Abstract
Extracellular ATP is a potent surfactant secretagogue but its origin in the alveolus, its mechanism(s) of release and its regulatory pathways remain unknown. Previously, we showed that hypotonic swelling of alveolar A549 cells induces Ca2+-dependent secretion of several adenosine and uridine nucleotides, implicating regulated exocytosis. In this study, we examined sources of Ca2+ for the elevation of intracellular Ca2+ concentration ([Ca2+]i) evoked by acute 50% hypotonic stress and the role of autocrine purinergic signalling in Ca2+-dependent ATP release. We found that ATP release does not directly involve Ca2+ influx from extracellular spaces, but depends entirely on Ca2+ mobilization from intracellular stores. The [Ca2+]i response consisted of slowly rising elevation, representing mobilization from thapsigargin (TG)-insensitive stores and a superimposed rapid spike due to Ca2+ release from TG-sensitive endoplasmic reticulum (ER) Ca2+ stores. The latter could be abolished by hydrolysis of extracellular triphospho- and diphosphonucleotides with apyrase; blocking P2Y2/P2Y6 receptors of A549 cells with suramin; blocking UDP receptors (P2Y6) with pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS); emptying TG-sensitive stores downstream with TG or caffeine in Ca2+-free extracellular solution; or blocking the Ca2+-release inositol 1,4,5-triphosphate receptor channel of the ER with 2-aminoethyldiphenylborinate. These data demonstrate that the rapid [Ca2+]i spike results from the autocrine stimulation of IP3/Ca2+-coupled P2Y, predominantly P2Y6, receptors, accounting for ∼70% of total Ca2+-dependent ATP release evoked by hypotonic shock. Our study reveals a novel paradigm in which stress-induced ATP release from alveolar cells is amplified by the synergistic autocrine/paracrine action of coreleased uridine and adenosine nucleotides. We suggest that a similar mechanism of purinergic signal propagation operates in other cell types.
Extracellular nucleotides, such as ATP and UTP, are important autocrine/paracrine mediators in most tissues. In the distal lung, ATP is a potent secretagogue that stimulates type II cell surfactant secretion (Rooney, 2001). In the airways, through interactions with purinergic receptors, ATP, UTP, UDP and adenosine control the volume of airway surface liquid by regulating transepithelial ion transport rates (Lazarowski et al. 2004), activating cilia beating (Geary et al. 1995) and mucin secretion (Lethem et al. 1993) and thereby mobilizing the mucociliary clearance process that removes noxious materials from the airways. Despite the physiological relevance of responses triggered by extracellular nucleotides in the lungs, little is known about their origin on the epithelial surface and the release pathways. Increasing evidence suggests that extracellular ATP functions as a stress-responsive molecule, and mechanically induced ATP release is a cell-regulated process that does not involve cell lysis. In particular, mechanical stresses, such as stretch, shear, medium change or osmotic stress, have been shown to evoke ATP release from many cell types. Except in freshwater drowning, lung epithelia are seldom exposed to hypotonic shock. It represents, however, an experimentally convenient and frequently used surrogate of mechanical stress, with which it shares many common characteristics, including induction of ATP release, transient cytoskeleton reorganization, elevation of intracellular Ca2+ concentration ([Ca2+]i) and stimulation of other signalling pathways (Koyama et al. 2001). We have shown recently that swelling-induced ATP release from lung alveolar A549 cells, bronchial epithelial 16HBE14o− cells and NIH 3T3 fibroblasts tightly correlates with [Ca2+]i elevation, indicating the involvement of Ca2+-dependent exocytosis (Boudreault & Grygorczyk, 2004a). We also demonstrated that besides ATP, significant amounts of ADP, UTP and UDP are coreleased from A549 cells by hypotonic shock (Tatur et al. 2007). Mechanical stresses and hypotonic cell swelling are known to induce elevations of [Ca2+]i, which may involve Ca2+ influx from extracellular spaces and/or mobilization from intracellular stores. Furthermore, once released, extracellular nucleotides could have paracrine/autocrine effects on metabotropic P2Y receptors expressed on the surface of airway epithelia. Because stimulation of P2Y receptors is coupled to elevation of [Ca2+]i, it may lead to nucleotide-induced enhancement of ATP release. Indeed, ATP-induced ATP release from astrocytes could play a role in Ca2+ wave propagation (Anderson et al. 2004).
In this study, we investigated hypotonic stress-induced ATP release from A549 cells and examined the role of Ca2+ influx and mobilization from intracellular stores. We also examined the contribution of the autocrine effects of released nucleotides on [Ca2+]i signalling and ATP release.
Methods
Cells
Human lung carcinoma A549 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 56 U ml−1 penicillin G and 56 μg ml−1 streptomycin sulphate. All culture medium constituents were from Gibco-BRL (Burlington, ON, Canada). ATP efflux was measured from cell monolayers grown to confluence (∼500 cells mm−2) on 24 mm × 60 mm glass coverslips. Cell volume was quantified from cells plated at low density on 22 mm × 22 mm glass coverslips, while Fura-2 calcium imaging experiments were performed with cells grown on circular 15 mm diameter glass coverslips.
Solutions and chemicals
Physiological isotonic solution (IS) contained (mm): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 glucose and 10 TES, pH 7.4, adjusted with NaOH. Hypotonic saline (HS) at 50% was prepared by reducing NaCl concentration to 70 mm while keeping divalent cation concentration constant. In some control experiments, solutions with the same reduced NaCl content were used, while isoosmolarity was maintained by adding 140 mm mannitol. Osmolarity of the solutions was checked with a freezing point osmometer (Micro Osmometer 3300, Advanced Instruments Inc., Norwood, MA, USA) and was 316 mosmol l−1 for IS, 161 mosmol l−1 for HS and 316 mosmol l−1 for HS with added mannitol. For studies in the absence of extracellular calcium ([Ca2+]o), CaCl2 was omitted and the solutions were supplemented with 0.1 mm ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) to chelate trace Ca2+. All reagents, including Pluronic F127 and probenecid, were obtained from Sigma-Aldrich Canada, Ltd (Oakville, ON, Canada). For calcium imaging, Fura-2-AM was procured from Invitrogen-Molecular Probes (Kingston, ON, Canada). Apyrase (2 U ml−1), bafilomycin (1 μm), glycyl-l-phenyl-β-naphthylamid (GPN, 100 μm), carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP, 10 μm), suramin (100 μm) as well as modulators of [Ca2+]i, 2-aminoethyl diphenylborinate (2-APB, 75 μm), caffeine (10 mm), ruthenium red (100 μm) and thapsigargin (TG, 1 μm), were purchased from Sigma-Aldrich Canada, Ltd. 6-N,N - Diethyl- β,γ - dibromomethylene - d - adenosine-5′-triphosphate (ARL 67156) was bought from Calbiochem (San Diego, CA, USA). In general, the cells were pretreated for 30 min in IS with the indicated concentration of the test compound; the experiments were then conducted in the absence of the inhibitor in the case of bafilomycin, GPN and TG. By contrast, 2-APB, caffeine, ruthenium red and suramin were also present during ATP efflux experiments, where they were included in the perfusate solutions. Thus, they were also present in perfusate aliquots used to evaluate ATP content by luciferase bioluminescence. Therefore, all these compounds were tested for their ability to directly interfere with luciferase bioluminescence (see below).
ATP efflux assay
ATP efflux during hypotonic challenge was measured with high temporal resolution using a custom-designed, low-volume (325 μl), flow-through chamber, as previously described (Boudreault & Grygorczyk, 2004a). Briefly, a 24 mm × 60 mm coverslip with confluent cell monolayer was mounted in the chamber and perfused with a warm (37°C, in-line SF-28 heater, Warner Instrument Co., Hamden, CT, USA) solution at 1.3 ml min−1. After an equilibration period in IS (5–15 min), 50% hypotonic shock was applied by HS perfusion (t= 0), and the perfusate was collected at 30 s intervals during the initial burst of ATP secretion (0–5 min) and every 1 min thereafter. ATP in the samples was quantified by a luciferase–luciferin-based assay, using ATP Assay Mix and ATP Assay Mix Dilution Buffer supplied by Sigma-Aldrich Canada, Ltd. ATP release rates were expressed in pmol min−1 (106 cells)−1. Total ATP release was calculated by summing all ATP values collected, starting from the application of hypotonic shock, until the release rate returned to baseline, typically after about 15 min.
