Abstract
There is increasing evidence that locally produced angiotensin AII (AII) regulates the function of many tissues, but the involvement of enterocyte-derived AII in the control of intestinal transport is unknown. This study examined whether there is a local renin–angiotensin system (RAS) in rat villus enterocytes and assessed the effects of AII on SGLT1-dependent glucose transport across the brush border membrane (BBM). Gene and protein expression of angiotensinogen, ACE, and AT1 and AT2 receptors were studied in jejunal and ileal enterocytes using immunocytochemistry, Western blotting and RT-PCR. Mucosal uptake of d-[14C]glucose by everted intestinal sleeves before and after addition of AII (0–100 nm) to the mucosal buffer was measured in the presence or absence of the AT1 receptor antagonist losartan (1 μm). Immunocytochemistry revealed the expression of angiotensinogen, ACE, and AT1 and AT2 receptors in enterocytes; immunoreactivity of AT1 receptor and angiotensinogen proteins was especially pronounced at the BBM. Expression of angiotensinogen and AT1 and AT2 receptors, but not ACE, was greater in the ileum than the jejunum. Addition of AII to mucosal buffer inhibited phlorizin-sensitive (SGLT1-dependent) jejunal glucose uptake in a rapid and dose-dependent manner and reduced the expression of SGLT1 at the BBM. Losartan attenuated the inhibitory action of AII on glucose uptake. AII did not affect jejunal uptake of l-leucine. The detection of RAS components at the enterocyte BBM, and the rapid inhibition of SGLT1-dependent glucose uptake by luminal AII suggest that AII secretion exerts autocrine control of intestinal glucose transport.
In addition to the Angiotensin II (AII) that is delivered throughout the body via blood circulation, many peripheral tissues are also regulated by AII produced by a local renin–angiotensin system (RAS). The RAS consists of several key components: the precursor angiotensinogen, two critical enzymes renin and angiotensin-converting enzyme (ACE), and the physiologically active peptide AII, as well as its receptors (Leung, 2004). There is a growing list of tissue-specific RAS functions exerted by paracrine and autocrine effects of locally secreted AII (Paul et al. 2006). In the gastrointestinal system, functional RAS has been identified in the pancreas and liver (Leung et al. 2003; Leung, 2004; Leung, 2007). In the intestine, local RAS components, including renin (Seo et al. 1991) and ACE (Erickson et al. 1992), have been detected in small intestinal mucosa; however, the role of the intestinal RAS has not been resolved. It has been suggested that mucosal ACE may function as a brush border membrane (BBM) peptidase (Yoshioka et al. 1987), but there is no evidence for the involvement of a local RAS in control of intestinal solute transport.
The effects of AII on peripheral tissues are mediated through two receptors: the more prevalent AT1 receptor and the AT2 receptor. It was shown two decades ago that AII binds the enterocyte membrane (Cox et al. 1986). More recent work demonstrated that vascular infusion of low concentrations of AII stimulates intestinal fluid transport via the AT2 receptor, whereas higher levels of AII inhibit fluid transport via an AT1 receptor-dependent process (Jin et al. 1998). However, the vascular infusion methodology used in these studies did not allow determination of the site of AII action at the enterocyte per se. Importantly, despite the finding that ACE is present at the intestinal BBM (Stevens et al. 1988; Naim, 1992), there is no information available on the transport actions of locally produced AII at this membrane.
Studies using proximal tubule cells, where the glucose uptake process is very similar to that in enterocytes, imply that locally secreted AII affects sugar transport. For example, AII inhibits uptake of the glucose analogue α-methyl-glucopyranoside (AMG) into LLC-PK1 cells (Kawano et al. 2002) and reduces expression of the sodium-dependent glucose transporter (SGLT1) at the apical membrane. Thus the aim of the present study was to test the hypothesis that locally produced AII regulates enterocyte glucose transport, particularly at the BBM. To this end, we first examined the localization of RAS components in intestinal epithelium; specifically, we compared the expression of angiotensinogen, AT1 and AT2 receptors, and ACE in jejunum and ileum. In parallel studies, we determined the effect of mucosal AII on the rate of glucose uptake across the BBM of isolated small intestine.
Methods
Animals
This study used male adult Sprague–Dawley rats (240–260 g) purchased from the Laboratory Animal Services Centre at The Chinese University of Hong Kong. All procedures were approved by the Animal Ethical Committee of the Chinese University of Hong Kong. Animals were maintained on food and water ad libitum up to the time of experimentation. Anaesthesia prior to removal of the intestine was achieved by intraperitoneal injection of sodium pentobarbital (50 mg kg−1 of body weight). The abdomen was immediately opened via midline laparotomy. After removal of the intestine, animals were killed by an overdose of pentobarbital, followed by cervical dislocation.
BBM vesicles and enterocytes were prepared from 20 cm long segments of small intestine either beginning 10 cm distal to the ligament of Treitz (jejunum) or ending 2 cm from the caecum (ileum). Intestinal segments 2–3 cm in length, taken from the mid-point of these regions were used for uptake experiments and for immunocytochemistry.
Isolation of enterocytes
Villus cells were harvested by a Ca2+-chelation technique (Del Castillo, 1987; Sundaram et al. 1991), a procedure which produces enterocytes with a high viability. Briefly, intestinal segments were washed thoroughly with ice-cold saline followed by air. The segment was tied off at one end and filled with Ca2+-free hypertonic isolation buffer (7 mm K2SO4, 44 mm K2HPO4, 9 mm NaHCO3, 10 mm Hepes, 2 mm l-glutamine, 0.5 mm dithiothreitol, 1 mm Na2EDTA, 180 mm glucose 180 mm, pH 7.4), equilibrated with 95% O2–5% CO2, avoiding over distention. The segment was then tied off to form a closed sac and incubated in 0.9% saline at 37°C with gentle shaking for 16 min. Cells were dislodged manually and the resulting suspension was collected and centrifuged for 30 s at 500 g. The pellet was resuspended in freshly prepared cold buffer and re-centrifuged, a procedure that was repeated twice. RNase Out (Gibco, Invitrogen) RNase inhibitor was added to all isolation solutions used for RNA isolation.
