Abstract
Stimulation of α2-noradrenergic (NA) receptors within the PFC improves working memory performance. This improvement is accompanied by a selective increase in the activity of PFC neurons during delay periods, although the cellular mechanisms responsible for this enhanced response are largely unknown. Here we used current and voltage clamp recordings to characterize the response of layer V–VI PFC pyramidal neurons to α2-NA receptor stimulation. α2-NA receptor activation produced a small hyperpolarization of the resting membrane potential, which was accompanied by an increase in input resistance and evoked firing. Voltage clamp analysis demonstrated that α2-NA receptor stimulation inhibited a caesium and ZD7288-sensitive hyperpolarization-activated (HCN) inward current. Suppression of HCN current by α2-NA stimulation was not dependent on adenylate cyclase but instead required activation of a PLC–PKC linked signalling pathway. Similar to direct blockade of HCN channels, α2-NA receptor stimulation produced a significant enhancement in temporal summation during trains of distally evoked EPSPs. These dual effects of α2-NA receptor stimulation – membrane hyperpolarization and enhanced temporal integration – together produce an increase in the overall gain of the response of PFC pyramidal neurons to excitatory synaptic input. The net effect is the suppression of isolated excitatory inputs while enhancing the response to a coherent burst of synaptic activity.
Pyramidal cells within the prefrontal cortex (PFC) are thought to be key processing elements in neuronal networks responsible for complex executive functions such as working memory. Through their recurrent synaptic connections, these networks are thought to hold information online in order to guide future behaviour (Fuster, 1997; Durstewitz et al. 2000). This action requires the suppression of other irrelevant stimuli that may interfere with the active maintenance of this memory trace. The ability to maintain such sustained attention is critically dependent on the proper functioning of noradrenergic (NA) afferents to the PFC (Aston-Jones & Cohen, 2005). Based on recordings from behaving primates, it has been postulated that NA acts to enhance the signal to noise ratio of PFC neuronal firing during working memory tasks by either increasing task-related activity (Li et al. 1999; Wang et al. 2007), or decreasing background activity (Sawaguchi et al. 1990). However, the mechanisms responsible for these effects are poorly understood.
Administration of the psychostimulant methylphenidate produces an enhancement in working memory performance in human subjects (Elliott et al. 1997; Mehta et al. 2000). We have recently shown that application of methylphenidate produces a substantial increase in the excitability of PFC pyramidal neurons recorded in vitro (Andrews & Lavin, 2006). This effect was produced by increased activation of α2-noradrenergic (α2-NA) receptors due to blockade of NA reuptake by methylphenidate. In the present paper, we sought to determine the ionic mechanisms responsible for the facilitating effects of α2-NA receptor stimulation on PFC pyramidal neurons. Utilizing a combination of voltage and current clamp studies in acute PFC slices, the experiments described here demonstrate that the effects of α2-NA receptor activation are mediated by the inhibition of hyperpolarization/cyclic nucleotide gated (HCN) channels through a PLC–PKC linked signalling cascade. Inhibition of these channels by α2-NA receptors produces a hyperpolarization of the resting membrane potential, but a significant enhancement in the temporal integration of distally evoked EPSPs. The net effect is the suppression of isolated excitatory inputs while enhancing the response to a coherent burst of synaptic activity. Thus, inhibition of HCN channels may be an important cellular mechanism mediating the enhanced signal to noise ratio produced by NA in the PFC.
Methods
Slice preparation and aCSF solutions
All experimental protocols were approved by the institutional animal care and use committee of the Medical University of South Carolina. Male Sprague–Dawley rats (P16–25) were deeply anaesthetized with chloral hydrate (400 mg kg−1i.p.) and rapidly decapitated. The brain was quickly removed and submerged in a 0°C sucrose solution containing (mm): sucrose, 200; KCl, 1.9; Na2HPO4, 1.2; NaHCO3, 33; MgCl2, 6; CaCl2, 0.5; dextrose, 10; ascorbic acid, 0.4. Coronal slices (300–350 μm) including the infralimbic and prelimbic cortices (Paxinos & Watson, 1998) were cut using an oscillating tissue slicer (Leica, VT1000) and transferred to a holding chamber for a minimum of 1 h at room temperature (22–24°C) prior to recording. The holding buffer contained (mm): NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; MgCl2, 4; CaCl2, 1; sucrose, 15; glucose, 10; ascorbic acid, 0.4; ∼310 mosmol l−1. Slices were transferred to a submersion-type recording chamber and perfused with aCSF containing (mm): NaCl 125; KCl 2.5; NaHCO3, 25; MgCl2, 1.3; CaCl2, 2; glucose 10; ascorbic acid, 0.4; ∼300 mosmol l−1 at a rate of 1–2 ml min−1. All aCSF solutions were constantly aerated with a mixture of 95% O2–5% CO2 to maintain pH ∼7.4.
Current clamp recordings
Deep layer pyramidal neurons (layers V–VI) were targeted for recording using an upright microscope equipped with Nomarski differential interference contrast optics, a 40× water immersion objective, and an infrared video imaging camera. Whole-cell current-clamp recordings of targeted cells were made using glass microelectrodes (3–7 MΩ) filled with internal solution containing (mm): potassium gluconate or KMeSO4, 125; KCl, 20; Hepes, 10; EGTA, 1; MgCl2, 2; ATP, 4.0; GTP, 0.3; pH 7.2–7.4; 298 mosmol l−1. After gigaohm seal formation and patch rupture, neurons were given at least 5 min to stabilize before data were collected. In experiments in which drugs were included in the internal solution, recordings began > 10 min after patch rupture to allow perfusion of the drug into the recorded cell. The signal was amplified using an AxoPatch 200B or MultiClamp 700B (Axon Instruments) and stored for off-line analysis using a custom-made, LabView based program. Recordings were performed at room temperature unless stated otherwise.
Voltage clamp recordings
For voltage clamp recordings, glass micropipettes were filled with an internal solution containing (mm): KMeSO4, 130; KCl, 5; MgATP, 2; Na2GTP, 0.5; Hepes, 5; CaCl2, 0.5; EGTA, 5; pH 7.3, 270 mosmol l−1. The pipette resistance, as measured in the bath, was typically 4 ± 0.5 MΩ. Following patch rupture, series resistance was compensated 50–70% and continually monitored throughout the experiment. Cells were discarded if series resistance increased by > 15%. Voltage clamp recordings were obtained using a Multiclamp 700B amplifier, a Digidata 1322 A/D converter and pCLAMP software (Axon Instruments). An inline heater was used to maintain the temperature of the aCSF in the bath at 33 ± 1°C.
Drugs
All reagents were obtained from Sigma (St Louis, MO) with the exception of KMeSO4 (ICN Biochemicals), ZD7288 (Tocris), SQ22536, MDL-12 330 A, 2′,5′-dideoxyadenosine, U73122, calphostin C, and chelrythrine chloride (Calbiochem). All adrenergics, channel blockers, and signal transduction reagents were prepared as concentrated stock solutions and either added immediately to the recording aCSF or internal solution at working concentrations or aliquotted and frozen at −20°C until use.
Catecolamine depletion
For the experiments with catecholamine depletions, animals were injected with reserpine (5 mg kg−1, i.p.) 24 h before slice procedure. Previous reports in the literature indicate that this procedure reduces basal levels of catecholamines by 60–96% (Kannari et al. 2000; Hatip-Al-Khatib et al. 2001; Yoshitake et al. 2004).
Statistics
Data are presented as means ± standard error unless stated otherwise. Statistical comparisons between groups were performed using either Student's paired t test or a one-way repeated measures ANOVA with Fisher's post hoc test. The significance level was P < 0.05 unless stated otherwise.