All test compounds that were added to the extracellular solution during the ATP efflux experiments were also examined for their ability to directly interfere with luciferase bioluminescence. We found strong inhibition of bioluminescence by suramin (see inset in Fig. 8A), moderate by caffeine, and weak by 2-APB. The colour of ruthenium red-containing solution also interfered with bioluminescence detection and contributed to inaccuracies in ATP evaluation. To correct for these inhibitory effects, calibration of luciferase–luciferin luminescence versus ATP standards was always performed in the presence of these reagents.
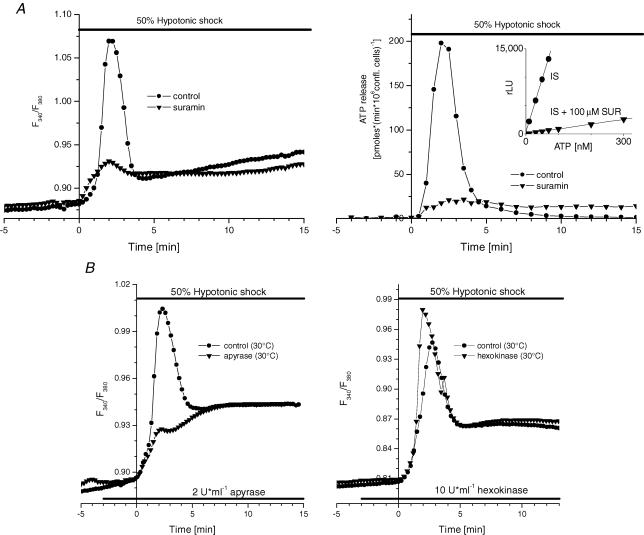
Figure 8. Role of autocrine/paracrine purinergic signalling.
A, effect of hypotonic shock on [Ca2+]i (left panel) and ATP release (right panel) after blocking purinergic receptors with suramin (▾); •, control response in the absence of suramin. Representative traces out of n= 3 independent experiments are shown. Inset in right panel, calibration curve of luciferase–luciferin luminescence (in relative light units, RLU) versus standard ATP concentrations: •, ATP standards in IS; ▪, ATP standards in IS containing 100 μm suramin. Similar effect of suramin on luciferase–luciferin luminescence was observed in HS. B, effect of apyrase (▾, left panel), or hexokinase (▾, right panel) on the hypotonic shock-induced [Ca2+]i response. Apyrase (or hexokinase) was added 3 min prior to and throughout hypotonic shock. On each panel, a representative trace out of n= 3 independent experiments is shown. Control (•), untreated cells. All experiments were performed in the presence of 1 mm extracellular Ca2+ and at 30°C, not as usually at 37°C, in accordance with the optimal temperature of the enzyme activity. Therefore, the extent of peak reduction by apyrase at 37°C might be slightly different.
Statistical significance was determined by Student's t test for paired data, and P < 0.05 was considered significant.
[Ca2+]i measurements
For [Ca2+]i measurements, cells were loaded (1 h, room temperature) with 10 μm Fura-2-AM in physiological solution containing 0.02% Pluronic F127 and 2.5 mm probenecid, followed by a 30 min de-esterification period in IS containing probenecid and the desired inhibitor (see below). For fluorescent imaging, a coverslip with Fura-2-loaded cells was mounted in an imaging/perfusion chamber (RC-20, volume 48 μl) attached to a heated platform (P-5, Warner Instrument Co.) on the stage of an inverted microscope (Nikon TE300). The imaging chamber was perfused continuously with a warm solution (37°C) via an in-line heater (SF-28, Warner Instrument Co.) at ∼0.5 ml min−1. The cells were illuminated for 100 ms with alternate light wavelengths of 340 and 380 nm, using a high-pressure mercury lamp (100 W) via interference filters (Chroma Technology, Brattleboro, VT) mounted on a filter wheel (Sutter Lambda 10-C, Sutter Instrument Co., Novato, CA) and a dichroic mirror (510/540 nm, Chroma Technology Corp., Brattleboro, VT, USA). Fluorescence images were recorded at 15 s intervals with the digital camera and stored for later analysis. Fura-2 measurements are presented as the fluorescence F340/F380 ratio. In some experiments, to chelate intracellular Ca2+, cells were loaded with BAPTA-AM (25 μm) for 30 min at room temperature in physiological solution.
All test compounds that were added to the extracellular solution during Ca2+-imaging experiments were examined for their potential interference with Fura-2 fluorescence. To do so, control cell-free experiments were performed with Fura-2 solution in the cuvette, and fluorescence was measured in the presence and absence of the test compound at 340 nm and 380 nm excitation wavelengths with a SPEX FluoroMax spectrofluorimeter (Edison, NJ, USA). Only ruthenium red affected fluorescence noticeably and was therefore not used in [Ca2+]i imaging experiments.
Cell volume evaluation
To evaluate the volume changes of substrate-attached cells, we deployed an upgraded version of the 3D imaging technique described in our previous work (Boudreault & Grygorczyk, 2004b; Groulx et al. 2006). Briefly, the method involves 3D reconstruction of cell shape based on cell images acquired in two perpendicular directions. Side-view and top-view cell images were acquired at 10–60 s intervals prior to hypotonic challenge, and at 5–30 s intervals during the challenge, to closely follow rapid cell volume changes. The 3D topography of the cell surface was reconstructed by a dual image surface reconstruction technique (Boudreault & Grygorczyk, 2004b). This technique generates a set of topographical curves of the cell surface from its digitized profile and base outline. Cell volume, surface and height were calculated from such a reconstructed cell topographical model. All calculations were carried out entirely with MatLab (MathWorks, Inc., Natick, MA, USA).
Results
We previously showed that hypotonic stress-induced ATP release from A549 cells was triggered by [Ca2+]i elevation, but the sources and mechanism of Ca2+ mobilization were not determined. In this study, we examined the contribution of different intracellular Ca2+ stores to hypotonic stress-evoked [Ca2+]i responses and their role in triggering ATP release from A549 cells. We also investigated the involvement of [Ca2+]i signals resulting from autocrine purinoreceptor stimulation by secreted nucleotides. Throughout this study, the kinetics of ATP release, [Ca2+]i responses and cell volume changes evoked by acute 50% hypotonic stress were measured in parallel experiments.
Role of extracellular Ca2+
We first examined the role of Ca2+ influx from the extracellular spaces in hypotonic shock-evoked ATP release and [Ca2+]i responses. Under control conditions, with 1 mm CaCl2 in the extracellular solution, 50% hypotonic shock rapidly increased the ATP release rate, reaching a peak at approximately 2 min, followed by decay that lasted approximately 15 min, before returning to baseline (Fig. 1). To abolish calcium influx from the extracellular spaces, experiments were carried out with nominally Ca2+-free extracellular solutions containing 0.1 mm EGTA to chelate any trace of Ca2+. When the cells were perfused with a Ca2+-free IS for up to 10 min, the kinetics and amount of released ATP, induced by subsequent application of a Ca2+-free HS, were indistinguishable from those observed in parallel control experiments performed in the presence of Ca2+ (Fig. 1). Longer, up to 40 min, preincubation in Ca2+-free IS slightly altered the time course of hypotonic shock-evoked ATP release, reducing the peak and total amount of secreted ATP, but the changes remained statistically insignificant. These experiments demonstrated that hypotonic shock-induced ATP release does not directly require Ca2+ influx from the extracellular spaces, but depends entirely on Ca2+ mobilization from intracellular stores.
Figure 1. Time course of ATP release induced by 50% hypotonic shock (t= 0) from confluent A549 cell monolayers.
•, ATP efflux observed with 1 mm Ca2+ present in the perfusate throughout the experiment (n= 4). ▪ and ▾, ATP efflux observed in the absence of extracellular Ca2+ when it was removed 10 min and 40 min before hypotonic shock, respectively (n= 3 and n= 4, respectively). Peak and total ATP releases were not significantly different between the three experimental groups.