BBM preparation
The methods used to prepare BBM vesicles have been previously described (Kessler et al. 1978). All procedures were carried out at 4°C. Jejunal and ileal segments were opened and the mucosal layers were scraped off and added to homogenization buffer (50 mm mannitol, 2 mm Hepes, 0.25 mm phenylmethylsulphonyl fluoride (PMSF), pH 7.1; 28 ml g−1 mucosa). The mixture was homogenized three times at 50% maximum speed for 20 s using an Ultra Turrax homogenizer (Janke & Kunkel, Germany). MgCl2 was added to a final concentration of 10 mm, and the mixture was stirred on ice for 20 min and then centrifuged at 3000 g for 15 min. The supernatant was re-centrifuged at 27 000 g for 30 min. The pellet was resuspended in buffer (100 mm mannitol, 0.1 mm MgSO4, and 0.4 mm Hepes-Tris, pH 7.2) and centrifuged for 15 min at 6000 g. The supernatant was then re-centrifuged at 27 000 g for 30 min. The resulting pellet was suspended in the final buffer (300 mm mannitol, 10 mm Hepes-Tris, 0.1 mm MgSO4, and 0.25 mm PMSF, pH 7.4) by passing six times through a 25-gauge needle. The purified BBM pellet was then re-suspended in the final buffer at a protein concentration of 3–6 mg ml−1. Protein concentration in the BBM preparation was determined as previously described (Bradford, 1976).
Immunocytochemistry
Immunofluorescent labelling was used to determine the mucosal localization of AT1 receptors and angiotensinogen (AO) as previously described (Leung et al. 2000). Intestinal sections were embedded in OCT medium (Tissue-Tek). Sections, 6 μm thick, were collected on Superforst slides (Menzel-Glaser). Sections were fixed in 4% (v/v) chilled paraformaldehyde and further treated with 0.5% Triton X-100 and incubated with 10% (w/v) normal goat serum (NGS) (Jackson ImmunoResearch, USA) for 1 h at room temperature to block non-specific antibody binding. Excess blocking solution was poured off and the slides were incubated overnight at 4°C with primary antibody (anti-AT1 receptor rabbit polyclonal antibodies (Santa Cruz Biotechnology, USA) (1 : 200) or anti-AO rabbit polyclonal antibody (1 : 12 000)) diluted in 0.1 m phosphate buffered saline (PBS) with 3% NGS and 0.3% Triton X-100 (pH 7.4). After three washes with PBS, bound primary antibodies were detected by incubating sections with FITC-conjugated antirabbit secondary antibodies (1 : 250) (Jackson ImmunoResearch) at room temperature for 1 h. Immunoreactivity was captured with a fluorescent microscope equipped with a DC480 digital camera (Leica Microsystems).
Real-time PCR analysis
Quantitative RT-PCR was performed using an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA, USA) as previously described (Chu & Leung, 2007). Briefly, total RNA was extracted from freshly prepared enterocytes using Trizol reagent (Gibco, Invitrogen) according to the manufacturer's instructions. RNase Out (Gibco, Invitrogen) was added to the RNA solutions to prevent degradation by RNase. Total RNA served as the template for cDNA preparation using the Bio-RAD one step cDNA preparation kit. Primers were designed from rat cDNA sequences using Primer Express software purchased from Applied Biosystems (Perkins-Elmer). β-Actin was used as a reference gene to normalize the relative expression of each RAS gene. The sequences of all primers used are shown in Table 1.
Table 1.
Sequences of specific primers for the RAS components and β-actin used for real-time quantitative RT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| β-Actin | TCCTCCTGAGCGCAAGTACTC | GTGGACAGTAGTGAGGCCAGGAT |
| AO | GCA AATCAGTGCCTTCACCC | AAACAAACCCTCACCCCAGGAG |
| AT1 | CTCAAGCCTGTCTACGAAAATGAG | TAGATCCTGAGGCAGGGTGAAT |
| AT2 | TGCTGTTGTGTTGGCATTC | GCATCCAAGAAGGTCAGAACATG |
| ACE | GGAGACGACTTACAGTGTAGCC | CACACCCAAAGCAATTCTTC |
Sybergreen reactions were set up in a volume of 25 μl with ABI two-step sybergreen PCR reagents. Each reaction consisted of 12.5 μl PCR master mix, 0.05–0.30 μm of each amplification primer and 1 μl cDNA. Each sample was run in duplicate with an initial 10 min period at 95°C to enable the reaction, followed by 40 cycles at 95°C for 15 s and 10°C for 10 s. The samples were heated to 60°C over 1 min, then to 95°C over the next minute, and finally cooled slowly from 95°C to 60°C over 20 min. Amplification data were collected by the 7700 Sequence Detector and analysed with Sequence Detection System software. The RNA concentration in each sample was determined from the threshold cycle (CT) at which fluorescence was first detected, the cycle number being inversely related to RNA concentration. The fold changes were calculated using the 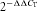 method (Lau et al. 2004).
method (Lau et al. 2004).
Western blotting
The methods used for immunoblotting have been previously described (Ip et al. 2003). Protein from BBM vesicles or enterocyte homogenate was extracted using the CytoBuster Protein Extraction Reagent (Novagen, Darmstadt, Germany). Protein content was determined by a Bradford protein assay kit (Bio-Rad, Munich, Germany). Proteins 10 μg lane−1 were subjected to electrophoresis on a 10% (w/v) polyacrylamide gel. The blotted protein was saturated by submersion in 5% (w/v) non-fat skimmed milk in PBS (pH 7.4) with 0.1% (v/v) Tween 20 for 1 h at room temperature. The membranes were sequentially and individually incubated with anti-AT1 receptor rabbit polyclonal antibodies (Santa Cruz Biotechnology) (1 : 200), anti-AT2 receptor goat polyclonal antibodies (Santa Cruz Biotechnology) (1 : 300), anti-Angiotensiongen rabbit polyclonal antibodies (1 : 20,000), anti-ACE goat polyclonal antibodies (Santa Cruz Biotechnology) (1 : 1300), anti-SGLT1 rabbit polyclonal antibodies (Abcam) (1 : 600) and anti-β-actin mouse polyclonal antibodies (Chemicon) (1 : 2500) overnight at 4°C. After being rinsed in PBS, the membranes were incubated with the following corresponding peroxidase-labelled secondary antibodies for 1 h at room temperature: anti-rabbit IgG antibody (Amersham) (1 : 1300), anti-goat IgG antibody (1 : 1,500) (Amersham) and anti-mouse IgG antibody (Amersham) (1 : 2500). The positive signal was revealed using ECL plus Western blotting detection reagent and autoradiography film (Amersham). The intensity of the bands was quantified using FluorChem™ software.