Results
α2-NA receptor stimulation increases the excitability of PFC pyramidal neurons
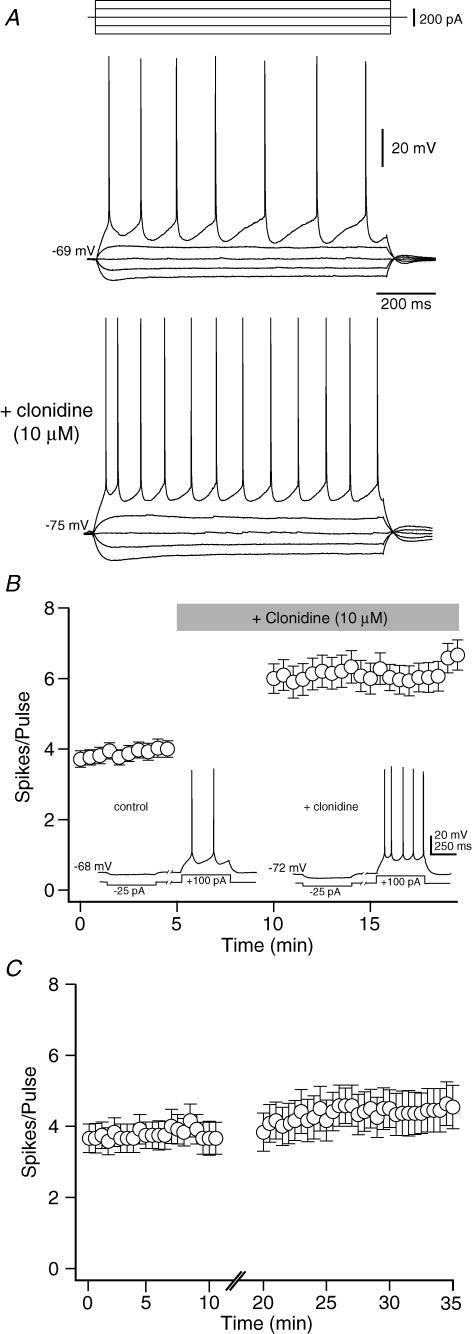
Bath application of the α2-NA agonist clonidine produced three consistent effects on deep layer mPFC pyramidal neurons recorded in current clamp: (1) a small (3–5 mV) hyperpolarization in resting membrane potential, (2) a significant increase in input resistance, and (3) an increase in the number of evoked spikes per depolarizing pulse (Fig. 1A). To quantify these changes in neuronal excitability, 1 s depolarizing current pulses were delivered every 30 s, with the current amplitude adjusted for each cell to elicit an average of three to four action potentials per pulse. Once a stable baseline was established (> 5 min) clonidine (10 μm) was bath applied for 5 min without stimulation. During this period, clonidine induced a small but significant hyperpolarization of the resting membrane potential (control: −68.3 ± 0.56 mV; clonidine: −71.8 ± 0.68 mV, P < 0.001, n= 18). However, when stimulation was resumed, a significant increase in the average number of spikes per depolarizing pulse was observed (baseline: 4.0 ± 0.7 spikes/pulse; clonidine: 8.8 ± 1.9 spikes/pulse, P < 0.05, n= 18; Fig. 1B). This increase in excitability was accompanied by an increase in input resistance, as measured by the peak amplitude of the hyperpolarization produced by a small (25–50 pA, 250 ms) negative current pulse (baseline: 245 ± 34 MΩ; clonidine: 286 ± 43 MΩ, P < 0.05; n= 7). The effects of clonidine were not due to the hyperpolarization of the resting membrane potential, as they persisted even when Vm was held constant during the experiment using DC injection (excitability – baseline: 3.7 ± 0.2 spikes/pulse; clonidine: 6.2 ± 0.4 spikes/pulse, P < 0.001, n= 39; input resistance – baseline: 243 ± 31 MΩ; clonidine: 321 ± 38 MΩ, P < 0.05, n= 15). These effects were also not due to repeated stimulation, intracellular perfusion, or any other time dependent process as no significant changes in Vm, Rin, or evoked firing were observed during repeated stimulation in the absence of clonidine (Fig. 1C). Similar results were also obtained if recordings were performed at 32 ± 1°C rather than room temperature (membrane potential – baseline: −68.9 ± 0.62 mV; clonidine: −73.2 ± 0.53 mV, P < 0.05, n= 6; input resistance – baseline: 138 ± 22; clonidine: 169 ± 31, P < 0.05, n= 6; excitability – baseline: 3.2 ± 0.5 spikes/pulse; clonidine: 7.1 ± 0.8 spikes/pulse, P < 0.05, n= 6).
Figure 1. The α2-NA receptor agonist clonidine increases excitability of deep layer PFC pyramidal neurons in vitro.
A, sweeps from a representative neuron showing the response to hyperpolarizing and depolarizing current injection before and after clonidine (10 μm). Clonidine produced a small hyperpolarization but an increase in the number of spikes evoked by depolarizing current pulses. The increase in cell excitability was accompanied by an increase in input resistance. B, timeline showing the change in excitability of 39 PFC pyramidal neurons following clonidine application (grey bar). Each point represents the mean ±s.e.m. For each cell, the amplitude of a 1 s depolarizing current pulse was adjusted to evoke 3–4 spikes per pulse and the membrane potential was held constant with DC injection (see text). Inset, sweeps from a representative neuron showing the response to bath applied clonidine when membrane potential was allowed to float. C, timeline showing no change in the average number of spikes per depolarizing current pulse during prolonged recording without drug application (n= 12).
The effects of clonidine are mediated by postsynaptic α2-NA receptors
Although clonidine is a potent α2-NA receptor agonist, it also exhibits affinity for both α1-NA receptors and imidazole receptors. To exclude the possibility that the observed increases in excitability elicited by clonidine application were mediated via non-α2-NA receptors, we assessed the effects of selective α2-NA receptor, α1-NA receptor and imidazole receptor antagonists.
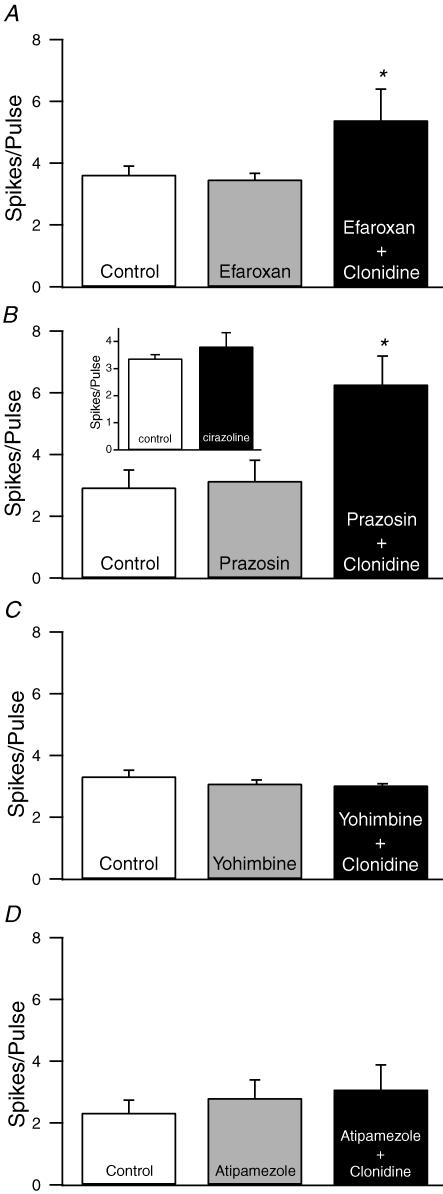
To determine if the effects of clonidine are mediated by imidazole receptors or α1-NA receptors, slices were incubated with either the imidazole antagonist efaroxan (4 nm, Haxhiu et al. 1994) or the α1-NA receptor antagonist prazosin (1 μm, Crepel et al. 1987; Arcos et al. 2003) prior to application of clonidine (10 μm). Neither antagonist produced any significant effect on cell excitability when applied alone or blocked the effects of clonidine (Fig. 2A and B). Further evidence against the involvement of α1-NA receptors in the effect of clonidine comes from the observation that the α1-NA receptor agonist cirazoline (10 μm, Croce et al. 2003) did not elicit any effect on the excitability of pyramidal neurons (Fig. 2B inset). In contrast, the α2-NA receptor antagonists yohimbine (2 μm) or atipamezole (1 μm) prevented the effects of clonidine on neuronal excitability, although neither antagonist produced any significant changes when applied alone (Fig. 2C and D).