Figure 2 shows the typical [Ca2+]i response of A549 cells to 50% hypotonic shock with Ca2+ present in extracellular solution. The response, represented by the Fura-2 fluorescence F340/F380 ratio, consisted of a slow, pre-spike elevation initiated at the onset of hypotonic shock (t= 0), lasting approximately 0.5 min (see Fig. 2B), and followed by a rapid spike at about 1.5 min. After the peak, [Ca2+]i decayed to a level that was significantly above baseline. This sustained [Ca2+]i elevation lasted as long as the cells remained in HS (at least 20 min) and returned to baseline only after re-perfusion with IS. Thus, kinetically, it appears that the [Ca2+]i response to hypotonic shock consists of two superimposed components, a slowly rising response that includes the prespike and postspike sustained elevation, and a rapid spike (see also Figs 5B and 6 below).
Figure 2. Time course of the hypotonic shock-induced [Ca2+]i response in A549 cells in Ca2+-containing HS.
A, the response consisted of a slow pre-spike elevation initiated at the onset of hypotonic shock (t= 0), a rapid spike at about 1.5 min, and a sustained after-spike [Ca2+]i elevation, which returned to baseline after the cells were re-perfused with physiological IS. B, the initial [Ca2+]i response is shown with higher temporal resolution, clearly demonstrating the existence that was acquired of a pre-spike.
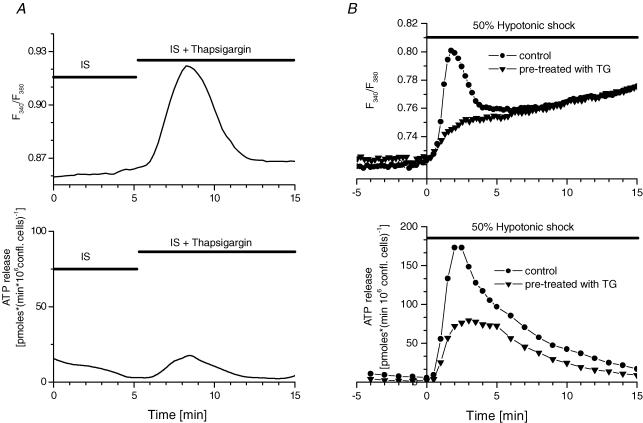
Figure 5. Modulation of the cell swelling-induced [Ca2+]i response and ATP release by TG.
A, time course of [Ca2+]i changes (top panel) and ATP release (bottom panel) in response to the acute addition of 1 μm TG in Ca2+-free IS. Each trace is representative of 3 independent experiments. B, top panel: Hypotonic shock-induced [Ca2+]i response in TG-treated cells, representative of 4 independent experiments. Bottom panel: Hypotonic shock-induced ATP release from TG-treated cells, representative of 6 independent experiments. •, control cells incubated for 30 min in Ca2+-free solution prior to the experiment; ▾, cells incubated for 30 min in Ca2+-free solution containing 1 μm TG.
Figure 6. Role of RyR and IP3R in the cell swelling-induced [Ca2+]i response and ATP release.
Hypotonic shock-induced [Ca2+]i response (A) and ATP release (B) in caffeine (▾) and 2-APB (▪) pretreated cells. Representative traces are shown out of n= 3 independent experiments; control (•) refers to the [Ca2+]i response in untreated cells. All experiments were performed in Ca2+-free solutions.
Figure 3A examines the impact of extracellular Ca2+ removal on basal [Ca2+]i under isotonic conditions and its response to 50% hypotonic shock. During short (3–5 min) incubation in Ca2+-free IS, basal [Ca2+]i decreased slowly, consistent with a steady Ca2+ leak from the extracellular spaces, but the subsequent response to Ca2+-free HS remained unaffected (data not shown). However, longer (> 10 min) incubation in Ca2+-free IS gradually reduced the peak [Ca2+]i response to HS, and after 30–40 min incubation, it was significantly but not completely diminished (Fig. 3A). Interestingly, the pre-spike and sustained post-spike [Ca2+]i elevation remained almost unaffected in these experiments. The results suggest that the rapid [Ca2+]i spike induced by hypotonic shock is due to Ca2+ mobilization from intracellular stores, which are gradually depleted during cell incubation in Ca2+-free solution. In contrast, the slow component of the [Ca2+]i response remained unchanged even after prolonged Ca2+-free incubation and, thus, likely represents Ca2+ mobilization from different intracellular source(s).
Figure 3. Effect of extracellular Ca2+ removal on the hypotonic shock-induced [Ca2+]i response.
A, three representative traces (out of n= 3–4 independent experiments) showing the Fura-2 fluorescence F340/F380 ratio response to hypotonic shock recorded with Ca2+ present throughout the experiment (•), or after removal of extracellular Ca2+ for 17 min (▪) or 40 min (▾) before Ca2+-free 50% HS was applied. Note the significant reduction of the peak [Ca2+]i response in cells incubated for 40 min in Ca2+-free extracellular solution. B, the [Ca2+]i and ATP responses to hypotonic shock are abolished in BAPTA-loaded (25 μm, 30 min) cells; representative of n= 3 independent experiments.
The fact that prolonged Ca2+-free incubation significantly reduced the rapid [Ca2+]i spike but had no effect on ATP release suggests that part of the ATP response is Ca2+ independent, or that the remaining [Ca2+]i signal is sufficient to trigger a full ATP response. Indeed, the latter seems to be the case, because BAPTA chelation of intracellular Ca2+ almost completely abolished both [Ca2+]i elevation and ATP release induced by 50% hypotonic shock (Fig. 3B). This experiment confirms the conclusion of our previous study that hypotonic shock-induced ATP release from A549 cells depends entirely on [Ca2+]i signalling (Boudreault & Grygorczyk, 2004a). Interestingly, we noticed that residual [Ca2+]i and ATP responses to hypotonic shock varied between individual experiments, likely reflecting the variable efficiency of BAPTA loading and/or the spatiotemporal dependence of intracellular Ca2+ buffering by BAPTA in A549 cells.
In our experiments, we applied hypotonic shock by perfusing cells with a HS made by reducing NaCl content. When the cells were presented with a solution of the same reduced NaCl content but with iso-osmolarity kept constant with added mannitol, no responses were observed (Fig. 4A). This result demonstrates that [Ca2+]i and ATP release responses evoked by HS are caused by reduced osmolarity and not by changes in ionic strength. During 50% hypotonic shock, cell swelling reached a peak at 2–3 min; this was followed by a regulatory volume decrease (RVD) (Fig. 4B). Removal of extracellular Ca2+ may change the kinetics of cell swelling or RVD, and could thus contribute to altered [Ca2+]i responses. To verify this, cell volume changes evoked by 50% hypotonic shock were investigated in Ca2+-containing and Ca2+-free solutions. As Fig. 4B shows, the kinetics, extent of hypotonic swelling and subsequent RVD in A549 cells were not different in the presence or absence of extracellular Ca2+.
Figure 4.
A, the [Ca2+]i and ATP responses, left and right panels, respectively, are evoked by reduced osmolarity, not ionic strength. The control traces (•) refer to responses triggered by HS that had 50% reduced NaCl content. When the cells were perfused with HS complemented with mannitol to maintain isoosmolarity (see Methods), no responses were observed (▪); representative of n= 3 independent experiments. B, 50% hypotonic shock-induced volume responses of single, substrate-attached A549 cells in Ca2+-containing (left panel) and Ca2+-free (right panel) solutions. Thin lines represent data from individual single-cell experiments, and thick lines represent a fit to the average data (±s.d.; n= 3–4). Cell swelling and RVD were similar for both experimental conditions.
