Abstract
The mechanism for exercise hyperaemia is a century old enigma. Much of the research on the topic has focused on the factors controlling skeletal muscle blood flow during steady-state dynamic exercise. It is likely that the factors which initiate the increase in blood flow are distinct from those which sustain the elevated blood flow. There is now convincing evidence that there is rapid vasodilatation following release of muscle contraction. Metabolic, neural and acetylcholine spillover mechanisms do not appear to explain the initial dilatation. Heretofore there has been only circumstantial evidence regarding the role of potassium released by skeletal muscle fibres. Studies which interrupt potassium-mediated dilatation are just emerging and are not conclusive. In addition, the latency of the vascular smooth muscle response to potassium makes it desirable to identify a mechanism that does not rely on diffusion of a vasoactive agent. Compression of the intramuscular arterioles during contraction could activate a mechanosensitive response by the vascular smooth muscle and/or endothelium. Recent in vitro and in vivo data support the notion that brief periods of mechanical compression elicit rapid vasodilatation. Thus, vascular compression could represent a feedforward mechanism for initiating skeletal muscle vasodilatation at the onset of exercise.
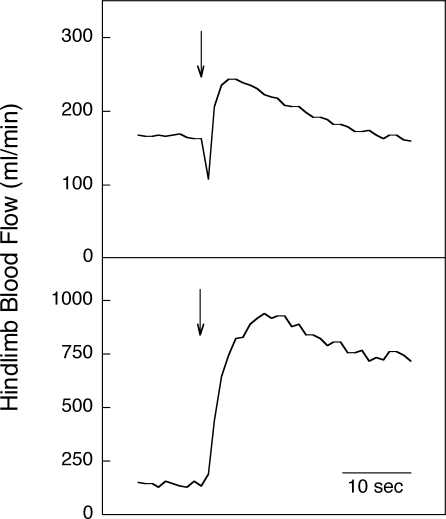
It has long been known that skeletal muscle blood flow increases in proportion to the metabolic demands of the tissue during steady-state dynamic exercise. However, little attention has been paid to the initial phase of increasing blood flow at the onset of exercise. The skeletal muscle blood flow response to contraction is rapid, increasing within the first second following release of a brief contraction in both human and animal models. This is demonstrated in Fig. 1 which shows prompt increases in hindlimb blood flow to a 1 s tetanic contraction in an anaesthetized dog (upper panel) and at the onset of mild dynamic exercise in a chronically instrumented dog (lower panel). Evaluation of the mechanism for the immediate increase in blood flow is facilitated in protocols employing a single contraction because there are no confounding effects of subsequent contractions on arterial impedance or venous filling and no systemic changes in arterial pressure with attendant reflex effects.
Figure 1.
Muscle blood flow response to a 1 s tetanic contraction (upper panel) and mild intensity dynamic exercise (lower panel) in dogs with perivascular flowprobes implanted around the iliac artery The initiation of contraction or exercise is denoted by the arrows. From Clifford & Hellsten (2004) with permission.
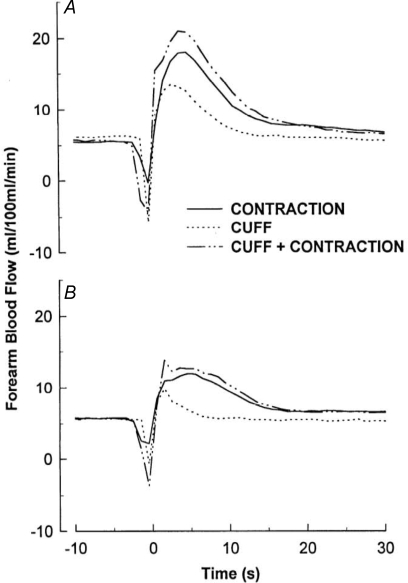
The earliest measurements of blood flow in contracting muscle were reported in this Journal well over a century ago. In anaesthetized dogs, Gaskell (1878) stimulated the motor nerve to produce a brief contraction and measured the venous outflow in a graduated cylinder over 5 s periods. As seen in Fig. 2, the initial expulsion of blood from the muscle was followed by a delayed increase in venous blood flow which peaked between 10 and 15 s. One hundred years later, Tschakovsky et al. (1996) made continuous measurements of the arterial inflow to contracting muscle in human volunteers. Figure 3 shows blood flow following a 1 s contraction with the arm below the heart (Fig. 3A) and with the arm above the heart (Fig. 3B). The rapid change in blood flow under both conditions was attributed to vasodilatation, and the difference between the two positions in the magnitude of increase was attributed to a muscle pump effect. In intact humans, it has been difficult to separate these two factors – vasodilatation versus the muscle pump. What was needed was direct measurement of arteriolar diameters.