Figure 2. The effects of clonidine are mediated by α2-NA receptors.
A–B, neither the imidazole receptor antagonist eferoxan (4 nm, A) nor the α1-NA receptor antagonist prazosin (1 μm, B) prevented the increase in spikes per pulse produced by clonidine (n= 6 for both groups). In addition, neither antagonist produced any changes when applied alone. B, inset). The selective α1-NA receptor agonist cirazoline (10 μm) did not mimic the effect of clonidine on cell excitability (n= 18). C–D, the α2-NA receptor antagonists yohimbine (2 μm, C) and atipamezole (1 μm, D) prevented the clonidine-mediated increases in excitability (n= 7 for both groups). However, neither antagonist produced any change when applied alone. *P < 0.05.
In addition to their localization on postsynaptic membranes, α2-NA receptors are also localized on noradrenergic terminals where they act as release modulating autoreceptors (Aoki et al. 1998). To test the hypothesis that the effects of α2-NA receptor stimulation are due to altered presynaptic NA release, animals were injected with reserpine (5 mg kg−1, i.p.) 24 h before sacrifice. Previous studies have found that this protocol reduces basal levels of catecholamines by 60–96% (Kannari et al. 2000; Yoshitake et al. 2004; Hatip-Al-Khatib et al. 2001). In tissue taken from these animals, clonidine still produced a significant increase in excitability, similar to the data obtained in untreated animals (baseline: 2.9 ± 0.4 spikes/pulse; clonidine: 5.7 ± 1.0 spikes/pulse, n= 8, P < 0.05) suggesting that the observed actions of clonidine are not mediated by altering presynaptic NA release.
α2-NA receptor stimulation inhibits HCN currents
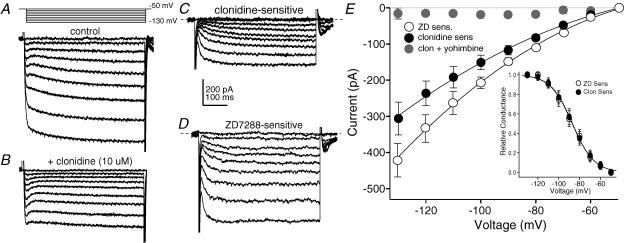
What is the mechanism through which α2-NA receptors enhance pyramidal cell excitability? The increase in input resistance by α2-NA receptor stimulation suggests the suppression of a current that is active at the resting membrane potential (∼−70 mV). The resting potential of PFC pyramidal cells is influenced by the balance between outward currents produced by constitutively active K+ channels (inwardly rectifying and K+leak channels) and inward currents produced by hyperpolarization/cyclic nucleotide gated cation (HCN) channels (Day et al. 2005). Similar to the effect of clonidine, inhibition of resting K+ channels would produce an increase in input resistance. However, as these channels mediate a standing outward current at −70 mV, their closure would result in membrane depolarization (Day et al. 2005; Taverna et al. 2005) rather than the hyperpolarization seen following α2-NA receptor activation. In contrast, blockade of HCN channels produces similar effects to those observed following α2-NA receptor stimulation (Day et al. 2005), suggesting that HCN channels may be an important downstream target of α2-NA receptors. Somatic voltage clamp recordings were performed to test this hypothesis. Cells were held at −50 mV in the presence of TTX (0.5 μm) and given 500 ms voltage steps from −50 to −130 mV in 10 mV increments to evoke hyperpolarization-activated currents. ZD7288 (40 μm) was used to block HCN channels. Subtracting the currents evoked following a 5 min perfusion with ZD from control records yields the ZD-sensitive current (Fig. 3D and E), which began to activate between −50 and −60 mV and showed relatively fast activation kinetics. This observation is consistent with previous findings that HCN current is primarily carried by HCN1 channels in PFC pyramidal cells (Day et al. 2005). Repeating these experiments, applying clonidine (10 μm) instead of ZD revealed a clonidine-sensitive current with similar characteristics to the current blocked by ZD (Fig. 3A–C). The amplitude of this clonidine-sensitive current was significantly attenuated if clonidine was applied following a 5 min incubation in the α2-NA receptor antagonist yohimbine (2 μm; Fig. 3E). The activation voltage dependence of these currents was determined by measuring the tail currents generated at the end of the hyperpolarizing pulses. The normalized tail current amplitudes were plotted as a function of voltage and fitted with a first-order Boltzmann equation (Fig. 3E inset). The half-activation voltage of the clonidine-sensitive current (−86.7 mV) was very similar to that of the ZD-sensitive current (−87.1 mV). It is important to note that, due to the incomplete control of dendritic membrane potential, these somatic voltage clamp data are likely to represent an underestimation of both HCN channel conductance and activation kinetics, particularly at more hyperpolarized potentials (Day et al. 2005). We thus sought to provide additional pharmacologicalal and nonpharmacological evidence that the effects of clonidine are mediated via inhibition of HCN currents.
Figure 3. α2-NA receptor stimulation inhibits hyperpolarization-activated currents in PFC pyramidal neurons.
A, currents evoked by 500 ms voltage steps from −50 mV to −130 mV in 10 mV increments in the presence of TTX (0.5 μm). B, bath application of clonidine (10 μm, 5 min) decreased the amplitude of currents evoked by this voltage protocol without affecting holding current. C, subtraction of the currents in B from A yields the clonidine-sensitive current. The dashed line shows the zero current point. D, the ZD7288 (40 μm, 5 min)-sensitive current evoked by the same protocol in a separate neuron. E, summary of the current–voltage relationship of the ZD7288- (40 μm, open symbols, n= 5) and clonidine- (10 μm, filled symbols, n= 6) sensitive currents evoked by the voltage protocol shown in A. Points are means ±s.e.m. The clonidine-sensitive current was significantly attenuated by prior application of the α2-NA receptor antagonist yohimbine (2 μm, grey symbols, n= 6). Inset, plot of the normalized amplitudes of the tail currents of the ZD- (open symbols, n= 6) and clonidine- (filled symbols, n= 6) sensitive currents. The values are fitted with a first-order Boltzmann equation (continuous line, ZD; dashed line, clonidine).