Role of TG-sensitive Ca2+ stores
To examine, in more detail, the nature of the intracellular Ca2+ stores contributing to the complex [Ca2+]i response induced by hypotonic shock, we applied pharmacological tools acting on specific stores or release mechanisms. The endoplasmic reticulum (ER), the most eminent and active Ca2+ store in most cells (Brini & Carafoli, 2000), was the first target of our investigation. In Ca2+-containing IS, acute addition of TG (1 μm), a specific, irreversible inhibitor of the sarco-endoplasmic reticulum Ca2+ pump (SERCA) (Thastrup et al. 1990), produced significant Ca2+ mobilization. However, despite large [Ca2+]i responses, it induced only negligible ATP release (Fig. 5A). When the cells were pretreated with TG (1 μm) for 30 min in Ca2+-free IS to empty the intracellular Ca2+ stores, and then challenged by Ca2+-free HS, the rapid [Ca2+]i spike was completely abolished, while the slow component of the [Ca2+]i response remained unaffected (Fig. 5B, top panel). This confirms that the rapid spike and the slow component of the hypotonic shock-induced [Ca2+]i response represent Ca2+ mobilization from two different stores, one of which is TG sensitive and the other TG insensitive. The peak rate of hypotonic shock-induced ATP release from TG-treated cells was significantly reduced to 42 ± 8% of that observed with control, untreated cells (n= 6; Fig. 5B, lower panel). As a result, total ATP release was also reduced but to a lesser degree (62 ± 3% of control, Table 1). These data also indicate that both Ca2+ stores contributed to ATP release; the rapid [Ca2+]i spike involving TG-sensitive stores was closely correlated with the rapid peak of ATP release. However, with cells having their TG-sensitive stores depleted, hypotonic shock induced only a slowly rising [Ca2+]i response, representing Ca2+ mobilization from TG-insensitive stores, and a significantly slower rate of ATP release was observed (Fig. 5B, lower panel).
Table 1.
Effect of Ca2+ signalling and cytoskeleton modulators on 50% hypotonic shock-induced ATP release from A549 cells
| Peak ATP release | Total ATP release | ||||
|---|---|---|---|---|---|
| Agent | Action | [Ca2+]o | Absence of [Ca2+]o | [Ca2+]o | Absence of [Ca2+]o |
| 2-APB | IP3R inhibition | 70 ± 2 (3)* | 58 ± 6 (3)* | 108 ± 1 (3)* | 69 ± 10 (3)* |
| Caffeine | RyR stimulation | 65 ± 12 (6)* | 56 ± 4 (3)* | 60 ± 15 (6)* | 42 ± 8 (3)* |
| Ruthenium Red | RyR inhibition | 51 ± 4 (3)* | 73 ± 5 (3)* | 72 ± 5 (3)* | 75 ± 12 (3) |
| Thapsigargin (TG) | ER-Ca2+-pump inhibition | 44 ± 14 (7)*** | 42 ± 8 (6)*** | 94 ± 31 (6) | 62 ± 3 (6)*** |
| 2-APB + caffeine + TG | — | 31 ± 9 (3)* | — | 30 ± 6 (3)* | |
| Bafilomycin | Ca2+/H+ exchanger inhibition | 104 ± 9 (3) | — | 98 ± 3 (3) | — |
| GPN | Lysosome disruption | 105 ± 28 (3) | — | 108 ± 13 (3) | — |
| BAPTA | [Ca2+]i chelating agent | 10 ± 2 (3)* | — | 11 ± 2 (3)* | — |
| Cytochalasin D | Inhibition of actin polymerization | 33 ± 6 (3)* | — | 56 ± 5 (3)* | — |
| Jasplakinolide + TG | Induction of actin polymerization | — | 97 ± 15 (3) | — | 135 ± 10 (3) |
| Latrunculin A + TG | Disruption of microfilament-mediated processes | — | 88 ± 9 (3) | — | 121 ± 9 (3) |
Data are means ±s.d. Values are a percentage of control, except for jasplakinolide and latrunculin where they are a percentage of TG. Values of n are given in parentheses.
P < 0.05
P < 0.001.
Using 3D cell imaging, we also verified that swelling and the RVD of TG-treated cells were similar to those seen with untreated cells (n= 4, data not shown). Therefore, the effects of TG treatment on ATP release could be entirely attributed to a specific action of TG on Ca2+ signalling.
Two major types of Ca2+-release receptor channels, ryanodine (RyR) and inositol 1,4,5-trisphosphate (IP3R), may be involved in Ca2+ mobilization from TG-sensitive stores such as the ER (Galione & Churchill, 2002). Cell pretreatment (30 min, 37°C) with caffeine (10 mm), or 2-APB (75 μm), modulators of RyR and IP3R, respectively, had a similar effect on [Ca2+]i responses to Ca2+-free hypotonic shock as TG, i.e. they completely abolished the rapid Ca2+ mobilization spike, while the slowly rising component remained unaffected (Fig. 6A). These two compounds also diminished the peak rate of ATP release to 56 ± 4% and 58 ± 6% of control values, respectively (Fig. 6B).
Table 1 summarizes the effects of several RyR and IP3R modulators on ATP secretion induced by 50% hypotonic shock in Ca2+-containing and Ca2+-free solutions. It confirms that both RyR and IP3R play a role in this process. From Table 1 it is also apparent that simultaneous application of TG, caffeine and 2-APB did not completely abolish ATP secretion. The residual ∼30% of total ATP release was therefore due to Ca2+ mobilization from TG-insensitive Ca2+ stores. When the ER Ca2+ store modulators (TG, 2-APB, caffeine, ruthenium red) were tested in the presence of extracellular Ca2+, the time course of cell swelling-induced ATP release displayed larger variability, with a slightly higher peak and a shallower decline, compared to experiments performed in the absence of extracellular Ca2+ (data not shown). This could be attributed to Ca2+ entry via store-operated plasma membrane Ca2+ channels (SOC), such as Trp1 and Trp6, which are expressed in A549 cells (Xue et al. 2000; Brough et al. 2001). However, the difference in total ATP released remained statistically insignificant (see Table 1). It should be remembered, however, that most of the pharmacological modulators in our study have limited specificity, e.g. 2-APB is likely to have many targets affecting not only IP3R-mediated Ca2+ release but also Ca2+ influx via SOC, phospholipase C (PLC) activity and IP3 production (Padar et al. 2005).
Role of extracellular ATP degradation and autocrine effects of secreted nucleotides
To minimize the hydrolysis of secreted ATP by ecto-nucleotidases, our ATP release experiments were carried out in a custom-made flow-through chamber (internal volume 325 μl), which was continuously perfused at the rate of 1.3 ml min−1, providing complete solution replacement in the experimental chamber every ∼15 s. This allowed us to maintain the bulk ATP concentration in the chamber below 100 nm, even at the peak of ATP secretion (Boudreault & Grygorczyk, 2004a). This approach, however, may not completely prevent ATP degradation or the autocrine effects of secreted nucleotides. If ecto-nucleotidases are located in close proximity to ATP release sites, a significant fraction of extracellular ATP could be hydrolysed before it is washed away from the cellular surface. Furthermore, the local cell surface concentration of ATP and other coreleased nucleotides may go much higher than in the bulk perfusate, and may thus be sufficient to activate P2Y receptors. To assess the extent of ATP degradation at the cell surface in our experiments, release was studied in the presence of 100 μm ARL 67156, an ATP analogue and selective inhibitor of ecto-ATPases, which shows 300-fold greater selectivity for ecto-ATPase versus P2Y receptors (Drakulich et al. 2004; Berra-Romani et al. 2004). As Fig. 7 demonstrates, ARL 67156 significantly enhanced ATP content in perfusate aliquots and, at the peak, the rate of ATP release observed in the presence of ARL reached 146 ± 5% of control values (n= 4, Table 2). Thus, in the absence of ARL 67156, despite continuous perfusion, a significant fraction of secreted ATP was hydrolysed by cell surface ecto-ATPases. However, we did not notice the contribution of secreted nucleotidases to ATP degradation in our experiments, because ATP concentration in perfusate aliquots remained stable at room temperature for at least 1–2 h after collection.
Figure 7. Blocking ecto-ATPases enhances peak ATP release.
Effect of the ecto-ATPase inhibitor ARL 67156 (100 μm) on hypotonic shock-induced ATP release. Control experiments (•) were performed in the presence of [Ca2+]o without the addition of inhibitor; ▪, the time course of ATP release in the presence of [Ca2+]o and ARL. Representative traces out of n= 4 are shown.
Table 2.