Figure 2.
Venous outflow following a single muscular contraction Vertical lines represent 5 s intervals. From Gaskell (1878).
Figure 3.
Forearm blood flow responses to contraction and cuff inflation Forearm blood flow response averaged across 10 subjects to a single 1 s cuff inflation (CUFF), a single 1 s contraction (CONTRACTION), and a single contraction within a cuff inflation (CUFF + CONTRACTION) with arm below (A) and above (B) the heart. From Tschakovsky et al. (1996) with permission.
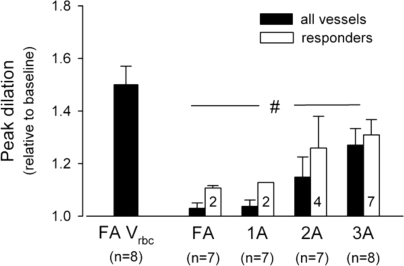
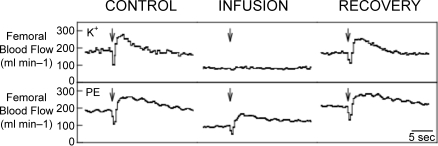
Until recently, direct observations of vessel diameter in situ have presented a confusing picture with latencies for vasodilatation ranging from 2 s (Marshall & Tandon, 1984) to 20 s (Gorczynski et al. 1978). These results were used by some researchers as evidence that instantaneous activation of the skeletal muscle pump must account for the initial increase in blood flow (Sheriff et al. 1993). However, recent data have provided unequivocal evidence of rapid vasodilatation. For hamster cremaster muscle stimulated at various frequencies and durations, Mihok & Murrant (2004) reported a significant dilation of transverse arterioles within 1 s following contraction (Fig. 4). VanTeeffelen & Segal, 2006) observed an immediate increase in red blood cell velocity at the level of the feed arteries following a single brief contraction of hamster retractor muscle. Furthermore, they observed rapid dilatation of arterioles with the incidence of dilatation increasing from proximal vessels to distal (Fig. 5). Seven out of eight terminal arterioles demonstrated an initial vasodilatation. Additional data supporting the idea of rapid vasodilatation come from experiments in which contractions were performed while changes in vascular smooth muscle membrane potential were prevented by raising the extracellular K+ concentration (Hamann et al. 2004a). Figure 6 shows the data from one such experiment in which a 1 s contraction elicited a marked increase in blood flow under control conditions, but not during intra-arterial infusion of K+. Infusion of the adrenergic agonist phenylephrine to achieve the same reduction in baseline blood flow failed to abolish the contraction-induced increase in blood flow. The most parsimonious explanation of these results is that muscle contractions elicit rapid dilatation of the intramuscular vasculature.
Figure 4.
Time course of the change in transverse arteriolar diameter frollowing a single contraction of 500 ms duration at a stimulus frequency of 60 Hz Modified from Mihok & Murrant (2004).
Figure 5.
Vasodilator responses to single tetanic contractions in hamster retractor muscle Data represent peak red blood cell velocity in the feed artery (FA) and peak dilatation in feed artery, first-order arteriole (1A), second-order arteriole (2A), and third order arteriole (3A). From VanTeeffelen & Segal (2006) with permission.