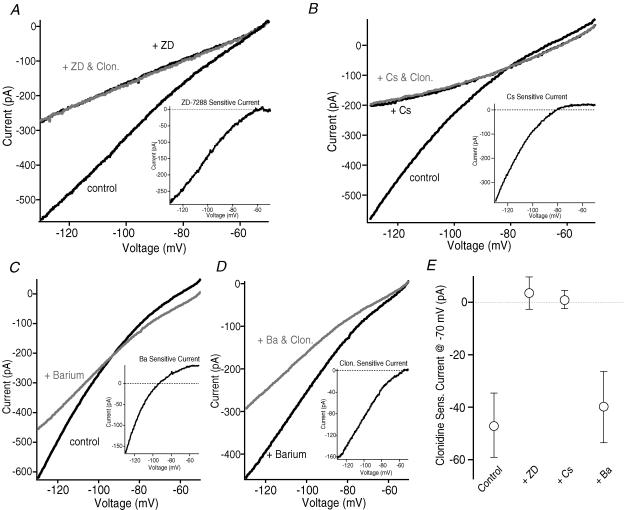
If the actions of clonidine are mediated by inhibition of HCN channels, then blockade of these channels should mimic and occlude the effects of clonidine. To test this hypothesis, clonidine was applied in the presence of the HCN channel blockers ZD7288 (40 μm) or caesium (5 mm) (Robinson & Siegelbaum, 2003). In voltage clamp recordings the α2-NA receptor agonist did not produce any change in the current response to 1 s voltage ramps from −50 to −130 mV when it was applied following a 5 min pretreatment with either HCN blocker (Fig. 4A, B and E). In addition to blocking HCN currents, caesium also blocks inwardly rectifying K+ (Kir2) channels in this voltage range (Stanfield et al. 2002). To test whether α2-NA receptor stimulation inhibits Kir2 channels, clonidine was applied following a 5 min application in barium at a concentration (100 μm) which blocks Kir2 channels while leaving HCN currents intact (Stanfield et al. 2002; Robinson & Siegelbaum, 2003). Consistent with previous observations (Day et al. 2005; Carr & Surmeier, 2007), Ba2+ blocked an inwardly rectifying current that reversed close to the predicted K+ equilibrium potential (Fig. 4C). However, contrary to the results obtained in the presence of ZD or Cs+, clonidine still produced a significant reduction in hyperpolarization-activated current when applied in the presence of Ba2+ (Fig. 4D and E).
Figure 4. Block of HCN, but not Kir2, currents occludes the effects of clonidine in voltage clamp.
A–D, current traces evoked by a 1 s voltage ramp from −50 to −130 mV. Each trace is the average of 5 consecutive sweeps. A, the HCN channel blocker ZD7288 (40 μm) produced a significant decrease in the amplitude of hyperpolarization-activated currents evoked by this voltage ramp protocol in a representative neuron. The ZD-sensitive current is shown in the inset. Subsequent addition of clonidine (10 μm, grey trace) did not produce any additional effect. B, clonidine (grey trace) also failed to produce any change in the hyperpolarization-activated current evoked by this voltage protocol when applied following a 5 min incubation in the HCN and Kir2 channel blocker Cs+ (5 mm) in a separate cell. The Cs+-sensitive current is shown in the inset. As Cs+ blocks both HCN and Kir2 channels, the Cs+-sensitive current reflects a mixture of both currents. C, the Kir2 channel blocker Ba2+ (100 μm, grey trace) reduced the amplitude of hyperpolarization-activated currents in a representative neuron. The Ba2+-sensitive current (inset) is inwardly rectifying with a reversal potential close to the predicted K+ reversal potential. D, in the same cell as C, subsequent addition of clonidine (10 μm, grey trace) produced an additional inhibition of hyperpolarization-activated current evoked by this voltage ramp protocol. Inset, the clonidine-sensitive current evoked in the presence of Ba2+ in this cell. Note the similarities to the ZD-sensitive current evoked in the cell in A. E, summary of the clonidine-sensitive current measured at −70 mV under control conditions (n= 6), or following incubation in ZD (n= 6), Cs+ (n= 5) or Ba2+ (n= 5).
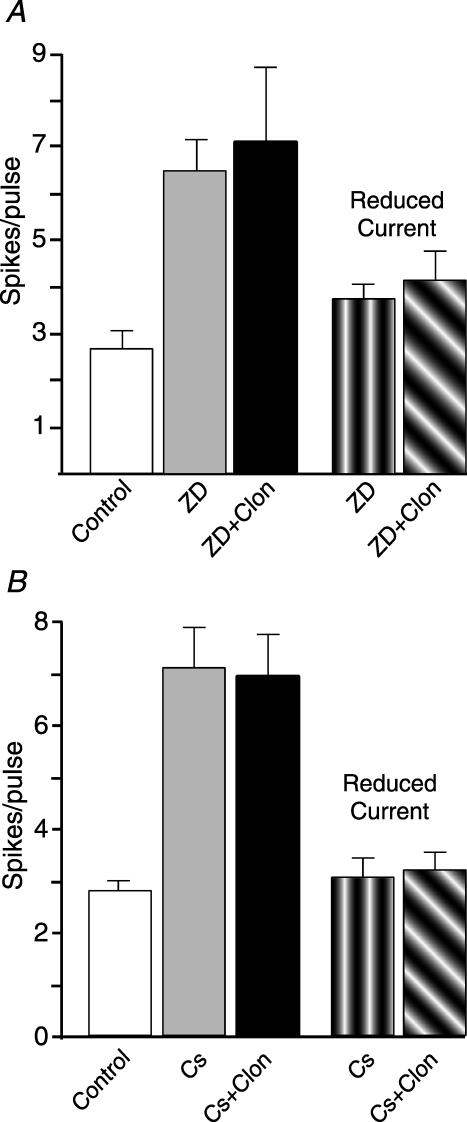
ZD also mimicked and occluded the actions of clonidine on evoked firing in current clamp recordings. Application of ZD produced a significant increase in evoked firing, but subsequent addition of clonidine did not produce any additional change (Fig. 5A). If the amplitude of the depolarizing test pulse was reduced so that the cell only fired three to four spikes in the presence of ZD, subsequent addition of clonidine still did not produce any significant change in excitability (Fig. 5A) arguing that the lack of a clonidine effect in the presence of ZD was not because the cell had reached its maximal firing rate in the presence of ZD. Repeating these experiments utilizing Cs+ rather than ZD yielded similar effects on evoked firing (Fig. 5B). As Cs+ also blocks Kir2 channels, whose closure leads to depolarization and tonic firing (Day et al. 2005), the membrane potential of cells was held at −70 mV using direct current (DC) injection during these experiments. Similar to the data obtained using ZD, Cs+ produced a significant increase in evoked firing, but subsequent addition of clonidine did not produce any additional change (Fig. 5B).
Figure 5. Blockade of HCN channels mimics and occludes the effects of clonidine on evoked firing.
A, in current clamp, ZD7288 (40 μm, grey bar) significantly increased the average number of evoked spikes per pulse (see text for protocol). Subsequent application of clonidine (10 μm, black bar) did not produce any additional change in cell excitability. If the amplitude of the current pulse was reduced following ZD application (vertically hatched bar), subsequent addition of clonidine still did not produce an increase in cell excitability (diagonally hatched bar). n= 5 for each condition. B, repeating the experiment in A using Cs+ (5 mm) instead of ZD yielded similar results. n= 5 for each condition.
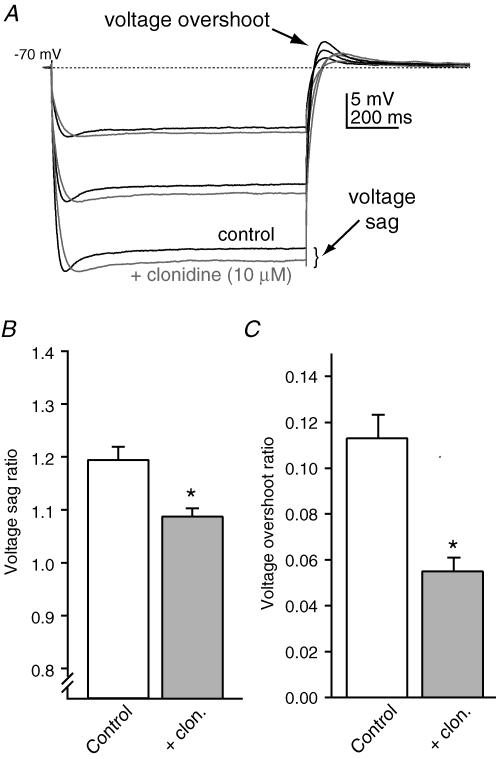
The slow kinetics of HCN currents are responsible for the voltage sag seen in cortical pyramidal cells in response to hyperpolarizing current pulses as well as the depolarizing voltage overshoot following the end of the pulse (Pape, 1996). To obtain additional evidence that α2-NA receptor stimulation produces a reduction in HCN current, the voltage sag ratio (peak voltage change/voltage change at steady state) and voltage overshoot ratio (voltage overshoot/voltage change at steady state) were determined under control conditions and in the presence of clonidine (Fig. 6). Figure 6A shows the effects of clonidine (10 μm) on the response to hyperpolarizing current pulses in a representative cell. For each cell tested, the resting potential was maintained at ∼−70 mV with DC injection and the amplitude of the current was adjusted to yield peak hyperpolarizing voltage deflections of 10, 20 and 30 mV. Under these conditions both the voltage sag ratio (Fig. 6A and B) and the normalized voltage overshoot (Fig. 6A and C) significantly decreased in the presence of clonidine.