Modulation of hypotonic shock-induced ATP release by selected inhibitors of ecto-nucleotidases and P2Y receptors
| Peak ATP release [Ca2+]o | Total ATP release [Ca2+]o | ||
|---|---|---|---|
| ARL | Ecto-nucleotidase inhibition | 146 ± 5 (4)* | — |
| Suramin | P2X, P2Y receptor inhibition | 10 ± 1 (3)*** | 32 ± 21 (3)* |
| PPADS | P2Y6 receptor inhibition | 24 ± 2 (3)*** | 35 ± 10 (3)* |
Data are mean ±s.d.[Ca2+]o as a percentage of control. Values of n are given in parentheses.
P < 0.05
P < 0.001.
We have previously reported that upon hypoosmotic challenge, several other nucleotides besides ATP are secreted from A549 cells, in particular, significant amounts of UTP and UDP have been detected (Tatur et al. 2007). These nucleotides may have autocrine/paracrine effects by acting on P2Y and P2X receptors. However, the contribution of ionotropic Na+- and Ca2+-permeable P2X receptors to the autocrine effects is unlikely, because the responses were independent of Ca2+ influx from the extracellular spaces, as demonstrated in Fig. 1 above. Therefore we focused on P2Y2 and P2Y6 receptors, which were found on the A549 cell surface (Xue et al. 2000; Schafer et al. 2003; White & Burnstock, 2006). Activation of these IP3/Ca2+-coupled receptors could provide a positive feedback loop, where nucleotide-induced [Ca2+]i elevation could further enhance Ca2+-dependent nucleotide release, a mechanism similar to ATP-induced ATP release reported in astrocytes (Anderson et al. 2004). To investigate this possibility, [Ca2+]i responses and ATP release induced by hypotonic shock were examined in the presence of 100 μm suramin, a nonspecific P2Y receptor antagonist.
Figure 8A (left panel) shows that the peak [Ca2+]i response, representing release from TG-sensitive stores, was drastically reduced in the presence of suramin. The peak rate and cumulative ATP release were also significantly reduced to 10 ± 1% and 32 ± 21%, respectively, of control values (Fig. 8A, right panel, and Table 2). The reduction of ATP release could not be attributed to the strong inhibitory effect of suramin on luciferase bioluminescence reaction, because this was corrected by using suramin-containing ATP standards (see inset in Fig. 8A). Thus, the above experiments strongly suggest that the autocrine stimulation of P2Y receptors and the IP3/Ca2+ signalling pathway contribute significantly (∼70%) to ATP secretion evoked by hypotonic shock. This was further confirmed in experiments performed in the presence of apyrase (2 U ml−1; 30°C), an enzyme which hydrolyses the triphospho- and diphosphonucleotides (Fig. 8B, left panel). The rapid [Ca2+]i spike, which represents Ca2+ mobilization from TG-sensitive stores, was almost completely abolished, demonstrating that it was, in fact, evoked by the autocrine effects of rapidly secreted extracellular tri- and diphosphate nucleotides acting on P2Y receptors. These experiments also showed that secreted nucleotides are susceptible to hydrolysis by exogenously added apyrase. When similar experiments were performed with hexokinase (10–20 U ml−1; 30°C), which, in the presence of 10 mm glucose, effectively converts nucleotide triphosphates (ATP, UTP) into diphosphates (ADP, UDP) (Lazarowski et al. 1997), no effect on the peak [Ca2+]i response was observed (Fig. 8B, right panel). If hexokinase was indeed effective in removing nucleotide triphosphates in these experiments, the result may suggest that autocrine stimulation of the peak [Ca2+]i response could be attributed mainly to UDP, acting on the P2Y6 receptor, and/or ADP, acting on the P2Y1 receptor. We addressed this point further in the experiments presented below.
Because of the wide and non-specific effects of suramin (Zhang et al. 1998), to examine the involvement of P2Y1, P2Y2 and P2Y6 receptors more explicitly, we tested their known antagonist and agonists. Figure 9A shows that PPADS, a more specific P2Y6 receptor antagonist, abolished the hypotonic shock-induced peak [Ca2+]i response and strongly inhibited peak ATP release (by ∼76%), effects that were similar to that of suramin and consistent with the prominent role of P2Y6 receptors. In the absence of hypotonic shock, direct bulk addition of 10 μm UDP, a selective P2Y6 receptor agonist, or 100 μm UTP, a selective P2Y2 receptor agonist, induced a significant [Ca2+]i response but small ATP release, which at the peak amounted only to ∼1% to 6%, respectively, compared to that induced by 50% hypotonic shock (Fig. 9B and C). We also verified that contaminating ATP levels in 100 μm UTP and 10-μM UDP samples were negligible (< 0.1 nm), assuring that ATP detected in the perfusate indeed originated from UTP- or UDP-induced cellular ATP release. Exogenous ATP, which is equipotent with UTP at P2Y2 receptors, also induced a significant [Ca2+]i response (Fig. 9D, left panel). These results confirm that both P2Y2 and P2Y6 receptors are functionally expressed in A549 cells. Recently, the presence of UDP-glucose (UDP-glc)-specific P2Y14 receptors was reported in A549 cells (Muller et al. 2005). In our hands, however, 10 μm UDP-glc did not evoke any detectable [Ca2+]i responses in A549 cells (Fig. 9D, left panel), suggesting that P2Y14 receptor expression may vary between different laboratories, e.g. due to different number of cell passages or culture conditions. Interestingly, 10 μm ADP or 2-MeSADP, a P2Y1 receptor agonist, evoked a strong, although transient, short-lasting (< 1 min) [Ca2+]i spike (Fig. 9D, right panel). Such a transient response is consistent with the reported fast desensitization of P2Y1 receptors observed in other cell types (Palmer et al. 1998). To determine if the P2Y1 receptor significantly contributes to autocrine [Ca2+]i responses during hypotonic shock, hexokinase experiments, such as those in the Fig. 8B (right panel), were repeated in the presence of the P2Y1 receptor antagonist A3P5PS (1 μm). We found no effect of the antagonist on the hypotonic shock-induced [Ca2+]i response in these experiments (not shown), demonstrating that P2Y1 receptors do not contribute significantly to the autocrine effects noted in our study.
Figure 9.
A, effect of the P2Y6 receptor antagonist PPADS on hypotonic shock-induced ATP release and the [Ca2+]i response. PPADS (100 μm) was added to cells 30 min before the application of hypotonic shock and was present throughout the experiment performed in Ca2+-containing solutions. For each condition, a representative experiment out of n= 3 is shown. •, untreated cells; ▪, PPADS-treated cells. B, effect of 10 μm UDP, a P2Y6 agonist, on the [Ca2+]i response (representative of n= 3) and ATP release (average ±s.d. of n= 3), left and right panels, respectively. C, effect of 100 μm UTP, a P2Y2 agonist, on the [Ca2+]i response (representative of n= 3) and ATP release (average ±s.d. of n= 3), left and right panels, respectively. D, left panel, effect of 10 μm ATP (•), a P2Y2 agonist, and 10 μm UDP-glc (▪), a P2Y14 agonist, added at t= 0, on the [Ca2+]i response. Note that UDP-glc had no effect, although the cells did respond robustly to the subsequent addition of UTP (▪). Right panel, 10 μm ADP or 2-MeSADP, agonists of P2Y1 receptors, evoked short-lasting [Ca2+]i responses that were kinetically different from those observed for other nucleotides, shown in B and C. For these experiments, ADP was pretreated with 10 U ml−1 of hexokinase in the presence of 10 mm glucose for 1 h at room temperature to remove any contaminating ATP and UTP. For each condition, a representative experiment out of n= 3 is shown.
Collectively, the above results confirm that A549 cells express functional P2Y2 and P2Y6 receptors and, in addition, suggest the functional expression of P2Y1 receptors. Although all three receptors are functionally present, only P2Y6 seems to play a prominent role in autocrine [Ca2+]i signalling during hypotonic shock-evoked nucleotide release.