Figure 6.
Original tracings of the mean femoral blood flow response to a 1 s contraction of the hindlimb of one dog during the K+ and phenylephrine infusion PE, phenylephrine. Arrows indicate the start of contraction. Note the rapid and marked increase in blood flow for all the contractions except for K+ infusion. From Hamann et al. (2004a).
The mechanism for the rapid vasodilatation is not well understood. Potential mechanisms are listed in Table 1 and form the basis for the following discussion.
Table 1.
Possible mechanisms for rapid contraction-induced vasodilatation
| Metabolic |
| Neural |
| Acetylcholine spillover |
| Potassium |
| Mechanical |
Metabolic
There is substantial evidence for a close association between metabolic rate and blood flow during steady-state exercise. This fact has led to a perpetual search for the elusive metabolite which links the two. Over the years, a number of candidates have been identified (Clifford & Hellsten, 2004). However, the time required for diffusion of a vasoactive substance from the skeletal muscle myocyte to the vascular smooth muscle makes metabolic vasodilatation seem an unlikely explanation for the initial increase in blood flow.
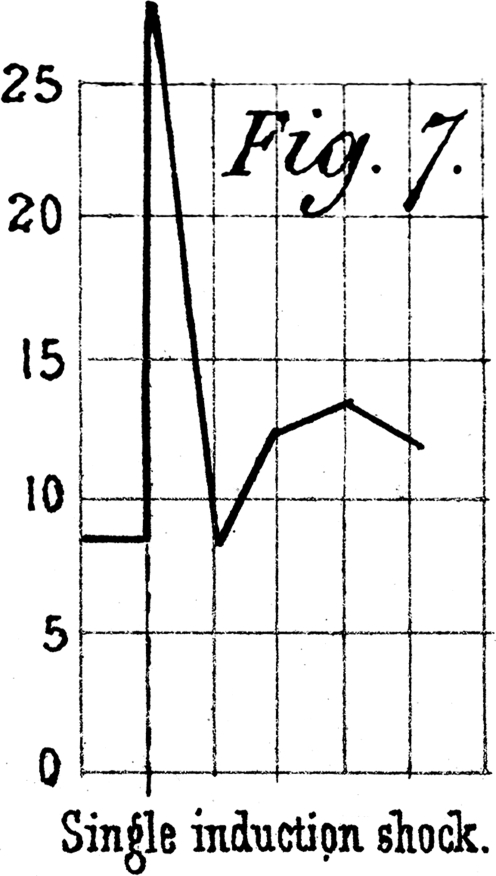
Neural
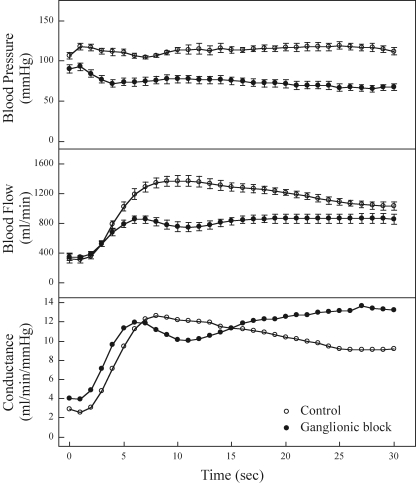
A neural mechanism seems appealing because of the fast transmission times for action potentials. Buckwalter & Clifford (1999) provided a direct test of this hypothesis by administration of a ganglionic blocker prior to the initiation of exercise. As shown in Fig. 7, absolute blood flows were lower during exercise after ganglionic blockade due to a lower perfusion pressure. But careful comparison of the changes in vascular conductance as an index of the magnitude of vasodilatation reveals that the rate of dilation is unaltered over the initial 5 s of exercise, indicating that there is no neural component to the initial vasodilatation.
Figure 7.
