548:859–874. doi: 10.1113/jphysiol.2002.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteau R, Errchidi S, Gauthier P, Hilaire G, Rega P. Pneumotaxic centre and apneustic breathing: interspecies differences between rat and cat. Neurosci Lett. 1989;99:311–316. doi: 10.1016/0304-3940(89)90465-5. [DOI] [PubMed] [Google Scholar]
- Monteau R, Morin D, Hennequin S, Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical motoneurons: an in vitro study on the newborn rat. Neurosci Lett. 1990;111:127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Nicholson C, ten Bruggencate G, Stockle H, Steinberg R. Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. J Neurophysiol. 1978;41:1026–1039. doi: 10.1152/jn.1978.41.4.1026. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Olsson Y, Sheardown MJ, Hansen AJ. Regional changes in interstitial K+ and Ca2+ levels following cortical compression contusion trauma in rats. J Cereb Blood Flow Metab. 1993;13:183–192. doi: 10.1038/jcbfm.1993.22. [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends Pharmacol Sci. 2004;25:158–163. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kuwana S, Kawai A, Muckenhoff K, Scheid P. Significance of extracellular potassium in central respiratory control studied in the isolated brainstem-spinal cord preparation of the neonatal rat. Respir Physiol Neurobiol. 2005;146:21–32. doi: 10.1016/j.resp.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflugers Arch. 1990;417:425–432. doi: 10.1007/BF00370663. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ballanyi K, Homma I. Contribution of Ca2+-dependent conductances to membrane potential fluctuations of medullary respiratory neurons of newborn rats in vitro. J Physiol. 2003;552:727–741. doi: 10.1113/jphysiol.2003.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987;403:380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Point : Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Point: The pFRG is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol. 2006;100:2094–2095. doi: 10.1152/japplphysiol.00119.2006. Discussion. 2097–2098, 2103–2108. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Oshima N, Kumagai H, Kawai A, Sakata K, Matsuura T, Saruta T. Three types of putative presympathetic neurons in the rostral ventrolateral medulla studied with rat brainstem-spinal cord preparation. Auton Neurosci. 2000;84:40–49. doi: 10.1016/S1566-0702(00)00179-X. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBotzinger complex neurons and respiratory rhythm generation. J Physiol. 2007;580:485–496. doi: 10.1113/jphysiol.2006.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Abdala AP, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci. 2006;9:311–313. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Hatton JD, Bloom FE. Spontaneous activity in perfused hypothalamic slices: dependence on calcium content of perfusate. Exp Brain Res. 1981;42:49–52. doi: 10.1007/BF00235728. [DOI] [PubMed] [Google Scholar]
- Puka-Sundvall M, Hagberg H, Andine P. Changes in extracellular calcium concentration in the immature rat cerebral cortex during anoxia are not influenced by MK-801. Brain Res Dev Brain Res. 1994;77:146–150. doi: 10.1016/0165-3806(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Zuperku EJ, Alheid GF, Lieske SP, Ptak K, McCrimmon DR. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol. 2002;131:43–56. doi: 10.1016/s1569-9048(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubie M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Richter DW, Acker H. Respiratory neuron behaviour during medullary hypoxia. In: Lahiri S, editor. Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. Oxford: Oxford University Press; 1989. pp. 267–274. [Google Scholar]
- Richter DW, Lalley PM, Pierrefiche O, Haji A, Bischoff AM, Wilken B, Hanefeld V. Intracellular signal pathways controlling respiratory neurons. Respir Physiol. 1997;110:113–123. doi: 10.1016/s0034-5687(97)00077-7. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Ballanyi K. Reversal by phosphodiesterase-4 blockers of in vitro apnea in the isolated brainstem-spinal cord preparation from newborn rats. Neurosci Lett. 2006;401:194–198. doi: 10.1016/j.neulet.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Ma Y, Poon B, Secchia L, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signalling pathways at physiological [K+] of confocally-imaged respiratory centre neurons in online-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzacher SW, Smith JC, Richter DW. Pre-Bötzinger complex in the cat. J Neurophysiol. 1995;73:1452–1461. doi: 10.1152/jn.1995.73.4.1452. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Wilhelm Z, Anders K, Richter DW. The medullary respiratory network in the rat. J Physiol. 1991;435:631–644. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu GS, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 2002;8:254–267. doi: 10.1177/1073858402008003011. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF, Leiter JC. Uncoupling of rhythmic hypoglossal from phrenic activity in the rat. Exp Physiol. 2004;89:727–737. doi: 10.1113/expphysiol.2004.028829. [DOI] [PubMed] [Google Scholar]
- St-John WM, Rudkin AH, Harris MR, Leiter JC, Paton JF. Maintenance of eupnea and gasping following alterations in potassium ion concentration of perfusates of in situ rat preparation. J Neurosci Methods. 2005;142:125–129. doi: 10.1016/j.jneumeth.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Goodchild AK, Chalmers JP, Pilowsky PM. The pre-Botzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippenbach T, Richter DW, Acker H. Hypoxia and ion activities within the brain stem of newborn rabbits. J Appl Physiol. 1990;68:2494–2503. doi: 10.1152/jappl.1990.68.6.2494. [DOI] [PubMed] [Google Scholar]
- Völker A, Ballanyi K, Richter DW. Anoxic disturbance of the isolated respiratory network of neonatal rats. Exp Brain Res. 1995;103:9–19. doi: 10.1007/BF00241960. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Vasilakos K, Remmers JE. Phylogeny of vertebrate respiratory rhythm generators: the oscillator homology hypothesis. Respir Physiol Neurobiol. 2006;154:47–60. doi: 10.1016/j.resp.2006.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.