D-Glucose and L-leucine uptake
Mucosal glucose and l-leucine uptake was measured by the everted sleeve technique (Karasov & Diamond, 1983), a procedure that has previously been fully validated, including the concentration dependency of glucose uptake which showed saturation of the uptake process at 50 mm glucose (Debnam et al. 1988). Although the affinity of SGLT1 for glucose uptake by isolated brush border membrane is < 1mM, all reported work using intact intestine to measure uptake of the sugar shows a much lower affinity for SGLT1-glucose binding (Debnam et al. 1988; Kellett, 2001). Jejunal and ileal segments were rinsed with cold saline containing trasylol (5000 kIU ml−1) and ‘Complete protease inhibitor’ (GE Healthcare) 1 pellet/500 ml saline and everted over a glass rod. The tissue was securely tied to the end of the rod and preincubated in gassed bicarbonate buffer (mm: NaCl 128, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2 and NaHCO3 20) for 2 min at 37°C followed by 4 min in the same buffer to which AII (0.1–100 nm; Bachem) was added. The tissue was transferred to fresh buffer containing 50 mm d-glucose and d-[14C]glucose (0.2 μCi ml−1) (GE Healthcare) with trace amounts of l-[3H]glucose (0.1 μCi ml−1) (Sigma-Aldrich, USA) to correct for non-specific uptake. The buffer contained AII with or without the AT1 receptor antagonist losartan (1 μm) (Merck, NJ, USA) or 0.3 mm phlorizin (Sigma-Aldrich, USA). After 2 min, the uptake process was stopped by the addition of ice-cold buffer containing 0.3 mm phlorizin. The tissue was removed from the rod, oven-dried and weighed. The dried residue was dissolved by incubation with 1 ml Soluene-350 (Perkin-Elmer, USA) per 50 mg of tissue at 60°C for 4 h. After the tissue solution had cooled to room temperature, 9 ml of Ultima Gold scintillation fluid (Perkin-Elmer, USA) per ml of soluene used was added. A similar procedure was used to measure uptake of l-[4,5-3H]leucine (5 mm, 0.4 μCi ml−1). Glucose and leucine uptake values were calculated as pmoles per milligram dry weight per second.
Statistical analysis
All results were analysed by Prism 3.0 software. The data are expressed as means ±s.e.m. The absence of vertical bars on bar charts indicates s.e.m. that are too small to be visible. Student's unpaired two-tailed t test and one-way analysis of variance (ANOVA) were used to detect significant differences between two groups and three or more groups, respectively. For all comparisons, P < 0.05 was considered statistically significant. For RT-PCR, the CT value of the target gene of a sample was first corrected for the CT value of β-actin, before being statistically analysed (Lau et al. 2004).
Results
Gene expression of RAS components
The real-time RT-PCR analysis results of mRNA expression of AT1 receptor, AT2 receptor, AO, and ACE normalized to β-actin from the jejunum and ileum are shown in Table 2. Enterocyte mRNA expression of AT1, AT2 and AO was 3.61-, 3.25- and 3.31-fold, respectively, greater in ileum than in jejunum (P < 0.01 in all cases). In contrast, expression of ACE mRNA in ileum was 16% of that in jejunum (P < 0.01).
Table 2.
Relative expression of RAS component mRNAs in enterocytes isolated from rat jejunum and ileum as determined by real-time RT-PCR
| Gene | CTa | ΔCTb | ΔΔCTc | Expression relative to jejunum (fold change)d |
|---|---|---|---|---|
| Jejunum | ||||
| β-actin | 16.13 ± 0.03 | — | — | — |
| AT1 | 25.31 ± 0.02 | 9.18 ± 0.05 | — | — |
| AT2 | 26.37 ± 0.01 | 10.24 ± 0.04 | — | — |
| AO | 26.99 ± 0.02 | 10.86 ± 0.05 | — | — |
| ACE | 18.58 ± 0.02 | 2.45 ± 0.05 | — | — |
| Distal Ileum | ||||
| β-actin | 15.60 ± 0.04 | — | — | — |
| AT1 | 22.93 ± 0.02 | 7.33 ± 0.06 | −1.85 ± 0.11 | 3.61 |
| AT2 | 24.14 ± 0.01 | 8.54 ± 0.05 | −1.7 ± 0.09 | 3.25 |
| AO | 24.73 ± 0.02 | 9.13 ± 0.03 | −1.73 ± 0.08 | 3.31 |
| ACE | 20.68 ± 0.01 | 5.08 ± 0.05 | 2.68 ± 0.10 | 0.16 |
The average CT data for each sample
The ΔCT value is calculated by subtraction of the β-actin CT from each sample CT
The ΔΔCT value is calculated by subtraction of the control ΔCT from each sample ΔCT
The expression relative to control is calculated using the expression 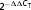 .
.
Western blotting
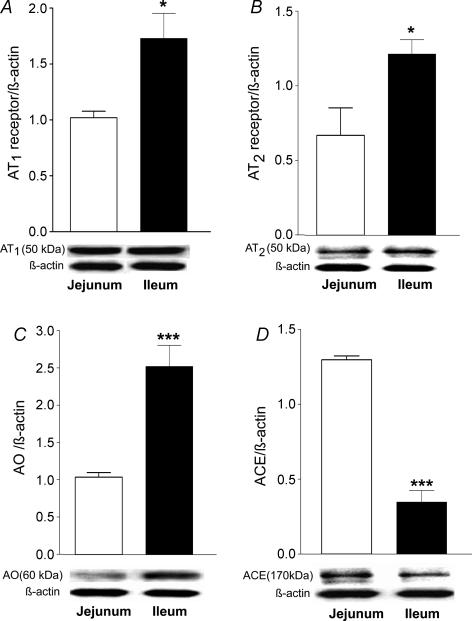
Western blotting of protein derived from isolated enterocytes revealed the presence of AT1 and AT2 receptors, AO and ACE proteins in both jejunum and ileum (Fig. 1). Levels of AT1 and AT2 receptor and AO protein in ileal enterocytes were some 0.7-, 0.9- and 1.4-fold, respectively, higher than in jejunal cells (Fig. 1A–C). However, jejunal expression of ACE was 3.5-fold higher than in ileum (Fig. 1D).