Role of TG-insensitive Ca2+ stores
The above experiments demonstrated an important role of purinoreceptor-mediated Ca2+ mobilization from TG-sensitive Ca2+ stores in hypotonic shock-induced ATP release. Here, we investigated the role of the TG-insensitive part of the [Ca2+]i response, which is seen as a slowly rising, sustained component during hypotonic shock (see e.g. Figs 5B and 6A). Acidic compartments, such as lysosomes, are being recognized as a part of the TG-insensitive Ca2+ stores (Galione & Churchill, 2002); therefore, we examined the effect of bafilomycin, an inhibitor of the lysosomal H+/Ca2+ exchanger, and GPN, which disrupts lysosomal organelles. Both of these agents did not affect the peak rate or total ATP released from A549 cells induced by hypotonic shock (Table 1), demonstrating that TG-insensitive Ca2+ mobilization in A549 cells involves stores other than acidic-lysosomal compartments. Next, we examined the modulators of mitochondrial function: FCCP, a proton ionophore which depolarizes the inner mitochondrial membrane and reduces the electrochemical driving force for Ca2+ uptake, and oligomycin, inhibitor of F1/F0-ATP synthase. Figure 10A shows that both modulators were without effect, suggesting that mitochondria also do not contribute to the TG-insensitive Ca2+ response evoked by hypotonic shock.
Figure 10. Role of mitochondria and actin in the TG-insensitive [Ca2+]i response to hypotonic shock.
A, effect of FCCP and oligomycin (OM), on the hypotonic shock-induced [Ca2+]i response. Cells were pretreated for 30 min with 10 μm FCCP + 1 μm TG, or 10 μm OM + 1 μm TG, in Ca2+-free IS for 30 min prior to the experiment. Experiments were performed in the absence of extracellular Ca2+. •, TG-treated cells; ▪, FCCP + TG-treated cells; ▾, OM + TG-treated cells. For each condition, a representative experiment out of n= 3 is shown. B, effect of jasplakinolide (JASP), an inducer of actin polymerization, and latrunculin A (LA), a microfilament-disrupting agent, on the hypotonic shock-induced [Ca2+]i response (top panel) and ATP release (bottom panel). The cells were pretreated for 2 h with 1 μm JASP, or 1 h with 1 μm LA, and with 1 μm TG for 30 min in Ca2+-free IS prior to the experiment. Experiments were performed in the absence of extracellular Ca2+. •, TG-treated cells; ▪, LA + TG-treated cells; ▾, JASP + TG-treated cells. For each condition, a representative experiment out of n= 3 is shown.
The actin cytoskeleton is known to regulate diverse cellular processes, including secretion and Ca2+ signalling. Some studies even suggest that the actin cytoskeleton itself may be part of the intracellular Ca2+ stores (Lange & Brandt, 1996; Lange, 1999; Janmey, 1998). We therefore explored the possibility that in our experiments the actin cytoskeleton may function as a TG-insensitive Ca2+ store. To perform such studies in isolation from TG-sensitive stores, the contribution of the latter was eliminated by pretreating cells with TG in Ca2+-free solution for 30 min to completely empty them. We found that disruption of actin filaments with latrunculin had no effect on hypotonic shock-induced, TG-insensitive Ca2+ responses or on corresponding ATP release (Fig. 10B). Disruption of actin filaments with cytocholasin D was also without effect (data not shown). Similarly, induction of actin polymerization with jasplakinolide had no effect on the Ca2+ response, while ATP release showed only slightly slower kinetics, with the peak rate and total ATP release not being affected significantly (Fig. 10B and Table 1). In contrast, in control cells not treated with TG, cytocholasin D had a significant (∼70%) inhibitory effect on peak ATP release (Table 1). Thus, the intact cytoskeleton has an important role in modulating ATP release from cells with functional TG-sensitive stores. However, it has no direct role in Ca2+ storage and mobilization from TG-insensitive stores and corresponding ATP release.
Discussion
The present study examined different sources of [Ca2+]i elevations induced by acute hypotonic stress in A549 cells and their role in stimulating Ca2+-dependent ATP release.
We found that ATP release depends entirely on Ca2+ mobilization from intracellular stores. Both TG-sensitive and TG-insensitive stores are involved, contributing approximately 70% and 30%, respectively, to total ATP released by hypotonic shock. Importantly, we also found that despite rapid perfusion of the experimental chamber, autocrine stimulation of P2Y receptors by coreleased uridine and adenosine nucleotides plays a major role in amplifying the initial hypotonic stress-induced [Ca2+]i response and further promoting ATP release.
Two components of the [Ca2+]i response to acute hypotonic stress
The [Ca2+]i response induced by acute 50% hypotonic shock in A549 cells consisted of two superimposed, kinetically different components. Both were due to Ca2+ mobilization from intracellular stores, while Ca2+ influx from extracellular spaces was not directly involved. The rapid [Ca2+]i spike originated from Ca2+ mobilization from TG-sensitive stores, such as the sarco-endoplasmic reticulum, and was abolished when the stores were depleted by blocking SERCA with TG, or by activating RyR Ca2+ release channels with caffeine, under Ca2+-free conditions to prevent refilling of the stores. Antagonists of IP3R (2-APB) and RyR (ruthenium red) also inhibited the [Ca2+]i spike and ATP release (Table 1), indicating that Ca2+ mobilization via both channels plays a role in triggering ATP secretion. RyR may contribute to the propagation of [Ca2+]i signalling via cyclic ADP-ribose and Ca2+-induced Ca2+ release mechanism (Galione & Churchill, 2002). Interestingly, full inhibition of the rapid [Ca2+]i peak by 2-APB, a noncompetitive IP3R Ca2+ release channel antagonist (Fig. 6), suggests that this component of the hypotonic shock-induced [Ca2+]i response results from activation of plasma membrane receptor(s) that couple with the IP3/Ca2+ signalling pathway. Indeed, autocrine activation of P2Y purinoreceptors was confirmed in a further study, and is discussed below.
The absence of the rapid spike after pretreatment with TG revealed the slowly rising [Ca2+]i component originating from a TG-insensitive intracellular source. Beside the ER, the mitochondria and the nucleus appear to be crucial for the generation of Ca2+ signals of high spatiotemporal complexity (Brini & Carafoli, 2000). However, both organelles are implicated in the buffering and uptake of cytosolic Ca2+ rather than initiating and extending Ca2+ signalling (Brini & Carafoli, 2000). Hence, they are unlikely to be responsible for the TG-insensitive part of the hypotonic shock-induced Ca2+ signal. Indeed, the modulators of mitochondrial function, FCCP and oligomycin, were without effect on the TG-insensitive Ca2+ response to hypotonic shock. Furthermore, experiments with bafilomycin and GPN, which interfere with the Ca2+-storage capability of acidic compartments, showed that they are not involved in triggering ATP release, suggesting the involvement of Ca2+ stores different from acidic lysosomal compartments. Interestingly, even prolonged ∼40 min absence of extracellular Ca2+ had no effect on the slow [Ca2+]i component, indicating that TG-insensitive stores may not be of an organellar nature and prompting us to examine the hypothesis that actin may act as a non-organellar Ca2+ store (Lange & Brandt, 1996; Lange, 1999). However, our experiments with agents that promote either actin polymerization or depolymerization were without significant effect on hypotonic shock-induced Ca2+ mobilization from TG-insensitive stores and corresponding ATP release. Thus, the nature of this slow [Ca2+]i response component remains incompletely understood.
Earlier reports with other cell types showed that [Ca2+]i elevations caused by hypotonic shock could result from Ca2+ influx across the plasma membrane and/or from Ca2+ release from internal stores (McCarty & O'Neil, 1992; Wu et al. 1997; Tinel et al. 2000; Wu et al. 2001; Sanchez et al. 2003). In our experiments, Ca2+ influx did not play a direct role in either the slow or rapid peak of the [Ca2+]i response to acute hypotonic shock, although the prolonged absence of extracellular Ca2+ led to partial depletion of TG-sensitive stores and reduction of the rapid [Ca2+]i peak. These differences may be due to the different cell types studied and experimental conditions. Our experiments were performed with substrate-attached cells at 37°C, while some other studies utilized cells in suspension or at room temperature. Because the rapid peak of [Ca2+]i responses results from the autocrine/paracrine purinergic loop in A549 cells, other factors may include the differential expression of P2Y receptors, differences in nucleotide release and the expression of different plasma membrane Ca2+-permeable channels.