Haemodynamic responses averaged over 1 s intervals at the onset of exercise under control (saline) and ganglionic blockade (hexamethonium) conditions There were no statistically significant differences in the conductance response between the two treatments at 5, 10 and 15 s of exercise. However, the conductance values were significantly elevated with ganglionic blockade at 20, 25 and 30 s of exercise (P < 0.01). From Buckwalter & Clifford (1999) with permission.
ACh spillover
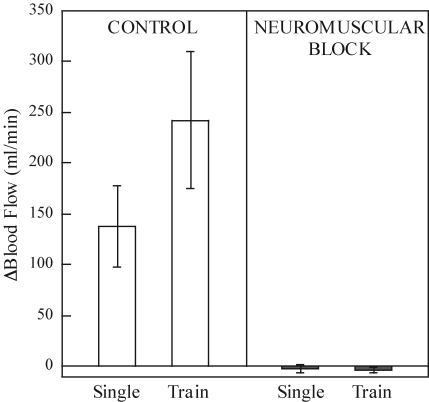
ACh spillover is the idea that ACh released by activation of the motor nerve spills over onto muscarinic receptors in the skeletal muscle vasculature, resulting in dilatation. This hypothesis, first proposed by Welsh & Segal (1997), is intriguing because it could explain both the promptness of the response and the close relationship of blood flow with force development. Two experimental approaches have been employed to test this hypothesis. One is to measure the blood flow response contraction after blockade of vascular muscarinic receptors. The second is to stimulate the motor nerve after blockade of nicotinic receptors at the neuromuscular junction. This would not alter the release of ACh, but would prevent muscle contractions. Administration of atropine in humans failed to change the blood flow response to a single contraction (Brock et al. 1998). Neuromuscular block abolished the increase in blood flow to a single contraction and to repeated contractions as shown in Fig. 8 (Naik et al. 1999; Clifford et al. 2000). The results of these experiments suggest that the ACh spillover mechanism does not explain rapid dilatation in the vasculature of large muscles.
Figure 8.
Summary of the blood flow response to sciatic nerve stimulation under control and neuromuscular blockade conditions Single refers to an individual 1 s sciatic nerve stimulation (30 Hz, 1 ms, 10× motor threshold). Train refers to a 30 s train of sciatic nerve of sciatic nerve stimulations (30 Hz, 1 ms, 10× motor threshold at 50% duty cycle). Following neuromuscular blockade the blood flow response to sciatic stimulation was completely abolished. From Naik et al. (1999) with permission.
Potassium
K+ is released through voltage-dependent K+ channels in active skeletal muscle fibres during the repolarization phase of the contraction/relaxation cycle and accumulates in the interstitial fluid surrounding the vasculature. Kjellmer's (1961) studies in the 1960s with a crude microdialysis technique substantiated the rise in interstitial [K+] with contraction. More recent and more elegant microdialysis studies confirm the elevation in interstitial [K+] with exercise (Green et al. 2000; Juel et al. 2000; Lott et al. 2001). Furthermore, the magnitude of elevation of [K+] is proportional to the duration (Hnik et al. 1976) and intensity (Green et al. 2000; Juel et al. 2000) of contractions. It is noteworthy that Hnik et al. (1976) demonstrated rapid accumulation of K+ in the interstitium following a single muscle contraction.
K+ has been considered a potential candidate for contraction-induced hyperaemia since Dawes (1941) observed vasodilatation in response to intra-arterial injection, although most of the evidence is circumstantial. Interestingly, the vascular response to elevated extracellular [K+] is bimodal, with smooth muscle hyperpolarization and dilation at low concentrations and depolarization and constriction at high concentrations. The response to high concentrations of K+ is predictable based on the Nernst equation. The deviation from the expected vasoconstrictor response at extracellular [K+] below ∼20 mm is the result of activation of inward rectifying K+ (Kir) channels (Knot et al. 1996; Sobey & Faraci, 2000) and/or the electrogenic Na+–K+ pump (Lombard & Stekiel, 1995; Burns et al. 2004).