Figure 6. Clonidine alters the voltage response to hyperpolarizing current pulses.
A, responses in a representative neuron to hyperpolarizing current steps. Cells were held at −70 mV using DC injection and the amplitude of the current pulses adjusted for each condition to yield peak voltage deflections of −10, −20 and −30 mV. Clonidine (10 μm, grey traces) reduced the amplitude of the voltage sag as well as the depolarizing overshoot at the end of the current pulse. B, summary of the voltage sag ratio (see text for definition) under control conditions and in the presence of clonidine (n= 7). C, summary of the voltage overshoot ratio (see text for definition) under control conditions and in the presence of clonidine (n= 7). *P < 0.05. The data presented in B and C are from −10 mV peak amplitude hyperpolarizations for each cell.
α2-NA receptor activation inhibits HCN currents via a PLC-PKC signalling pathway
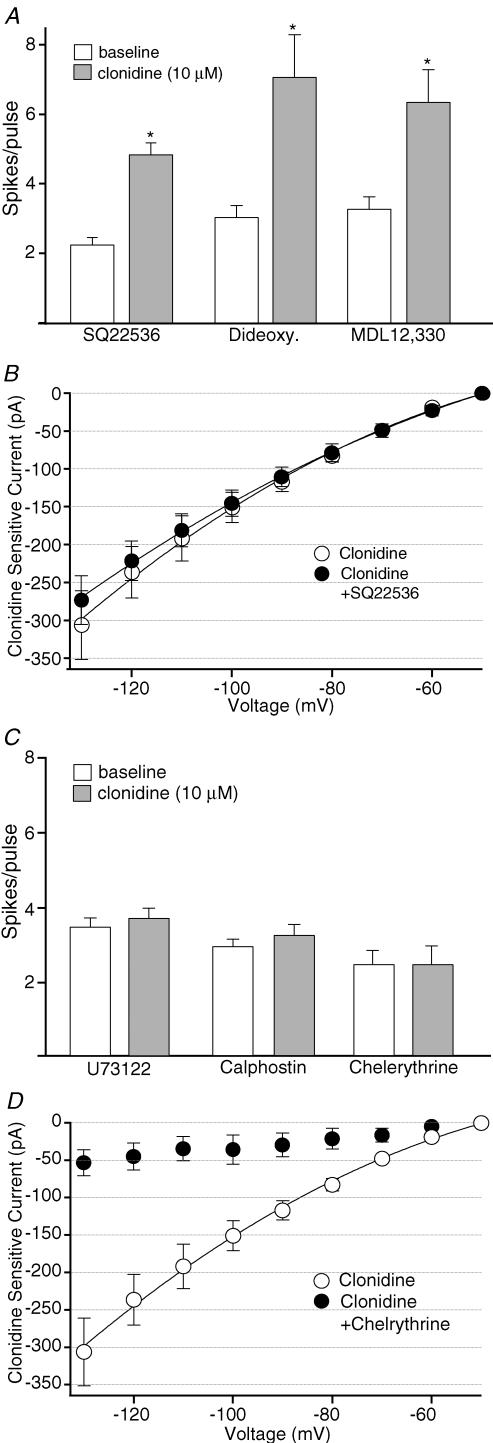
How does activation of α2-NA receptors lead to inhibition of HCN currents? HCN channels are sensitive to intracellular levels of cAMP, with a reduction in cAMP resulting in a hyperpolarizing shift in activation voltage dependence (Robinson & Siegelbaum, 2003). As α2-NA receptors are classically coupled to inhibition of adenylate cyclase via Gαi we tested the hypothesis that the observed effects of α2-NA receptor stimulation are mediated via this signalling pathway. In current clamp, recorded neurons were dialysed with the adenylate cyclase inhibitor SQ22536 (1 mm in pipette, Rosenkranz & Johnston, 2006). However, in the presence of this adenylate cyclase inhibitor, clonidine still continued to produce a significant increase in evoked firing (Fig. 7A). Similar results were also obtained in experiments utilizing two other adenylate cyclase inhibitors, MDL-12 330A (0.5 mm in pipette) and 2′,5′-dideoxyadenosine (0.1 mm in pipette, Pape, 1992; Jiang et al. 1993; Fig. 7A). Dialysis with SQ22536 also failed to prevent the effect of clonidine on hyperpolarization-activated currents evoked in voltage clamp (Fig. 7B). Taken together, these data do not support the hypothesis that the α2-NA receptor mediated inhibition of HCN channels is mediated by inhibition of adenylate cyclase.
Figure 7. α2-NA receptor stimulation inhibits HCN currents via a PLC–PKC signalling pathway.
A, dialysis with the adenylate cyclase inhibitors SQ22536 (1 mm, n= 5), 2′,5′-dideoxyadenosine (0.1 mm, n= 5), or MDL 12 330 (0.5 mm, n= 5) did not prevent the increase in evoked spikes per pulse produced by clonidine (10 μm) in current clamp. B, summary of the current–voltage relationship of the clonidine-sensitive current under control conditions (^, n= 6, data from Fig. 3E) or from cells dialysed with SQ22536 (1 mm, •, n= 6). Points are means ±s.e.m.C, the effects of clonidine on evoked firing were blocked by the PLC inhibitor U73122 (10 μm in pipette, n= 5). The PKC antagonists calphostin C (1 μm in pipette, n= 6) and chelerythrine (1 μm in bath, n= 7) also prevented the increase in evoked spikes per pulse produced by clonidine in current clamp. D, summary of the current–voltage relationship of the clonidine-sensitive current under control conditions (^, n= 6, data from Fig. 3E) or in the presence of chelerythrine (•, n= 6). *P-value < 0.05.
HCN currents have also been reported to be inhibited by receptors coupled to increased phosphoinositide turnover and stimulation of PKC (Cathala & Paupardin-Tritsch, 1997). Although it is classically coupled to inhibition of adenylate cyclase via Gαi, α2-NA receptors have also been linked to increased phosphoinositide turnover via Gβγ stimulation of phospholipase C (PLC) and subsequent activation of PKC (Boehm et al. 1996; Gesek, 1996; Dorn et al. 1997; Talaia et al. 2006). Consistent with the hypothesis that the observed effects of α2-NA receptor activation are mediated via this signalling pathway, the effects of clonidine in current clamp were prevented by either intracellular perfusion with the PLC inhibitor U73122 (10 μm, Fig. 7C) or pretreatment with the PKC inhibitors chelerythrine chloride (1 μm in bath, Fig. 7C) or calphostin C (1 μm in pipette, Fig. 7C). Pretreatment with chelerythrine chloride also blocked the effect of clonidine on hyperpolarization-activated currents when tested in voltage clamp (Fig. 7D).