Figure 1. Western blot analysis showing the expression of AT1 receptor (A), AT2 receptor (B), angiotensinogen (C) and ACE (D) in homogenates prepared from jejunal or ileal enterocytes.
Results are expressed as means ±s.e.m.A, n= 5, *P < 0.05 versus jejunum; B, n= 5, *P < 0.05 versus jejunum; C, n= 5, ***P < 0.001 versus jejunum; D, n= 5, ***P < 0.001 versus jejunum.
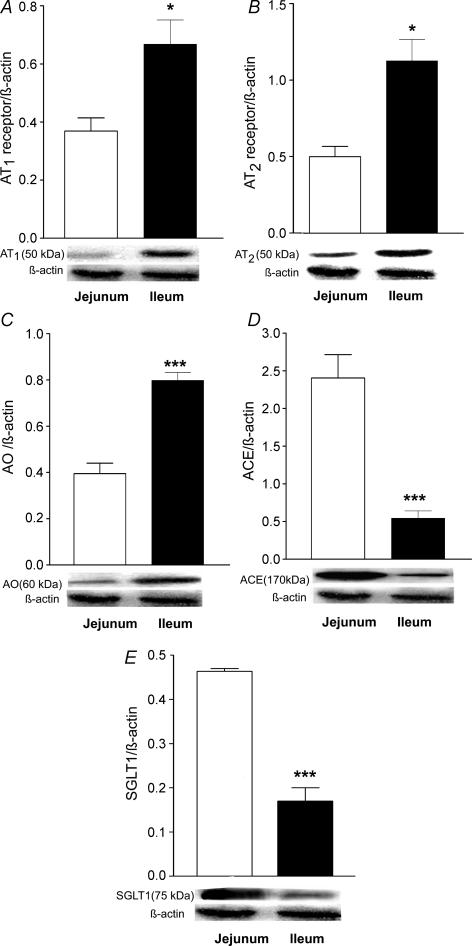
RAS proteins were also detected in isolated BBM (Fig. 2). In keeping with the results from isolated enterocytes, levels of AT1 and AT2 receptors and of AO in ileal BBM all showed an approximate doubling of those seen in jejunal BBM (Fig. 2A–C). Expression of ACE in ileal BBM was significantly lower than in jejunal BBM (Fig. 2D). The expression of SGLT1 protein was markedly lower in ileal compared to jejunal BBM (Fig. 2E).
Figure 2. Western blot analysis showing the expression of AT1 receptors (A), AT2 receptors (B), angiotensinogen (C), ACE (D), and SGLT1 (E) in BBM prepared from jejunal and ileal mucosa.
A, n= 6, *P < 0.05 versus jejunum; B, n= 5, *P < 0.05 versus jejunum; C, n= 5, ***P < 0.001 versus jejunum; D, n= 6, ***P < 0.001 versus jejunum; E, n= 5, ***P < 0.001 versus jejunum.
Villus localization of RAS components
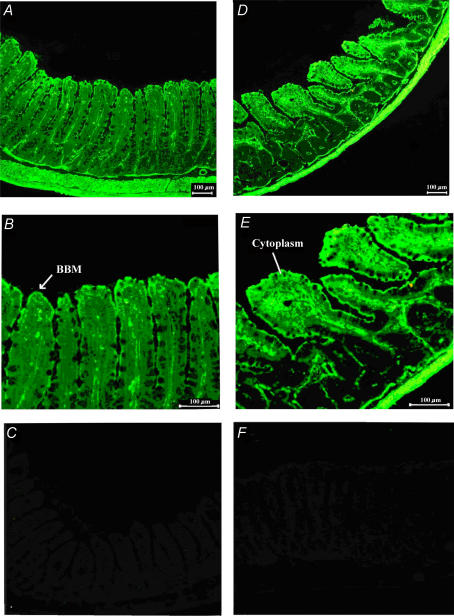
Immunocytochemistry revealed the presence of AT1 receptors along the entire villus length at both brush border and basolateral membranes (Fig. 3A and B). BBM expression of the AT1 receptor was more pronounced than at the basolateral membrane. AT1 receptor was also expressed in the lamina propria, muscularis mucosa, muscle layers and submucosal blood vessels. AO was expressed at the BBM (Fig. 3D and E), but had a relatively diffuse cytosolic expression. AO was also expressed in the lamina propria, the muscularis mucosa, the muscle layer and submucosal blood vessels. Figure 3C and F depict the negative controls of AT1 receptor and AO immunolabelling experiments, respectively, in which no primary antibodies were added.
Figure 3. Immunodetection of AT1 receptors and angiotensinogen in rat jejunum.
A and B, immunodetection of AT1 receptors; D and E, immunodetection of angiotensinogen. AT1 is highly expressed at the BBM (B); angiotensinogen is highly expressed in the cytoplasm in addition to the BBM (E). Control slides were prepared using preabsorption of antibody by the antigens for AT1 receptor (C) and angiotensinogen (F).
Effects of AII on glucose and leucine uptake
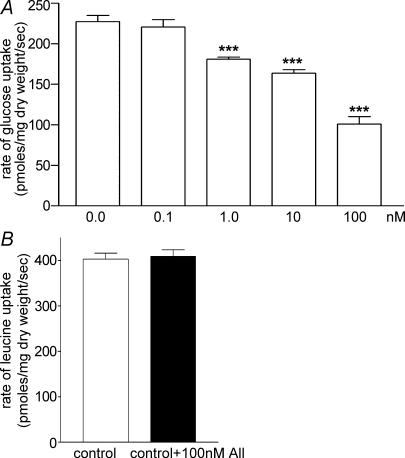
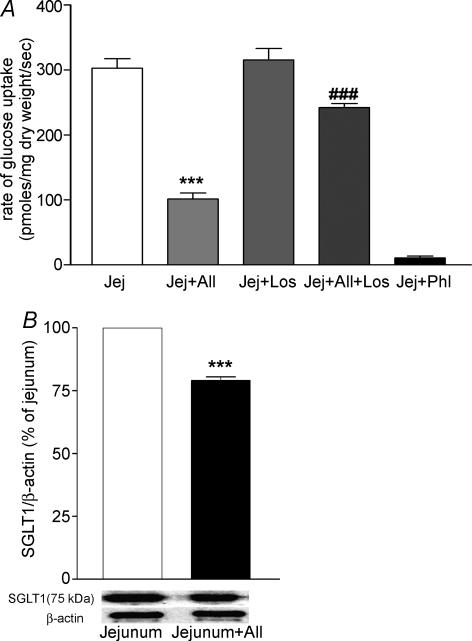
Addition of AII to mucosal fluid caused a dose-dependent inhibition of jejunal glucose uptake (Fig. 4A) which became significant at a concentration of 1 nm. At the maximum concentration of AII used (100 nm), glucose uptake was decreased from 225 to 90 pmol glucose (mg dry weight)−1 s−1, a reduction of about 60%. Pretreatment of the tissue with 1 μm losartan, a specific AT1 receptor antagonist, did not affect jejunal glucose uptake in the absence of AII, but abolished the inhibitory effect of 100 nm AII (Fig. 5A). Phlorizin (0.3 mm) in the mucosal fluid almost completely inhibited glucose uptake. Western blotting of BBM samples revealed that jejunal exposure to 100 nm AII for 4 min reduced the expression of SGLT1 (Fig. 5B). Despite these clear effects on glucose uptake, AII (1 μm) did not affect l-leucine uptake (Fig. 4B).