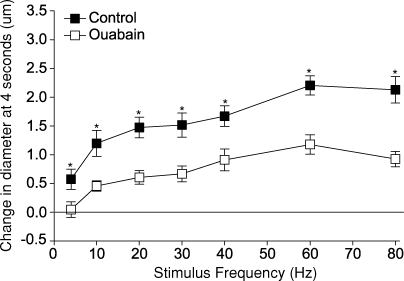
Studies which examine the involvement of K+ in exercise hyperaemia are just emerging. The release of K+ by skeletal muscle fibres and its potential effects on vascular diameter were studied in the hamster cremaster preparation (Armstrong et al. 2007). The initial dilation to contraction was attenuated by application of 3,5 diaminopyridine (to inhibit release of K+ from voltage-dependent K+ channels in skeletal muscle), barium chloride (to block Kir channels in smooth muscle), or ouabain (to inhibit the Na+–K+ pump in smooth muscle). The effect of ouabain is depicted in Fig. 9. In our laboratory, simultaneous administration of ouabain and barium chloride is required to abolish the response to infused K+ in the canine hindlimb. Preliminary data (Valic et al. 2006) show that the blood flow response to contraction persists despite the absence of a response to K+. In other words, it is possible to block the dilator response to K+ without abolishing the blood flow response to contraction. Thus, these preliminary data are not in accord with the findings of Armstrong et al. (2007).
Figure 9.
Effect of 10−4 M ouabain on the change in diameter of transverse arterioles at 4 s across all stimulus frequencies tested *Significantly different with ouabain. From Armstrong et al. (2007).
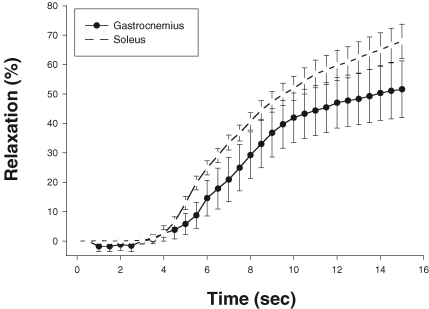
One issue that must be addressed is the time course of the vascular smooth muscle response to any vasodilator substance released from skeletal muscle. Wunsch et al. (2000) showed that there is almost a 4 s latency between delivery of K+ to gastrocnemius and soleus arterioles and the start of dilation (Fig. 10). The enigma of a 4 s latency and contraction-induced vasodilatation within 1 s provides impetus to search for a mechanism that does not rely on diffusion of a vasoactive agent.
Figure 10.
Time course of vasodilatation following application of 10 mM KCl at time 0 Data are presented as means ±s.e.m. No differences were detected between vasodilatory responses of soleus (open symbols) and gastrocnemius (filled symbols) arterioles. From Wunsch et al. (2000) with permission.
Mechanical
Intramuscular pressures of 270 mmHg have been recorded at moderate running speeds in humans (Ballard et al. 1998) and can reach 570 mmHg during maximal contraction (Sejersted et al. 1984). Using ultrasound methods, it can be observed that the arteries of the human forearm are compressed and deformed during forceful contractions (unpublished observations). Both smooth muscle cells and endothelial cells are known to be mechanosensitive. Smooth muscle cells alter myogenic tone in response to changes in intravascular pressure and endothelial cells cause dilatation in response to changes in intraluminal shear stress. Despite the widespread attention given to these mechanosensitive responses, little consideration has been given in recent years to a mechanical mechanism to explain vasodilatation during exercise.