α2-NA receptor stimulation enhances temporal integration of distal EPSPs evoked by layer I stimulation
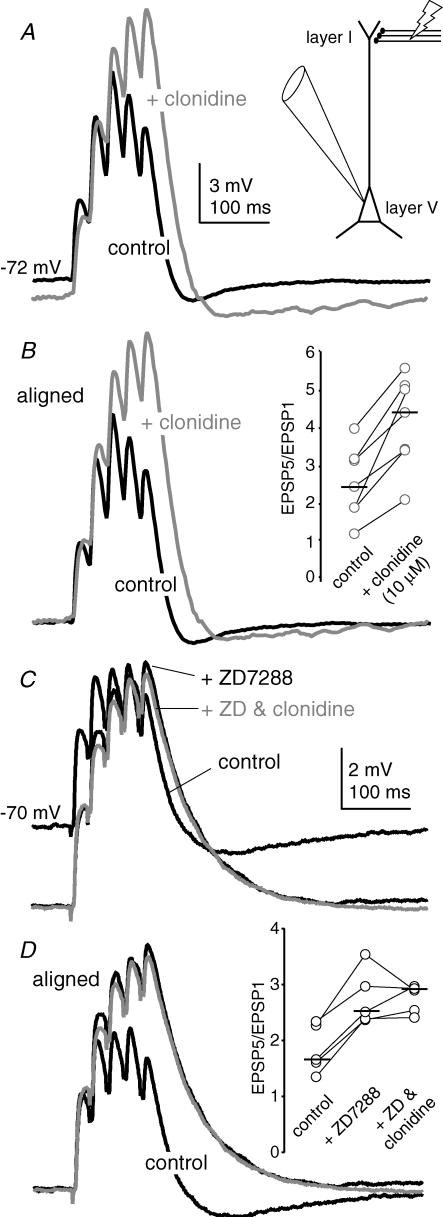
HCN channels have been shown to play an important role in the temporal integration of distal synaptic inputs onto the apical dendrites of pyramidal cells (Magee, 1998; Berger et al. 2001; Day et al. 2005; Rosenkranz & Johnston, 2006). Deactivation of HCN channels by distal EPSPs results in a net outward current that opposes subsequent EPSPs, resulting in sublinear summation during EPSP trains. To determine if the inhibition of HCN currents by α2-NA receptors influences synaptic integration, distal EPSPs were generated by a bipolar stimulating electrode placed in layer I, ∼500 μm dorsal to the recorded cell in layer V (Fig. 8A inset). The temperature of the aCSF was maintained at 33 ± 1°C. Under control conditions, a 40 Hz train of five stimuli produced EPSPs that showed sublinear temporal summation (Fig. 8A). The ratio of the amplitude of the fifth to the first EPSP in the train (EPSP5/EPSP1) had a median value of 2.4 (n= 7; Fig. 8B inset). In the presence of clonidine (10 μm), this ratio increased significantly to a median value of 4.4 (n= 7; P < 0.05, Wilcoxon's signed rank test; Fig. 8B inset). Moreover, although clonidine typically produced a 2–4 mV hyperpolarization of the resting membrane potential, the concurrent enhancement in temporal summation resulted in a larger absolute depolarization by the end of the train (Fig. 8A). This increase in temporal summation was not due to the hyperpolarization of the membrane potential as it persisted when Vm was subsequently adjusted to control levels by DC injection (median EPSP5/EPSP1 value: 4.5; n= 7; data not shown).
Figure 8. α2-NA receptor stimulation enhances temporal integration of EPSPs evoked by layer I stimulation.
A, current clamp recordings from a representative layer V PFC pyramidal neuron during a 40 Hz train of 5 EPSPs generated via a stimulating electrode (50 μA stim.) placed in layer I, ∼500 μm dorsal to the recorded neuron (inset). Each record is the average of 10 consecutive sweeps under control conditions (black trace), or in the presence of clonidine (10 μm, grey trace). B, the traces in A are aligned to the resting membrane potential. Inset, summary of the change in the EPSP5/EPSP1 ratio produced by clonidine (n= 7). The horizontal bars represent the median value for each group. C, in a separate neuron, application of the HCN blocker ZD7288 (40 μm) hyperpolarizes the membrane potential, but enhances the temporal summation during the 40 Hz, 5 pulse train. Subsequent addition of clonidine (grey trace) did not produce any additional effect on either membrane potential or EPSP summation. D, the traces in C are aligned to the resting membrane potential. Inset, summary of the change in the EPSP5/EPSP1 ratio produced by ZD7288. Subsequent addition of clonidine did not produce any additional change in the EPSP5/EPSP1 ratio (n= 5).
To determine if these effects of α2-NA receptor activation on temporal summation were mediated through inhibition of HCN channels, the experiment was repeated in the presence of the HCN channel blocker ZD7288 (40 μm). Consistent with previous observations (Magee, 1998; Berger et al. 2001; Day et al. 2005; Rosenkranz & Johnston, 2006), ZD produced a significant increase in temporal summation during the EPSP train (Fig. 8C and D). When clonidine (10 μm) was subsequently applied in the presence of ZD, there was no further enhancement in the EPSP5/EPSP1 ratio (Fig. 8C and D), suggesting that inhibition of HCN channels is the primary mechanism through which α2-NA receptor stimulation enhances temporal synaptic summation.
Discussion
The studies reported here indicate that α2-NA receptor activation results in the inhibition of HCN channels, which are active at resting membrane potentials in PFC pyramidal neurons (Day et al. 2005). Closure of these HCN channels produces a hyperpolarization of the resting membrane potential, but a significant increase in evoked spiking due to an increase in input resistance. The inhibition of HCN channels also produces a significant enhancement in the temporal integration of distal excitatory inputs. These combined effects result in an enhancement in the gain function of the pyramidal neuron's integrate and fire properties and may thus be an important cellular mechanism through which NA enhances task related activity while suppressing background firing during the performance of working memory tasks.
α2-NA receptors target HCN channels in PFC pyramidal cells
The effects of clonidine in current clamp were blocked by antagonists of α2-NA, but not other adrenergic or imidazole receptors. Both α2A- and α2C-NA receptor subtypes are expressed by PFC pyramidal neurons (Scheinin et al. 1994; Aoki et al. 1998). Of these subtypes, α2A displays the highest level of expression (Scheinin et al. 1994; Aoki et al. 1998) and likely mediates the observed effects of clonidine. This contention is based on the inability to block the effects of clonidine with prazosin, which shows considerable affinity for α2C, but not α2A receptors (Renouard et al. 1994). This finding is of note as the improvement in working memory performance produced by α2-NA receptor agonists is lost in α2A but not in α2C receptor knockout mice (Tanila et al. 1999; Franowicz et al. 2002). In addition, a recent report indicates that α2A receptors are colocalized with HCN1 subunits in dendritic spines of PFC pyramidal cells (Wang et al. 2007) further supporting a link between the two.
Our conclusion that the effects of α2-NA receptor stimulation are mediated by the inhibition of HCN channels is based on several observations. First, the effects of clonidine in current clamp recordings (hyperpolarization, increased input resistance, increased temporal integration, decreased voltage sag, decreased voltage overshoot) are very similar to the effects of HCN blockade using Cs+ or ZD7288 (Magee, 1998; Berger et al. 2001; Day et al. 2005). Second, the clonidine-sensitive current evoked by hyperpolarizing steps in voltage clamp exhibits similar kinetics and voltage dependence to the current that is blocked by the HCN channel blocker ZD7288. Most significantly, blockade of HCN channels with either ZD or Cs+ occluded any additional effects of clonidine in either current clamp or voltage clamp recordings. Thus, while we do not exclude additional downstream targets of α2-NA receptor stimulation, our data suggest that the observed effects of clonidine are mediated primarily through inhibition of HCN channels. Inhibition of HCN currents by α2-NA receptor stimulation has also been observed in hypoglossal motoneurons (Parkis & Berger, 1997) and dorsal root ganglion neurons (Yagi & Sumino, 1998).