Ca2+ and ATP release
Our previous study demonstrated a tight correlation between [Ca2+]i elevations and ATP release in A549, 16HBE14o− and NIH-3T3 fibroblasts during the initial ∼5 min of hypotonic stress (Boudreault & Grygorczyk, 2004a). The present work shows correspondingly that manoeuvres which eliminated the rapid [Ca2+]i spike also significantly reduced the rate of ATP release. Complete emptying of TG-sensitive stores by pharmacological manoeuvres reduced, by up ∼70%, the total ATP release evoked by subsequent 50% hypotonic shock (Table 1). The remaining ∼30% of ATP release could be attributed to the slow [Ca2+]i signal originating from TG-insensitive stores. Interestingly, TG on its own provoked quite significant [Ca2+]i elevation, but in contrast to hypotonic shock, induced only minor ATP release. We previously observed that the strong [Ca2+]i signal generated by application of the Ca2+-ionophore ionomycin produced relatively minor ATP release (Boudreault & Grygorczyk, 2004a). Both experiments imply that other factor(s) besides global [Ca2+]i elevation modulate ATP release from A549 cells. For example, the spatiotemporal dependence of [Ca2+]i signalling is now a widely recognized feature of this universal second messenger, which restricts the Ca2+ action to specific intracellular microdomains, allowing the fine control of cell function (Spat, 2006; Oheim et al. 2006). Thus, relatively minor ATP release might indicate that ATP release sites are distant from TG-induced Ca2+ mobilization sites. Moreover, besides Ca2+, other signalling pathways not investigated in this study have been implicated in the regulation of ATP release, including tyrosine kinase, Rho/Rho kinase and PI3-kinase (Koyama et al. 2001; Grygorczyk & Guyot, 2001).
Some studies found that only a fraction of hypotonic shock-induced ATP release could be inhibited by loading cells with the intracellular Ca2+ chelator BAPTA-AM, which suggests a Ca2+-independent release mechanism (Okada et al. 2006). Mobile Ca2+ buffers, such as BAPTA, provide an efficient mechanism to spatially restrict the [Ca2+]i increase (Oheim et al. 2006), but its efficiency in preventing Ca2+-triggered vesicular exocytosis will critically depend on the distance between Ca2+ release sites and secretory vesicles. Ca2+ transients might be highly localized, reaching as high as 5 μm to 30 μm at submembrane microdomains (reviewed in Oheim et al. 2006). Thus, despite a high Ca2+-binding rate, BAPTA may not completely prevent localized [Ca2+]i increases. This may be especially the case for highly differentiated primary epithelial cells, where secretory vesicles may colocalize with submembrane Ca2+ stores. Further studies are required to explore the effectiveness of BAPTA in preventing hypotonic shock-induced submembrane [Ca2+]i transients and ATP release from such cells.
Role of the autocrine/paracrine loop in ATP release
Our data demonstrate that the rapid [Ca2+]i peak induced by hypotonic shock results entirely from the autocrine/paracrine effects of released nucleotides acting on IP3/Ca2+ signalling pathway-coupled P2Y receptors. This conclusion is supported by the following experimental evidence. The rapid [Ca2+]i peak could be almost completely abolished by: (i) the hydrolysis of extracellular triphospho- and diphosphonucleotides with apyrase, but not hexokinase; (ii) blocking P2Y receptors with the non-specific P2Y receptor antagonist suramin; (iii) blocking the P2Y6 receptor with its more specific antagonist PPADS, but not the P2Y1 receptor antagonist A3P5PS; (iv) blocking the ER Ca2+-release IP3R channel with its antagonist 2-APB downstream of the P2Y receptor; or (v) completely emptying ER stores with TG or caffeine.
We have recently reported that several adenine and uridine nucleotides are transiently released from A549 cells in response to hypotonic shock (Tatur et al. 2007). Importantly, apart from ATP, significant amounts of UTP, UDP and ADP could be detected in cell perfusates (ATP > UTP > UDP > ADP). Therefore, released nucleotides, by interacting with their specific receptors, could contribute to autocrine/paracrine signalling in A549 cells. In this study, by investigating [Ca2+]i responses to the bulk addition of UTP, ATP, or UDP, we confirmed the functional expression of P2Y2 and P2Y6 receptors, respectively (Clunes & Kemp, 1996; Zhao et al. 2000), but not UDP-glc-specific P2Y14 receptors (Muller et al. 2005) in A549 cells. Interestingly, we also found evidence for the functional expression of the ADP receptor P2Y1. Based on their functional presence, we conclude that, in principle, the three P2Y receptor subtypes may contribute to the autocrine effects of released nucleotides. In particular, the involvement of P2Y6 receptor was further confirmed by using its more specific antagonist PPADS, which almost completely abolished the hypotonic shock-induced rapid [Ca2+]i peak and significantly diminished ATP release, while the P2Y1 receptor antagonist A3P5PS had no detectable effect on the rapid [Ca2+]i response. This suggests an important role for P2Y6 receptor in the autocrine/paracrine loop. The relative contribution of P2Y2 receptors could not be independently determined in complementary experiments because selective P2Y2 receptor antagonists are not available commercially. However, complementary support that P2Y6 but not P2Y2 receptors are the main players in the autocrine [Ca2+]i response was provided by experiments with apyrase and hexokinase. Apyrase, by hydrolysing nucleotide tri- and diphosphates, eliminates the agonists of both P2Y2 and P2Y6 receptors, completely abolishing the peak [Ca2+]i response. However, hexokinase, which eliminates only agonists of P2Y2 receptors by converting UTP and ATP into UDP and ADP (Lazarowski et al. 1997), had no noticeable effect.
Based on our data, we propose the following mechanism of hypotonic shock-induced ATP release. Upon hypotonic shock, initial Ca2+ mobilization likely occurs from TG-insensitive stores, seen as the slowly rising pre-spike in Fig. 2. This initial [Ca2+]i elevation triggers the vesicular exocytosis of adenosine and uridine nucleotides which, via autocrine/paracrine activation of Gq protein-coupled P2Y receptors, stimulates the PLC/IP3 signalling pathway, leading to subsequent activation of IP3R Ca2+ release channels of the ER. At this point, Ca2+-inducd Ca2+ release via RyR will also contribute to [Ca2+]i signal propagation. The enhanced [Ca2+]i signal, seen as the rapid [Ca2+]i spike in Fig. 2 will, in turn, further promote Ca2+-dependent ATP release. Such nucleotide-promoted nucleotide release likely leads to almost full depletion of exocytosis-available vesicular pools in A549 cells, since after ∼5 min, the rate of ATP release does not tightly correlate with the [Ca2+]i signal any longer. As a result, after the initial peak, the rate of ATP release decays towards background, despite significantly elevated [Ca2+]i (Boudreault & Grygorczyk, 2004a; Grygorczyk & Boudreault, 2005).
The proposed mechanism might be a general scheme of hypotonic or mechanical stress-induced ATP release from other cell types, although the specific players of the autocrine loop may vary, depending on the type of P2Y receptor expressed by a given cell and its ability to release different adenosine and uridine nucleotides. In the particular case of A549 cells, UDP acting on P2Y6 receptors seems to have a prominent role. As exemplified by the effect of the ecto-ATPase inhibitor ARL in our experiments, extracellular nucleotide metabolism, and possibly their interconversion, may also have an important role in regulating nucleotide release and autocrine signalling and requires future investigation. Our findings may have interesting implications for the regulation of surfactant secretion by alveolar type II cells. Although the stimulatory effect of stretch and involvement of P2Y2 receptors in surfactant secretion are well-established (Dietl & Haller, 2005; Rooney, 2001), the contribution of other P2Y receptors remains unclear. Our study suggests an important role of P2Y6 receptors in synergistic autocrine/paracrine stimulation of the IP3/Ca2+ signalling pathway after stress-induced nucleotide release from A549 cells, a model of type II pneumocytes. The resulting amplification and spatiotemporal extension of the [Ca2+]i signal may influence several steps of surfactant secretion, including lamellar body fusion, pore-opening and surfactant dispersion (Dietl & Haller, 2005).