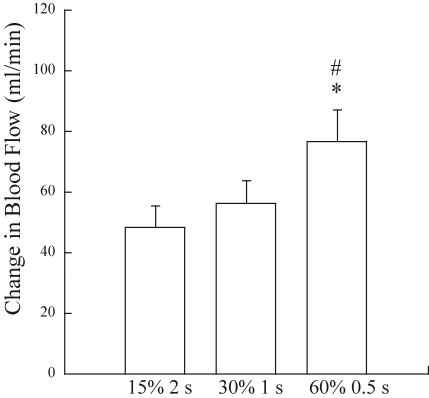
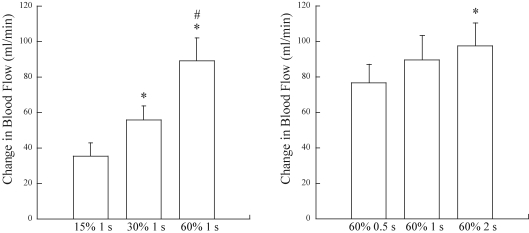
Our first hint about this mechanism came from data collected in human volunteers who performed single contractions at three different intensities (Hamann et al. 2004b). The duration of contraction was varied to maintain a constant time-tension integral. For static contractions, the time-tension integral is the equivalent of work. Notice in Fig. 11 that blood flow was highest for the highest force, that is, not solely dependent on the work performed. In Fig. 12 results from two sets of trials are juxtaposed; in both sets of trials there was a fourfold increase in work – in the left panel due to increased force and in the right panel due to increased duration at a constant force. Note that the increment in blood flow was greater under conditions which would have produced graded compression of the intramuscular vessels (left panel) than under conditions in which the amount of compression would be constant (right panel). These findings suggest that the degree of compression of the skeletal muscle vasculature contributes to the blood flow response to contraction. Two older studies bolster this argument. Mohrman & Sparks (1974) measured changes in intramuscular pressure and vascular conductance in the gastrocnemius muscle during contraction and inflation of a cuff placed around the muscle. After observing vasodilatation in response to muscle compression, they concluded that extravascular compression may play a role in causing exercise hyperaemia. Although most often cited for their negative results with very brief pulsatile changes in extravascular pressure of 10–50 mmHg, Bacchus et al. (1981) reported vasodilatation in gracilis muscle subjected to 1 s pulses when the pressure exceeded 100 mmHg.
Figure 11.
Peak blood flow responses to a brief contraction obtained at three intensities in which the time-tension integral was held constant Despite the lack of change in work performed, blood flow was augmented in relation with contraction intensity. From Hamann et al. (2004b) with permission.
Figure 12.
Effect of increases in time-tension integral Peak blood flow responses to a brief contraction obtained at three intensities in which there were graded increases in time-tension integral due to increased contraction intensity (left) and graded increases in time-tension integral due to increased contraction duration (right). From Hamann et al. (2004b) with permission.
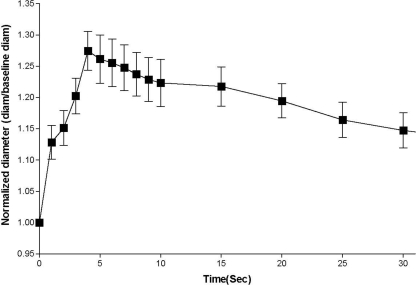
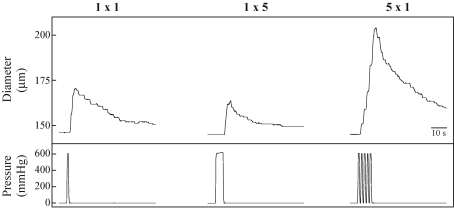
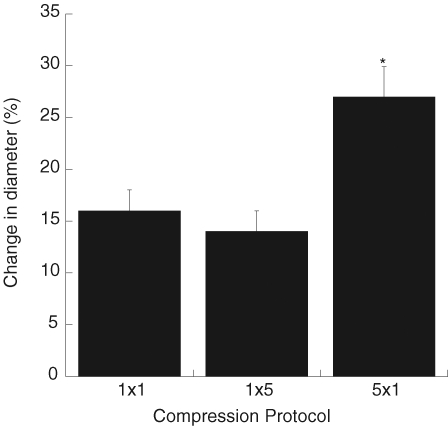
We recently performed studies to directly test the postulate that mechanical deformation of the skeletal muscle vasculature during contraction can elicit vasodilatation (Clifford et al. 2006). Rat soleus feed arteries were mounted on micropipettes in a chamber with luminal diameter measured using an inverted microscope. The design of the chamber allowed it to be sealed so that the extravascular pressure could be varied to mimic the compression due to muscle contraction. Pressure pulses of 600 mmHg were delivered for 1 s, 5 s, and as a series of five repeated 1 s pulses with 1 s between pulses. During application of external pressure the lumen of the artery was completely closed, but immediately following release of pressure, diameter was significantly increased. A typical example is shown in Fig. 13. As seen in this figure, the time course is remarkably similar to that for the change in blood flow after a brief muscle contraction. The magnitude of dilatation was not affected by increasing the duration of compression but was enhanced by increasing the number of compressions (Fig. 14). Removal of the endothelium reduced but did not abolish the dilatation to mechanical compression, thus indicating that the dilatation is mediated by both endothelium-dependent and independent signalling pathways. These data provide evidence that mechanical deformation of the vascular wall during contraction can elicit dilation of intramuscular arteries and arterioles.