HCN channels play important roles in regulating resting membrane potential, synaptic integration and synaptic plasticity (Pape, 1996; Magee, 1999; Nolan et al. 2004; Day et al. 2005). Given the importance of HCN channels in shaping neural activity, it is perhaps not surprising that they are the target of many neuromodulatory systems including dopamine (Jiang et al. 1993; Rosenkranz & Johnston, 2006), serotonin (Bobker & Williams, 1989; Pape & McCormick, 1989), and noradrenaline (Pape & McCormick, 1989; Parkis & Berger, 1997; Yagi & Sumino, 1998). The most well characterized signalling pathway for altering HCN channel function is the depolarizing shift in activation voltage dependence produced by elevations in cAMP (Robinson & Siegelbaum, 2003). As α2-NA receptors are classically coupled to inhibition of adenylate cyclase via Gαi, one might predict that the inhibition of HCN currents by this receptor is mediated via this signalling pathway. However, intracellular dialysis with three different adenylate cyclase inhibitors failed to block the effects of clonidine in current and voltage clamp recordings. In addition, if the effects of α2-NA receptor stimulation were mediated primarily through a hyperpolarizing shift in activation voltage dependence, one would expect that clonidine would produce a much smaller effect on HCN current amplitude at very hyperpolarized potentials where conductance is maximal. However, this was not the case for the clonidine-sensitive current. Although these observations were contrary to our original hypothesis, they are consistent with the finding that HCN currents in PFC pyramidal cells are primarily carried by HCN1 channels (Day et al. 2005), which are considerably less sensitive to changes in cAMP than HCN2 channels (Chen et al. 2001). However, it is also possible that basal AC activity is low under our recording conditions, precluding any additional reduction by Gαi.
Our finding that the α2-NA receptor-mediated inhibition of HCN channels requires activation of a PLC–PKC pathway is consistent with other reports that α2-NA receptors are linked to increased phosphoinositide turnover via Gβγ stimulation of PLC and subsequent activation of PKC (Boehm et al. 1996; Gesek, 1996; Dorn et al. 1997; Talaia et al. 2006). Our findings are also consistent with a previous report that PKC activation produced an inhibition of HCN currents in substantia nigra dopamine neurons (Cathala & Paupardin-Tritsch, 1997). North and colleagues (Jiang et al. 1993) have also shown that stimulation of another Gi/o linked receptor (dopamine D2) in ventral tegmental area neurons produces a cAMP-independent decrease in the maximal conductance of HCN currents without changing the activation voltage dependence. Although the involvement of PKC was not tested in this paper, D2, like other Gi/o coupled receptors (Selbie & Hill, 1998), has been shown to couple to PLC-linked signalling cascades in other neurons (Hernández-López et al. 2000; Maurice et al. 2004). Although our data indicate that the α2-NA receptor inhibition of HCN current requires PKC activation, it is not known if PKC acts directly on HCN channels or via an intermediary, such as the transactivation of a protein tyrosine kinase (Shah & Catt, 2004).
In addition to their regulation by cAMP and PKC, recent data have demonstrated that HCN channels are also regulated by a host of other factors, including p38 MAP kinase (Poolos et al. 2006), Src kinase (Zong et al. 2005), membrane phosphoinositides (Zolles et al. 2006), and elevations in intracellular Ca2+ produced by neural activity (van Welie et al. 2004). Thus HCN channels are potentially under the dynamic control of a host of different intracellular signalling pathways. One important task for future studies will be to determine how these different signalling pathways interact to regulate the activity of HCN channels in native cells.
α2-NA receptor modulation of HCN channels enhances temporal integration
HCN channels are predominantly expressed within the dendrites of pyramidal neurons where they influence the temporal integration of distally generated synaptic inputs (Magee, 1998; Berger et al. 2001; Day et al. 2005). The depolarization produced by trains of EPSPs deactivates HCN channels, generating a net outward current that opposes further depolarization and leads to sublinear summation. Inhibition of these channels prevents this sublinear summation and increases the probability that a train of excitatory input will bring the neuron to spike threshold. Inhibition of HCN channels may thus be an important cellular mechanism for enhancing the gain of a neuron's response to excitatory synaptic input. Due to the relatively slow deactivation kinetics of HCN channels, their influence on the amplitude of individual EPSPs is minimal (Magee, 1998; Berger et al. 2001; Day et al. 2005). However, because inhibition of HCN channels hyperpolarizes the resting potential away from spike threshold, the net effect of an isolated EPSP is reduced. In contrast, by preventing sublinear summation, inhibition of HCN channels enhances the amplitude and duration of the response to a burst of excitatory synaptic input. Thus the overall effect of HCN channel inhibition is to suppress the response to isolated excitatory inputs while enhancing the response to a coherent burst of synaptic activity.
By enhancing the response to trains of excitatory input, it is likely that α2-NA receptor-mediated inhibition of HCN channels plays a significant role in the maintenance of delay activity within a recurrent network of PFC neurons during the performance of a working memory task. For example, previous work in behaving primates has shown that intra-PFC administration of an α2-NA agonist improved working memory performance (Franowicz & Arnsten, 1999) while selectively increasing delay-related activity in PFC neurons (Li et al. 1999; Wang et al. 2007). In contrast, administration of an α2-NA antagonist impairs working memory performance (Li & Mei, 1994) as well as selectively reducing delay-related activity in PFC neurons (Sawaguchi, 1998; Li et al. 1999; Wang et al. 2007). Evidence that these α2-NA effects are mediated by inhibition of HCN channels is provided by a recent report by Arnsten and colleagues (Wang et al. 2007). These authors report that iontophoresis of ZD7288 enhanced the delay related firing of PFC neurons during a working memory task, similar to the application of the α2-NA agonist guanfascine. In addition, the improvement in working memory performance produced by intra-PFC administration of α2-NA agonists (Franowicz & Arnsten, 1999) could be mimicked by either intra-PFC injections of ZD or RNAi induced down-regulation of HCN1 expression (Wang et al. 2007).
Deficits in executive functioning are found in a number of neuropsychiatric disorders, including schizophrenia, depression, and attention deficit hyperactivity disorder. It is hoped that better understanding of the cellular mechanisms that mediate these executive functions of the PFC, combined with the knowledge of how these mechanisms are influenced by neuromodulators and intracellular signalling pathways, will highlight novel targets in the search for new treatments to enhance cognitive function in these patient populations.
Acknowledgments
We thank Dr J. David Jentsch for insightful discussions. This work was supported by NIDA (14698, A.L.) and the Tourette Syndrome Association (A.L.). This work was conducted in a facility constructed with support from the National Institutes of Health Extramural Research Facilities Program (C06 RR015455).