Figure 4. Effects of AII on glucose and leucine uptake in jejunum.
A, the AII effect on jejunal glucose uptake is dose dependent. Data are given as means +s.e.m., n= 5 for each concentration of AII. At 1 nm AII, ***P < 0.001 versus control; at 10 nm, ***P < 0.001 versus control; and at 100 nm, ***P < 0.001 versus control. B, AII (100 nm) did not affect l-leucine uptake; n= 5 per group, P > 0.05 versus no AII.
Figure 5. Effects of losartan on jejunal glucose uptake (A) and SGLT1 expression of jejunal BBM in response to AII (B).
A, AII reduced jejunal glucose uptake and this decrease was attenuated in the presence of Losartan. Jej (Jejunum + 0 nm AII), Jej + AII (Jejunum + 100 nm AII), Jej + los (Jejunum + 1 μm Losartan), Jej + AII + los (Jejunum + 100 nm AII + 1 μm Losartan), Jej + Phl (Jejunum + 0.3 mm phlorizin). Results are means +s.e.m., n= 5; ***P < 0.001 versus Jej group and ###P < 0.001 versus Jej + AII. B, Western blot analysis showing relative expression of SGLT1 in jejunal BBM. Jej (jejunum), Jej + AII (Jejunum + 100 nm AII). Results are given as means +s.e.m., n= 5; ***P < 0.001 versus Jej group.
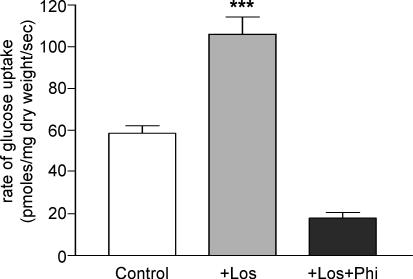
Consistent with our finding of lower SGLT1 expression in ileum than jejunum (Fig. 2E), a reduced rate of ileal glucose uptake was observed relative to jejunum (Fig. 6). Interestingly, in contrast to its effect in jejunum, the addition of 1 μm losartan alone to mucosal fluid resulted in a doubling of ileal glucose uptake, an action that was blocked by 0.3 mm phlorizin.
Figure 6. Losartan (1 μM) increased ileal glucose uptake and this increase was blocked in the presence of phlorizin (0.3 mM).
Control (Ileum), + los (Control + 1 μm Losartan), + los + Phl (Control + 1 μm Losartan + 0.3 mm phlorizin). Results are means +s.e.m., n= 5; ***P < 0.001 versus Control group.
Discussion
Intestinal glucose transport is a highly regulated process that is altered by both systemic and luminal stimuli. Adaptation can occur very rapidly, over a timeframe of minutes to hours (Karasov & Debnam, 1987), and may modulate transport at the BBM or basolateral membrane or at both loci. Changes in glucose uptake may be a consequence of alterations in levels or activities of membrane transporters and, in the case of SGLT1, an altered BBM electrochemical gradient. Established systemic influences include stimulatory or inhibitory actions of endocrine hormones, e.g. insulin (Pennington et al. 1994), pancreatic glucagon (Debnam & Sharp, 1993), GIP (Cheeseman & Tsang, 1996), GLP-2 (Cheeseman, 1997; Maenz & Cheeseman, 1986) and CCK (Hirsh & Cheeseman, 1998), or the level of blood glucose (Maenz & Cheeseman, 1986; Karasov & Debnam, 1987). Glucose is also an important luminal stimulus for both SGLT1- and GLUT2-mediated transport at the BBM (Sharp et al. 1996; Debnam et al. 1998; Dyer et al. 2003), a response that serves to match uptake capacity to the fluctuations in luminal glucose level during the normal progression of carbohydrate digestion (Ferraris et al. 1990). Less information is available concerning local control of glucose transport. Mucosal release of the proinflammatory interleukins IL-6, IL-1α, and IL-8 may promote glucose absorption by an action at the serosal side of the enterocyte (Hardin et al. 2000); epidermal growth factor enhances SGLT1-mediated glucose uptake (Bird et al. 1996).
Gene and protein detection techniques used in our study provide unequivocal evidence for enterocyte expression of an RAS system; the intestinal epithelium should now be added to the growing list of tissues that express a RAS. Experiments further revealed that AII, the final RAS product, modulates glucose uptake across the BBM. Taken together, the data provide the first evidence for autocrine regulation of intestinal glucose transport. Previous studies also localized ACE to the BBM (Stevens et al. 1988; Naim, 1992), and a particularly high expression of the enzyme in upper small intestine suggested an involvement in BBM dipeptidase activity (Yoshioka et al. 1987). However, the fact that intestinal ACE has the pharmacological and kinetic properties of vascular ACE (Stevens et al. 1988) implies a more specific role in enterocyte function. The expression of both angiotensinogen and ACE at the enterocyte BBM suggests that these proteins have a role in transport function.