In summary, our study demonstrates an important role of autocrine purinergic signalling in hypotonic stress-induced ATP release. The autocrine loop amplifies the initial stress-evoked Ca2+ response, accounting for the majority (∼70%) of released ATP. Our study also provides a novel paradigm in which stress-induced ATP release from alveolar A549 cells is amplified predominantly by the autocrine/paracrine action of coreleased UDP acting on P2Y6 receptors. We propose that such synergistic effects of coreleased nucleotides may be a general mechanism of purinergic signal propagation and amplification in other cell types.
Acknowledgments
This study was supported in part by the Canadian Institutes of Health Research and the Canadian Cystic Fibrosis Foundation (CCFF). S.T. was the recipient of a CCFF studentship. The authors acknowledge the technical work of Heléne Chabot, Cedric Mawle and Laura De Benedetti, and the editorial assistance of Ovid Da Silva, Research Support Office, Research Centre, CHUM. Thanks are due to Dr Eduardo Lazarowski for his comments on the manuscript.
References
- Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Berra-Romani R, Rinaldi C, Raqeeb A, Castelli L, Magistretti J, Taglietti V, Tanzi F. The duration and amplitude of the plateau phase of ATP- and ADP-evoked Ca2+ signals are modulated by ectonucleotidases in in situ endothelial cells of rat aorta. J Vasc Res. 2004;41:166–173. doi: 10.1159/000077146. [DOI] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol. 2004a;561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Evaluation of rapid volume changes of substrate-adherent cells by conventional microscopy 3D imaging. J Microsc. 2004b;215:302–312. doi: 10.1111/j.0022-2720.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- Brini M, Carafoli E. Calcium signalling: a historical account, recent developments and future perspectives. Cell Mol Life Sci. 2000;57:354–370. doi: 10.1007/PL00000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001;15:1727–1738. [PubMed] [Google Scholar]
- Clunes MT, Kemp PJ. P2u purinoceptor modulation of intracellular Ca2+ in a human lung adenocarcinoma cell line: down-regulation of Ca2+ influx by protein kinase C. Cell Calcium. 1996;20:339–346. doi: 10.1016/s0143-4160(96)90039-1. [DOI] [PubMed] [Google Scholar]
- Dietl P, Haller T. Exocytosis of lung surfactant: from the secretory vesicle to the air–liquid interface. Annu Rev Physiol. 2005;67:595–621. doi: 10.1146/annurev.physiol.67.040403.102553. [DOI] [PubMed] [Google Scholar]
- Drakulich DA, Spellmon C, Hexum TD. Effect of the ecto-ATPase inhibitor, ARL 67156, on the bovine chromaffin cell response to ATP. Eur J Pharmacol. 2004;485:137–140. doi: 10.1016/j.ejphar.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Galione A, Churchill GC. Interactions between calcium release pathways: multiple messengers and multiple stores. Cell Calcium. 2002;32:343–354. doi: 10.1016/s0143416002001902. [DOI] [PubMed] [Google Scholar]
- Geary CA, Davis CW, Paradiso AM, Boucher RC. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am J Physiol Lung Cell Mol Physiol. 1995;268:L1021–L1028. doi: 10.1152/ajplung.1995.268.6.L1021. [DOI] [PubMed] [Google Scholar]
- Groulx N, Boudreault F, Orlov SN, Grygorczyk R. Membrane reserves and hypotonic cell swelling. J Membr Biol. 2006;214:43–56. doi: 10.1007/s00232-006-0080-8. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Boudreault F. The emerging role of calcium-dependent exocytosis in ATP release from nonexcitable cells. Physiol News. 2005;60:21–22. [Google Scholar]
- Grygorczyk R, Guyot A. Osmotic swelling-induced ATP release: a new role for tyrosine and Rho-kinases? J Physiol. 2001;532:582. doi: 10.1111/j.1469-7793.2001.0582e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol. 2001;532:759–769. doi: 10.1111/j.1469-7793.2001.0759e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K. Microvillar Ca++ signaling: a new view of an old problem. J Cell Physiol. 1999;180:19–34. doi: 10.1002/(SICI)1097-4652(199907)180:1<19::AID-JCP3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lange K, Brandt U. Calcium storage and release properties of F-actin. Evidence for the involvement of F-actin in cellular calcium signaling. FEBS Lett. 1996;395:137–142. doi: 10.1016/0014-5793(96)01025-3. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc Natl Acad Sci U S A. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Tarran R, Grubb BR, Van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethem MI, Dowell ML, Van Scott M, Yankaskas JR, Egan T, Boucher RC, Davis CW. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am J Respir Cell Mol Biol. 1993;9:315–322. doi: 10.1165/ajrcmb/9.3.315. [DOI] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiol Rev. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Muller T, Bayer H, Myrtek D, Ferrari D, Sorichter S, Ziegenhagen MW, Zissel G, Virchow JC, Jr, Luttmann W, Norgauer J, Di Virgilio F, Idzko M. The P2Y14 receptor of airway epithelial cells: coupling to intracellular Ca2+ and IL-8 secretion. Am J Respir Cell Mol Biol. 2005;33:601–609. doi: 10.1165/rcmb.2005-0181OC. [DOI] [PubMed] [Google Scholar]
- Oheim M, Kirchhoff F, Stuhmer W. Calcium microdomains in regulated exocytosis. Cell Calcium. 2006;40:423–439. doi: 10.1016/j.ceca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padar S, Bose DD, Livesey JC, Thomas DW. 2-Aminoethoxydiphenyl borate perturbs hormone-sensitive calcium stores and blocks store-operated calcium influx pathways independent of cytoskeletal disruption in human A549 lung cancer cells. Biochem Pharmacol. 2005;69:1177–1186. doi: 10.1016/j.bcp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Rooney SA. Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:233–243. doi: 10.1016/s1095-6433(01)00320-8. [DOI] [PubMed] [Google Scholar]
- Sanchez JC, Danks TA, Wilkins RJ. Mechanisms involved in the increase in intracellular calcium following hypotonic shock in bovine articular chondrocytes. Gen Physiol Biophys. 2003;22:487–500. [PubMed] [Google Scholar]
- Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L376–L385. doi: 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]
- Spat A. Calcium microdomains and the fine control of cell function: an introduction. Cell Calcium. 2006;40:403–404. doi: 10.1016/j.ceca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Tatur S, Kreda SM, Lazarowski ER, Grygorczyk R. Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal. 2007 doi: 10.1007/s11302-007-9059-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel H, Kinne-Saffran E, Kinne RK. Calcium signalling during RVD of kidney cells. Cell Physiol Biochem. 2000;10:297–302. doi: 10.1159/000016375. [DOI] [PubMed] [Google Scholar]
- White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Wu MM, Grabe M, Adams S, Tsien RY, Moore HP, Machen TE. Mechanisms of pH regulation in the regulated secretory pathway. J Biol Chem. 2001;276:33027–33035. doi: 10.1074/jbc.M103917200. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang H, Iserovich P, Fischbarg J, Reinach PS. Regulatory volume decrease by SV40-transformed rabbit corneal epithelial cells requires ryanodine-sensitive Ca2+-induced Ca2+ release. J Membr Biol. 1997;158:127–136. doi: 10.1007/s002329900250. [DOI] [PubMed] [Google Scholar]
- Xue HH, Zhao DM, Suda T, Uchida C, Oda T, Chida K, Ichiyama A, Nakamura H. Store depletion by caffeine/ryanodine activates capacitative Ca2+ entry in nonexcitable A549 cells. J Biochem (Tokyo) 2000;128:329–336. doi: 10.1093/oxfordjournals.jbchem.a022757. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Keng YF, Zhao Y, Wu L, Zhang ZY. Suramin is an active site-directed, reversible, and tight-binding inhibitor of protein-tyrosine phosphatases. J Biol Chem. 1998;273:12281–12287. doi: 10.1074/jbc.273.20.12281. [DOI] [PubMed] [Google Scholar]
- Zhao DM, Xue HH, Chida K, Suda T, Oki Y, Kanai M, Uchida C, Ichiyama A, Nakamura H. Effect of erythromycin on ATP-induced intracellular calcium response in A549 cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L726–L736. doi: 10.1152/ajplung.2000.278.4.L726. [DOI] [PubMed] [Google Scholar]