References
- Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cerebral Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Arcos D, Sierra A, Nunez A, Flores G, Aceves J, Arias-Montano JA. Noradrenaline increases the firing rate of a subpopulation of rat subthalamic neurones through the activation of α1-adrenoceptors. Neuropharmacology. 2003;45:1070–1079. doi: 10.1016/s0028-3908(03)00315-0. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Luscher H-R. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. Serotonin augments the cationic current Ih in central neurons. Neuron. 1989;2:1535–1540. doi: 10.1016/0896-6273(89)90041-x. [DOI] [PubMed] [Google Scholar]
- Boehm S, Huck S, Freissmuth M. Involvement of a phorbol ester-insensitive protein kinase C in the α2-adrenergic inhibition of voltage-gated calcium current in chick sympathetic neurons. J Neurosci. 1996;16:4596–4603. doi: 10.1523/JNEUROSCI.16-15-04596.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–3438. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- Cathala L, Paupardin-Tritsch D. Neurotensin inhibition of the hyperpolarization-activated cation current (Ih) in the rat substantia nigra pars compacta implicates the protein kinase C pathway. J Physiol. 1997;503:87–97. doi: 10.1111/j.1469-7793.1997.087bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F, Debono M, Flores R. α-Adrenergic inhibition of rat cerebellar Purkinje cells in vitro: a voltage-clamp study. J Physiol. 1987;383:487–498. doi: 10.1113/jphysiol.1987.sp016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce A, Astier H, Recasens M, Vignes M. Opposite effects of α1- and β-adrenoceptor stimulation on both glutamate- and γ-aminobutyric acid-mediated spontaneous transmission in cultured rat hippocampal neurons. J Neurosci Res. 2003;71:516–525. doi: 10.1002/jnr.10516. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW, Oswald KJ, McCluskey TS, Kuhel DG, Liggett SB. α2A-Adrenergic receptor stimulated calcium release is transduced by Gi-associated Gβγ-mediated activation of phospholipase C. Biochemistry. 1997;36:6415–6423. doi: 10.1021/bi970080s. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology. 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AFT. Treatment with the noradrenergic α-2 agonist clonidine, but not diazepam, improves spatial working memory in normal young rhesus monkeys. Neuropsychopharmacology. 1999;21:611–621. doi: 10.1016/S0893-133X(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the α2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. New York: Lippincott-Raven Publishers; 1997. [Google Scholar]
- Gesek FA. α2-Adrenergic receptors activate phospholipase C in renal epithelial cells. Mol Pharmacol. 1996;50:407–414. [PubMed] [Google Scholar]
- Hatip-Al-Khatib I, Mishima K, Iwasaki K, Fujiwara M. Microdialysates of amines and metabolites from core nucleus accumbens of freely moving rats are altered by dizocilpine. Brain Res. 2001;902:108–118. doi: 10.1016/s0006-8993(01)02382-4. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Dreshaj I, Schafer SG, Ernsberger P. Selective antihypertensive action of moxonidine is mediated mainly by I1-imidazoline receptors in the rostral ventrolateral medulla. J Cardiovasc Pharmacol. 1994;24(Suppl. 1):S1–S8. doi: 10.1097/00005344-199424001-00002. [DOI] [PubMed] [Google Scholar]
- Hernández-López S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC1-IP3calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Pessia M, North RA. Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurones. J Physiol. 1993;462:753–764. doi: 10.1113/jphysiol.1993.sp019580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannari K, Tanaka H, Maeda T, Tomiyama M, Suda T, Matsunaga M. Reserpine pretreatment prevents increases in extracellular striatal dopamine following L-DOPA administration in rats with nigrostriatal denervation. J Neurochem. 2000;74:263–269. doi: 10.1046/j.1471-4159.2000.0740263.x. [DOI] [PubMed] [Google Scholar]
- Li B-M, Mao Z-M, Wang M, Mei Z-T. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Li BM, Mei ZT. Delayed-response deficit induced by local injection of the alpha 2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic Ih normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/12229. [DOI] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptormediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Pape H-C. Adenosine promotes burst activity in guinea-pig geniculocortical neurones through two different ionic mechanisms. J Physiol. 1992;447:729–753. doi: 10.1113/jphysiol.1992.sp019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H-C. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Berger AJ. Clonidine reduces hyperpolarization-activated inward current (Ih) in rat hypoglossal motoneurons. Brain Res. 1997;769:108–118. doi: 10.1016/s0006-8993(97)00677-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Poolos NP, Bullis JB, Roth MK. Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. J Neurosci. 2006;26:7995–8003. doi: 10.1523/JNEUROSCI.2069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renouard A, Widdowson PS, Millan MJ. Multiple alpha2 adrenergic receptor subtypes. I. Comparison of [3H]RX821002-labeled rat Ralpha−2A adrenergic receptors in cerebral cortex to human Halpha2A adrenergic receptor and other populations of alpha-2 adrenergic subtypes. J Pharmacol Exp Ther. 1994;270:946–957. [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarizationactivated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci. 2006;26:3229–3244. doi: 10.1523/JNEUROSCI.4333-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T. Attenuation of delay-period activity of monkey prefrontal neurons by an α2-adrenergic antagonist during an oculomotor delayed-response task. J Neurophysiol. 1998;80:2200–2205. doi: 10.1152/jn.1998.80.4.2200. [DOI] [PubMed] [Google Scholar]
- Sawaguchi B, Matsumura M, Kubota K. Catecholaminergic effects on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63:1385–1400. doi: 10.1152/jn.1990.63.6.1385. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT., Jr Distribution of α2-adrenergic receptor subtype expression in rat brain. Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- Shah BH, Catt KJ. GPCR-mediated transactivation of RTKs in the CNS: mechanisms and consequences. Trends Neurosci. 2004;27:48–53. doi: 10.1016/j.tins.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima S, Nakajima Y. Constituatively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir 3.0. Rev Physiol Biochem Pharmacol. 2002;145:47–179. doi: 10.1007/BFb0116431. [DOI] [PubMed] [Google Scholar]
- Talaia C, Queiroz G, Pinheiro H, Moura D, Goncalves J. Involvement of G-protein βγ subunits on the influence of inhibitory α2-autoreceptors on the angiotensin AT1-receptor modulation of noradrenaline release in the rat vas deferens. Neurochem Int. 2006;49:698–707. doi: 10.1016/j.neuint.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Tanila H, Mustonen K, Sallinen J, Scheinin M, Riekkinen P., Jr Role of α2C-adrenoceptor subtype in spatial working memory as revealed by mice with targeted disruption of the α2C-adrenoceptor gene. Eur J Neurosci. 1999;11:599–603. doi: 10.1046/j.1460-9568.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- Taverna S, Tkatch T, Metz AE, Martina M. Differential expression of TASK channels between horizontal interneurons and pyramidal cells of rat hippocampus. J Neurosci. 2005;25:9162–9170. doi: 10.1523/JNEUROSCI.2454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Welie I, van Hooft JA, Wadman WJ. Homeostatic scaling of neuronal excitability by synaptic modulation of somatic hyperpolarization-activated Ih channels. Proc Natl Acad Sci U S A. 2004;101:5123–5128. doi: 10.1073/pnas.0307711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. α2A-Adrenoceptors strengthen working memory networks by inhibiting cAMPHCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Yagi J, Sumino R. Inhibition of a hyperpolarizationactivated current by clonidine in rat dorsal root ganglion neurons. J Neurophysiol. 1998;80:1094–1104. doi: 10.1152/jn.1998.80.3.1094. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Fujino K, Nohta H, Yamaguchi M, Kehr J. High-sensitive liquid chromatographic method for determination of neuronal release of serotonin, noradrenaline and dopamine monitored by microdialysis in the rat prefrontal cortex. J Neurosci Methods. 2004;140:163–168. doi: 10.1016/j.jneumeth.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Zolles G, Klocker N, Wenzel D, Weisser-Thomas J, Fleischmann BK, Roeper J, Fakler B. Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron. 2006;52:1027–1036. doi: 10.1016/j.neuron.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Zong X, Eckert C, Yuan H, Wahl-Schott C, Abicht H, Fang L, Li R, Mistrik P, Gerstner A, Much B, Baumann L, Michalakis S, Zeng R, Chen Z, Biel M. A novel mechanism of modulation of hyperpolarization-activated cyclic nucleotidegated channels by Src kinase. J Biol Chem. 2005;280:34224–34232. doi: 10.1074/jbc.M506544200. [DOI] [PubMed] [Google Scholar]