The addition of AII to rat jejunum mucosal fluid rapidly (within 4 min) almost completely blocked glucose uptake. The fact that glucose uptake in the absence of AII was inhibited by phlorizin indicates that the peptide blocks SGLT1-mediated uptake. As shown in Fig. 5, the inhibitory effect of AII on SGLT1 expression at the BBM was relatively small (∼20%) compared to its effect on glucose uptake, an observation that might be due to reduced activity of SGLT1 protein at the BBM or to the fact that the blotting procedure detected SGLT1 that was bound to the inner surface of the membrane and would therefore not be detected in uptake studies. Further studies will be needed to elucidate the reason for the discrepancy between uptake and SGLT1 expression. However, it is of interest that the inhibitory effect was specific for glucose since under the same experimental conditions l-leucine uptake was unaffected by AII. In contrast to that occurring in isolated small intestine, glucose movement across the BBM in vivo involves both SGLT1 and GLUT2 proteins (Kellett, 2001). The action of AII on GLUT2-mediated glucose transport is therefore an important area for future study.
Previous work has reported changes in intestinal glucose transport over a similar time course to that observed following AII reported here. For example, leptin inhibits glucose uptake by rat jejunal mucosa within 2–3 min (Ducroc et al. 2005), whilst cAMP (Stumpel et al. 1998), AMP (Kimura et al. 2005), PGE2 (Scholtka et al. 1999) and enteric-derived glucagon (Stumpel et al. 1998) all stimulate phlorizin-sensitive glucose transport within 5 min of exposure to these agents. These very rapid changes in glucose transport are likely to be the result of alterations in membrane insertion of SGLT1 from a submembrane storage compartment. Changes in the electrochemical driving force for Na+–glucose movement across the BBM can be excluded since uptake of l-leucine was unaffected by AII.
The detection of both AT1 and AT2 receptor subtypes at the BBM in this intestinal region extends the conclusions of a previous autoradiographic study in which AII binding to jejunal mucosa was blocked by both AT1 and AT2 receptor antagonists (Sechi et al. 1993). The ability of losartan to block the inhibitory effect of AII on jejunal glucose uptake indicates that the inhibition was mediated by AT1 receptors. The lack of an AT1 receptor blockade effect alone indicates that there is negligible basal release of AII at the jejunal BBM.
Glucose uptake across the renal proximal tubule BBM is strikingly similar to that seen in enterocytes. Interestingly, it has been reported that AII decreases SGLT-mediated glucose uptake by proximal tubule cells albeit with a different time scale than that observed in small intestine (Han et al. 2004), an AT1 receptor-mediated pathway being responsible for this action. The fact that proximal tubule cells can synthesize and secrete AII into tubular fluid (Wang et al. 2003) further suggests a transport action of locally secreted AII. Increased glucose uptake across proximal tubule cells in experimental Type 1 diabetes mellitus has been linked to reduced AII receptor expression in these cells (Cheng et al. 1994), a finding that raises the issue of whether AII is involved in the enhanced SGLT1-mediated glucose transport that is a feature of diabetic small intestine (Debnam et al. 1995).
As expected, the rate of ileal glucose uptake was lower than in jejunum, a likely consequence of the dependence of uptake capacity on local luminal levels of the sugar (Debnam et al. 1998). However, unlike the jejunum, addition of losartan alone to the ileal mucosal fluid increased glucose uptake implying a basal luminal secretion of AII in the distal small intestine. This feature may be linked to our finding of higher ileal expression of angiotensinogen and AT1 receptor. The relevance of these observations for normal ileal absorptive function is unclear at the present time.
The cellular events that link AT1 receptor stimulation to suppression of jejunal glucose uptake are unknown. The AT1 receptor-mediated effects of AII in many peripheral tissues involve reduced activity of the protein kinase A (PKA) signalling pathway (Glossman et al. 1974). Moreover, levels of AII that cause an AT1 receptor mediated reduction in fluid transport across rat jejunum are associated with decreased epithelial production of cAMP (Jin et al. 1998). Studies have shown that SGLT1 activity is dependent in part on PKA signalling (Wright et al. 1997) and that cAMP promotes SGLT1-mediated glucose uptake in rat small intestine (Sharp & Debnam, 1994; Williams & Sharp, 2002). It is possible therefore that interruption of the PKA signalling pathway may be responsible, at least in part, for the inhibitory effects of AII on SGLT1-mediated glucose transport.
The concentration of AII used in our uptake studies was chosen on the basis of the AII dose–response relationship shown in Fig. 4. An obvious question to be addressed is the process by which low levels of locally produced AII escape degradation at the BBM, bearing in mind the high protease activity that is crucial to the digestive function of this membrane. Degradation of AII may be in keeping with the transport function of the peptide in that a short half-life would permit rapid changes in SGLT1 level in response to luminal or blood-borne signals. It is also possible that degraded fragments of biologically active peptides may retain the receptor binding properties of the parent molecule. Recent studies indicate that addition of leptin to mucosal buffer also acutely inhibits SGLT1-mediated glucose uptake in rat jejunum in vitro (Ducroc et al. 2005) and in vivo (Iñigo et al. 2007), an effect that is likely to involve BBM leptin receptors (Barrenetxe et al. 2002) and cellular signalling pathways (Barrenetxe et al. 2004). The response to leptin is intriguing, not only because it is another example of control of enterocyte glucose transport by a luminal peptide with an established circulatory role, but also because it implies that like AII, leptin must also be resistant to digestive enzyme action.
In conclusion, our work indicates that enterocytes are able to synthesize AII. It is likely that binding of the peptide to AT1 receptors at the BBM initiates cellular responses that culminate in the rapid inhibition of SGLT1-mediated glucose uptake. Future studies are needed to determine the physiological role of this autocrine control of glucose uptake and the cellular signalling pathways that mediate the transport response.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Competitive Earmarked Research Grant from the Research Grants Council of Hong Kong (Ref. no. CUHK4537/05 m), awarded to PS Leung, and The Royal Society's International Joint Project (Ref. no. 2005/R3-JP), awarded to ES Debnam and PS Leung. Losartan was generously provided by Merck, Whitehouse Station, New Jersey.