Figure 13.
Response of a single soleus feed artery to external pressure 1 × 1 signifies one pressure pulse of 1 s duration. 1 × 5 signifies one pressure pulse of 5 s duration. 5 × 1 signifies five separate 1 s pulses with 1 s between each pulse. Diameters were tracked manually by moving a cursor on the video screen. From Clifford et al. (2006).
Figure 14.
Peak dilatation produced by compression of soleus feed arteries (n = 6) 1 × 1 signifies one pressure pulse of 1 s duration. 1 × 5 signifies one pressure pulse of 5 s duration. 5 × 1 signifies five separate 1 s pulses with 1 s between each pulse. *P < 0.01 compared to 1 × 1 and 1 × 5. From Clifford et al. (2006).
Recent data from Kirby et al. (2007) provide proof of principle in human subjects. Brief increases in extravascular pressure, achieved by rapid inflation of a custom-designed cuff around the forearm, increased brachial artery blood flow and vascular conductance by as much as 185%. Furthermore, a dose–response relationship was observed over a range of cuff pressures between 25 and 100 mmHg. Taken together, the in vitro and in vivo data make a plausible case that mechanical compression could be involved in initiating skeletal muscle vasodilatation at the onset of exercise.
Much more work needs to be done to assess the contribution of mechanical compression to the overall picture of exercise hyperaemia. Our in vitro studies were performed at a single extravascular pressure designed to mimic the maximum pressure that would be encountered. What is the pressure threshold for eliciting dilatation? The initial studies were performed in feed arteries. Is there a differential sensitivity of different vessel generations? The in vitro data show that there is a greater response to multiple compressions. As exercise continues beyond a single contraction, does this mechanism still contribute to the observed hyperaemia? Finally, the signalling mechanisms within the vascular smooth muscle and endothelium remain to be elucidated. Despite these limitations to our current level of understanding, it appears that vascular compression could represent a feedforward mechanism for initiating skeletal muscle vasodilatation at the onset of exercise.
Acknowledgments
The author gratefully acknowledges the indispensable contributions of colleagues and research support from the National Heart Lung and Blood Institute.