References
- Barrenetxe J, Sainz N, Barber A, Lostao MP. Involvement of PKC and PKA in the inhibitory effect of leptin on intestinal galactose absorption. Biochem Biophys Res Commun. 2004;317:717–721. doi: 10.1016/j.bbrc.2004.03.106. [DOI] [PubMed] [Google Scholar]
- Barrenetxe J, Villaro AC, Guembe L, Pascual I, Muñoz-Navas M, Barber A, Lostao MP. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut. 2002;50:797–802. doi: 10.1136/gut.50.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AR, Croom WJ, Jr, Fan YK, Black BL, McBride BW, Taylor IL. Peptide regulation of intestinal glucose absorption. J Anim Sci. 1996;74:2523–2540. doi: 10.2527/1996.74102523x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheeseman C. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1965–R1971. doi: 10.1152/ajpregu.1997.273.6.R1965. [DOI] [PubMed] [Google Scholar]
- Cheeseman CI, Tsang R. The effect of GIP and glucagon-like peptides on intestinal basolateral membrane hexose transport. Am J Physiol Gastrointest Liver Physiol. 1996;271:G477–G482. doi: 10.1152/ajpgi.1996.271.3.G477. [DOI] [PubMed] [Google Scholar]
- Cheng HF, Burns KD, Harris RC. Reduced proximal tubule angiotensin II receptor expression in streptozotocininduced diabetes mellitus. Kidney Int. 1994;46:1603–1610. doi: 10.1038/ki.1994.458. [DOI] [PubMed] [Google Scholar]
- Chu KY, Leung PS. Angiotensin II type 1 receptor antagonism mediates uncoupling protein 2-driven oxidative stress and ameliorates pancreatic islet β-cell function in young type 2 diabetic mice. Antioxid Redox Signal. 2007;9:869–878. doi: 10.1089/ars.2007.1590. [DOI] [PubMed] [Google Scholar]
- Cox HM, Munday KA, Poat JA. Identification of selective, high affinity [125I]-angiotensin and [125I]-bradykinin binding sites in rat intestinal epithelia. Br J Pharmacol. 1986;87:201–209. doi: 10.1111/j.1476-5381.1986.tb10172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam ES, Denholm EE, Grimble GK. Acute and chronic exposure of rat intestinal mucosa to dextran promotes SGLT1-mediated glucose transport. Eur J Clin Invest. 1998;28:651–658. doi: 10.1046/j.1365-2362.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Debnam ES, Karasov WH, Thompson CS. Nutrient uptake by rat enterocytes during diabetes mellitus: evidence for an increased sodium electrochemical gradient. J Physiol. 1988;397:503–512. doi: 10.1113/jphysiol.1988.sp017015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam ES, Sharp PA. Acute and chronic effects of pancreatic glucagon on sugar transport across the brush-border and basolateral membranes of rat jejunal enterocytes. Exp Physiol. 1993;78:197–207. doi: 10.1113/expphysiol.1993.sp003680. [DOI] [PubMed] [Google Scholar]
- Debnam ES, Smith MW, Sharp PA, Srai SK, Turvey A, Keable SJ. The effects of streptozotocin diabetes on sodiumglucose transporter (SGLT1) expression and function in rat jejunal and ileal villus-attached enterocytes. Pflugers Arch. 1995;430:151–159. doi: 10.1007/BF00374645. [DOI] [PubMed] [Google Scholar]
- Del Castillo JR. The use of hyperosmolar, intracellularlike solutions for the isolation of epithelial cells from guinea-pig small intestine. Biochim Biophys Acta. 1987;901:201–208. doi: 10.1016/0005-2736(87)90116-7. [DOI] [PubMed] [Google Scholar]
- Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes. 2005;54:348–354. doi: 10.2337/diabetes.54.2.348. [DOI] [PubMed] [Google Scholar]
- Dyer J, Vayro S, King TP, Shirazi-Beechey SP. Glucose sensing in the intestinal epithelium. Eur J Biochem. 2003;270:3377–3388. doi: 10.1046/j.1432-1033.2003.03721.x. [DOI] [PubMed] [Google Scholar]
- Erickson RH, Suzuki Y, Sedlmayer A, Song IS, Kim YS. Rat intestinal angiotensin-converting enzyme: purification, properties, expression, and function. Am J Physiol Gastrointest Liver Physiol. 1992;263:G466–G473. doi: 10.1152/ajpgi.1992.263.4.G466. [DOI] [PubMed] [Google Scholar]
- Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol Gastrointest Liver Physiol. 1990;259:G822–G837. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- Glossman H, Baukal A, Catt KJ. Angiotensin II receptors in bovine adrenal cortex: modification of angiotensin II binding by guanine nucleotides. J Biol Chem. 1974;249:664–666. [PubMed] [Google Scholar]
- Han HJ, Park SH, Lee YJ. Signaling cascade of ANG II-induced inhibition of α-MG uptake in renal proximal tubule cells. Am J Physiol Renal Physiol. 2004;286:F634–F642. doi: 10.1152/ajprenal.00217.2003. [DOI] [PubMed] [Google Scholar]
- Hardin J, Kroeker K, Chung B, Gall DG. Effect of proinflammatory interleukins on jejunal nutrient transport. Gut. 2000;47:184–191. doi: 10.1136/gut.47.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh AJ, Cheeseman CI. Cholecystokinin decreases intestinal hexose absorption by a parallel reduction in SGLT1 abundance in the brush border membrane. J Biol Chem. 1998;273:14545–14549. doi: 10.1074/jbc.273.23.14545. [DOI] [PubMed] [Google Scholar]
- Iñigo C, Patel N, Kellett GL, Barber A, Lostao MP. Luminal leptin inhibits intestinal sugar absorption in vivo. Acta Physiol. 2007;190:303–310. doi: 10.1111/j.1748-1716.2007.01707.x. [DOI] [PubMed] [Google Scholar]
- Ip SP, Wong TP, Tsai SJ, Leung PS. The recovery of some components of the renin angiotensin system in the rat pancreas after chronic exposure to hypoxic condition. J Mol Endocrinol. 2003;31:563–571. doi: 10.1677/jme.0.0310563. [DOI] [PubMed] [Google Scholar]
- Jin XH, Wang ZQ, Siragy HM, Guerrant RL, Carey RM. Regulation of jejunal sodium and water absorption by angiotensin subtype receptors. Am J Physiol Regul Integr Comp Physiol. 1998;275:R515–R523. doi: 10.1152/ajpregu.1998.275.2.R515. [DOI] [PubMed] [Google Scholar]
- Karasov WH, Debnam ES. Rapid adaptation of intestinal glucose transport: a brush-border or basolateral phenomenon? Am J Physiol Gastrointest Liver Physiol. 1987;253:G54–G61. doi: 10.1152/ajpgi.1987.253.1.G54. [DOI] [PubMed] [Google Scholar]
- Karasov WH, Diamond JM. A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol. 1983;152:105–116. [Google Scholar]
- Kawano K, Ikari A, Nakano M, Suketa Y. Phosphatidylinositol 3-kinase mediates inhibitory effect of angiotensin II on sodium/glucose cotransporter in renal epithelial cells. Life Sci. 2002;71:1–13. doi: 10.1016/s0024-3205(02)01573-4. [DOI] [PubMed] [Google Scholar]
- Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Acuto O, Storelli C, Murer H, Müller M, Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes: Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978;506:136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Turner JR, Braasch DA, Buddington RK. Lumenal adenosine and AMP rapidly increase glucose transport by intact small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1007–G1014. doi: 10.1152/ajpgi.00085.2005. [DOI] [PubMed] [Google Scholar]
- Lau T, Carlsson PO, Leung PS. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004;47:240–248. doi: 10.1007/s00125-003-1295-1. [DOI] [PubMed] [Google Scholar]
- Leung PS. The peptide hormone angiotensin II: its new functions in tissues and organs. Curr Protein Pept Sci. 2004;5:267–273. doi: 10.2174/1389203043379693. [DOI] [PubMed] [Google Scholar]
- Leung PS. The physiology of a local renin–angiotensin system in the pancreas. J Physiol. 2007;580:31–37. doi: 10.1113/jphysiol.2006.126193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung PS, Chan WP, Nobiling R. Regulated expression of pancreatic renin-angiotensin system in experimental pancreatitis. Mol Cell Endocrinol. 2000;166:121–128. doi: 10.1016/s0303-7207(00)00275-6. [DOI] [PubMed] [Google Scholar]
- Leung PS, Suen PM, Ip SP, Yip CK, Chen G, Lai BS. Expression and localization of AT1 receptors in hepatic Kupffer cells: its potential role in regulating a fibrogenic response. Regul Pept. 2003;116:61–69. doi: 10.1016/s0167-0115(03)00192-7. [DOI] [PubMed] [Google Scholar]
- Maenz DD, Cheeseman CI. Effect of hyperglycaemia on d-glucose transport across the brush border and basolateral membrane of rat small intestine. Biochim Biophys Acta. 1986;860:277–285. doi: 10.1016/0005-2736(86)90524-9. [DOI] [PubMed] [Google Scholar]
- Naim HY. Angiotensin-converting enzyme of the human small intestine. Subunit and quaternary structure, biosynthesis and membrane association. Biochem J. 1992;286:451–457. doi: 10.1042/bj2860451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M, Poyan MA, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Pennington AM, Corpe CP, Kellett GL. Rapid regulation of rat jejunal glucose transport by insulin in a luminally and vascularly perfused preparation. J Physiol. 1994;478:187–193. doi: 10.1113/jphysiol.1994.sp020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtka B, Stumpel F, Jungermann K. Acute increase stimulated by prostaglandin E2 in glucose absorption via the sodium-dependent glucose transporter-1 in rat intestine. Gut. 1999;44:490–496. doi: 10.1136/gut.44.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi LA, Valentin JP, Griffin CA, Schambelan M. Autoradiographic characterization of angiotensin II receptor subtypes in rat intestine. Am J Physiol Gastrointest Liver Physiol. 1993;265:G21–G27. doi: 10.1152/ajpgi.1993.265.1.G21. [DOI] [PubMed] [Google Scholar]
- Seo MS, Fukamizu A, Saito T, Murakami K. Identification of a previously unrecognized production site of human renin. Biochim Biophys Acta. 1991;1129:87–89. doi: 10.1016/0167-4781(91)90216-9. [DOI] [PubMed] [Google Scholar]
- Sharp PA, Debnam ES. The role of cAMP in the control of sugar transport across the brush border and basolateral membranes of rat jejunal enterocytes. Exp Physiol. 1994;79:203–214. doi: 10.1113/expphysiol.1994.sp003753. [DOI] [PubMed] [Google Scholar]
- Sharp PA, Debnam ES, Srai SK. Rapid enhancement of brush border glucose uptake following exposure of rat jejunal mucosa to glucose. Gut. 1996;39:545–550. doi: 10.1136/gut.39.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens BR, Fernandez A, Kneer C, Cerda JJ, Phillips MI, Woodward ER. Human intestinal brush border angiotensin-converting enzyme activity and its inhibition by antihypertensive Ramipril. Gastroenterology. 1988;94:942–947. doi: 10.1016/0016-5085(88)90551-3. [DOI] [PubMed] [Google Scholar]
- Stumpel F, Scholtka B, Hunger A, Jungermann K. Enteric glucagon rather than pancreatic glucagon stimulates glucose absorption in rat intestine. Gastroenterology. 1998;115:1163–1171. doi: 10.1016/s0016-5085(98)70087-3. [DOI] [PubMed] [Google Scholar]
- Sundaram U, Knickelbein RG, Dobbins JW. pH regulation in ileum: Na+-H+ and Cl−-HCO− exchange in isolated crypt and villus cells. Am J Physiol Gastrointest Liver Physiol. 1991;260:G440–G449. doi: 10.1152/ajpgi.1991.260.3.G440. [DOI] [PubMed] [Google Scholar]
- Wang CT, Navar LG, Mitchell KD. Proximal tubular fluid angiotensin II levels in angiotensin II-induced hypertensive rats. J Hypertens. 2003;21:353–360. doi: 10.1097/00004872-200302000-00027. [DOI] [PubMed] [Google Scholar]
- Williams M, Sharp P. Regulation of jejunal glucose transporter expression by forskolin. Biochim Biophys Acta. 2002;1559:179–185. doi: 10.1016/s0005-2736(01)00449-7. [DOI] [PubMed] [Google Scholar]
- Wright EM, Hirsch JR, Loo DD, Zampighi GA. Regulation of Na+/glucose cotransporters. J Exp Biol. 1997;200:287–293. doi: 10.1242/jeb.200.2.287. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Erickson RH, Woodley JF, Gulli R, Guan D, Kim YS. Role of rat intestinal brush-border membrane angiotensin-converting enzyme in dietary protein digestion. Am J Physiol Gastrointest Liver Physiol. 1987;253:G781–G786. doi: 10.1152/ajpgi.1987.253.6.G781. [DOI] [PubMed] [Google Scholar]