References
- Armstrong ML, Dua A, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchus A, Gamble G, Anderson D, Scott J. Role of the myogenic response in exercise hyperemia. Microvasc Res. 1981;21:92–102. doi: 10.1016/0026-2862(81)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard RE, Watenpaugh DE, Breit GA, Murthy G, Holley DC, Hargens AR. Leg intramuscular pressures during locomotion in humans. J Appl Physiol. 1998;84:1976–1981. doi: 10.1152/jappl.1998.84.6.1976. [DOI] [PubMed] [Google Scholar]
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol. 1998;85:2249–2254. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. Autonomic control of skeletal muscle blood flow at the onset of exercise. Am J Physiol Heart Circ Physiol. 1999;277:H1872–H1877. doi: 10.1152/ajpheart.1999.277.5.H1872. [DOI] [PubMed] [Google Scholar]
- Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11:279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572:561–567. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Valic Z, Naik JS, Buckwalter JB. Effect of vecuronium on the release of acetylcholine after nerve stimulation. J Appl Physiol. 2000;89:1249–1251. [PubMed] [Google Scholar]
- Dawes GS. The vaso-dilator action of potassium. J Physiol. 1941;99:224–238. doi: 10.1113/jphysiol.1941.sp003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell WH. Further researches on the vasomotor nerves of ordinary muscles. J Physiol. 1878;1:262–302. doi: 10.1113/jphysiol.1878.sp000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski RJ, Klitzman B, Duling BR. Interrelations between contracting striated muscle and precapillary microvessels. Am J Physiol Heart Circ Physiol. 1978;235:H494–H504. doi: 10.1152/ajpheart.1978.235.5.H494. [DOI] [PubMed] [Google Scholar]
- Green S, Langberg H, Skovgaard D, Bulow J, Kjaer M. Interstitial and arterial–venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J Physiol. 2000;529:849–861. doi: 10.1111/j.1469-7793.2000.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol. 2004a;557:1013–1020. doi: 10.1113/jphysiol.2004.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS, Shoemaker JK. Is the blood flow response to a single contraction determined by work performed? J Appl Physiol. 2004b;96:2146–2152. doi: 10.1152/japplphysiol.00779.2003. [DOI] [PubMed] [Google Scholar]
- Hnik P, Holas M, Krekule I, Kriz N, Mejsnar J, Smiesko V, Ujec E, Vyskocil F. Work-induced potassium changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchange microelectrodes. Pflugers Arch. 1976;362:85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Reg Integ Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–874. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellmer I. The role of potassium ions in exercise hyperaemia. Med Exp. 1961;5:56–60. doi: 10.1159/000135055. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Zimmerman PA, Nelson MT. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard JH, Stekiel WJ. Responses of cremasteric arterioles of spontaneously hypertensive rats to changes in extracellular K+ concentration. Microcirculation. 1995;2:355–362. doi: 10.3109/10739689509148279. [DOI] [PubMed] [Google Scholar]
- Lott MEJ, Hogeman CS, Vickery L, Kunselman AR, Sinoway LI, MacLean DA. Effects of dynamic exercise on mean blood velocity and muscle interstitial metabolite responses in humans. Am J Physiol Heart Circ Physiol. 2001;281:H1734–H1741. doi: 10.1152/ajpheart.2001.281.4.H1734. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Tandon HC. Direct observations of muscle arterioles and venules following contraction of skeletal muscle fibres in the rat. J Physiol. 1984;350:447–459. doi: 10.1113/jphysiol.1984.sp015211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol. 2004;82:282–287. doi: 10.1139/y04-016. [DOI] [PubMed] [Google Scholar]
- Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol. 1974;227:531–535. doi: 10.1152/ajplegacy.1974.227.3.531. [DOI] [PubMed] [Google Scholar]
- Naik J, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol. 1999;87:1741–1746. doi: 10.1152/jappl.1999.87.5.1741. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol. 1984;56:287–295. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol. 1993;265:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Faraci FM. Knockout blow for channel identity crisis: vasodilation to potassium is mediated via Kir2.1. Circ Res. 2000;87:83–84. doi: 10.1161/01.res.87.2.83. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol. 1996;271:H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- Valic Z, Hamann JJ, DeLorey DS, Kluess HA, Buckwalter JB, Clifford PS. Is the blood flow response to contraction attributable to potassium? FASEB J. 2006;20:A1401. [Google Scholar]
- VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–H127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol Heart Circ Physiol. 1997;273:H156–H163. doi: 10.1152/ajpheart.1997.273.1.H156. [DOI] [PubMed] [Google Scholar]
- Wunsch SA, Muller-Delp J, Delp MD. Time course of vasodilatory responses in skeletal muscle arterioles: role in hyperemia at onset of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1715–H1723. doi: 10.1152/ajpheart.2000.279.4.H1715. [DOI] [PubMed] [Google Scholar]